Abstract
Mouse 3T3 fibroblasts derived from fetuses lacking c-Jun were used to define an essential role of c-Jun, a main component of the transcription factor AP-1, in the cellular response to the alkylating agent methyl methanesulfonate (MMS). MMS represents the most potent and selective activator of the stress-induced kinases JNK/SAPK and p38, resulting in very efficient induction of c-Jun hyperphosphorylation and c-jun transcription. This agent induced apoptosis with high efficiency in wild-type cells but not in c-jun−/− cells. Resistance to apoptosis was accompanied by impaired expression of CD95 ligand (CD95-L), a well-known inducer of apoptosis. The addition of recombinant CD95-L restored apoptosis sensitivity in c-jun−/− fibroblasts. MMS-induced apoptosis in wild-type fibroblasts or human lymphocytes was strongly reduced by neutralizing CD95-L antibodies or transdominant negative FADD, confirming the importance of CD95 signalling in MMS-induced apoptosis. The loss-of-function approach in fibroblasts allowed the identification and dissection of c-Jun-dependent and -independent processes upstream or downstream of CD95 activation. We have found that c-Jun can act as a proapoptotic regulator in cells exposed to DNA damage via induction of CD95-L. Once activated, CD95-induced death signalling is not affected by the loss of c-Jun, demonstrating that only the initiation and not the execution of stress-induced apoptosis depends on c-Jun.
Mammalian cells are exposed to many environmental cues, including chemical carcinogens, tumor promoters, and radiation, that commonly induce damage to DNA. Cells respond to these agents by activating DNA repair enzymes and other protection functions or by inducing the apoptotic program (for reviews, see references 16 and 28). A critical role of the transcription factor AP-1 in the induction of the genetic programs regulating cell survival and apoptosis was suggested. For example, transcription of the members of the jun, fos, and ATF gene families, encoding AP-1 subunits, is highly induced in response to UV irradiation and alkylating agents. The enhanced de novo synthesis and the activation of preexisting and newly synthesized proteins by phosphorylation are required for the subsequent induction of the two main types of AP-1 target genes: those regulated by the c-Fos–c-Jun-specific 7-bp consensus AP-1 sequence 5′-TGAGTCA-3′, as found in the collagenase and stromelysin genes (1, 2), and genes such as c-jun, harboring the c-Jun–ATF-2-specific 8-bp motive sequence 5′-TTACCTCA-3′ in the promoter (3, 9, 22, 31, 49). However, neither the type of AP-1 target genes nor the specific function of individual members of the Jun, Fos, and ATF families in apoptosis and cell survival has been identified conclusively.
The best evidence for a specific function of AP-1 subunits in the mammalian response to DNA-damaging agents was provided by fibroblasts lacking c-fos. These cells exhibited an increased rate of apoptosis and, in consequence, reduced cell survival upon UV irradiation (44). Thus, c-Fos-regulated genes play a role in protecting cells from the cytotoxic effects of UV irradiation. On the other hand, the lack of c-Fos results in the loss of light-induced apoptosis of photoreceptors in retinal degeneration (21), demonstrating proapoptotic and antiapoptotic functions of c-Fos, depending on the cell type and extracellular stimuli. Inhibition of c-Jun either by a dominant negative mutant or by a neutralizing antibody led to reduced apoptosis upon nerve growth factor withdrawal in rat sympathetic neurons. Similarly, interference with Jun activity reduced apoptosis in human monoblastic leukemia cells upon induction of stress, indicating that c-Jun is required for programmed cell death (15, 23, 52). Correspondingly, ectopic expression of c-Jun in 3T3 fibroblasts increased apoptosis (10). The proposed role of c-Jun as a mediator of apoptosis is further supported by recent data describing the regulation and function of components of the mitogen-activated protein kinase (MAPK) cascades regulating AP-1 activity. Two types of MAPKs, JNK/SAPKs and p38, as well as common upstream kinases, including MEKK, are activated by genotoxic agents, such as UV and the alkylating agent methyl methanesulfonate (MMS), to phosphorylate and thereby activate transcription factors, including c-Jun and ATF-2 (reviewed in references 30 and 40). Persistent activation of JNK/SAPKs has been shown to induce apoptosis (11). Persistent activation of JNK/SAPKs by dominant active MEKK-1 resulted in hyperphosphorylation and activation of c-Jun and increased apoptosis in PC12 cells (37). Vice versa, inhibition of JNK/SAPK activity by a transdominant negative mutant conferred resistance to apoptosis induced by various genotoxic agents (57). In mice lacking the neuron-specific JNK isoform, JNK3, stimulation of the glutamate receptors does not result in excitotoxicity and apoptosis of hippocampal neurons (56). In line with these data, neuronal apoptosis induced by the excitatory amino acid kainate is absent in mice expressing a c-Jun mutant protein which contains amino acid substitutions at the critical JNK phosphorylation sites (8).
Despite these different lines of evidence suggesting an important role of AP-1 proteins in cell death, however, a direct link between AP-1 activity and the induction of specific initiators or executors of apoptosis has not yet been identified by functional means. Induction of apoptosis may be initiated by activation of the cell surface receptor CD95 through binding of its ligand, CD95-L. The subsequent cross-linking of CD95 results in the binding of adapter molecules, such as FADD and caspase 8, to the intracellular death domain, leading to the activation of the death-signalling cascade (5, 20, 34, 41, 46). Interestingly, transient transfection analyses with human T-lymphocytes showed that the induction of CD95-L by DNA-damaging agents depends on AP-1 and NF-κB activities (33). Moreover, a c-Jun–ATF-2-like binding site which mediates transcriptional activation of CD95-L and apoptosis in lymphocytes upon overexpression of MEKK1 was defined in the CD95-L promoter (17).
To elucidate the specific function of c-Jun and c-Jun-regulated target genes in apoptosis in response to genotoxic agents, we used immortalized 3T3 fibroblast cell lines with a targeted disruption of the c-jun gene (45). We analyzed the cellular response to the monofunctional alkylating agent MMS because it represents one of the most potent activators of c-jun transcription and c-Jun or ATF-2 hyperphosphorylation (38, 50) and the most selective inducer of the stress-induced signalling pathways involving JNK/SAPK and p38 (38, 50, 54). We found that c-Jun-deficient cells, in contrast to wild-type cells, failed to induce the apoptotic program upon MMS treatment. Lack of apoptosis was accompanied by a strongly reduced induction of AP-1 target genes, including the CD95-L gene. Apoptosis sensitivity in mutant cells could be restored upon the addition of recombinant CD95-L, demonstrating that c-Jun is not required for the expression and activity of downstream components of the CD95 death-signalling cascade. Reduction of MMS-induced apoptosis by dominant negative FADD or by neutralizing CD95-L antibodies further underlined the critical role of c-Jun-dependent CD95-L expression and CD95 signalling in the induction of apoptosis by genotoxic agents.
MATERIALS AND METHODS
Cell culture.
Wild-type and c-jun−/− 3T3 fibroblasts were cultured in Dulbecco's modified Eagle's medium containing 10% fetal calf serum. The following human cell lines (described in reference 26) were used: JURKAT (acute human T-cell leukemia); JAPO, a JURKAT subclone resistant to antibody to APO-1 (α-APO-1); BJAB (human B lymphoma); and BJAB-FADD-DN (human B lymphoma transfected with pcDNA3-FADD-DN). These cell lines were cultured in RPMI medium containing 10% fetal calf serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 25 mM HEPES, and 2 mM l-glutamine.
RNA isolation, Northern blot analysis, and Southern blot analysis.
Total RNA was prepared as described by Chomczynski and Sacchi (13). Northern blot and Southern blot analyses were performed as previously described (44). For the amplification of CD95-L and β-tubulin, the following primers were used: CD95-L, 5′-CAGCAGTGCCACTTCATCTTGG-3′ and 5′-TTCACTCCAGAGATCAGAGCGG-3′ (amplified fragment, 550 bp); and β-tubulin, 5′-TCACTGTGCCTGAACTTACC-3′ and 5′-GGAACATAGCCGTAAACTGC-3′ (amplified fragment, 317 bp).
Analysis of apoptosis and flow cytometry.
Cells were incubated with various doses of MMS. To measure DNA content (apoptotic nuclei), cells were harvested, washed with phosphate-buffered saline, and lysed in a hypotonic buffer containing 0.1% sodium citrate, 0.1% Triton X-100, and 50 μg of propidium iodide per ml. The fluorescence intensity of propidium iodide-stained nuclei was determined by flow cytometry (FACScan; Becton Dickinson, Heidelberg, Germany) with Cell Quest software. Segmented apoptotic nuclei were recognized by subdiploid DNA content. Early apoptotic changes were identified by staining of cells with fluorescein thiocyanate-conjugated annexin V (Bender, Vienna, Austria) and analysis by flow cytometry as described previously (25).
To measure the expression of CD95, cells were incubated with Jo2, an agonistic antibody specific for mouse CD95 raised in hamsters (Pharmingen, Hamburg, Germany), or nonspecific polyclonal hamster immunoglobulin G (Pharmingen) and analyzed by flow cytometry with a fluorescein isothiocyanate-conjugated secondary antihamster antibody.
Stimulation of cells.
Recombinant CD95-L was derived from supernatants of stably transfected 293 cells (27). Neutralizing CD95-L monoclonal antibodies MFL3 and NOK-1 were purchased from Pharmingen. Antibody α-APO-1 was prepared as previously described (14).
RESULTS
Reduced expression of AP-1 target genes in fibroblasts lacking c-Jun.
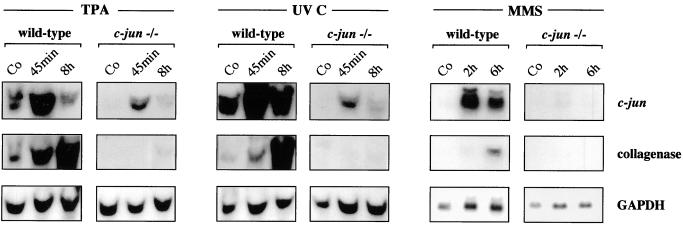
The interstitial collagenase (collagenase-3) gene and c-jun represent the most extensively analyzed AP-1 target genes used to measure changes in Jun- Fos- and Jun-ATF-dependent gene expression, respectively, in response to phorbol esters and genotoxic agents (1, 2, 9, 29, 31, 44, 49). To confirm the requirement of c-Jun in AP-1-dependent gene expression, we compared the mRNA levels of both genes in immortalized 3T3 fibroblasts derived from wild-type mouse embryos and embryos lacking c-Jun (45) in response to well-known inducers of AP-1 activity, such as the phorbol ester tetradecanoyl phorbol acetate (TPA), UV, and MMS. In c-jun−/− cells, part of the coding sequence of c-jun is replaced by the neomycin resistance gene, yielding a c-jun–neo fusion transcript whose expression is controlled by the intact c-jun promoter (45 and references therein). Since previous studies have shown that maximal induction of c-jun is reached after 45 min (for TPA and UV) (3, 47) or 2 h (for MMS) (54), RNA was prepared at these times. In addition, RNA was prepared 6 h posttreatment, representing the time point of maximal induction of collagenase (1, 19, 42). As shown in Fig. 1, the induction of c-jun and the collagenase gene was very efficient in wild-type cells. Importantly, the absence of c-Jun resulted in a decrease in basal-level expression and a strong reduction or complete loss of induction of the c-jun and collagenase genes, respectively (Fig. 1). Induction of the stromelysin-1 gene, representing another c-Jun–c-Fos-regulated target gene (29, 44), was observed in wild-type but not mutant cells (data not shown). These data demonstrate that the induction of both classes of c-Jun target genes regulated by either c-Jun–c-Fos or c-Jun–ATF-2 heterodimeric complexes is greatly impaired in c-Jun-deficient cells. Residual induction of the c-jun promoter in mutant cells might be explained by the ability of ATF-2 (or ATFa) homodimers to bind to the c-jun promoter and functionally compensate, at least in part, for c-Jun–ATF heterodimers (50).
FIG. 1.
Expression of AP-1 target genes is reduced in c-jun−/− cells. Wild-type and c-jun−/− cells were treated with TPA (100 ng/ml), UV-C (30 J/m2), or MMS (1 mM) or left untreated (Co), and total RNA was prepared at the indicated times. Probes specific for c-jun and the collagenase gene were used for Northern blot analysis. Levels of expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were determined as an internal control for equal loading.
Reduced apoptosis of c-jun−/− fibroblasts after treatment with MMS.
Previously, we have observed reduced AP-1 DNA binding and transactivation of AP-1 target genes in fibroblasts lacking c-Fos, leading to increased cell death, in response to UV irradiation (44). To analyze the consequences of defects in c-Jun-dependent gene expression for the cellular response to genotoxic agents, we compared the rate of MMS-induced apoptosis in wild-type and c-jun−/− fibroblasts.
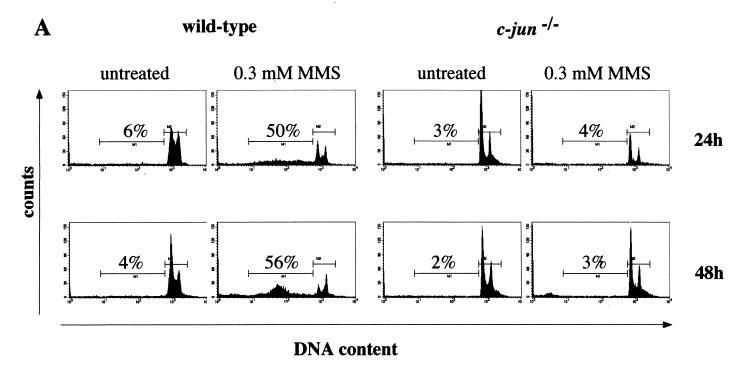
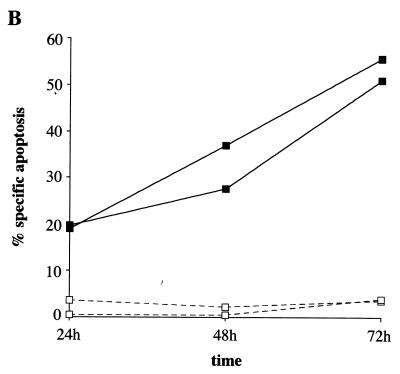
Apoptosis was measured by propidium iodide staining and flow cytometry. At 24 and 48 h after treatment with 300 μM MMS, 50 and 56%, respectively, of wild-type cells showed a hypodiploid (sub-G1) DNA content, reflecting apoptosis (Fig. 2A). Apoptosis was also induced at 200 μM MMS, although at a lower efficiency (data not shown). Surprisingly, c-jun−/− cells did not exhibit a significant increase in apoptosis at 24 h (4%) and 48 h (3%), even at 300 μM MMS (Fig. 2A). Also, at 72 h following MMS treatment, wild-type cells showed a much higher rate of apoptosis than c-Jun-deficient cells (data not shown). To confirm these differences in apoptosis by an independent assay, we measured staining of phosphatidylserine exposed on the outer cell membrane by flow cytometry. In agreement with the data obtained by propidium iodide incorporation, annexin V staining showed that at 24, 48 and 72 h following MMS treatment, only wild-type and not mutant cells exhibited a higher percentage of apoptosis (Fig. 2B). To rule out the possibility that the reduced rate of proliferation of c-jun−/− cells (45) is responsible for the reduced rate of apoptosis, we measured apoptosis 6 days after MMS treatment by propidium iodine staining. At this late time point, apoptosis in c-jun−/− cells was still below the rate of apoptosis in wild-type cells measured at any time point after MMS treatment (data not shown). These data demonstrate that c-Jun is required for the efficient induction of apoptosis in response to the alkylating agent MMS.
FIG. 2.
The absence of c-Jun confers resistance to apoptosis after treatment with MMS. (A) DNA fluorescence histograms of wild-type and c-jun−/− cells stained with propidium iodide 24 and 48 h after treatment with 300 μM MMS. The percentage of cells with a hypodiploid (sub-G1) DNA content indicative of apoptosis is shown. The two horizontal bars represent apoptotic cells (M1) and intact cells in the different phases of the cell cycle (M2). (B) Apoptosis of MMS-treated (400 μM) wild-type (■) and c-jun−/− (□) cells 24, 48, or 72 h posttreatment was measured by annexin V staining and flow cytometry. Specific apoptosis was calculated as described previously (27) (see also Fig. 4B). Each point represents the average of three independent experiments for which the deviation was not more than 20%.
Efficient induction of CD95-L expression by MMS in wild-type but not in c-jun−/− cells.
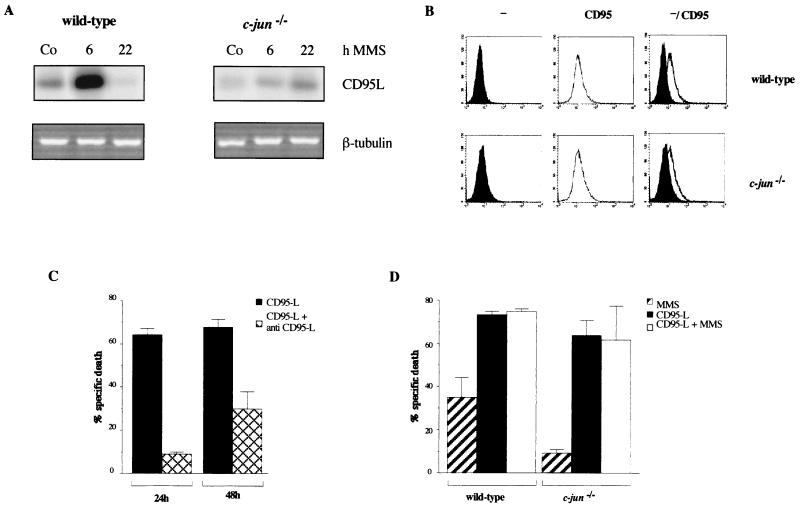
Recently, in lymphoid cells, the induction of a master regulator of apoptosis, CD95-L, by DNA-damaging agents was found to depend on the presence of both NF-κB- and AP-1-binding sites in the CD95-L promoter (33). Having found defects in the induction of known AP-1-target genes in c-jun−/− cells (Fig. 1), we compared the expression of CD95-L in wild-type and mutant cells in response to MMS. For wild-type fibroblasts, we observed a strong increase in CD95-L expression upon MMS treatment (Fig. 3A) which resembled the kinetics of induction of collagenase and stromelysin, reaching maximal levels at 6 h and returning to basal levels or below at 22 h poststimulation (Fig. 3A and data not shown). In contrast, basal-level expression of CD95-L in c-jun−/− cells was reduced, and induction by MMS was almost completely absent (Fig. 3A). Only at late time points after MMS treatment was a small increase observed. However, the level of CD95-L transcripts in MMS-treated mutant cells was still below the basal level seen in untreated wild-type cells (Fig. 3A). In contrast to those of CD95-L, the levels of expression of its receptor, CD95, were similar in wild-type and mutant cells (Fig. 3B). These data identify the CD95-L gene as a novel c-Jun-regulated target gene and open the possibility that the loss of induction of this gene is responsible for the defects in MMS-induced apoptosis in c-jun−/− cells.
FIG. 3.
Expression of CD95-L is impaired in c-jun−/− fibroblasts. (A) Wild-type and c-jun−/− cells were treated with 400 μM MMS, and total RNA was prepared 6 and 22 h later. The expression of CD95-L was detected by reverse transcription-PCR and quantified by Southern blotting. As an internal control, the PCR product of β-tubulin is shown. (B) Levels of CD95 in wild-type and c-jun−/− cells were measured by staining the cells with nonspecific hamster immunoglobulin G (−) or a CD95-specific antibody (Jo2) (CD95) followed by flow cytometry. (C) Wild-type cells were treated with supernatant from 293 cells containing recombinant CD95-L protein in the presence or absence of a neutralizing CD95-L antibody (NOK-1) (see also Fig. 4). After 24 or 48 h, apoptosis was measured by annexin V staining and flow cytometry. The experiment was performed three times in duplicate, and averages (± standard deviations) are shown. The rate of apoptosis in wild-type or c-jun−/− cells treated with supernatant from 293 cells transfected with an empty vector was below 10% (data not shown). (D) Wild-type and c-jun−/− cells were treated with either 400 μM MMS, supernatant from 293 cells containing recombinant CD95-L protein, or both (CD95-L plus MMS). After 24 h, apoptosis was measured by annexin V staining and flow cytometry. The experiment was performed three times in duplicate, and averages (± standard deviations) are shown.
Addition of exogenous CD95-L restores the apoptotic program in cells lacking c-Jun.
To analyze whether c-Jun-dependent expression of CD95-L might be a rate-limiting step in MMS-induced apoptosis that is missing in c-jun−/− cells, we examined the rate of apoptosis upon the addition of recombinant CD95-L protein. As shown in Fig. 3C for wild-type fibroblasts, apoptosis was very efficiently induced by recombinant CD95-L. The apoptotic effect of CD95-L could be reversed by the addition of neutralizing CD95-L antibodies, which prevent the binding of the ligand to its receptor (Fig. 3C), demonstrating the specificity of the recombinant protein. Importantly, in wild-type and c-jun−/− cells, the rate of apoptosis was greatly increased with similar efficiencies after the addition of CD95-L (Fig. 3D). These results imply that the expression or activity of cellular components acting downstream of activated CD95 is not significantly affected by the lack of c-Jun expression. The induction of apoptosis in both wild-type and mutant cells was also observed through agonistic antibody (Jo2) binding to CD95 (data not shown). Interestingly, neither in wild-type nor in mutant cells was CD95-induced apoptosis significantly amplified in the presence of MMS (Fig. 3D), suggesting that CD95 signalling is the major pathway which mediates MMS-induced apoptosis.
MMS-induced apoptosis can be inhibited by blocking of the CD95 signalling pathway.
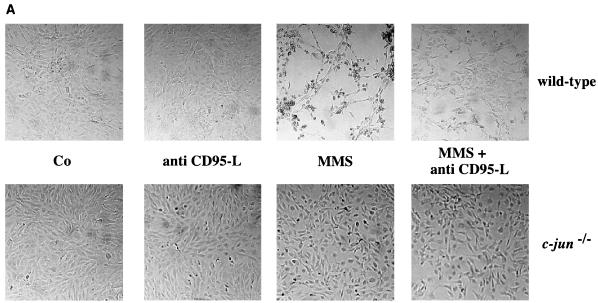
The strong induction of CD95-L by MMS in wild-type cells and the ability of recombinant CD95-L to induce apoptosis in c-jun−/− cells suggest an important role of this protein in MMS-induced apoptosis. To confirm this assumption, we measured the efficiency of MMS-induced apoptosis in the presence of neutralizing anti-human or anti-mouse CD95-L-specific antibodies. MMS treatment (200 or 400 μM) resulted in increased apoptosis in wild-type but not in mutant cells which was easily detectable by changes in cell morphology (Fig. 4A) or differences in annexin V staining (Fig. 4B). In wild-type cells, the MMS-induced apoptosis was strongly reduced by neutralizing CD95-L antibodies (Fig. 4). In line with the lack of CD95-L expression and induction of apoptosis in c-jun−/− cells, neither untreated nor MMS-treated mutant cells were significantly affected by neutralizing CD95-L antibodies (Fig. 4).
FIG. 4.
MMS-induced apoptosis can be inhibited by neutralizing CD95-L antibodies. (A) Wild-type and c-jun−/− cells were left untreated (Co) or were treated with 300 μM MMS in the presence or absence of a neutralizing anti–mouse CD95-L antibody (30 μg of MFL3 per ml) (anti CD95-L). Apoptosis was detected by microscopy. Very similar results were obtained with a neutralizing anti–human CD95-L antibody (NOK-1). (B) Wild-type and c-jun−/− cells were left untreated (Co) or were treated with 200 μM MMS in the presence (□) or absence (■) of a neutralizing CD95-L antibody (30 μg of NOK-1 per ml). Apoptosis was measured by annexin V staining and flow cytometry. A representative of three experiments with similar outcomes is shown. The standard deviation was less than 10%. When we calculated the percentage of specific apoptosis according to the formula 100 × [(percent experimental apoptosis − percent spontaneous apoptosis in the control/(100 − percent spontaneous apoptosis in the control)], MMS-induced apoptosis in wild-type cells in the absence and presence of the neutralizing antibody was 38 and 18%, respectively.
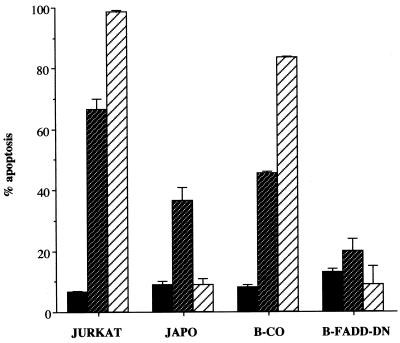
Additional evidence for the importance of the CD95 pathway for MMS-induced apoptosis was obtained with an acute T-cell leukemia cell line (JURKAT) or a human B-lymphoma cell line (BJAB). In both cell lines, apoptosis was induced by direct triggering of CD95 with a CD95-specific agonistic antibody (α-APO-1) or, to a lesser extent, by MMS (Fig. 5). A subclone of JURKAT cells that is resistant to apoptosis induced by α-APO-1 exhibited a decrease in the rate of apoptosis in response to MMS (Fig. 5). Residual apoptosis in these cells may be explained by MMS-dependent induction of other death-inducing ligands, such as tumor necrosis factor alpha (TNF-α) and TNF-related apoptosis-inducing ligand (TRAIL; for reviews, see references 5, 34, and 41), or triggering of death receptor-independent pathways. Correspondingly, BJAB cells stably expressing transdominant negative FADD (12), which blocks CD95-induced death signalling (Fig. 5), exhibited an almost complete block of MMS-induced apoptosis (Fig. 5).
FIG. 5.
MMS-induced apoptosis in human T and B cells. JURKAT cells, a resistant subclone (JAPO), B-lymphoma cells (B-CO), or B-lymphoma cells expressing a dominant negative version of FADD (B-FADD-DN) were treated with 400 μM MMS ( ) or a CD95-specific agonistic antibody (α-APO-1; 1 μg/ml) (▨). Apoptosis was measured by annexin V staining and flow cytometry 48 h following treatment. The numbers are averages of three experiments, each performed in duplicate. Error bars show standard deviations. ■, untreated cells.
Taken together, the data obtained for different cell types demonstrate that the binding of CD95-L to its receptor and signalling via the CD95 pathway are essential for efficient MMS-induced apoptosis. Thus, the absence of CD95-L induction in c-jun−/− fibroblasts very likely is responsible for the failure of these cells to undergo apoptosis.
DISCUSSION
AP-1 has been suggested to play an essential role in the cellular responses to genotoxic agents. This role includes the regulation of genetic programs associated with protection and survival functions and the induction of apoptosis. Here we have genetically defined the function of a specific subunit of AP-1, c-Jun, in apoptosis induced by alkylating agents, such as MMS. This class of genotoxic agents represents the most potent inducer of c-jun expression and the transactivation function of c-Jun protein (38, 50, 54). Fibroblasts with a targeted null mutation in c-jun exhibit a defect in MMS-induced apoptosis. We provide different lines of evidence that this phenotype is due to reduced expression of a major initiator of apoptosis, CD95-L, whereas events downstream of CD95 signalling function in a c-Jun-independent manner.
First, the expression of the CD95-L gene is highly induced by MMS in wild-type fibroblasts but is almost completely abolished in c-Jun-deficient cells, identifying the CD95-L gene as a novel c-Jun target gene. This conclusion is in line with previous findings showing strongly reduced CD95-L induction in cells expressing a c-Jun mutant protein which lacks the critical JNK/SAPK phosphorylation sites in its transactivation domain (8) and a reduction of apoptosis and CD95-L expression in PC12 cells upon overexpression of a c-Jun mutant lacking the JNK/SAPK phosphorylation sites (37). Second, the addition of recombinant CD95-L induced apoptosis with a high efficiency in both wild-type and mutant fibroblasts. Upon binding, trimerization of the receptor, CD95, is induced, leading to the recruitment of adaptor molecules, such as FADD and procaspase molecules. In turn, a cascade of downstream caspases is induced, leading to degradation of chromosomal DNA and cell death (for reviews, see references 20, 34, and 41). Obviously, c-Jun is not absolutely required for the expression and activity of these cellular components located downstream of CD95, because we were able to restore CD95-L-induced apoptosis in mutant cells. In agreement with our findings, in JURKAT T cells the overexpression of a dominant negative c-Jun mutant which blocked nonselectively total AP-1 activity interfered with AP-1-dependent gene expression but not with CD95-induced apoptosis (36). Induction of the apoptotic program by recombinant CD95-L demonstrates that the lack of apoptosis in the mutant cells cannot be explained by a constitutive upregulation of antiapoptotic genes. We have found the activity of the transcription factor NF-κB, which has been described to induce the expression of survival genes, depending on the cell type and treatment (7, 39, 48, 53), even to be slightly reduced in c-jun−/− cells. Moreover, we did not detect major differences in the expression of members of the Bcl-2 family in wild-type and c-jun−/− cells (unpublished data).
In the presence of neutralizing CD95-L antibodies, MMS-induced apoptosis is not completely blocked. At present, we cannot exclude the possibility that the activation of other death-inducing ligands, such as TRAIL and TNF-α, also contributes to MMS-induced apoptosis. However, when we measured the expression of these ligands, only very low levels of TRAIL were found in both wild-type and mutant fibroblasts, and these were not further increased upon MMS treatment. Moreover, neither in wild-type nor in c-jun−/− fibroblasts was the induction of apoptosis by recombinant TRAIL observed. The amount of TNF-α expressed in wild-type and c-jun−/− cells was below the level of detection, even after MMS treatment (unpublished data). These data, together with the previous finding that TNF-α treatment of embryonic fibroblasts does not induce apoptosis (55), strongly suggest that neither TNF-α nor TRAIL contributes to MMS-induced apoptosis in fibroblasts. In contrast, in T (JURKAT) and B (BJAB) cell lines, apoptosis can be efficiently induced by activation with TNF-α, TRAIL, and CD95-L (26). We found these cells also highly susceptible to MMS-induced apoptosis. Whether or not c-Jun is required for apoptosis in these cells remains to be determined. Nevertheless, the reduction of MMS-induced apoptosis in cells expressing a dominant negative mutant of FADD further underlines the critical role of death receptor-induced signalling in alkylating agent-induced apoptosis, a role which is not restricted to fibroblasts but can be extended to lymphoid cells and most likely other cell types.
What are the mechanisms of MMS-induced expression of CD95-L? Alkylating agents, such as MMS, are very efficient inducers of the JNK/SAPK and p38 pathways in many cells, including fibroblasts and JURKAT cells, but do not affect the growth factor-induced Ras-Raf-MAPKK-MAPK pathway (50, 54; D. Wilhelm, A. Dieckmann, and P. Angel, unpublished data). In the presence of an inhibitor of p38, MMS-induced expression of c-jun is significantly reduced and correlates with a reduced rate of apoptosis (I. Herr, D. Wilhelm, and P. Angel, unpublished data). These data strongly suggest that both JNK/SAPK and p38 MAPKs are required for the full activation of MMS-induced c-jun transcription and c-Jun-dependent CD95-L expression. In fact, numerous reports describe a correlation among JNK/SAPK activation, CD95-L expression, and the induction of apoptosis (8, 18, 24, 33, 35, 56; for a review, see reference 6).
Most likely, the critical transcription factors serving as a substrate of JNK/SAPKs and p38 to regulate c-jun and CD95-L gene expression are c-Jun and ATF-2. These proteins are most efficiently phosphorylated and, in turn, activated by alkylating agents, and c-Jun–ATF-2 heterodimers have been identified as binding to and activating the c-jun promoter (50). They also bind to the CD95-L promoter to mediate the induction of CD95-L and apoptosis in response to MEKK-1 overexpression (17, 18). On the other hand, in the human CD95-L promoter, NF-κB and Jun-Fos recognition sequences have been defined as being required for induction by genotoxic agents (33). However, in contrast to c-jun, c-fos was only weakly induced by alkylating agents (unpublished data). Treatment of cells with activators of protein kinase C, such as the phorbol ester TPA, which hardly activate JNK/SAPK and p38 kinase activities (54) and which induce neither CD95-L expression nor apoptosis in wild-type or c-jun−/− fibroblasts (A. Kolbus, I. Herr, and P. Angel, unpublished data) are very efficient inducers of c-fos expression (4). These data strongly suggest that c-Jun–ATF-2 rather than c-Jun–Fos is responsible for JNK/SAPK- and p38-mediated transcriptional activation of c-jun and, subsequently, CD95-L in response to alkylating agents.
While JNK/SAPKs and p38 are required for the induction of CD95-L, consensus has not been reached for the requirement of JNK/SAPK (and p38) activation downstream of activated death receptors. Depending on the cell type and death-inducing ligand, data either supporting a function of JNK/SAPKs in apoptosis or describing a lack of correlation between JNK activation and cell death have been obtained. In some cases, JNK activation even interfered with apoptosis (for a review, see reference 6 and references therein). When we measured JNK/SAPK activity in wild-type and c-jun−/− cells, no induction was detectable in response to recombinant CD95-L (unpublished data). Treatment of wild-type and mutant cells with MMS induced a characteristic transient activation of kinase activity that was seen in other cell types (50) and that returned to basal levels after 6 h. No additional increase in JNK activity was observed at later time points, when CD95-L induction reached maximal levels and apoptosis became detectable (unpublished data). These data represent another line of evidence that JNK/SAPKs activation is required for the initial phase of the apoptotic program in response to alkylating agents, transcriptional activation of CD95-L, but is not absolutely required for the cellular events downstream of activated CD95 leading to cell death. In agreement with this concept, in thymocytes from JNK2 knockout mice, apoptosis induced by an agonistic CD95 antibody is not affected (43).
In addition to MMS, we also observed an almost complete loss of CD95-L induction in response to UV irradiation, as measured by reverse transcription-PCR and Western blot analyses. Accordingly, the rate of apoptosis is reduced in c-jun−/− fibroblasts, as determined by measurement of lactate dehydrogenase release, sub-G1 DNA content, and annexin V staining (unpublished data). These data are in agreement with the reduction of UV-induced apoptosis in fibroblasts expressing a c-Jun mutant which lacks the critical JNK/SAPK phosphorylation sites (8). Interestingly, others have observed even enhanced rates of UV-induced apoptosis in primary fibroblasts from c-Jun-deficient mouse embryos (55), suggesting that c-Jun, like c-Fos (21, 44), can exhibit either proapoptotic or antiapoptotic activities, depending on the cell type and intracellular and extracellular conditions.
Most recently, c-Jun-deficient fibroblasts have been used to establish a critical role of c-Jun in the regulation of cell proliferation (32, 45, 55). Obviously, c-Jun represents an intersection of multiple pathways which regulate cell proliferation and apoptosis, two apparently opposing phenotypes. Some of the specificity of c-Jun function is presumably based on the choice of the heterodimeric partner, dictating sequence specificity and, in turn, the subset of AP-1 target genes to be addressed. Reintroduction of c-Jun mutants that select and sequester a preferred partner subunit (51) may help to identify subgroups of c-Jun target genes involved in either cell growth and/or apoptosis. A shift in the equilibrium of the expression of such distinct classes of c-Jun target genes, in conjunction with alterations in c-Jun-independent pathways, will contribute to the decision of the cell to proliferate, to activate survival factors, or to induce the genetic program of cell death in response to extracellular signals, including genotoxic agents.
ACKNOWLEDGMENTS
We thank Jan-Paul Medema, Christian Behrens, and Klaus Hexel for help with cytometry; Melanie Sator-Schmidt and Sibylle Teurich for technical assistance; Dagmar Wilhelm and Andreas Dieckmann for sharing unpublished data; and Marina Schorpp-Kistner, Axel Behrens, and Kanaga Sabapathy for critical reading of the manuscript.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (He-551/8-2) and the German-Israeli Cooperation in Cancer Research and the TMR and Biomedicine and Health programs (CT96-0044 and CT BMH4-98-3505) of the European Economic Community.
REFERENCES
- 1.Angel P, Baumann I, Stein B, Delius H, Rahmsdorf H J, Herrlich P. 12-O-Tetradecanoyl-phorbol-13-acetate induction of the human collagenase gene is mediated by an inducible enhancer element located in the 5′-flanking region. Mol Cell Biol. 1987;7:2256–2266. doi: 10.1128/mcb.7.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angel P, Imagawa M, Chiu R, Stein B, Imbra R J, Rahmsdorf H J, Jonat C, Herrlich P, Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987;49:729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- 3.Angel P, Hattori K, Smeal T, Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988;55:875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- 4.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 5.Ashkenazi A, Dixit V M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 6.Basu S, Kolesnick R. Stress signals for apoptosis: ceramide and c-Jun kinase. Oncogene. 1998;17:3277–3285. doi: 10.1038/sj.onc.1202570. [DOI] [PubMed] [Google Scholar]
- 7.Beg A A, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 8.Behrens A, Sibilia M, Wagner E F. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat Genet. 1999;21:326–329. doi: 10.1038/6854. [DOI] [PubMed] [Google Scholar]
- 9.Benbrook D M, Jones N C. Heterodimer formation between CREB and JUN proteins. Oncogene. 1990;5:295–302. [PubMed] [Google Scholar]
- 10.Bossy-Wetzel E, Bakiri L, Yaniv M. Induction of apoptosis by the transcription factor c-Jun. EMBO J. 1997;16:1695–1709. doi: 10.1093/emboj/16.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y R, Wang X, Templeton D, Davis R J, Tan T H. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J Biol Chem. 1996;271:31929–31936. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- 12.Chinnaiyan A M, Tepper C G, Seldin M F, O'Rourke K, Kischkel F C, Hellbardt S, Krammer P H, Peter M E, Dixit V M. FADD/MORT1 is a common mediator of CD95 (Fas/APO-1) and tumor necrosis factor receptor-induced apoptosis. J Biol Chem. 1996;271:4961–4965. doi: 10.1074/jbc.271.9.4961. [DOI] [PubMed] [Google Scholar]
- 13.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol/chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 14.Dhein J, Walczak H, Baumler C, Debatin K M, Krammer P H. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 15.Estus S, Zaks W J, Freeman R S, Gruda M, Bravo R, Johnson E M. Altered gene expression in neurons during programmed cell death: identification of c-jun as necessary for neuronal apoptosis. J Cell Biol. 1994;127:1717–1727. doi: 10.1083/jcb.127.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evan G, Littlewood T. A matter of life and cell death. Science. 1998;281:1317–1322. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]
- 17.Faris M, Latinis K M, Kempiak S J, Koretzky G A, Nel A. Stress-induced Fas ligand expression in T cells is mediated through a MEK kinase 1-regulated response element in the Fas ligand promoter. Mol Cell Biol. 1998;18:5414–5424. doi: 10.1128/mcb.18.9.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faris M, Kokot N, Latinis K, Kasibhatla S, Green D R, Koretzky G A, Nel A. The c-Jun N-terminal kinase cascade plays a role in stress-induced apoptosis in Jurkat cells by up-regulating Fas ligand expression. J Immunol. 1998;160:134–144. [PubMed] [Google Scholar]
- 19.Gack S, Vallon R, Schaper J, Rüther U, Angel P. Phenotypic alterations in Fos-transgenic mice correlate with changes in Fos/Jun-dependent collagenase type I expression. J Biol Chem. 1994;269:10363–10369. [PubMed] [Google Scholar]
- 20.Green D R. Apoptotic pathways: the roads to ruin. Cell. 1998;94:695–698. doi: 10.1016/s0092-8674(00)81728-6. [DOI] [PubMed] [Google Scholar]
- 21.Hafezi F, Steinbach J P, Marti A, Munz K, Wang Z Q, Wagner E F, Aguzzi A, Reme C E. The absence of c-fos prevents light-induced apoptotic cell death of photoreceptors in retinal degeneration in vivo. Nat Med. 1997;3:346–349. doi: 10.1038/nm0397-346. [DOI] [PubMed] [Google Scholar]
- 22.Hai T A, Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci USA. 1991;88:3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ham J, Babij C, Whitfield J, Pfarr C M, Lallemand D, Yaniv M, Rubin L L. A c-Jun dominant negative mutant protects sympathetic neurons against programmed cell death. Neuron. 1995;14:927–939. doi: 10.1016/0896-6273(95)90331-3. [DOI] [PubMed] [Google Scholar]
- 24.Herdegen T, Claret F-X, Kallunki T, Villalba M A, Hunter T, Karin M. Lasting N-terminal phosphorylation of c-Jun and activation of c-Jun N-terminal kinases after neuronal injury. J Neurosci. 1998;18:5124–5135. doi: 10.1523/JNEUROSCI.18-14-05124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herr I, Wilhelm D, Bohler T, Angel P, Debatin K M. Activation of CD95 (APO-1/Fas) signaling by ceramide mediates cancer therapy-induced apoptosis. EMBO J. 1997;16:6200–6208. doi: 10.1093/emboj/16.20.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herr I, Wilhelm D, Bohler T, Angel P, Debatin K M. JNK/SAPK activity is not sufficient for anticancer therapy-induced apoptosis involving CD95-L, TRAIL and TNF-alpha. Int J Cancer. 1999;80:417–424. doi: 10.1002/(sici)1097-0215(19990129)80:3<417::aid-ijc14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 27.Herr I, Villalba M A, Kurz E, Roncaioli P, Schenkel J, Cifone M G, Debatin K M. FK506 prevents stroke-induced generation of ceramide and apoptosis signaling. Brain Res. 1999;826:210–219. doi: 10.1016/s0006-8993(99)01288-3. [DOI] [PubMed] [Google Scholar]
- 28.Herrlich P, Blattner C, Knebel A, Bender K, Rahmsdorf H J. Nuclear and non-nuclear targets of genotoxic agents in the induction of gene expression. Shared principles in yeast, rodents, man and plants. Biol Chem. 1997;378:1217–1229. doi: 10.1515/bchm.1997.378.11.1217. [DOI] [PubMed] [Google Scholar]
- 29.Hu E, Mueller E, Oliviero S, Papaioannou V E, Johnson R, Spiegelman B M. Targeted disruption of the c-fos gene demonstrates c-fos-dependent and -independent pathways for gene expression stimulated by growth factors or oncogenes. EMBO J. 1994;13:3094–3103. doi: 10.1002/j.1460-2075.1994.tb06608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ip Y T, Davis R J. Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- 31.Ivashkiv L B, Liou H C, Kara C J, Lamph W W, Verma I M, Glimcher L H. mXBP/CRE-BP2 and c-Jun form a complex which binds to the cyclic AMP, but not to the 12-O-tetradecanoylphorbol-13-acetate, response element. Mol Cell Biol. 1990;10:1609–1621. doi: 10.1128/mcb.10.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson R S, van Lingen B, Papaioannou V E, Spiegelman B M. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev. 1993;7:1309–1317. doi: 10.1101/gad.7.7b.1309. [DOI] [PubMed] [Google Scholar]
- 33.Kasibhatla S, Brunner T, Genestier L, Echeverri F, Mahboubi A, Green D R. DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-kappaB and AP-1. Mol Cell. 1998;1:543–551. doi: 10.1016/s1097-2765(00)80054-4. [DOI] [PubMed] [Google Scholar]
- 34.Krammer P H. CD95(APO-1/Fas)-mediated apoptosis: live and let die. Adv Immunol. 1999;71:163–210. doi: 10.1016/s0065-2776(08)60402-2. [DOI] [PubMed] [Google Scholar]
- 35.Kuan C-Y, Yang D, Samata Roy D R, Davis R J, Rakic P, Flavell R A. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22:667–676. doi: 10.1016/s0896-6273(00)80727-8. [DOI] [PubMed] [Google Scholar]
- 36.Lenczowski J M, Dominiguez L, Eder A M, King L B, Zacharchuk C M, Ashwell J D. Lack of a role for Jun kinase and AP-1 in Fas-induced apoptosis. Mol Cell Biol. 1997;17:170–181. doi: 10.1128/mcb.17.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le-Niculescu H, Bonfoco E, Kasuya Y, Claret F X, Green D R, Karin M. Withdrawal of survival factors results in activation of the JNK pathway in neuronal cells leading to fas ligand induction and cell death. Mol Cell Biol. 1999;19:751–763. doi: 10.1128/mcb.19.1.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Z G, Baskaran R, Lea Chou E T, Wood L D, Chen Y, Karin K, Wang J Y. Three distinct signalling responses by murine fibroblasts to genotoxic stress. Nature. 1996;384:273–276. doi: 10.1038/384273a0. [DOI] [PubMed] [Google Scholar]
- 39.Liu Z G, Hsu H, Goeddel D V, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 40.Minden A, Karin M. Regulation and function of the JNK subgroup of MAP kinases. Biochim Biophys Acta. 1997;1333:85–104. doi: 10.1016/s0304-419x(97)00018-8. [DOI] [PubMed] [Google Scholar]
- 41.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 42.Reichardt H M, Kaestner K H, Tuckermann T, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schütz G. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- 43.Sabapathy K, Hu Y, Kallunki T, Schreiber M, David J-P, Jochum W, Wagner E F, Karin M. JNK2 is required for efficient T-cell activation and apoptosis but not for normal lymphocyte development. Curr Biol. 1999;9:116–125. doi: 10.1016/s0960-9822(99)80065-7. [DOI] [PubMed] [Google Scholar]
- 44.Schreiber M, Baumann B, Cotten M, Angel P, Wagner E F. Fos is an essential component of the mammalian UV response. EMBO J. 1995;14:5338–5349. doi: 10.1002/j.1460-2075.1995.tb00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schreiber M, Kolbus A, Piu F, Szabowski A, Möhle-Steinlein U, Tian F, Karin M, Angel P, Wagner E F. Control of cell cycle progression by c-Jun is p53 dependent. Genes Dev. 1999;13:607–613. doi: 10.1101/gad.13.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter M E. Apoptosis signaling by death receptors. Eur J Biochem. 1998;254:439–459. doi: 10.1046/j.1432-1327.1998.2540439.x. [DOI] [PubMed] [Google Scholar]
- 47.Stein B, Angel P, van Dam H, Ponta H, Herrlich P, van der Eb A, Rahmsdorf H J. UV induced c-Jun gene transcription: two AP-1 like binding sites mediate the response. Photochem Photobiol. 1992;55:409–415. doi: 10.1111/j.1751-1097.1992.tb04255.x. [DOI] [PubMed] [Google Scholar]
- 48.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 49.van Dam H, Duyndam M, Rottier R, Bosch A, de Vries Smits L, Herrlich P, Zantema A, Angel P, van der Eb A. Heterodimer formation of cJun and ATF-2 is responsible for induction of c-junby the 243 amino acid adenovirus E1A protein. EMBO J. 1993;12:479–487. doi: 10.1002/j.1460-2075.1993.tb05680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Dam H, Wilhelm D, Herr I, Steffen A, Herrlich P, Angel P. ATF-2 is preferentially activated by stress-activated protein kinases to mediate c-jun induction in response to genotoxic agents. EMBO J. 1995;14:1798–1811. doi: 10.1002/j.1460-2075.1995.tb07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Dam H, Huguier S, Kooistra K, Baguet J, Vial E, van der Eb A, Herrlich P, Castellazzi M. Autocrine growth and anchorage independence: two complementing Jun-controlled genetic programs of cellular transformation. Genes Dev. 1998;12:1227–1239. doi: 10.1101/gad.12.8.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verheij M, Bose R, Lin X H, Yao B, Jarvis W D, Grant S, Birrer M J, Szabo E, Zon L I, Kyriakis J M, Haimovitz-Friedmann A, Fuks Z, Kolesnick R N. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 53.Wang C Y, Mayo M W, Baldwin A S., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 54.Wilhelm D, Bender K, Knebel A, Angel P. The level of intracellular glutathione is a key regulator for the induction of stress-activated signal transduction pathways including Jun N-terminal protein kinases and p38 kinase by alkylating agents. Mol Cell Biol. 1997;17:4792–4800. doi: 10.1128/mcb.17.8.4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wisdom R, Johnson R S, Moore C. c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. EMBO J. 1999;18:188–197. doi: 10.1093/emboj/18.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang D D, Kuan C Y, Whitmarsh A J, Rincon M, Zheng T S, Davis R J, Rakic P, Flavell R A. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- 57.Zanke B W, Boudreau K, Rubie E, Winnett E, Tibbles L A, Zon L, Kyriakis J, Liu F F, Woodgett J R. The stress-activated protein kinase pathway mediates cell death following injury induced by cis-platinum, UV irradiation or heat. Curr Biol. 1996;6:606–613. doi: 10.1016/s0960-9822(02)00547-x. [DOI] [PubMed] [Google Scholar]