Abstract
Objectives
We aimed to construct an induction system for polyploid giant cancer cells (PGCCs), as well as to investigate PGCC features and clinical significance.
Methods
A laryngeal neoplasm‐PGCC induction system was constructed using paclitaxel liposomes (PTX). We used western blots to compare expression of epithelial‐mesenchymal transition‐related proteins, stem cell interrelated proteins, and cyclin‐associated proteins. We then measured PGCC count in tissue samples of patients with laryngeal neoplasms and analyzed its relationship with prognosis. Statistical significance was determined using t‐tests.
Results
PTX successfully induced PGCCs. Western blotting showed that CyclinB1, CDC25C, CDK1, E‐cadherin, and EIF‐4A expression decreased in PGCCs compared with normal cancer cells, whereas vimentin and CD133 expression increased. Number of PGCCs in laryngeal cancer tissues and overall survival time were inversely correlated (P < .05).
Conclusions
PTX successfully induces PGCC formation in laryngeal carcinoma, which may be the cause of poor prognosis in patients with laryngeal cancer.
Level of Evidence: 4.
Keywords: epithelial mesenchymal transformation, laryngeal neoplasms, polyploid giant cancer cells, prognosis
1. INTRODUCTION
Laryngeal cancer has a high incidence, accounting for 26% of malignant head and neck tumors worldwide, of which 95% are squamous cell carcinomas. 1 Although early diagnosis is straightforward, relapse and tumor metastasis can still occur after treatment, with a 5‐year survival rate of only 50%. 2 Therefore, exploring the specific mechanisms of recurrence and metastasis in laryngeal cancer patients is of great importance for improving prognosis.
Available research indicates that polyploid giant cancer cells (PGCCs) are important factors in tumorigenesis, invasion, development, metastasis, and chemotherapy resistance. 3 , 4 This subgroup has characteristics of cancer stem cells and contain either a large nucleus or multiple nuclei. Previously, PGCCs were thought to be undivided and incapable of surviving. However, recent studies have revealed instead that these cells exhibit a clear dormant period with stagnant proliferation. After the dormant period, a large number of offspring PGCCs rapidly divide through budding and explosive division. Thus, they are no longer considered “doomed” cells but living “transitional” cells.
The nuclei of PGCCs are usually irregular and at least three times the size of normal diploid neoplasm nuclei. 5 These features may cause DNA over‐replication, cancelation of mitotic checkpoints, and cytokinesis failure. Studies have also shown that PGCCs have tumor stem cell‐like characteristics, 3 , 6 specifically the capacity to express normal stem‐cell and tumor stem‐cell markers. They can also differentiate into other tissues using differentiation medium, including cartilage, bone, adipose, red blood cells, and fibrous cells. 7 , 8 This differentiation ability is considered an important cause of tumor recurrence after radiotherapy and chemotherapy. 9 Several factors greatly elevate the clinical significance of PGCCs. First, PGCC count varies across patient tissue samples, increasing significantly with high pathological grade and during late disease stages or after chemotherapy. 10 The nuclear features of PGCCs are useful as prognostic predictors of patients with ovarian cancer. 11
Patients with laryngeal cancer are usually treated with neoadjuvant chemotherapy, specifically using paclitaxel liposome (PTX), a microtubule poison that inhibits cancer‐cell mitosis and proliferation. In this study, we aimed to explore the process, characteristics, and clinical value of PGCC formation after PTX treatment of laryngeal cancer cells.
2. MATERIALS AND METHODS
The study protocol was approved by the Ethics Committee for Human Experiments of the Affiliated Hospital of Nantong University (2020‐L160). Informed consent was obtained from all patients.
2.1. Cell culture
Human laryngeal tumor cell line TU212 was obtained from the Laboratory of Medical College of Shanghai Jiao‐tong University. Cells were cultured in IMDM medium containing 10% FBS and 1% penicillin‐streptomycin, using an incubator maintained at 5% CO2 and 37°C.
2.2. Induction of laryngeal cancer cell‐PGCCs
TU212 was inoculated in a cell culture dish and cultured until cell fusion reached 80% to 90%. After 16 hours of induction with a base culture of PTX (Paclitaxel liposome for injection, China, 0.04 mg/mL), cells were gently rinsed using phosphate buffered saline (PBS). Culturing continued in a complete medium, with the solution changing every 2 days. Approximately 20 to 25 days after PTX removal, ordinary TU212 cells experienced 100% mortality, and only PGCCs survived. Surviving PGCCs were collected for follow‐up experiments.
2.3. Immunofluorescence
TU212 cells were cultured on glass coverslips. After rinsing with fresh PBS for 20 minutes, cells were fixed using 4% paraformaldehyde at room temperature. Next, they were blocked in 4% BSA for 2 hours at 4°C, followed by overnight incubation with primary antibody against vimentin (Cell Signaling Technology, USA, 5741; dilution ratio: 1:100) at 4°C. Cells were then washed with fresh 1× PBS for 15 minutes and incubated in a solution of Alexa Fluor 488 (Beyotime, China, A0423; dilution ratio: 1:200). After three PBS washes and staining with DAPI (Invitrogen, D1306) for 15 minutes, samples were observed under a fluorescence microscope (Zeiss, Germany).
2.4. Live/dead assay
Live/dead fluorescent staining was performed using a LIVE/DEAD assay kit (BestBio, China, BB‐4101‐1). Calcein AM emits green fluorescence in living cells, whereas propidium iodide causes red fluorescence in dead cells. First, PGCCs were cultured in cell culture dishes, then rinsed twice with PBS before using the assay kit. Subsequently, they were incubated for 25 minutes. Samples were observed under a fluorescence microscope (Zeiss, Germany).
2.5. Western blotting
Three groups of cells were used: control (normal diploid laryngeal cancer cells), drug‐withdrawal (PTX removed), and PGCC. After extraction using RIPA buffer with protease inhibitors, protein concentration was measured with BCA and electrophoresis using a 6% to 10% SDS‐PAGE gel. Proteins were then transferred onto PVDF membranes and blocked with 5% non‐fat milk powder for 1 hour. Membranes were incubated with primary antibodies overnight at 4°C, washed three times with TBST, incubated with secondary antibody for 1 hour, rewashed, and subjected to the ECL kit (Millipore, USA, WBKLS0500). Resultant signals were exposed to X‐ray film for development and scanned into a computer using radiographic film. Antibodies used were β‐Actin (Proteintech, Wuhan, China, 66009‐1‐1g, dilution ratio: 1:600), CDC25C (Proteintech, Wuhan, China, 16485‐1‐AP; dilution ratio: 1:1000), CDK1 (Proteintech, Wuhan, China, 19532‐1‐AP; dilution ratio: 1:1000), CyclinB1 (Proteintech, Wuhan, China, 55004‐1‐AP; dilution ratio: 1:1000), and CD133 (Proteintech, Wuhan, China, 18470‐1‐AP; dilution ratio: 1:1000), E‐cadherin (Cell Signaling Technology, USA, 3195; dilution ratio: 1:2000), and vimentin(Cell Signaling Technology, USA, 5741; dilution ratio: 1:2000).
2.6. Detection of PGCCs in pathological sections
Tissue samples from 102 patients diagnosed with laryngeal carcinoma during 2013 to 2020 were collected and stored at the Department of Surgery and Pathology, Affiliated Hospital of Nantong University. Hematoxylin‐eosin (HE)‐stained sections from each sample were subjected to a count of PGCCs by three independent senior pathologists. For counting, nine fields of view were randomly selected for each slice under a 200‐fold microscope (Zeiss, Germany).
2.7. Statistical analyses
Data were analyzed using t‐tests in SPSS 19.0. Kaplan‐Meier survival curves were used to express survival results in GraphPad Prism 8.0 (GraphPad Prism Software, Inc). Statistical significance was defined as P < .05.
3. RESULTS
3.1. PTX treatment can induce PGCC formation
To investigate the influence of PTX treatment on laryngeal carcinoma, we first treated TU212 cells with PTX for 16 hours (Figure 1A). Microscopic observation revealed a few TU212 cells that became round and bright when PTX was withdrawn (Figure 1B‐a,B‐b). At 20 days posttreatment, most normal‐sized diploid cells were killed, and only a minority of PGCCs survived. These PGCCs were multinucleated and much larger than normal TU212 cells (Figure 1B‐c,B‐d). Normal Tu212 cells were around 50 μm and grew adherent to the wall, with a polygonal shape and clear outline (Figure 1B). In contrast, PGCCs were irregular and about 160 μm in size, with stagnant proliferation. We then investigated changes to nucleocytoplasmic distribution after PTX treatment. After labeling chromosomes with DAPI (blue) and cytoplasm with vimentin (green), immunofluorescence identified different nucleus sizes in PGCCs (Figure 1C). In addition, we observed one large nucleus and two small nuclei in the same cell. This asymmetrical division indicates failed mitosis, and could also be the start of splitting genetic material before the daughter cell buds.
FIGURE 1.
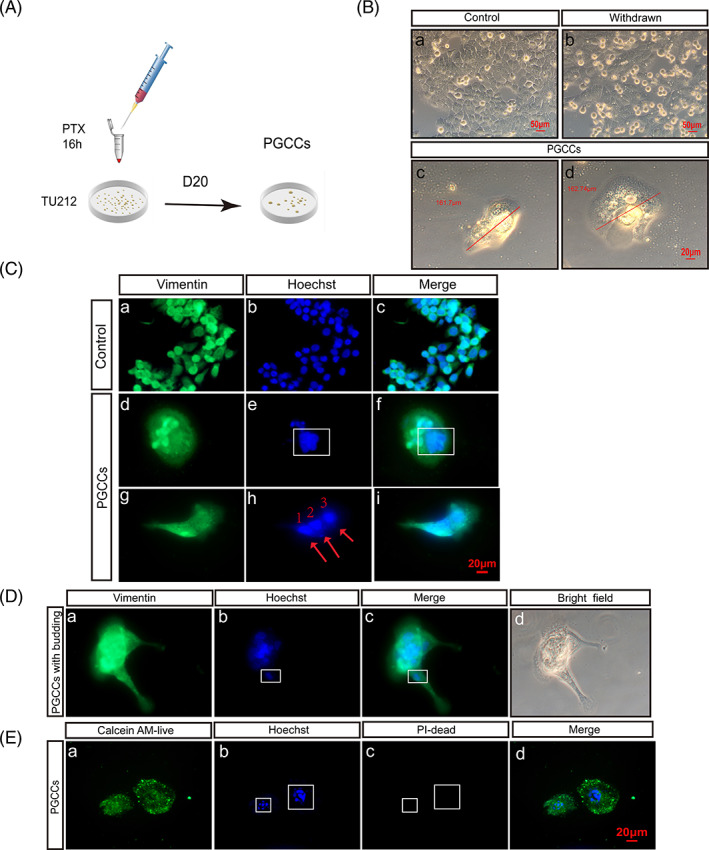
PTX treatment can induce PGCC formation. (A) After PTX treatment for 16 hours and at 20 days posttreatment, TU212 was induced to form PGCCs. (B) (a) Normal TU212 without PTX treatment. (b) Cell morphology was observed after 16 hours of PTX treatment. (c,d) After 20 days, giant nuclei developed. Scale: 20 μm. (C) Cytoplasm was stained with vimentin (green) and nuclei were stained with Hoechst (blue). (a‐c) Control group. (d‐i) Multicore PGCCs; red arrow indicates nucleus. Scale: 20 μm. (D) (a‐d) Budding PGCCs, with DNA present in PGCC branches. Red arrow indicates nucleus. Scale: 20 μm. (E) PGCCs were alive. Images taken under 400× microscope
At 20 to 25 days post‐PTX treatment, daughter cells budded from surviving PGCCs (Figure 1D), specifically through extending long branches containing DNA. Daughter cell nuclei are derived from PGCC nuclei via these branches, consistent with our observations. In addition, we confirmed that PGCCs were alive using live/dead cell double‐staining (Figure 1E).
3.2. Characteristics of PGCCs
We found that PGCCs have the capability to form colonies, also known as the intrinsic of stem cells. We used western blotting to measure changes in stemness. Compared with control TU212, CD133 expression was significantly higher in PGCCs (Figure 2A), suggesting that PGCCs present cancer stem‐like cell characteristics.
FIGURE 2.
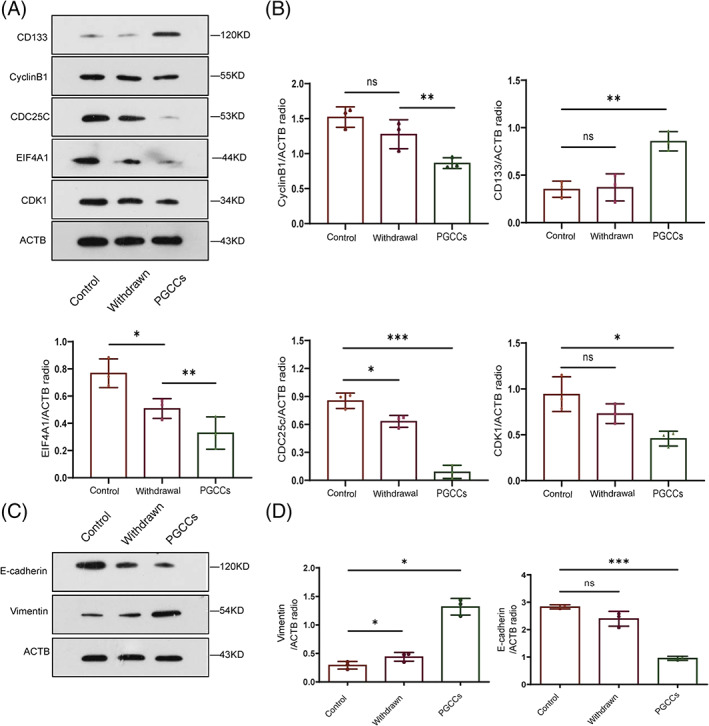
Characteristics of PGCCs. (A and B) Western blots of CD133, CyclinB1, CDC25C, EIF4A1, and CDK1 expression in TU212, cells after PTX withdrawal, as well as in PGCCs. (C and D) E‐cadherin and vimentin expression in TU212, cells after PTX withdrawal, as well as in PGCCs
Because PTX acts on tubulin to stabilize microtubules and cause cell cycle arrest, we investigated expression of cell cycle‐related proteins. CDC25C, CDK1, and cyclinB1 expression was lower in PGCCs than in control TU212. CDC25C expression also decreased upon PTX withdrawal. Both control TU212 and PTX‐withdrawal groups had lower EIF‐4A expression (Figure 2A,B).
Epithelial‐to‐mesenchymal transition (EMT) refers to the loss of intercellular adhesion in epithelial cells, followed by acquiring the ability to migrate and invade. Some studies have shown that EMT promotes tumor invasion and metastasis. To investigate the complex biological behaviors of PGCCs, we detected protein cleavage with immunoblotting (Figure 2C,D). Decreased E‐cadherin expression and increased vimentin expression in PGCCs suggested that EMT had occurred.
3.3. Clinical significance of PGCC expression in laryngeal carcinoma specimens
Examination of patient tissue specimens revealed PGCCs in pathologic sections, based on known morphological features (Figure 3A). HE staining revealed significant correlations between PGCC expression and histological differentiation (Figure 3B). Concurrently, we followed up on overall survival times (Table 1) and analyzed them using Kaplan‐Meier plots. Number of PGCCs and prognosis time were inversely correlated (Figure 3C‐a). Additionally, patients with a high PGCC count had relatively poor prognosis. Thus, PGCCs may be an element indicating poor prognosis in patients with laryngeal carcinoma. In addition, we counted the 3‐year survival rate of 51 patients with laryngeal cancer from 2013 to 2016 (Table 2), and demonstrated that those who developed lymph node metastasis had a poor prognosis (P < .05) (Figure 3C‐b).
FIGURE 3.
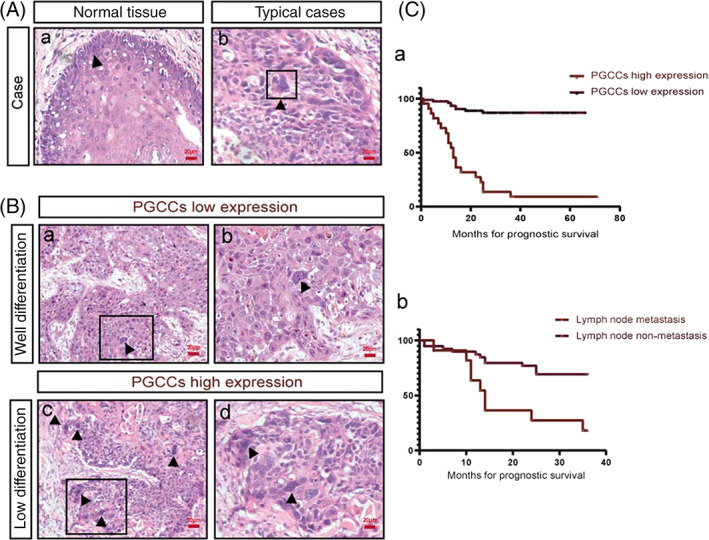
Number of PGCCs in tissue sections of patients with laryngeal carcinoma. (A) (a) HE staining of normal epithelial tissues. (b) HE staining of PGCCs, indicated with the black arrow. Scale: 20 μm. (B) (a and b) PGCCs in tissue sections from patients with well‐differentiated laryngeal carcinoma. (c and d) PGCCs in tissue sections of patients with lowly differentiated laryngeal carcinoma. (C) (a) Relationship between total survival time and PGCC count in patients with laryngeal cancer from 2013 to 2020. (b) Relationship between 3‐year survival rate and lymph node metastasis in patients with laryngeal cancer from 2013 to 2016, P < .05
TABLE 1.
Quantitative analysis of PGCCs in 102 cases of laryngeal carcinoma
| Parameters | Total (102) | PGCCs high expression (22) | PGCCs low expression (80) | P‐value |
|---|---|---|---|---|
| Age (years) | .22 | |||
| ≤63 | 53 | 8 | 45 | |
| >63 | 49 | 14 | 35 | |
| Gender | ||||
| Male | 102 | 22 | 80 | |
| Female | 0 | 0 | 0 | |
| Lymph node metastasis | .06 | |||
| Positive | 22 | 8 | 14 | |
| Negative | 80 | 14 | 66 | |
| Prognosis | .0001 | |||
| Alive | 80 | 9 | 71 | |
| Dead | 22 | 13 | 9 |
TABLE 2.
Statistical analysis of 3‐year survival rate and lymph node metastasis in 51 patients with laryngeal carcinoma
| Parameters | Lymph node metastasis | Lymph node metastasis | P‐value | |
|---|---|---|---|---|
| Total (51) | Positive (21) | Negative (30) | ||
| Prognosis | ||||
| Alive | 51 | 12 | 24 | .0009 |
| Dead | 9 | 6 |
4. DISCUSSION
Paclitaxel, docetaxel, CoCl2, radiotherapy and targeted‐therapy agents can induce PGCC formation from tumor diploid cells. 3 , 12 , 13 , 14 , 15 Clinically, PTX is commonly used for chemotherapy in patients who suffer from laryngeal carcinoma, with greatly improved prognosis. However, some patients still develop “explosive” recurrence and distant metastasis, accompanied by poor prognosis. 7 , 12 Therefore, we need to understand the mechanism of this poor prognosis after PTX treatment.
First, we performed long‐term observation of TU212 cells after PTX treatment and found that a small number of them did not die, instead generating giant nuclei or multinucleated PGCCs. These cells then generate daughter cells through budding out of branch‐like structures. PGCCs are thought to arise from failed mitosis, in a process called endoreplication. 16 During the normal cancer cell cycle, CDC25C activates CDK1. Subsequently, the binding and translocation of CDK1 and cyclin B1 to the nucleus triggers the G2/M transition of cell cycle. 17 , 18 Here, we found that upon PTX withdrawal, CDC25C expression decreased, suggesting a block in cell cycle initiation. Meanwhile, CDC25C, CDK1, and cyclinB1 expression was lower in PGCCs than in control TU212, possibly accounting for this mitotic block and causing the formation of multiple giant nuclei in PGCCs. 19 , 20
We also discovered that PGCCs had significantly lower growth rates than normal TU212 cells. We showed that EIF‐4A1 expression decreased in PGCCs. EIF4A is a dead‐box helicase that is a part of the translation initiation complex, and lowered EIF‐4A1 expression inhibits cell proliferation ability. 21 Our findings suggested that PTX caused stress in TU212 cells, shutting down protein translation. Upon PTX withdrawal, the cell cycle progressed, but more slowly.
Studies have indicated that PGCCs can express CD133, 22 a marker of cancer stem cells. CD133 regulates tumorigenesis, self‐renewal, angiogenesis, and chemoradiotherapy resistance. Here, we confirmed that CD133 expression is significantly elevated in PGCCs.
Some scholars suggest that PGCCs have a mesenchymal phenotype, meaning a stronger ability to migrate and invade than common diploid cancer cells. 5 , 23 Here, we examined the expression of two EMT‐associated proteins in PGCCs and TU212 cells without PTX treatment. The first protein is E‐cadherin, which promotes epithelial cell adhesion and is widely recognized as a molecular marker of EMT in epithelial tissues. 24 The second protein is vimentin, an essential part of the cytoskeleton (distributed widely in mesenchymal cells). 25 We found that PGCCs had lower E‐cadherin and higher vimentin expression than normal cancer cells, indicating greater migratory capacity in PGCCs. This trait may be responsible for the development of distant metastases years after treatment.
On this basis, we collected clinical data and counted PGCCs in pathological sections of patients with laryngeal cancer over a period of nearly 7 years. Our results suggest that the number of PGCCs is related to 3‐year survival. Furthermore, PGCCs appear to be a poor prognostic factor for patients.
In conclusion, our study demonstrated that a fraction of laryngeal carcinoma cells generate PGCCs after PTX treatment. This occurrence is likely an extremely significant reason for posttreatment tumor recurrence and distant metastasis. Therefore, PGCCs may be a novel therapeutic target for the treatment of laryngeal cancer. Prevention of PGCC formation, as well as exploring the molecular mechanisms of their behaviors, will benefit the refinement of laryngeal cancer therapy.
CONFLICT OF INTEREST
The authors declare no potential conflict of interests.
Liu H‐T, Xia T, You Y‐W, et al. Characteristics and clinical significance of polyploid giant cancer cells in laryngeal carcinoma. Laryngoscope Investigative Otolaryngology. 2021;6(5):1228‐1234. doi: 10.1002/lio2.667
Hui‐Ting Liu and Tian Xia contributed equally to this study.
Funding information National Natural Science Foundation of China, Grant/Award Numbers: 81972554, 81672682, 81602385; Clinical Frontier Technology of Jiangsu, Grant/Award Number: BE2017680; The Heath Research Foundation for Clinical Oncology, Grant/Award Number: Y‐XD2019‐213; Innovative Research Project for Postgraduate Students of Jiangsu Province, Grant/Award Number: SJCX19_0871
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Forastiere AA, Ismaila N, Lewin JS, et al. Use of larynx‐preservation strategies in the treatment of laryngeal cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36:1143‐1169. [DOI] [PubMed] [Google Scholar]
- 3. Niu N, Mercado‐Uribe I, Liu J. Dedifferentiation into blastomere‐like cancer stem cells via formation of polyploid giant cancer cells. Oncogene. 2017;36:4887‐4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Amend SR, Torga G, Lin KC, et al. Polyploid giant cancer cells: unrecognized actuators of tumorigenesis, metastasis, and resistance. Prostate. 2019;79:1489‐1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fei F, Zhang D, Yang Z, et al. The number of polyploid giant cancer cells and epithelial‐mesenchymal transition‐related proteins are associated with invasion and metastasis in human breast cancer. J Exp Clin Cancer Res. 2015;34:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang S, Mercado‐Uribe I, Xing Z, Sun B, Kuang J, Liu J. Generation of cancer stem‐like cells through the formation of polyploid giant cancer cells. Oncogene. 2014;33:116‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang S, Mercado‐Uribe I, Liu J. Tumor stroma and differentiated cancer cells can be originated directly from polyploid giant cancer cells induced by paclitaxel. Int J Cancer. 2014;134:508‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang S, Mercado‐Uribe I, Liu J. Generation of erythroid cells from fibroblasts and cancer cells in vitro and in vivo. Cancer Lett. 2013;333:205‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mirzayans R, Andrais B, Murray D. Roles of polyploid/multinucleated giant cancer cells in metastasis and disease relapse following anticancer treatment. Cancers. 2018;10:118. 10.3390/cancers10040118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen J, Niu N, Zhang J, et al. Polyploid giant cancer cells (PGCCs): the evil roots of cancer. Curr Cancer Drug Targets. 2019;19:360‐367. [DOI] [PubMed] [Google Scholar]
- 11. Zhang L, Ding P, Lv H, et al. Number of polyploid giant cancer cells and expression of EZH2 are associated with VM formation and tumor grade in human ovarian tumor. Biomed Res Int. 2014;2014:903542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mittal K, Donthamsetty S, Kaur R, et al. Multinucleated polyploidy drives resistance to Docetaxel chemotherapy in prostate cancer. Br J Cancer. 2017;116:1186‐1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lopez‐Sanchez LM, Jimenez C, Valverde A, et al. CoCl2, a mimic of hypoxia, induces formation of polyploid giant cells with stem characteristics in colon cancer. PLoS One. 2014;9:e99143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. White‐Gilbertson S, Lu P, Jones CM, et al. Tamoxifen is a candidate first‐in‐class inhibitor of acid ceramidase that reduces amitotic division in polyploid giant cancer cells—Unrecognized players in tumorigenesis. Cancer Medicine. 2020;9:3142‐3152. 10.1002/cam4.2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tagal V, Roth MG. Loss of Aurora Kinase Signaling Allows Lung Cancer Cells to Adopt Endoreplication and Form Polyploid Giant Cancer Cells That Resist Antimitotic Drugs. Cancer Research. 2021;81:400‐413. 10.1158/0008-5472.can-20-1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shu Z, Row S, Deng WM. Endoreplication: the good, the bad, and the ugly. Trends Cell Biol. 2018;28:465‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang G, Meng L, Tsai RYL. p53 Configures the G2/M arrest response of nucleostemin‐deficient cells. Cell Death Discovery. 2015;1. 10.1038/cddiscovery.2015.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lv H, Shi Y, Zhang L, et al. Polyploid giant cancer cells with budding and the expression of cyclin E, S‐phase kinase‐associated protein 2, stathmin associated with the grading and metastasis in serous ovarian tumor. BMC Cancer. 2014;14:576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fei F, Qu J, Liu K, et al. The subcellular location of cyclin B1 and CDC25 associated with the formation of polyploid giant cancer cells and their clinicopathological significance. Lab Invest. 2019;99:483‐498. [DOI] [PubMed] [Google Scholar]
- 20. Liu K, Lu R, Zhao Q, et al. Association and clinicopathologic significance of p38MAPK‐ERK‐JNK‐CDC25C with polyploid giant cancer cell formation. Med Oncol. 2019;37:6. [DOI] [PubMed] [Google Scholar]
- 21. Harms U, Andreou AZ, Gubaev A, Klostermeier D. eIF4B, eIF4G and RNA regulate eIF4A activity in translation initiation by modulating the eIF4A conformational cycle. Nucleic Acids Res. 2014;42:7911‐7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiang Q, Zhang Q, Wang S, et al. A fraction of CD133+ CNE2 cells is made of giant cancer cells with morphological evidence of asymmetric mitosis. J Cancer. 2015;6:1236‐1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang X, Zheng M, Fei F, et al. EMT‐related protein expression in polyploid giant cancer cells and their daughter cells with different passages after triptolide treatment. Med Oncol. 2019;36:82. [DOI] [PubMed] [Google Scholar]
- 24. Derksen PWB, Liu X, Saridin F, et al. Somatic inactivation of E‐cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 2006;10:437‐449. [DOI] [PubMed] [Google Scholar]
- 25. Yao X, Wang X, Wang Z, et al. Clinicopathological and prognostic significance of epithelial mesenchymal transition‐related protein expression in intrahepatic cholangiocarcinoma. Onco Targets Ther. 2012;5:255‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]


