The Coronavirus Disease (COVID-19) pandemic imposes a high burden of morbidity and mortality worldwide. Several vaccines have been developed to curb the plague and currently represent the most important weapon for this fight. The currently available SARS-CoV-2 vaccines are either lipid nanoparticle/mRNA-based or adenoviral vector vaccines. These vaccines proved effective in preventing severe disease and death in phase-III randomized clinical trials, without significant safety warnings [1], [2]. This led to worldwide approval and mass administration.
Shortly after, a rare syndrome characterized by atypical thrombosis and thrombocytopenia was described [3], [4], [5], [6]. This syndrome, defined as “vaccine-induced immune thrombotic thrombocytopenia” (VITT) [7], [8], mainly occurred among otherwise healthy adults – mostly women, all vaccinated with SARS-CoV-2 adenoviral vector vaccines [3], with an estimated prevalence of approximately 1 every 100.000 vaccinated adults [9]. Cerebral venous thrombosis was the most frequent type of thrombosis reported, with splanchnic thrombosis described only in exceptional cases [5].
The pathogenesis, diagnosis, and optimal treatment of VITT are largely unknown. A role for anti-platelet factor 4 (PF4) antibodies in its pathogenesis has been postulated [10], with high uncertainty on the underlying mechanisms, and poor knowledge of the platelet reactivity throughout the disease course. Interim guidance has been developed to aid physicians in treating this syndrome, with anticoagulation and high dose intravenous immunoglobulin (IVIG) currently proposed as first-line therapies [11]. Plasma exchange (PE) has been described once as a tentative treatment for patients with refractory VITT [12], but further confirmation are urgently needed to validate its use.
We report a case of severe splanchnic thrombosis caused by VITT in a middle-aged man, successfully treated with PE; we also describe the activation state of circulating platelets during the disease course.
A Caucasian 59-years-old man presented to the emergency department complaining of abdominal pain that worsened over five days. The patient received the first dose of the adenoviral vector anti-SARS-CoV-2 vaccine (ChAdOx1 nCoV-19) 8 days prior symptoms onset. His medical history was characterized by well-controlled arterial hypertension and obesity (BMI = 31.8 kg/m2, weight 115 kg), without previous history of venous or arterial thrombosis; he reported two siblings with history of myocardial infarction in their forties. Physical examination showed cutaneous purpura with a blister in the right arm, and diffuse tenderness of the abdomen, without rebound or rigidity; bowel sounds were present, without jaundice or pallor.
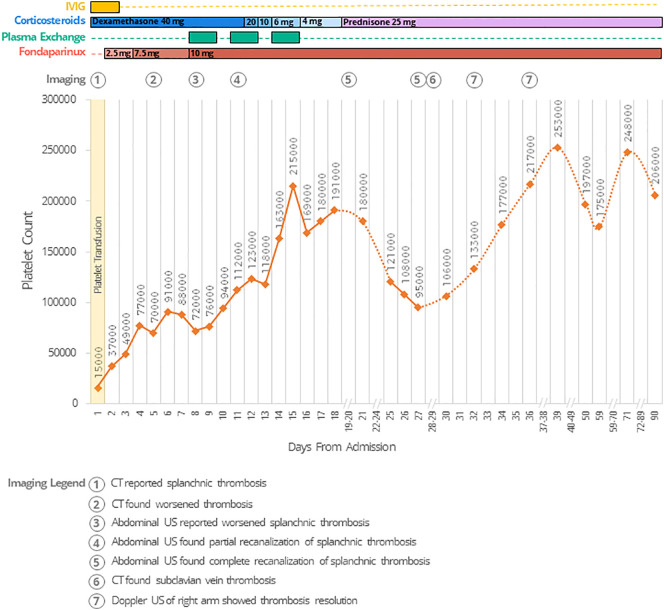
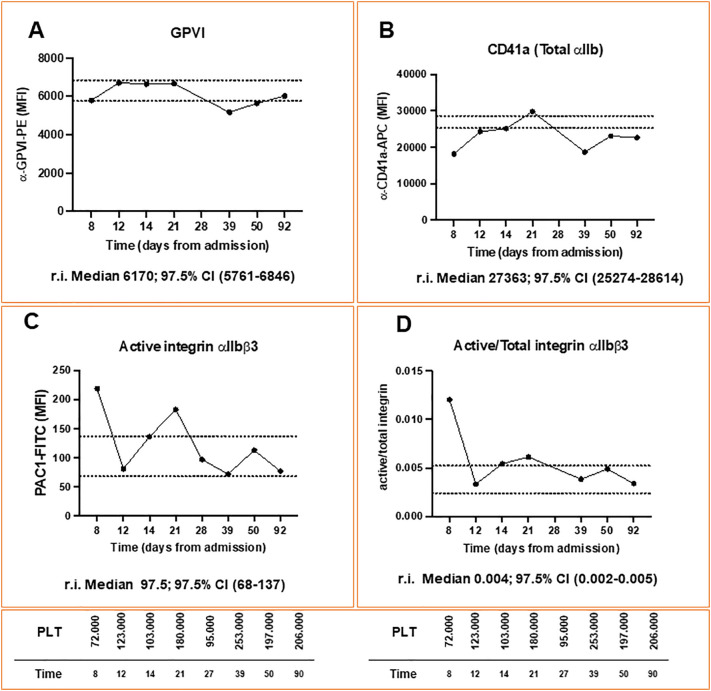
A graphical representation of the clinical course and laboratory analyses of the patient is reported in Fig. 1 . At admission, severe thrombocytopenia was observed (Platelet (PLT) count: 15.000/mm3), along with increased D-dimer (4309 μg/L- reference interval (r.i.) 0-550), low fibrinogen levels (117 mg/dL, r.i. 200-400), and slightly altered INR (1.28). Other laboratory tests were normal. An abdomen computed tomography (CT) showed complete portal vein thrombosis, with partial thrombosis of the superior mesenteric vein; subsequent CT scans excluded other sites of thrombosis, and a suspected diagnosis of VITT was made. Due to the bleeding risk, the patient underwent PLTs transfusion; in accordance with the Italian Society for the Study of Haemostasis and Thrombosis Guidelines [11], the patient started treatment with Dexamethasone (40 mg/die), and IVIG (1 g/kg) for two consecutive days. On day 2, PLTs were 37.000/mm3, and we introduced fondaparinux 2.5 mg/die. On day 4, PLTs levels rose to 77.000/mm3, and fondaparinux was further increased to 7.5 mg/die. An enzyme-immunoassay (EIA) revealed high titre of anti-PF4 antibodies (day 4 – optical density (OD): 1.985), confirmed by functional test [13]; both tests were strongly reduced in the presence of high concentrations of heparin (100 IU/mL), in accordance with a diagnosis of VITT. On day 5, a new abdomen CT scan showed progression of the splanchnic thrombosis, with complete occlusion of the splenic vein, superior mesenteric vein, and the splenomesenteric confluence; spleen and colon appeared congested and increase in total bilirubin levels (1.4 mg/dL) was also observed. From day 5 to day 8, the platelet count fluctuated between 70.000 and 90.000/mm3. On day 8, flow cytometric analysis of platelet surface receptors expression showed relatively normal levels of the stimulatory receptor glycoprotein (GP)VI and low levels of total integrin αIIb (CD41a) (Fig. 2 , panel A and B, respectively), which fluctuated within the normal ranges throughout the clinical course and follow-up; on the contrary, circulating platelets expressed high levels of active integrin αIIbβ3 on their surface (PAC1-FITC median fluorescence intensity (MFI) = 219 -Fig. 2, Panel C), and a high ratio of active/total integrin (0.012) (Fig. 2, Panel D), compared to the levels found among 34 healthy controls (median 97.5, 97.5% Confidence Interval (CI) 68-137, and median 0.004, 97.5% CI 0.002-0.005 respectively). The anti-PF4 quantification assay (EIA) was repeated and confirmed a high antibody titre (OD: 1.782). The patient was considered refractory to treatment, and a rescue-treatment with PE was started on day 8; fondaparinux was also increased to 10 mg. On day 11, PLTs increased to 112.000/mm3, and an abdominal ultrasound (US) revealed partial recanalization of portal and superior mesenteric veins, with complete recanalization of splenic vein. This was accompanied by a reduction of the active integrin αIIbβ3 detected on the surface of circulating platelets (PAC1-FITC MFI = 81). A total of 6 cycles of PE were completed by day 15, when the platelet count reached 215.000/mm3. Contextually, Dexamethasone was tapered during the following days. Another US showed complete resolution of the splanchnic thrombosis on day 19. Platelet count remained stable above 150.000/mm3. In the meanwhile, inherited and acquired thrombophilia (including antiphospholipid syndrome) were excluded. However, on day 14 and day 21 we detected a progressive increase of platelet integrin activation (PAC1-FITC MFI = 136.5 and 183, respectively) and, on day 25, we observed a sudden drop of the platelet count. The patient did not report new symptoms, and his blood exam showed no significant new findings. On day 27, anti-PF4 antibodies levels were strongly reduced (OD:0.910), the functional test became negative, and an abdominal US excluded the recurrence of splanchnic thrombosis. On day 28, the integrin activation status of circulating platelets had decreased again (PAC1-FITC MFI = 97). A whole-body CT scan showed thrombosis of the right subclavian and axillar veins, in which a peripherally inserted central catheter had been placed on day 6. The device was promptly removed, and the patient was closely monitored, without change in therapy. On day 32, an US scan showed complete resolution of the right arm thrombosis, consistent with an increase in PLT count. The patient was discharged on day 39 in good clinical conditions, on treatment with fondaparinux 10 mg/die, prednisolone 25 mg, and is now attending scheduled follow-up visits. PLTs count remained above 170.000/mm3 during follow-up, and we also observed normal levels of PAC1-FITC and active/total integrin ratio. The patient is still treated with fondaparinux and is currently undergoing steroids tapering. No relapsing or new thrombotic events were observed during follow-up.
Fig. 1.
Platelets levels and clinical course during hospital stay and follow-up.
Legend: CT: Computed tomography; IVIG: Intravenous Immunoglobulin; US: Ultrasound.
Fig. 2.
Platelet surface receptor expression during hospital stay and follow-up. Panel A: glycoprotein GPVI; Panel B: integrin CD41a (total integrin αIIb); Panel C: PAC1 (active integrin αIIbβ3); Panel D: active/total integrin ratio.
Legend: CI = Confidence Intervals; GPVI = Glycoprotein VI; MFI = Median Fluorescence Intensity, PLT = Platelets, r.i.: reference intervals (compared to the levels found among 34 healthy controls).
VITT is a rare adverse event of viral vectored SARS-CoV-2 vaccines. Most cases occurred in young adults (typically <60 years old), particularly women, without overt risk factors or known thrombophilia [14]. In our case, three major remarks should be made: first, the epidemiology and natural history of VITT is still largely unknown (our case occurred in a middle-aged man, and the thrombosis involved an uncommon and extensive vein district); second, refractory VITT may be treated effectively with PE, as previously described in a recent case series [12]; third, knowledge on the platelet activation state during the course of the disease is urgently needed and may help to understand what causes VITT, its evolution, and what treatments may be useful.
Beyond that, the mechanisms underlying VITT are still largely unknown [15]. Similar platelet signaling patterns have been reported among patients with COVID-19-related thrombosis and VITT, suggesting that common mechanisms may be involved [16], [17]. A recent report demonstrated that the different binding properties of anti-PF4 antibodies detected in VITT and heparin-induced thrombocytopenia patients could explain why in the former, but not in the latter, antibodies do not need heparin to crosslink the FcγIIA receptor and activate platelets [18]. Alternatively, one could envisage that the nucleic acids contained in the vaccines promote the antibody crosslinking since previous reports showed that nucleic acids can bind PF4 [19]. All these hypotheses, however, still need confirmation in vivo, and insights on platelet activation during VITT course are urgently needed. In our case, the median fluorescence intensity of PAC1-FITC (that binds active αIIbβ3 on platelets), and the ratio of active/total integrin followed the thrombotic evolution mediated by anti-PF4 antibodies. Thus, we postulate that platelet integrin activation could represent a promising tool to diagnose and monitor the progression of VITT.
This case underlines the high uncertainty surrounding our knowledge of VITT, particularly regarding risk factors, clinical presentation, and treatment, as for COVID-19 [20]. While VITT is a rare event, and further studies are required to confirm our findings on PE efficacy and platelet reactivity, we believe that granular reporting of VITT cases is helpful to inform clinical practice and tackle the burden of VITT during this vaccination campaign. This case contributes to expand the knowledge on VITT and PE approach and suggests that flow cytometric analysis of integrin activation could be used to improve the surveillance of vaccinated individuals and the diagnosis of suspected VITT.
Declaration of competing interest
SB received a research grant from MSD, outside the scope of this study.
Acknowledgments
We thank the patient, who reviewed the manuscript, for giving his consent to publication. We thank Prof. Antonio Angeloni, Dr. Shafii Bafti Mahnaz, Dr. Martina Di Palma and the Immunohematology and Transfusion Medicine Unit of Policlinico Umberto I, Sapienza University of Rome, Italy for their involvement in the case management.
References
- 1.Folegatti P.M., Ewer K.J., Aley P.K., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greinacher A., Thiele T., Warkentin T.E., et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N. Engl. J. Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umbrello M., Brena N., Vercelli R., et al. Successful treatment of acute spleno-Porto-mesenteric vein thrombosis after ChAdOx1 nCoV-19 vaccine. A case report. J. Crit. Care. 2021;65:72–75. doi: 10.1016/j.jcrc.2021.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavin M., Elder P.T., O’Keeffe D., et al. Vaccine-induced immune thrombotic thrombocytopenia (VITT) – a novel clinico-pathological entity with heterogeneous clinical presentations. Br. J. Haematol. 2021;195(1):76–84. doi: 10.1111/bjh.17613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schultz N.H., Sørvoll I.H., Michelsen A.E., et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 2021;384(22):2124–2130. doi: 10.1056/NEJMoa2104882. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cines D.B., Bussel J.B. SARS-CoV-2 vaccine–induced immune thrombotic thrombocytopenia. N. Engl J. Med. 2021;384(23):2254–2256. doi: 10.1056/NEJMe2106315. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Afshar Z.M., Babazadeh A., Janbakhsh A., et al. Vaccine-induced immune thrombotic thrombocytopenia after vaccination against Covid-19: a clinical dilemma for clinicians and patients. Rev. Med. Virol. 2021 doi: 10.1002/rmv.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Medical Agency AstraZeneca’s COVID-19 vaccine benefits and risks in context European Medicines Agency [Internet] 2021. https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-benefits-risks-context [cited 2021 Jul 12]. Available from:
- 10.Vayne C., Rollin J., Gruel Y., et al. PF4 immunoassays in vaccine-induced thrombotic thrombocytopenia. N. Engl. J. Med. 2021;385(4):376–378. doi: 10.1056/NEJMc2106383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gresele P., Marietta M., Ageno W., et al. Management of cerebral and splanchnic vein thrombosis associated with thrombocytopenia in subjects previously vaccinated with Vaxzevria (AstraZeneca): a position statement from the Italian Society for the Study of Haemostasis and Thrombosis (SISET) Blood Transfus. 2021;19(4):281–283. doi: 10.2450/2021.0117-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patriquin C.J., Laroche V., Selby R., et al. Therapeutic Plasma Exchange in Vaccine-Induced Immune Thrombotic Thrombocytopenia. N. Engl. J. Med. 2021;385:857–859. doi: 10.1056/NEJMc2109465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guarino M.L., Massimi I., Mardente S., et al. New platelet functional method for identification of pathogenic antibodies in HIT patients, 28. Platelets Taylor and Francis Ltd. 2017:728–730. doi: 10.1080/09537104.2017.1293803. [DOI] [PubMed] [Google Scholar]
- 14.Schultz N.H., Sørvoll I.H., Michelsen A.E., et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021;384:2124–2130. doi: 10.1056/NEJMoa2104882. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rzymski P., Perek B., Flisiak R. Thrombotic thrombocytopenia after covid-19 vaccination: In search of the underlying mechanism [Internet]. Vaccines. Multidisciplinary Digital Publishing Institute. 2021. https://www.mdpi.com/2076-393X/9/6/559/htm [cited 2021 Jul 15]. p. 559. Available from: [DOI] [PMC free article] [PubMed]
- 16.Kowarz E., Krutzke L., Reis J., et al. “Vaccine-Induced Covid-19 Mimicry” Syndrome:Splice reactions within the SARS-CoV-2 Spike open reading frame result in Spike protein variants that may cause thromboembolic events in patients immunized with vector-based vaccines. PREPRINT (Version 1) available at Research Square. 2021 doi: 10.21203/rs.3.rs-558954/v1. [DOI] [Google Scholar]
- 17.Apostolidis S.A., Sarkar A., Giannini H.M., et al. Signaling through FcγRIIA and the C5a-C5aR pathway mediates platelet hyperactivation in COVID-19. bioRxiv Prepr Serv Biol [Internet] Cold Spring Harbor Laboratory. 2021. https://www.biorxiv.org/content/10.1101/2021.05.01.442279v1 [cited 2021 Jul 15]; 2021.05.01.442279. Available from:
- 18.Huynh A., Kelton J.G., Arnold D.M., et al. Nature [Internet] Nature Publishing Group; 2021. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopenia; pp. 1–7.https://www.nature.com/articles/s41586-021-03744-4 Available from: [DOI] [PubMed] [Google Scholar]
- 19.Jaax M.E., Krauel K., Marschall T., et al. Complex formation with nucleic acids and aptamers alters the antigenic properties of platelet factor 4. Blood. 2013;122:272–281. doi: 10.1182/blood-2013-01-478966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romiti G.F., Talerico G. Embracing the uncertainty: an important lesson from COVID-19. J. Gen. Int. Med. 2021:1–3. doi: 10.1007/s11606-021-06809-2. [DOI] [PMC free article] [PubMed] [Google Scholar]