Abstract
Through stress and injury to tissues, the cell membrane is damaged and can lead to cell death and a cascade of inflammatory events. Soluble factors that mitigate and repair membrane injury are important to normal homeostasis and are a potential therapeutic intervention for regenerative medicine. A myokine is a type of naturally occurring factors that come from muscle and have impact on remote organs. MG53, a tripartite motif-containing family protein, is such a myokine which has protective effects on lungs, kidneys, liver, heart, eye, and brain. Three mechanisms of action for the beneficial regenerative medicine potential of MG53 have been identified and consist of 1) repair of acute injury to the cellular membrane, 2) anti-inflammatory effects associated with chronic injuries, and 3) rejuvenation of stem cells for tissue regeneration. As such, MG53 has the potential to be a novel and effective regeneration medicine therapeutic.
Keywords: MG53, TRIM protein, Cell membrane repair, Myokine, Regenerative medicine
Introduction
The cell membrane is a dynamic barrier that serves as the interface between the intracellular and extracellular environments. The complex interactions of trafficking intracellular to extracellular contents and vice versa is influenced by autocrine, paracrine, and direct contact stimuli. Injury to the cell membrane has critical implications on cell function, regulation, and overall survival. Inherent in cells is a membrane repair mechanism to counteract any perturbations caused by stress, whether that be mechanical, chemical, ischemia reperfusion (IR), pharmacologic, or oxidative stress. Timely repair of the cell membrane is critical to cell survival and regeneration from injury.
In the field of regenerative medicine, soluble factors secreted from muscles have been found to have important impacts on aging and regeneration, often associated with exercise [1,2]. These soluble factors are now known as myokines. Myokines can have an impact on immunologic function [3], aging, and organ protection. Mitsugumin-53 (MG53) is one such endogenously secreted myokine which has beneficial effects on repair and regeneration of remote vital organs [4–17].
MG53 secretion from skeletal muscle
MG53 is a tripartite motif-containing (TRIM) family protein and plays a key role in repairing cell membrane damage and tissue regeneration. Mechanistically, MG53 facilitates the trafficking of vesicles to the cell membrane to begin the repair process [4,18,19]. First identified as a novel protein in excitation-contraction coupling and muscle physiology [20] regulated by zinc binding to RING and B-box motifs [21], MG53 was quickly identified to be a key cell membrane regeneration factor [4,5,19,22]. Not entirely unique, there have been more than 80 identified TRIM proteins in humans and all share the RING and B-Box motifs [23]. The cellular functions of these TRIM proteins encompass a wide array of critical processes [23–25]. There is a close association among MG53 within species and it is highly conserved. The human MG53 shares homology particularly with Pongo abelii (99.6%), Gorilla (99.4%) and Hylobates moloch (99.2%) [26], implying that this degree of conservation in diverse animals denotes a common ancestry.
While MG53 is primarily found and expressed in striated muscles [4,19,20], it is also found in other cells throughout the body, such as the lung and kidney [7,8] and has circulating effects throughout the body [27,28]. As circulating MG53 could play a role in tissue regeneration, targeted effects of extracellular MG53 could be translated into a treatment of injured tissues in people. To facilitate its regenerative medicine potential, recombinant human MG53 (rhMG53) has been developed as a novel cell membrane repair therapeutic [7–11,13–17,27,29–40].
Several stimulations could contribute to release of MG53 from skeletal muscle. First, noninjurious treadmill running can trigger elevation of circulating MG53 in murine model [29], suggesting the beneficial role of exercise might involve promotion of regenerative factors such as MG53. Second, several studies have shown that insulin treatment could induce release of MG53 from skeletal muscle [27,41], thus potential therapeutics could involve stimulation of the metabolic process of MG53 secretion. In addition, given the elevation of circulating MG53 after IR stress in striated muscles [10,30], noninvasive manipulations of repetitive short-term IR treatment to mimic ischemic preconditioning has emerged as an attractive means to enhance MG53 secretion to preserve remote organ function associated with surgery or chemotherapy [42].
Mechanism of MG53 function in tissue repair and regeneration
As our understanding of MG53 has grown, we have come to identify three general mechanisms of action in tissue repair and regeneration: a) repair of acute injury to cell membrane, b) anti-inflammation, and c) rejuvenation of stem cells.
These three modes are separate yet complementary to each other. On one hand, MG53’s function in repair of membrane injury could have direct implication to facilitate treatment of acute injury to multiple organs [5,7–11,13,29,31]. In addition, several studies from our laboratory and other investigators have revealed an anti-inflammatory function of MG53 associated with chronic tissue injury, sepsis, or viral infection [14–16,33,40,43,44]. Targeting the anti-inflammatory role of MG53 could have broader application in regenerative medicine. Finally, another important physiological role of MG53 has been linked to protection and rejuvenation of stem cells [14,35,40,44,45]. Preservation of stem cell lineage and function associated with aging or degenerative human diseases is another important area of MG53 research.
MG53 protection in vital organs
MG53 as a myokine has been found to have protective and regenerative effects in several vital organ tissues (Figure 1). Here we summarize the various applications of MG53 in multiorgan protection.
Figure 1.
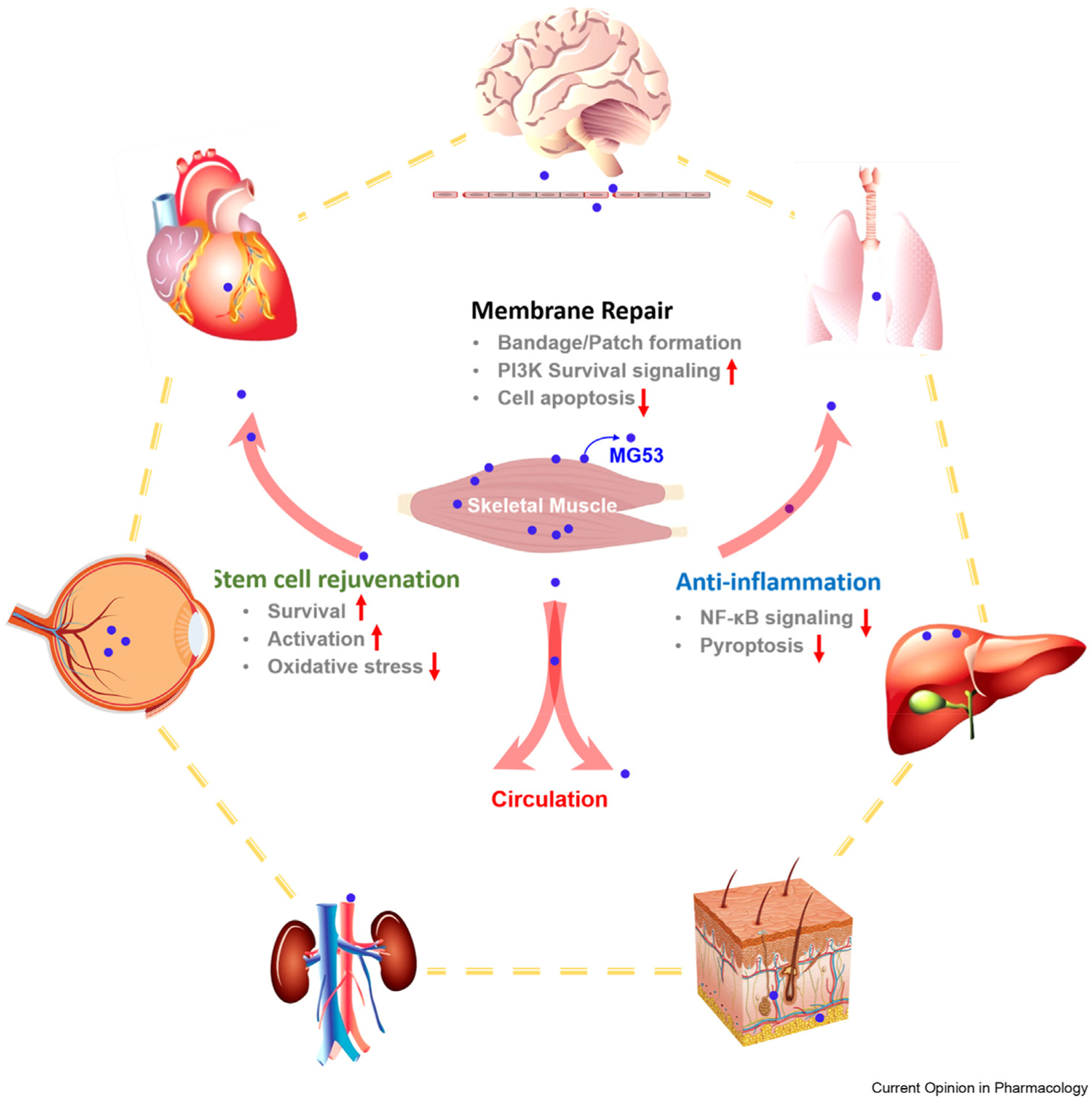
MG53 is abundantly expressed in skeletal muscle and circulates in the blood stream. As a myokine, MG53 has benefits on protection against injury to multiple organs, including heart, lung, kidney, liver, brain, cornea, and skin. In addition to facilitating tissue repair, MG53 has anti-inflammation function to preserve organ function under chronic injury conditions. Moreover, MG53 can preserve stem cells to improve the regenerative capacity of multiple tissues.
a. MG53 and the lung
Numerous stresses can induce acute lung injury (ALI)—trauma, transfusion, ventilator-induced lung injury, and infection being the most notable. MG53 has been identified in type I and II murine, rat, and human alveolar epithelial cells [7,31,46]. Through the use of the mg53−/− mice, researchers have been able to demonstrate that a lack of endogenous MG53 leads to increased susceptibility to lung injury caused by various stresses including IR injury, ventilator-induced lung injury, and lipopolysaccharide [7,31,46]. MG53 over-expression, intravenous delivery, or inhalation of rhMG53 had protective effects in repair of alveolar epithelial cells and prevented lung injury by facilitating cell membrane repair [7,31,46].
Trauma and poly-trauma has direct deleterious effects on the lung through direct contact trauma, contusive blast effect, inhalational injury, and hemorrhage/resuscitative injury. This mechanism of trauma-related injury is relevant to military service personnel as well as civilian populations. Through live cell imaging, cell membrane repair in human bronchial epithelial cells is preserved with MG53 [17]. In addition, in a porcine model of combined chest contusive lung injury and hemorrhagic shock, exogenous rhMG53 reduced percentages of circulating neutrophils and red blood cells in bronchioalveolar lavage fluid of injured animals [17].
Recent and timely critical discoveries in ALI have found that rhMG53 may have a dual therapeutic effect in treating viral associated ALI [16]. With the ongoing global SARS-CoV-2 (COVID-19) pandemic and the annual viral pneumonia cases predominated by influenza virus, the concomitant action of rhMG53 on mitigating the infectious inflammasome and inhibiting pyroptosis and the cell membrane repair properties, has seen a significantly positive beneficial effect in animal models [15,16]. These positive effects in viral associated ALI are not by a direct antiviral effect of MG53. In mice that were exposed to a lethal dose of influenza virus and treated with exogenous rhMG53, a 92% survival rate with preservation of normal body weight was seen, as compared with universal death in the control mice [16]. In these contexts, rhMG53 may be a promising directed therapeutic to suppress an inflammatory cytokine storm and facilitate lung regeneration.
b. MG53 and acute kidney injury
Although expressed at low concentrations in the kidney, MG53 is significantly expressed in areas, particularly the proximal tubular epithelium [8]. This is important since the proximal tubules are very sensitive to injury from nephrotoxic agents, IR, and infection. The MG53 deficient mice develop pathologic renal phenotypes with interstitial cell injury and are at increased risk of IR associated renal injury. This renal IR injury in mice is able to be mitigated through exogenous rhMG53 administration [8].
rhMG53 has also been demonstrated to significantly reduce acute kidney injury (AKI) from cisplatin (without reducing the oncologic efficacy of cisplatin) [8], from radiologic contrast associated AKI [36], and from renal insufficiency associated with a 30% total body surface area burn [47]. From these many animal models of AKI ameliorated by exogenous rhMG53, one can see that MG53 is a critical component to prevent tubular epithelial cell injury from several etiologies.
c. MG53 and the liver
Liver or hepatocyte injury can occur during trauma, drug effect, resection, or IR injury associated with transplantation and surgical manipulation in nontransplant settings. In a hepatic IR model, Yao et al were able to demonstrate that exogenous rhMG53 administration reduced lactate dehydrogenase, ALT, and AST, associated with liver injury [12]. Exactly how muscle-derived MG53 in circulation contributes to hepatic protection under physiological or pathological conditions remains to be studied.
d. MG53 in myocardial protection
Myocardial injury can manifest as acute, chronic, and acute on chronic pathophysiology. Typically, an acute injury is attributed to myocardial ischemia which may sometimes progress to infarction and scar. MG53 has shown a broad ability to be protective in myocardial injury [6,42,48,49,56]. Ischemic preconditioning of myocardium is able to promote MG53 release [42]. This injury/preconditioning effect may be augmented to be protective through exogenous rhMG53. Liu et al. demonstrated that exogenous administration of rhMG53 to injured cardiomyocytes is able to stimulate the prosurvival PI3K pathway in pigs [10]. It appears that there may be a common pathway irrespective of the mode of stress that is affected during myocardial infarction, which can be targeted by MG53. This is further reinforced by work reported by Han et al. who demonstrated myocardial dysfunction protection by rhMG53 in the setting of sepsis [43].
e. MG53 in neuronal injury protection
While MG53 is undetectable in the neurons, overexpression of MG53 can enhance cell membrane repair in cultured neuronal cells [11,29,50]. Yao et al. used a mouse model with sustained elevation of MG53 in circulation, and demonstrated that increased MG53 levels could protect the brain against IR injury [11]. In addition, they found that MG53 could permeate the blood-brain barrier under brain injury condition, and rhMG53 administration promotes prosurvival RISK pathway in response to IR injury to the brain [11].
Guan et al. used a mouse model of contusion-induced traumatic brain injury (TBI) and observed neuroprotective function of rhMG53 [14]. They found that TBI caused transfer of MG53 from circulation to the injured brain, and further elevation of rhMG53 was detected in the brain after intravenous administration to mice subjected to TBI. The neuroprotective function of MG53 is linked to decreased brain edema and lesion volume through activation of PI3K/Akt and ERK survival pathway. Additional studies showed that rhMG53 could protect against lipopolysaccharide-induced neuroinflammation and neurotoxicity in mice, via mitigation of the proinflammatory TLR4/NF-κB signal cascade [33].
f. MG53 protection of the cornea
In addition to circulating in the blood stream, MG53 is also found in tears and aqueous humor in murine, canine, and human [13], suggesting a physiologic role of MG53 in cornea homeostasis and wound healing. Chandler et al. showed that the mg53−/− mice exhibited delayed re-epithelialization, pronounced fibrosis and neovascularization as compared with wild-type mice following alkaline-induced injury to the cornea [13]. Topical application of rhMG53 to the eye was effective to treat alkaline-induced corneal wounds in mice [13,27] and rats [13]. Furthermore, daily application of rhMG53 had additive benefits in corneal healing in db/db mice, a type 2 diabetic model [27], without measurable side effects.
Mechanistically, endogenous MG53 is present in the human corneal epithelial cells that can preserve corneal integrity against injury. MG53 in tears and aqueous humor could enter corneal fibroblasts to inhibit their activation by TGF-β [13], thus contributing to reduced neovascularization. Whether or not MG53 plays a role in anti-inflammation associated with cornea ulceration remains to be investigated. It is also important to determine if MG53 can preserve the regenerative capacity of the eye through modulation of the limbal stem cells.
g. Dermal protection of MG53
Given the similarity between corneal epithelial and dermal epithelial, rhMG53 was also used to treat dermal wounds and found to be effective [9]. GFP-MG53 could nucleate to mechanical injury site and protect human keratinocytes. Similar to corneal wounding, mg53−/− mice are more susceptible to incisional dermal wounding than wild-type mice. Formulated rhMG53 protein in hydrogel promoted healing of the dermal wounds with reduced scar formation [9,51]. More importantly, mice with elevated levels of MG53 in circulation displayed enhanced healing capacity, as revealed by the faster rate of wound closure following punch-hole injury to their ears [40].
h. MG53’s role in preservation of stem cells associated with tissue injury
As a mechanical active tissue, skeletal muscle experiences constant injuries in daily activity. The abundant expression of MG53 in skeletal muscle ensures protection against sarcolemma injury induced by extensive exercise [29], genetic alterations [37,52,53], IR injury [30], and toxin-induced injury [29,40]. We know that MG53 could interact with dysferlin and caveolin-3 for intracellular repair vesicle trafficking [19,52]. Nonmuscle myosin IIA is a motor protein for MG53-containing vesicle trafficking [54], and PTRF serves as an anchor protein to help targeting MG53-containing vesicles to the cell membrane injury site [55].
In addition to tissue repair, MG53 also promotes regeneration of skeletal muscle following injury. Muscle satellite cells derived from the mg53−/− mice proliferate slower than those from wild-type mice, and those derived from mice with increased MG53 expression grow faster than wild-type satellite cells [40]. In vivo eccentric skeletal muscle injury experiments revealed that delayed and repetitive rhMG53 administration had extended benefits to facilitate muscle recovery, demonstrating a role of rhMG53 in skeletal muscle regeneration [40].
Multiple studies also support a role for MG53 in preservation of stem cell function associated with tissue injury. Li et al. reported that cell membrane damage is involved in the impaired survival of bone marrow stem cells by oxidized low-density lipoprotein, and rhMG53 could protect oxidative stress–induced injury to these stem cells [45]. Moreover, n-acetylcysteine, an antioxidant, could prevent oxidized low-density lipoprotein-induced reduction of MG53 and enhance MG53’s protective effect on bone marrow stem cells [35]. Guan et al. found that rhMG53 could enhance survival and therapeutic benefits of human umbilical cord-derived mesenchymal stem cells to treat traumatic brain injury [14,44].
Conclusion and perspectives
MG53 is a highly conserved myokine derived from striated muscle and has the potential to be an effective biologic in regenerative medicine. rhMG53 has robust and beneficial effects on cell membrane repair through well-defined mechanisms of action. These positive reparative effects are seen in many tissues and cell types. Repetitive administration of rhMG53 may have beneficial effects on sustained viability and anti-aging. Evolving impact of MG53 on anti-inflammation and mitigating pyroptosis have encouraging implications for the use of rhMG53 as a broad therapeutic in regenerative medicine [16].
As the benefit of MG53 in tissue repair and regenerative medicine continues to evolve and mature, we formulate these questions for future directions of investigation and intervention: a) How is MG53 released from skeletal muscle? b) Is there an “injury receptor” for MG53 on the plasma membrane that facilitates the extracellular action of MG53 into promotion of cellular survival under stress conditions? c) Does the dual action of MG53 in tissue repair and inflammation control share a similar “injury receptor”, and where do they diverge at the intracellular signaling cascade? d) Can we design therapeutic means to target both anti-inflammation and tissue repair functions of MG53 to elicit long term benefits in degenerative human disease? e) As MG53 contains the conserved E3-ligase activity, how does this function participate in the quality control and stress-response of the cells under physiologic and pathologic conditions? f) Are there genetic roots for MG53-associated repair machinery in degenerative human diseases that can be exploited; and how do developmental changes impact MG53’s role in the aging aspect of regenerative medicine ? Finally, from a surgical point of view, how might MG53 be used as a novel reagent for organ preservation during surgery or transplantation?
Funding
BAW is supported through NHLBI R01HL143000 and DOD grant W81XWH-18-1-0787. JM is supported by NIH grants AR061385, AR070752, DK106394, and AG056919 as well as by DOD grant W81XWH-18-1-0787. TT is supported by R44GM123887.
Footnotes
Conflict of interest statement
JM and TT hold equity interest in TRIM-edicine, Inc., a university spin-off biotechnology company that develops MG53 for regenerative medicine application. Patents on the use of MG53 are held by Rutgers University and The Ohio State University.
References
- 1.Petersen AM, Pedersen BK: The anti-inflammatory effect of exercise. J Appl Physiol 2005, 98:1154–1162. [DOI] [PubMed] [Google Scholar]
- 2.Gorgens SW, Eckardt K, Jensen J, Drevon CA, Eckel J: Exercise and regulation of adipokine and myokine production. Prog Mol Biol Transl Sci 2015, 135:313–336. [DOI] [PubMed] [Google Scholar]
- 3.Pillon NJ, Bilan PJ, Fink LN, Klip A: Cross-talk between skeletal muscle and immune cells: muscle-derived mediators and metabolic implications. Am J Physiol Endocrinol Metab 2013, 304:E453–E465. [DOI] [PubMed] [Google Scholar]
- 4.Cai C, Masumiya H, Weisleder N, Matsuda N, Nishi M, Hwang M, Ko JK, Lin P, Thornton A, Zhao X, Pan Z, Komazaki S, Brotto M, Takeshima H, Ma J: MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol 2009, 11:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a milestone study that describes the cell memebrane repair function of MG53.
- 5.McNeil P: Membrane repair redux: redox of MG53. Nat Cell Biol 2009, 11:7–9. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Lv F, Jin L, Peng W, Song R, Ma J, Cao CM, Xiao RP: MG53 participates in ischaemic postconditioning through the RISK signalling pathway. Cardiovasc Res 2011, 91:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia Y, Chen K, Lin P, Lieber G, Nishi M, Yan R, Wang Z, Yao Y, Li Y, Whitson BA, Duann P, Li H, Zhou X, Zhu H, Takeshima H, Hunter JC, McLeod RL, Weisleder N, Zeng C, Ma J: Treatment of acute lung injury by targeting MG53-mediated cell membrane repair. Nat Commun 2014, 5:4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duann P, Li H, Lin P, Tan T, Wang Z, Chen K, Zhou X, Gumpper K, Zhu H, Ludwig T, Mohler PJ, Rovin B, Abraham WT, Zeng C, Ma J: MG53-mediated cell membrane repair protects against acute kidney injury. Sci Transl Med 2015, 7:279ra36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Duann P, Lin PH, Zhao L, Fan Z, Tan T, Zhou X, Sun M, Fu M, Orange M, Sermersheim M, Ma H, He D, Steinberg SM, Higgins R, Zhu H, John E, Zeng C, Guan J, Ma J: Modulation of wound healing and scar formation by MG53 protein-mediated cell membrane repair. J Biol Chem 2015, 290:24592–24603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Zhu H, Zheng Y, Xu Z, Li L, Tan T, Park KH, Hou J, Zhang C, Li D, Li R, Liu Z, Weisleder N, Zhu D, Lin P, Ma J: Cardioprotection of recombinant human MG53 protein in a porcine model of ischemia and reperfusion injury. J Mol Cell Cardiol 2015, 80:10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]; In the large animal model of myocardial infarction, rhMG53 has demonstrated effecacy for cardiac protection.
- 11.Yao Y, Zhang B, Zhu H, Li H, Han Y, Chen K, Wang Z, Zeng J, Liu Y, Wang X, Li Y, He D, Lin P, Zhou X, Park KH, Bian Z, Chen Z, Gong N, Tan T, Zhou J, Zhang M, Ma J, Zeng C: MG53 permeates through blood-brain barrier to protect ischemic brain injury. Oncotarget 2016, 7:22474–22485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao W, Li H, Han X, Chen C, Zhang Y, Tai WL, Xia Z, Hei Z: MG53 anchored by dysferlin to cell membrane reduces hepatocyte apoptosis which induced by ischaemia/reperfusion injury in vivo and in vitro. J Cell Mol Med 2017, 21: 2503–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandler HL, Tan T, Yang C, Gemensky-Metzler AJ, Wehrman RF, Jiang Q, Peterson CMW, Geng B, Zhou X, Wang Q, Kaili D, Adesanya TMA, Yi F, Zhu H, Ma J: MG53 promotes corneal wound healing and mitigates fibrotic remodeling in rodents. Commun Biol 2019, 2:71. [DOI] [PMC free article] [PubMed] [Google Scholar]; MG53 is present in the eye and serves important physiological function to preserve cornea integrity following injury.
- 14.Guan F, Huang T, Wang X, Xing Q, Gumpper K, Li P, Song J, Tan T, Yang GL, Zang X, Zhang J, Wang Y, Yang Y, Liu Y, Zhang Y, Yang B, Ma J, Ma S: The TRIM protein Mitsugumin 53 enhances survival and therapeutic efficacy of stem cells in murine traumatic brain injury. Stem Cell Res Ther 2019, 10: 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sermersheim M, Kenney AD, Lin PH, McMichael TM, Cai C, Gumpper K, Adesanya TMA, Li H, Zhou X, Park KH, Yount JS, Ma J: MG53 suppresses interferon-beta and inflammation via regulation of ryanodine receptor-mediated intracellular calcium signaling. Nat Commun 2020, 11:3624. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports the anti-inflammatory function of MG53.
- 16.Kenney AD, Li Z, Bian Z, Zhou X, Li H, Whitson BA, Tan T, Cai C, Ma J, Yount JS: Recombinant MG53 protein protects mice from lethal influenza virus infection. Am J Respir Crit Care Med 2021, 203:254–257. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report demonstrates the dual function of MG53 as a tissue repair protein with anti-inflammatory property to protect lung function associated with viral infection.
- 17.Whitson BA, Mulier K, Li H, Zhou X, Cai C, Black SM, Tan T, Ma J, Beilman JG: MG53 as a novel therapeutic protein to treat acute lung injury. Mil Medicine 2021. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai C, Masumiya H, Weisleder N, Pan Z, Nishi M, Komazaki S, Takeshima H, Ma J: MG53 regulates membrane budding and exocytosis in muscle cells. J Biol Chem 2009, 284: 3314–3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisleder N, Takeshima H, Ma J: Mitsugumin 53 (MG53) facilitates vesicle trafficking in striated muscle to contribute to cell membrane repair. Commun Integr Biol 2009, 2: 225–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weisleder N, Takeshima H, Ma J: Immuno-proteomic approach to excitation–contraction coupling in skeletal and cardiac muscle: molecular insights revealed by the mitsugumins. Cell Calcium 2008, 43:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]; A good review article that summerizes the history behind the identification of the mitsugumin proteins.
- 21.Cai C, Lin P, Zhu H, Ko JK, Hwang M, Tan T, Pan Z, Korichneva I, Ma J: Zinc binding to MG53 protein facilitates repair of injury to cell membranes. J Biol Chem 2015, 290:13830–13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisleder N, Lin P, Zhao X, Orange M, Zhu H, Ma J: Visualization of MG53-mediated cell membrane repair using in vivo and in vitro systems. J Vis Exp 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatakeyama S: TRIM family proteins: roles in autophagy, immunity, and carcinogenesis. Trends Biochem Sci 2017, 42: 297–311. [DOI] [PubMed] [Google Scholar]
- 24.Gushchina LV, Kwiatkowski TA, Bhattacharya S, Weisleder NL: Conserved structural and functional aspects of the tripartite motif gene family point towards therapeutic applications in multiple diseases. Pharmacol Ther 2018, 185:12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HR, Lee MK, Kim CW, Kim M: TRIM proteins and their roles in the influenza virus life cycle. Microorganisms 2020, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, Wang L, Yue H, Whitson BA, Haggard E, Xu X, Ma J: MG53, A tissue repair protein with broad applications in regenerative medicine. Cells 2021, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Q, Bian Z, Jiang Q, Wang X, Zhou X, Park KH, Hsueh W, Whitson BA, Haggard E, Li H, Chen K, Cai C, Tan T, Zhu H, Ma J: MG53 does not manifest the development of diabetes in db/db mice. Diabetes 2020, 69:1052–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study utilizes db/db mice with ablation of MG53 or elevation of MG53 and reports negative impact of MG53 on insulin signaling and glucose handling in skeletal muscle.
- 28.Ma H, Liu J, Bian Z, Cui Y, Zhou X, Zhou X, Zhang B, Adesanya TM, Yi F, Park KH, Tan T, Chen Z, Zhu H: Effect of metabolic syndrome on mitsugumin 53 expression and function. PloS One 2015, 10, e0124128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weisleder N, Takizawa N, Lin P, Wang X, Cao C, Zhang Y, Tan T, Ferrante C, Zhu H, Chen PJ, Yan R, Sterling M, Zhao X, Hwang M, Takeshima M, Cai C, Cheng H, Takeshima H, Xiao RP, Ma J: Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Sci Transl Med 2012, 4:139ra85. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study establishes the safety and effecacy of rhMG53 protein to treat muscle injury.
- 30.Zhu H, Hou J, Roe JL, Park KH, Tan T, Zheng Y, Li L, Zhang C, Liu J, Liu Z, Ma J, Walters TJ: Amelioration of ischemia-reperfusion-induced muscle injury by the recombinant human MG53 protein. Muscle Nerve 2015, 52:852–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagre N, Cong X, Ji HL, Schreiber JM, Fu H, Pepper I, Warren S, Sill JM, Hubmayr RD, Zhao X: Inhaled TRIM72 protein protects ventilation injury to the lung through injury-guided cell repair. Am J Respir Cell Mol Biol 2018, 59:635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adesanya TMA, Russell M, Park KH, Zhou X, Sermersheim MA, Gumpper K, Koenig SN, Tan T, Whitson BA, Janssen PML, Lincoln J, Zhu H, Ma J: MG 53 protein protects aortic valve interstitial cells from membrane injury and fibrocalcific remodeling. J Am Heart Assoc 2019, 8, e009960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guan F, Zhou X, Li P, Wang Y, Liu M, Li F, Cui Y, Huang T, Yao M, Zhang Y, Ma J, Ma S: MG53 attenuates lipopolysaccharide-induced neurotoxicity and neuroinflammation via inhibiting TLR4/NF-kappaB pathway in vitro and in vivo. Prog Neuro-Psychopharmacol Biol Psychiatry 2019, 95:109684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cong X, Nagre N, Herrera J, Pearson AC, Pepper I, Morehouse R, Ji HL, Jiang D, Hubmayr RD, Zhao X: TRIM72 promotes alveolar epithelial cell membrane repair and ameliorates lung fibrosis. Respir Res 2020, 21:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Jiang M, Tan T, Narasimhulu CA, Xiao Y, Hao H, Cui Y, Zhang J, Liu L, Yang C, Li Y, Ma J, Verfaillie CM, Parthasarathy S, Zhu H, Liu Z: N-acetylcysteine prevents oxidized low-density lipoprotein-induced reduction of MG53 and enhances MG53 protective effect on bone marrow stem cells. J Cell Mol Med 2020, 24:886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu C, Hu YH, Han Y, Wang YB, Zhang Y, Zhang XQ, He DF, Ren HM, Liu YK, Wang HY, Tan T, Lin PH, Li HC, Rovin BH, Ma JJ, Zeng CY: MG53 protects against contrast-induced acute kidney injury by reducing cell membrane damage and apoptosis. Acta Pharmacol Sin 2020, 41:1457–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He B, Tang RH, Weisleder N, Xiao B, Yuan Z, Cai C, Zhu H, Lin P, Qiao C, Li J, Mayer C, Ma J, Xiao X: Enhancing muscle membrane repair by gene delivery of MG53 ameliorates muscular dystrophy and heart failure in delta-Sarcoglycan-deficient hamsters. Mol Ther : J Am Soc Gene Therap 2012, 20:727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]; AAV delivery of MG53 can be a potential approach to treat degenerative human diseases.
- 38.Corona BT, Garg K, Roe JL, Zhu H, Park KH, Ma J, Walters TJ: Effect of recombinant human MG53 protein on tourniquet-induced ischemia-reperfusion injury in rat muscle. Muscle Nerve 2014, 49:919–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gushchina LV, Bhattacharya S, McElhanon KE, Choi JH, Manring H, Beck EX, Alloush J, Weisleder N: Treatment with recombinant human MG53 protein increases membrane integrity in a mouse model of limb girdle muscular dystrophy 2B. Mol Ther : J Am Soc Gene Therap 2017, 25:2360–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bian Z, Wang Q, Zhou X, Tan T, Park KH, Kramer HF, McDougal A, Laping NJ, Kumar S, Adesanya TMA, Sermersheim M, Yi F, Wang X, Wu J, Gumpper K, Jiang Q, He D, Lin PH, Li H, Guan F, Zhou J, Kohr MJ, Zeng C, Zhu H, Ma J: Sustained elevation of MG53 in the bloodstream increases tissue regenerative capacity without compromising metabolic function. Nat Commun 2019, 10:4659. [DOI] [PMC free article] [PubMed] [Google Scholar]; Aside from increased tissue regenerative capacity, elevated MG53 in circulation can preserve muscle satellite cell function.
- 41.Wu HK, Zhang Y, Cao CM, Hu X, Fang M, Yao Y, Jin L, Chen G, Jiang P, Zhang S, Song R, Peng W, Liu F, Guo J, Tang L, He Y, Shan D, Huang J, Zhou Z, Wang DW, Lv F, Xiao RP: Glucose-sensitive myokine/cardiokine MG53 regulates systemic insulin response and metabolic homeostasis. Circulation 2019, 139: 901–914. [DOI] [PubMed] [Google Scholar]
- 42.Shan D, Guo S, Wu HK, Lv F, Jin L, Zhang M, Xie P, Wang Y, Song Y, Wu F, Lan F, Hu X, Cao CM, Zhang Y, Xiao RP: Cardiac ischemic preconditioning promotes MG53 secretion through H2O2-activated protein kinase C-delta signaling. Circulation 2020, 142:1077–1091. [DOI] [PubMed] [Google Scholar]
- 43.Han X, Chen D, Liufu N, Ji F, Zeng Q, Yao W, Cao M: MG53 protects against sepsis-induced myocardial dysfunction by upregulating peroxisome proliferator-activated receptor-alpha. Oxid Med Cell Longev 2020, 2020:7413693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma S, Wang Y, Zhou X, Li Z, Zhang Z, Wang Y, Huang T, Zhang Y, Shi J, Guan F: MG53 protects hUC-MSCs against inflammatory damage and synergistically enhances their efficacy in neuroinflammation injured brain through inhibiting NLRP3/caspase-1/IL-1beta Axis. ACS Chem Neurosci 2020, 11:2590–2601. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Xiao Y, Cui Y, Tan T, Narasimhulu CA, Hao H, Liu L, Zhang J, He G, Verfaillie CM, Lei M, Parthasarathy S, Ma J, Zhu H, Liu Z: Cell membrane damage is involved in the impaired survival of bone marrow stem cells by oxidized low-density lipoprotein. J Cell Mol Med 2014, 18:2445–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]; MG53 can protect against injury to stem cells.
- 46.Kim SC, Kellett T, Wang S, Nishi M, Nagre N, Zhou B, Flodby P, Shilo K, Ghadiali SN, Takeshima H, Hubmayr RD, Zhao X: TRIM72 is required for effective repair of alveolar epithelial cell wounding. Am J Physiol Lung Cell Mol Physiol 2014, 307:L449–L459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Y, Huang J, Liu D, Tan J, Peng Y, Yang J, Cui Y, He W, Luo G, Wu J: Mitsugumin 53 protects the kidney from severe burn injury in mice. Burns Trauma 2013, 1:128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao CM, Zhang Y, Weisleder N, Ferrante C, Wang X, Lv F, Zhang Y, Song R, Hwang M, Jin L, Guo J, Peng W, Li G, Nishi M, Takeshima H, Ma J, Xiao RP: MG53 constitutes a primary determinant of cardiac ischemic preconditioning. Circulation 2010, 121:2565–2574. [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Xie W, Zhang Y, Lin P, Han L, Han P, Wang Y, Chen Z, Ji G, Zheng M, Weisleder N, Xiao RP, Takeshima H, Ma J, Cheng H: Cardioprotection of ischemia/reperfusion injury by cholesterol-dependent MG53-mediated membrane repair. Circ Res 2010, 107:76–83. [DOI] [PubMed] [Google Scholar]
- 50.Paleo BJ, Madalena KM, Mital R, McElhanon KE, Kwiatkowski TA, Rose AL, Lerch JK, Weisleder N: Enhancing membrane repair increases regeneration in a sciatic injury model. PloS One 2020, 15, e0231194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li M, Li H, Li X, Zhu H, Xu Z, Liu L, Ma J, Zhang M: A bioinspired alginate-gum Arabic hydrogel with micro-/nanoscale structures for controlled drug release in chronic wound healing. ACS Appl Mater Interfaces 2017, 9: 22160–22175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cai C, Weisleder N, Ko JK, Komazaki S, Sunada Y, Nishi M, Takeshima H, Ma J: Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. J Biol Chem 2009, 284: 15894–15902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waddell LB, Lemckert FA, Zheng XF, Tran J, Evesson FJ, Hawkes JM, Lek A, Street NE, Lin P, Clarke NF, Landstrom AP, Ackerman MJ, Weisleder N, Ma J, North KN, Cooper ST: Dysferlin, annexin A1, and mitsugumin 53 are upregulated in muscular dystrophy and localize to longitudinal tubules of the T-system with stretch. J Neuropathol Exp Neurol 2011, 70: 302–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin P, Zhu H, Cai C, Wang X, Cao C, Xiao R, Pan Z, Weisleder N, Takeshima H, Ma J: Nonmuscle myosin IIA facilitates vesicle trafficking for MG53-mediated cell membrane repair. Faseb J : Off Publ Federation Am Soc Exp Biol 2012, 26:1875–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu H, Lin P, De G, Choi KH, Takeshima H, Weisleder N, Ma J: Polymerase transcriptase release factor (PTRF) anchors MG53 protein to cell injury site for initiation of membrane repair. J Biol Chem 2011, 286:12820–12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gumpper K, Ma H, Krishnamurthy K, Zhou X, Park KH, Sermersheim M, Zhou J, Tan T, Lin PH, Li L, Liu J, Zhu H, Ma J: Recombinant human MG53 protein preserves mitochondria integrity in cardiomyocytes during ischemia reperfusion-induced oxidative stress. bioRixv 2020, 10.1101/2020.02.06.936278. [DOI] [PMC free article] [PubMed] [Google Scholar]


