Abstract
Objective
Botulinum toxin (BT) therapy is a first‐line treatment for spasmodic dysphonia (SD). However, a detailed chronological course and clinical factors that affect the therapeutic effect have been vague. In this study, we analyzed the data from our placebo‐controlled, randomized, double‐blinded parallel‐group comparison/open‐label clinical trial of BT (Botox) to clarify these.
Methods
A total of 22 patients with abductor SD (ADSD) were enrolled. The female‐to‐male ratio was 20:2 with a mean age of 40.0 ± 10.3 years and a median duration of symptoms of 7.5 years. The therapeutic effect was evaluated based on the change in the number of aberrant morae (phonemes), GRBAS scale, Voice Handicap Index (VHI), and Visual Analogue Scale (VAS).
Results
The change in the number of aberrant morae peaked at 2 weeks and lasted for 12 weeks in the BT group with significance (P < .01) compared to the placebo group. Objective improvement (number of aberrant morae and [S] element in GRBAS) preceded subjective improvement (VHI and VAS). The change in number of aberrant morae and VHI showed a significant correlation (P < .01). The changes in the number of aberrant morae, VHI, and VAS in younger subjects were greater than in older subjects. Patients who presented with post‐treatment breathy hoarseness or dysphagia showed better therapeutic effects.
Conclusions
BT therapy was effective for ADSD based on both objective and subjective assessments. Improvements in subjective parameters were delayed compared to objective measures due to post‐treatment breathy hoarseness. However, this adverse event was believed to reflect the treatment effect.
Level of Evidence
1b.
Keywords: aberrant mora, adductor spasmodic dysphonia, botulinum toxin, double‐blinded clinical trial, voice handicap index

Comparisons of post‐treatment change values between subjects with and without adverse events (AEs). Change values of number of aberrant morae, (S) in GRBAS, Voice Handicap Index and Visual analogue scale were greater in subjects with AEs than those without AEs.
1. INTRODUCTION
Spasmodic dysphonia (SD) is a rare form of focal dystonia that occurs in the absence of paralysis or other structural pathology in the phonatory organs. It is characterized by involuntary intermittent spasms of the intrinsic laryngeal muscles. 1 , 2 Depending on the muscles involved, SD is divided into adductor, abductor, and mixed types. Among them, 80‐95% are adductor SD (ADSD) followed by abductor SD (ABSD) and mixed SD. 3 , 4 , 5 In ADSD, intermittent glottal closure blocks expiratory airflow during phonation causing voice breaks, which makes the voice strained and strangled. SD may affect females more, with a 1.1:1 to 4.1:1 female: male ratio. 2 , 4 , 5 , 6 The mean age of onset ranges from 31 to 51 years. 2 , 5 , 6 , 7 Treatment modalities for ADSD aim to prevent vocal fold hyperadduction and include voice therapy, botulinum toxin (BT) injection into the thyroarytenoid muscle, 8 , 9 , 10 and surgical interventions that include thyroarytenoid myectomy, selective adductor denervation‐reinnervation surgery, and type 2 thyroplasty. 11 , 12 , 13 , 14 , 15
Among them, BT injection therapy is generally the treatment of choice for SD. 2 , 10 , 16 , 17 However, few well‐controlled clinical studies have been performed. 18 In addition, BT for SD treatment has not been approved in the USA, Europe, or Asian countries. 5 Therefore, we conducted a multi‐center placebo‐controlled, randomized, double‐blinded parallel‐group comparison/open‐label clinical trial of BT (Botox) therapy (the “BOtulinum toxin Injection therapy for Spasmodic dySphonia” [BOISS] study) in Japan. That study confirmed the effectiveness and safety of BT therapy for both ADSD and ABSD, 19 and BT therapy for SD was approved in Japan in 2018.
Although BT injection is an effective treatment modality, there remains an issue that a chronological course of post‐treatment voice change and clinical factors to affect the therapeutic effect have not been clarified by high‐quality clinical studies. Therefore, we further analyzed data from the BOISS study to increase our understanding of clinical pictures after BT therapy for ADSD by evaluating disease specific voice parameters.
2. MATERIALS AND METHODS
The BOISS study was performed as an investigator‐initiated clinical trial approved by the Pharmaceuticals and Medical Devices Agency (PMDA) of Japan under Good Clinical Practice (GCP) guidelines. It was conducted at eight Japanese institutes. In the present study, we analyzed data of ADSD subjects to explore the post‐treatment clinical course in detail.
2.1. Participants
The major inclusion criteria of ADSD patients were aged 12 years or older, duration of SD voice symptoms ≥6 months, and moderate to severe SD (≥12/25 in aberrant morae). The exclusion criteria were coexisting systemic neuromuscular disease excluding dystonia, vocal fold paralysis or symptomatic swallowing disorder, prior surgical treatment for SD, and having received voice therapy within the last 8 weeks or BT therapy within the last 24 weeks. Participants were randomly assigned to either the BT or placebo group at a 1:1 ratio using a computerized randomization method. The patients and physicians were both blinded until the key code was opened 16 weeks after the initial injections and data were fixed in all patients.
2.2. Study design
For the initial injections in ADSD patients, we followed a double‐blinded and randomized procedure, and up to two re‐injections of BT were allowed as an open‐label study. The drug and placebo were dissolved in saline and transcutaneously injected into the thyroarytenoid muscle through the cricothyroid membrane under electromyography guidance. The initial injection dose was 2.5 U and the first re‐injection dose was 1.0‐2.5 U depending on the initial response. These two injections were administered unilaterally to prevent possible severe adverse events (AEs) by BT. The second re‐injection dose was 1.0‐2.5 U unilaterally or bilaterally. The interval between injections was at least 12 weeks. After each injection, patients were followed‐up at 2 and 4 weeks, and every 4 weeks thereafter, during the entire 48‐week study period. At each visit, the number of aberrant morae, GRBAS scale, VHI, and VAS scores were collected.
2.3. Mora method
In Japanese, mora refers to a minimum rhythmic sound unit (phonemes) as represented by a single Japanese vowel or consonant‐vowel complex. 20 Japanese words are composed of morae, analogous to syllables in English. Phonatory disorder in SD is represented by aberrant mora production. According to a previous report by Kumada et al, 21 the patients were asked to read the following sentence aloud: “mu/ka/shi/a/ru/to/ko/ro/ni/ja/k/ku/to/i/u/o/to/ko/no/ko/ga/i/ma/shi/ta” (many years ago, there lived a boy named Jack). It contains 25 morae with many vowels and voiced consonants, which are likely disturbed in ADSD. Voices were recorded using a digital sound recorder (ICD‐UX543F, Sony, Japan) without any personal information. The voice data were sent to a central evaluation committee online and three phoniatric experts separately evaluated each mora as normal or aberrant and counted the numbers of aberrant morae. Median values of the three were used for analysis.
2.4. GRBAS scale
The GRBAS scale developed by the Japan Society of Logopedics and Phoniatrics 22 , 23 was used to objectively evaluate the characteristics of dysphonia. Grade (G) indicates the severity of hoarseness, roughness (R) for a rasping or rattling voice, breathiness (B) for a whispery voice, asthenia (A) for a weak voice, and strain (S) for an effortful or constricted voice. Each element of the GRBAS is scored as follows: 0 (normal), 1 (mild), 2 (moderate), or 3 (severe). Voice disorder in ADSD is characterized by a high score of the (S) element. In this study, each physician evaluated the GRBAS scale by listening to patient voices.
2.5. Voice Handicap Index
The VHI is a patient‐rated scale developed by Jacobson et al to rate the severity of disability caused by verbal communication impairment. 24 The questionnaire of VHI includes 30 items and a 5‐point scale is used for each item: 0 (never), 1 (almost never), 2 (sometimes), 3 (almost always), and 4 (always). Total scores range from 0 to 120; the more severe the subjective voice disorder, the higher the total score. We used the Japanese version of the VHI 25 with minor modifications.
2.6. Visual analogue scale
Participants subjectively assessed their dysphonia severity using a 100 mm VAS; higher scores indicated that phonation was more affected. The left and right anchor points corresponded to no dysphonia (0) and the worst dysphonia (100), respectively.
2.7. Safety evaluation
As safety evaluations, laboratory blood tests were examined and vital signs were recorded. Any undesirable or unexpected signs, symptoms, diseases, or accidents arising after injection throughout the study period were collected as AEs, based on thorough interviews by clinical research coordinators.
2.8. Statistical analysis
The outcomes included the changes in number of aberrant morae, GRBAS scale, VHI, and VAS at each evaluation timepoint. Differences in least‐squares mean values between groups were calculated with two‐sided 95% confidence intervals (CIs) and P‐values. Data were analyzed using the Wilcoxon sign rank test, and the Wilcoxon rank sum test was used to compare differences between the two groups. For all comparisons, a P‐value < .05 was considered significant.
2.9. ETHICS STATEMENT
The clinical trial was performed in accordance with GCP guidelines and the ethical principles of the Declaration of Helsinki. The study protocol and informed consent form were reviewed and approved by the Institutional Review Board of Kochi Medical School (ID: 1492501) and of other institutions. Written informed consent was obtained from each patient prior to randomization. The trial was registered with the Center for Clinical Trials of the Japan Medical Association (Registry ID: JMA‐IIA00176).
3. RESULTS
A total of 22 ADSD patients were enrolled and 11 were assigned to each of the BT and placebo groups. The ages, female‐to‐male ratios, and disease durations did not differ between the two groups (Table 1); neither did the number of aberrant morae and (S) scale, VHI, and VAS at enrollment.
TABLE 1.
Subject demographics
| BT (n = 11) | Placebo (n = 11) | P | |
|---|---|---|---|
| Age (y) | 38.5 ± 11.2 | 41.6 ± 10.0 | .429 |
| Female:male | 10:1 | 10:1 | 1.000 |
| Disease duration (y) | 10.5 ± 10.0 | 5.9 ± 3.4 | .575 |
| Number of aberrant morae | 19.2 ± 1.36 | 21.3 ± 1.86 | .263 |
| (S) in GRBAS | 2.1 ± 0.21 | 1.9 ± 0.34 | .366 |
| VHI | 78.5 ± 5.69 | 72.5 ± 5.01 | .093 |
| VAS | 71.9 ± 3.56 | 72.9 ± 5.45 | .843 |
Abbreviations: BT, botulinum toxin; VHI, Voice Handicap Index; VAS, visual analogue scale.
Changes in aberrant morae at 4 weeks after injection (primary endpoint) were −7.0 ± 2.30 and −0.2 ± 0.46 in the BT and placebo groups, respectively. The least mean‐squares difference (95% CI) between the two groups, of −6.5 (−11.6, −1.4), was statistically significant (P = .0148) (Figure 1). In the BT group (n = 11), dysphonic parameters after BT/placebo administration were chronologically compared to baseline (Table 2). Aberrant morae significantly decreased from the baseline for 2‐12 weeks post‐injection with a peak at 2 weeks. The (S) element in GRBAS also decreased after 2 to 8 weeks. VHI significantly improved from 4 to 12 weeks but was nonsignificant at 2 weeks. VAS showed post‐injection improvement; however, it was statistically nonsignificant. The placebo group did not show any apparent changes throughout the study period.
FIGURE 1.
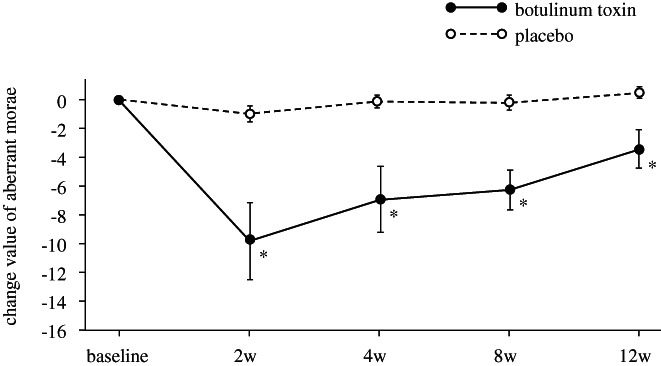
Changes in the number of aberrant morae in the ADSD patients. The number of aberrant morae significantly decreased from the baseline for 2‐12 weeks after botulinum toxin injection with a peak at 2 weeks. Placebo group showed nonsignificant change. *P < .05
TABLE 2.
Chronological change values of the dysphonic parameters
| (Mean ± SE) | |||||
|---|---|---|---|---|---|
| 2w | 4w | 8w | 12w | ||
| Aberrant morae | BT | −9.9 ± 2.66 b | −7.0 ± 2.30 a | −6.3 ± 1.90 a | −3.5 ± 1.42 a |
| Placebo | −1.1 ± 0.68 | −0.2 ± 0.46 | −0.3 ± 0.62 | 0.4 ± 0.43 | |
| (S) in GRBAS | BT | −1.18 ± 0.33 b | −0.91 ± 0.37 a | −0.73 ± 0.36 a | −0.36 ± 0.24 |
| Placebo | −0.18 ± 0.18 | −0.27 ± 0.36 | −0.00 ± 0.19 | −0.27 ± 0.30 | |
| VHI | BT | −14.6 ± 7.35 | −24.0 ± 9.63 a | −20.6 ± 9.91 a | −16.7 ± 7.59 a |
| Placebo | −9.8 ± 3.32 | −5.3 ± 3.43 | −8.0 ± 3.52 | −5.7 ± 4.90 | |
| VAS | BT | −11.6 ± 8.67 | −20.5 ± 8.74 | −18.6 ± 10.53 | −15.6 ± 8.68 |
| Placebo | −2.0 ± 4.09 | −6.2 ± 4.67 | −0.2 ± 4.70 | −3.2 ± 3.95 |
Abbreviations: BT, botulinum toxin; VHI, Voice Handicap Index; VAS, visual analogue scale.
<0.05 (vs baseline).
<0.01 (vs baseline).
All 22 subjects received at least one BT injection. The parameters significantly decreased (vs baseline) at 4 weeks after initial BT injection: number of aberrant morae, −8.7 ± 7.8 (P < .01); (S), −0.82 ± 1.03 (P < .01); VHI, −24.1 ± 26.7 (P < .01); and VAS, −21.9 ± 28.3 (P < .01). The decrease in number of aberrant morae and VHI showed significant correlations (P < .01, Spearman's rank correlation coefficient) (Figure 2).
FIGURE 2.
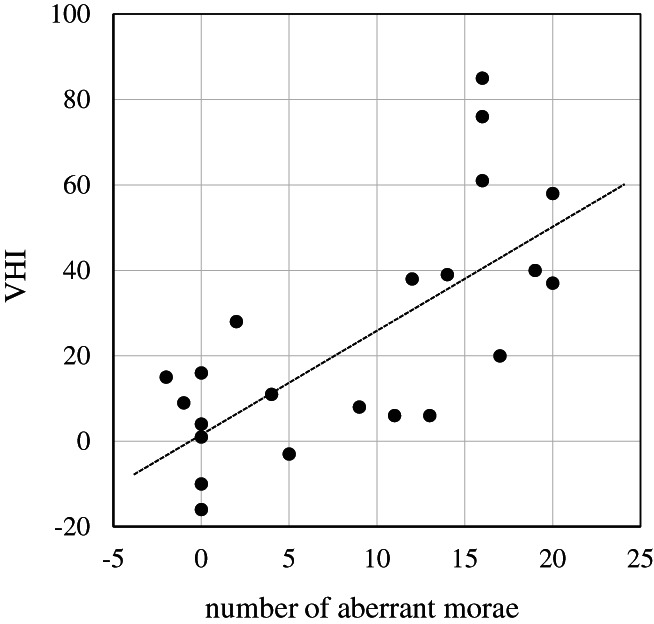
Correlation between the number of aberrant morae and VHI at 4 weeks after botulinum toxin injection. The decreases of number of aberrant morae and VHI showed significant correlation. (P < .01)
Subjects were divided into two groups according to their ages: younger (<40 years, n = 12) and older (≥40 years, n = 10) groups. Although there was no statistical significance, the changes in all parameters were greater in the younger group than in the older group (Table 3). Disease durations of the subjects ranged from 6 months to 30 years with a median of 7.5 years. Dividing the data into two groups based on disease duration, 11 subjects had durations of <8 years and another 11 had durations of ≥8 years. Although the changes in the parameters showed nonsignificant differences between the two groups, aberrant morae, VHI, and VAS tended to decrease more in those with durations of ≥8 years (Table 3).
TABLE 3.
Change values at 4 weeks by age and disease duration after BT injection
| (Mean ± SE) | ||||||
|---|---|---|---|---|---|---|
| n | Aberrant morae | (S) in GRBAS | VHI | VAS | ||
| Age (years) | <40 | 12 | −10.8 ± 2.07 | −1.00 ± 0.22 | −29.8 ± 8.16 | −27.9 ± 7.94 |
| ≥40 | 10 | −6.2 ± 2.44 | −0.60 ± 0.35 | −17.1 ± 7.21 | −14.7 ± 8.68 | |
| Disease Duration (years) | <8 | 11 | −6.5 ± 2.29 | −0.82 ± 0.32 | −16.8 ± 8.28 | −18.3 ± 8.30 |
| ≥8 | 11 | −10.9 ± 2.18 | −0.82 ± 0.30 | −31.3 ± 7.19 | −25.5 ± 8.61 |
Abbreviations: BT, botulinum toxin; VHI, Voice Handicap Index; VAS, visual analogue scale.
Nineteen subjects received at least two BT injections. We subsequently compared the changes in parameters at 4 weeks after the initial and second administrations of BT in them. The (S) element in GRBAS showed a significantly greater change after the second BT injection than after the initial injection (Table 4). Changes in aberrant morae number, VHI, and VAS did not show significant differences.
TABLE 4.
Change values at 4 weeks after initial and second BT injection
| (Mean ± SE) | |||||
|---|---|---|---|---|---|
| Aberrant morae | (S) in GRBAS | VHI | VAS | ||
| Initial | −8.5 ± 1.88 | −0.79 ± 0.24 | a | −25.7 ± 6.31 | −23.1 ± 6.62 |
| Second | −10.2 ± 2.01 | −1.37 ± 0.23 | −23.6 ± 5.75 | −28.0 ± 7.56 |
Abbreviations: BT, botulinum toxin; VHI, Voice Handicap Index; VAS, visual analogue scale.
<0.05.
In the BOISS study, three subjects (2 in the BT group and 1 in the placebo group) showed no relevant change in aberrant morae after any sessions of BT injection. We investigated these non‐responders to explore the cause of the negative response. Among them, the (S) element, VHI, and VAS showed a tendency to improve after BT injection (Table 5). In the protocol of the trial, the mora method was conducted using a two‐step evaluation of normal or abnormal; therefore, we reevaluated each mora of the three subjects using a three‐step (normal, mildly disturbed, severely disturbed) evaluation. The results showed a gradual increase in mildly disturbed morae and a decrease in severely disturbed morae as the number of injections increased, although the number of normal morae was unchanged.
TABLE 5.
Re‐evaluation of morae after BT injection in non‐responders
| Morae | ||||||
|---|---|---|---|---|---|---|
| Case ID | BT inj. | Normal/abnormal | Normal/mild/severe | (S) in GRBAS | VHI | VAS |
| 01‐01 (BT group) | Baseline | 1/24 | 1/0/24 | 3 | 95 | 100 |
| Initial inj. | 1/24 | 1/0/24 | 3 | 79 | 73 | |
| 1st re‐inj. | 1/24 | 1/6/18 | 1 | 62 | 50 | |
| 2nd re‐inj. | 1/24 | 1/16/8 | 1 | 78 | 75 | |
| 02‐03 (Placebo group) | Baseline | 1/24 | 1/0/24 | 2 | 53 | 64 |
| Initial inj. | 1/24 | 1/0/24 | 3 | 61 | 66 | |
| 1st re‐inj. | 1/24 | 1/5/19 | 1 | 40 | 43 | |
| 07‐02 (BT group) | Baseline | 3/22 | 1/2/22 | 2 | 82 | 47 |
| Initial inj. | 1/24 | 1/9/15 | 2 | 67 | 43 | |
| 1st re‐inj. | 2/23 | 2/14/9 | 2 | 63 | 39 | |
| 2nd re‐inj. | 1/24 | 1/19/5 | 2 | 65 | 41 | |
Abbreviations: BT, botulinum toxin; inj., injection.
After the initial BT injection, 12 subjects presented with breathy hoarseness and/or aspiration of drinking water as AEs. Ten subjects presented with neither symptom. The changes in the parameters (number of aberrant morae, [S], VHI, and VAS) at 4 weeks after injection were compared between the groups with/without AEs, and all were greater in the group with AEs (P < .01, P < .05, P < .05, and P < .05, respectively) (Wilcoxon's rank sum test) (Figure 3).
FIGURE 3.
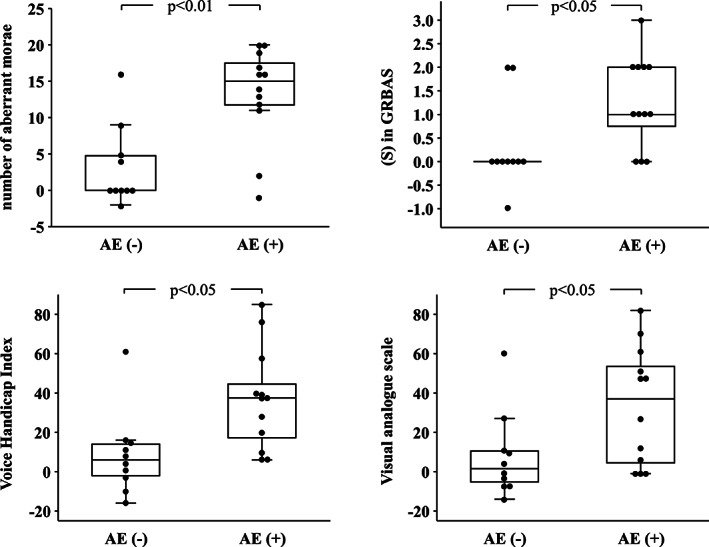
Comparisons of post‐treatment change values between subjects with and without adverse events (AEs). Change values of number of aberrant morae, (S) in GRBAS, VHI and VAS were greater in subjects with AEs than those without AEs
4. DISCUSSION
Laryngeal injection of BT for SD was first introduced in 1984 by Blitzer et al. 1 Since then, several studies have supported the effectiveness of this therapy. Blitzer et al reported a 91.2% response rate in a large series of 1300 patients. 3 Tisch et al treated 144 patients, of whom 81.9% showed excellent or very good outcomes. 6 However, most previous studies were case accumulation series and the quality of evidence was limited. Boutsen et al 26 analyzed 30 studies and reported that BT therapy led to a moderate improvement; however, patient cohorts, measurements, and treatment conditions varied. A systematic review 27 found only one study with high methodological quality on BT therapy for ADSD. In that study, Troung et al, 28 which was a prospective, randomized, double‐blinded clinical trial with blinded outcome assessments, the BT group showed a significant reduction in voice perturbation and fundamental frequency range. In addition, the spectrographic voice characteristics and speech scores improved. However, these parameters are not specific for SD, and subjective parameters were not included in their study. Therefore, we performed a placebo‐controlled, randomized, double‐blinded parallel‐group comparison/open‐label clinical trial for ADSD to create high‐quality evidence of the therapy. 19 It showed that BT therapy led to significant improvement in voices in terms of aberrant morae. In addition, the (S) component of the GRBAS, as well as VHI and VAS, improved. These results demonstrated that both objective and subjective parameters improved.
In this study, the aberrant morae number and (S) scale showed a peak of improvement at 2 weeks after BT therapy and the improvements lasted for around 12 weeks. These results indicate that objective improvement in the voice was achieved in early phase. However, VHI and VAS as subjective parameters showed peaks of improvement at 4 weeks. The time lag of the peak therapeutic effect between objective and subjective parameters may be explained by post‐treatment breathiness of the voice, which impairs verbal function. The breathy voices gradually disappeared within 4 weeks leading to further subjective improvements in VHI and VAS. Although there was a time lag, the decrease in aberrant morae and VHI showed a significant correlation at 4 weeks. We believe that a combination of objective and subjective assessments provides a detailed picture of post‐therapeutic clinical course after BT therapy.
The present study showed that improvement in objective and subjective parameters lasted for 12 weeks or longer. This is consistent with previous studies reporting a duration of therapeutic effect of 11 to 16 weeks. 6 , 9 , 17 , 29 On the other hand, the change in VAS was not significant, although it showed an improvement. This suggests that VHI is a better subjective indicator than VAS. Previous studies have also reported that VHI is commonly used to assess the treatment effect for SD. 30 , 31 , 32
In younger patients or those with longer disease duration, the therapeutic effect tended to be greater. This result is seemingly contradictory, as younger patients likely have a shorter disease duration. However, in subjects of this clinical trial, there was no significant correlation between age and disease duration. It is possible that younger patients have more plasticity of the disordered central nervous system network. Patients with longer disease duration may have stable or little progression of SD. This may explain why they tended to show better responses, although further study is required. The (S) element showed greater change after the second administration than after the initial administration. The number of aberrant morae, and VHI and VAS scores, after second injection tended to show better improvement. Namin et al 33 reported that BT dosing decreases as the treatment is repeated. This is suggestive of an increase in the therapeutic effect over time. Patients with SD usually have secondarily acquired strained phonation. Repeated BT administration may address this issue and lead to an increased therapeutic effect.
In the BOISS study, the primary endpoint was evaluated by aberrant mora, and three subjects did not respond to any of the administrations. The original mora method evaluated each phoneme using a two‐step assessment of normal or abnormal. Therefore, we reevaluated the voice data using a three‐step assessment. The results indicated a gradual shift of severely to mildly disturbed morae. It indicated that a partial response was achieved by BT therapy, even in these non‐responders. The improvements in (S), VHI, and VAS also support this partial response. Severely advanced SD or secondarily acquired hypertonic phonation may lead to incomplete restoration of disturbed morae.
Temporal breathy hoarseness and liquid aspiration are common AEs after BT therapy. Brin et al 9 reported that these AEs are mild and generally restored within 2 weeks and thus are not clinically problematic. In the present study, patients presenting with these AEs showed a significantly better therapeutic effect in terms of objective and subjective assessments. Previous studies have also reported that patients with post‐treatment hoarseness or aspiration show better or longer treatment effects. 6 , 17 , 34 These AEs are believed to reflect the therapeutic effect.
The major limitation of this study was the small number of subjects due to the strict inclusion criteria and rarity of the disease. In addition, the BOISS study was conducted as a multi‐center clinical trial and BT injection was performed by multiple investigators. This may lead to technical variance among injections. However, we believe that the high‐quality study design and thorough observation address these limitations. Also, disease specific objective evaluation in terms of aberrant morae and GRBAS provides a detailed understanding of clinical picture after BT injections in patients with ADSD.
5. CONCLUSION
We conducted further analysis of outcomes after BT injection therapy for ADSD. Significant voice improvement was achieved for around 12 weeks after treatment. Younger patients or those with longer disease durations tended to show better treatment effects. Due to post‐treatment breathy hoarseness, improvements in subjective parameters (VHI and VAS) followed improvements in objective parameters (aberrant mora number and [S] scale). However, patients who showed breathy hoarseness or liquid aspiration had greater treatment effects.
CONFLICT OF INTEREST
No author has any competing interest. Allergan supported the clinical trial by providing the investigational drug and placebo. GlaxoSmithKline K.K. provided pharmacological and safety information on Botox and scientific advice pertaining to the conduct of the study. However, no author or institution received any financial support from either company, nor were they involved in the data collection, analysis and interpretation, study management, or manuscript preparation.
ACKNOWLEDGMENT
This investigator‐initiated clinical trial was conducted with the grant from Center for Clinical Trial, Japan Medical Association (ID: CCT‐A‐2403, CCT‐B‐2504, CCT‐C‐2601).
Hirose K, Asano K, Sakaguchi M, et al. Post‐treatment clinical course following botulinum toxin injection therapy for adductor spasmodic dysphonia: Analysis of data from a placebo‐controlled, randomized, double‐blinded clinical trial in Japan. Laryngoscope Investigative Otolaryngology. 2021;6(5):1088‐1095. doi: 10.1002/lio2.669
Funding information Center for Clinical Trial, Japan Medical Association, Grant/Award Numbers: CCT‐A‐2403, CCT‐B‐2504, CCT‐C‐2601
BIBLIOGRAPHY
- 1. Blitzer A, Brin MF, Fahn S, Lovelace RE. Clinical and laboratory characteristics of laryngeal dystonia: Study of 110 cases. Laryngoscope. 1988;98(6 Pt 1):636‐640. [DOI] [PubMed] [Google Scholar]
- 2. Aronson AE, Bless DM. Spasmodic Dysphonia (Clinical Voice Disorders, 4th ed). New York: Thieme; 2009:101‐133. [Google Scholar]
- 3. Blitzer A. Spasmodic dystonia and botulinum toxin: experience from the largest treatment series. Eur J Neurol. 2010;17(suppl 1):28‐30. [DOI] [PubMed] [Google Scholar]
- 4. Patel AB, Bansberg SF, Adler CH, Lott DG, Crujido L. The Mayo Clinic Arizona spasmodic dysphonia experience: a demographic analysis of 718 patients. Ann Otol Rhinol Laryngol. 2015;124:859‐863. [DOI] [PubMed] [Google Scholar]
- 5. Hyodo M, Hisa Y, Nishizawa N, et al. The prevalence and clinical features of spasmodic dysphonia: a review of epidemiological surveys conducted in Japan. Auris Nasus Larynx. 2021;48:179‐184. [DOI] [PubMed] [Google Scholar]
- 6. Tisch SH, Brake HM, Law M, Cole IE, Darveniza P. Spasmodic dysphonia: clinical features and effects of botulinum toxin therapy in 169 patients – an Australian experience. J Clin Neurosci. 2003;10:434‐438. [DOI] [PubMed] [Google Scholar]
- 7. Creighton FX, Hapner E, Klein A, Rosen A, Jinnah HA, Johns MM. Diagnostic delays in spasmodic dysphonia: a call for clinician education. J Voice. 2015;29:592‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watts CR, Truong DD, Nye C. Evidence for the effectiveness of botulinum toxin for spasmodic dysphonia from high‐quality research designs. J Neural Transm. 2008;115:625‐630. [DOI] [PubMed] [Google Scholar]
- 9. Brin MF, Blitzer A, Stewart C. Laryngeal dystonia (spasmodic dysphonia): observations of 901 patients and treatment with botulinum toxin. Adv Neurol. 1998;78:237‐252. [PubMed] [Google Scholar]
- 10. Stachler RJ, Francis DO, Schwartz SR, et al. Clinical practice guideline: hoarseness (dysphonia) (update). Otolaryngol Head Neck Surg. 2018;158(1 suppl):S1‐S42. [DOI] [PubMed] [Google Scholar]
- 11. Koufman JA, Rees CJ, Halum SL, Blalock D. Treatment of adductor‐type spasmodic dysphonia by surgical myectomy: a preliminary report. Ann Otol Rhinol Laryngol. 2006;115:97‐102. [DOI] [PubMed] [Google Scholar]
- 12. Nakamura K, Muta H, Watanabe Y, Mochizuki R, Yoshida T, Suzuki M. Surgical treatment for adductor spasmodic dysphonia—efficacy of bilateral thyroarytenoid myectomy under microlaryngoscopy. Acta Otolaryngol. 2008;128:1348‐1353. [DOI] [PubMed] [Google Scholar]
- 13. Berke GS, Blackwell KE, Gerratt BR, Verneil A, Jackson KS, Sercarz JA. Selective laryngeal adductor denervation‐reinnervation: a new surgical treatment for adductor spasmodic dysphonia. Ann Otol Rhinol Laryngol. 1999;108:227‐231. [DOI] [PubMed] [Google Scholar]
- 14. Isshiki N, Haji T, Yamamoto Y, Mahieu HF. Thyroplasty for adductor spasmodic dysphonia: further experiences. Laryngoscope. 2001;111(4 Pt 1):615‐621. [DOI] [PubMed] [Google Scholar]
- 15. Sanuki T, Yumoto E. Long‐term evaluation of type 2 thyroplasty with titanium bridges for adductor spasmodic dysphonia. Otolaryngol Head Neck Surg. 2017;157:80‐84. [DOI] [PubMed] [Google Scholar]
- 16. Sulica L. Contemporary management of spasmodic dysphonia. Curr Opin Otolaryngol Head Neck Surg. 2004;12:543‐548. [DOI] [PubMed] [Google Scholar]
- 17. Novakovic D, Waters HH, D'Elia JB, Blitzer A. Botulinum toxin treatment of adductor spasmodic dysphonia: longitudinal functional outcomes. Laryngoscope. 2011;121:606‐612. [DOI] [PubMed] [Google Scholar]
- 18. Watts C, Whurr R, Nye C. Botulinum toxin injections for the treatment of spasmodic dysphonia. Cochrane Database Syst Rev. 2004;3:CD004327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hyodo M, Nagao A, Asano K, et al. Botulinum toxin injection into the intrinsic laryngeal muscles to treat spasmodic dysphonia: a multicenter, placebo‐controlled, randomized, double‐blinded, parallel‐group comparison/open‐label clinical trial. Eur J Neurol. 2021;28:1548‐1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Warner N, Arai T. Japanese mora‐timing: a review. Phonetica. 2001;58:1‐25. [DOI] [PubMed] [Google Scholar]
- 21. Kumada M, Kobayashi T, Kosaki H, Niimi S. ‘Mora method’ for objective evaluation of severity of spasmodic dysphonia. Jpn J Logop Phoniatr. 1997;38:176‐181. [Google Scholar]
- 22. Hirano M. Psycho‐Acoustic Evaluation of Voice. (Clinical Examination of Voice). New York: Springer‐Verlag; 1981:81‐84. [Google Scholar]
- 23. Yamaguchi H, Shrivastav R, Andrews ML, Niimi S. A comparison of voice quality ratings made by Japanese and American listeners using the GRBAS scale. Folia Phoniatr Logop. 2003;55:147‐157. [DOI] [PubMed] [Google Scholar]
- 24. Jacobson BH, Johnson A, Grywalski C, et al. The voice handicap index (VHI): development and validation. Am J Speech Lang Pathol. 1997;6:66‐70. [Google Scholar]
- 25. Taguchi A, Mise K, Nishikubo K, Hyodo M, Shiromoto O. Japanese version of voice handicap index for subjective evaluation of voice disorder. J Voice. 2012;26:668.e15‐9. [DOI] [PubMed] [Google Scholar]
- 26. Boutsen F, Cannito MP, Taylor M, Bender B. Botox treatment in adductor spasmodic dysphonia: a meta‐analysis. J Speech Lang Hear Res. 2002;45:469‐481. [DOI] [PubMed] [Google Scholar]
- 27. van Esch BF, Wegner I, Stegeman I, Grolman W. Effect of botulinum toxin and surgery among spasmodic dysphonia patients. Otolaryngol Head Neck Surg. 2017;156:238‐254. [DOI] [PubMed] [Google Scholar]
- 28. Troung D, Rontal M, Rolnick M, Aronson AE, Mistura K. Double blind controlled study of botulinum toxin in adductor spasmodic dysphonia. Laryngoscope. 1991;101:630‐634. [DOI] [PubMed] [Google Scholar]
- 29. Lundy DS, Lu FL, Casiano RR, Xue JW. The effect of patient factors on response outcomes to Botox treatment of spasmodic dysphonia. J Voice. 1998;12:460‐466. [DOI] [PubMed] [Google Scholar]
- 30. Morzaria S, Damrose EJ. A comparison of the VHI, VHI‐10, and V‐RQOL for measuring the effect of botox therapy in adductor spasmodic dysphonia. J Voice. 2012;26:378‐380. [DOI] [PubMed] [Google Scholar]
- 31. Rumbach A, Aiken P, Novakovic D. Outcome measurement in the treatment of spasmodic dysphonia: a systematic review of the literature. J Voice. 2019;33:810.e13‐810.e39. [DOI] [PubMed] [Google Scholar]
- 32. Faham M, Ahmadi A, Silverman E, Harouni GG, Dabirmoghaddam P. Patients with adductor spasmodic dysphonia; a systematic review and meta‐analysis. J Voice. 2021;35:271‐283. [DOI] [PubMed] [Google Scholar]
- 33. Namin AW, Christopher KM, Eisenbeis JF. Botulinum toxin dosing trends in spasmodic dysphonia over a 20‐year period. J Voice. 2017;31:107‐110. [DOI] [PubMed] [Google Scholar]
- 34. Blitzer A, Brin MF, Stewart C, Aviv JE, Fahn S. Abductor laryngeal dystonia: a series treated with botulinum toxin. Laryngoscope. 1992;102:163‐167. [DOI] [PubMed] [Google Scholar]


