Abstract
Purpose
The COVID‐19 pandemic gave rise to renewed concerns of the transmission risks posed by surgeries on sites of high viral colonization such as the nasopharynx. Endoscopic dacryocystorhinostomy (DCR) involves the creation of a new tear duct from the lacrimal sac to the nasal cavity. The purpose of this project is to determine if endoscopic DCR is an aerosol generating procedure (AGP).
Methods
An optical particle sizer (OPS) was used to intraoperatively quantify aerosol concentrations during four cases of endoscopic DCR. The OPS sampled the air once every 60 seconds throughout the operations. The time of important operative steps were documented and correlated with OPS readings. Particle concentrations during each major surgical step were compared to baseline readings by the Mann Whitney U Test.
Results
There were statistically significant increases in median particle concentrations during laryngeal mask airway intubations for both particles 0.3 to 5.0 μm and >5.0 μm (P < .001 and P = .023, respectively). Median particle concentrations during nasolacrimal duct probing, middle meatal debridement, drilling, balloon insertion, tube insertion, and Posisef insertion were not statistically different from baseline.
Conclusions
Endoscopic DCR in itself does not appear to be an AGP. It is, however, associated with other aerosol generating events such as laryngeal mask intubation, and thus requires appropriate personal protective equipment. Cautious interpretation of the results is encouraged given the limitations of OPS.
Level of Evidence
4.
Keywords: aerosol, dacryocystorhinostomy, transmission
An optical particle sizer quantified aerosols during key steps of endoscopic 42 dacryocystorhinostomy, showing increases in 0.3‐5.0 μm and >5.0 μm particles during 43 intubation, but no increases in particles associated with any of the surgical steps.
1. INTRODUCTION
The COVID‐19 pandemic has highlighted the risk to health care workers posed by surgical procedures that are potentially aerosol generating. Endoscopic dacryocystorhinostomy (DCR) corrects nasolacrimal duct obstruction and involves the use of a surgical drill to remove bone from the lacrimal sac fossa. 1 The SARS‐CoV‐2 virus is a highly virulent pathogen that colonizes the nasopharynx and has been a common point of concern for endoscopic nasal procedures. 2 , 3 Drilling within the nasopharynx in other procedures such as endoscopic sinus skull base surgery has been shown to generate high levels of aerosols, potentially putting those in the operating room at risk for infection. 4 Endoscopic DCR may pose a similar theoretical risk, but this conjecture has yet to be investigated.
In the context of the COVID‐19 pandemic, guidelines have been issued for both otolaryngologists and oculoplastic surgeons for aerosol generating procedures (AGPs). 2 , 5 As has been discussed in recent commentary, oculoplastic surgeons regularly probe and irrigate the nasolacrimal duct system. 5 There has been concern that probing could lead to aerosolization of viral particles from the nasal mucosa and a similar concern has been raised regarding drilling for osteotomies during orbital cases. There is still an open question of whether these procedures should be deferred during an active pandemic.
The purpose of this project is to determine whether or not endoscopic DCR is a high‐risk AGP. Particular attention is paid to aerosolization risk during intubation, probing of nasolacrimal system, and drilling at the osteotomy site. We utilized an optical particle sizer (OPS) intraoperatively to quantify the changes in aerosol concentrations during endoscopic DCR.
2. METHODS
This project was granted approval from the Weill Cornell institutional review board under protocol number 20‐08022558. All participants gave informed consent to participate in the study. We utilized an OPS [AeroTrak 9306, TSI Incorporated; Shoreview, Minnesota] to quantify aerosol concentrations during four separate endoscopic DCRs. OPS has been used to quantify aerosols during laryngoscopy procedures in the previous literature. 6 It takes in air through an isokinetic inlet at 2.84 L/min ± 5%; light scatter is then measured by an internal laser system to give an approximate count of air particles every 60 seconds. All procedures were carried out in a standard operating room.
Baseline measurements from the OPS were taken prior to the patient coming into the room. The isokinetic inlet of the OPS was placed 18 in. away from the nose of the patient, maintaining sterility. The OPS collected particle counts in one‐minute intervals for the duration of the surgery. Specific operative steps of interest included laryngeal mask intubation, probing of the nasolacrimal system, middle meatal debridement, surgical drilling, balloon insertion, tube insertion, and Posisef insertion. The times of these steps were documented for later correlation to measured particle counts. Probing of nasolacrimal duct was done using a lacrimal metal probe. Debridement of nasal mucosa off the underlying bone was carried out. Drilling through the lacrimal sac fossa was done using a low speed Medtronic drill at 12 000 rpm with suction attached for approximately 1 and 2 minutes. Rate of oscillation during microdebridement was 5 000 rpm with suction on medium setting at 60 to 105 mm Hg. Once an adequate osteotomy was created, a sickle blade was utilized to open the lacrimal sac widely into the nose. A 5 mm lacrimal balloon was placed through lacrimal sac and into the nose and inflated for 90 seconds in the osteotomy site at the middle meatus. Bicanalicular intubation was performed with Crawford tubes that were tied to themselves and allowed to retract into the nose. A 1 cm × 1 cm segment of Posisef was placed at the site of the osteotomy and injected with local anesthesia (lidocaine 1% with 1:100,000 epinephrine). Particles were stratified according to size, 0.3 to 5.0 μm and >5 μm for data analysis. The 5 μm cutoff was chosen due to the existing convention that particles less than this size are thought to represent aerosols and those larger than 5 μm are considered droplets; it is important to note that there is still existing controversy over these cutoffs. 6 , 7 For statistical analysis, we first utilized Kruskall‐Wallis testing to evaluate for differences in median particle counts between any intraoperative event including baseline. Where Kruskall‐Wallis was significant, we utilized Mann‐Whitney pairwise comparison between each event and baseline conditions. All statistical analysis was done using Microsoft Excel and Prism. All P‐values calculated were two‐tailed, evaluated at the .05 alpha level for significance.
3. RESULTS
There were statistically significant differences in median particle concentrations during the operations for both particles 0.3 to 5.0 μm and >5.0 μm (Figures 1 and 2). Results of pairwise multiple comparisons is presented in Table 1. A statistically significant increase in median particle concentration was observed specifically during laryngeal mask airway intubation for both particles 0.3 to 5.0 μm and >5.0 μm (P < .001 and P = .023, respectively). For particles 0.3 to 5.0 μm, there was an approximately 1.5‐fold increase, whereas particles >5.0 μm saw a 1.6‐fold increase during intubation. Median particle concentrations during nasolacrimal duct probing, middle meatal debridement, drilling, balloon insertion, tube insertion, and Posisef insertion were not significantly changed from baseline.
FIGURE 1.
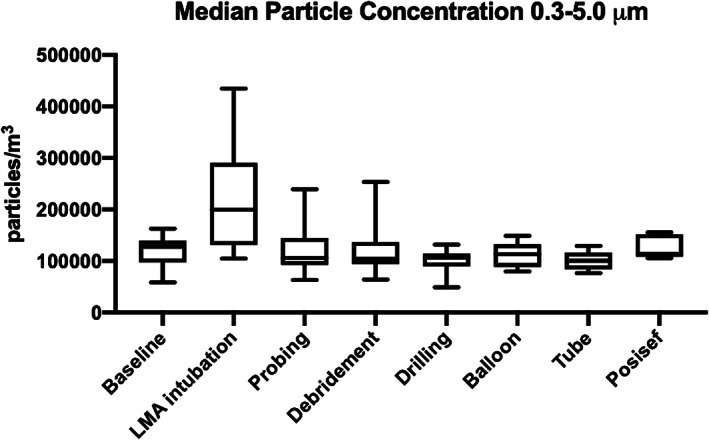
Concentrations of particles between 0.3 and 5.0 μm during endoscopic dacryocystorhinostomy (Kruskal‐Wallis test: P < .001)
FIGURE 2.
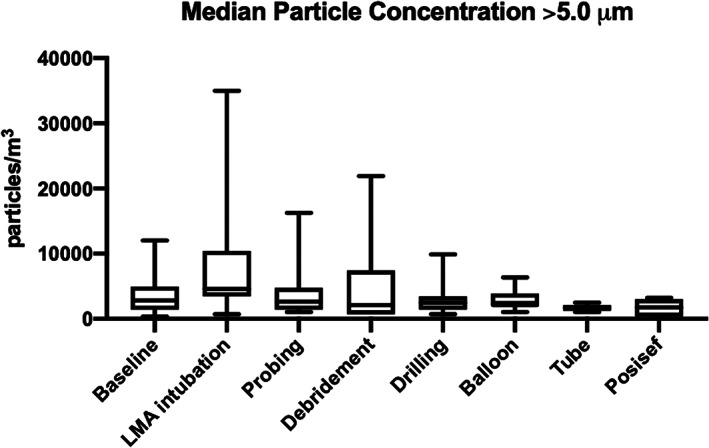
Concentrations of particles >5.0 μm during endoscopic dacryocystorhinostomy (Kruskal‐Wallis test: P = .030)
TABLE 1.
Particle concentrations stratified by size during endoscopic dacryocystorhinostomy. Concentrations during each step of interest compared to baseline by Mann‐Whitney U Test
| 0.3–5.0 μm | >5.0 μm | |||||
|---|---|---|---|---|---|---|
| N | Median concentration (particles/m3) | P‐value | N | Median concentration (particles/m3) | P‐value | |
| Baseline | 16 | 127 561.84 | 16 | 2826.86 | ||
| LMA intubation | 23 | 199 646.64 | <.001 | 23 | 4593.64 | .023 |
| Nasolacrimal duct probing | 8 | 106 007.07 | .522 | 8 | 2650.18 | .976 |
| Middle meatal debridement | 12 | 104 416.96 | .471 | 12 | 2120.14 | .834 |
| Drilling | 12 | 106 360.42 | .069 | 12 | 2473.50 | .596 |
| Balloon insertion | 7 | 113 074.20 | .401 | 7 | 2120.14 | .990 |
| Tube insertion | 5 | 100 706.71 | .147 | 5 | 1766.78 | .230 |
| Posisef insertion | 5 | 113 579.01 | .741 | 5 | 1766.78 | .162 |
Bold values indicate significance at the p < 0.05 level.
4. DISCUSSION
With the rising number of COVID‐19 cases, ophthalmologists are at continued risk for infection due to close contact with patients both in the outpatient setting and in operating room. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) can be transmissible through fomites, droplets, and aerosols. 8 AGPs are traditionally defined as events that lead to significantly more air particles than baseline risks such as breathing, talking, coughing, and sneezing. 9 AGPs have been shown to produce particles <10 μm in size, 10 and other surgical procedures with an endoscopic nasal approach can generate significant aerosols in the immediate surgical field. 11 , 12 Many of these studies in the past have utilized OPS technology due to its ease‐of‐use and accessibility. It is important to note, however, that OPS has limitations in its sensitivity, and there are more developed and complex methods of quantifying aerosols including microscopy, interferometric Mie imaging, aerodynamic particle sizing, and others that could complement OPS. 13
Our data suggest that endoscopic dacryocystorhinostomy in itself is not likely an aerosolizing generating procedure. Debridement, low‐speed drilling, tube insertion, balloon insertion, and Posisef insertion do not result in any significant increase in particulate compared to baseline to a level detectible by OPS. A particularly concerning aerosolization scenario for endoscopic DCR is laryngeal mask airway (LMA) intubation—a finding of our study and confirmed in previous literature. 14 Based on the existing literature, LMA placement has less risk of aerosolization though given the fact that there are less instances of coughing and there is a more complete airway seal. 15 , 16 Further benefits include faster insertion and maintaining a further distance by the anesthetist. It is difficult to say definitely though if differences in aerosolization would be generated with endotracheal tube (ETT) intubation without directly comparing LMA with ETT in a future study. This work builds on previous research using a similar methodology with an OPS for endoscopic endonasal skull base surgery which utilizes high‐speed drilling (75 000 rpm) and has been shown to generate up to a 37‐fold increase in aerosol particles under 5 μm. 17 Compared to such procedures, the low‐speed drilling used in endoscopic dacryocystorhinostomy is likely of lower risk and is comforting to know for surgeons. As described in the literature, further mitigation of any aerosol production during drilling can also be addressed with the use of additional suction or with the addition of a built‐in suction to the low speed drill as was done in our cases. 18 , 19 Surgical smoke evacuators have also been shown to be the most effective in reducing aerosols. 18 The results herein suggest that DCR is not aerosol generating; however, it is associated with an aerosol‐generating event in laryngeal mask intubation.
There are limitations to this study that should be addressed. As has been reported in the past literature, use of this specific OPS does not measure the aerodynamics, rates of settling, or material makeup of the particles. 11 The OPS only measures particles 0.5 to 10 μm in diameter, and there is the possibility of systematic error with repeat testing if calibration of the machine is not done in the same way. Under‐sampling of particulate generation is also unavoidable given the nature of the sampling method of the OPS—a limitation addressed by other authors as well. 6 , 20 , 21 The OPS also cannot detect presence of virus within generated particles and presence within droplets. Furthermore, recording of the data was done in an active operating room; even though the isokinetic inlet of the OPS was directed toward the patients and placed very close, there is still the possibility of background noise affecting the final counts.
Lastly, the definition of AGPs requires that the intervention lead to increased air particles compared to baseline risks including breathing, coughing, sneezing, and talking. 6 , 9 Our project does not specifically address the risk of DCR compared to all of these activities since patients were asleep during surgery, and this was considered the baseline. It is still unknown what magnitude of increase in aerosols would be necessary to lead to an increased clinical risk of infection and future work is needed to properly address this. Although endoscopic DCR was not detected to be strictly aerosol generating, very miniscule amounts of aerosols may be infective, even those not detected by OPS. Given that the minimum infective dose of SARs‐CoV‐2 is still unknown, results should be interpreted with caution. 22 , 23
5. CONCLUSION
The COVID‐19 pandemic has highlighted the risks of AGPs. We used an OPS in a small number of endoscopic dacryocystorhinostomies to evaluate for generation of aerosols; our results suggest that DCR does not appear to be an AGP but is associated with an aerosol generating event in laryngeal mask intubation. Surgeons should don standard personal protective equipment during these procedures and consider the use of an N95 if there is concern for virulent transmission.
FINANCIAL DISCLOSURES & CONFLICTS OF INTEREST
Provisional patent EFS ID 39386708, Application #63021722 submitted by Cornell University on May 8, 2020. The authors report no conflicts of interest.
Chen DA, Lee M, Lelli GJ, Kacker A. Evaluation of the aerosol generating potential of endoscopic dacryocystorhinostomy. Laryngoscope Investigative Otolaryngology. 2021;6(5):948‐951. doi: 10.1002/lio2.639
BIBLIOGRAPHY
- 1. Yakopson VS, Flanagan JC, Ahn D, Luo BP. Dacryocystorhinostomy: history, evolution and future directions. Saudi J Ophthalmol. 2011;25(1):37‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thamboo A, Lea J, Sommer DD, et al. Clinical evidence based review and recommendations of aerosol generating medical procedures in otolaryngology–head and neck surgery during the COVID‐19 pandemic. J Otolaryngol Head Neck Surg. 2020;49(1):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patel ZM, Fernandez‐Miranda J, Hwang PH, et al. Precautions for endoscopic transnasal skull base surgery during the COVID‐19 pandemic. Neurosurgery. 2020;87(1):E66‐E67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mick P, Murphy R. Aerosol‐generating otolaryngology procedures and the need for enhanced PPE during the COVID‐19 pandemic: a literature review. J Otolaryngol Head Neck Surg. 2020;49:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nguyen AX, Gervasio KA, Wu AY. COVID‐19 recommendations from ophthalmic and plastic reconstructive surgery societies worldwide. Ophthal Plast Reconstr Surg. 2020;36(4):334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rameau A, Lee M, Enver N, Sulica L. Is office laryngoscopy an aerosol‐generating procedure? Laryngoscope. 2020;130(11):2637‐2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davies A, Thomson G, Walker J, Bennett A. A review of the risks and disease transmission associated with aerosol generating medical procedures. J Infect Prev. 2009;10(4):122‐126. [Google Scholar]
- 8. Van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. N Engl J Med. 2020;382(16):1564‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Judson SD, Munster VJ. Nosocomial transmission of emerging viruses via aerosol‐generating medical procedures. Viruses. 2019;11:940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tran K, Cimon K, Severn M, Pessoa‐Silva CL, Conly J. Aerosol‐generating procedures and risk of transmission of acute respiratory infections: a systematic review. CADTH Technol Overv. 2013;3(1):E3101. [PMC free article] [PubMed] [Google Scholar]
- 11. Workman AD, Jafari A, Welling DB, et al. Airborne aerosol generation during endonasal procedures in the era of COVID‐19: risks and recommendations. Otolaryngol Neck Surg. 2020;163(3):465‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Workman AD, Welling DB, Carter BS, et al. Endonasal instrumentation and aerosolization risk in the era of COVID‐19: simulation, literature review, and proposed mitigation strategies. Int Forum Allergy Rhinol. 2020;10. Wiley Online Library:798‐805. [DOI] [PubMed] [Google Scholar]
- 13. Dudalski N. Experimental measurements of human cough airflows from healthy subjects and those infected with respiratory viruses. Published online 2018.
- 14. Xiao R, Workman AD, Puka E, Juang J, Naunheim MR, Song PC. Aerosolization during common ventilation scenarios. Otolaryngol Neck Surg. 2020;163(4):702‐704. [DOI] [PubMed] [Google Scholar]
- 15. Lim WY, Wong P. Supraglottic airways in the management of COVID‐19 patients. Anaesth Crit Care Pain Med. 2020;39(5):589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brimacombe J. The advantages of the LMA over the tracheal tube or facemask: a meta‐analysis. Can J Anaesth. 1995;42(11):1017‐1023. [DOI] [PubMed] [Google Scholar]
- 17. Snyderman CH, Gardner PA. Endonasal drilling may be employed safely in the COVID‐19 era. Int Forum Allergy Rhinol. Wiley‐Blackwell. 2020;10(9):1118‐1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sharma D, Ye MJ, Campiti VJ, et al. Mitigation of aerosols generated during rhinologic surgery: a pandemic‐era cadaveric simulation. Otolaryngol Neck Surg. 2021;164(2):433‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chari DA, Workman AD, Chen JX, et al. Aerosol dispersion during mastoidectomy and custom mitigation strategies for otologic surgery in the COVID‐19 era. Otolaryngol Neck Surg. 2021;164(1):67‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rivera‐Rosario H, Lee M, Kim M, et al. Flow disturbances reduce the effectiveness of a face‐mounted negative pressure antechamber for Endonasal surgery. In: APS Division of Fluid Dynamics Meeting Abstracts. 2020:H01.013.
- 21. Workman AD, Xiao R, Feng A, et al. Suction mitigation of airborne particulate generated during sinonasal drilling and cautery. Int Forum Allergy Rhinol. 2020;10. Wiley Online Library:1136‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yezli S, Otter JA. Minimum infective dose of the major human respiratory and enteric viruses transmitted through food and the environment. Food Environ Virol. 2011;3(1):1‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thompson K‐A, Pappachan JV, Bennett AM, et al. Influenza aerosols in UKhospitals during the H1N1 (2009) pandemic–the risk of aerosol generation during medical procedures. PloS One. 2013;8(2):e56278. [DOI] [PMC free article] [PubMed] [Google Scholar]


