Abstract
Conventional lateral flow assay (LFA) is typically performed by observing the color changes in the test lines by naked eyes, which achieves considerable commercial success and has a significant impact on the fields of food safety, environment monitoring, disease diagnosis, and other applications. However, this qualitative detection method is not very suitable for low levels of disease biomarkers’ detection. Although many nanomaterials are used as new labels for LFA, additional readers limit their application to some extent. Fortunately, a lot of work has been done for improving the sensitivity of LFA. In this review, currently reported LFA sensitivity enhancement methods with an objective evaluation are summarized, such as sample pretreatment, the change of flow rate, and label evolution, and future development direction and challenges of LFAs are discussed.
Keywords: Lateral flow assay, Sensitivity enhancement, Signal amplification
Introduction
The rapid, portable, sensitive, and inexpensive detection of analytes from complex samples is essential for in vitro diagnostics [1, 2]. It is estimated that improving the technique of diagnostic tests for infectious diseases in developing countries can annually save at least 1.2 million deaths [3]. Especially when facing the outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a rapid and facile screening strategy, which is employed in airports, customs, and community, shows the great significance to prevent epidemic and resume shipping and economic development.
Lateral flow assay (LFA) deployed as point-of-care testing (POCT), owing to its rapidity, simplicity, stability, and visual characteristics [4], has been vital in enabling faster diagnosis, directing medical interventions, and mitigating the transmission of infectious diseases [2].
Conventionally, the results of LFA are read out by naked eyes, by measuring the color change due to the accumulation of gold nanoparticles (AuNPs). This detection strategy is simple and rapid, and the early pregnancy test is the outstanding representative. However, these results are qualitative and lack of sensitivity and may function only for certain applications. As for the detection of critical biochemical markers present in extremely small amounts in a sample, such as myocardial infarction and cancer, these methods do not afford sufficient sensitivity, which restrict their applications. In recent years, the development of new nanomaterials has broadened the type of labels available for LFA to enhance sensitivity. These nanomaterials can be roughly divided into three categories according to the type of readout [5], that is, naked-eye detection, fluorescence detection, and non-optical readout detection. Carbon nanoparticles [6, 7], carbon nanotubes [8, 9], and dye-loaded latex beads [10, 11] can provide an alternative to AuNPs for naked-eye detection. Fluorescent labels are generally recommended for low concentrations of targets and quantitative detection. Suitable fluorescent nanoparticles include fluorescent microspheres (FMs) [12], quantum dots (QDs) [11], upconverting nanoparticles (UCNPs) [13], and liposomes with fluorescent dyes [14]. LFA with non-optical readout can be comparable with that of LFA with fluorescent labels, such as magnetic nanoparticles [15–17] and nanoparticles for electrochemical readings[18–20]. However, they either cannot provide a strong signal as AuNPs for naked-eye detection or comes with a higher cost and the need for an external reader.
In order to enhance the signal–noise ratio, a lot of signal amplification methods have been employed in POCT, such as gas-propelled [21, 22], enzyme-mimicking accelerated signal enhancement [23, 24], and cascade amplification [25, 26]. As for AuNP-based LFA, significant efforts have been focused on highly sensitive detection, such as sample pretreatment, changes in structure, materials, and labels. In this review, currently reported LFA sensitivity enhancement methods with an objective evaluation are summarized, and future development direction and challenges of LFAs are also discussed.
Sensitivity enhancement based on sample pretreatment
Serum and saliva are the common sample matrix for disease diagnosis. AuNPs may aggregate together caused by the mixture of proteins, nucleic acids, and other substances in the matrix of serum and saliva [27], which interferes with the sensitivity and specificity for detection. To get rid of interfering components, a lot of sample pretreatment methods are introduced in LFA, such as isothermal amplification for nucleic acid detection [28] or sample enrichment for proteins detection [29]. The following sections will highlight such sample pretreatment integrated with LFA.
Sensitivity enhancement based on isothermal nucleic acid amplification
Comparing with other biomarkers, nucleic acids are more stable under harsh environments and are gradually classified as biomarkers for disease diagnosis [30], microbial detection [31], and environmental monitoring [32]. With an increasing demand for diagnosis under resource-limited conditions, LFA for nucleic acid detection has gained greater attention owing to the lower cost and user-friendly [33]. However, one major drawback of the current LFA for nucleic acid detection is a low sensitivity, limiting its practical applications. Different from protein biomarkers present at the level of nanomolar or picomolar, the amounts of pathogenic bacteria that cause disease can be as low as a few CFU/mL [34]. In order to enhance the sensitivity of LFA for nucleic acid detection, various isothermal amplification methods are used, including rolling circle amplification (RCA) [35], loop-mediated isothermal amplification (LAMP) [36], recombinase polymerase amplification (RPA) [37], nucleic acid sequence–based amplification (NASBA) [38], helicase-dependent isothermal DNA amplification (HDA) [39], and hybridization chain reaction (HCR) [40].
Sensitivity enhancement based on RCA
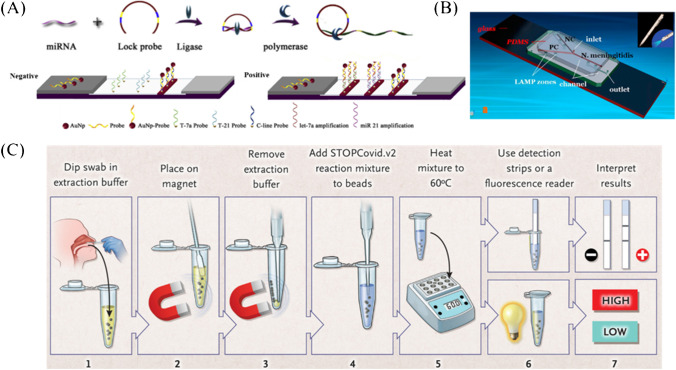
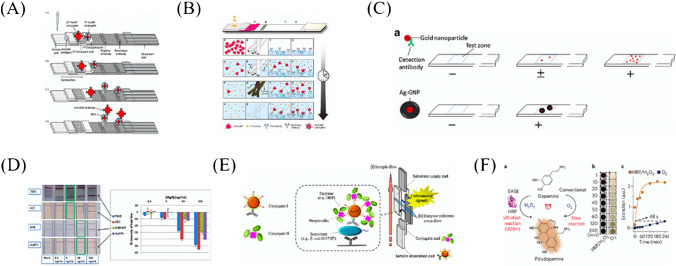
RCA is an isothermal enzymatic process where a short DNA or RNA primer is amplified to form a long single-stranded DNA or RNA under the function of a circular DNA template and special DNA or RNA polymerases [41, 42]. The RCA product is a concatemer containing tens to hundreds of tandem repeats that are complementary to the circular template [43], which gives a huge amount of capturing sites or signal generating sites. Yao et al. [35] combined RCA with AuNP-based LFA for simultaneous detection of miRNA 21 and miRNA let-7a. The limit of detection (LOD) was as low as 40 pM and 20 pM, respectively (Fig. 1A). Compared with the results obtained by Kor et al. [44], the sensitivity of miRNA21 was enhanced 7.5 times. Moreover, RCA integrated with LFA were designed for the detection of Karenia mikimotoi (hyperbranched RCA, HRCA [45]) and Karlodinium veneficum (exponential RCA, E-RCA [46]), respectively. Inspiringly, the sensitivity of HRCA-LFA was 100 times that of polymerase chain reaction (PCR) [45], and E-RCA-LFA was more sensitive than that of the conventional PCR and reached a LOD of 0.01 cell/mL [46]. In addition, RCA-based LFA has also been used in the detection of metal ions [47], small molecules [48], enzymes [49], and antibodies [50].
Fig. 1.
Sensitivity enhancement based on isothermal nucleic acid amplification. A Schematic illustration of RCA integrated with LFA for miRNA detection [35]. B Layout of the PDMS/paper hybrid microfluidic device integrated with on-chip LAMP system [51]. C The whole process for STOPCovid, Version 2 (STOPCovid.v2) Test [52]
Sensitivity enhancement based on RPA
RPA is regarded as an isothermal PCR method. Recombinase and single-stranded binding protein are used to replace the programmed temperature changing of PCR [53]. The recombinase is used to complete the dissociation of the template and the combination of primers and templates, while single-stranded binding protein is used for maintaining the single chain so that the DNA polymerase completes the primer extension process. Most importantly, it is an effective approach in terms of sensitivity, specificity, and multiplexing [54, 55].
Zhang’s group [56] established a clustered regularly interspaced short palindromic repeats (CRISPR)–based diagnostic platform, which combined RPA and LFA with CRISPR–Cas enzymology for specific recognition of desired DNA or RNA sequences, called specific high-sensitivity enzymatic reporter unlocking (SHERLOCK). The template underwent the isothermal amplification reaction of RPA to amplify the signal. At the same time, a T7 promoter was added for in vitro transcription by RPA, which was used for converting the target from DNA to RNA for detection. After recognizing the target site of RNA, Cas13a would non-specifically cleave molecular beacons to generate signals. For RNA target, reverse transcription was necessary.
To date, RPA integrated with LFA has been widely used in the detection of pathogenic bacteria and viruses in food safety [57–59], environmental monitoring [37], and other biomedical fields [60, 61].
Sensitivity enhancement based on LAMP
The core principle of LAMP is to design 2 or 3 pairs of primers for 6 regions of the target gene fragment to achieve rapid and efficient amplification [62]. Similar to nested PCR, multiple primers of LAMP strictly and efficiently ensured specificity. And, it is not affected by non-target DNA and PCR inhibitors with high similarity to the target DNA [63, 64].
Dou and co-workers [51] integrated polydimethylsiloxane (PDMS)/paper hybrid microfluidic chip with LAMP for Neisseria meningitidis detection (Fig. 1B). Compared with the results of previous studies [65], the introduction of paper into the microfluidic device for LAMP enabled more stable results than that of a paper-free microfluidic system. The LOD was about 3 copies of DNA, which was lower than that of other studies [66, 67].
Facing the COVID-19, Zhang’s group [52] developed an upgraded version of the new coronavirus detection process based on SHERLOCK, called STOPCovid (Fig. 1C). The RNA in the sample was enriched by adding magnetic beads during the sample preparation process, thereby increasing the amount of initial RNA in LMAP and further improving the detection sensitivity. In addition, STOPCovid version 2.0 streamlined the operating steps of RNA enrichment with magnetic beads, removed the ethanol washing and eluting process, and shortened the whole time of the entire RNA enrichment process to less than 15 min.
Furthermore, LAMP integrated with LFA has also been used in the detection of E. coli [68], plasmodium [64], P. aeruginosa [69], and SARS-CoV-2[70].
Sensitivity enhancement based on sample enrichment
Unlike nucleic acid, protein can not achieve quantity increasing by amplification. Fortunately, enrichment strategies, such as isoelectric electrophoresis, dialysis, and magnetic enrichment, can be integrated with protein detection to increase the concentration and enhance the detection sensitivity.
Sensitivity enhancement based on electrophoresis
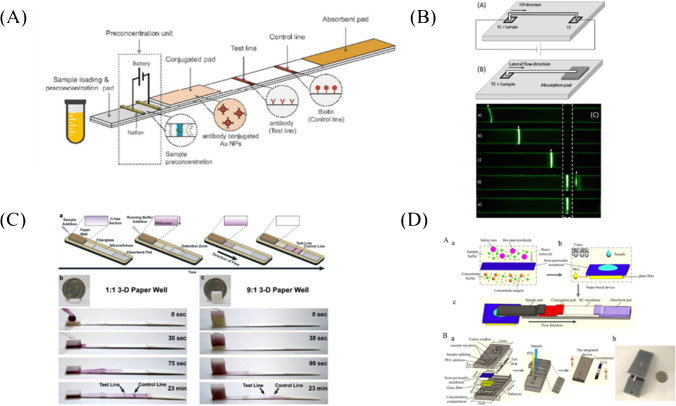
Paper-based ion concentration polarization (ICP) pre-concentrators were integrated with LFA by Kim et al. [71] to enhance the detecting sensitivity of β-human chorionic gonadotropin (β-hCG) (Fig. 2A). Through a simple 9 V battery and low power consumption (about 81 μW), a preconcentration factor of 25-folds was obtained, and the detection sensitivity was enhanced by 2.69 times compared with that of the commercial LFA for β-hCG.
Fig. 2.
Sensitivity enhancement based on sample enrichment. A Paper-based ICP pre-concentrators integrated with LFA for β-hCG detection [71]. B Experimental setup for (a) ITP-enhanced LFA and (b) conventional LFA. (c) Experimental snapshots were taken at 5 different time points [72]. C The 3-D paper is well combined with ATPS and LFA for transferrin detection [73]. (a) The 3-D paper well device was combined with a transferrin competition assay on nitrocellulose paper. Samples containing no transferrin were correctly diagnosed when using the (b) 1:1 or (c) 9:1 volume ratio of ATPS solutions. D Schematic of the integrated device of the semi-permeable membrane, glass fiber, and PEG buffer into with LFA [74]
Isotachophoresis (ITP) was used by Moghadam et al. [72] to focus target analytes into a thin band and then was transported to the test line of LFA, resulting in a dramatic increase in the surface reaction rate and equilibrium binding (Fig. 2B). This strategy can improve the sensitivity of LFA by 400-fold compared with that of the commercial LFAs for β-hCG.
Sensitivity enhancement based on extraction
Kamei’s group [53] firstly developed an aqueous two-phase system (ATPS) to concentrate a target biomarker into a smaller volume before loading it onto LFA. Using this method, a tenfold improvement in the overall detection sensitivity of LFA was achieved for bacteriophage M13 [75] and transferrin (Tf) [76]. In addition, this newly discovered concentrating phenomenon suggested that the paper membrane sped up the macroscopic phase separation of ATPS.
Chiu et al. [73] expanded the paper device vertically, thereby increasing the cross-sectional area of flow and exploiting the effects of gravity on macroscopic separation (Fig. 2C). In addition to accelerating phase separation, this 3-D component also enhanced the ability to process larger and more diluted volumes of sample. The novel integration of ATPS and LFA within a 3-D paper well successfully yielded a tenfold improvement in the detection of transferrin.
Sensitivity enhancement based on dialysis
Tang et al. [74] integrated the semi-permeable membrane, glass fiber, and PEG buffer with LFA for samples enrichment (Fig. 2D). PEG-loaded glass fiber was used as the dialysate for sample concentrating. Compared with that of the conventional LFA, tenfold signal enhancement in HIV detection and fourfold signal enhancement in myoglobin detection have been achieved, respectively.
Sensitivity enhancement based on magnetic enrichment
Taking advantage of supermagnetism, magnetic bead-based separation is used as a convenient way to perform sample pretreatment and eliminate the interference of food matrices in LFA. Generally, aptamers [77, 78] or antibodies [79, 80] are labeled with magnetic beads for target capturing.
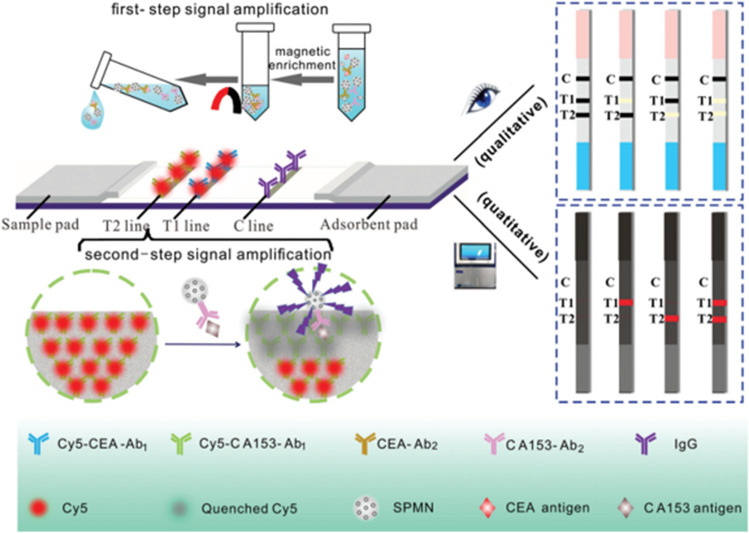
Besides the capability of fast separation in the magnetic field, magnetic nanoparticles or magnetic beads possess the color of brown and can provide low background noise, which make them ideal label materials of LFA [6]. Zhang et al. [81] developed a multiple immunoassay test strip based on Fe3O4 superparamagnetic nanosphere (SPMN) probes, which was used to enrich samples and quench the fluorescence of multiple fluorescer on the test line (Fig. 3). Simultaneous detection of carcinoembryonic antigen (CEA) and carbohydrate antigen (CA153) was realized with LOD of 0.06 ng/mL and 0.6 U/mL, respectively.
Fig. 3.
Mechanism of magnetic fluorescence-based LFA platform for CEA and CA153 detection [81]
Sensitivity enhancement based on the change of flow rate
Another critical factor affecting the sensitivity of LFA is the time for the immunoreaction between the analyte and the capture antibody pre-deposited on the test line, which depends on the migration time of the sample over the test line [82]. The decrease in the reaction time causes insufficient time for the antibody-antigen reaction [83], so a test should be long enough for a sufficient antibody-antigen reaction. An increase in the distance from the conjunction pad to the test line can increase the reaction time [84]. An increase in the pore size of a nitrocellulose membrane can decrease the amount of bound protein and increase the flow rate. And, the rapid migration times caused a high signal–noise ratio [85]. More importantly, some engineering methods, such as flow block and NC membrane size change, have been developed for LFA sensitivity enhancement.
Sensitivity enhancement based on flow block
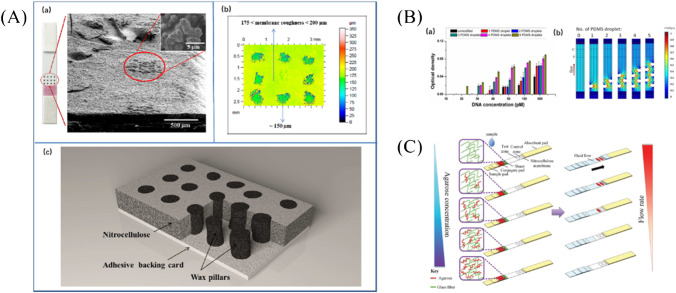
Rivas et al. [86] developed delay hydrophobic barriers fabricated by wax printing to improve LFA sensitivity (Fig. 4A). When running buffer flows through the wax barrier, microfluidics delay and pseudo-turbulent flow were generated in the columnar region, which improved the sensitivity of almost 3-folds in comparison to a commercial barrier-free LFA.
Fig. 4.
Sensitivity enhancement based on flow block. A (a)SEM image for the transversal cut of wax pillars area on LFA. (b) Surface profile roughness of LFA modified with wax pillars. (c) Schematic of a transversal cut of pillars zone on nitrocellulose membrane [86]. B Sensitivity enhancement in LFA by creating a PDMS barrier. (a) Effects of PDMS droplets numbers on LOD. (b) Results of flow velocity simulation of LFA [87]. C Schematic diagram of fluidic control in a paper–agarose hybrid material–based LFA [88]
However, the wax barrier on the NC membrane of LFA might melt during the process of heating [89]. A piece of paper-based shunt and a polydimethylsiloxane (PDMS) barrier were integrated with LFA by Choi et al.[87] to achieve optimal fluidic delays, and tenfold signal enhancement was obtained for detecting hepatitis B virus (HBV) nucleic acid (Fig. 4B). In further studies, Choi et al. [88] used agarose as the barrier due to its strong permeability, excellent mechanical properties, easier fluid control, and tunable pore size and porosity (Fig. 4C). Remarkably, this detecting strategy enhanced the sensitivity nearly 10-folds with a detection limit of 100 copies of dengue viral RNA, which yielded comparable or more sensitive result than that of the published techniques of enzyme enhancement [90], temperature and humidity control [91], and fluidic control by hydrophobic barriers [86].
Sensitivity enhancement based on NC membrane size change
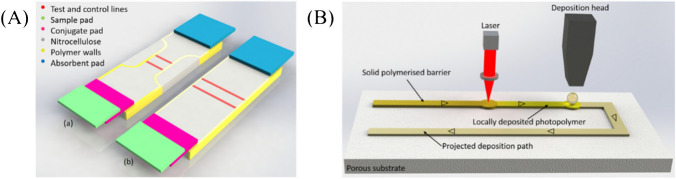
Katis et al. [92] used spatial constrictions in the flow path as a route to increase the sensitivity and lower the LOD of LFA, due to the slower flow rate and the smaller test zone area (Fig. 5A). The liquid photopolymer was locally deposited onto the paper substrate with a deposition nozzle. A laser beam subsequently followed the deposition head, illuminated the deposited patterns, and induced the photo-polymerization of the polymer (Fig. 5B). The polymerized patterns defined the fluidic walls, which served as demarcation barriers to confine and transport the liquids within the paper device. The LOD of C-reactive protein (CRP) was 5 ng/mL with a 1-mm-wide constriction, which was a 30-fold enhancement compared with that of the standard LFA with the width of 5 mm.
Fig. 5.
Sensitivity enhancement based on NC membrane size change [92]. A Schematic of the flow path constriction device (a) and a standard lateral flow device (b). B Schematic of the modified laser-based direct-write procedure
Sensitivity enhancement based on label evolution
Sensitivity enhancement based on colorimetric methods
Sensitivity enhancement based on dual AuNPs
With an increase in the particle size of AuNPs, the molar extinction coefficient is significantly enhanced [93, 94]. Therefore, a larger particle size of AuNPs as the labeling nanomaterial is more conducive to improve the sensitivity [95].
Choi et al. [96] used two different sizes of AuNPs to realize the sensitive detection of cardiac troponin I (cTnI) by LFA (Fig. 6A). The first conjugate was AuNPs (10 nm) coated with anti-troponin I antibody and blocked with bovine serum albumin (BSA), and the second conjugate was AuNPs (40 nm) coated with anti-BSA antibody and blocked with human serum albumin. Double AuNP-based LFA can detect as low as 0.01 ng/mL of cTnI within 10 min, which was enhanced about 100 times compared with that of Posthuma-Trumpie’ research [97]. This dual-labeling strategy was subsequently optimized for the on-site and sensitive detection of melamine in milk, offering a 10- to 25-fold improvement [98]. Later, this method was used in detecting bisphenol A (BPA) [99], Hg2+ [100], and procalcitonin (PCT) [101].
Fig. 6.
Sensitivity enhancement based on colorimetric methods. A Dual AuNPs LFA was used for cTnI detection; the sizes of AuNPs were 10 nm and 40 nm, respectively [96]. B Schematic of a hybrid nanofiber-deposited LFA kit and the time-dependent changes of the conjugate pad (P), electrospun nanofibers E, test line (T), and control line C during the signal-enhanced assay [102]. C Expected effects of silver staining on LFA sensitivity enhancement [103]. D (Left) Results of LFA for different concentrations of human IgG and the different substrates. (Right) Results were obtained with the strip reader [90]. E Schematic of AuNP-assisted signal amplification on LFA for pathogen detection [104]. F (a) Schematic of EASE. (b) The relationship between dopamine accumulation and time in the presence of HRP [105]
Sensitivity enhancement based on silver staining
Inspired by GNSs, the method of silver staining was developed to change the shape of AuNPs.
Silver staining is another method for signal amplification via the color change at the test line based on chemical reactions [106]. These reactions involve the catalytic reduction of silver ions on gold nanoparticles, which produces larger absorbance values for gold nanoparticles and darker colors at the test lines of LFA [107].
Yang et al. [108] used silver staining in LFA for the first time. When a visible red color appeared on the detection area, the NC membrane was covered with an AgNO3 pad, and on which the reducer pad was placed. The produced larger size of silver particles was used to change the color of the detection line from red to black which made the contrast more obvious. A 100-fold improvement in sensitivity with a detection limit of 0.1 ng/mL for abrin-a was obtained. Kim et al. [102] used core–shell hybrid nanofibers and silver staining technique to detect cTnI. AgNO3 and silver-reducing reagent of hydroquinone solutions were separately encapsulated by electrospinning (Fig. 6C). The silver ions were reduced to metallic silver around AuNPs and the color of the test line was darkened. The obtained detection limit for cTnI was 0.24 ng/mL, and the sensitivity was enhanced by up to 10 times compared with that of the commercial LFA. Anfossi et al. [103] used this method to realize the detection of ochratoxin A (OTA) by competitive LFA (Fig. 6D), and the sensitivity was enhanced by 10 times compared with that of the traditional LFA for OTA detection [109].
Sensitivity enhancement based on enzymatic amplification
Inspired by the wide use of enzymes as labels in bioassays, the nano-carrier-enzyme probes have been used in LFA to improve the detecting performance of various optical and electronic biosensing systems [110–113].
In the study of Parolo et al. [90], AuNPs were used not only as labels for antibodies, but also as carriers for enzymes (Fig. 6E). When acting as direct labels, AuNPs turned red at the test line and the control line of LFA. However, after AuNPs conjugating with antibody and blocking with horseradish peroxidase (HRP), the produced insoluble chromogens cannot move by the flow and then darken the color of the lines. By comparing three substrates (TMB for blue-violet, AEC for red [114], and DAB for gray-black [115]), it was found that TMB is optimal for signal enhancement maintaining at an order of magnitude.
Cho et al. [104] used the abovementioned signal amplification technique for E. coli O157:H7 detection (Fig. 6F). The obtained detection limit was 100 CFU/mL, which was about 1000 times lower than that of the traditional AuNP-based LFA.
Li et al. [105] developed a universal “add-on” technique called enzyme-accelerated signal enhancement (EASE) (Fig. 6G). EASE depended on the ultrafast and localized deposition of polydopamine (PDA) at the test line [94], permitting a large number of signal molecules to be captured and leading to sensitivity enhancement over three orders of magnitude. Under the normal conditions of using DAB as the substrate, p24 at the concentration of 10 ng/mL can be detected by LFA, while by EASE, the detection limit can be obtained at the level of 10 pg/mL, allowing the ultrasensitive detection of HIV antigens with naked eyes.
Sensitivity enhancement based on surface-enhanced Raman scattering (SERS)
In the excitation region of some specially prepared metal conductor surfaces or solutions, a stronger Raman signal caused by surface roughness can be observed, which is called surface-enhanced Raman scattering (SERS). Compared with traditional Raman scattering, SERS can realize 6 orders of magnitude or more [116].
Covian et al. [117] developed SERS-based LFA to detect pneumolysin by using gold-core-silver-shell nanoparticles as the plasmonic platform and rhodamine B isothiocyanate as Raman tag. Compared with the results of the electrochemical immunosensor with the detection limit of 0.6 ng/mL [118] and the chemiluminescence immunoassay with the detection limit of 5.5 pg/mL [119], the sensitivity of SERS-based LFA was enhanced with the detection limit of 1 pg/mL.
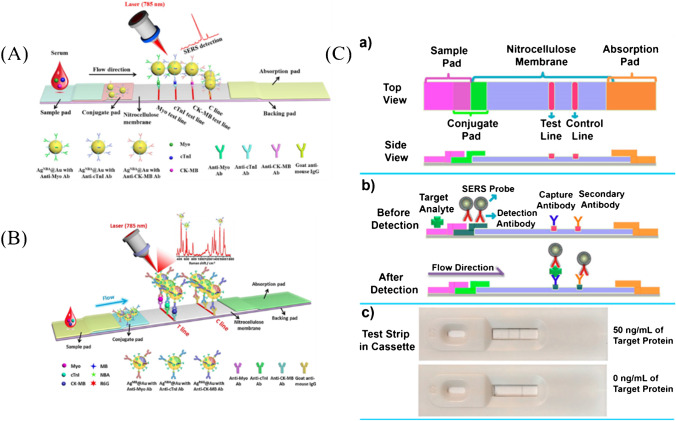
It has been proved that SERS nanotags have the coding capacity by absorbing different Raman dyes on the surface of the metal [120], which made it possible to realize multiple detections. Zhang et al. [121] encapsulated Raman dye Nile Blue A (NBA) in the interface of the core–shell structure (Fig. 7A), and based on the core–shell SERS nanotags, a novel LFA was developed to realize rapid quantification of creatine kinase isoenzyme (CK-MB), cTnI, and myoglobin (Myo) on three test lines with a detection limit of 0.55, 0.44, and 3.2 pg/mL, respectively, which decreased the LOD nearly three orders of magnitude than that of the colorimetric detection[122, 123]. In the follow-up study, Zhang et al. [124] encapsulated Raman dyes methylene blue (MB), Nile blue A (NBA), and rhodamine 6G (R6G) in the core–shell interface to achieve rapid quantification of three cardiac biomarkers on a single test line with the similar detection limit (Fig. 7B).
Fig. 7.
Sensitivity enhancement based on SERS. A Schematic illustration of the core–shell SERS nanotag–based multiplex LFA for three cardiac biomarkers detection [121]. B Schematic illustration of quantitative LFA for three cardiac biomarkers detection on a single T line with different roman dyes encoded core–shell SERS nanotags [124]. C Schematic illustration of the principle of SERS paper-based lateral flow strip (PLFS) based on HGN [125]. (a) Top and side views; (b) side view before and after detection of protein biomarker; (c) optical results of PLFS assembled in cassettes in the presence (upper) and absence (bottom) of the target protein
The stability of Raman dye plays a pivotal role in sensitive and accurate detection. Gao et al. [126] developed Au nanostar @ Raman dye @ silica sandwich nanoparticles for the detection of neuron-specific enolase (NSE), a marker of traumatic brain injury (TBI), with the LOD of 0.86 ng/mL. By wrapped between AuNPs and thin silica, Raman dyes malachite green isothiocyanate (MGITC) can be effectively stabilized. Compared with the traditional colorimetric method, the SERS-based method exhibited excellent performance especially in the matrix of plasma [127]. Hwang et al. [125] combined hollow gold nanospheres (HGN) with Raman dye MGTTC to achieve the detection of staphylococcal enterotoxin B (SEB) (Fig. 7C). With the advantage of HGN enhancing SERS signal, high-sensitivity detection with a LOD of 1 pg/mL was realized, and more than 30 times enhancement was obtained compared with that of the traditional ELISA.
Sensitivity enhancement based on photothermal methods
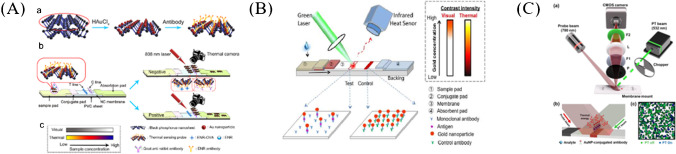
Li et al. [128] developed a quantitative photothermal-sensing LFA for enrofloxacin detection with the detection limit of 0.023 ng/mL (Fig. 8A). Black phosphorus (BP)-Au nanosheets showed good photothermal properties at the wavelength of 808 nm, and the photothermal conversion efficiency was enhanced by 12.9% compared with that of the black phosphorus nanosheets alone. Qin et al. [129] developed a thermal contrast-based technique to improve the sensitivity of LFA (Fig. 8B). The sensitivity can be enhanced by 32 times compared with that of the FDA-approved LFA for cryptococcal antigen (CrAg) detection.
Fig. 8.
Sensitivity enhancement based on the photothermal method. A Schematic of PT-ICSs [128]. (a) Preparation of BP-Au-Ab photothermal-sensing probe. (b) Structure and procedures of PT-ICSs. (c) Comparison of colorimetric results with photothermal results. B Principle of thermal contrast for LFA [129]. C Schematic of the PT-LSI sensor [130]. (a) A 780-nm light illuminated the NC membrane of LFA. (b) The absorbed light energy was converted into heat, and the resultant temperature increase altered the speckle pattern. (c) Exemplary speckle images before (green) and after (blue) photothermal illumination
Wang et al. [131] developed a thermal contrast magnification (TCA) reader, which was composed of an emitter for lasers of multiple wavelengths emitting, an infrared camera for the generated heat reading, and software for data reading and analysis. The reader can significantly increase the accuracy of antigen quantification by LFA. Compared with naked eyes or colorimetric readers (such as BD VeritorTM system readers), the TCA reader possessed a higher sensitivity of 8 times for detection of malaria and C. difficile [97, 132].
Laser speckle is the high-contrast random granular pattern, which is extremely sensitive to the refractive index and physical displacement of the medium [133, 134]. NC membrane is composed of randomly oriented nanofibers, so it can be used as a diffusion medium to generate high-contrast speckle patterns. Song et al. [130] developed a photothermal laser speckle imaging (PT-LSI)–based LFA (Fig. 8c). The sensitivity can be enhanced by 68 times compared with that of the FDA-approved LFA for CrAg detection. The detecting ability of the developed PT-LSILFA was verified for CrAg detection by US FDA-approved LFA, and it was found that the detection sensitivity of PT-LSI was enhanced by 68 times.
All the parameters of the abovementioned LFA for rapid detection are compared and summarized in Table 1.
Table 1.
Comparison of sensitivity enhancing effect of LFA based on different principles
| Method | Labels | LOD | Promotion degree | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|
| Isothermal nucleic acid amplification based | AuNPs | Single copy or lower 10 copies | / | Sensitivity | Enzyme inactivation | [38, 135] |
| Electrophoresis based | AuNPs | ng or pg/mL level | One or two order of magnitude | Without substances interference | Equipment dependent | [71, 72] |
| Extraction based | AuNPs | ng or pg/mL | An order of magnitude | Without substances interference | Non-portable | [73, 75, 76] |
| Dialysis based | AuNPs | ng or pg/mL level | An order of magnitude | Without substances interference | Equipment dependent | [74] |
| Magnetic enrichment based | AuNPs | ng or pg/mL level | An order of magnitude | Without substances interference and easy to separate | Expensive for functional magnet based | [136, 137] |
| Dual AuNPs based | AuNPs | ng or pg/mL level | An order of magnitude | Low cost, rapidness, easy to operation, and naked-eye readout | Disability to quantification, low sensitivity | [96, 98–101] |
| Silver staining based | AuNPs, AgNPs | ng or pg/mL level | An order of magnitude | Low cost, rapidness, and naked-eye readout | Multiple steps, low sensitivity | [102, 103, 108] |
| Enzymatic amplification based | AuNPs | ng or pg/mL level | An order of magnitude | Low cost, rapidness, and naked-eye readout | Multiple steps, enzyme inactivation | [90, 104, 105] |
| SERS based | MGITC, rhodamine B, rhodamine 6 G Nile blue A, methylene blue | Close to fg/mL level | 3–4 orders of magnitude | Sensitivity, rapidness, and quantification | Equipment dependent | [125, 138, 139] |
| Photothermal illumination based | AuNPs | Close to fg/mL level | 3–4 orders of magnitude | Sensitivity, rapidness, and quantification | Equipment dependent | [128–131] |
Conclusions and future perspectives
LFA has been proven to be a rapid, sensitive, and cost-effective method for point-of-care and in-field diagnosis in resource-limited areas such as developing countries and rural areas. However, there are long-standing criticisms of LFA as POCT, such as limited sensitivity, limited ability for quantification, inability for multistep performing, and inability to multiplexing.
In this paper, recent development and breakthroughs of the sensitivity enhancement for LFA are reviewed, such as sample pretreatment, changes in structure, materials, and labels, with an objective evaluation. For sample pretreatment, various isothermal nucleic acid amplification techniques, such as RCA, LAMP, and RPA, have been used in LFA, which makes the detection more sensitive. However, there are still challenges associated with the integration of amplification and detection into a single device. In addition, the reproducibility of complex enzymatic reactions should be considered. Changing the flow rate to enhance sensitivity through the flow barrier extends the time for antibody-antigen reaction; however, structural changes increase the difficulty of engineering and undoubtedly limit the application of LFA to some extent. AuNPs are the most popular labels for LFA. AuNPs integrated with other materials such as silver, gold, enzymes, or catalytic metals can further enhance the sensitivity of LFA, but this strategy has limitations regarding the preparation, purification, storage, and detection steps.
Accordingly, the latest research indicates that there is still a large development space for LFA with smaller demand volume, shorter analysis time and the absence of hook effect, and higher accuracy and sensitivity. Here, several possible directions for sensitivity enhancement of LFA are summarized as follows.
Firstly, the development of new materials, including new labels, probes, and paper-based materials. The super-strong signal characteristics of aggregation-induced emission (AIE) molecules in the solid phase are promising to become an excellent label for LFA. Novel capturing/detecting probes, such as nanobodies, short peptides, and aptamers, are also being developed to enhance stability, to increase detection sensitivity, and to minimize the cross reaction. In addition, with the smaller molecular weight, they can be used to target small molecules or epitopes that are inaccessible for conventional antibodies.
Secondly, for the detection of nucleic acid, a certain nucleic acid amplification method is currently used for sample pretreatment. However, a single nucleic acid amplification method may not satisfy the detecting requirements. Therefore, a cascade amplification detection method that uses two or even multiple nucleic acid amplification methods integrated with LFA will be great development potential.
Finally, miniaturization and optimization of LFA devices are another vital goal for the sensitivity enhancement of LFA. It must be noted that among LFA detection methods, optical methods based on AuNPs and their derivatives are most likely to be one of the most viable POCT devices, given their simplicity and easy integration in conventional LFA. Therefore, significant engineering efforts should be focused on miniaturizing the whole process to be used in portable devices, which will be affordable and portable in field conditions and resource-limited settings.
Acknowledgements
This work was financially supported by the Space Medical Experiment Project of China Manned Space Program (HYZHXM04003).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wu YH, Zhou YF, Leng YK, Lai WH, Huang XL, Xiong YH. Emerging design strategies for constructing multiplex lateral flow test strip sensors. Biosens Bioelectron. 2020;157:13. doi: 10.1016/j.bios.2020.112168. [DOI] [PubMed] [Google Scholar]
- 2.Soh JH, Chan HM, Ying JY. Strategies for developing sensitive and specific nanoparticle-based lateral flow assays as point-of-care diagnostic device. Nano Today. 2020;30:17. doi: 10.1016/j.nantod.2019.100831. [DOI] [Google Scholar]
- 3.Drain PK, Hyle EP, Noubary F, Freedberg KA, Wilson D, Bishai WR, Rodriguez W, Bassett IV. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect Dis. 2014;14:239–249. doi: 10.1016/S1473-3099(13)70250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gervais L, De Rooij N, Delamarche E. Microfluidic chips for point-of-care immunodiagnostics. Adv Mater. 2011;23:H151–H176. doi: 10.1002/adma.201100464. [DOI] [PubMed] [Google Scholar]
- 5.Parolo C, Sena-Torralba A, Bergua JF, Calucho E, Fuentes-Chust C, Hu LM, Rivas L, Alvarez-Diduk R, Nguyen EP, Cinti S, Quesada-Gonzalez D, Merkoci A. Tutorial: Design and fabrication of nanoparticle-based lateral-flow immunoassays. Nat Protoc. 2020;15:3788–3816. doi: 10.1038/s41596-020-0357-x. [DOI] [PubMed] [Google Scholar]
- 6.Oliveira-Rodriguez M, Serrano-Pertierra E, Garcia AC, Martin SL, Mo MY, Cernuda-Morollon E, Blanco-Lopez MC. Point-of-care detection of extracellular vesicles: sensitivity optimization and multiple-target detection. Biosens Bioelectron. 2017;87:38–45. doi: 10.1016/j.bios.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Noguera P, Posthuma-Trumpie GA, Van Tuil M, Van Der Wal FJ, De Boer A, Moers A, Van Amerongen A. Carbon nanoparticles in lateral flow methods to detect genes encoding virulence factors of shiga toxin-producing escherichia coli. Anal Bioanal Chem. 2011;399:831–838. doi: 10.1007/s00216-010-4334-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiu WW, Xu H, Takalkar S, Gurung AS, Liu B, Zheng YF, Guo ZB, Baloda M, Baryeh K, Liu GD. Carbon nanotube-based lateral flow biosensor for sensitive and rapid detection of DNA sequence. Biosens Bioelectron. 2015;64:367–372. doi: 10.1016/j.bios.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 9.Yao L, Teng J, Zhu MY, Zheng L, Zhong YH, Liu GD, Xue F, Chen W. Mwcnts based high sensitive lateral flow strip biosensor for rapid determination of aqueous mercury ions. Biosens Bioelectron. 2016;85:331–336. doi: 10.1016/j.bios.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 10.Greenwald R, Esfandiari J, Lesellier S, Houghton R, Pollock J, Aagaard C, Andersen P, Hewinson RG, Chambers M, Lyashchenko K. Improved serodetection of mycobacterium bovis infection in badgers (meles meles) using multiantigen test formats. Diagn Micr Infec Dis. 2003;46:197–203. doi: 10.1016/S0732-8893(03)00046-4. [DOI] [PubMed] [Google Scholar]
- 11.Morales-Narvaez E, Naghdi T, Zor E, Merkoci A. Photo luminescent lateral-flow immunoassay revealed by graphene oxide: highly sensitive paper-based pathogen detection. Anal Chem. 2015;87:8573–8577. doi: 10.1021/acs.analchem.5b02383. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Lv XF, Feng W, Li XQ, Li KJ, Deng YL. Aptamer-based fluorometric lateral flow assay for creatine kinase mb. Microchim Acta. 2018;185:364. doi: 10.1007/s00604-018-2905-4. [DOI] [PubMed] [Google Scholar]
- 13.Kim J, Kwon JH, Jang J, Lee H, Kim S, Hahn YK, Kim SK, Lee KH, Lee S, Pyo H, Song CS, Lee J. Rapid and background-free detection of avian influenza virus in opaque sample using nir-to-nir upconversion nanoparticle-based lateral flow immunoassay platform. Biosens Bioelectron. 2018;112:209–215. doi: 10.1016/j.bios.2018.04.047. [DOI] [PubMed] [Google Scholar]
- 14.Edwards KA, Baeumner AJ. Liposome-enhanced lateral-flow assays for the sandwich-hybridization detection of rna. Methods Mol Biol (Clifton, N.J.) 2009;504:185–215. doi: 10.1007/978-1-60327-569-9_13. [DOI] [PubMed] [Google Scholar]
- 15.Panferov VG, Safenkova IV, Zherdev AV, Dzantiev BB. Setting up the cut-off level of a sensitive barcode lateral flow assay with magnetic nanoparticles. Talanta. 2017;164:69–76. doi: 10.1016/j.talanta.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 16.Lago-Cachon D, Oliveira-Rodriguez M, Rivas M, Blanco-Lopez MC, Martinez-Garcia JC, Moyano A, Salvador M, Garcia JA. Scanning magneto-inductive sensor for quantitative assay of prostate-specific antigen. IEEE Magn Lett. 2017;8:5. doi: 10.1109/LMAG.2017.2702108. [DOI] [Google Scholar]
- 17.Moyano A, Salvador M, Martinez-Garcia JC, Socoliuc V, Vekas L, Peddis D, Alvarez MA, Fernandez M, Rivas M, Blanco-Lopez MC. Magnetic immunochromatographic test for histamine detection in wine. Anal Bioanal Chem. 2019;411:6615–6624. doi: 10.1007/s00216-019-02031-6. [DOI] [PubMed] [Google Scholar]
- 18.Ruiz-Vega G, Kitsara M, Pellitero MA, Baldrich E, Del Campo FJ. Electrochemical lateral flow devices: towards rapid immunomagnetic assays. ChemElectroChem. 2017;4:880–889. doi: 10.1002/celc.201600902. [DOI] [Google Scholar]
- 19.Cinti S, Moscone D, Arduini F. Preparation of paper-based devices for reagentless electrochemical (bio)sensor strips. Nat Protoc. 2019;14:2437–2451. doi: 10.1038/s41596-019-0186-y. [DOI] [PubMed] [Google Scholar]
- 20.Li ZD, Li F, Xing Y, Liu Z, You ML, Li YC, Wen T, Qu ZG, Li XL, Xu F. Pen-on-paper strategy for point-of-care testing: rapid prototyping of fully written microfluidic biosensor. Biosens Bioelectron. 2017;98:478–485. doi: 10.1016/j.bios.2017.06.061. [DOI] [PubMed] [Google Scholar]
- 21.Liu XL, Wang YP, Gao YF, Song YJ. Gas-propelled biosensors for quantitative analysis. Analyst. 2021;146:1115–1126. doi: 10.1039/D0AN02154G. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Xuan J, Song YJ, Qi WJ, He BS, Wang P, Qin LD. Nanoporous glass integrated in volumetric bar-chart chip for point-of-care diagnostics of non-small cell lung cancer. ACS Nano. 2016;10:1640–1647. doi: 10.1021/acsnano.5b07357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun L, Zhao Q, Liu XL, Pan YC, Gao YF, Yang JJ, Wang YZ, Song YJ. Enzyme-mimicking accelerated signal enhancement for visually multiplexed quantitation of telomerase activity. Chem Commun. 2020;56:6969–6972. doi: 10.1039/D0CC01951H. [DOI] [PubMed] [Google Scholar]
- 24.Dervisevic M, Senel M, Cevik E. Novel impedimetric dopamine biosensor based on boronic acid functional polythiophene modified electrodes. Mater Sci Eng C-Mater Biol Appl. 2017;72:641–649. doi: 10.1016/j.msec.2016.11.127. [DOI] [PubMed] [Google Scholar]
- 25.Shen JJ, Zhou XM, Shan YY, Yue HH, Huang R, Hu JM, Xing D. Sensitive detection of a bacterial pathogen using allosteric probe-initiated catalysis and crispr-cas13a amplification reaction. Nat Commun. 2020;11:10. doi: 10.1038/s41467-019-14135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang WY, Hao WH, Liu XT, Sun XR, Yan JL, Wang YC. Visual detection of mirnas using enzyme-free amplification reactions and ratiometric fluorescent probes. Talanta. 2020;219:6. doi: 10.1016/j.talanta.2020.121332. [DOI] [PubMed] [Google Scholar]
- 27.Cheng Y-SL, Rees T, Wright J. A review of research on salivary biomarkers for oral cancer detection. Clin Transl Med. 2014;3:3–3. doi: 10.1186/2001-1326-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukama O, Nie CR, Habimana JD, Meng XG, Ting Y, Songwe F, Al Farga A, Mugisha S, Rwibasira P, Zhang YH, Zeng LW. Synergetic performance of isothermal amplification techniques and lateral flow approach for nucleic acid diagnostics. Anal Biochem. 2020;600:13. doi: 10.1016/j.ab.2020.113762. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez JM, Foley MW, Bieber NM, Bourdelle PA, Niedbala RS. Development of an ultrasensitive immunochromatography test to detect nicotine metabolites in oral fluids. Anal Bioanal Chem. 2011;400:3655–3664. doi: 10.1007/s00216-011-5051-y. [DOI] [PubMed] [Google Scholar]
- 30.Niemz A, Ferguson TM, Boyle DS. Point-of-care nucleic acid testing for infectious diseases. Trends Biotechnol. 2011;29:240–250. doi: 10.1016/j.tibtech.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Cao JJ, Zhu MS, Xu MG, Shi F. Loop-mediated isothermal amplification-lateral-flow dipstick (lamp-lfd) to detect mycoplasma ovipneumoniae. World J Microb Biot. 2019;35:10. doi: 10.1007/s11274-019-2601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu JQ, Wang Y, Su HJ, Ding HM, Sun XC, Gao H, Geng Y, Wang ZC. Rapid analysis of escherichia coli o157:H7 using isothermal recombinase polymerase amplification combined with triple-labeled nucleotide probes. Mol Cell Probe. 2020;50:6. doi: 10.1016/j.mcp.2019.101501. [DOI] [PubMed] [Google Scholar]
- 33.Mahmoudi T, De La Guardia M, Baradaran B. Lateral flow assays towards point -of -care cancer detection: a review of current progress and future trends. Trac-trend Anal Chem. 2020;125:20. doi: 10.1016/j.trac.2020.115842. [DOI] [Google Scholar]
- 34.Luo K, Kim HY, Oh MH, Kim YR. Paper-based lateral flow strip assay for the detection of foodborne pathogens: principles, applications, technological challenges and opportunities. Crit Rev Food Sci. 2020;60:157–170. doi: 10.1080/10408398.2018.1516623. [DOI] [PubMed] [Google Scholar]
- 35.Yao MD, Lv XF, Deng YL, Rasheed M. Specific and simultaneous detection of micro rna 21 and let-7a by rolling circle amplification combined with lateral flow strip. Anal Chim Acta. 2019;1055:115–125. doi: 10.1016/j.aca.2018.12.040. [DOI] [PubMed] [Google Scholar]
- 36.Mei XR, Zhai XW, Lei CW, Ye XL, Kang ZZ, Wu X, Xiang R, Wang YL, Wang HN. Development and application of a visual loop-mediated isothermal amplification combined with lateral flow dipstick (lamp-lfd) method for rapid detection of salmonella strains in food samples. Food Control. 2019;104:9–19. doi: 10.1016/j.foodcont.2019.04.014. [DOI] [Google Scholar]
- 37.Li TT, Jalbani YM, Zhang GL, Zhao ZY, Wang ZY, Zhao Y, Zhao XY, Chen AL. Rapid authentication of mutton products by recombinase polymerase amplification coupled with lateral flow dipsticks. Sensor Actuat B-chem. 2019;290:242–248. doi: 10.1016/j.snb.2019.03.018. [DOI] [Google Scholar]
- 38.Niazi A, Jorjani O-N, Nikbakht H, Gill P. A nanodiagnostic colorimetric assay for 18s rrna of leishmania pathogens using nucleic acid sequence-based amplification and gold nanorods. Mol Diagn Ther. 2013;17:363–370. doi: 10.1007/s40291-013-0044-5. [DOI] [PubMed] [Google Scholar]
- 39.Kolm C, Martzy R, Fuhrer M, Mach RL, Krska R, Baumgartner S, Farnleitner AH, Reischer GH. Detection of a microbial source tracking marker by isothermal helicase-dependent amplification and a nucleic acid lateral-flow strip test. Sci Rep. 2019;9:9. doi: 10.1038/s41598-018-36749-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ying N, Ju CJ, Sun XW, Li LT, Chang HB, Song GP, Li ZY, Wan JY, Dai EY. Lateral flow nucleic acid biosensor for sensitive detection of micrornas based on the dual amplification strategy of duplex-specific nuclease and hybridization chain reaction. PLoS ONE. 2017;12:12. doi: 10.1371/journal.pone.0185091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuhn H, Demidov VV, Frank-Kamenetskii MD. Rolling-circle amplification under topological constraints. Nucleic Acids Res. 2002;30:574–580. doi: 10.1093/nar/30.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao WA, Ali MM, Brook MA, Li YF. Rolling circle amplification: applications in nanotechnology and biodetection with functional nucleic acids. Angew Chem Int Edit. 2008;47:6330–6337. doi: 10.1002/anie.200705982. [DOI] [PubMed] [Google Scholar]
- 43.Ali MM, Li F, Zhang ZQ, Zhang KX, Kang DK, Ankrum JA, Le XC, Zhao WA. Rolling circle amplification: a versatile tool for chemical biology, materials science and medicine. Chem Soc Rev. 2014;43:3324–3341. doi: 10.1039/c3cs60439j. [DOI] [PubMed] [Google Scholar]
- 44.Kor K, Turner APF, Zarei K, Atabati M, Beni V, Mak WC. Structurally responsive oligonucleotide-based single-probe lateral-flow test for detection of mirna-21 mimics. Anal Bioanal Chem. 2016;408:1475–1485. doi: 10.1007/s00216-015-9250-9. [DOI] [PubMed] [Google Scholar]
- 45.Zhang CY, Chen GF, Wang YY, Zhou J, Li CH. Establishment and application of hyperbranched rolling circle amplification coupled with lateral flow dipstick for the sensitive detection of karenia mikimotoi. Harmful Algae. 2019;84:151–160. doi: 10.1016/j.hal.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 46.Liu FG, Chen GF, Zhang CY, Wang YY, Zhou J. Exponential rolling circle amplification coupled with lateral flow dipstick strips as a rapid and sensitive method for the field detection of karlodinium veneficum. J Appl Phycol. 2019;31:2423–2436. doi: 10.1007/s10811-019-01762-4. [DOI] [Google Scholar]
- 47.Kim TY, Lim MC, Woo MA, Jun BH. Radial flow assay using gold nanoparticles and rolling circle amplification to detect mercuric ions. Nanomaterials. 2018;8:13. doi: 10.3390/nano8020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu M, Hui CY, Zhang Q, Gu J, Kannan B, Jahanshahi-Anbuhi S, Filipe CDM, Brennan JD, Li YF. Target-induced and equipment-free DNA amplification with a simple paper device. Angew Chem Int Edit. 2016;55:2709–2713. doi: 10.1002/anie.201509389. [DOI] [PubMed] [Google Scholar]
- 49.Hui CY, Liu M, Li YF, Brennan JD. A paper sensor printed with multifunctional bio/nano materials. Angew Chem Int Edit. 2018;57:4549–4553. doi: 10.1002/anie.201712903. [DOI] [PubMed] [Google Scholar]
- 50.Wu LD, Ma C, Zheng XX, Liu HY, Yu JH. Paper-based electrochemiluminescence origami device for protein detection using assembled cascade DNA-carbon dots nanotags based on rolling circle amplification. Biosens Bioelectron. 2015;68:413–420. doi: 10.1016/j.bios.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 51.Dou MW, Dominguez DC, Li XJ, Sanchez J, Scott G. A versatile pdms/paper hybrid microfluidic platform for sensitive infectious disease diagnosis. Anal Chem. 2014;86:7978–7986. doi: 10.1021/ac5021694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joung J, Ladha A, Saito M, Kim N-G, Woolley AE, Segel M, Barretto RPJ, Ranu A, Macrae RK, Faure G, Ioannidi EI, Krajeski RN, Bruneau R, Huang M-LW, Yu XG, Li JZ, Walker BD, Hung DT, Greninger AL, Jerome KR, Gootenberg JS, Abudayyeh OO, Zhang F. Detection of sars-cov-2 with sherlock one-pot testing. New Engl J Med. 2020;383:1492. doi: 10.1056/NEJMc2026172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piepenburg O, Williams CH, Stemple DL, Armes NA. DNA detection using recombination proteins. PLoS Biol. 2006;4:1115–1121. doi: 10.1371/journal.pbio.0040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zasada AA, Zacharczuk K, Forminska K, Wiatrzyk A, Ziolkowski R, Malinowska E. Isothermal DNA amplification combined with lateral flow dipsticks for detection of biothreat agents. Anal Biochem. 2018;560:60–66. doi: 10.1016/j.ab.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 55.Karakkat BB, Hockemeyer K, Franchett M, Olson M, Mullenberg C, Koch PL. Detection of root-infecting fungi on cool-season turfgrasses using loop-mediated isothermal amplification and recombinase polymerase amplification. J Microbiol Meth. 2018;151:90–98. doi: 10.1016/j.mimet.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 56.Kellner MJ, Koob JG, Gootenberg JS, Abudayyeh OO, Zhang F. Sherlock: nucleic acid detection with crispr nucleases. Nat Protoc. 2019;14:2986–3012. doi: 10.1038/s41596-019-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fu MQ, Chen GF, Zhang CY, Wang YY, Sun R, Zhou J. Rapid and sensitive detection method for karlodinium veneficum by recombinase polymerase amplification coupled with lateral flow dipstick. Harmful Algae. 2019;84:1–9. doi: 10.1016/j.hal.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 58.Saxena A, Pal V, Tripathi NK, Goel AK. Development of a rapid and sensitive recombinase polymerase amplification-lateral flow assay for detection of burkholderia mallei. Transbound Emerg Dis. 2019;66:1016–1022. doi: 10.1111/tbed.13126. [DOI] [PubMed] [Google Scholar]
- 59.Sun N, Wang Y, Yao XY, Chen FF, Gao DY, Wang WP, Li XJ. Visual signal generation for the detection of influenza viruses by duplex recombinase polymerase amplification with lateral flow dipsticks. Anal Bioanal Chem. 2019;411:3591–3602. doi: 10.1007/s00216-019-01840-z. [DOI] [PubMed] [Google Scholar]
- 60.Du XJ, Zang YX, Liu HB, Li P, Wang S. Recombinase polymerase amplification combined with lateral flow strip for listeria monocytogenes detection in food. J Food Sci. 2018;83:1041–1047. doi: 10.1111/1750-3841.14078. [DOI] [PubMed] [Google Scholar]
- 61.Xu YC, Wei YJ, Cheng N, Huang KL, Wang WR, Zhang L, Xu WT, Luo YB. Nucleic acid biosensor synthesis of an all-in-one universal blocking linker recombinase polymerase amplification with a peptide nucleic acid-based lateral flow device for ultrasensitive detection of food pathogens. Anal Chem. 2018;90:708–715. doi: 10.1021/acs.analchem.7b01912. [DOI] [PubMed] [Google Scholar]
- 62.Choi JR, Hu J, Gong Y, Feng SS, WaW A, Pingguan-Murphy B, Xu F. An integrated lateral flow assay for effective DNA amplification and detection at the point of care. Analyst. 2016;141:2930–2939. doi: 10.1039/C5AN02532J. [DOI] [PubMed] [Google Scholar]
- 63.Seok Y, Joung HA, Byun JY, Jeon HS, Shin SJ, Kim S, Shin YB, Han HS, Kim MG. A paper-based device for performing loop-mediated isothermal amplification with real-time simultaneous detection of multiple DNA targets. Theranostics. 2017;7:2220–2230. doi: 10.7150/thno.18675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu GL, Nolder D, Reboud J, Oguike MC, Van Schalkwyk DA, Sutherland CJ, Cooper JM. Paper-origami-based multiplexed malaria diagnostics from whole blood. Angew Chem Int Edit. 2016;55:15250–15253. doi: 10.1002/anie.201606060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zuo P, Li XJ, Dominguez DC, Ye BC. A pdms/paper/glass hybrid microfluidic biochip integrated with aptamer-functionalized graphene oxide nano-biosensors for one-step multiplexed pathogen detection. Lab Chip. 2013;13:3921–3928. doi: 10.1039/c3lc50654a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fang XE, Chen H, Xu LJ, Jiang XY, Wu WJ, Kong JL. A portable and integrated nucleic acid amplification microfluidic chip for identifying bacteria. Lab Chip. 2012;12:1495–1499. doi: 10.1039/c2lc40055c. [DOI] [PubMed] [Google Scholar]
- 67.Safavieh M, Ahmed MU, Sokullu E, Ng A, Braescuac L, Zourob M. A simple cassette as point-of-care diagnostic device for naked-eye colorimetric bacteria detection. Analyst. 2014;139:482–487. doi: 10.1039/C3AN01859H. [DOI] [PubMed] [Google Scholar]
- 68.Connelly JT, Rolland JP, Whitesides GM. “Paper machine” for molecular diagnostics. Anal Chem. 2015;87:7595–7601. doi: 10.1021/acs.analchem.5b00411. [DOI] [PubMed] [Google Scholar]
- 69.Chen YT, Cheng N, Xu YC, Huang KL, Luo YB, Xu WT. Point-of-care and visual detection of p. Aeruginosa and its toxin genes by multiple lamp and lateral flow nucleic acid biosensor. Biosens Bioelectron. 2016;81:317–323. doi: 10.1016/j.bios.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 70.Zhu X, Wang X, Han L, Chen T, Wang L, Li H, Li S, He L, Fu X, Chen S, Xing M, Chen H, Wang Y (2020) Multiplex reverse transcription loop-mediated isothermal amplification combined with nanoparticle-based lateral flow biosensor for the diagnosis of covid-19. Biosens Bioelectron 166. [DOI] [PMC free article] [PubMed]
- 71.Kim C, Yoo YK, Il Han S, Lee J, Lee D, Lee K, Hwang KS, Lee KH, Chung S, Lee JH. Battery operated preconcentration-assisted lateral flow assay. Lab Chip. 2017;17:2451–2458. doi: 10.1039/C7LC00036G. [DOI] [PubMed] [Google Scholar]
- 72.Moghadam BY, Connelly KT, Posner JD. Two orders of magnitude improvement in detection limit of lateral flow assays using lsotachophoresis. Anal Chem. 2015;87:1009–1017. doi: 10.1021/ac504552r. [DOI] [PubMed] [Google Scholar]
- 73.Chiu RYT, Jue E, Yip AT, Berg AR, Wang SJ, Kivnick AR, Nguyen PT, Kamei DT. Simultaneous concentration and detection of biomarkers on paper. Lab Chip. 2014;14:3021–3028. doi: 10.1039/C4LC00532E. [DOI] [PubMed] [Google Scholar]
- 74.Tang RH, Yang H, Choi JR, Gong Y, Hu J, Feng SS, Pingguan-Murphy B, Mei QB, Xu F. Improved sensitivity of lateral flow assay using paper-based sample concentration technique. Talanta. 2016;152:269–276. doi: 10.1016/j.talanta.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 75.Mashayekhi F, Chiu RYT, Le AM, Chao FC, Wu BM, Kamei DT. Enhancing the lateral-flow immunoassay for viral detection using an aqueous two-phase micellar system. Anal Bioanal Chem. 2010;398:2955–2961. doi: 10.1007/s00216-010-4213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mashayekhi F, Le AM, Nafisi PM, Wu BM, Kamei DT. Enhancing the lateral-flow immunoassay for detection of proteins using an aqueous two-phase micellar system. Anal Bioanal Chem. 2012;404:2057–2066. doi: 10.1007/s00216-012-6278-y. [DOI] [PubMed] [Google Scholar]
- 77.Chen F, Ming X, Chen XX, Gan M, Wang BG, Xu F, Wei H. Immunochromatographic strip for rapid detection of cronobacter in powdered infant formula in combination with silica-coated magnetic nanoparticles separation and 16s rrna probe. Biosens Bioelectron. 2014;61:306–313. doi: 10.1016/j.bios.2014.05.033. [DOI] [PubMed] [Google Scholar]
- 78.Fang ZY, Wu W, Lu XW, Zeng LW. Lateral flow biosensor for DNA extraction-free detection of salmonella based on aptamer mediated strand displacement. Amplification Biosens Bioelectron. 2014;56:192–197. doi: 10.1016/j.bios.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 79.Wang DB, Tian B, Zhang ZP, Wang XY, Fleming J, Bi LJ, Yang RF, Zhang XE. Detection of bacillus anthracis spores by super-paramagnetic lateral-flow immunoassays based on “road closure”. Biosens Bioelectron. 2015;67:608–614. doi: 10.1016/j.bios.2014.09.067. [DOI] [PubMed] [Google Scholar]
- 80.Lu XW, Liang XL, Dong JH, Fang ZY, Zeng LW. Lateral flow biosensor for multiplex detection of nitrofuran metabolites based on functionalized magnetic beads. Anal Bioanal Chem. 2016;408:6703–6709. doi: 10.1007/s00216-016-9787-2. [DOI] [PubMed] [Google Scholar]
- 81.Zhang B, Ma WJ, Li FX, Gao WC, Zhao Q, Peng WP, Piao JF, Wu XL, Wang HJ, Gong XQ, Chang J. Fluorescence quenching-based signal amplification on immunochromatography test strips for dual-mode sensing of two biomarkers of breast cancer. Nanoscale. 2017;9:18711–18722. doi: 10.1039/C7NR06781J. [DOI] [PubMed] [Google Scholar]
- 82.Bahadir EB, Sezginturk MK. Lateral flow assays: principles, designs and labels. Trac-trend Anal Chem. 2016;82:286–306. doi: 10.1016/j.trac.2016.06.006. [DOI] [Google Scholar]
- 83.Kumar R, Singh CK, Kamle S, Sinha RP, Bhatnagar RK, Kachru DN. Development of nanocolloidal gold based immunochromatographic assay for rapid detection of transgenic vegetative insecticidal protein in genetically modified crops. Food Chem. 2010;122:1298–1303. doi: 10.1016/j.foodchem.2010.03.086. [DOI] [Google Scholar]
- 84.Blazkova M, Mickova-Holubova B, Rauch P, Fukal L. Immunochromatographic colloidal carbon-based assay for detection of methiocarb in surface water. Biosens Bioelectron. 2009;25:753–758. doi: 10.1016/j.bios.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 85.Zeng Q, Mao X, Xu H, Wang S, Liu G (2009) Quantitative immunochromatographic strip biosensor for the detection of carcinoembryonic antigen tumor biomarker in human plasma.
- 86.Rivas L, Medina-Sanchez M, De La Escosura-Muniz A, Merkoci A. Improving sensitivity of gold nanoparticle-based lateral flow assays by using wax-printed pillars as delay barriers of microfluidics. Lab Chip. 2014;14:4406–4414. doi: 10.1039/C4LC00972J. [DOI] [PubMed] [Google Scholar]
- 87.Choi JR, Liu Z, Hu J, Tang RH, Gong Y, Feng SS, Ren H, Wen T, Yang H, Qu ZG, Pingguan-Murphy B, Xu F. Polydimethylsiloxane-paper hybrid lateral flow assay for highly sensitive point-of-care nucleic acid testing. Anal Chem. 2016;88:6254–6264. doi: 10.1021/acs.analchem.6b00195. [DOI] [PubMed] [Google Scholar]
- 88.Choi JR, Yong KW, Tang RH, Gong Y, Wen T, Yang H, Li A, Chia YC, Pingguan-Murphy B, Xu F. Lateral flow assay based on paper-hydrogel hybrid material for sensitive point-of-care detection of dengue virus. Adv Healthc Mater. 2017;6:9. doi: 10.1002/adhm.201600920. [DOI] [PubMed] [Google Scholar]
- 89.Xu ZG, Zhao Y, Dai LM, Lin T. Multi-responsive janus liquid marbles: The effect of temperature and acidic/basic vapors. Part Part Syst Char. 2014;31:839–842. doi: 10.1002/ppsc.201400009. [DOI] [Google Scholar]
- 90.Parolo C, De La Escosura-Muniz A, Merkoci A. Enhanced lateral flow immunoassay using gold nanoparticles loaded with enzymes. Biosens Bioelectron. 2013;40:412–416. doi: 10.1016/j.bios.2012.06.049. [DOI] [PubMed] [Google Scholar]
- 91.Choi JR, Hu J, Feng SS, WaW A, Pingguan-Murphy B, Xu F. Sensitive biomolecule detection in lateral flow assay with a portable temperature-humidity control device. Biosens Bioelectron. 2016;79:98–107. doi: 10.1016/j.bios.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 92.Katis IN, He PJW, Eason RW, Sones CL. Improved sensitivity and limit-of-detection of lateral flow devices using spatial constrictions of the flow-path. Biosens Bioelectron. 2018;113:95–100. doi: 10.1016/j.bios.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 93.Urusov AE, Petrakova AV, Kuzmin PG, Zherdev AV, Sveshnikov PG, Shafeev GA, Dzantiev BB. Application of gold nanoparticles produced by laser ablation for immunochromatographic assay labeling. Anal Biochem. 2015;491:65–71. doi: 10.1016/j.ab.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 94.Yao YY, Guo WS, Zhang J, Wu YD, Fu WH, Liu TT, Wu XL, Wang HJ, Gong XQ, Liang XJ, Chang J. Reverse fluorescence enhancement and colorimetric bimodal signal readout immunochromatography test strip for ultrasensitive large-scale screening and postoperative monitoring. ACS Appl Mater Interfaces. 2016;8:22963–22970. doi: 10.1021/acsami.6b08445. [DOI] [PubMed] [Google Scholar]
- 95.Kong MM, Yang B, Gong CJ, Wang H, Li X, Zhao KS, Li JJ, Wu F, Liu X, Hu Z. Development of immunochromatographic colloidal gold test strip for rapid detection of haemophilus influenzae in clinical specimens. J Appl Microbiol. 2017;123:287–294. doi: 10.1111/jam.13489. [DOI] [PubMed] [Google Scholar]
- 96.Choi DH, Lee SK, Oh YK, Bae BW, Lee SD, Kim S, Shin YB, Kim MG. A dual gold nanoparticle conjugate-based lateral flow assay (lfa) method for the analysis of troponin i. Biosens Bioelectron. 2010;25:1999–2002. doi: 10.1016/j.bios.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 97.Posthuma-Trumpie GA, Korf J, Van Amerongen A. Lateral flow (immuno) assay: its strengths, weaknesses, opportunities and threats. A literature survey. Anal Bioanal Chem. 2009;393:569–582. doi: 10.1007/s00216-008-2287-2. [DOI] [PubMed] [Google Scholar]
- 98.Zhong YH, Chen YJ, Yao L, Zhao DP, Zheng L, Liu GD, Ye YW, Chen W. Gold nanoparticles based lateral flow immunoassay with largely amplified sensitivity for rapid melamine screening. Microchim Acta. 2016;183:1989–1994. doi: 10.1007/s00604-016-1812-9. [DOI] [Google Scholar]
- 99.Mei ZL, Qu W, Deng Y, Chu HQ, Cao JX, Xue F, Zheng L, El-Nezamic HS, Wu YC, Chen W. One-step signal amplified lateral flow strip biosensor for ultrasensitive and on-site detection of bisphenol a (bpa) in aqueous samples. Biosens Bioelectron. 2013;49:457–461. doi: 10.1016/j.bios.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 100.Zhu MY, Wang Y, Deng Y, Yao L, Adeloju SB, Pan DD, Xue F, Wu YC, Zheng L, Chen W. Ultrasensitive detection of mercury with a novel one-step signal amplified lateral flow strip based on gold nanoparticle-labeled ssdna recognition and enhancement probes. Biosens Bioelectron. 2014;61:14–20. doi: 10.1016/j.bios.2014.04.049. [DOI] [PubMed] [Google Scholar]
- 101.Serebrennikova K, Samsonova J, Osipov A. Hierarchical nanogold labels to improve the sensitivity of lateral flow immunoassay. Nano-Micro Lett. 2018;10:8. doi: 10.1007/s40820-017-0180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim W, Lee S, Jeon S. Enhanced sensitivity of lateral flow immunoassays by using water-soluble nanofibers and silver-enhancement reactions. Sensor Actuat B-chem. 2018;273:1323–1327. doi: 10.1016/j.snb.2018.07.045. [DOI] [Google Scholar]
- 103.Anfossi L, Di Nardo F, Giovannoli C, Passini C, Baggiani C. Increased sensitivity of lateral flow immunoassay for ochratoxin a through silver enhancement. Anal Bioanal Chem. 2013;405:9859–9867. doi: 10.1007/s00216-013-7428-6. [DOI] [PubMed] [Google Scholar]
- 104.Cho IH, Bhunia A, Irudayaraj J. Rapid pathogen detection by lateral-flow immunochromatographic assay with gold nanoparticle-assisted enzyme signal amplification. Int J Food Microbiol. 2015;206:60–66. doi: 10.1016/j.ijfoodmicro.2015.04.032. [DOI] [PubMed] [Google Scholar]
- 105.Li JW, Baird MA, Davis MA, Tai WY, Zweifel LS, Waldorf KMA, Gale M, Rajagopal L, Pierce RH, Gao XH. Dramatic enhancement of the detection limits of bioassays via ultrafast deposition of polydopamine. Nat Biomed Eng. 2017;1:12. doi: 10.1038/s41551-017-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Panferov VG, Safenkova IV, Varitsev YA, Drenova NV, Kornev KP, Zherdev AV, Dzantiev BB. Development of the sensitive lateral flow immunoassay with silver enhancement for the detection of ralstonia solanacearum in potato tubers. Talanta. 2016;152:521–530. doi: 10.1016/j.talanta.2016.02.050. [DOI] [PubMed] [Google Scholar]
- 107.Rodriguez MO, Covian LB, Garcia AC, Blanco-Lopez MC. Silver and gold enhancement methods for lateral flow immunoassays. Talanta. 2016;148:272–278. doi: 10.1016/j.talanta.2015.10.068. [DOI] [PubMed] [Google Scholar]
- 108.Yang W, Li XB, Liu GW, Zhang BB, Zhang Y, Kong T, Tang JJ, Li DN, Wang Z. A colloidal gold probe-based silver enhancement immunochromatographic assay for the rapid detection of abrin-a. Biosens Bioelectron. 2011;26:3710–3713. doi: 10.1016/j.bios.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 109.Anfossi L, Giovannoli C, Giraudi G, Biagioli F, Passini C, Baggiani C. A lateral flow immunoassay for the rapid detection of ochratoxin a in wine and grape must. J Agr Food Chem. 2012;60:11491–11497. doi: 10.1021/jf3031666. [DOI] [PubMed] [Google Scholar]
- 110.Ambrosi A, Castaneda MT, Killard AJ, Smyth MR, Alegret S, Merkoci A. Double-codified gold nanolabels for enhanced immunoanalysis. Anal Chem. 2007;79:5232–5240. doi: 10.1021/ac070357m. [DOI] [PubMed] [Google Scholar]
- 111.Tang DP, Yuan R, Chal YQ. Ultrasensitive electrochemical immunosensor for clinical immunoassay using thionine-doped magnetic gold nanospheres as labels and horseradish peroxidase as enhancer. Anal Chem. 2008;80:1582–1588. doi: 10.1021/ac702217m. [DOI] [PubMed] [Google Scholar]
- 112.Lai GS, Yan F, Ju HX. Dual signal amplification of glucose oxidase-functionalized nanocomposites as a trace label for ultrasensitive simultaneous multiplexed electrochemical detection of tumor markers. Anal Chem. 2009;81:9730–9736. doi: 10.1021/ac901996a. [DOI] [PubMed] [Google Scholar]
- 113.Li J, Song SP, Li D, Su Y, Huang Q, Zhao Y, Fan CH. Multi-functional crosslinked au nanoaggregates for the amplified optical DNA detection. Biosens Bioelectron. 2009;24:3311–3315. doi: 10.1016/j.bios.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 114.He YQ, Zhang SQ, Zhang XB, Baloda M, Gurung AS, Xu H, Zhang XJ, Liu GD. Ultrasensitive nucleic acid biosensor based on enzyme-gold nanoparticle dual label and lateral flow strip biosensor. Biosens Bioelectron. 2011;26:2018–2024. doi: 10.1016/j.bios.2010.08.079. [DOI] [PubMed] [Google Scholar]
- 115.Mao X, Ma YQ, Zhang AG, Zhang LR, Zeng LW, Liu GD. Disposable nucleic acid biosensors based on gold nanoparticle probes and lateral flow strip. Anal Chem. 2009;81:1660–1668. doi: 10.1021/ac8024653. [DOI] [PubMed] [Google Scholar]
- 116.Jia XF, Wang CW, Rong Z, Li J, Wang KL, Qie ZW, Xiao R, Wang SQ. Dual dye-loaded au@ag coupled to a lateral flow immunoassay for the accurate and sensitive detection of mycoplasma pneumoniae infection. RSC Adv. 2018;8:21243–21251. doi: 10.1039/C8RA03323D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Blanco-Covian L, Montes-Garcia V, Girard A, Fernandez-Abedul MT, Perez-Juste J, Pastoriza-Santos I, Faulds K, Graham D, Blanco-Lopez MC. Au@ag serrs tags coupled to a lateral flow immunoassay for the sensitive detection of pneumolysin. Nanoscale. 2017;9:2051–2058. doi: 10.1039/C6NR08432J. [DOI] [PubMed] [Google Scholar]
- 118.Cima-Cabal MD, Mendez FJ, Vazquez F, Garcia-Suarez MD, De Los Toyos JR. A specific and ultrasensitive chemiluminescent sandwich ELISA test for the detection and quantitation of pneumolysin. J Immunoass Immunoch. 2001;22:99–112. doi: 10.1081/IAS-100103223. [DOI] [PubMed] [Google Scholar]
- 119.Bastus NG, Comenge J, Puntes V. Kinetically controlled seeded growth synthesis of citrate-stabilized gold nanoparticles of up to 200 nm: Size focusing versus ostwald ripening. Langmuir. 2011;27:11098–11105. doi: 10.1021/la201938u. [DOI] [PubMed] [Google Scholar]
- 120.Wang R, Kim K, Choi N, Wang X, Lee J, Jeon JH, Rhie GE, Choo J. Highly sensitive detection of high-risk bacterial pathogens using sers-based lateral flow assay strips. Sensor Actuat B-chem. 2018;270:72–79. doi: 10.1016/j.snb.2018.04.162. [DOI] [Google Scholar]
- 121.Zhang D, Huang L, Liu B, Ni HB, Sun LD, Su EB, Chen HY, Gu ZZ, Zhao XW. Quantitative and ultrasensitive detection of multiplex cardiac biomarkers in lateral flow assay with core-shell sers nanotags. Biosens Bioelectron. 2018;106:204–211. doi: 10.1016/j.bios.2018.01.062. [DOI] [PubMed] [Google Scholar]
- 122.Xu QF, Xu H, Gu HC, Li JB, Wang YY, Wei M. Development of lateral flow immunoassay system based on superparamagnetic nanobeads as labels for rapid quantitative detection of cardiac troponin i. Mat Sci Eng C-bio S. 2009;29:702–707. doi: 10.1016/j.msec.2009.01.009. [DOI] [Google Scholar]
- 123.Zhu JM, Zou NL, Zhu DN, Wang J, Jin QH, Zhao JL, Mao HJ. Simultaneous detection of high-sensitivity cardiac troponin i and myoglobin by modified sandwich lateral flow immunoassay: proof of principle. Clin Chem. 2011;57:1732–1738. doi: 10.1373/clinchem.2011.171694. [DOI] [PubMed] [Google Scholar]
- 124.Zhang D, Huang L, Liu B, Su EB, Chen HY, Gu ZZ, Zhao XW. Quantitative detection of multiplex cardiac biomarkers with encoded sers nanotags on a single t line in lateral flow assay. Sensor Actuat B-chem. 2018;277:502–509. doi: 10.1016/j.snb.2018.09.044. [DOI] [PubMed] [Google Scholar]
- 125.Hwang J, Lee S, Choo J. Application of a sers-based lateral flow immunoassay strip for the rapid and sensitive detection of staphylococcal enterotoxin b. Nanoscale. 2016;8:11418–11425. doi: 10.1039/C5NR07243C. [DOI] [PubMed] [Google Scholar]
- 126.Gao XF, Zheng P, Kasani S, Wu S, Yang F, Lewis S, Nayeem S, Engler-Chiurazzi EB, Wigginton JG, Simpkins JW, Wu NQ. Paper-based surface-enhanced raman scattering lateral flow strip for detection of neuron-specific enolase in blood plasma. Anal Chem. 2017;89:10104–10110. doi: 10.1021/acs.analchem.7b03015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.North SH, Shriver-Lake LC, Taitt CR, Ligler FS, Rapid analytical methods for on-site triage for traumatic brain injury, in: R.G. Cooks,E.S. Yeung (Eds.) Annual review of analytical chemistry, vol 5, Annual Reviews, Palo Alto, 2012, pp. 35–56. [DOI] [PubMed]
- 128.Li SJ, Zhang Y, Wen WJ, Sheng W, Wang JY, Wang S, Wang JP. A high-sensitivity thermal analysis immunochromatographic sensor based on au nanoparticle-enhanced two-dimensional black phosphorus photothermal-sensing materials. Biosens Bioelectron. 2019;133:223–229. doi: 10.1016/j.bios.2019.03.039. [DOI] [PubMed] [Google Scholar]
- 129.Qin ZP, Chan WCW, Boulware DR, Akkin T, Butler EK, Bischof JC. Significantly improved analytical sensitivity of lateral flow immunoassays by using thermal contrast. Angew Chem Int Edit. 2012;51:4358–4361. doi: 10.1002/anie.201200997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Song S, Choi S, Ryu S, Kim S, Kim T, Shin J, Jung HI, Joo C. Highly sensitive paper-based immunoassay using photothermal laser speckle imaging. Biosens Bioelectron. 2018;117:385–391. doi: 10.1016/j.bios.2018.06.024. [DOI] [PubMed] [Google Scholar]
- 131.Wang YR, Qin ZP, Boulware DR, Pritt BS, Sloan LM, Gonzalez IJ, Bell D, Rees-Channer RR, Chiodini P, Chan WCW, Bischof JC. Thermal contrast amplification reader yielding 8-fold analytical improvement for disease detection with lateral flow assays. Anal Chem. 2016;88:11774–11782. doi: 10.1021/acs.analchem.6b03406. [DOI] [PubMed] [Google Scholar]
- 132.Ge XX, Asiri AM, Du D, Wen W, Wang SF, Lin YH. Nanomaterial-enhanced paper-based biosensors. Trac-trend. Anal Chem. 2014;58:31–39. [Google Scholar]
- 133.Nguyen VT, Song S, Park S, Joo C. Recent advances in high-sensitivity detection methods for paper-based lateral-flow assay. Biosens Bioelectron. 2020;152:17. doi: 10.1016/j.bios.2020.112015. [DOI] [PubMed] [Google Scholar]
- 134.Zhao YF, Huang Y, Zhao XW, Mcclelland JF, Lu M. Nanoparticle-based photoacoustic analysis for highly sensitive lateral flow assays. Nanoscale. 2016;8:19204–19210. doi: 10.1039/C6NR05312B. [DOI] [PubMed] [Google Scholar]
- 135.Park C, Kwon EY, Shin NY, Choi SM, Kim SH, Park SH, Lee DG, Choi JH, Yoo JH. Evaluation of nucleic acid sequence based amplification using fluorescence resonance energy transfer (fret-nasba) in quantitative detection of aspergillus 18s rrna. Med Mycol. 2011;49:73–79. doi: 10.3109/13693786.2010.507604. [DOI] [PubMed] [Google Scholar]
- 136.Sharma A, Tok AIY, Lee C, Ganapathy R, Palaniappan A, Liedberg B. Magnetic field assisted preconcentration of biomolecules for lateral flow assaying. Sensor Actuat B-chem. 2019;285:431–437. doi: 10.1016/j.snb.2019.01.073. [DOI] [Google Scholar]
- 137.Ren W, Mohammed SI, Wereley S, Irudayaraj J. Magnetic focus lateral flow sensor for detection of cervical cancer biomarkers. Anal Chem. 2019;91:2876–2884. doi: 10.1021/acs.analchem.8b04848. [DOI] [PubMed] [Google Scholar]
- 138.Fu X, Cheng Z, Yu J, Choo P, Chen L, Choo J. A sers-based lateral flow assay biosensor for highly sensitive detection of hiv-1 DNA. Biosens Bioelectron. 2016;78:530–537. doi: 10.1016/j.bios.2015.11.099. [DOI] [PubMed] [Google Scholar]
- 139.Choi S, Hwang J, Lee S, Lim DW, Joo H, Choo J. Quantitative analysis of thyroid-stimulating hormone (tsh) using sers-based lateral flow immunoassay. Sensor Actuat B-chem. 2017;240:358–364. doi: 10.1016/j.snb.2016.08.178. [DOI] [Google Scholar]