Abstract
Introduction
Radiotherapy for head and neck cancer (HNC) has evolved rapidly in the past decades from conformal three‐dimensional technique (3D‐CRT) to intensity‐modulated radiotherapy (IMRT) and volumetric‐modulated arc therapy (VMAT). This paper presents a dosimetric comparative study between VMAT and IMRT delivery based on current literature, while also presenting the potential challenges encountered with volumetric arc therapy.
Methods
A systematic search of the scientific literature was conducted within Medline/Pubmed databases. A number of 13 papers fulfilled the search criteria which was based on the main objective to evaluate dosimetric characteristics of comparative treatment delivery with VMAT vs IMRT in HNC.
Results
Overall, from a dosimetric perspective, dose delivery via VMAT and IMRT present comparable results. Beside the delivery technique, target volume coverage also depends on the planner's expertise as well as the employed planning algorithm. At times, the superiority of VMAT emerges from the improved sparing of normal tissue, reduction of monitor units (MU) and of treatment delivery time. Similar to IMRT, one of the most important challenges of VMAT is the risk of developing secondary cancer due to the higher number of MUs compared to 3D‐CRT.
Conclusions
Based on the comparative results with the more established IMRT, VMAT in HNC can be safely delivered either as a single treatment or combined with other techniques.
Keywords: dosimetry, head and neck carcinoma, IMRT, organ sparing, VMAT
The aim of this work is to summarize and analyze the status of knowledge in the field of volumetric‐modulated arc therapy, focusing on the progress, dosimetric challenges and prospects of the increasing employment of this technique as compared to intensity modulated radiotherapy, for the management of head and neck cancer.

1. INTRODUCTION
External beam radiotherapy is a standard treatment for a large number of patients diagnosed with head and neck cancer (HNC) as either single treatment or in combination with surgery and/or chemotherapy. Despite its effectiveness, external beam radiotherapy has its challenges, owing to the organs at risk (OAR) located in close proximity to target volumes, that often make the delivery of an optimum radiation dose to the target difficult. There are several radiotherapy treatment planning options available for HNC, starting from the traditional conformal therapy (3D‐CRT) to the modern intensity‐modulated radiotherapy (IMRT) and volumetric modulated arc therapy (VMAT), each of them having advantages and challenges. 1
The factor that led to the development of modern treatment planning techniques was the need to increase conformity and homogeneity of the delivered dose, to increase sparing of OAR, to reduce the monitor units (MU) and to shorten the delivery time. All these requirements were satisfied by the development of the multileaf collimator (MLC) which offered the possibility of treating patients with the modulation of intensity during treatment delivery. Compared to the classic 3D‐CRT, the IMRT technique uses multiple intensity‐modulated beams to deliver non‐uniform dose to the target, thus improving OAR sparing. However, fixed angle IMRT has its own disadvantages compared to 3D‐CRT, which include longer radiation delivery time, increased patient exposure to low dose radiation and high MU. 2 To overcome these drawbacks a new version of IMRT was developed in the form of volumetric modulated arc therapy (VMAT), first presented by Karl Otto in 2008 as a novel plan optimization platform, where treatment is delivered efficiently and accurately in a single dynamically modulated arc. 3 Although the sparing of OAR is almost the same as with IMRT, VMAT entails a significantly reduced treatment delivery time and reduced number of MU. All these improvements are due to the fact that in VMAT, instead of using a certain number of fixed‐angle fields, the radiation is delivered in a continuous arc as the gantry of the linear accelerator rotates around the patient, the intensity being modulated via the MLC. VMAT is considered to be more efficient in the treatment of HNC due to its improved delivery efficiency over IMRT, as this modality introduces extra degrees of freedom in the optimization process in the form of dose rate and gantry rotation speed modulation. 4
Although the main goal of radiotherapy is to ensure that the target volume is fully covered by the prescribed dose, one must try to improve patients' quality of life (QOL) by sparing the OAR as much as possible. In HNC treatment‐related acute toxicities such as mucositis and edema commonly disrupt normal swallowing functions during treatment, while late toxicities that occur more than 90 days after radiotherapy and mostly includes xerostomia, dysphagia, and osteoradionecrosis of the jaws, all affect notably QOL. 5 , 6 The employment of VMAT can assure a better sparing of OAR which could reduce the extent of both acute and late toxicities.
Considering that VMAT is a highly advanced and complex irradiation technique, one of the most challenging issues regarding accurate treatment delivery consists of a very precise quality assurance (QA) and dosimetry. QA in radiotherapy must be conducted for all the procedures influencing the consistency or accuracy of a treatment plan. Therefore, as a first step, the delineation of the target and organ at risk (OAR) requires standardization across treatment centers and quality control. The second step is the proof of dose specification with different treatment planning systems (TPS), followed by a dummy run including target volume and OAR contouring. Checking a treatment plan requires a thorough QA that is often time consuming. Every step from the QA must be followed, otherwise the clinical outcome of head and neck cancer patients is compromised. 7
In view of the above, the aim of this systematic review is to present in more details any dosimetric advantages as well as possible shortcomings of using VMAT as compared to IMRT for HNC treatment delivery.
2. METHODS—LITERATURE SEARCH
A systematic search of the scientific literature was conducted within Medline/Pubmed databases using the following key terms: volumetric arc therapy, VMAT, intensity modulated radiotherapy, IMRT, head and neck. Only full text articles written in English were considered for this overview, therefore reviews, abstracts or conference papers were excluded. A number of 189 articles fulfilled the search criteria. However, for the purpose of the current study having as primary objective the evaluation of dosimetric aspects of comparative treatment delivery with VMAT and IMRT the keyword dosimetry was added to the search and therefore, the number of the articles was reduced to 31. Additional papers were identified by pearling recent publications as well as the reference lists of the relevant articles. All the papers underwent further eligibility criteria verification and the following inclusion / exclusion criteria was employed in view of the above: (1) the number of patients must be conclusive (≥6); (2) the studies must contain information about dose conformity and homogeneity, (3) studies must include information on the number of monitor units and treatment delivery time, (4) quantitative dosimetric aspects of OAR for both VMAT and IMRT plans must be reported. Overall, 13 papers met these requirements (see Figure 1). Some of the studies offered additional information about helical tomotherapy, but those sections were excluded due to the fact that the aim of this paper is focused on the comparison between VMAT and IMRT.
FIGURE 1.
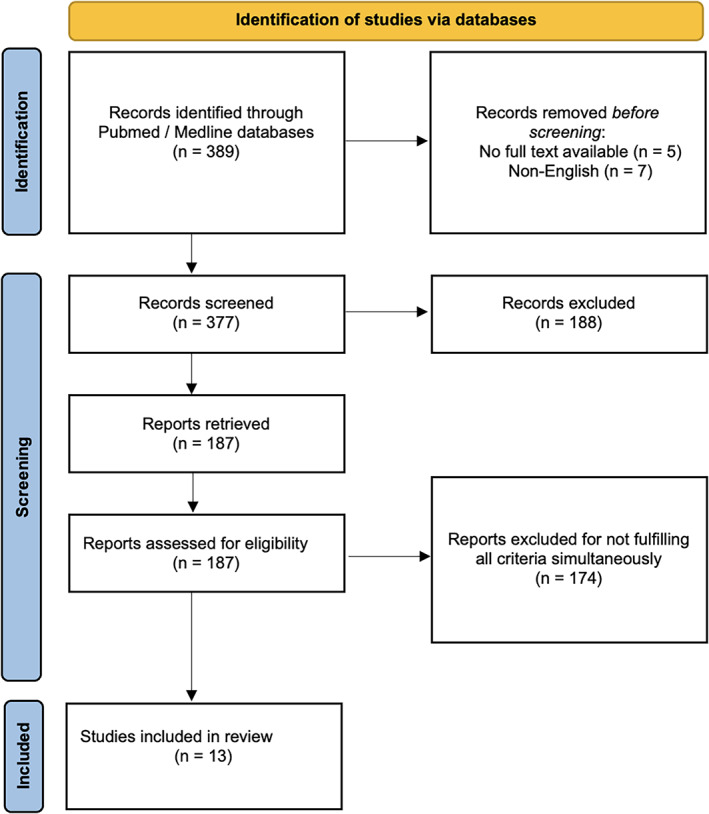
PRISMA flow diagram for search criteria
While the exclusion criteria were rather strict, a systematic analysis of the reported dosimetric aspects was not achievable due to the high heterogeneity of data across the studies.
3. RESULTS
3.1. Dose conformity and homogeneity
Achieving a better conformity and homogeneity of plans is a key aspect of radiotherapy, irrespective of the treatment technique used. In most papers discussed in this review, PTV coverage was evaluated using conformity index (CI), and homogeneity index (HI), however some of the studies used quality index (QI) and coverage factor (CVF) in addition. The conformity of dose distributions is often measured in terms of a conformity index (CI), which is defined as the ratio between the volume to receive 95% of the prescribed dose and the volume of the PTV. The smaller the value of CI, the better the conformal fitness.
Homogeneity index (HI) basically indicates the ratio between the maximum and minimum dose in the target volume. A higher HI indicates a poorer homogeneity. Quality index (QI) is used to measure the quality of the PTV coverage and consists of two components: one considering the underdosage (below 95%) and another the overdosed regions (above 107%). 4 Studies showed no significant difference between coverage of PTV in VMAT and IMRT, concluding that a similar tumor control can be achieved with the tow techniques. 4
To further improve VMAT planning, developments of treatment optimization are undergoing. An example is the study of Klippel et al. who showed that a plan quality for treatment of HNC patients with VMAT can be substantially improved using the so‐called PRO3 (Progressive Resolution Optimizer PRO3) optimization algorithm which was first introduced with the clinical system Aria 10 from Varian Medical Systems. Both, the PTV coverage and the sparing of organs at risk are better with PRO3 plans compared to PRO2 (previous version) plans. 8
Table 1 presents the findings of the 13 studies that compared dose conformity and homogeneity between VMAT and IMRT treatment techniques. Two studies that enrolled 20 HNC patients each, both delivering the same range of doses (54‐70Gy) in 33 fractions, aimed to compare target coverage conformity between VMAT and IMRT treatment techniques for simultaneous integrated boost (SIB) planning. 9 , 10 While Lee et al. found no significant difference in conformity index between VMAT and IMRT, Lu et al. showed that both CI and HI were higher for IMRT. The study led by Wiehle et al. showed an average QI of 45% lower than with IMRT. However, since the QI is calculated from the DVH it cannot give any information about the position of hot and cold areas and the large difference suggest a non‐existent superiority. Therefore, QI is a very useful tool when it comes to comparing the coverage of the target volume, but at the same time is a very sensitive tool that could lead to misleading interpretations.
TABLE 1.
Comparison of PTV coverage for different studies that evaluated dose homogeneity and conformity for VMAT plans vs IMRT plans
| Study (reference) | Dose fractionation | Coverage of PTV | Observations |
|---|---|---|---|
|
VMAT vs IMRT for carcinomas of the oro‐pharynx, larynx hypopharynx 29 patients, (Vanetti, 2009) 19 |
SIB: 66 Gy, 60 Gy, 54 Gy/30 fr |
Degree of plan conformality measured with a Conformity Index; Slight improvement in target dose homogeneity and coverage with VMAT |
Difficult to control all sources of bias influencing plan results such as optimization performed by different planners and institutes |
|
Single and double arc VMAT vs conventional IMRT 12 patients (Verbakel, 2009) 26 |
SIB: 70 Gy; 57.7 Gy/35 fr |
CI for IMRT plans were 1.14 for PTVboost and 1.54 PTVelective CI for single arc were 1.21 for PTVboost and 1.60 for PTVelective CI for double arc were 1.24 for PTVboost and 1.59 for PTVelective The dose homogeneity to PTVboost was largely improved by the double arc VMAT compared with the single‐arc one |
Fewer objectives for OAR used for RapidArc optimizations vs IMRT. No objectives for oral cavity, larynx, upper back of the neck, lower back of the neck, brain, or lungs; all replaced by a simple ring structure around the PTV and the normal tissue objective in the optimizer |
|
Single arc volumetric modulated arc therapy of HNC 25 patients (Bertelsen, 2010) 27 |
SIB: 68/66 Gy, 60 Gy, 50 Gy/33 fr |
CI for PTV50: IMRT: 1.65 and VMAT: 1.57 CI for PTV60: IMRT: 1.66 and VMAT: 1.59 CI for PTV66/68: IMRT: 1.71 and VMAT: 1.69 |
All plans were forced to use only one single arc |
|
VMAT and step‐and‐shoot IMRT in head and neck Cancer 15 patients (Wiehle, 2011) 4 |
70 Gy, 60 Gy, and 50 Gy/total of 35 fr |
QI average VMAT: 36.3 and IMRT: 66.5 CVF [95%] average for VMAT: 0.09 and IMRT:0.25 CVF [80%] average for VMAT 0.45 and IMRT: 0.87 |
Comparison between different planning systems and linacs. Only treatment plans for PTV1 (encompassed draining lymph nodes) were considered in this study. |
|
VMAT vs IMRT SiB of nasopharyngeal Carcinomas 20 patients (Lee, 2011) 9 |
SIB: 70 Gy 59.4 Gy, 54 Gy Gy/33 fr |
For all target volumes, CI was higher for VMAT, and in some case was equal with CI of IMRT There is no significant difference between VMAT and IMRT |
Bias minimized by cross‐planning by two equally experienced planners and dose protocols approved by an oncologist specialized in nasopharyngeal carcinomas |
|
VMAT vs TomoTherapy vs step‐and‐shoot IMRT 20 patients (Lu, 2011) 10 |
SIB: 70 Gy; 60 Gy; 54 Gy/33 fr |
Plans compared with conformity index (CI), homogeneity index (HI) Both CI and HI were higher for IMRT |
Limitation: the use of a coplanar beam for VMAT/IMRT planning. For VMAT parameter settings affects planning quality (collimator angles, arc numbers, rotation angles, use of noncoplanar beam). |
|
Smartarc‐based VMAT vs IMRT vs tomotherapy 8 patients (Clemente, 2011) 11 |
SIB: 70 Gy; 63 Gy; 58.1 Gy/35 fr |
Average CI for VMAT: 1.5 Average CI for IMRT: 1.6 |
Limited number of patients |
|
A comparison of several modulated RT techniques 10 patients (Stieler, 2011) 12 |
60 Gy for PTVhigh 56 Gy for PTVlow |
Average CI for VMAT: 1.82 Average CI for IMRT: 2.23 HI for VMAT for PTVhigh 1.20 HI for VMAT for PTVhigh 1.11 |
Limited number of patients |
|
Clinical experience transitioning from IMRT to VMAT for HNC 20 patients (Studenski, 2012) 15 |
All cases were rescaled to a dose of 70 Gy in 2 Gy fractions for consistency in the comparison. | VMAT provides comparable coverage of target volumes to IMRT |
No specific comparison for target volumes coverage. IMRT optimizer Elekta XIO v4.62 and VMAT optimizer Nucletron Oncentra MasterPlan v4.1 |
|
VMAT vs conventional intensity modulated radiation therapy 20 patients (Fung‐Kee‐Fung, 2012) 2 |
For patients with unresected tumor: SIB 70 Gy/66–60 Gy/56 Gy in 35 fractions For patients receiving post‐operative radiation: SIB 66 Gy/56 Gy |
Plans were compared for dose conformity and homogeneity VMAT plans trended towards better dose homogeneity but ultimately were found to have statistically significant less conformity in PTV irradiation compared to IMRT plans. |
Optimization and dose calculations performed with Eclipse version 8.1 for IMRT. VMAT planning performed in Eclipse version 8.5, using the AAA calculation algorithm, and the Progressive Resolution optimization algorithm |
|
Static and rotational intensity modulated techniques for HNC 18 patients (Broggi, 2014) 13 |
SIB 66 Gy/54 Gy/30 fr |
For IMRT homogeneity of PTVhigh 1.12 and for PTVlow was 1.21 For VMAT (Eclipse) homogeneity of PTVhigh 1.12 and for PTVlow was 1.20; For RapidArc (Varian) homogeneity of PTVhigh 1.11 and for PTVlow was 1.19 |
Dosimetric differences due to variations among various optimization/objective function/leaf sequencing approaches used in different planning systems. |
|
Equally spaced beam, beam angle optimization, and VMAT in HNC 119 patients (Leung, 2019) 17 |
66–70 Gy for high‐risk PTV/60 Gy for intermediate risk PTV/54 Gy for low‐risk PTV |
For the target volumes the dose parameters were the homogeneity index (HI) and conformation number (CN). HI was lower for both VMAT plans CN was higher for both VMAT plans |
Five hypothetical plans computed for each patient using the Eclipse treatment planning system Version 13.6 by the same dosimetrist, significantly reducing bias. |
|
VMAT, IMRT and helical tomotherapy for the ESCALOX‐trial pre‐study 6 patients (Pigorsch, 2020) 7 |
SIB: 77 Gy, 70 Gy, 56 Gy/35 fr |
CVF was equal for IMRT and VMAT for PTV77 and PTV56, while it was higher for IMRT for PTV70; CN was higher in IMRT for PTV77 and PTV56 and higher for VMAT for PTV70; HI was equal for PTV77 and higher for VMAT for PTV70 and PTV56 |
Limited number of patients |
Abbreviations: CI, conformity index; CN, conformation number; CVF, coverage factor; fr, fractions; HI, homogeneity index; HNC, head and neck cancer; IMRT, intensity modulated radiation therapy; PTV, planning target volume; QI, quality index; RT, radiotherapy; SIB, simultaneous integrated boost; VMAT, volumetric modulated arc therapy.
Although a number of studies enrolled a limited number of patients, they all succeeded to show the differences regarding PTV coverage. 7 , 11 , 12 While Clemente et al. and Stieler et al. achieved lower values for CI for VMAT plans, thus demonstrating the superiority of VMAT over IMRT, Pigorsch et al. obtained equal coverage factors (CVF) for IMRT and VMAT for PTV77 and PTV56. 7 , 11 , 12
3.2. Monitor units and treatment delivery time
Despite the similarities in PTV coverage as shown in the above comparative studies, a significant difference was observed in terms of Monitor Units (MU) and treatment delivery time. The values of MU of the selected studies that were presented in the previous section can be found in Table 2. The study led by Pigorsch was excluded from this table due to lack of information regarding MU and treatment delivery time. 7 In all the studies, the number of monitor units was lower for VMAT plans compared to IMRT. The maximum reduction was obtained by Broggi et al., of around 73% for rapid arc. 13 Since the amount of scattered radiation that reaches the healthy tissues is directly proportional to the number of MU delivered, a lower number of MUs equates to a dose reduction to the distant healthy tissues. 14
TABLE 2.
Comparison of monitor units and treatment delivery time for the selected studies (IMRT vs VMAT)
| Study (reference) | Monitor units | Treatment delivery time |
|---|---|---|
|
VMAT vs IMRT oropharynx, larynx hypopharynx 29 patients, (Vanetti, 2009) 19 |
IMRT plans showed values of MUs at least roughly doubled compared to RapidArc | Overall time for IMRT of 15 minutes, while for VMAT the beam‐on time was estimated to be less than 1.5 minutes per arc |
|
Single and double arc VMAT vs conventional IMRT 12 patients (Verbakel, 2009) 26 |
IMRT: 1108 MU Single‐arc VMAT: 439 MU Double arc VMAT: 459 MU |
Single arc delivery of 2 Gy requiring less than 80 seconds, and double arc plans in less than 3 minutes. IMRT sliding window delivery for seven fields requires 8‐12 minutes for a typical plan |
|
Single arc VMAT of HNC 25 patients (Bertelsen, 2010) 27 |
Average IMRT: 503 Average VMAT: 460 |
IMRT: 372 seconds VMAT: 241 seconds |
|
VMAT and step‐and‐shoot IMRT in HNC 15 patients (Wiehle, 2011) 4 |
Not specified |
For VMAT treatment times of less than 3 minutes (for 2 Gy) IMRT treatments take about 10 minutes, depending on number of fields |
|
VMAT vs. TomoTherapy vs step‐and‐shoot IMRT 20 patietnts (Lu, 2011) 10 |
Average 693.1 for VMAT Average 949.3 for IMRT |
5.7 minutes for VMAT 9.2 minutes for IMRT |
|
VMAT vs IMRT SiB of nasopharynx 20 patients (Lee, 2011) 9 |
VMAT average 445 ± 33 for single arc and 493 ± 36 for double arc IMRT average 773 ± 48 and 903 ± 98 |
8.2 ± 0.4 minute for IMRT Reduction of delivery time for both single and double arc VMAT by 51 and 41% which means 4.0 ± 0.6 minute and 4.8 ± 0.4 minute respectively |
|
Smartarc‐based VMAT vs IMRT vs tomotherapy 8 patients (Clemente, 2011) 11 |
IMRT: 931.3 ± 228.7 VMAT: 672.4 ± 64.5 |
487.6 ± 178.7 seconds IMRT: 836.2 ± 61.3 seconds |
|
A comparison of several modulated RT techniques 10 patients (Stieler, 2011) 12 |
IMRT: 935 VMAT: 521.5 |
IMRT: 8.05 minutes VMAT: 6.2 minutes |
|
Clinical experience transitioning from IMRT to VMAT for HNC 20 patients (Studenski, 2012) 15 |
Reduction of MU by 289.3 ± 179.9 MU (32.9 ± 14.3%) for the VMAT The maximum reduction was 541 MU (48.9%) and the minimum was 54 MU (7.4%) |
Treatment time was reduced by 9.2 ± 3.9 minutes for VMAT over IMRT with an average reduction was 51.4 ± 15.6%. The maximum time reduction was 15 minutes (78.8%) and the minimum was 2.9 minutes (17.5%). |
|
VMAT vs conventional IMRT 20 patients (Fung‐Kee‐Fung, 2012) 2 |
VMAT MU = 542.85 IMRT MU = 1612.58 |
Faster treatment time of VMAT |
|
Static and rotational IMRT for HNC 18 patients (Broggi, 2014) 13 |
Average reduction of around 73% for rapid arc (Varian) and around 59% for VMAT (Elekta) |
RA beam‐on time (around 2 minutes) was reduced on average by 53% compared to IMRT (around 5 minutes) VMAT (around 4 minutes) beam‐on time by an average of 15% compared to IMRT |
|
Equally spaced beam, beam angle optimization, and VMAT in HNC 119 patients (Leung, 2019) 17 |
Lower MU for VMAT | Shorter treatment time of VMAT |
Abbreviations: HNC, head and neck cancer; IMRT, intensity modulated radiation therapy; min, minutes; MU, monitor units; RA, RapidArc; RT, radiotherapy; s, seconds; VMAT, volumetric modulated arc therapy.
Another parameter analyzed is treatment delivery time, which is shorter by an average of 30%‐60% in each studied case for VMAT plans as compared to IMRT (Table 2), making the rapid treatment delivery one of the greatest benefits of VMAT. This allows improved patient comfort, reduced intrafraction motion, and increased patient throughput. 15
3.3. Management of OAR toxicities
Radiotherapy of HNC is usually associated with significant toxicity both during and post treatment. During the first weeks of radiotherapy, acute effects such as mucositis or/and edema can be noticed, while after treatment completion, numerous long‐term side effects can occur such as dysphagia, xerostomia or osteoradionecrosis of the jaws.
There is clinical evidence showing a direct relationship between dose distribution in HNC radiotherapy and loss of saliva production or dysphagia. 6 , 16 Mendez et al. showed that 44 months post‐radiotherapy with IMRT a greater salivary flow recovery occurs, reducing xerostomia and thus improving quality of life (QoL). 16 As with xerostomia, other acute side effects such as edema or mucositis affect the QoL by disrupting swallowing functions during radiotherapy, but these symptoms improve in a few months after the completion of treatment. However, there are situations which include persistence of side effects and involve longer recovery time such as the case of neuropathy and fibrosis of the oral, laryngeal, and pharyngeal musculature. 6
To evaluate the dosimetric advantages of VMAT over IMRT the same 13 studies as in the previous sections were analyzed, though this time only the dosimetric aspects were taken into account. The prescribed dose differed from case to case, varying between 66/70/77 Gy for high‐risk PTV, 66/60/59.4 Gy for intermediate risk PTV and 56/54 Gy for low‐risk PTV, variations that do not allow for a significant statistical analysis of these data.
All but the study of Lee et al. included very precise dosimetric information regarding OAR. Parotid glands, spinal cord and brain stem doses were reported in all studies, and the average values obtained for these OAR are presented in Table 3. Excepting Leung's and Stieler's study all the other studies reported a lower mean dose for parotid glands in case of VMAT plans. 12 , 17 A mean dose ≥ 26 Gy and V30 ≥ 50% are correlated with acute xerostomia with an increasing risk of 1.06 times for every Gy over 26 Gy and late xerostomia at 6 and 12 months with an increasing risk of 1.04 times for every Gy over 26 Gy. 18
TABLE 3.
Dosimetric comparison for organs at risk for VMAT and IMRT plans
| Study (reference) | Parotid glands average D mean (Gy) | Spinal cord (Gy) | Brainstem (Gy) | |||
|---|---|---|---|---|---|---|
| VMAT | IMRT | VMAT | IMRT | VMAT | IMRT | |
| VMAT vs IMRT for oropharynx, larynx hypopharynx, 29 patients, (Vanetti, 2009) 19 | 31.3 | 35.35 | D 2% = 39.0 | D 2% = 42.8 | D 2% = 38.2 | D 2% = 24.8 |
| Single arc volumetric modulated arc therapy of head and neck cancer 25 patients (Bertelsen, 2010) 27 | 27.85 | 28.2 | D max = 44.2 | D max = 44.8 | — | — |
| VMAT and step‐and‐shoot IMRT in HNC 15 patients (Wiehle, 2011) 4 | 20.7 | 22.2 | D 2% = 36.7 | D 2% = 37.4 | — | — |
| VMAT vs TomoTherapy vs step‐and‐shoot IMRT 20 patietnts (Lu, 2011) 10 | 26.3 | 31.3 | D max = 30.5 | D max = 36.1 | D max = 48.8 | D max = 53.1 |
| VMAT vs IMRT SiB of nasopharynx 20 patients (Lee, 2011) 9 | Dose reduction of D mean by 1.22 ± 1.38 Gy from IMRT to VMAT | Dose reduction of D max by 1.34 ± 2.29 Gy from IMRT to VMAT | Dose reduction of D max by 1.58 ± 1.75 Gy from IMRT to VMAT | |||
| Smartarc‐based VMAT vs IMRT vs tomotherapy 8 patients (Clemente, 2011) 11 | 30.85 | 31.1 | D 0.1cc = 35.9 | D 0.1cc 35.8 | D 0.1cc = 44.3 | D 0.1cc = 44.5 |
| A comparison of several modulated RT techniques 10 patients (Stieler, 2011) 12 | 14.9 | 14 | D max = 42.6 | D max = 41.2 | D max = 47.8 | D max = 44.85 |
| Clinical experience transitioning from IMRT to VMAT in HNC 20 patients (Studenski, 2012) 15 | 26.6 | 29.3 | D max = 44 | D max = 43 | D max = 48 | D max = 51 |
| VMAT vs conventional IMRT 20 patients (Fung‐Kee‐Fung, 2012) 2 | 46.6 | 47.05 | D mean = 20.9 | D mean = 20.4 | D mean = 14.3 | D mean = 15.3 |
| Static and rotational IMRT for HNC 18 patients (Broggi, 2014) 13 | 34.8 (Electa)/36.6 (Varian) | 32 Gy | D max = 40.8(Electa) D max = 38 (Varian) | D max = 40.4 | D max = 47.5 (Electa) D max = 46 (Varian) | D max = 48 |
| Equally spaced beam, beam angle optimization, and VMAT in HNC 119 patients (Leung, 2019) 17 | 28.22 | 27.75 | D 2% = 41.65 | D 2% = 41.3 | D 2% = 45.81 | D 2% = 46.05 |
| VMAT, IMRT and helical tomotherapy for the ESCALOX‐trial pre‐study 6 patients (Pigorsch, 2020) 7 | 29.55 | 29.65 | D max = 50.5 | D max = 51.9 | D max = 27.4 | D max = 29.07 |
Abbreviations: HNC, head and neck cancer; IMRT, intensity modulated radiation therapy; RT, radiotherapy; VMAT, volumetric modulated arc therapy.
The studies by Clemente et al., Stieler et al., Studenski et al., Fung‐Kee‐Fung et al. and Leung et al. presented a higher value of the maximum dose for the spinal cord for VMAT plans, the rest of the studies showing a reduction of D max. 2 , 15 , 17 For the brainstem only the study by Vanetti et al. reported a higher value of VMAT plans for the maximum dose, while the others presented a dose reduction of D max with VMAT when compared to IMRT. 19
Although the doses to the parotid glands and spinal cord were unanimously reported, there were studies that presented dosimetric aspects of other OAR, such as larynx, esophagus, eyes, cochlea, optic nerves, or oral cavity. In most cases VMAT plans obtained a slightly better sparing of these OAR. Radiotherapy techniques that are focused on sparing the organs that may cause dysphagia have the potential to reduce late dysphagia by constraining dose to critical structures such as the pharyngeal constrictors, larynx, oral cavity, and esophageal inlet. In this respect, the studies by Lu et al. and Studenski et al. presented a decrease of the mean dose for larynx from 41.7 Gy with IMRT to 37.8 Gy with VMAT, and from 41 Gy with IMRT to 40 Gy with VMAT delivery, respectively. 10 , 15
The brachial plexus was delineated in 2 of the mentioned studies. The maximum dose obtained was higher in case of VMAT (58.5 Gy VMAT and 58.1 Gy IMRT); 71 Gy VMAT and 70 Gy IMRT. 7 , 15 Dosimetric parameters for the cochlea were reported in 3 studies, obtaining a lower mean dose with VMAT as follows: 39.1 for VMAT vs 39.6 for IMRT 10 ; 18 Gy left cochlea, 19.3 Gy right cochlea for VMAT vs 23.7 Gy left cochlea and 22.4 Gy right cochlea for IMRT, 2 whereas Studenski et al. 15 reported very similar doses for the two techniques: 27 Gy for VMAT vs 28 Gy for IMRT.
3.4. Secondary cancer risk
The occurrence of secondary, radio‐induced cancer is a very controversial subject due to the uncertainties of its nature: patients undergoing radiotherapy are often at high risk of a second cancer because of their lifestyles or genetic predisposition, which could be more dominant than the risk from radiation. Numerous studies proved that radiation has a significant, though very small, contribution to the risk of second malignancies, particularly in long‐term survivors. 20 , 21
Irradiation techniques underwent great developments over the last decades, from the simple two‐dimensional radiotherapy to 3D‐CRT and later IMRT, VMAT and helical tomotherapy, aiming to increase the conformity of the dose and minimize the exposure of healthy tissue to radiation and thus reduce the risk of secondary malignancies. Therefore, while modern techniques such as IMRT or VMAT reduce the dose in the proximity of the target, large volumes of normal tissue are exposed to lower doses due to the scattered radiation from the treatment head. Healthy organs can be exposed both within the in‐field volume (IFV) or outside de‐irradiated field (OFV), due to scattered photons or neutrons (for higher photon energies). Compared to the OFV, the risk of developing a second malignancy is typically higher in the IFV because of the higher doses delivered to organs at risk in‐field. 22
Due to the increase in dose conformity, IMRT and VMAT reduce the risk of a second malignancy in IFV compared to conventional 3D‐CRT. However, since scattered photons are the main source of secondary cancer post radiotherapy for OFV organs, with the adoption of the IMRT techniques the amount of scattered radiation can be expected to increase due to the high amount of monitor units delivered during the treatment. On the other hand, when using VMAT, the reduction of MU leads to a significant decrease of the whole‐body integral dose and, as a result, the risk of radiation‐induced carcinogenesis decreases as well. 22 , 23
Dörr et al. observed that the majority of secondary tumors appear in the region receiving <6 Gy and within the margin of the planning target volume defined as the volume 2.5 cm inside to 5 cm outside the proper field margin. Dörr's study also showed that the risk of developing a second malignancy is higher in the in IFV compared to the OFV because of the higher doses delivered to organs at risk in the proximity of the target volume. 24
In an HNC study, Sakthivel reported lower doses to OAR close to the PTV using VMAT compared to IMRT and less secondary dose from VMAT than from conventional IMRT. However, as the distance from the field edge increased, secondary dose became similar between IMRT and VMAT and the incidence of second cancer for VMAT and 9‐field IMRT was comparable. VMAT resulted in reduced relative second cancer risk in all organs except skin and soft tissue close to PTV, while in IMRT, the risk of second cancer was significantly influenced by increasing the energy from 6 MV to 10 MV. 23
4. CONCLUSIONS
Radiotherapy is a key technique for the management of advanced head and neck cancer. The remarkable evolution of irradiation techniques is due to the desire to produce better dose conformity and thus to obtain a better coverage of the target volume. This goal was achieved by the volumetric‐modulated arc therapy technique.
In addition to improved conformity and coverage, VMAT has further advantages over other radiotherapy techniques. Dose delivery time was found to be shorter for VMAT compared to IMRT in all the analyzed studies, thus improving patients' comfort and reducing the possibility of intrafraction motion. Furthermore, the decreased number of monitor units diminishes scattered radiation which was indicated to be the cause of second malignancies. Several studies showed that compared to 3D‐CRT, modern radiotherapy techniques such as IMRT or VMAT reduce the dose in the proximity of the target volume, while large volumes of normal tissue will be exposed to a low dose due to the scattered radiation from treatment head, thus increasing the risk of secondary radio‐induced cancer in the regions that are distant from the target volume.
It was shown that toxicities to organs at risk are reduced when using VMAT for HNC, a particularly important aspect when exposing organs that can lead to xerostomia, dysphagia, or other side effects that influence patients' quality of life. With a more critical eye it is to be noted, that differences in dose among the two techniques were often small and possibly planner dependent, an important aspect that is not mentioned in the studies. Over the years, treatment delivery via both IMRT and VMAT have improved due to continuous developments of planning algorithms and the expertise of planners. Differences between VMAT and IMRT are becoming smaller and remain patient‐, operator‐ and planning technique‐dependent (number of beam directions for IMRT). Variations in treatment planning quality have been observed both among planners and between treatment centers, requiring quantification of these differences and standardization of planning to reduce differences in output. For instance, a solution to reduce these variations is the Knowledge Based Planning—a machine learning process that assists the planner to optimize dose distribution in prostate cancer IMRT. 25
Nowadays, the optimization strategy will have the largest effect on plan quality together with the skills of the planner. These factors could further reduce the dosimetric differences between IMRT and VMAT delivery.
When compared with IMRT or older techniques, VMAT has proven its dosimetric value in the management of head and neck cancer, and it can be safely used either as a single treatment or in combination with other methods to increase the therapeutic ratio in these challenging tumors.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Buciuman N, Marcu LG. Dosimetric justification for the use of volumetric modulated arc therapy in head and neck cancer—A systematic review of the literature. Laryngoscope Investigative Otolaryngology. 2021;6(5):999‐1007. doi: 10.1002/lio2.642
BIBLIOGRAPHY
- 1. Shang Q, Liu Shen Z, Ward MC, Joshi NP, Koyfman SA, Xia P. Evolution of treatment planning techniques in external‐beam radiation therapy for head and neck cancer. Appl Rad Oncol. 2015;4(3):18‐25. [Google Scholar]
- 2. Fung‐Kee‐Fung SD, Hackett R, Hales L, Warren G, Singh AK. 2012 Baishideng, a prospective trial of volumetric intensity‐modulated arc therapy vs conventional intensity modulated radiation therapy in advanced head and neck cancer. World J Clin Oncol. 2012;3(4):57‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. 2008;35(1):310‐317. [DOI] [PubMed] [Google Scholar]
- 4. Wiehle R, Knippen S, Grosu AL, Bruggmoser G, Hodapp N. VMAT and step‐and‐shoot IMRT in head and neck cancer: a comparative plan analysis. Strahlenther Onkol. 2011;187:820‐825. [DOI] [PubMed] [Google Scholar]
- 5. Baudelet M, Van den Steen L, Tomassen P, et al. Very late xerostomia, dysphagia, and neck fibrosis after head and neck radiotherapy. Head Neck. 2019;41(10):3594‐3603. [DOI] [PubMed] [Google Scholar]
- 6. Hutcheson KA, Lewin JS, Barringer DA, et al. Late dysphagia after radiotherapy‐based treatment of head and neck cancer. Cancer. 2012;118(23):5793‐5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pigorsch SU, Kampfer S, Oechsner M, et al. Report on planning comparison of VMAT, IMRT and helical tomotherapy for the ESCALOX‐trial pre‐study. Radiat Oncol. 2020;15:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klippel N, Schmücking M, Terribilini D, Geretschläger A, Aebersold DM, Manser P. Improved VMAT planning for head and neck tumors with an advanced optimization algorithm. Z Med Phys. 2015;25(4):333‐340. [DOI] [PubMed] [Google Scholar]
- 9. Lee TF, Ting HM, Chao PJ, Fang FM. Dual arc volumetric‐modulated arc radiotherapy (VMAT) of nasopharyngeal carcinomas: a simultaneous integrated boost treatment plan comparison with intensity‐modulated radiotherapies and single arc VMAT. Clin Oncol (R Coll Radiol). 2012;24(3):196‐207. [DOI] [PubMed] [Google Scholar]
- 10. Lu SH, Cheng JCH, Kuo SH, Lee JJS, Chen LH, Wu JK. Volumetric modulated arc therapy for nasopharyngeal carcinoma: a dosimetric comparison with TomoTherapy and step‐and‐shoot IMRT. Radiother Oncol. 2012;104:324‐330. [DOI] [PubMed] [Google Scholar]
- 11. Clemente S, Wu B, Sanguineti G, et al. SmartArc‐based volumetric modulated arc therapy for oropharyngeal cancer: a dosimetric comparison with both intensity‐modulated radiation therapy and helical tomotherapy. Int J Radiat Oncol Biol Phys. 2011;80(4):1248‐1255. [DOI] [PubMed] [Google Scholar]
- 12. Stieler F, Wolff D, Schmid H, Welzel G, Wenz F, Lohr F. A comparison of several modulated radiotherapy techniques for head and neck cancer and dosimetric validation of VMAT. Radiother Oncol. 2011;101(3):388‐393. [DOI] [PubMed] [Google Scholar]
- 13. Broggi S, Perna L, Bonsignore F, et al. Static and rotational intensity modulated techniques for head‐neck cancer radiotherapy: a planning comparison. Phys Med. 2014;30(8):973‐979. [DOI] [PubMed] [Google Scholar]
- 14. Osborn J. Is VMAT beneficial for patients undergoing radiotherapy to the head and neck? Radiography (Lond). 2017;23(1):73‐76. [DOI] [PubMed] [Google Scholar]
- 15. Studenski MT, Bar‐Ad V, Siglin J, et al. Clinical experience transitioning from IMRT to VMAT for head and neck cancer. Med Dosim. 2013;38(2):171‐175. [DOI] [PubMed] [Google Scholar]
- 16. Mendez LC, Moraes FY, Poon I, Marta GN. The management of head and neck tumors with high technology radiation therapy. Expert Rev Anticancer Ther. 2016;16(1):99‐110. [DOI] [PubMed] [Google Scholar]
- 17. Leung WS, Wu VWC, Liu CYW, Cheng ACK. A dosimetric comparison of the use of equally spaced beam (ESB), beam angle optimization (BAO), and volumetric modulated arc therapy (VMAT) in head and neck cancers treated by intensity modulated radiotherapy. J Appl Clin Med Phys. 2019;20(11):121‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mazzola R, Ricchetti F, Fiorentino A, et al. Dose‐volume‐related dysphagia after constrictor muscles definition in head and neck cancer intensity‐modulated radiation treatment. Br J Radiol. 2014;87(1044):20140543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vanetti E, Clivio A, Nicolini G, et al. Volumetric modulated arc radiotherapy for carcinomas of the oro‐pharynx, hypo‐pharynx and larynx: a treatment planning comparison with fixed field IMRT. Radiother Oncol. 2009;92(1):111‐117. [DOI] [PubMed] [Google Scholar]
- 20. Hall EJ, Wuu CS. Radiation‐induced second cancers: the impact of 3D‐CRT and IMRT. Int J Radiat Oncol Biol Phys. 2003;56(1):83‐88. [DOI] [PubMed] [Google Scholar]
- 21. Rehman JU, Muhammad Isa M, Ahmad N, et al. Dosimetric, radiobiological and secondary cancer risk evaluation in head‐and‐neck three‐dimensional conformal radiation therapy, intensity‐modulated radiation therapy, and volumetric modulated arc therapy: a phantom study. J Med Phys. 2018;43(2):129‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paganetti H, Athar BS, Moteabbed M, Adams JA, Schneider UI, Yock TI. Assessment of radiation‐induced second cancer risks in proton therapy and IMRT for organs inside the primary radiation field. Phys Med Biol. 2012;57(19):6047‐6061. [DOI] [PubMed] [Google Scholar]
- 23. Sakthivel V, Mani GK, Mani S, Boopathy R. Radiation‐induced second cancer risk from external beam photon radiotherapy for head and neck cancer: impact on in‐field and out‐of‐field organs. Asian Pac J Cancer Prev. 2017;18(7):1897‐1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dörr W, Herrmann T. Second primary tumors after radiotherapy for malignancies. Treatment‐related parameters. Strahlenther Onkol. 2002;178(7):357‐362. [DOI] [PubMed] [Google Scholar]
- 25. Good D, Lo J, Lee WR, Wu QJ, Yin FF, Das SK. A knowledge‐based approach to improving and homogenizing intensity modulated radiation therapy planning quality among treatment centers: an example application to prostate cancer planning. Int J Radiat Oncol Biol Phys. 2013;87(1):176‐181. [DOI] [PubMed] [Google Scholar]
- 26. Verbakel W, Cuijpers JP, Hoffmans D, Bieker M, Slotman BJ, Senan S. Volumetric intensity‐modulated arc therapy vs. conventional IMRT in head‐and‐neck cancer: a comparative planning and dosimetric study. Int J Radiat Oncol Biol Phys. 2009;74(1):252‐259. [DOI] [PubMed] [Google Scholar]
- 27. Bertelsen A, Hansen CR, Johansen J, Brink C. Single arc volumetric modulated arc therapy of head and neck cancer. Radiother Oncol. 2010;95(2):142‐148. [DOI] [PubMed] [Google Scholar]


