Abstract
Objective
To examine the associations between dietary intake and tissue biomarkers of alpha linolenic acid (ALA) and risk of mortality from all causes, cardiovascular disease (CVD), and cancer.
Design
Systematic review and meta-analysis of prospective cohort studies.
Data sources
PubMed, Scopus, ISI Web of Science, and Google Scholar to 30 April 2021.
Study selection
Prospective cohort studies that reported the risk estimates for death from all causes, CVD, and cancer.
Data synthesis
Summary relative risks and 95% confidence intervals were calculated for the highest versus lowest categories of ALA intake using random effects and fixed effects models. Linear and non-linear dose-response analyses were conducted to assess the dose-response associations between ALA intake and mortality.
Results
41 articles from prospective cohort studies were included in this systematic review and meta-analysis, totalling 1 197 564 participants. During follow-up ranging from two to 32 years, 198 113 deaths from all causes, 62 773 from CVD, and 65 954 from cancer were recorded. High intake of ALA compared with low intake was significantly associated with a lower risk of deaths from all causes (pooled relative risk 0.90, 95% confidence interval 0.83 to 0.97, I2=77.8%, 15 studies), CVD (0.92, 0.86 to 0.99, I2=48.2%, n=16), and coronary heart disease (CHD) (0.89, 0.81 to 0.97, I2=5.6%, n=9), and a slightly higher risk of cancer mortality (1.06, 1.02 to 1.11, I2=3.8%, n=10). In the dose-response analysis, a 1 g/day increase in ALA intake (equivalent to one tablespoon of canola oil or 0.5 ounces of walnut) was associated with a 5% lower risk of all cause (0.95, 0.91 to 0.99, I2=76.2%, n=12) and CVD mortality (0.95, 0.91 to 0.98, I2=30.7%, n=14). The pooled relative risks for the highest compared with lowest tissue levels of ALA indicated a significant inverse association with all cause mortality (0.95, 0.90 to 0.99, I2=8.2%, n=26). Also, based on the dose-response analysis, each 1 standard deviation increment in blood concentrations of ALA was associated with a lower risk of CHD mortality (0.92, 0.86 to 0.98, I2=37.1%, n=14).
Conclusions
The findings show that dietary ALA intake is associated with a reduced risk of mortality from all causes, CVD, and CHD, and a slightly higher risk of cancer mortality, whereas higher blood levels of ALA are associated with a reduced risk of all cause and CHD mortality only.
Systematic review registration
PROSPERO CRD42021229487.
Introduction
Cardiovascular disease (CVD) and cancer remain two leading causes of death worldwide.1 2 Adherence to a healthy diet can delay the onset of such diseases and improve longevity.3 Low consumption of saturated fatty acids and trans fatty acids and high consumption of plant oils rich in polyunsaturated fatty acids, particularly omega 3 fatty acids, instead of animal fats has been a cornerstone of worldwide dietary guidelines.4 5 Although many studies have shown beneficial properties of omega 3 polyunsaturated fatty acids, findings from different studies are still conflicting.6 7 Some reports are available on the increased risk of certain cancers after intake of omega 3 fatty acids.6 Questions therefore still remain about the health benefits of omega 3 polyunsaturated fatty acids and this topic has received research interest for decades.
Alpha linolenic acid (ALA) and linoleic acid are the most common essential polyunsaturated fatty acids available in plant sources.8 ALA is an omega 3 all-cis-9,12,15-octadecatrienoic acid that is metabolised to eicosapentaenoic acid and, to a lesser extent, docosahexaenoic acid, which both have anti-inflammatory properties, whereas linoleic acid is an omega 6 all-cis-9,12-octadecadienoic acid that is metabolised to arachidonic acid and subsequently to some prostaglandins, leukotrienes, and thromboxanes, which are involved in inflammatory responses.8 9 Among the two important fatty acids, much attention has been given to the health benefits of ALA.10 This fatty acid is readily available in soybean, nuts, canola oils, flaxseed, and other plant food sources.10 In addition to its role in the production of eicosapentaenoic acid and docosahexaenoic acid, it provides independent and specific effects on health.11 ALA might have a preventive effect against CVD and some cancers through its anti-inflammatory properties.12 13 However, some evidence suggests that high consumption of ALA increases oxidative stress in body tissues.6 This inconsistency was also observed after reviewing the results of observational studies that assessed dietary intake and tissue biomarkers of ALA in relation to chronic diseases or longevity.14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Some studies reported a statistically significant inverse association between ALA and risk of chronic diseases and mortality,16 18 20 24 38 41 50 51 whereas others showed no significant15 17 19 21 22 25 26 27 28 29 30 31 32 33 39 40 42 43 44 45 46 47 48 49 52 53 and even positive associations.14 23 34 35 36 37 A recent meta-analysis of cohort studies concluded that a higher intake of ALA is associated with a lower risk of composite and fatal coronary heart disease (CHD).55 Findings from another meta-analysis indicated a statistically significant positive association between dietary ALA intake and risk of prostate cancer.56
Although some meta-analyses are available on the link between ALA and risk of chronic diseases,55 56 no study has examined the association between ALA and risk of all cause mortality. Moreover, previous meta-analyses have mainly focused on dietary intake of ALA, with little attention to tissue biomarkers of ALA.55 57 In addition, no information is available about the strength and shape of the dose-response association between intake of ALA and risk of mortality. We performed a systematic review and dose-response meta-analysis of prospective cohort studies to review available findings on the associations between ALA and risk of mortality from all causes, CVD, and cancer by considering both dietary intake and tissue biomarkers of ALA.
Methods
This systematic review and meta-analysis was reported according to the preferred reporting items for systematic review and meta-analysis guidelines.58
Search strategy
We performed a systematic search using online databases, including PubMed, Scopus, and ISI Web of Science to identify relevant papers published to 30 April 2021 that assessed dietary intake or tissue biomarkers of ALA in relation to mortality due to all causes, CVD, CHD, and cancer. In the search strategy, a combination of MeSH (medical subject heading terms) and non-MeSH terms were used (supplementary table 1). Also, we conducted a web based search in Google Scholar using a combination of “linolenic acid” and “mortality” terms. In this engine, we screened the first 500 relevancy ranked papers. No restrictions were applied for publication time or language of articles. In addition, the reference lists of the selected papers and recent reviews were cross checked to identify any articles that might have been missed.
Inclusion and exclusion criteria
Studies were included that were prospective (eg, prospective cohort, nested case-control, case-cohort studies), concerned adults (≥18 years), and reported relative risk estimates, including hazard ratios and odds ratios, with 95% confidence intervals for the association between dietary intake or tissue biomarkers (plasma or serum, phospholipids, cholesteryl esters, adipose tissue, and erythrocyte) of ALA and risk of mortality from all causes, CVD, CHD, or cancer. If the results from one study were published in more than one paper, we selected the most recent paper; otherwise, the study with the greatest number of cases or with higher quality was included.
Letters, comments, reviews, meta-analyses, and ecological studies were excluded. We also did not include studies that were conducted on children and adolescents, enrolled patients with chronic kidney disease or critically ill participants, had a retrospective design, considered genetically predicted dietary intake or tissue levels of polyunsaturated fatty acids, or had insufficient data (ie, did not report relative risks or 95% confidence intervals for the link between ALA and mortality).
Data extraction
Two independent investigators (SN and OS) extracted data from each paper, with disagreements resolved by discussion and consensus. For the meta-analysis, we extracted any reported relative risk estimates, including odds ratios, relative risks, or hazard ratios, along with 95% confidence intervals for the relation between ALA and mortality. If an included study reported several effect sizes, we chose the one adjusted for the most confounding variables. Moreover, from each included article we extracted additional information on the first author name, publication year, study design, sample size, number of cases, personal characteristics of participants (age range or mean age and sex), study location, follow-up (years), health of participants at baseline, methods used to assess dietary intake or tissue levels of ALA, and confounding variables adjusted in the statistical analysis.
Risk of bias assessment
To assess risk of bias among included studies, we used the risk of bias in non-randomised studies of exposures tool.59 This tool comprises seven domains through which bias might be introduced. The questions of these domains include bias due to confounding, bias in the selection of participants into the study, bias in the classification of exposures, bias due to departure from intended exposures, bias due to missing data, bias in the measurement of outcomes, and bias in the selection of reported results. Under each domain we categorised studies as having a low, moderate, serious, and critical risk of bias.
Statistical analysis
We included the relative risks (including hazard ratios and odds ratios) and 95% confidence intervals of mortality for the comparison between the highest and lowest intakes of ALA into the meta-analysis. However, some studies reported relative risks of mortality per 1 standard deviation (SD) increment in ALA intake. To include such studies in the meta-analysis, we converted the per SD increment risk estimates to the relative risks for the comparison of the top versus bottom third of ALA intakes using the method suggested by Danesh et al in which the log risk estimates reported for the comparison between the top and bottom third are equivalent to 2.18 times the log risk estimates for a 1 SD increase.60 This method assumes that the exposure is a normally distributed variable and that the association with disease risk is log-linear.
Since the relative risks (and 95% confidence intervals) are non-normally distributed variables, we included the natural log form (and its standard error) of these effect sizes into statistical analyses. To calculate the overall relative risk of mortality for the highest compared with lowest intakes or tissue levels of ALA, a random effects model was used. Random effects models take into consideration different sources of uncertainties, including within study (sampling or estimation) error and variance between studies.61 However, since these models tend to give disproportionally more weight to smaller studies, particularly when the outcome is binary (eg, death), fixed effects models might present more reliable results compared with the random effects models.62 Therefore, in addition to the random effects model, we used a fixed effects model for the statistical analyses. In addition to the overall relative risks, we calculated absolute risk differences based on the obtained overall relative risks and baseline risks using the formula: absolute risk difference=baseline risk×(relative risk−1). Baseline risks for all cause, CVD, and CHD mortality were obtained from the Emerging Risk Factors Collaboration that contained data from 102 international cohorts.63 For cancer mortality, baseline risk was based on the GLOBOCAN that described cancer incidence and mortality from 20 large areas of the world.64
Heterogeneity was assessed using Cochran’s Q test and the I2 statistic. For the I2 statistic, we considered values of <25%, 25-50%, 50-75%, and >75% as low, moderate, high, and severe heterogeneity between studies, respectively.65 Subgroup and meta-regression analyses were conducted to identify possible sources of heterogeneity. If a study reported subgroup risk estimates stratified by sex or other variables, we first pooled the subgroup estimates by using a fixed effects model and then included the obtained pooled risk estimate in the main meta-analysis. Studies that investigated only CHD mortality were considered in the meta-analysis of CVD mortality. Publication bias was determined using Egger’s linear regression test.66 In the case of substantial publication bias, the trim-and-fill method was used to detect the effect of potential missing studies on the overall relative risk.67 To assess the dependency of overall effect size on one study, sensitivity analysis was done using a random effects model in which each study was excluded to examine the influence of that study on the overall estimate.
We used the generalised least squares trend estimation method68 69 for the linear dose-response analysis. Firstly, we estimated study specific slopes, and then these slopes were combined to obtain an overall average slope. We combined the study specific slopes using random effects and fixed effects models. In this method, the distribution of deaths, the total number of participants, and the effect sizes with the variance estimates for ≥3 quantitative categories of exposure were required. We assigned the median or mean amount of ALA intake in each category to the corresponding effect size for each study. For studies that reported the intakes of ALA as the percentage of energy, we converted them to g/day. For studies that reported the intake as ranges, we estimated the midpoint in each category by calculating the mean of the lower and upper bound. When the highest category was open ended, we assumed the length of the open ended interval to be the same as that of the adjacent interval. We also examined a possible non-linear dose-response association using restricted cubic splines with three knots at centiles of 10%, 50%, and 90% of the distribution. The correlation within each set of provided risk estimates was accounted for and the study specific estimates were combined by using a one stage linear mixed effects meta-analysis. This method estimates the study specific slopes and combines them to obtain an overall average slope in a single stage, providing a more precise, flexible, and efficient method than the traditional two stage method. The significance for non-linearity was calculated by null hypothesis testing, in which we considered the coefficient of the second spline to be equal to zero. For tissue levels of ALA and mortality, we performed linear and non-linear dose-response analyses when possible based on the weight percentage of ALA among total fatty acids. Statistical analyses were conducted using STATA version 14.0. P<0.05 was considered as statistically significant for all tests, including Cochran’s Q test.
Patient and public involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. No patients were asked to advise on interpretation or writing up of results. There are no plans to disseminate the results of the research to study participants or the relevant patient community.
Results
In total, 12 873 articles were identified in the initial search. After excluding duplicate papers (n=5991) and studies that did not meet the inclusion criteria (n=6829), 53 full articles of potentially relevant studies were assessed for eligibility (fig 1). After full text reviews, eight articles were excluded for being conducted on patients who required haemodialysis (n=1)70 or critically ill patients (n=1),71 having a case-control design (n=1),72 assessing genetically predicted ALA levels rather than tissue levels (n=1),73 and not reporting relative risk estimates (n=1) or 95% confidence intervals (n=3).74 75 76 In addition, two different publications were found for the Nurses’ Health Study,34 77 three for the Health Professionals Follow-up Study,34 78 79 and two for the Cardiovascular Health Study.20 80 As these articles assessed similar intake and outcome variables, only the one of higher quality or with the most number of cases for each dataset were included20 34 and the duplicate papers excluded.77 78 79 80 Moreover, two studies that were conducted on the Nurses’ Health Study43 and Health Professionals Follow-up Study44 were included in the dose-response analysis of CHD mortality. The articles of Zhuang et al37 and Pelser et al45 were published on the National Institutes of Health-American Association of Retired Persons (NIH-AARP) dataset.37 45 Although the paper of Zhuang et al was more complete than that of Pelser et al’s, the latter provided information for the non-linear dose-response analysis that was not available in the duplicate paper. Both articles were therefore included; however, to avoid double counting data, the findings of Pelser et al article were used only for the non-linear dose-response analyses. Papers from the same studies that assessed different indicators of ALA (dietary or tissue biomarkers) or different types of deaths (eg, CVD, cancer) were not considered duplicates and were included in the meta-analysis.22 32 34 46 Also, a partial overlap in populations was found between the study of Harris et al and some of the included studies on tissue biomarkers of ALA.54 As this overlap was incomplete, the Harris et al study was included in the main analysis. A sensitivity analysis was, however, carried out for that study. After these exclusions, 41 papers were included in the current systematic review and meta-analysis14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54: 26 papers assessed the association between ALA and all cause mortality (11 on dietary intake,16 19 22 23 32 34 36 37 38 40 50 13 on tissue levels of ALA,18 21 27 28 31 35 39 47 48 49 51 52 54 and two on both20 26), 26 articles described the relation between ALA and CVD mortality (13 on dietary ALA intake,16 22 24 29 30 34 36 37 38 42 43 44 46 nine on tissue levels of ALA,15 18 21 35 41 47 48 49 54 and four on both14 20 25 26), and 14 papers investigated the link between ALA and cancer mortality (10 on dietary ALA intake16 17 22 23 32 34 36 37 45 53 and four on tissue levels of ALA21 33 48 54).
Fig 1.
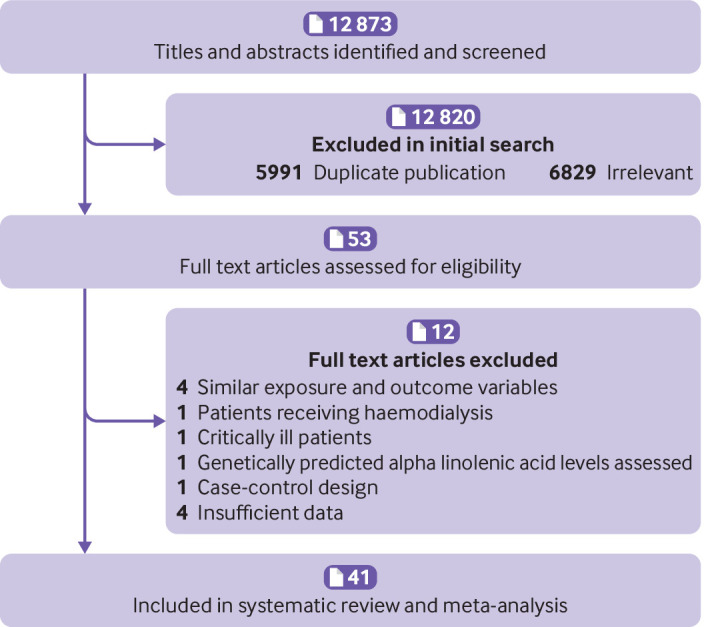
Flow diagram of study selection
Characteristics of included studies
Supplementary tables 2-5 show the characteristics of studies included in the systematic review and meta-analysis, and supplementary tables 6-9 show the data extracted for the dose-response analysis. The studies were published between 1991 and 2021 and had a prospective cohort design. The number of participants in these studies ranged from 162 to 521 120, with an age range between 18 and 98 years. In total, 1 197 564 participants were included in the 41 papers selected. During the follow-up periods ranging from two to 32 years, 198 113 deaths from all causes, 62 773 CVD deaths, and 65 954 cancer deaths were recorded.
Of the 41 included articles, 12 included only men16 17 25 29 30 32 33 35 44 45 47 53 and four included only women.21 23 43 46 Among the remaining articles, one reported risk estimates for men and women separately.34 In total, 17 papers described studies that were conducted in the US,16 20 21 22 23 31 32 33 34 37 43 44 45 46 48 50 19 described studies that were performed in non-US countries,14 15 17 18 19 24 25 26 27 28 29 30 35 38 39 40 47 49 51 52 and the remaining five described studies that were conducted on populations from different countries.36 41 42 53 54 To examine dietary ALA intake, 19 papers used food frequency questionnaires,14 17 19 20 22 23 24 26 30 32 34 37 38 40 42 43 44 45 46 four applied food recall or record,16 25 36 50 one applied food frequency questionnaires or diet histories,53 and one used cross checked dietary history.29 ALA was measured in adipose tissue (n=3) and blood (n=27), and the biomarkers included phospholipids (n=14), cholesteryl esters (n=6), total serum or plasma ALA (n=6), and whole blood ALA (n=1) as a weight percentage of total fatty acids. In the Harris et al study that was done on different datasets, ALA levels were measured in different parts of blood.54 All included studies used gas chromatography or gas-liquid chromatography to measure ALA concentrations. In the most included publications, some important confounders, including age (n=34), body mass index (BMI) (n=34), smoking status (n=35), alcohol consumption (n=29), physical activity (n=24), and energy intake (n=23) were adjusted in the analysis of ALA and mortality. Based on the risk of bias in non-randomised studies of exposures tool, 33 articles had a low risk of bias in all components (supplementary table 10).
Random effects meta-analysis: all cause mortality
Dietary ALA
Fifteen studies (13 publications) examined the association between dietary intake of ALA and all cause mortality.16 19 20 22 23 26 32 34 36 37 38 40 50 These studies included a total of 745 122 participants and 176 694 deaths. The summary relative risk for all cause mortality, comparing the highest categories of dietary ALA intake (median 1.59 g/day, interquartile range 1.26-2.25) with the lowest (0.73 g/day, 0.36-0.89), was 0.90 (95% confidence interval 0.83 to 0.97, I2=77.8%, P<0.001 for heterogeneity), indicating a significant inverse association (table 1 and supplementary figure 1). The corresponding absolute risk difference for this association was −113 (95% confidence interval −192 to −34), which showed a reduction of 113 deaths from all causes per 10 000 person years for the highest versus lowest categories of dietary ALA intake (supplementary table 11).
Table 1.
Summary risk estimates for association between alpha linolenic acid (ALA) intake and risk of all cause mortality in adults aged ≥18 years
| No of effect sizes | Pooled relative risk (95% CI)* | I2 (%)† | P values | ||
|---|---|---|---|---|---|
| Heterogeneity‡ | Meta-regression | ||||
| Highest v lowest ALA intake | |||||
| Dietary ALA intake: | |||||
| Overall | 15 | 0.90 (0.83 to 0.97) | 77.8 | <0.001 | |
| Study location | |||||
| US | 10 | 0.91 (0.84 to 0.98) | 80.6 | <0.001 | 0.82 |
| Western countries§ | 11 | 0.90 (0.83 to 0.97) | 79.6 | <0.001 | |
| Eastern/Mediterranean countries | 4 | 0.85 (0.60 to 1.21) | 77.9 | 0.004 | |
| Sex | |||||
| Men and women | 10 | 0.85 (0.74 to 0.97) | 82.7 | <0.001 | 0.67 |
| Men | 3 | 0.87 (0.68 to 1.10) | 74.9 | 0.01 | |
| Women | 2 | 0.98 (0.93 to 1.04) | 0 | 0.36 | |
| Follow-up duration (years) | |||||
| ≥10 | 8 | 0.98 (0.91 to 1.05) | 76.1 | <0.001 | 0.03 |
| <10 | 7 | 0.77 (0.70 to 0.85) | 0 | 0.57 | |
| Dietary assessment tools | |||||
| Food frequency questionnaire | 11 | 0.92 (0.86 to 0.99) | 73.8 | <0.001 | 0.68 |
| Food recall or record | 4 | 0.84 (0.64 to 1.11) | 83.5 | <0.001 | |
| Adjustment for energy | |||||
| Yes | 12 | 0.92 (0.85 to 0.99) | 78.6 | <0.001 | 0.18 |
| No | 3 | 0.75 (0.59 to 0.95) | 26.9 | 0.25 | |
| Adjustment for BMI | |||||
| Yes | 13 | 0.90 (0.84 to 0.98) | 78.2 | <0.001 | 0.90 |
| No | 2 | 0.87 (0.52 to 1.47) | 82.7 | 0.01 | |
| Adjustment for other nutrients¶ | |||||
| Yes | 10 | 0.96 (0.90 to 1.03) | 70 | <0.001 | 0.05 |
| No | 5 | 0.76 (0.63 to 0.92) | 53 | 0.07 | |
| Adjustment for trans fatty acids | |||||
| Yes | 5 | 1.00 (0.94 to 1.05) | 63.8 | 0.02 | 0.10 |
| No | 10 | 0.82 (0.70 to 0.95) | 67.7 | 0.001 | |
| Health status** | |||||
| Healthy | 12 | 0.89 (0.82 to 0.97) | 81 | <0.001 | 0.56 |
| Unhealthy | 3 | 0.91 (0.80 to 1.04) | 10.6 | 0.32 | |
| Tissue ALA levels: | |||||
| Overall | 15 | 0.95 (0.90 to 0.99) | 8.2 | 0.36 | |
| Study location | |||||
| US | 5 | 0.95 (0.86 to 1.05) | 0 | 0.98 | 0.84 |
| Western countries§ | 11 | 0.97 (0.91 to 1.03) | 0 | 0.88 | |
| Eastern/Mediterranean countries | 3 | 0.54 (0.31 to 0.93) | 43.8 | 0.16 | |
| Sex | |||||
| Men and women | 12 | 0.93 (0.89 to 0.99) | 10.7 | 0.34 | 0.25 |
| Men | 2 | 1.01 (0.87 to 1.16) | 24.8 | 0.24 | |
| Women | 1 | 0.95 (0.81 to 1.11) | - | - | |
| Follow-up duration (years) | |||||
| ≥10 | 7 | 0.95 (0.91 to 0.99) | 0 | 0.56 | 0.06 |
| <10 | 8 | 0.86 (0.74 to 1.00) | 19.5 | 0.27 | |
| Tissue biomarkers | |||||
| Adipose tissue | 1 | 0.91 (0.73 to 1.13) | - | - | 0.43 |
| Blood | 14 | 0.95 (0.89 to 0.99) | 14.1 | 0.29 | |
| Total serum or plasma | 1 | 0.75 (0.53 to 1.07) | - | - | |
| Phospholipids (plasma or RBC) | 8 | 0.92 (0.85 to 1.01) | 0 | 0.68 | |
| Cholesteryl esters | 3 | 1.02 (0.78 to 1.34) | 58.6 | 0.08 | |
| Adjustment for BMI | |||||
| Yes | 10 | 0.95 (0.90 to 1.01) | 17 | 0.28 | 0.48 |
| No | 5 | 0.87 (0.72 to 1.07) | 0 | 0.43 | |
| Health status** | |||||
| Healthy | 9 | 0.95 (0.91 to 0.99) | 0 | 0.55 | 0.27 |
| Unhealthy | 6 | 0.84 (0.68 to 1.04) | 28.8 | 0.21 | |
| Linear dose-response association | |||||
| Dietary ALA intake (per 1 g/day increase): | |||||
| Overall | 12 | 0.95 (0.91 to 0.99) | 76.2 | <0.001 | |
| Study location | |||||
| US | 9 | 0.96 (0.92 to 1.01) | 79 | <0.001 | 0.30 |
| Western countries§ | 10 | 0.96 (0.92 to 1.00) | 78 | <0.001 | |
| Eastern/Mediterranean countries | 2 | 0.89 (0.74 to 1.08) | 26.6 | 0.24 | |
| Sex | |||||
| Men and women | 8 | 0.93 (0.87 to 0.99) | 77.6 | <0.001 | 0.73 |
| Men | 2 | 0.93 (0.77 to 1.13) | 92.6 | 0.001 | |
| Women | 2 | 1.01 (0.94 to 1.08) | 9.9 | 0.29 | |
| Follow-up duration (years) | |||||
| ≥10 | 8 | 0.98 (0.94 to 1.01) | 65.2 | 0.005 | 0.10 |
| <10 | 4 | 0.91 (0.78 to 1.05) | 81.6 | 0.001 | |
| Dietary assessment tools | |||||
| Food frequency questionnaire | 8 | 0.98 (0.94 to 1.02) | 62.6 | 0.009 | 0.57 |
| Food recall or record | 4 | 0.93 (0.84 to 1.03) | 80.3 | 0.002 | |
| Adjustment for energy | |||||
| Yes | 10 | 0.97 (0.93 to 1.00) | 71.6 | <0.001 | 0.09 |
| No | 2 | 0.83 (0.76 to 0.92) | 0 | 0.47 | |
| Adjustment for BMI | |||||
| Yes | 10 | 0.96 (0.93 to 1.00) | 72.6 | <0.001 | 0.64 |
| No | 2 | 0.96 (0.71 to 1.30) | 80.1 | 0.02 | |
| Adjustment for other nutrients¶ | |||||
| Yes | 7 | 0.99 (0.96 to 1.02) | 64.4 | 0.01 | 0.03 |
| No | 5 | 0.88 (0.81 to 0.94) | 31.7 | 0.21 | |
| Adjustment for trans fatty acids | |||||
| Yes | 4 | 1.00 (0.97 to 1.03) | 57.7 | 0.06 | 0.21 |
| No | 8 | 0.92 (0.85 to 0.99) | 66.1 | 0.004 | |
| Health status** | |||||
| Healthy | 10 | 0.96 (0.92 to 0.99) | 77.3 | <0.001 | 0.91 |
| Unhealthy | 2 | 0.98 (0.77 to 1.25) | 70.1 | 0.06 | |
| Tissue ALA levels (per 1 SD increase): | |||||
| Overall | 11 | 0.97 (0.94 to 1.01) | 29.5 | 0.16 | |
| Study location | |||||
| US | 3 | 0.97 (0.93 to 1.02) | 0 | 0.97 | 0.97 |
| Western countries§ | 9 | 0.99 (0.96 to 1.01) | 0 | 0.44 | |
| Eastern/Mediterranean countries | 2 | 0.82 (0.59 to 1.15) | 67.8 | 0.07 | |
| Sex | |||||
| Men and women | 8 | 0.96 (0.92 to 1.00) | 38.5 | 0.12 | 0.32 |
| Men | 2 | 1.01 (0.94 to 1.07) | 28 | 0.23 | |
| Women | 1 | 0.98 (0.91 to 1.05) | - | - | |
| Follow-up duration (years) | |||||
| ≥10 | 6 | 0.99 (0.96 to 1.03) | 19.5 | 0.28 | 0.06 |
| <10 | 5 | 0.94 (0.90 to 0.99) | 0 | 0.42 | |
| Tissue biomarkers | |||||
| Adipose tissue | 1 | 0.96 (0.87 to 1.06) | - | - | 0.79 |
| Blood | 10 | 0.97 (0.94 to 1.01) | 35.7 | 0.12 | |
| Total serum or plasma | 0 | - | - | - | |
| Phospholipids (plasma or RBC) | 6 | 0.95 (0.91 to 0.99) | 15.6 | 0.31 | |
| Cholesteryl esters | 3 | 1.00 (0.95 to 1.06) | 55.7 | 0.01 | |
| Adjustment for BMI | |||||
| Yes | 7 | 0.99 (0.96 to 1.01) | 16.9 | 0.30 | 0.21 |
| No | 4 | 0.91 (0.82 to 1.01) | 35.9 | 0.19 | |
| Health status** | |||||
| Healthy | 6 | 0.99 (0.96 to 1.03) | 19.5 | 0.28 | 0.09 |
| Unhealthy | 5 | 0.94 (0.90 to 0.99) | 0 | 0.42 | |
BMI=body mass index; RBC=red blood cells; SD=standard deviation.
Obtained from random effects model.
Inconsistency—percentage of variation across studies due to heterogeneity.
Obtained from Q test.
Includes studies from the US.
Studies that controlled for at least one of dietary nutrients, including lipids, vitamins, and minerals.
Studies that recruited apparently healthy adults (without a previous diagnosis of cancer, cardiovascular disease, or any other chronic diseases) were included in the healthy subgroup, and studies that recruited participants with at least one chronic disease were included in the unhealthy subgroup.
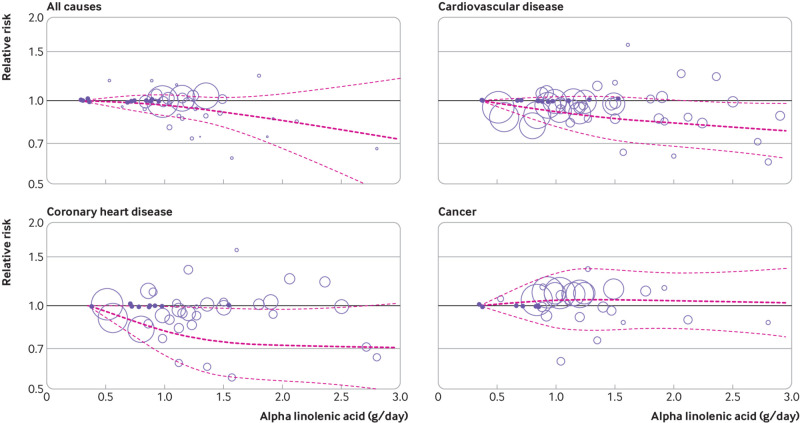
Twelve studies from 10 papers with sufficient data were included in the dose-response analysis of dietary ALA intake and all cause mortality.16 19 20 22 23 34 36 37 40 50 In the linear dose-response analysis, a significant inverse association was found between a 1 g/day increase in ALA intake and all cause mortality (pooled relative risk 0.95, 95% confidence interval 0.91 to 0.99, I2=76.2%, P<0.001 for heterogeneity) (table 1 and supplementary figure 2). The absolute risk for this linear relation was −57 deaths (95% confidence interval −102 to −11) per 10 000 person years (supplementary table 11). In the non-linear dose-response analysis, no evidence was found of a non-linear association between ALA intake and all cause mortality (P=0.58 for non-linearity) (fig 2 and supplementary table 12).
Fig 2.
Non-linear dose-response association of alpha linolenic acid intake (g/day) with risk of mortality from all causes, cardiovascular disease, coronary heart disease, and cancer in adults aged ≥18 years. Dark (light) dashes represent results from spline model (95% confidence intervals), respectively
Tissue ALA
Fifteen articles were included in the analysis for tissue levels of ALA and all cause mortality.18 20 21 26 27 28 31 35 39 47 48 49 51 52 54 These studies included a total of 53 935 participants and 18 881 deaths from all causes. The summary relative risk for all cause mortality, comparing the highest with lowest levels of ALA intake, was 0.95 (95% confidence interval 0.90 to 0.99, I2=8.2%; P=0.36 for heterogeneity), indicating a significant inverse association between ALA levels and risk of all cause mortality (table 1 and supplementary figure 3). The pooled relative risk provided an absolute risk of −57 deaths (95% confidence interval −113 to −11) per 10 000 person years (supplementary table 11).
Eleven of the 15 papers were included in the linear dose-response analysis.18 20 21 27 28 31 35 39 47 49 51 No significant linear association was found between a 1 SD increment in ALA intake and all cause mortality (pooled relative risk 0.97, 95% confidence interval 0.94 to 1.01, I2=29.5%; P=0.16 for heterogeneity) (table 1 and supplementary figure 4). The absolute risk difference for this association was −34 deaths (95% confidence interval −68 to 11) per 10 000 person years (supplementary table 11). It was not possible to perform a non-linear dose-response analysis as the articles had insufficient data.
Random effects meta-analysis: CVD mortality
Dietary ALA
Sixteen cohort studies from 15 papers investigated the association between dietary ALA intake and CVD mortality and included 965 668 participants and 56 921 CVD deaths.14 16 20 22 24 25 26 29 30 34 36 37 38 42 46 When the relative risks comparing the highest intakes of ALA with the lowest were combined from these studies, a significant inverse association was found between dietary ALA intake and CVD mortality (pooled relative risk 0.92, 95% confidence interval 0.86 to 0.99, I2=48.2%; P=0.01 for heterogeneity) (table 2 and supplementary figure 5). The absolute risk for this association was −33 CVD deaths (95% confidence interval −57 to −4) per 10 000 person years (supplementary table 11). In this association, a median ALA intake of 1.77 g/day (interquartile range 1.32-2.65) was classified as high and 0.88 (0.70-1.06) g/day as low, with two studies not providing quantification.26 37
Table 2.
Summary risk estimates for association between alpha linolenic acid (ALA) intake and risk of cardiovascular disease mortality in adults aged ≥18 years
| No of effect sizes | Pooled relative risk (95% CI)* | I2 (%)† | P values | ||
|---|---|---|---|---|---|
| Heterogeneity‡ | Meta-regression | ||||
| Highest v lowest ALA intake | |||||
| Dietary ALA intake: | |||||
| Overall | 16 | 0.92 (0.86 to 0.99) | 48.2 | 0.01 | |
| Study location | |||||
| US | 10 | 0.91 (0.83 to 0.99) | 65 | 0.002 | 0.52 |
| Western countries§ | 13 | 0.96 (0.90 to 1.02) | 27.4 | 0.16 | |
| Eastern/Mediterranean countries | 3 | 0.82 (0.74 to 0.90) | 0 | 0.82 | |
| Sex | |||||
| Men and women | 9 | 0.93 (0.84 to 1.04) | 58.9 | 0.01 | 0.75 |
| Men | 5 | 0.89 (0.72 to 1.10) | 45.7 | 0.11 | |
| Women | 2 | 0.90 (0.80 to 1.00) | 0 | 0.89 | |
| Follow-up duration (years) | |||||
| ≥10 | 10 | 0.93 (0.85 to 1.01) | 61.3 | 0.006 | 0.93 |
| <10 | 6 | 0.92 (0.82 to1.05) | 5.8 | 0.38 | |
| Dietary assessment tools | |||||
| Food frequency questionnaire | 13 | 0.94 (0.88 to1.01) | 45.6 | 0.03 | 0.08 |
| Food recall or record | 3 | 0.73 (0.57 to 0.94) | 2.5 | 0.35 | |
| Adjustment for energy | |||||
| Yes | 15 | 0.94 (0.87 to 1.00) | 42 | 0.04 | 0.07 |
| No | 1 | 0.60 (0.39 to 0.92) | - | - | |
| Adjustment for BMI | |||||
| Yes | 15 | 0.94 (0.87 to 1.00) | 42 | 0.04 | 0.07 |
| No | 1 | 0.60 (0.39 to 0.92) | - | - | |
| Adjustment for other nutrients¶ | |||||
| Yes | 14 | 0.93 (0.87 to 0.99) | 46.1 | 0.01 | 0.32 |
| No | 2 | 0.78 (0.49 to 1.23) | 67.5 | 0.07 | |
| Adjustment for trans fatty acids | |||||
| Yes | 7 | 1.00 (0.96 to 1.04) | 1.6 | 0.04 | 0.005 |
| No | 9 | 0.83 (0.76 to 0.90) | 0 | 0.57 | |
| Health status** | |||||
| Healthy | 15 | 0.91 (0.85 to 0.98) | 49.5 | 0.04 | 0.23 |
| Unhealthy | 1 | 1.13 (0.85 to 1.51) | - | - | |
| Tissue ALA levels: | |||||
| Overall | 21 | 0.98 (0.86 to 1.11) | 45.6 | 0.01 | |
| Study location | |||||
| US | 7 | 1.03 (0.74 to 1.45) | 40.1 | 0.12 | 0.83 |
| Western countries§ | 17 | 1.02 (0.86 to 1.21) | 50.7 | 0.009 | |
| Eastern/Mediterranean countries | 3 | 0.68 (0.46 to 1.00) | 0 | 0.82 | |
| Sex | |||||
| Men and women | 14 | 0.92 (0.78 to 1.09) | 51.3 | 0.01 | 0.12 |
| Men | 5 | 1.18 (1.01 to 1.39) | 0 | 0.18 | |
| Women | 2 | 0.91 (0.65 to 1.27) | 0.3 | 0.31 | |
| Follow-up duration (years) | |||||
| ≥10 | 16 | 0.97 (0.85 to 1.10) | 46 | 0.02 | 0.07 |
| <10 | 3 | 0.84 (0.42 to 1.66) | 35.7 | 0.21 | |
| Tissue biomarkers | |||||
| Adipose tissue | 2 | 1.48 (0.61 to 3.59) | 85.5 | 0.008 | 0.17 |
| Blood | 19 | 0.95 (0.84 to 1.07) | 34.5 | 0.07 | |
| Total serum or plasma | 5 | 0.84 (0.61 to 1.15) | 0 | 0.49 | |
| Phospholipids (plasma or RBC) | 9 | 0.92 (0.74 to 1.14) | 50.4 | 0.04 | |
| Cholesteryl esters | 4 | 1.12 (0.88 to 1.41) | 7.7 | 0.35 | |
| Adjustment for BMI | |||||
| Yes | 21 | 0.98 (0.86 to 1.11) | 45.6 | 0.01 | - |
| No | 0 | - | - | - | |
| Health status** | |||||
| Healthy | 19 | 0.99 (0.85 to 1.14) | 49.2 | 0.008 | 0.72 |
| Unhealthy | 2 | 0.93 (0.72 to 1.19) | 1.8 | 0.31 | |
| Linear dose-response association | |||||
| Dietary ALA intake (per 1 g/day increase): | |||||
| Overall | 14 | 0.95 (0.91 to 0.98) | 30.7 | 0.13 | |
| Study location | |||||
| US | 10 | 0.94 (0.90 to 0.98) | 42.6 | 0.05 | 0.74 |
| Western countries§ | 12 | 0.97 (0.94 to 0.99) | 0 | 0.73 | |
| Eastern/Mediterranean countries | 1 | 0.79 (0.70 to 0.89) | - | - | |
| Sex | |||||
| Men and women | 7 | 0.94 (0.88 to 1.01) | 53.4 | 0.04 | 0.92 |
| Men | 4 | 0.94 (0.84 to 1.04) | 32.1 | 0.22 | |
| Women | 3 | 0.95 (0.91 to 0.99) | 0 | 0.52 | |
| Follow-up duration (years) | |||||
| ≥10 | 10 | 0.95 (0.91 to 0.99) | 38.6 | 0.10 | 0.84 |
| <10 | 4 | 0.94 (0.87 to 1.02) | 24 | 0.26 | |
| Dietary assessment tools | |||||
| Food frequency questionnaire | 11 | 0.95 (0.92 to 0.99) | 35.6 | 0.11 | 0.35 |
| Food recall or record | 3 | 0.90 (0.83 to 0.99) | 0 | 0.44 | |
| Adjustment for energy | |||||
| Yes | 13 | 0.96 (0.92 to 0.99) | 23.9 | 0.20 | 0.17 |
| No | 1 | 0.85 (0.74 to 0.97) | - | - | |
| Adjustment for BMI | |||||
| Yes | 13 | 0.96 (0.92 to 0.99) | 23.9 | 0.20 | 0.17 |
| No | 1 | 0.85 (0.74 to 0.97) | - | - | |
| Adjustment for other nutrients¶ | |||||
| Yes | 12 | 0.95 (0.92 to 0.99) | 30 | 0.15 | 0.23 |
| No | 2 | 0.88 (0.78 to 0.98) | 0 | 0.43 | |
| Adjustment for trans fatty acids | |||||
| Yes | 6 | 0.97 (0.94 to 0.99) | 0 | 0.66 | 0.34 |
| No | 8 | 0.93 (0.86 to 1.00) | 48.6 | 0.05 | |
| Health status** | |||||
| Healthy | 13 | 0.94 (0.91 to 0.98) | 32.6 | 0.12 | 0.33 |
| Unhealthy | 1 | 1.05 (0.87 to 1.27) | - | - | |
| Tissue ALA levels (per 1 SD increase): | |||||
| Overall | 17 | 0.97 (0.91 to 1.03) | 54.2 | 0.004 | |
| Study location | |||||
| US | 6 | 0.96 (0.87 to 1.05) | 19.3 | 0.28 | 0.89 |
| Western countries§ | 15 | 0.97 (0.91 to 1.04) | 59 | 0.002 | |
| Eastern/Mediterranean countries | 2 | 0.97 (0.86 to 1.08) | 0 | 0.51 | |
| Sex | |||||
| Men and women | 10 | 0.97 (0.89 to 1.04) | 54.9 | 0.01 | 0.75 |
| Men | 5 | 0.99 (0.83 to 1.18) | 70.7 | 0.008 | |
| Women | 2 | 0.96 (0.83 to 1.11) | 0 | 0.49 | |
| Follow-up duration (years) | |||||
| ≥10 | 14 | 0.96 (0.89 to 1.03) | 58.7 | 0.003 | 0.78 |
| <10 | 1 | 0.97 (0.87 to 1.09) | - | - | |
| Tissue biomarkers | |||||
| Adipose tissue | 2 | 1.07 (0.97 to 1.17) | 30.9 | 0.22 | 0.19 |
| Blood | 15 | 0.95 (0.89 to 1.02) | 45.1 | 0.03 | |
| Total serum or plasma | 4 | 0.76 (0.63 to 0.91) | 0 | 0.83 | |
| Phospholipids (plasma or RBC) | 7 | 0.95 (0.88 to 1.02) | 37 | 0.14 | |
| Cholesteryl esters | 4 | 1.03 (0.95 to 1.12) | 14.8 | 0.31 | |
| Adjustment for BMI | |||||
| Yes | 17 | 0.97 (0.91 to 1.03) | 54.2 | 0.004 | - |
| No | 0 | - | - | - | |
| Health status** | |||||
| Healthy | 16 | 0.97 (0.91 to 1.04) | 56.7 | 0.003 | 0.99 |
| Unhealthy | 1 | 0.97 (0.87 to 1.09) | - | - | |
BMI=body mass index; RBC=red blood cells; SD=standard deviation.
Obtained from random effects model.
Inconsistency—percentage of variation across studies due to heterogeneity.
Obtained from Q test.
Includes studies from the US.
Studies that controlled for at least one of dietary nutrients, including lipids, vitamins, and minerals.
Studies that recruited apparently healthy adults (without a previous diagnosis of cancer, cardiovascular disease, or any other chronic diseases) were included in the healthy subgroup, and studies that recruited participants with at least one chronic disease were included in the unhealthy subgroup.
Fourteen studies from 13 papers were eligible for the dose-response analysis of dietary ALA intake and CVD mortality.14 16 20 22 24 25 29 30 34 36 37 42 46 Each additional gram of ALA intake per day was significantly associated with a 5% lower risk of CVD mortality (pooled relative risk 0.95, 95% confidence interval 0.91 to 0.98, I2=30.7%; P=0.13 for heterogeneity) (table 2 and supplementary figure 6). The absolute risk based on each additional gram of ALA intake per day was −21 CVD deaths (95% confidence interval −37 to −8) per 10 000 person years (supplementary table 11). No evidence was found of a non-linear association (P=0.56 for non-linearity; fig 2 and supplementary table 12).
Tissue ALA
Twenty five studies from 13 publications examined the association between tissue levels of ALA and risk of CVD mortality and included 59 180 people and 7264 CVD deaths.14 15 18 20 21 25 26 35 41 47 48 49 54 Meta-analysis comparing the highest with the lowest levels of ALA showed no clear significant association with CVD mortality (pooled relative risk 0.98, 95% confidence interval 0.86 to 1.11, I2=45.6%; P=0.01 for heterogeneity) (table 2 and supplementary figure 7). The absolute risk difference for this association was −8 CVD deaths (95% confidence interval −57 to 45) per 10 000 person years (supplementary table 11).
Seventeen studies from eight publications were included in the linear dose-response analysis, When the relative risks were combined, no significant linear association was found between a 1 SD increase in ALA levels and CVD mortality (pooled relative risk 0.97, 95% confidence interval 0.91 to 1.03, I2=54.2%; P=0.004 for heterogeneity) (table 2 and supplementary figure 8).14 15 18 20 21 35 41 47 The corresponding absolute risk difference per 10 000 person years was −12 CVD deaths (95% confidence interval −37 to 12) (supplementary table 11). Non-linear dose-response analyses were not possible owing to limited data.
Random effects meta-analysis: CHD mortality
Dietary ALA
Nine studies of dietary ALA and CHD mortality were included in the meta-analysis.14 16 20 24 29 30 36 38 These studies included a total of 408 033 participants and 7613 CHD deaths. 42 When the highest intake of dietary ALA (median 2.31 g/day, interquartile range 1.30-2.69) was compared with the lowest intake (0.92 g/day, 0.52 to 1.02), a significant inverse association was found between dietary ALA intake and CHD mortality (pooled relative risk 0.89, 95% confidence interval 0.81 to 0.97, I2=5.6%; P=0.38 for heterogeneity) (table 3 and supplementary figure 9). The corresponding absolute risk difference showed a risk reduction of −23 CHD deaths (95% confidence interval −40 to −6) per 10 000 person years (supplementary table 11). In the dose-response analysis of 10 eligible studies,14 16 20 24 29 30 36 42 43 44 a marginally significant inverse association was observed between a 1 g/day increase in ALA intake and CHD mortality (pooled relative risk 0.95, 95% confidence interval 0.90 to 1.01, I2=10.8%; P=0.34 for heterogeneity) (table 3 and supplementary figure 10). The absolute risk difference for this association was −11 CHD deaths (95% confidence interval −21 to 2) per 10 000 person years (supplementary table 11). The test for non-linearity was not significant (P=0.13; fig 2 and supplementary table 12).
Table 3.
Summary risk estimates for association between alpha linolenic acid (ALA) and risk of coronary heart disease mortality in adults aged ≥18 years
| No of effect sizes | Pooled relative risk (95% CI)* | I2 (%)† | P values | ||
|---|---|---|---|---|---|
| Heterogeneity‡ | Meta-regression | ||||
| Highest v lowest ALA intake | |||||
| Dietary ALA intake: | |||||
| Overall | 9 | 0.89 (0.81 to 0.97) | 5.6 | 0.38 | |
| Study location | |||||
| US | 5 | 0.81 (0.73 to 0.91) | 0 | 0.71 | 0.07 |
| Western countries§ | 6 | 0.94 (0.83 to 1.05) | 1.4 | 0.41 | |
| Eastern/Mediterranean countries | 2 | 0.82 (0.73 to 0.93) | 0 | 0.81 | |
| Sex | |||||
| Men and women | 6 | 0.88 (0.80 to 0.96) | 0 | 0.46 | 0.72 |
| Men | 3 | 0.92 (0.63 to 1.33) | 44.8 | 0.16 | |
| Women | 0 | - | - | - | |
| Follow-up duration (years) | |||||
| ≥10 | 4 | 0.82 (0.73 to 0.93) | 0 | 0.49 | 0.10 |
| <10 | 5 | 0.95 (0.84 to 1.08) | 0 | 0.52 | |
| Dietary assessment tools | |||||
| Food frequency questionnaire | 7 | 0.89 (0.81 to 0.99) | 9.3 | 0.35 | 0.65 |
| Food recall or record | 2 | 0.81 (0.55 to 1.22) | 39.5 | 0.19 | |
| Adjustment for energy | |||||
| Yes | 8 | 0.89 (0.82 to 0.98) | 0 | 0.44 | 0.25 |
| No | 1 | 0.65 (0.40 to 1.05) | - | - | |
| Adjustment for BMI | |||||
| Yes | 8 | 0.89 (0.82 to 0.98) | 0 | 0.44 | 0.25 |
| No | 1 | 0.65 (0.40 to 1.05) | - | - | |
| Adjustment for other nutrients¶ | |||||
| Yes | 7 | 0.90 (0.81 to 0.99) | 11.2 | 0.34 | 0.35 |
| No | 2 | 0.76 (0.57 to 1.03) | 0 | 0.39 | |
| Adjustment for trans fatty acids | |||||
| Yes | 2 | 1.00 (0.86 to 1.17) | 0 | 0.33 | 0.10 |
| No | 7 | 0.84 (0.76 to 0.93) | 0 | 0.67 | |
| Health status** | |||||
| Healthy | 9 | 0.89 (0.81 to 0.97) | 5.6 | 0.38 | - |
| Unhealthy | 0 | - | - | - | |
| Tissue ALA levels: | |||||
| Overall | 16 | 0.90 (0.70 to 1.15) | 57 | 0.003 | |
| Study location | |||||
| US | 5 | 0.85 (0.43 to 1.70) | 57.3 | 0.05 | 0.90 |
| Western countries§ | 14 | 0.93 (0.71 to 1.23) | 61.2 | 0.001 | |
| Eastern/Mediterranean countries | 2 | 0.68 (0.40 to 1.14) | 0 | 0.54 | |
| Sex | |||||
| Men and women | 10 | 0.85 (0.62 to 1.15) | 59.5 | 0.008 | 0.52 |
| Men | 5 | 1.05 (0.66 to 1.66) | 58.3 | 0.04 | |
| Women | 1 | 0.21 (0.01 to 3.80) | - | - | |
| Follow-up duration (years) | |||||
| ≥10 | 13 | 0.85 (0.67 to 1.09) | 56.5 | 0.006 | 0.47 |
| <10 | 1 | 0.44 (0.10 to 1.93) | - | - | |
| Tissue biomarkers | |||||
| Adipose tissue | 2 | 1.68 (0.77 to 3.66) | 62.2 | 0.10 | 0.04 |
| Blood | 14 | 0.81 (0.65 to 1.01) | 40.7 | 0.05 | |
| Total serum or plasma | 4 | 0.66 (0.45 to 0.97) | 5.1 | 0.36 | |
| Phospholipids (plasma or RBC) | 6 | 0.81 (0.55 to 1.18) | 57.9 | 0.03 | |
| Cholesteryl esters | 4 | 1.03 (0.79 to 1.35) | 0 | 0.52 | |
| Adjustment for BMI | |||||
| Yes | 16 | 0.90 (0.70 to 1.15) | 57 | 0.003 | - |
| No | 0 | - | - | - | |
| Health status** | |||||
| Healthy | 15 | 0.91 (0.71 to 1.17) | 58.9 | 0.002 | 0.44 |
| Unhealthy | 1 | 0.44 (0.10 to 1.93) | - | - | |
| Linear dose-response association | |||||
| Dietary ALA intake (per 1 g/day increase): | |||||
| Overall | 10 | 0.95 (0.90 to 1.01) | 10.8 | 0.34 | |
| Study location | |||||
| US | 7 | 0.93 (0.86 to 1.00) | 22 | 0.26 | 0.62 |
| Western countries§ | 9 | 0.97 (0.92 to 1.03) | 0 | 0.62 | |
| Eastern/Mediterranean countries | 1 | 0.82 (0.70 to 0.96) | - | - | |
| Sex | |||||
| Men and women | 5 | 0.93 (0.86 to 1.02) | 21.3 | 0.27 | 0.54 |
| Men | 4 | 0.98 (0.89 to 1.07) | 11.2 | 0.33 | |
| Women | 1 | 0.82 (0.61 to 1.10) | - | - | |
| Follow-up duration (years) | |||||
| ≥10 | 5 | 0.91 (0.81 to 1.02) | 30.4 | 0.21 | 0.86 |
| <10 | 5 | 0.97 (0.91 to 1.04) | 0 | 0.47 | |
| Dietary assessment tools | |||||
| Food frequency questionnaire | 8 | 0.95 (0.88 to 1.02) | 18.1 | 0.28 | 0.67 |
| Food recall or record | 2 | 0.93 (0.82 to 1.06) | 26.9 | 0.24 | |
| Adjustment for energy | |||||
| Yes | 9 | 0.96 (0.90 to 1.02) | 8.1 | 0.36 | 0.33 |
| No | 1 | 0.87 (0.74 to 1.02) | - | - | |
| Adjustment for BMI | |||||
| Yes | 9 | 0.96 (0.90 to 1.02) | 8.1 | 0.36 | 0.33 |
| No | 1 | 0.87 (0.74 to 1.02) | - | - | |
| Adjustment for other nutrients¶ | |||||
| Yes | 8 | 0.96 (0.90 to 1.03) | 15.8 | 0.30 | 0.25 |
| No | 2 | 0.88 (0.76 to 1.00) | 0 | 0.88 | |
| Adjustment for trans fatty acids | |||||
| Yes | 2 | 0.93 (0.74 to 1.17) | 0 | 0.32 | 0.92 |
| No | 8 | 0.95 (0.89 to 1.01) | 22.9 | 0.24 | |
| Health status** | |||||
| Healthy | 10 | 0.95 (0.90 to 1.01) | 10.8 | 0.34 | - |
| Unhealthy | 0 | - | - | - | |
| Tissue ALA levels (per 1 SD increase): | |||||
| Overall | 16 | 0.94 (0.87 to 1.01) | 61.1 | 0.001 | |
| Study location | |||||
| US | 5 | 0.87 (0.79 to 0.97) | 19.5 | 0.29 | 0.34 |
| Western countries§ | 14 | 0.94 (0.86 to 1.02) | 65.9 | <0.001 | |
| Eastern/Mediterranean countries | 2 | 0.97 (0.86 to 1.02) | 0 | 0.51 | |
| Sex | |||||
| Men and women | 10 | 0.94 (0.86 to 1.03) | 68 | 0.001 | 0.84 |
| Men | 5 | 0.97 (0.79 to 1.18) | 59.5 | 0.04 | |
| Women | 1 | 0.75 (0.37 to 1.54) | - | - | |
| Follow-up duration (years) | |||||
| ≥10 | 13 | 0.92 (0.84 to 0.99) | 64.9 | 0.001 | 0.89 |
| <10 | 1 | 0.97 (0.87 to 1.09) | - | - | |
| Tissue biomarkers | |||||
| Adipose tissue | 2 | 1.10 (1.02 to 1.08) | 0 | 0.82 | 0.04 |
| Blood | 14 | 0.92 (0.86 to 0.98) | 37.1 | 0.08 | |
| Total serum or plasma | 4 | 0.76 (0.63 to 0.91) | 0 | 0.83 | |
| Phospholipids (plasma or RBC) | 6 | 0.90 (0.82 to 0.98) | 44.3 | 0.11 | |
| Cholesteryl esters | 4 | 0.99 (0.92 to 1.07) | 0 | 0.87 | |
| Adjustment for BMI | |||||
| Yes | 16 | 0.94 (0.87 to 1.01) | 61.1 | 0.001 | - |
| No | 0 | - | - | - | |
| Health status** | |||||
| Healthy | 15 | 0.94 (0.86 to 1.02) | 63.6 | <0.001 | 0.79 |
| Unhealthy | 1 | 0.97 (0.87 to 1.09) | - | - | |
BMI=body mass index; RBC=red blood cells; SD=standard deviation.
Obtained from random effects model.
Inconsistency—percentage of variation across studies due to heterogeneity.
Obtained from Q test.
Includes studies from the US.
Studies that controlled for at least one of dietary nutrients, including lipids, vitamins, and minerals.
Studies that recruited apparently healthy adults (without a previous diagnosis of cancer, cardiovascular disease, or any other chronic diseases) were included in the healthy subgroup, and studies that recruited participants with at least one chronic disease were included in the unhealthy subgroup.
Tissue ALA
Sixteen studies from four publications investigated the association between tissue levels of ALA and CHD mortality.14 15 18 41 These studies included a total of 30 818 participants and 3380 CHD deaths. Combining data from these studies, comparing the highest and lowest levels of ALA, indicated no significant association between tissue levels of ALA and CHD mortality (pooled relative risk 0.90, 95% confidence interval 0.70 to 1.15, I2=57%; P=0.003 for heterogeneity) (table 3 and supplemental figure 11). In the linear dose-response analysis, no significant association was observed (pooled relative risk 0.94, 95% confidence interval 0.87 to 1.01, I2=61.1%; P=0.001 for heterogeneity) (table 3 and supplementary figure 12). The corresponding absolute risks for the highest versus lowest comparison and the linear dose-response analysis were −21 (95% confidence interval −63 to 32) and −13 (−27 to 2) deaths per 10 000 person years, respectively (supplementary table 11). Non-linear dose-response analyses were not possible owing to a limited number of papers with sufficient data.
Random effects meta-analysis: cancer mortality
Dietary ALA
Ten studies from nine papers reported on the association between dietary ALA intake and cancer mortality.16 17 22 23 32 34 36 37 53 These studies included a total of 849 711 participants with 61 259 cancer deaths. Combining data from these studies, comparing the highest intakes of ALA (median 1.35 g/day, interquartile range 1.20-2.12) with the lowest (0.70 g/day, 0.38-0.80), showed a significant positive association between ALA intake and cancer mortality (pooled relative risk 1.06, 95% confidence interval 1.02 to 1.11, I2=3.8%; P=0.40 for heterogeneity; table 4 and supplementary figure 13). In the dose-response meta-analysis, no significant association was found between a 1 g/day increase in ALA intake and cancer mortality (pooled relative risk 1.03, 95% confidence interval 0.98 to 1.08, I2=51%; P=0.04 for heterogeneity; table 4 and supplementary figure 14). The estimated absolute risks for the highest versus lowest comparison and the linear dose-response association for cancer mortality were 63 (95% confidence interval 21 to 116) and 42 (−21 to 95) cases per 10 000 person years, respectively (supplementary table 11). No evidence was found of a non-linear association (P=0.72 for non-linearity) (fig 2 and supplementary table 12).
Table 4.
Summary risk estimates for association between alpha linolenic acid (ALA) and risk of cancer mortality in adults aged ≥18 years
| No of effect sizes | Pooled relative risk (95% CI)* | I2 (%)† | P values | ||
|---|---|---|---|---|---|
| Heterogeneity‡ | Meta-regression | ||||
| Highest v lowest comparison | |||||
| Dietary ALA intake: | |||||
| Overall | 10 | 1.06 (1.02 to 1.11) | 3.8 | 0.40 | |
| Study location | |||||
| US | 8 | 1.06 (1.00 to 1.12) | 18.6 | 0.28 | 0.70 |
| Western countries§ | 10 | 1.06 (1.02 to 1.11) | 3.8 | 0.40 | |
| Eastern/Mediterranean countries | 0 | - | - | - | |
| Sex | |||||
| Men and women | 3 | 0.94 (0.77 to 1.14) | 57.8 | 0.09 | 0.25 |
| Men | 5 | 1.10 (1.00 to 1.21) | 0 | 0.79 | |
| Women | 2 | 1.10 (1.01 to 1.19) | 0 | 0.79 | |
| Follow-up duration (years) | |||||
| ≥10 | 7 | 1.07 (1.01 to 1.13) | 18.7 | 0.28 | 0.08 |
| <10 | 3 | 0.91 (0.72 to 1.14) | 0 | 0.94 | |
| Dietary assessment tools | |||||
| Food frequency questionnaire | 8 | 1.07 (1.02 to 1.13) | 7.1 | 0.37 | 0.26 |
| Food recall or record | 2 | 0.89 (0.68 to 1.15) | 0 | 0.94 | |
| Adjustment for energy | |||||
| Yes | 8 | 1.06 (1.01 to 1.12) | 20.2 | 0.27 | 0.51 |
| No | 2 | 0.93 (0.65 to 1.33) | 0 | 0.77 | |
| Adjustment for BMI | |||||
| Yes | 8 | 1.06 (1.01 to 1.12) | 21.3 | 0.26 | 0.64 |
| No | 2 | 0.96 (0.66 to 1.39) | 0 | 0.66 | |
| Adjustment for other nutrients¶ | |||||
| Yes | 6 | 1.06 (0.99 to 1.13) | 38.5 | 0.14 | 0.57 |
| No | 4 | 1.01 (0.85 to 1.19) | 0 | 0.83 | |
| Adjustment for trans fatty acids | |||||
| Yes | 5 | 1.07 (1.00 to 1.14) | 40.5 | 0.15 | 0.29 |
| No | 5 | 0.98 (0.85 to 1.13) | 0 | 0.84 | |
| Health status** | |||||
| Healthy | 6 | 1.06 (1.02 to 1.11) | 0.5 | 0.41 | 0.28 |
| Unhealthy | 4 | 0.94 (0.77 to 1.15) | 0.2 | 0.39 | |
| Tissue ALA levels: | |||||
| Overall | 4 | 0.93 (0.86 to 1.01) | 0 | 0.39 | |
| Linear dose-response association | |||||
| Dietary ALA intake (per 1 g/day increase) | |||||
| Overall | 8 | 1.03 (0.98 to 1.08) | 51 | 0.04 | |
| Study location | |||||
| US | 7 | 1.03 (0.97 to 1.09) | 57.3 | 0.02 | 0.83 |
| Western countries§ | 8 | 1.03 (0.98 to 1.08) | 51 | 0.04 | |
| Eastern/Mediterranean countries | 0 | - | - | - | |
| Sex | |||||
| Men and women | 3 | 0.96 (0.85 to 1.07) | 64 | 0.06 | 0.31 |
| Men | 3 | 1.05 (0.98 to 1.13) | 13 | 0.31 | |
| Women | 2 | 1.10 (1.03 to 1.17) | 0 | 0.96 | |
| Follow-up duration (years) | |||||
| ≥10 | 6 | 1.04 (0.98 to 1.10) | 58 | 0.03 | 0.02 |
| <10 | 2 | 0.95 (0.86 to 1.06) | 0 | 0.93 | |
| Dietary assessment tools | |||||
| Food frequency questionnaire | 6 | 1.04 (0.98 to 1.10) | 58 | 0.03 | 0.27 |
| Food recall or record | 2 | 0.95 (0.86 to 1.06) | 0 | 0.93 | |
| Adjustment for energy | |||||
| Yes | 7 | 1.03 (0.98 to 1.09) | 56.2 | 0.03 | 0.60 |
| No | 1 | 0.96 (0.79 to 1.16) | - | - | |
| Adjustment for BMI | |||||
| Yes | 6 | 1.03 (0.97 to 1.09) | 63.3 | 0.01 | 0.74 |
| No | 2 | 0.98 (0.83 to 1.17) | 0 | 0.56 | |
| Adjustment for other nutrients¶ | |||||
| Yes | 5 | 1.03 (0.97 to 1.10) | 70.1 | 0.01 | 0.67 |
| No | 3 | 0.99 (0.89 to 1.11) | 0 | 0.84 | |
| Adjustment for trans fatty acids | |||||
| Yes | 4 | 1.04 (0.97 to 1.10) | 74 | 0.009 | 0.30 |
| No | 4 | 0.98 (0.90 to 1.06) | 0 | 0.89 | |
| Health status** | |||||
| Healthy | 5 | 1.04 (0.99 to 1.10) | 53 | 0.07 | 0.22 |
| Unhealthy | 3 | 0.94 (0.79 to 1.11) | 40.4 | 0.18 | |
| Tissue ALA levels (per 1 SD increase): | |||||
| Overall | 2 | 0.99 (0.88 to 1.11) | 0 | 0.83 | |
BMI=body mass index; RBC=red blood cells; SD=standard deviation.
Obtained from random effects model.
Inconsistency—percentage of variation across studies due to heterogeneity.
Obtained from Q test.
Includes studies from the US.
Studies that controlled for at least one of dietary nutrients, including lipids, vitamins, and minerals.
Studies that recruited apparently healthy adults (without a previous diagnosis of cancer, cardiovascular disease, or any other chronic diseases) were included in the healthy subgroup, and studies that recruited participants with at least one chronic disease were included in the unhealthy subgroup.
Tissue ALA
Sixteen studies from four publications examined the association between tissue levels of ALA and cancer mortality.21 33 48 54 These studies included a total of 38 250 participants and 4153 cancer deaths. No significant association was found between ALA levels and cancer mortality in either the comparison of the highest versus lowest ALA levels (pooled relative risk 0.93, 95% confidence interval 0.86 to 1.01, I2=0%; P=0.39 for heterogeneity) or the dose-response analysis (0.99, 0.88 to 1.11, I2=0%; P=0.83 for heterogeneity) (table 4 and supplementary figures 15 and 16). The corresponding absolute risks for these associations were −74 (95% confidence interval −147 to 11) and −11 (−126 to 116) deaths per 10 000 person years, respectively (supplementary table 11).
Subgroup analysis, meta-regression, sensitivity analyses, and publication bias
Table 1, table 2, table 3, and table 4 show findings from subgroup analyses and meta-regression of ALA and mortality. In the meta-regression, follow-up duration and adjustments for dietary intakes of trans fatty acids and other nutrients appeared to be the main factors for heterogeneity in the observed associations between dietary ALA and mortality. For tissue levels of ALA and mortality, follow-up duration and sample type for measuring ALA levels (adipose tissue or total serum, plasma, blood phospholipids, or blood cholesteryl esters) appeared to be the main factors for heterogeneity among studies.
Based on subgroup analyses, a significant inverse association was seen between dietary ALA intake and all cause mortality among studies recruiting apparently healthy adults (without a previous diagnosis of cancer, CVD, or any other chronic diseases at study entry) and those that included both men and women, were conducted in the US, had a follow-up duration of <10 years, used food frequency questionnaire for dietary assessment, and presented BMI and energy adjusted risk estimates. In the subgroups of studies that controlled for dietary intakes of other nutrients, ALA intake showed a significant inverse relation with CVD and CHD mortality but not with all cause mortality. In studies that adjusted for dietary trans fatty acids, a positive association was found between dietary ALA intake and cancer mortality. For tissue ALA levels, we found a significant protective association between blood levels of ALA and all cause and CHD mortality, whereas such an inverse association was not found for ALA levels in adipose tissue. Each 1 SD increment in blood levels of ALA was associated with an 8% reduced risk of CHD mortality. Such a linear inverse association was also seen for serum and plasma levels of ALA and CVD mortality. We conducted a sex stratified non-linear dose-response analysis for the association between ALA intake and all cause mortality but found no non-linearity either in men (P=0.44 non-linearity) or in women (P=0.31 non-linearity) (supplementary figure 17 and supplementary table 12). Owing to a limited number of studies, we were unable to perform sex stratified non-linear analysis for other associations.
In the sensitivity analyses based on a random effect model, when the Harris et al study was excluded, the inverse association between tissue levels of ALA and all cause mortality (pooled relative risk 0.95, 95% confidence interval 0.90 to 0.99) lost statistical significance (0.95, 0.88 to 1.02).54 Sensitivity analyses for other associations showed that the pooled relative risks obtained in the current meta-analysis were robust and not driven by single studies. In addition, by assessing publication bias using Egger’s linear regression test, we found no substantial publication bias in all associations except for ALA intake and all cause mortality, in which there was evidence of publication bias. Nevertheless, the application of the trim-and-fill method did not change the average effect size, further suggesting that results were not affected by publication bias.
Fixed effects meta-analysis: mortality
Findings from the overall associations of dietary intakes and tissue levels of ALA with mortality based on the fixed effects analyses were similar to those obtained in the random effects analyses except for two associations (supplementary table 11): firstly, the inverse association between dietary ALA intake and all cause mortality, either in the linear dose-response analysis or in the highest versus lowest intake, that was significant in the random effects analysis but became non-significant in the fixed effects analysis (linear analysis: pooled relative risk 0.99, 95% confidence interval 0.98 to 1.01, high versus low intake: 1.01, 0.99 to 1.03); and, secondly, the non-significant linear association between dietary ALA and cancer mortality that became significant in the fixed effects analysis such that a 1 g/day increase in ALA intake was associated with a 4% increased risk of cancer mortality (pooled relative risk 1.04, 95% confidence interval 1.01 to 1.06). In the sensitivity analysis based on a fixed effect model, however, the non-significant association between dietary ALA intake and all cause mortality was sensitive to the NIH-AARP study37 (comprising 62.1% of the total weight in the fixed effects analysis) so that by excluding that study, the inverse association became significant in the highest versus lowest comparison (pooled relative risk 0.87, 95% confidence interval 0.80 to 0.95) and in the linear dose-response analysis (0.98, 0.96 to 0.99).
Discussion
In this systematic review and meta-analysis, we found that a higher intake of ALA was significantly associated with a 10%, 8%, and 11% lower risk of mortality from all causes, CVD, and CHD, respectively. A higher intake of ALA, however, was associated with a slightly higher risk of cancer mortality. No evidence was found of non-linear associations between ALA intake and all cause, CVD, CHD, and cancer mortality. A linear dose-response association was, however, found between dietary ALA intake and CVD mortality, such that a 1 g/day increase in ALA intake was associated with a 5% lower risk of CVD mortality. For tissue ALA levels, we found significant inverse associations between blood levels of ALA and all cause and CHD mortality and between serum and plasma levels of ALA and CVD mortality.
Comparison with other studies
The current study provides a comprehensive assessment of the health effects of ALA intake compared with previous meta-analyses that only focused on CHD mortality.55 81
Potential health benefits of polyunsaturated fatty acids have been reported in numerous cohort studies.82 83 It seems, however, that different types of polyunsaturated fatty acids have different effects.84 The current meta-analysis of prospective cohort studies showed a significant inverse association between dietary ALA intake and risk of mortality from all causes, CVD, and CHD based on analyses that compared the highest intakes with the lowest. The medians for the highest intakes of ALA among the studies included for these causes of death were more than 1.6 g/day (equivalent to 1.6 tablespoons of canola oil or 0.8 ounces of walnut) and medians for the lowest categories were <0.8 g/day (equivalent to 0.8 tablespoons of canola oil or 0.4 ounces of walnut). Our findings were in agreement with a meta-analysis of randomised clinical trials, which showed that ALA might slightly reduce the risk of CVD events, CHD mortality, and arrhythmia.85 Also, another meta-analysis showed a higher dietary ALA intake to be associated with a reduced risk of composite and fatal CHD.55 Concerning the biological mechanisms involved in the prevention of CVD or CHD, it is well known that ALA is a precursor to the long chain n-3 polyunsaturated fatty, including eicosapentaenoic acid and docosahexaenoic acid, that are critically responsible for producing various classes of anti-inflammatory eicosanoids.86 In contrast with n-3 polyunsaturated fatty acids, n-6 polyunsaturated fatty acids such as linoleic acid and arachidonic acid favour the production of pro-inflammatory eicosanoids that can regulate different homoeostatic and inflammatory processes.86 However, epidemiological studies have shown inverse associations between linoleic acid and CVD, cancer, and all cause mortality.83 Overall, previous studies have shown that a balanced intake of n-6 and n-3 polyunsaturated fatty acids is necessary to reduce the risk of CVD and maintain good health.11 ALA also possesses cardiovascular benefits regardless of conversion products. It is suggested that the intrinsic role of ALA linked to its CVD preventive effects include anticoagulant properties, regulation of eicosanoid production from arachidonic acid, triglyceride and blood pressure lowering effects, regulation of ion flux in cardiac cells, and the regulation of gene expression through the peroxisomal proliferation system.11 86 87 Also, evidence suggests that ALA might improve CVD risk factors more favourably than other n-3 polyunsaturated fatty acids such as eicosapentaenoic acid and docosahexaenoic acid.88
In the current meta-analysis, higher tissue levels of ALA were associated with a reduced risk of all cause mortality, whereas this was not significant for CVD and CHD mortality. This might be explained by ALA being measured in different tissues. When we confined the analysis to studies that considered blood ALA, particularly total plasma or serum ALA, the non-significant inverse associations between ALA and CVD and CHD mortality became significant. It seems that blood levels of ALA are objective biomarkers of circulating levels of ALA over the past 1-2 months that reflect diet together with the metabolism of dietary ALA.20 Therefore, findings from ALA blood levels compared with adipose tissue levels are more consistent with the findings from dietary ALA intake. Furthermore, ALA concentrations in tissue samples can be affected by metabolism and variations that occur in sample storage and laboratory assays, and these issues are other potential reasons for the lack of association between tissue levels of ALA and CVD and CHD mortality in the overall analysis. In total, our findings support the need for further investigation of the association between tissue ALA levels and mortality.
Based on the meta-analysis of the highest intake of ALA (median 1.35 g/day, equivalent to 1.35 tablespoons of canola oil or 0.7 ounces of walnut) compared with lowest (0.70 g/day, equivalent to 0.7 tablespoons of canola oil or 0.35 ounces of walnut), a high intake was associated with a slightly higher risk of cancer mortality. A meta-analysis conducted by Hanson et al showed that increasing ALA intake probably had little effect on the risk of death due to cancer but a slightly increased risk of prostate cancer.89 Nevertheless, that meta-analysis reported that this positive association was due to publication bias and possibly low quality evidence. In the present study, although the Egger test found no substantial publication bias in the relation between ALA and cancer mortality, we cannot fully reject the existence of such bias. Also, of nine studies included in this association, six had a moderate-to-high risk of bias.16 17 23 32 36 53 It should be taken into account that based on the non-linear dose-response analysis, we found no significant positive association between ALA and cancer mortality in the dietary intakes from 0.27 to 3 g/day. It seems that findings from the dose-response analyses are more reliable than those from the highest versus lowest intake comparison in which estimates might encounter misclassification bias because of the different ranges of the highest and lowest categories of ALA intakes among different studies. Also, such a positive association was not seen for tissue levels of ALA.
The positive association between ALA intake and cancer mortality might be due to the existence of trans forms of ALA in certain foods. This claim was confirmed in the subgroup analysis in which after combining trans fatty acid adjusted relative risk, the significant positive association between ALA intake and cancer mortality became marginally significant (pooled relative risk 1.07, 95% confidence interval 1.00 to 1.14). In addition, ALA is susceptible to oxidation and can produce oxidative species such as oxylipins.6 90 These species can induce DNA damage and increase the risk of cancer and have an adverse impact on cancer mortality.90 Overall, recommendations for ALA consumption or intakes of ALA-rich foods need to weigh the benefits and any risks cautiously. Our findings from the current meta-analysis cannot fully prove the deleterious association between ALA intake and cancer mortality. Other studies have found that high intakes of nuts and walnuts is associated with reduced all cause, CVD, and cancer mortality,91 92 suggesting that unless the observed association between ALA and cancer mortality is due to bias, it could differ by food source. Additional studies might over time provide extra evidence to help determine the association more definitively and determine whether particular food sources of ALA have a differential impact on cancer mortality.
People received ALA in combination with other nutrients in the form of a mixed diet. Therefore, intakes of nutrients in ALA-rich foods, particularly other lipids, could be important confounders for the association between ALA and mortality. By combining studies that controlled analyses for other nutrients in the subgroup analysis, the significant associations between ALA intake and all cause and cancer mortality disappeared, but the associations for CVD and CHD mortality remained significant. The number of studies with adjustment for other nutrients was, however, limited and we cannot exclude the possibility that other study specific factors might contribute to these subgroup differences. We also found a difference in the association between ALA intake and all cause mortality according to the length of follow-up, with a stronger association for those studies that had less than 10 years of follow-up compared with 10 years or more. This might at least in part be explained by reverse causation bias or regression dilution bias. For instance, participants might have changed their diet to a healthy one containing a high amount of ALA after developing chronic diseases or other conditions related to early death. Healthy diets often contain a high amount of ALA-rich plant based foods such as canola oil, soybean, nuts, and seeds. The mentioned reverse causation might be a reason for the small effect size obtained for the association between ALA and mortality. We also found regional differences in the relation between ALA intake and mortality that might be explained by different dietary sources of ALA among different regions. Another reason might be differences in cooking and processing methods of ALA-rich foods among cultures. Our findings on the relation between ALA intake and mortality should be interpreted with the previously mentioned points in mind.
Based on the non-linear dose-response analysis (supplementary table 12), intakes of ALA between 1.0 and 2.5 g/day were best for the prevention of CHD mortality. These intakes were equivalent to those of 1-2.5 tablespoons of canola oil or 0.5-1.25 ounces of walnut each day. We also found a significant risk reduction for CVD mortality associated with intakes of ALA of 2.25 g/day and higher. Although we found a risk reduction in these intakes of ALA for all cause mortality, this finding was not statistically significant. We can therefore conclude that intakes of ALA of 1 g/day and higher are safe and have no adverse effects on the risk of these causes of death. However, since a high intake of ALA (median 1.35 g/day, interquartile range 1.20-2.12) was associated with a slightly increased risk of cancer mortality, based on the analyses of the highest versus lowest intakes, recommendations for ALA intake in this range should be made cautiously. Based on the non-linear analysis in men and women, no significant relation was found between ALA intake and all cause mortality at intakes of 0-2.5 g/day, and thus a protective intake cannot be established. Owing to a limited number of studies, we were unable to perform a sex stratified analysis for the other causes of death. Also, we did not have detailed enough data to perform non-linear analyses based on some other important variables, including age groups and anthropometric measures, to give recommendations for ALA based on these variables.
In a usual dietary pattern, ALA intake is so small that statistical analyses might encounter difficulties in finding an association with health outcomes. Based on dietary reference intake, however, the recommended intake of ALA is 1.1 g/day for women and 1.6 g/day for men.93 ALA intake much more than 1.6 g/day or much less than 1.1 g/day might have beneficial or deleterious effects on health. For example, in a randomised clinical trial, intervention with 1.9 g/day ALA was associated with a reduction in serum levels of low density lipoprotein and total cholesterol in a population with metabolic syndrome and diabetes.12 Among the studies included in the current meta-analysis, ALA intakes varied between 0.35 and 3.0 g/day and therefore these variations helped us to detect the association between ALA intake and mortality at different high versus low intakes. Another difficulty is the estimation of ALA intake using dietary questionnaires because of its low intake. For this reason, we assessed tissue biomarkers of ALA that are good indicators of ALA intake in relation to mortality. For some associations, the findings of ALA intake and mortality were in line with those obtained for tissue levels of ALA and mortality.
Strengths and weaknesses of this study
In the current meta-analysis, the prospective design of included studies and many participants and deaths were the major strengths, which allowed us to quantitatively evaluate the association between ALA and mortality. Also, the linear and non-linear dose-response analyses provided compelling evidence for quantitative evaluation of associations and enabled us to determine the strength and shape of the dose-response relations. We also analysed studies that examined ALA concentrations in blood and serum and adipose tissues, which are useful for a more objective assessment of ALA intake. Finally, we evaluated the associations separately for cause specific mortality.
Our findings also need to be interpreted by considering several limitations. Firstly, since our included studies were observational, causality cannot be established. Secondly, the role of residual confounders resulting from unmeasured behavioural and biological factors or errors in the measurement of covariates cannot be entirely excluded owing to the observational design of included studies. Thirdly, measurement errors are inevitable in estimates of food and nutrient intakes. Misclassification due to measurement errors could result in an underestimation of the association between ALA intake and mortality. Fourthly, evidence of considerable heterogeneity among included studies might be explained by variations in the amount of ALA intake, follow-up, exposure assessment methods, frequency of dietary assessments, and adjustments for confounding factors. Fifthly, as is usually the case in nutritional studies, most had estimated ALA intake based on a single measurement at study baseline, and changes in diet during follow-up were not considered. In addition, regional differences in dietary ALA intake might have been a problem in this meta-analysis and could have affected the highest and lowest categories of ALA intake and the results from these comparisons. These categories were, however, not so different among studies from different regions, and accordingly we performed a subgroup analysis to control for these differences. We also conducted the dose-response analysis as another strategy to control for these differences and also the overlap between the ranges of ALA intake among different studies.
Conclusions, policy implications, and future research
Dietary intake of ALA was associated with a lower risk of mortality from all causes, CVD, and CHD and a slightly higher risk of cancer mortality. We found a significant inverse association between blood levels of ALA and risk of all cause mortality. Additionally, each 1 SD increment in blood and total plasma or serum levels of ALA was associated with a reduced risk of CHD and CVD mortality, respectively. No significant association was, however, observed between ALA levels and cancer mortality. As most of the studies included in the meta-analysis were from western nations, extrapolation of our findings to the worldwide population should be done cautiously. Further studies should examine the association between ALA and a wider range of causes of death to provide a more comprehensive assessment of the potential health effects of ALA as well as to examine whether specific foods rich in ALA are differentially associated with mortality from cancer and other causes.
What is already known on this topic
A high intake of alpha linolenic acid (ALA) has been associated with a lower risk of fatal coronary heart disease
Findings from epidemiological studies on ALA and risk of mortality have been inconclusive
What this study adds
A high intake of ALA is associated with a lower risk of mortality from all causes, cardiovascular disease (CVD), and coronary heart disease, and a slightly higher risk of cancer mortality
Each 1 g/day increase in ALA intake was associated with a 5% lower risk of CVD mortality
Each 1 SD increment in blood levels of ALA was associated with an 8% lower risk of coronary heart disease mortality
Acknowledgments
Joseph Beyene holds the John D Cameron endowed chair in the genetic determinants of chronic diseases, McMaster University.
Web extra.
Extra material supplied by authors
Web appendix: Supplementary material
Contributors: OS and MA (Asadi.masoomeh@ymail.com) are joint corresponding authors and were equally involved in the study. OS and SN searched the literature and extracted data. SN and DA analysed the data. OS and SN drafted the manuscript, which was critically revised for important intellectual content by all authors. JB drafted the manuscript and analysed the data. SM and MA obtained funding and edited the manuscript. OS supervised the study. All authors have read and approved the final manuscript. OS is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was funded by the Abadan University of Medical Sciences, Abadan, Iran (grant No 871). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the Abadan University of Medical Sciences, Abadan, Iran, for the submitted work; no financial relationships with any organisation that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The corresponding authors (OS and MA) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported. No important aspects of the study have been omitted and any discrepancies from the study as planned have been disclosed.
Dissemination to participants and related patient and public communities: We plan to disseminate these findings to participants in our annual newsletter.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Not required.
Data availability statement
No additional data available.
References
- 1. Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals. Nat Rev Cardiol 2018;15:230-40. 10.1038/nrcardio.2017.154. [DOI] [PubMed] [Google Scholar]
- 2. Balakumar P, Maung-U K, Jagadeesh G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol Res 2016;113(Pt A):600-9. 10.1016/j.phrs.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 3. Bauer UE, Briss PA, Goodman RA, Bowman BA. Prevention of chronic disease in the 21st century: elimination of the leading preventable causes of premature death and disability in the USA. Lancet 2014;384:45-52. 10.1016/S0140-6736(14)60648-6. [DOI] [PubMed] [Google Scholar]
- 4. Mori TA. Marine OMEGA-3 fatty acids in the prevention of cardiovascular disease. Fitoterapia 2017;123:51-8. 10.1016/j.fitote.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 5. Watanabe Y, Tatsuno I. Omega-3 polyunsaturated fatty acids for cardiovascular diseases: present, past and future. Expert Rev Clin Pharmacol 2017;10:865-73. 10.1080/17512433.2017.1333902. [DOI] [PubMed] [Google Scholar]
- 6. Aucoin M, Cooley K, Knee C, et al. Fish-Derived Omega-3 Fatty Acids and Prostate Cancer: A Systematic Review. Integr Cancer Ther 2017;16:32-62. 10.1177/1534735416656052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fritsche KL. The science of fatty acids and inflammation. Adv Nutr 2015;6:293S-301S. 10.3945/an.114.006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spector AA, Kim HY. Discovery of essential fatty acids. J Lipid Res 2015;56:11-21. 10.1194/jlr.R055095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kumar NG, Contaifer D, Madurantakam P, et al. Dietary Bioactive Fatty Acids as Modulators of Immune Function: Implications on Human Health. Nutrients 2019;11:E2974. 10.3390/nu11122974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shahidi F, Ambigaipalan P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu Rev Food Sci Technol 2018;9:345-81. 10.1146/annurev-food-111317-095850. [DOI] [PubMed] [Google Scholar]
- 11. Stark AH, Reifen R, Crawford MA. Past and Present Insights on Alpha-linolenic Acid and the Omega-3 Fatty Acid Family. Crit Rev Food Sci Nutr 2016;56:2261-7. 10.1080/10408398.2013.828678. [DOI] [PubMed] [Google Scholar]
- 12. Lee TC, Ivester P, Hester AG, et al. The impact of polyunsaturated fatty acid-based dietary supplements on disease biomarkers in a metabolic syndrome/diabetes population. Lipids Health Dis 2014;13:196. 10.1186/1476-511X-13-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mozaffarian D. Does alpha-linolenic acid intake reduce the risk of coronary heart disease? A review of the evidence. Altern Ther Health Med 2005;11:24-30, quiz 31, 79. [PubMed] [Google Scholar]
- 14. Bork CS, Jakobsen MU, Lundbye-Christensen S, Tjønneland A, Schmidt EB, Overvad K. Dietary intake and adipose tissue content of α-linolenic acid and risk of myocardial infarction: a Danish cohort study. Am J Clin Nutr 2016;104:41-8. 10.3945/ajcn.115.127019. [DOI] [PubMed] [Google Scholar]
- 15. de Goede J, Verschuren WM, Boer JM, Verberne LD, Kromhout D, Geleijnse JM. N-6 and N-3 fatty acid cholesteryl esters in relation to fatal CHD in a Dutch adult population: a nested case-control study and meta-analysis. PLoS One 2013;8:e59408. 10.1371/journal.pone.0059408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dolecek TA, Granditis G. Dietary polyunsaturated fatty acids and mortality in the Multiple Risk Factor Intervention Trial (MRFIT). World Rev Nutr Diet 1991;66:205-16. 10.1159/000419291. [DOI] [PubMed] [Google Scholar]
- 17. Epstein MM, Kasperzyk JL, Mucci LA, et al. Dietary fatty acid intake and prostate cancer survival in Örebro County, Sweden. Am J Epidemiol 2012;176:240-52. 10.1093/aje/kwr520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Erkkilä AT, Lehto S, Pyörälä K, Uusitupa MI. n-3 Fatty acids and 5-y risks of death and cardiovascular disease events in patients with coronary artery disease. Am J Clin Nutr 2003;78:65-71. 10.1093/ajcn/78.1.65. [DOI] [PubMed] [Google Scholar]
- 19. Fortes C, Forastiere F, Farchi S, Rapiti E, Pastori G, Perucci CA. Diet and overall survival in a cohort of very elderly people. Epidemiology 2000;11:440-5. 10.1097/00001648-200007000-00013. [DOI] [PubMed] [Google Scholar]
- 20. Fretts AM, Mozaffarian D, Siscovick DS, et al. Plasma phospholipid and dietary α-linolenic acid, mortality, CHD and stroke: the Cardiovascular Health Study. Br J Nutr 2014;112:1206-13. 10.1017/S0007114514001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harris WS, Luo J, Pottala JV, et al. Red blood cell polyunsaturated fatty acids and mortality in the Women’s Health Initiative Memory Study. J Clin Lipidol 2017;11:250-259.e5. 10.1016/j.jacl.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiao J, Liu G, Shin HJ, et al. Dietary fats and mortality among patients with type 2 diabetes: analysis in two population based cohort studies. BMJ 2019;366:l4009. 10.1136/bmj.l4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khankari NK, Bradshaw PT, Steck SE, et al. Dietary intake of fish, polyunsaturated fatty acids, and survival after breast cancer: A population-based follow-up study on Long Island, New York. Cancer 2015;121:2244-52. 10.1002/cncr.29329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koh AS, Pan A, Wang R, et al. The association between dietary omega-3 fatty acids and cardiovascular death: the Singapore Chinese Health Study. Eur J Prev Cardiol 2015;22:364-72. 10.1177/2047487313517576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laaksonen DE, Nyyssönen K, Niskanen L, Rissanen TH, Salonen JT. Prediction of cardiovascular mortality in middle-aged men by dietary and serum linoleic and polyunsaturated fatty acids. Arch Intern Med 2005;165:193-9. 10.1001/archinte.165.2.193. [DOI] [PubMed] [Google Scholar]
- 26. Lelli D, Antonelli Incalzi R, Ferrucci L, Bandinelli S, Pedone C. Association between PUFA intake and serum concentration and mortality in older adults: A cohort study. Clin Nutr 2020;39:510-5. 10.1016/j.clnu.2019.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lindberg M, Saltvedt I, Sletvold O, Bjerve KS. Long-chain n-3 fatty acids and mortality in elderly patients. Am J Clin Nutr 2008;88:722-9. 10.1093/ajcn/88.3.722. [DOI] [PubMed] [Google Scholar]
- 28. Marklund M, Leander K, Vikström M, et al. Polyunsaturated Fat Intake Estimated by Circulating Biomarkers and Risk of Cardiovascular Disease and All-Cause Mortality in a Population-Based Cohort of 60-Year-Old Men and Women. Circulation 2015;132:586-94. 10.1161/CIRCULATIONAHA.115.015607. [DOI] [PubMed] [Google Scholar]
- 29. Oomen CM, Ocké MC, Feskens EJ, Kok FJ, Kromhout D. alpha-Linolenic acid intake is not beneficially associated with 10-y risk of coronary artery disease incidence: the Zutphen Elderly Study. Am J Clin Nutr 2001;74:457-63. 10.1093/ajcn/74.4.457. [DOI] [PubMed] [Google Scholar]
- 30. Pietinen P, Ascherio A, Korhonen P, et al. Intake of fatty acids and risk of coronary heart disease in a cohort of Finnish men. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Epidemiol 1997;145:876-87. 10.1093/oxfordjournals.aje.a009047. [DOI] [PubMed] [Google Scholar]
- 31. Pottala JV, Garg S, Cohen BE, Whooley MA, Harris WS. Blood eicosapentaenoic and docosahexaenoic acids predict all-cause mortality in patients with stable coronary heart disease: the Heart and Soul study. Circ Cardiovasc Qual Outcomes 2010;3:406-12. 10.1161/CIRCOUTCOMES.109.896159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Richman EL, Kenfield SA, Chavarro JE, et al. Fat intake after diagnosis and risk of lethal prostate cancer and all-cause mortality. JAMA Intern Med 2013;173:1318-26. 10.1001/jamainternmed.2013.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Simon JA, Fong J, Bernert JT, Jr, Browner WS. Serum fatty acids and the risk of fatal cancer. MRFIT Research Group. Multiple Risk Factor Intervention Trial. Am J Epidemiol 1998;148:854-8. 10.1093/oxfordjournals.aje.a009710. [DOI] [PubMed] [Google Scholar]
- 34. Wang DD, Li Y, Chiuve SE, et al. Association of Specific Dietary Fats With Total and Cause-Specific Mortality. JAMA Intern Med 2016;176:1134-45. 10.1001/jamainternmed.2016.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Warensjö E, Sundström J, Vessby B, Cederholm T, Risérus U. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: a population-based prospective study. Am J Clin Nutr 2008;88:203-9. 10.1093/ajcn/88.1.203. [DOI] [PubMed] [Google Scholar]
- 36. Zhuang P, Wang W, Wang J, Zhang Y, Jiao J. Polyunsaturated fatty acids intake, omega-6/omega-3 ratio and mortality: Findings from two independent nationwide cohorts. Clin Nutr 2019;38:848-55. 10.1016/j.clnu.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 37. Zhuang P, Zhang Y, He W, et al. Dietary Fats in Relation to Total and Cause-Specific Mortality in a Prospective Cohort of 521 120 Individuals With 16 Years of Follow-Up. Circ Res 2019;124:757-68. 10.1161/CIRCRESAHA.118.314038. [DOI] [PubMed] [Google Scholar]
- 38. Sala-Vila A, Guasch-Ferré M, Hu FB, et al. PREDIMED Investigators . Dietary α-Linolenic Acid, Marine ω-3 Fatty Acids, and Mortality in a Population With High Fish Consumption: Findings From the PREvención con DIeta MEDiterránea (PREDIMED) Study. J Am Heart Assoc 2016;5:e002543. 10.1161/JAHA.115.002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miura K, Hughes MCB, Ungerer JP, Green AC. Plasma eicosapentaenoic acid is negatively associated with all-cause mortality among men and women in a population-based prospective study. Nutr Res 2016;36:1202-9. 10.1016/j.nutres.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 40. Gopinath B, Buyken AE, Flood VM, Empson M, Rochtchina E, Mitchell P. Consumption of polyunsaturated fatty acids, fish, and nuts and risk of inflammatory disease mortality. Am J Clin Nutr 2011;93:1073-9. 10.3945/ajcn.110.009977. [DOI] [PubMed] [Google Scholar]
- 41. Del Gobbo LC, Imamura F, Aslibekyan S, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCe) . ω-3 Polyunsaturated Fatty Acid Biomarkers and Coronary Heart Disease: Pooling Project of 19 Cohort Studies. JAMA Intern Med 2016;176:1155-66. 10.1001/jamainternmed.2016.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vedtofte MS, Jakobsen MU, Lauritzen L, et al. Association between the intake of α-linolenic acid and the risk of CHD. Br J Nutr 2014;112:735-43. 10.1017/S000711451400138X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Albert CM, Oh K, Whang W, et al. Dietary alpha-linolenic acid intake and risk of sudden cardiac death and coronary heart disease. Circulation 2005;112:3232-8. 10.1161/CIRCULATIONAHA.105.572008. [DOI] [PubMed] [Google Scholar]
- 44. Ascherio A, Rimm EB, Giovannucci EL, Spiegelman D, Stampfer M, Willett WC. Dietary fat and risk of coronary heart disease in men: cohort follow up study in the United States. BMJ 1996;313:84-90. 10.1136/bmj.313.7049.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pelser C, Mondul AM, Hollenbeck AR, Park Y. Dietary fat, fatty acids, and risk of prostate cancer in the NIH-AARP diet and health study. Cancer Epidemiol Biomarkers Prev 2013;22:697-707. 10.1158/1055-9965.EPI-12-1196-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rhee JJ, Kim E, Buring JE, Kurth T. Fish Consumption, Omega-3 Fatty Acids, and Risk of Cardiovascular Disease. Am J Prev Med 2017;52:10-9. 10.1016/j.amepre.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Iggman D, Ärnlöv J, Cederholm T, Risérus U. Association of Adipose Tissue Fatty Acids With Cardiovascular and All-Cause Mortality in Elderly Men. JAMA Cardiol 2016;1:745-53. 10.1001/jamacardio.2016.2259. [DOI] [PubMed] [Google Scholar]
- 48. Harris WS, Tintle NL, Etherton MR, Vasan RS. Erythrocyte long-chain omega-3 fatty acid levels are inversely associated with mortality and with incident cardiovascular disease: The Framingham Heart Study. J Clin Lipidol 2018;12:718-727.e6. 10.1016/j.jacl.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kleber ME, Delgado GE, Lorkowski S, März W, von Schacky C. Omega-3 fatty acids and mortality in patients referred for coronary angiography. The Ludwigshafen Risk and Cardiovascular Health Study. Atherosclerosis 2016;252:175-81. 10.1016/j.atherosclerosis.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 50. Jayanama K, Theou O, Godin J, Cahill L, Rockwood K. Association of fatty acid consumption with frailty and mortality among middle-aged and older adults. Nutrition 2020;70:110610. 10.1016/j.nut.2019.110610. [DOI] [PubMed] [Google Scholar]
- 51. Lázaro I, Rueda F, Cediel G, et al. Circulating Omega-3 Fatty Acids and Incident Adverse Events in Patients With Acute Myocardial Infarction. J Am Coll Cardiol 2020;76:2089-97. 10.1016/j.jacc.2020.08.073. [DOI] [PubMed] [Google Scholar]
- 52. Harris WS, Kennedy KF, O’Keefe JH, Jr, Spertus JA. Red blood cell fatty acid levels improve GRACE score prediction of 2-yr mortality in patients with myocardial infarction. Int J Cardiol 2013;168:53-9. 10.1016/j.ijcard.2012.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Perez-Cornago A, Huybrechts I, Appleby PN, et al. Intake of individual fatty acids and risk of prostate cancer in the European prospective investigation into cancer and nutrition. Int J Cancer 2020;146:44-57. 10.1002/ijc.32233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Harris WS, Tintle NL, Imamura F, et al. Fatty Acids and Outcomes Research Consortium (FORCE) . Blood n-3 fatty acid levels and total and cause-specific mortality from 17 prospective studies. Nat Commun 2021;12:2329. 10.1038/s41467-021-22370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wei J, Hou R, Xi Y, et al. The association and dose-response relationship between dietary intake of α-linolenic acid and risk of CHD: a systematic review and meta-analysis of cohort studies. Br J Nutr 2018;119:83-9. 10.1017/S0007114517003294. [DOI] [PubMed] [Google Scholar]
- 56. Simon JA, Chen YH, Bent S. The relation of alpha-linolenic acid to the risk of prostate cancer: a systematic review and meta-analysis. Am J Clin Nutr 2009;89:1558S-64S. 10.3945/ajcn.2009.26736E. [DOI] [PubMed] [Google Scholar]
- 57. Brouwer IA, Katan MB, Zock PL. Dietary alpha-linolenic acid is associated with reduced risk of fatal coronary heart disease, but increased prostate cancer risk: a meta-analysis. J Nutr 2004;134:919-22. 10.1093/jn/134.4.919. [DOI] [PubMed] [Google Scholar]
- 58. Shamseer L, Moher D, Clarke M, et al. PRISMA-P Group . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647. 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 59. Bero L, Chartres N, Diong J, et al. The risk of bias in observational studies of exposures (ROBINS-E) tool: concerns arising from application to observational studies of exposures. Syst Rev 2018;7:242. 10.1186/s13643-018-0915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA 1998;279:1477-82. 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 61. DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials 2015;45(Pt A):139-45. 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Borenstein M, Hedges LV, Higgins JP, et al. Fixed-effect versus random-effects models. Introduction to Meta-analysis 2009;77:85. [DOI] [PubMed] [Google Scholar]
- 63. Sarwar N, Gao P, Seshasai SR, et al. Emerging Risk Factors Collaboration . Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215-22. 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 65. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. van Enst WA, Ochodo E, Scholten RJ, Hooft L, Leeflang MM. Investigation of publication bias in meta-analyses of diagnostic test accuracy: a meta-epidemiological study. BMC Med Res Methodol 2014;14:70. 10.1186/1471-2288-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Abudurexiti M, Zhu W, Wang Y, et al. Targeting CPT1B as a potential therapeutic strategy in castration-resistant and enzalutamide-resistant prostate cancer. Prostate 2020;80:950-61. 10.1002/pros.24027. [DOI] [PubMed] [Google Scholar]
- 68. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301-9. 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 69. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stata J 2006;6:40-57 10.1177/1536867X0600600103. [DOI] [Google Scholar]
- 70. Huang X, Stenvinkel P, Qureshi AR, et al. Essential polyunsaturated fatty acids, inflammation and mortality in dialysis patients. Nephrol Dial Transplant 2012;27:3615-20. 10.1093/ndt/gfs132. [DOI] [PubMed] [Google Scholar]
- 71. Zelniker TA, Morrow DA, Scirica BM, et al. Plasma Omega-3 Fatty Acids and the Risk of Cardiovascular Events in Patients After an Acute Coronary Syndrome in MERLIN-TIMI 36. J Am Heart Assoc 2021;10:e017401. 10.1161/JAHA.120.017401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lemaitre RN, King IB, Sotoodehnia N, et al. Red blood cell membrane alpha-linolenic acid and the risk of sudden cardiac arrest. Metabolism 2009;58:534-40. 10.1016/j.metabol.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liyanage UE, Ong JS, An J, Gharahkhani P, Law MH, MacGregor S. Mendelian Randomization Study for Genetically Predicted Polyunsaturated Fatty Acids Levels on Overall Cancer Risk and Mortality. Cancer Epidemiol Biomarkers Prev 2019;28:1015-23. 10.1158/1055-9965.EPI-18-0940. [DOI] [PubMed] [Google Scholar]
- 74. Folsom AR, Demissie Z. Fish intake, marine omega-3 fatty acids, and mortality in a cohort of postmenopausal women. Am J Epidemiol 2004;160:1005-10. 10.1093/aje/kwh307. [DOI] [PubMed] [Google Scholar]
- 75. Dolecek TA. Epidemiological evidence of relationships between dietary polyunsaturated fatty acids and mortality in the multiple risk factor intervention trial. Proc Soc Exp Biol Med 1992;200:177-82. 10.3181/00379727-200-43413. [DOI] [PubMed] [Google Scholar]
- 76. Kilander L, Berglund L, Boberg M, Vessby B, Lithell H. Education, lifestyle factors and mortality from cardiovascular disease and cancer. A 25-year follow-up of Swedish 50-year-old men. Int J Epidemiol 2001;30:1119-26. 10.1093/ije/30.5.1119. [DOI] [PubMed] [Google Scholar]
- 77. Hu FB, Stampfer MJ, Manson JE, et al. Dietary intake of alpha-linolenic acid and risk of fatal ischemic heart disease among women. Am J Clin Nutr 1999;69:890-7. 10.1093/ajcn/69.5.890. [DOI] [PubMed] [Google Scholar]
- 78. Wu J, Wilson KM, Stampfer MJ, Willett WC, Giovannucci EL. A 24-year prospective study of dietary α-linolenic acid and lethal prostate cancer. Int J Cancer 2018;142:2207-14. 10.1002/ijc.31247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Leitzmann MF, Stampfer MJ, Michaud DS, et al. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am J Clin Nutr 2004;80:204-16. 10.1093/ajcn/80.1.204. [DOI] [PubMed] [Google Scholar]
- 80. Lemaitre RN, King IB, Mozaffarian D, Kuller LH, Tracy RP, Siscovick DS. n-3 Polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: the Cardiovascular Health Study. Am J Clin Nutr 2003;77:319-25. 10.1093/ajcn/77.2.319. [DOI] [PubMed] [Google Scholar]
- 81. Pan A, Chen M, Chowdhury R, et al. α-Linolenic acid and risk of cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr 2012;96:1262-73. 10.3945/ajcn.112.044040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mazidi M, Mikhailidis DP, Sattar N, et al. International Lipid Expert Panel (ILEP) & Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group . Association of types of dietary fats and all-cause and cause-specific mortality: A prospective cohort study and meta-analysis of prospective studies with 1,164,029 participants. Clin Nutr 2020;39:3677-86. 10.1016/j.clnu.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 83. Li J, Guasch-Ferré M, Li Y, Hu FB. Dietary intake and biomarkers of linoleic acid and mortality: systematic review and meta-analysis of prospective cohort studies. Am J Clin Nutr 2020;112:150-67. 10.1093/ajcn/nqz349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 2008;233:674-88. 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 85. Abdelhamid AS, Brown TJ, Brainard JS, et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev 2018;7:CD003177. 10.1002/14651858.CD003177.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Saini RK, Keum YS. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance - A review. Life Sci 2018;203:255-67. 10.1016/j.lfs.2018.04.049. [DOI] [PubMed] [Google Scholar]
- 87. Freese R, Mutanen M, Valsta LM, Salminen I. Comparison of the effects of two diets rich in monounsaturated fatty acids differing in their linoleic/alpha-linolenic acid ratio on platelet aggregation. Thromb Haemost 1994;71:73-7. 10.1055/s-0038-1642387 [DOI] [PubMed] [Google Scholar]
- 88. Goyens PL, Mensink RP. Effects of alpha-linolenic acid versus those of EPA/DHA on cardiovascular risk markers in healthy elderly subjects. Eur J Clin Nutr 2006;60:978-84. 10.1038/sj.ejcn.1602408. [DOI] [PubMed] [Google Scholar]
- 89. Hanson S, Thorpe G, Winstanley L, Abdelhamid AS, Hooper L, PUFAH group . Omega-3, omega-6 and total dietary polyunsaturated fat on cancer incidence: systematic review and meta-analysis of randomised trials. Br J Cancer 2020;122:1260-70. 10.1038/s41416-020-0761-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Serini S, Calviello G. Long-chain omega-3 fatty acids and cancer: any cause for concern? Curr Opin Clin Nutr Metab Care 2018;21:83-9. 10.1097/MCO.0000000000000439. [DOI] [PubMed] [Google Scholar]
- 91. Aune D, Keum N, Giovannucci E, et al. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective studies. BMC Med 2016;14:207. 10.1186/s12916-016-0730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Guasch-Ferré M, Bulló M, Martínez-González MÁ, et al. PREDIMED study group . Frequency of nut consumption and mortality risk in the PREDIMED nutrition intervention trial. BMC Med 2013;11:164. 10.1186/1741-7015-11-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Trumbo P, Schlicker S, Yates AA, Poos M, Food and Nutrition Board of the Institute of Medicine, The National Academies . Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc 2002;102:1621-30. 10.1016/S0002-8223(02)90346-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary material
Data Availability Statement
No additional data available.