Abstract
Background
We investigated the alarming trend of curable head and neck cancer (HNC) patients forgoing conventional treatment to pursue alternative medicine (AM).
Methods
A prospectively maintained database identified HNC patients with ≥12 weeks from diagnosis to treatment initiation between 2012 and 2017. Reasons for delay were categorized and clinical stages and outcomes of AM patients were assessed through chart review by December 2019.
Results
Among 1462 patients with primary HNC, 68 patients (4.7%) were confirmed to delay initiation of potentially curative treatment, and 19 of these patients (28%) delayed treatment to pursue AM. Eleven of 19 AM patients transitioned from curative intent to palliation while exploring AM. Continued treatment rejection was common and outcomes corresponded to patients' degree of treatment adherence.
Conclusions
AM caused treatment delay and poor outcomes in potentially curable HNC. Improved knowledge among physicians regarding AM and complementary approaches is urgently needed to improve patient counseling.
Level of Evidence
Level 2c outcomes research.
Keywords: alternative medicine, clinical outcomes, disease progression, head and neck cancer, treatment delay
1. INTRODUCTION
Rejecting a recommended treatment is a patient's unalienable right in healthcare. Many reasons exist as to why certain patients choose to modify, postpone, or refuse care; however, the increasing popularity of unconventional therapeutic approaches 1 , 2 is potentially contributing to this decision. 3 , 4 , 5 , 6 When used in conjunction with conventional medicine, these approaches are deemed “complementary medicine” (CM). When used in the place of evidence‐based care, however, those same approaches are now classified as “alternative medicine” (AM). 7 , 8 Of particular concern are those patients who rely solely on AM with the intention of treating their disease. This is especially true when a disease that is potentially curable with appropriate conventional treatment is at risk of progression to an incurable stage when conventional treatment is delayed in favor of alternative therapies. We investigated the observation made at our institution that an increasing number of head and neck cancer (HNC) patients in the curative setting are delaying or rejecting evidence‐based treatment in favor of pursuing AM. To our knowledge, the clinical outcomes of patients with curable HNC that opt to pursue AM have not been previously reported.
Several factors complicate the independent study of AM. 2 , 9 , 10 For a variety of reasons, 11 patient nondisclosure rates are reported at around 40%‐50%, 12 meaning it is difficult to retrospectively elucidate AM from other reasons for rejection or modification of recommended conventional treatment. Additionally, there is no consensus as to what qualifies as AM vs CM, the challenge being it is the context of use, rather than the approach itself, that distinguishes the two. For clarity in this paper, the term complementary and alternative medicine (CAM) will be used when no distinction was made with regards to concurrent or exclusive use of unconventional approaches. Quantifying the prevalence of CAM use among cancer patients is difficult and is reported to be somewhere between 11% and 95%, 12 , 13 , 14 and strategies for guidance has been specifically called for. 12 , 13 , 15 , 16
Outcomes in cancer patients forgoing the primary treatment for AM have been studied in cancer subsets outside of HNC. In those reports, it has been associated with disease progression, increased recurrence, shorter survival, and death. 17 , 18 , 19 , 20 , 21 In HNC, prior research states that HNC patients commonly use some form of CAM with the intention to treat their cancer or related impacts, such as side‐effects and well‐being. 22 , 23 , 24 , 25 While the survival impact of following nonstandard treatments in HNC has been described, 6 no study has looked directly at the consequences of AM use in HNC.
The aim of this study was to report a single institution's frequency and clinical outcomes of patients that significantly delay or reject the recommended conventional, potentially curative HNC treatment to pursue AM. A prospectively maintained database comprising all HNC cancer patients attending our institution was used to identify patients displaying no treatment start date or unexpectedly long intervals from first consult to the initiation of treatment. A retrospective chart review was completed to determine when that interval was attributed to AM pursuit and to document the clinical outcomes. The long‐term aim was to aid physicians in patient counseling by providing documentation from a population‐based setting of the potential consequences of treatment refusal or delay in favor of AM pursuit.
2. MATERIALS AND METHODS
2.1. Defining the study population of interest: Otobase and chart review
Our HNC database, Otobase, was used for patient identification. This database includes patient and tumor characteristics as well as treatments and clinical outcomes for the HNC patients treated at our tertiary cancer center (Foothills Medical Centre and Tom Baker Cancer Centre, Calgary, Alberta, Canada). It is prospectively maintained and includes all patients evaluated by our multidisciplinary HNC team. 26
The study population included all patients in Otobase aged 18 years or older that were offered initial treatment for any primary HNC except thyroid malignancies, basal cell carcinomas, ameloblastomas, and tumors without a histological diagnosis. The study population included patients evaluated between January 1, 2012, and December 31, 2017. The date of the first multidisciplinary consult was used as a proxy for date of diagnosis and related treatment recommendation to identify all patients with an unusually long interval from treatment recommendation to initiation, arbitrarily defined as 12 weeks or more. A retrospective chart review was performed on all such patients to identify the subset that may have rejected the recommended treatment for AM. The patients that initiated their first treatment within 12 weeks from their first consult were excluded. Any case that required further diagnostic workup before making a final treatment recommendation had the time interval recalculated from when the actual treatment recommendation was given to the start of the initial treatment. Of note, patients with no recorded treatment start date were also included in this patient selection.
The chart review was performed using the local electronic medical records. Following the identification of treatment delay, those patients recommended palliative treatment, that died prior to start of initial treatment, or that were referred to another institution for treatment were excluded. Thus, the remaining patients were those offered treatment of curative intent at our institution and who subsequently delayed the initiation of that treatment. These cases were defined as our study population of interest.
2.2. Identifying reasons for delayed curative treatment
During the chart review, detailed notes were taken on the reasons for delayed or absent start date of curative treatment. The common themes for delay or refusal that emerged during the review were used to create categories. The only predefined category was for those patients that explicitly stated an intention to pursue AM instead of consenting to conventional cancer treatments. AM was defined as all modalities outside of allopathic medicine used as a replacement for conventional Western medicine. Specific substances and regimens were listed and categorized according to the AM categories developed by the National Center for Complementary and Integrative Health, formerly the National Center for Complementary and Alternative Medicine, which have been commonly referenced. 10 , 27 , 28 , 29
Basic demographic and tumor characteristics (age, gender, diagnosis, and primary tumor site) were tabulated (as medians, ranges, and percentages as appropriate), for both the patients following a normal timeline (<12 weeks) and for the AM group.
2.3. AM patients: Characteristics, clinical course, and follow‐up
For the AM patients, the AM modality pursued, tumor histopathology, p16‐receptor status (positive, negative, not available), initial clinical stage (AJCC seventh edition), and initial treatment recommendation (surgery, chemotherapy, radiation therapy, and any combination thereof) were recorded. Records were also kept of other specifics that may have affected outcome, for example, the tonsillectomy for other reasons at which a patient was diagnosed with his or her current cancer. Following treatment refusal, adverse disease progression was recorded (yes/no). In the case of re‐presentation to pursue conventional cancer care, the time passed (months) and any discrepancies in the initial treatment recommendation, the revised treatment recommendation, and the final treatment consented by the patient were recorded. The TNM staging at representation, if available, was also documented. Patient vital status at the end of follow‐up by December 31, 2019 was registered. Vital status was assessed through charts as well as through the Alberta death registry, and listed as: no evidence of disease (NED), alive with disease (AWD) specified as local (L), regional (R), and/or distant (D) disease if possible; dead of disease (DOD), or dead of other causes. All calculated time intervals were rounded off to the previous full month and averages were presented as median and interquartile range (IQR) as appropriate.
2.4. Ethics approval
The study was approved by A pRoject Ethics Community Consensus Initiative (ARECCI) framework. 30 To ensure de‐identification ages are presented in 5‐year intervals only, and gender and specific AM use are presented on group level only.
3. RESULTS
Among the 1462 patients with primary HNC in Otobase that were initially evaluated and offered treatment at our institution, 1336 patients (91.4%) started treatment within 12 weeks (Figure 1). Of the 126 (8.6%) patients not receiving treatment within 12 weeks, 50 were offered palliative treatment or died before initiation of treatment. Eight patients were referred to other institutions for treatment. The remaining 68 patients (4.7%) were offered curative therapy but did not receive treatment within the identified 12 week period.
FIGURE 1.
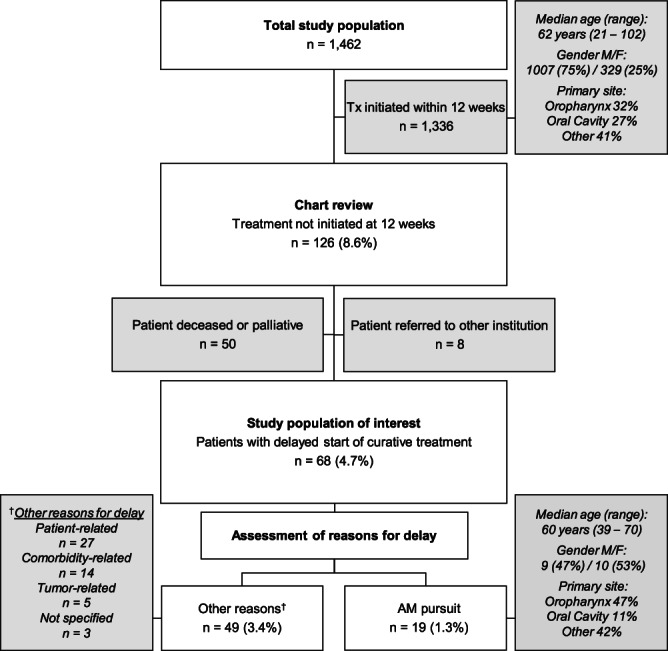
Flow chart of study population. The total study population included all adult patients with primary head and neck cancers offered treatment at our institution between January 2012 and December 2017 (n = 1462). A chart review was performed for the 126 patients that delayed their start of treatment for 12 weeks or more. Following exclusion of palliative patients, patients that died prior to start of treatment, and patients referred to other institutions, the remaining patients were considered the study population of interest (n = 68) since they were offered treatment of curative intent and delayed such treatment. The reasons for treatment delay, or no start of treatment, were explored and categorized. Nineteen patients pursued Alternative Medicine (AM), and 49 patients delayed treatment due to other reasons, as listed in the attached box to the left. To facilitate comparisons of the AM patients with the normal timeline (<12 weeks) patients, brief characteristics such as age, gender and the most common tumor primary sites are outlined in the attached boxes to the right. AM, alternative medicine; F, female; M, male; n, number; Tx, treatment
3.1. The reasons for delayed or rejected treatment
Five common themes emerged to explain treatment delay, the central one being pursuing AM (n = 19, 19/68 = 28%, or 1.3% of study total, Figure 1). Other reasons were categorized as patient‐related (such as logistic difficulties or family priorities: n = 27, 27/68 = 40%), comorbidity‐related (dementia, severe systemic illness: n = 14, 14/68 = 21%), and tumor‐related (low‐grade tumors, nonurgent medical management: n = 6, 6/68 = 9%). Finally, in three cases (3/68 = 4%), management was delayed without any clearly stated reason and was therefore termed “Not Specified.”
3.2. Characterizing the AM patients
Nineteen patients either delayed or rejected conventional curative treatment in favor of AM. When compared with the patients who started conventional treatment without delay, the AM patients were younger (median age 60 years [range 39‐70] vs 62 years [range 21‐102], Figure 1), more commonly female (53% vs 24%), and more commonly had oropharyngeal cancers (47% vs 32%).
In relation to AM pursuit, 15 of 19 patients (79%) reported to use more than one specific AM substance or regimen. Table 1 presents an overview of available AM categories, including their definitions and related examples, and how many patients used regimens from each AM category. Multimodal use was common. The most commonly pursued category was biologically based therapies (BBT; n = 16), followed by alternative medical systems (AMS; n = 13). Combining regimens from these two categories was common (n = 10). One patient concurrently used BBT, AMS, and mind‐body interventions (MBI). Further details, such as specific regimens and substances pursued by each individual, are presented on group level only (Appendix S1).
TABLE 1.
Definition of alternative medicine (AM) categories, related examples and AM categories pursued by the 19 study patients
| AM categories pursued | Definition | Examples a | Patient total (n) b |
|---|---|---|---|
| AMS | Whole medical systems are complete systems that include a defined philosophy and explanation of disease, diagnosis, and therapy |
Ayurveda Homeopathy Naturopathy Traditional Chinese medicine Alternative Medicine Clinics |
13 |
| BBT | Biologically based therapies use naturally occurring substances to affect health |
Botanical medicine Cannabis Chelation therapy Diet therapy Natural products and supplements |
16 |
| MBI | Mind‐body techniques are based on the theory that mental and emotional factors can influence physical health. Behavioral, psychologic, social, and spiritual methods are used |
Biofeedback Faith healing Guided imagery Hypnotherapy (hypnosis) Meditation Relaxation techniques including mindfulness |
1 |
| Manipulative and Body‐Based Methods (MBBM) | Manipulative and body‐based therapies treat various conditions through bodily manipulation |
Chiropractic Cupping Massage Moxibustion Osteopathic manipulation Reflexology Scraping (eg, coining, spooning) |
0 |
| Energy therapies (ET) | Energy therapies focus on the energy fields thought to exist in and around the body (biofields). These therapies also encompass the use of external energy sources (electromagnetic fields) to influence health and healing |
Acupuncture Magnets Qi gong and Tai chi Reiki Therapeutic touch |
0 |
| Not specified (NS) | 1 |
Note: The categories employed were developed by the former National Center for Complementary and Alternative Medicine, 28 , 29 with the addition of a Not Specified category. In addition to the presented examples of regimens, each AM category contains multiple regimens/substances, and their use may overlap between categories. The complete list of specific substances and regimens pursued by the study patients is found in Appendix S1.
Abbreviation: AM, alternative medicine.
The presented examples were selected to give an overview of each category. Italic letters indicate a regimen not pursued by any of the patients in the study.
Patients may have pursued treatments from more than one category, as specified as follows: Use of AM from one category; AMS: n = 2 (Patient 12, 18); BBT: n = 5 (Patient 10, 11, 15, 16, 19); NS: n = 1 (Patient 1). Concurrent use of AM from two categories; AMS and BBT: n = 10 (Patient 2, 4, 5, 6, 7, 8, 9, 13, 14, 17). Concurrent use of AM from three categories; AMS, BBT, and MBI: n = 1 (Patient 3).
3.3. Clinical outcomes for the AM pursuit patients
The characteristics, clinical course, and outcomes of the 19 AM pursuit patients are outlined in Table 2. At initial presentation, 4 of 19 patients had stage II disease. Among the 15 patients with stage IVa disease, 7 patients had confirmed p16 + oropharyngeal cancers. Patients returned to the medical system on average 10 months later (median interval time, IQR 5‐17), at which time tumor progression had occurred in 15 patients (79%). At re‐presentation, only eight patients (42%) remained candidates for curative intent treatment. Only two patients fully followed the curative intent treatment recommendations at re‐presentation, and these two patients remained disease‐free at 5 years and at 2 years follow‐up, respectively (patient 3 and patient 15, Table 2). The proportion of patients that either partially or fully consented to the recommended treatments at re‐presentation was similar in both curative and palliative setting. All five patients who partially accepted treatment consented to radiotherapy but rejected either chemotherapy (n = 4) or surgery (n = 1). Further assessments at end of follow‐up revealed that 11/19 patients (58%) were dead of disease and six patients (32%) had evidence of persistent disease. Median follow‐up time was 23 months (IQR 14‐33).
TABLE 2.
Case summaries of AM pursuit patients
| Patient N° age range | Tumor primary site | Histopathology | TNM stage | Initial recommended treatment | Time interval | Adverse progression during interval | Final Recommended Treatment | Status at end of F/u | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N° | (5 years) | (mo) | Yes/No | TNM Stage | Time F/u (mo) | Status | ||||||
| 1 | 60 | Oropharynx | p16 + SCC a | T1N2cM0 | Stage IVa | Chemo + Rt | 5 | No | n/a | Chemo + Rt b | 27 | AWD (LR) |
| 2 | 45 | Oropharynx | Cribriform Adenocarcinoma | T4aN1M0 | Stage IVa | Surgery + Rt | 17 | No | T4aN1M0 | Surgery + Rt | 40 | AWD |
| 3 | 65 | Oropharynx | p16 + SCC | T2N2bM0 | Stage IVa | Chemo + Rt | 7 | Yes | T4aN2cM0 | Chemo + Rt c | 60 | NED |
| 4 | 70 | Oropharynx | p16 + SCC | T2N2bM0 | Stage IVa | Chemo + Rt | 15 | Yes | T4N3bM0 | Palliative Rt c | 16 | DOD |
| 5 | 60 | Oropharynx | p16 + SCC | T2N2bM0 | Stage IVa | Chemo + Rt | 10 | Yes | T4bN2cM1 | Palliative Chemo + Rt b | 22 | DOD |
| 6 | 60 | Oropharynx | p16 + SCC | T4aN2cM0 | Stage IVa | Chemo + Rt | 16 | Yes | T4aN2cM1 | Palliative Chemo + Rt b , d | 78 | AWD (LRD) |
| 7 | 65 | Oropharynx | p16 + SCC | T2N2cM0 | Stage Iva | Chemo + Rt | 17 | Yes | T4aN3M1 | Palliative Chemo | 23 | DOD |
| 8 | 70 | Oropharynx | p16 + SCC a | T1N2bM0 | Stage IVa | Chemo + Rt | 17 | Yes | TXN2bM1 | Palliative Rt | 21 | DOD |
| 9 | 55 | Oropharynx | SCC | TXN2aM0 | Stage IVa | Chemo + Rt | 20 | Yes | T2N3M0 | Palliative Rt | 33 | DOD |
| 10 | 70 | Unknown | p16 + SCC | TXN2bM0 | Stage IVa | Chemo + Rt | 9 | No | TXN2bM0 | Chemo + Rt | 28 | AWD |
| 11 | 60 | Unknown | p16 − SCC | TXN2cM0 | Stage IVa | Chemo + Rt | 4 | Yes | n/a | Palliative Rt b | 6 | DOD |
| 12 | 60 | Larynx | SCC | T2bN2aM0 | Stage IVa | Chemo + Rt | 17 | No | T2bN2aM0 | Chemo + Rt | 33 | DOD |
| 13 | 60 | Larynx | SCC | T4aN0M0 | Stage IVa | Surgery + Rt | 3 | Yes | T4aN2cM0 | Palliative Rt b | 10 | DOD |
| 14 | 65 | Larynx | SCC | T4aN0M0 | Stage IVa | Surgery + Rt | 13 | Yes | T4N2cM0 | Palliative Rt | 14 | DOD |
| 15 | 50 | Oral cavity | SCC | T2N0M0 | Stage II | Surgery + Rt | 3 | Yes | T4aN0M0 | Surgery c | 24 | NED |
| 16 | 60 | Oral cavity | SCC | T2N0M0 | Stage II | Surgery + Rt | 10 | Yes | n/a | n/a e | 10 | DOD |
| 17 | 40 | Nasopharynx | Undifferentiated carcinoma | T1N1M0 | Stage IIb | Chemo + Rt | 5 | Yes | T1N1M0 | Chemo + Rt b | 18 | AWD (LR) |
| 18 | 50 | Nasopharynx | Undifferentiated carcinoma | T1N1M0 | Stage IIb | Chemo + Rt | 7 | Yes | TXN2bM1 | Palliative Chemo + Rt | 8 | DOD |
| 19 | 75 | Paranasal sinus | Adenoid cystic carcinoma | T4aN0M0 | Stage IVa | Surgery + Rt | 67 | Yes | n/a | Surgery + Rt b | 75 | AWD (L) |
Note: Each patient's clinical course is presented as a simplified clinical timeline. Tumor characteristics at diagnosis are presented for each case, followed by the treatment modality that was initially recommended, and rejected in pursuit of AM. Thereafter, the time interval until return to conventional care is listed (months), together with any adverse disease progression (yes/no), new TNM stage when available, and final treatment recommendation and whether consented or not. Outcomes are presented as vital status at end of follow up, December 31, 2019. Order of appearance, patient 1 through 19: Patients are presented by primary tumor site. Within each such group, patients with no adverse disease progression are presented prior to patients with adverse progression. Patients recommended curative treatments are listed prior to those recommended palliative treatments. Finally, any fully consented treatments are listed prior to any partially consented treatments, or no consented treatment.
Abbreviations: AM, alternative medicine; AWD, alive with disease; chemo, chemotherapy; D, distant; DOD, dead of disease; F/u, follow‐up; L, local; mo, months; n/a, not available; NED, no evidence of disease; R, regional; Rt, radiation therapy; SCC, squamous cell carcinoma; TNM, tumor node metastasis stage.
Diagnosis from prior surgery, such as tonsillectomy for other reasons.
Treatment partially consented. Partially consented treatments were radiotherapy in all patients.
Treatment fully consented. No symbol Recommended treatment not consented.
Prior off‐label use of low dose capecitabine.
Admitted for palliation prior to death.
4. DISCUSSION
In our study, more than one in four potentially curable HNC patients with delayed treatment initiation pursued AM. While AM patients typically returned to conventional care over time, their transition to palliation commonly took place while exploring AM. At re‐presentation, partial or total treatment rejection rates were still high. Clinical outcomes were poor compared to projected outcomes at diagnosis, and corresponded to the degree of treatment adherence.
Patients with oropharyngeal cancers were surprisingly common. In addition to the nine confirmed cases, two unknown primaries were most likely also of oropharyngeal origin, totaling 11 of 19 patients (58%), the majority of which had T1 or T2 p16 + stage IVa disease. Such human papillomavirus‐related cancers have a relatively good prognosis compared to their formal cancer stage in the AJCC seventh edition, 31 , 32 with three‐year survival rates of almost 90%. 33 Herein, however, already at 16 months of median follow‐up for oropharyngeal AM patients, only four out of nine (45%) of these patients were still alive. Similar depressing results were seen for other tumor sites.
Irrespective of primary tumor site, continued treatment rejection at return to care was common. Of the eight patients offered curative intent therapy at re‐presentation, only two accepted the suggested treatment. Those same two patients were those who remained alive with NED at the end of follow‐up. Thus, the patients with the most favorable outcomes were those who fully complied with the physician's treatment recommendations upon re‐presentation. Those who fared the worst were those who refused treatment entirely in favor of AM, a consequence which has previously been reported in other cancer settings. 17 , 19 , 21
While only 1.3% of our total head and neck cancer population chose AM, why these patients eschew proven treatment paradigms in search of experimental or poorly studied options to treat their disease remains an important question. In the vast majority of previous studies, prior justification for CAM use among cancer patients has mainly been to modulate end‐stage cancer, such as measures to reduce side effects or to improve quality of life. 24 , 34 , 35 , 36 Herein, however, the AM use has encroached on a new subset of potentially curable HNC patients. Over an average of 10 months, 11 of our 19 patients transitioned from curative intent to palliation while they were exploring or receiving AM. This is alarming, but not unexpected, as a strong relationship between time to treatment initiation and mortality in early HNC has previously been established. 37
The sample size of the present study permitted mainly descriptive findings, which was a weakness of the study. Also, the methodology did not mitigate the common knowledge of high non‐disclosure rates in relation to AM pursuit. 11 , 12 , 15 Thus, while our results are already alarming and need to be taken seriously, the true figure of AM pursuit in the curative HNC setting is likely greater than presented. The relatively long follow‐up and the use of a prospectively maintained database strengthened the study. The study can also be considered population‐based, since patients of this region are rarely referred outside of our institution for treatment. Patients who followed the normal timeline were similar in terms of characteristics to a general HNC population. Patient characteristics among AM patients, such as these patients more commonly being younger and female, were in line with earlier CAM studies. 14 , 21 , 23 , 24 , 25 , 36 , 38 The study findings may also be generalizable to other North American settings, with the reservation that CAM use, albeit common, varies between countries. 1 , 2
The lack of consensus on proper CM and AM definitions renders categorization prone to overlap and/or insufficiency. The categories used in this study are general, a problem commonly described in previous literature. 2 , 9 , 10 Regardless, these definitions have been previously successfully employed 10 , 14 , 39 , 40 and only one patient was unable to be categorized. To protect confidentiality, AM regimens were purposefully categorized rather than listed with individual patients. In line with previous CAM studies, multimodal use of AM was common. The most common category pursued was BBT, due to the exceedingly common reports of ingesting various vitamin, herbal, or cannabis supplements, oftentimes concurrently and in combination with one another. Previous studies on CAM use also report herbal and other oral supplements, components of BBT, as the most common choice. 15 , 18 , 25 AMS pursuit was also very common in the present study, whereas the remaining AM categories were rarely or not at all pursued. This difference between categories may be a true difference or related to higher rates of nondisclosure for the latter categories, which would be in line with a previous report on CAM disclosure among breast cancer patients that reported highest disclosure rates for AMS followed by BBT. 41 However, larger studies that distinctly separate CM and AM are needed to further quantify the issue and investigate formal outcome comparisons.
In HNC, the primary tumor site relates to the type and severity of patient symptoms. We hypothesize that less severe symptomatology, as is common with oropharyngeal malignancies, is a predictor of AM pursuit. Patients experiencing acute symptoms, such as pain, may be more likely to accept timely and proven approaches than those asymptomatic patients, where treatment acceptance carries its own symptoms and side effects. We also suggest that the curable status itself may impart a false sense of security for the patient and reinforce their motivation to continue using AM in the curative setting, until clinical progression of disease or presentation of symptoms.
Finally, all living patients returned to conventional care providers during follow‐up. The patient that rejects the recommended treatment is also very likely to return to care; patient's re‐presentation should be both expected and encouraged by the care team. Even though continued treatment rejection at re‐presentation was common, this does present an additional opportunity to further counsel patients on their AM use. All consultations should factor in an opportunity for these subjects to be discussed, especially in cases where the patient appears reluctant to accept treatment. Increased transparency from both physicians and patients regarding AM use is required, as fear of disapproval and lack of knowledge on behalf of the doctor have been reported as explanations for nondisclosure. 11 , 12 , 42 Contact nurses should be encouraged to seek permission early‐on for regular follow‐up visits from these patients. Consultations should include counseling on the credibility of certain sources of AM information, and suggestions of more reliable resources instead. 35 , 43 Nonjudgemental, objective discussion about the consequences of AM and cancer care is warranted, as well as the beneficial, neutral, and harmful effects of CM. 4 This communication has been reported as beneficial for the patient‐doctor relationship 12 , 13 and a deterrent from engaging in dangerous or unproven therapies. 16 , 36
In conclusion, AM was a significant contributor to treatment delay in this potentially curable HNC population. Patients who pursued AM instead of evidence‐based treatment fared much worse than their projected outcome at initial presentation, and often transitioned into the palliative setting while pursuing AM. Our study highlights the importance of maintaining contact with the patient to monitor progression and facilitate follow‐up appointments, which will benefit patient retention. Increased knowledge on AM and CM on behalf of the physician, and an honest and empathetic discussion during initial consultation may provide the proactive counseling needed to prevent total treatment rejection. The clinical course of these 19 AM patients may be considered representative for a single institution's six‐year experience, and the presented results may thereby be used to support informed counseling.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest.
Supporting information
Appendix S1. Specific substances and regimens pursued. The specific substances and regimens pursued by the 19 patients in the present study are presented by NCCAM category, 28 , 29 and in alphabetical order by administration type. Abbreviations: AM alternative medicine, Alpha‐GPC alpha‐glycerylphosphorylcholine, CBD cannabidiol, THC tetrahydrocannabinol, EPA/DHA eicosapentaenoic acid/docosahexaenoic acid
ACKNOWLEDGMENT
Assistance provided by Steven Nakoneshny of the Ohlson Research Initiative in data management and the ethics approval process was greatly appreciated.
Balogh LC, Matthews TW, Schrag C, Elebro KA. Clinical outcomes of head and neck cancer patients who refuse curative therapy in pursuit of alternative medicine. Laryngoscope Investigative Otolaryngology. 2021;6(5):991‐998. doi: 10.1002/lio2.636
Funding information Medicinska Fakulteten, Lunds Universitet
BIBLIOGRAPHY
- 1. World Health Organization . WHO global report on traditional and complementary medicine. 2019.
- 2. Horneber M, Bueschel G, Dennert G, Less D, Ritter E, Zwahlen M. How many cancer patients use complementary and alternative medicine: a systematic review and metaanalysis. Integr Cancer Ther. 2012;11(3):187‐203. [DOI] [PubMed] [Google Scholar]
- 3. Chotipanich A, Sooksrisawat C, Jittiworapan B. Association between complementary and alternative medicine use and prolonged time to conventional treatment among Thai cancer patients in a tertiary‐care hospital. Peer J. 2019;7:e7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnson SB, Park HS, Gross CP, Yu JB. Complementary medicine, refusal of conventional cancer therapy, and survival among patients with curable cancers. JAMA Oncol. 2018;4(10):1375‐1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davis GE, Bryson CL, Yueh B, McDonell MB, Micek MA, Fihn SD. Treatment delay associated with alternative medicine use among veterans with head and neck cancer. Head Neck. 2006;28(10):926‐931. [DOI] [PubMed] [Google Scholar]
- 6. Dronkers EA, Mes SW, Wieringa MH, van der Schroeff MP, Baatenburg de Jong RJ. Noncompliance to guidelines in head and neck cancer treatment; associated factors for both patient and physician. BMC Cancer. 2015;15:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. National Center for Complementary and Integrative Health . Complementary, alternative, or integrative health: what's in a name? https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name. Accessed April 5, 2021.
- 8. Cancer Council Australia . Cancer Information complementary therapies. https://www.cancer.org.au/cancer-information/treatment/complementary-therapies. Accessed April 5, 2021.
- 9. Adams M, Jewell AP. The use of complementary and alternative medicine by cancer patients. Int Semin Surg Oncol. 2007;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wieland LS, Manheimer E, Berman BM. Development and classification of an operational definition of complementary and alternative medicine for the Cochrane collaboration. Altern Ther Health Med. 2011;17(2):50‐59. [PMC free article] [PubMed] [Google Scholar]
- 11. Foley H, Steel A, Cramer H, Wardle J, Adams J. Disclosure of complementary medicine use to medical providers: a systematic review and meta‐analysis. Sci Rep. 2019;9(1):1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davis EL, Oh B, Butow PN, Mullan BA, Clarke S. Cancer patient disclosure and patient‐doctor communication of complementary and alternative medicine use: a systematic review. Oncologist. 2012;17(11):1475‐1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alsharif F. Discovering the use of complementary and alternative medicine in oncology patients: a systematic literature review. Evid Based Compl Alternat Med. 2021;2021:6619243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Judson PL, Abdallah R, Xiong Y, Ebbert J, Lancaster JM. Complementary and alternative medicine use in individuals presenting for care at a comprehensive cancer center. Integr Cancer Ther. 2017;16(1):96‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shakeel M, Newton JR, Bruce J, Ah‐See KW. Use of complementary and alternative medicine by patients attending a head and neck oncology clinic. J Laryngol Otol. 2008;122(12):1360‐1364. [DOI] [PubMed] [Google Scholar]
- 16. Latte‐Naor S. Managing patient expectations: integrative, not alternative. Cancer J. 2019;25(5):307‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Joseph K, Vrouwe S, Kamruzzaman A, et al. Outcome analysis of breast cancer patients who declined evidence‐based treatment. World J Surg Oncol. 2012;10:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Han E, Johnson N, DelaMelena T, Glissmeyer M, Steinbock K. Alternative therapy used as primary treatment for breast cancer negatively impacts outcomes. Ann Surg Oncol. 2011;18(4):912‐916. [DOI] [PubMed] [Google Scholar]
- 19. Chang EY, Glissmeyer M, Tonnes S, Hudson T, Johnson N. Outcomes of breast cancer in patients who use alternative therapies as primary treatment. Am J Surg. 2006;192(4):471‐473. [DOI] [PubMed] [Google Scholar]
- 20. Risberg T, Vickers A, Bremnes RM, Wist EA, Kaasa S, Cassileth BR. Does use of alternative medicine predict survival from cancer? Eur J Cancer. 2003;39(3):372‐377. [DOI] [PubMed] [Google Scholar]
- 21. Johnson SB, Park HS, Gross CP, Yu JB. Use of alternative medicine for cancer and its impact on survival. J Natl Cancer Inst. 2018;110(1):121‐124. [DOI] [PubMed] [Google Scholar]
- 22. Vyas T, Hart RD, Trites JR, et al. Complementary and alternative medicine use in patients presenting to a head and neck oncology clinic. Head Neck. 2010;32(6):793‐799. [DOI] [PubMed] [Google Scholar]
- 23. Lim CM, Ng A, Loh KS. Use of complementary and alternative medicine in head and neck cancer patients. J Laryngol Otol. 2010;124(5):529‐532. [DOI] [PubMed] [Google Scholar]
- 24. Amin M, Glynn F, Rowley S, et al. Complementary medicine use in patients with head and neck cancer in Ireland. Eur Arch Otorhinolaryngol. 2010;267(8):1291‐1297. [DOI] [PubMed] [Google Scholar]
- 25. Warrick PD, Irish JC, Morningstar M, Gilbert R, Brown D, Gullane P. Use of alternative medicine among patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 1999;125(5):573‐579. [DOI] [PubMed] [Google Scholar]
- 26. The Otobase™ clinical outcomes database , Ohlson research initiative, University of Calgary. https://cumming.ucalgary.ca/research/ohlson/home. Accessed April 5, 2021.
- 27. National Center for Complementary and Integrative Health (NCCIH) . https://www.nccih.nih.gov. Accessed April 5, 2021.
- 28. National Center for Complementary and Alternative Medicine (NCCAM) . Expanding horizons of health care. Strategic plan 2005‐2009. https://files.nccih.nih.gov/s3fspublic/about/plans/2005/strategicplan.pdf. Accessed April 5, 2021.
- 29. Millstine D. Types of complementary and alternative medicine. Merck Manual (Professional version). https://www.merckmanuals.com/en‐ca/professional/special‐subjects/integrative‐complementary‐and‐alternative‐medicine/types‐of‐complementary‐and‐alternative‐medicine. Accessed June 21, 2021.
- 30. Alberta Innovates (2017). ARECCI ethics guideline tool. albertainnovates.ca/wp‐content/uploads/2017/11/ARECCI‐Ethics‐Guideline‐Tool.pdf.
- 31. Machczyński P, Majchrzak E, Niewinski P, Marchlewska J, Golusiński W. A review of the 8th edition of the AJCC staging system for oropharyngeal cancer according to HPV status. Eur Arch Otorhinolaryngol. 2020;277(9):2407‐2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O'Sullivan B, Huang SH, Su J, et al. Development and validation of a staging system for HPV‐related oropharyngeal cancer by the international collaboration on Oropharyngeal cancer network for staging (ICON‐S): a multicentre cohort study. Lancet Oncol. 2016;17(4):440‐451. [DOI] [PubMed] [Google Scholar]
- 33. Schiff BA. Oropharyngeal Squamous Cell Carcinoma. Merck Manual (Professional version). https://www.merckmanuals.com/professional/ear,‐nose,‐and‐throat‐disorders/tumors‐of‐the‐head‐and‐neck/oropharyngeal‐squamous‐cell‐carcinoma. Accessed June 21, 2021.
- 34. Risberg T, Kaasa S, Wist E, Melsom H. Why are cancer patients using non‐proven complementary therapies? A cross‐sectional multicentre study in Norway. Eur J Cancer. 1997;33(4):575‐580. [DOI] [PubMed] [Google Scholar]
- 35. Matovina C, Birkeland AC, Zick S, Shuman AG. Integrative medicine in head and neck cancer. Otolaryngol Head Neck Surg. 2017;156(2):228‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oh B, Butow P, Mullan B, et al. The use and perceived benefits resulting from the use of complementary and alternative medicine by cancer patients in Australia. Asia Pac J Clin Oncol. 2010;6(4):342‐349. [DOI] [PubMed] [Google Scholar]
- 37. Murphy CT, Galloway TJ, Handorf EA, et al. Survival impact of increasing time to treatment initiation for patients with head and neck cancer in the United States. J Clin Oncol. 2016;34(2):169‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wode K, Henriksson R, Sharp L, Stoltenberg A, Hök NJ. Cancer patients' use of complementary and alternative medicine in Sweden: a cross‐sectional study. BMC Compl Altern Med. 2019;19(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Molassiotis A, Fernández‐Ortega P, Pud D, et al. Use of complementary and alternative medicine in cancer patients: a European survey. Ann Oncol. 2005;16(4):655‐663. [DOI] [PubMed] [Google Scholar]
- 40. Miller MC, Pribitkin EA, Difabio T, Keane WM. Prevalence of complementary and alternative medicine use among a population of head and neck cancer patients: a survey‐based study. Ear Nose Throat J. 2010;89(10):E23‐E27. [DOI] [PubMed] [Google Scholar]
- 41. Saxe GA, Madlensky L, Kealey S, Wu DPH, Freeman KL, Pierce JP. Disclosure to physicians of CAM use by breast cancer patients: findings from the women's healthy eating and living study. Integr Cancer Ther. 2008;7(3):122‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shumay DM, Maskarinec G, Kakai H, Gotay CC. Why some cancer patients choose complementary and alternative medicine instead of conventional treatment. J Fam Pract. 2001;50(12):1067. [PubMed] [Google Scholar]
- 43. Rogge AA, Baur I, Blettner G, et al. Defining criteria for guiding cancer patients to find a reputable complementary medicine provider: results of a literature review and a consensus procedure. Patient Prefer Adherence. 2020;14:747‐755. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Specific substances and regimens pursued. The specific substances and regimens pursued by the 19 patients in the present study are presented by NCCAM category, 28 , 29 and in alphabetical order by administration type. Abbreviations: AM alternative medicine, Alpha‐GPC alpha‐glycerylphosphorylcholine, CBD cannabidiol, THC tetrahydrocannabinol, EPA/DHA eicosapentaenoic acid/docosahexaenoic acid


