Abstract
Background
A variety of smartphone apps and wearables are available both to help patients monitor their health and to support health care professionals (HCPs) in providing clinical care. As part of the RADAR-CNS consortium, we have conducted research into the application of wearables and smartphone apps in the care of people with multiple sclerosis, epilepsy, or depression.
Methods
We conducted a large online survey study to explore the experiences of HCPs working with patients who have one or more of these conditions. The survey covered smartphone apps and wearables used by clinicians and their patients, and how data from these technologies impacted on the respondents' clinical practice. The survey was conducted between February 2019 and March 2020 via a web-based platform. Detailed statistical analysis was performed on the answers.
Results
Of 1009 survey responses from HCPs, 1006 were included in the analysis after data cleaning. Smartphone apps are used by more than half of responding HCPs and more than three quarters report that their patients use smartphone apps or wearable devices for health-related purposes. HCPs widely believe the data that patients collect using these devices impacts their clinical practice. Subgroup analyses show that views on the impact of this data on different aspects of clinical work varies according to whether respondents use apps themselves, and, to a lesser extent, according to their clinical setting and job role.
Conclusions
Use of smartphone apps is widespread among HCPs participating in this large European survey and caring for people with epilepsy, multiple sclerosis and depression. The majority of respondents indicate that they treat patients who use wearables and other devices for health-related purposes and that data from these devices has an impact on clinical practice.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12911-021-01640-5.
Keywords: Smartphone apps, Healthcare professionals, Depression, Multiple sclerosis, Epilepsy, Remote measurement technology, Survey
Introduction
The easy availability and reduced cost of mobile and wearable technologies is enabling individuals to record more data about their physical and mental health than ever before. Digital health smartphone apps (software installed and run on smartphones) are known to be used for a variety of healthcare-related purposes by both patients and clinicians. For example, a multi-national online survey found that diabetes apps are used by over half of those with type 1 and one third of those with type 2 diabetes [1]. In a recent US study, just under half of survey respondents living with hypertension reported using apps to help manage it [2]. In relation to central nervous system disorders, their use has been explored in the management of depressive and bipolar disorders for mood monitoring and contextual prompting (‘THINC-IT’ [3], ‘True Colours’ [4], ‘Wear-IT’ [5]), and systematic reviews of depression apps in 2015 and 2020 identified digitized therapeutic intervention, symptom tracking and psychoeducation as main functions of apps available for this market [6, 7]. In epilepsy, apps are available for a range of purposes, including to enter details of seizures to create a record (e.g. ‘Seizure Tracker’) and to notify an emergency contact if a person living with epilepsy does not respond to a daily prompt (‘Snug Safety’ app). A systematic review of the Apple app store for epilepsy or seizure-related apps also identified education and seizure trigger recording as functions of the available apps [8]. Smartphone apps for multiple sclerosis include functions such as medication reminders and symptom trackers, in addition to assessment of function through games and questionnaires [9].
There are also a wide variety of apps available which are targeted at health care professionals (HCPs) [10], including apps providing clinical guidelines [11], apps with clinical task specific calculators [12], prescribing apps [13], and apps providing the ability for HCPs to communicate with each other and/or with patients and to inform decision-making. Both digital health apps aimed at patients and apps aimed at HCPs can be subject to regulation, depending on their intended purpose, as they may be designated ‘software as a medical device’ [14].
A large variety of off-the-shelf wearable sensing devices are commercially available, aimed at the public for the purpose of monitoring health and wellbeing. These include electronic wristbands, pedometers and devices designed for specific conditions such as epilepsy seizure detection wristbands. Many of these interface with smartphone apps to provide greater functionality. Wearable sensing devices allow their wearers to monitor many aspects of their daily lives, including steps taken, heart rate, sleep quality and duration, and exercise. As such, their use is generally for the ‘passive’ collection of data, without the user’s active input. Recently, their use as tools for monitoring patients with central nervous system disorders has garnered interest [15]. Prior work has shown that connected digital products are used increasingly in clinical trials [16], demonstrating their value as tools to accurately record aspects of patient functioning. Patients themselves have a variety of views on the use of apps and wearables for monitoring specific conditions, including both benefits and challenges [17, 18].
When wearable devices and smartphone apps are used to collect data on human behaviour and physical parameters, these information-gathering tools are collectively described as remote measurement technology (RMT). The RADAR-CNS project, funded by Horizon 2020 and the Innovative Medicines Initiative, explores the potential of RMT in the clinical management of epilepsy, MS and depression. The consortium consists of a team of researchers, clinicians and a patient advisory board of individuals with lived experience of epilepsy, multiple sclerosis (MS), or depression (www.radar-cns.org). Project outputs to date have provided insight into the potential benefits of incorporating RMT in clinical pathways, via qualitative surveys of patients and healthcare professionals within the consortium [19] and interviews with HCPs outside the consortium [20]. In these small studies, HCPs and patients envisaged multiple ways that RMT could permit both ‘active’ (direct user input of data, such as mood diaries) and ‘passive’ (collection of data without the requirement of the user to input data, e.g. step count, GPS location) collection of data to remotely assess patients with these conditions.
This paper adds to this body of work by exploring the experiences of a much larger number of HCPs working with patients with epilepsy, MS, or depression outside the consortium. In studies relating to health care technology, exploring views from a wide range of health care professionals working across different job roles has the benefit of reducing bias in the self-selection of research participants with a particular interest in or positive disposition toward health care technology [21]. As such, the present research study aimed to elicit opinions and experiences from a broad sample of HCPs working in the care of patients with epilepsy, MS or depression.
The Covid-19 pandemic has seen the typical organisation of healthcare in England change to increase remote consultations in both primary [22] and secondary care [23] to reduce the risk of infection between patients and staff. It is therefore timely to explore opinions and experiences of HCPs on the use of smartphone apps and RMT.
Methods
Surveys are often used to elicit data from large populations and have been used to capture the views of clinicians [24, 25]. Since they require a short amount of time to administer and provide the opportunity to collect both quantitative and qualitative data, a survey was considered to be the most appropriate tool to learn more about HCP experiences, where these individuals have busy schedules and value the ability to complete these at a time of their choosing.
We conducted a large-scale, online survey, administered using the JISC Online Surveys platform [26]. Informed consent was gathered through an information sheet and consent form at the beginning of the survey form. Ethical approval for this study was granted by the University of Nottingham research ethics committee (ref 277-1802) and by the UK Health Research Authority (ref 19/HRA/5041). Inclusion criteria were that respondents should be currently working in the care of people with epilepsy, MS or depression, within adult services.
Our main objectives in the present work were: (i) to determine the extent of smartphone app usage among health care professionals; (ii) to determine the extent of HCP experience of their patients’ use of RMT (including smartphone apps and wearables); and (iii) to understand the impact of patient RMT usage on HCPs’ work, including how this may vary by demographic factors.
There were nine pages of questions in the survey (see Additional file 1), in two broad sections. Textbox 1 shows a breakdown of sections of the survey. Survey questions were designed by the research team to reflect the goals of the research programme. Questions 1–7 concerned participant consent. Questions 8–16 were developed through consultation with expert clinicians from each of the three specialties, together with consideration of existing literature, in order to measure the value of various parameters for target conditions. Questions 17–26 were developed with consideration of potential scenarios of use between patients and clinicians and considering key challenges highlighted by clinical and HCI researchers. Early questions prompted respondents to think about their existing use of apps in their work role before continuing with more focused aspects relevant to making RMT work in clinical practice. The survey was designed pragmatically to elicit data whilst ensuring an accessible survey to encourage participation by busy healthcare professionals.
The survey respondents took a mean of 12 minutes to complete the survey, and were required to do so in a single sitting. In addition to collecting demographic information (questions 3–7), the focus of the first section of the survey was on current and past experience of HCPs’ use of smartphone apps and their patients’ use of RMT (smartphone apps and wearables). The second section had a different focus, exploring the future potential of RMT and the requirements for its future implementation. In this paper, we present results relating only to HCPs’ current/past experience of using smartphone apps and the impact of their patients’ use of RMT on their clinical role.
| Textbox 1. Survey overview |
|---|
| SECTION 1: |
| Page 1: Participant Information and Consent |
| Page 2: Demographics/Healthcare Professional Role |
| Page 3: Current use of digital services and devices in your role as a Health Care Professional |
| SECTION 2: |
| Page 4: Your thoughts about using digital devices for long term monitoring of medical conditions |
| Page 5: Value of RMT |
| Page 6: Accessing and using the data |
| Page 7: Technical Support requirements |
| Page 8: Closing remarks |
Demographic questions in the survey consisted of respondent age group; specialism; job role; clinical setting; and country of employment. Job role was collected by free text box and during data analysis, participant-provided job roles were sorted into the seven categories reported here. Ambiguous terms were categorised as ‘not specified’.
Question 8 required respondents to indicate whether they had used any kind of smartphone apps in their daily clinical practice (and if so, what genre of app). Question 9 asked respondents to indicate whether they had experienced their patients using different types of RMT devices (smartphone apps, wearable sensing devices or other devices) to improve or increase awareness of their health across six categories of purpose. Question 10 invited respondents to evaluate to what extent the data from patient’s RMT devices had an impact on the respondent’s clinical practice in five areas, each scored on a five-point Likert-style scale. The survey instrument is provided in Additional file 1.
The survey was disseminated via multiple routes:
The UK National Institute for Health Research (NIHR) Clinical Research Network
A targeted social media campaign across multiple European countries
Through the stakeholder group of the Innovative Medicines Institute (IMI)
Snowballed through professional contacts of the RADAR-CNS consortium members working in five European countries.
The survey was translated from English into five European languages spoken in the countries where the research consortium for the project operated. The first response was received on the 4th February 2019 and the last was received on the 30th March 2020.
Statistical analysis
Data were analysed using SPSS v.26 and Stata SE 16.1. We used descriptive statistics to understand the rates of responses from different demographic groups. Chi-squared tests were used to compare the rate of reported app usage (question 8) between different job roles and age groups, as well as to compare reported use of apps and devices by respondents’ patients. Responses to items in question 10, on the impact of patient RMT data on five aspects of clinical work, were dichotomised as 1 for responses of ‘definitely’ or ‘sometimes’, and 0 for responses of ‘unsure’, ‘hardly’, and ‘never’. ‘Unsure’ was grouped with the negative polarity items since respondents may have responded in this way if they did not have experience of using these technologies. Dichotomising the data rather than treating it as a 5-point scale had the benefit of resolving this ambiguity. For question 10 on the impact of RMT data on respondents’ clinical practice, univariate, then multivariate logistic regression was used to explore the effect of demographic variables and app usage on views of the impact of RMT data. A p-value of 0.05 was considered significant. For each categorical variable, the reference category chosen was the category with the largest number of responses. Missing responses were accounted for using multiple imputation, also conducted in STATA. 83 imputations were calculated for each missing entry, as the highest fraction of missing information (FMI) found in the analysis was 0.83 [27]. A sensitivity analysis was used to examine the influence of missingness on parameter estimates by comparing the consistency of parameters estimates between models with imputed and observed datasets.
Results
Recruitment
Survey responses were received from 1009 HCPs. The greatest proportion of these were completed as a result of efforts to recruit via the UK National Institute for Health Research (NIHR) Clinical Research Network: 21 National Health Service (NHS) healthcare trusts and Clinical Commissioning Groups (CCGs) across the UK advertised the survey to healthcare staff working with patients with epilepsy, MS or depression in their area. These trusts and CCGs reported that 650 staff had notified them that they had completed the survey, however it is likely a greater proportion of the total survey responses were obtained via this recruitment method, as it was not compulsory for respondents to notify their trust of their involvement. We did not include a survey item to ask where respondents had heard about the survey to preserve anonymity, so it is not possible to further attribute respondents to different recruitment methods.
Despite the survey being available in 5 European languages in addition to English, and efforts made to disseminate the survey to clinicians working in other countries (via social media, LinkedIn groups, contacts of the consortium, and via the Innovative Medicines Initiative steering group), the number of respondents reporting to be working in countries outside the UK was small (n = 30, 3% of total). These were analysed together with the UK responses as there were too few from each individual country to viably compare them statistically against the UK responses.
Data cleaning
Of the 1009 completed surveys, 1006 were retained for analysis. Reasons for exclusion were insincere completion of the survey (n = 1) and improper completion of the consent form (n = 2). Figure 1 shows a STROBE flow diagram.
Fig. 1.
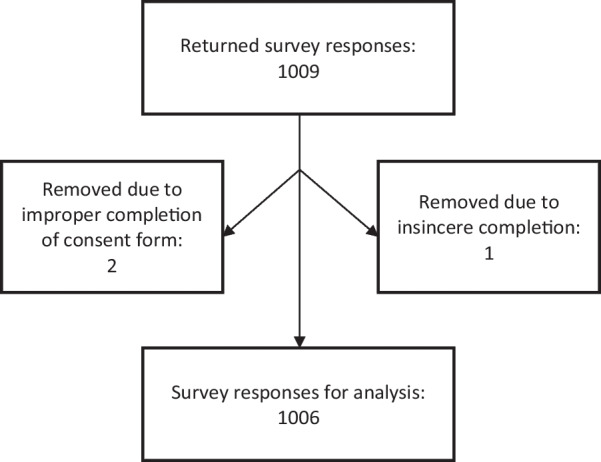
STROBE flow chart showing included responses in the dataset
Demographics
Table 1 shows a breakdown of the included survey responses by demographics. Age group categories followed a normal distribution, with the largest number of respondents in the middle category (ages 41–50, n = 293, 29% of respondents) and progressively fewer in older and younger age groups. The most frequently selected item under specialism was ‘mental health’ (n = 587, 38%), with the fewest selecting ‘social care’ (n = 32, 2%). Although relatively few respondents reported a specialism of ‘multiple sclerosis’ (n = 55, 4%) or ‘epilepsy’ (n = 73, 5%), a larger number reported working in neurology (n = 112, 7%), which is a specialism covering both these conditions. Among job roles, ‘nursing’ was the category with the largest proportion of responses. Doctors would have been the second most numerous category, however doctors were split into two categories of ‘GPs’ (n = 118, 12%) and ‘Doctor (excluding GP)’, (n = 138, 14%). ‘Clinical psychology professionals’ were therefore the second most numerous category, with 157 responses (16%). There was good representation from all clinical settings, with fewest (5%) from ‘specialist tertiary care’ settings, which might be expected due to their specialised nature. Most responses (97%) were from HCPs working in the UK. The second most reported country was Portugal, with 21 survey completions (2%).
Table 1.
Demographics of respondents to the survey
| Category | n | % |
|---|---|---|
| Age | ||
| 18–30 | 161 | 16.0 |
| 31–40 | 248 | 24.7 |
| 41–50 | 293 | 29.1 |
| 51–60 | 247 | 24.6 |
| 60+ | 52 | 5.2 |
| No response | 5 | |
| Specialism | ||
| Neurology | 112 | 11.1 |
| Mood disorders | 121 | 12.0 |
| Mental health | 587 | 58.3 |
| Epilepsy | 73 | 7.3 |
| Multiple sclerosis | 55 | 5.5 |
| Depression | 165 | 16.4 |
| General practice | 152 | 15.1 |
| Psychology | 126 | 12.5 |
| Social care | 32 | 3.2 |
| Other | 119 | 11.8 |
| Job role | ||
| Allied health professionals | 112 | 11.1 |
| Doctor (excl GP) | 138 | 13.7 |
| GP | 118 | 11.7 |
| Research/healthcare science | 24 | 2.4 |
| Management | 40 | 4.0 |
| Nursing | 268 | 26.6 |
| Pharmacy | 15 | 1.5 |
| Psychological professions | 157 | 15.6 |
| Student | 10 | 1.0 |
| Wider healthcare team | 76 | 7.6 |
| Not clear | 48 | 4.8 |
| Clinical setting | ||
| Primary care/general practice | 193 | 19.2 |
| Secondary care—hospital trust, inpatients | 91 | 9.0 |
| Secondary care—hospital trust, outpatients | 82 | 8.2 |
| Secondary care—mental health trust, inpatients | 127 | 12.6 |
| Secondary care—mental health trust, outpatients | 254 | 25.2 |
| Specialist tertiary care centre | 54 | 5.3 |
| Community care | 173 | 17.2 |
| Other | 32 | 3.2 |
| Country worked in | ||
| United Kingdom | 974 | 96.8 |
| Portugal | 21 | 2.1 |
| Belgium | 2 | 0.1 |
| Italy | 2 | 0.1 |
| Germany | 1 | 0.1 |
| Ireland | 1 | 0.1 |
| Israel | 1 | 0.1 |
| Mexico | 1 | 0.1 |
| Switzerland | 1 | 0.1 |
| No response | 2 | |
Use of smartphone apps by clinicians
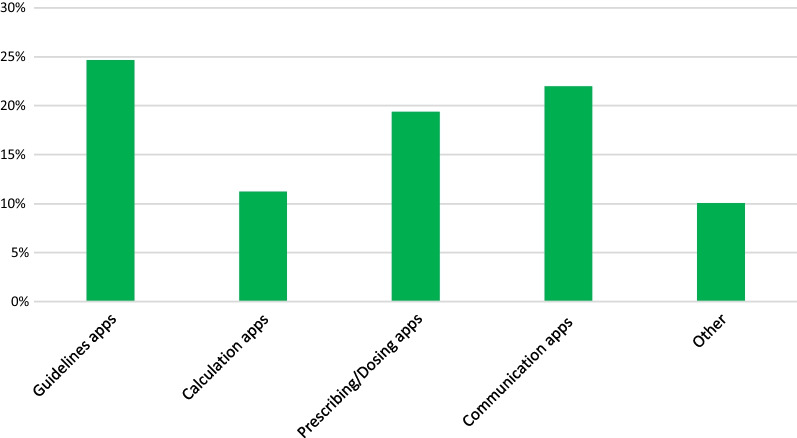
559 respondents (56%) indicated that they use digital health smartphone apps for at least one purpose in their clinical role. Figure 2 shows the percentages of respondents who reported using smartphone apps of each of the five types listed in this question. Guidelines apps were the most used (25%), while calculation apps were least used (11%). There was a spread of responses across all app types, with no app type receiving fewer than 10% of total responses.
Fig. 2.
Percentage of survey respondents who report using smartphone apps of each of these types on a daily basis in their professional role. Data were collected in survey question 8: ‘Are there any apps that you currently use on a daily basis in your clinical practice?'
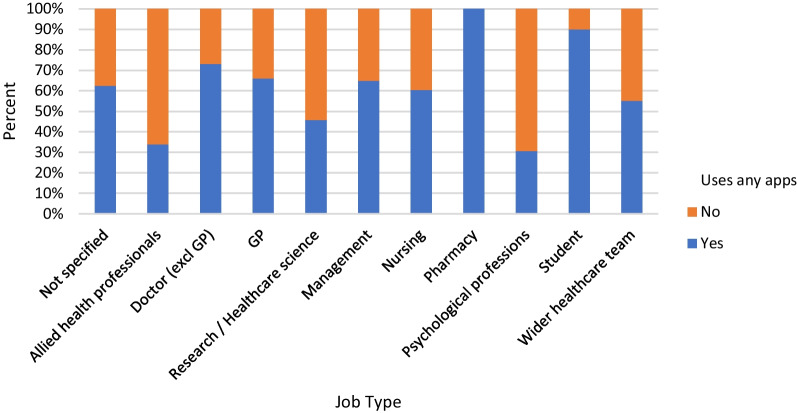
Figure 3 shows the percentage of respondents in each job category who reported using smartphone apps in their daily clinical practice. A chi-squared test showed that there were significant differences between job roles for this question (χ2 = 106.348, p < 0.01). Pharmacists and medical students were most likely to report using any apps, although there were fewer survey completions overall from these job roles. Further analysis revealed that of the different types of app listed, pharmacists were most likely to report using prescribing/dosing apps (87% of pharmacist respondents), and medical students were most likely to use guidelines apps (80% of student respondents). 40% of GP respondents and 38% of doctors excluding GPs reported using guidelines apps. Clinical psychology professionals and allied health professionals were least likely to report using smartphone apps of any kind.
Fig. 3.
Percentage of respondents in each job category who report using smartphone apps on a daily basis in their clinical practice
Question 8a invited respondents to provide examples of other types of app that they used in their daily clinical practice. Table 2 provides a random subsample of the responses, with information on the publisher, category, cost and target condition of these, where relevant. Responses covered apps published by the NHS, charities, commercial organisations, and public bodies. Apps mentioned included a large number targeting mental health, with fewer focussed on neurological conditions, which could be expected given the larger proportion of responses from mental health specialists in the sample. Many of these apps are either patient-facing or have a focus on wellbeing and it is possible that respondents understood this question to mean apps they recommend to their patients, rather than apps they use themselves to facilitate their job role.
Table 2.
A sample of names and types of smartphone apps reported by respondents who selected ‘other’ in response to a question on types of apps used in their daily clinical practice
| App name/genre (as reported) | Category | Target condition (MH/neurology/general) | Publisher | Cost |
|---|---|---|---|---|
| Survey apps | Communication apps | General | N/A | N/A |
| 3d brain app | Other | Neurology | DNA Learning Centre | Free |
| British National Formulary | Prescribing/dosing app | General | Indextra AB | $69.99 |
| Consultant-connect | Communication | General | Consultant Connect | Requires subscription by service |
| Headspace | Consumer | Wellbeing | Headspace (company) | Free, in-app subscription for more content |
| Calm | Consumer | Wellbeing | Calm (company) | Free, in-app subscription for more content |
| Medscape | Other | General | WebMD | Free, contains ads |
| Mindfulness apps | Consumer | Wellbeing | N/A | N/A |
| Observations apps | Other | General | N/A | N/A |
| distrACT | Patient-facing | Mental health | Expert Self Care Ltd | Free |
| Rise Up | Patient-facing | Mental health | Recovery warriors | Free |
| Rio | Other | General | Servelec | Requires subscription by service |
| Silvercloud | Communication/patient-facing | Mental health | SilverCloud Health | Free, requires referral |
| Stay Alive | Patient-facing | Mental health | Grassroots Suicide Prevention | Free |
| Symptom tracker | Patient-facing | General | N/A | N/A |
| Wellmind | Patient-facing | Mental health | Blue Step Solutions | Free |
| Young epilepsy | Patient-facing | Neurology | Young epilepsy charity | No longer available |
Across all age categories, between 50 and 60 percent of respondents reported using apps. The highest percentage was reported by those in the over-60 category. However, a chi-squared test on these age groupings was non-significant (χ2 = 1.848, p = 0.76).
Patient use of RMT for health-related purposes
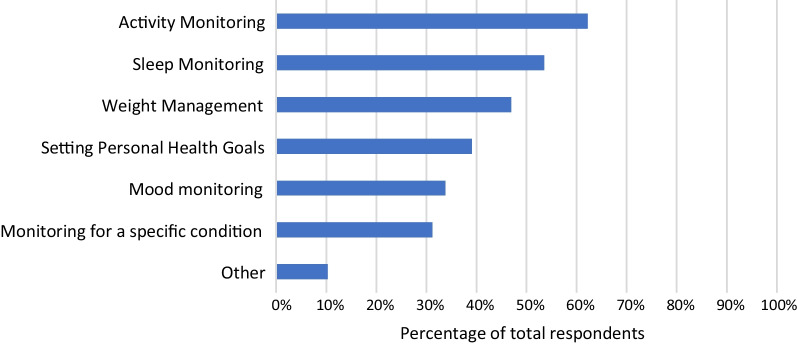
78% of respondents indicated that their patients used RMT (one or more of smartphone apps, wearables, or other type of device) to improve or increase awareness of their health. Figure 4 shows the relevant percentages for different purposes. Activity monitoring was selected by the largest proportion of respondents (62%). Sleep monitoring was also selected by more than half of respondents (53%). Of the given categories, monitoring for a specific condition was least selected (31%).
Fig. 4.
Graph showing percentages of respondents who reported that their patients use smartphone apps, wearable sensing devices or other devices for different purposes
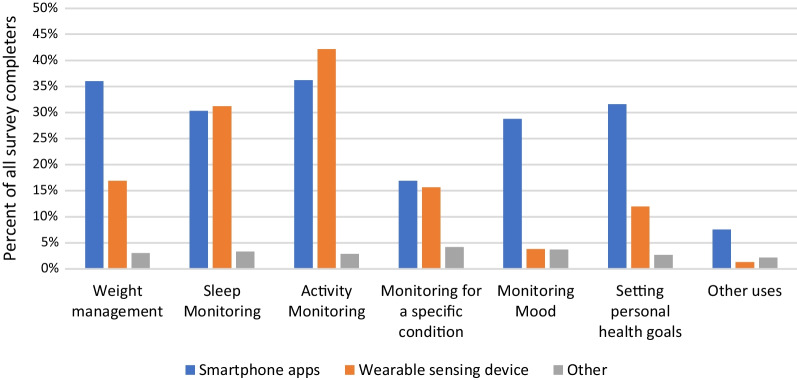
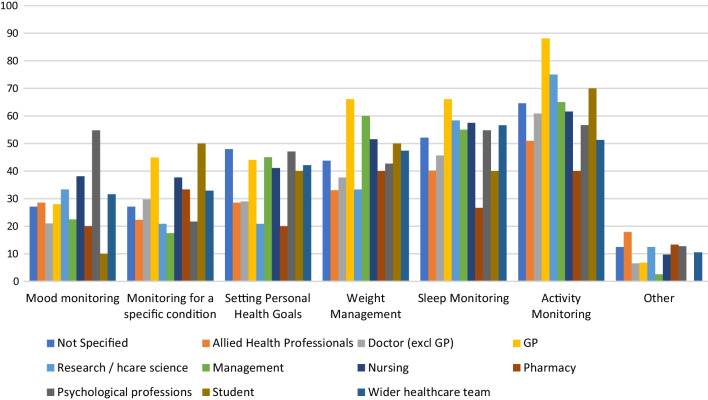
Figure 5 shows the split of these responses across different device types. Further to the above, this shows that wearable sensing devices are most commonly reported to be used for passive monitoring of activity and sleep, while smartphone apps were used more often than wearable devices for monitoring mood, setting personal health goals and weight management.
Fig. 5.
Percentages of survey respondents indicating that their patients use devices of these types for different purposes
A sub-group analysis by job role showed that there were significant differences between job roles for this question (χ2 = 154.952, p < 0.01). This is shown in Fig. 6. A greater proportion of GPs than other types of health professional had experienced their patients using devices to monitor activity (88% of GP respondents), monitor sleep (66%) and manage weight (66%).
Fig. 6.
Percentage of each job type indicating they had experience of their patients using RMT for each of these purposes
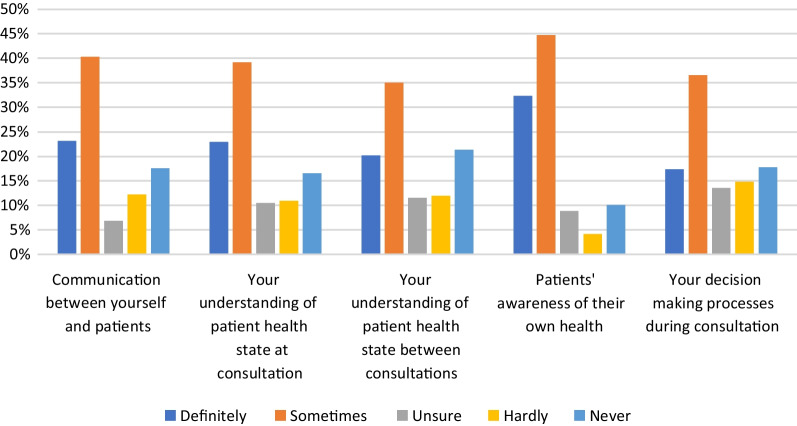
Impact of RMT data on respondents’ clinical work
Sub-questions 10.1–10.5 asked respondents about the impact of the data from patients’ RMT devices on different aspects of respondents’ clinical work. Figure 7 shows these results. Across all sub-questions, the mean percentage of respondents indicating that the data from RMT ‘definitely’ or ‘sometimes’ had an impact on their work was 62%. The mean percentage of those indicating that it ‘hardly’ or ‘never’ had an impact was 27%. A greater proportion of respondents selected ‘definitely’ or ‘sometimes’ for sub-question 10.4 (Does RMT data impact patients’ awareness of their own health?) than for any of the other sub-questions.
Fig. 7.
Percentages of item respondents for question items 10.1–10.6 indicating whether RMT data has an impact on five different aspects of their work
Results from univariate and multivariate logistic regression analyses with multiple imputation and using questions 3–8 as independent variables and sub-questions 10.1–10.5 as dependent variables are provided in Tables 3, 4, 5, 6 and 7. An analysis for multicollinearity revealed no variance inflation factor (VIF) greater than 3.5, suggesting that multicollinearity did not affect the results. Results from multiple imputation and observed datasets were consistent in term of the parameter estimate and its precision.
Table 3.
Question 10.1—Does data from patients' RMT impact your communication with patients?
| Question content/variable/category | Multivariate model | Univariate model |
|---|---|---|
| Impact of data on communication with patient | OR (95% CI), p-value | OR (95% CI), p-value |
| Q3—age | ||
| 18–30 years | 0.694 (0.432, 1.115), 0.1310 | 0.753 (0.494, 1.150), 0.1900 |
| 31–40 years | 1.050 (0.697, 1.582), 0.8140 | 1.045 (0.705, 1.549), 0.8280 |
| 41–50 years | Reference category | Reference category |
| 51–60 years | 1.167 (0.755, 1.802), 0.4870 | 1.136 (0.746, 1.729), 0.5510 |
| Over 60 years | 1.121 (0.538, 2.336), 0.7600 | 1.198 (0.596, 2.405), 0.6120 |
| Q4—specialism | ||
| Mental health | Reference category | Reference category |
| Neurology | 0.999 (0.643, 1.553), 0.9980 | 1.031 (0.675, 1.574), 0.8880 |
| Not specified | 1.152 (0.789, 1.682), 0.4630 | 1.163 (0.810, 1.670), 0.4140 |
| Q5—job role | ||
| Doctor excluding GP | Reference category | Reference category |
| Allied health professional | 1.356 (0.712, 2.582), 0.3540 | 0.909 (0.510, 1.621), 0.7470 |
| Not specified | 1.269 (0.606, 2.657), 0.5280 | 1.065 (0.517, 2.194), 0.8640 |
| GP | 1.453 (0.624, 3.382), 0.3860 | 2.006 (1.125, 3.577), 0.0180 |
| Research/healthcare scientist | 0.592 (0.214, 1.638), 0.3130 | 0.475 (0.182, 1.240), 0.1280 |
| Management | 0.730 (0.322, 1.658), 0.4520 | 0.685 (0.314, 1.495), 0.3420 |
| Nursing | 1.289 (0.776, 2.141), 0.3270 | 1.055 (0.664, 1.676), 0.8220 |
| Pharmacy | 1.364 (0.286, 6.494), 0.6960 | 1.598 (0.356, 7.184), 0.5400 |
| Clinical psychology professional | 1.272 (0.718, 2.256), 0.4100 | 0.910 (0.548, 1.512), 0.7170 |
| Student | 2.822 (0.427, 18.635), 0.2810 | 1.996 (0.330, 12.062), 0.4510 |
| Wider healthcare team | 1.166 (0.571, 2.380), 0.6730 | 0.956 (0.496, 1.843), 0.8930 |
| Q6—setting | ||
| Primary care | Reference category | Reference category |
| Secondary Care—hospital inpatient | 0.688 (0.322, 1.470), 0.3350 | 0.584 (0.317, 1.073), 0.0830 |
| Secondary care—hospital outpatient | 0.750 (0.338, 1.666), 0.4800 | 0.621 (0.340, 1.133), 0.1210 |
| Secondary care—MH inpatient | 0.846 (0.411, 1.742), 0.6500 | 0.694 (0.407, 1.183), 0.1790 |
| Secondary care—MH outpatient | 0.588 (0.323, 1.069), 0.0820 | 0.460 (0.299, 0.706), 0.0000 |
| Specialist tertiary care | 1.013 (0.408, 2.515), 0.9780 | 0.747 (0.363, 1.540), 0.4290 |
| Community care | 0.462 (0.237, 0.900), 0.0230 | 0.414 (0.256, 0.668), 0.0000 |
| Other | 0.505 (0.187, 1.361), 0.1760 | 0.400 (0.172, 0.933), 0.0340 |
| Q8—Uses apps in daily clinical practice | ||
| No | Reference category | Reference category |
| Yes | 1.697 (1.202, 2.398), 0.0030 | 1.727 (1.262, 2.365), 0.0010 |
Results from logistic regression with multiple imputation. Multiple imputation was used to impute data for this question item for 301 missing entries out of total 1006. Bold font indicates significant result
Table 4.
Question 10.2—Does data from patients' RMT impact your understanding of patient health state at consultation?
| Question content/variable/category | Multivariate model | Univariate model |
|---|---|---|
| Impact of data on understanding of patient health state at consultations | OR (95% CI), p | OR (95% CI), p |
| Q3—age | ||
| 18–30 years | 0.721 (0.435, 1.196), 0.2050 | 0.757 (0.480, 1.193), 0.2290 |
| 31–40 years | 0.857 (0.557, 1.321), 0.4850 | 0.814 (0.542, 1.225), 0.3240 |
| 41–50 years | Reference category | Reference category |
| 51–60 years | 1.236 (0.827, 1.847), 0.3020 | 1.174 (0.799, 1.725), 0.4150 |
| Over 60 years | 1.285 (0.596, 2.772), 0.5220 | 1.307 (0.639, 2.672), 0.4620 |
| Q4—specialism | ||
| Mental health | Reference category | Reference category |
| Neurology | 0.720 (0.467, 1.108), 0.1350 | 0.756 (0.503, 1.137), 0.1790 |
| Not specified | 0.983 (0.670, 1.442), 0.9300 | 1.006 (0.700, 1.446), 0.9750 |
| Q5—job role | ||
| Doctor excluding GP | Reference category | Reference category |
| Allied health professional | 0.898 (0.490, 1.647), 0.7280 | 0.599 (0.343, 1.048), 0.0730 |
| Not specified | 1.160 (0.532, 2.526), 0.7090 | 0.958 (0.449, 2.045), 0.9130 |
| GP | 1.051 (0.459, 2.409), 0.9060 | 1.453 (0.820, 2.574), 0.2000 |
| Research/healthcare scientist | 0.460 (0.161, 1.314), 0.1470 | 0.340 (0.124, 0.933), 0.0360 |
| Management | 0.624 (0.258, 1.506), 0.2940 | 0.598 (0.260, 1.378), 0.2270 |
| Nursing | 1.282 (0.770, 2.133), 0.3390 | 1.071 (0.669, 1.715), 0.7740 |
| Pharmacy | 1.057 (0.245, 4.565), 0.9410 | 1.333 (0.327, 5.428), 0.6880 |
| Clinical psychology professional | 0.856 (0.471, 1.556), 0.6100 | 0.637 (0.376, 1.078), 0.0930 |
| Student | 2.520 (0.343, 18.501), 0.3630 | 1.867 (0.277, 12.579), 0.5210 |
| Wider healthcare team | 0.950 (0.477, 1.891), 0.8840 | 0.778 (0.413, 1.466), 0.4370 |
| Q6—setting | ||
| Primary care | Reference category | Reference category |
| Secondary care—hospital inpatient | 0.763 (0.347, 1.676), 0.5000 | 0.732 (0.397, 1.351), 0.3190 |
| Secondary care—hospital outpatient | 0.680 (0.321, 1.438), 0.3120 | 0.658 (0.375, 1.157), 0.1460 |
| Secondary care—MH inpatient | 0.754 (0.371, 1.535), 0.4360 | 0.719 (0.431, 1.201), 0.2070 |
| Secondary care—MH outpatient | 0.656 (0.356, 1.209), 0.1760 | 0.558 (0.364, 0.856), 0.0070 |
| Specialist tertiary care | 0.858 (0.367, 2.005), 0.7240 | 0.752 (0.373, 1.514), 0.4240 |
| Community care | 0.471 (0.241, 0.920), 0.0280 | 0.464 (0.288, 0.745), 0.0020 |
| Other | 0.419 (0.153, 1.149), 0.0910 | 0.395 (0.166, 0.941), 0.0360 |
| Q8—uses apps in daily clinical practice | ||
| No | Reference category | Reference category |
| Yes | 1.886 (1.341, 2.654), 0.0000 | 2.010 (1.469, 2.749), 0.0000 |
Results from logistic regression with multiple imputation. Multiple imputation was used to impute data for this question item for 301 missing entries out of total 1006. Bold font indicates significant result
Table 5.
Question 10.3—Does data from patients' RMT impact your understanding of patient health state between consultations?
| Question content/variable/category | Multivariate model | Univariate model |
|---|---|---|
| Impact of data on understanding of patient health state between consultations | OR (95% CI), p | OR (95% CI), p |
| Q3—age | ||
| 18–30 years | 1.088 (0.647, 1.830), 0.7490 | 1.097 (0.680, 1.770), 0.7040 |
| 31–40 years | 1.244 (0.821, 1.884), 0.3030 | 1.233 (0.827, 1.838), 0.3030 |
| 41–50 years | Reference category | Reference category |
| 51–60 years | 1.116 (0.766, 1.627), 0.5670 | 1.101 (0.766, 1.584), 0.6030 |
| Over 60 years | 1.433 (0.675, 3.038), 0.3480 | 1.553 (0.764, 3.158), 0.2240 |
| Q4—specialism | ||
| Mental health | Reference category | Reference category |
| Neurology | 1.077 (0.707, 1.641), 0.7280 | 1.105 (0.740, 1.651), 0.6240 |
| Not specified | 1.421 (0.973, 2.074), 0.0690 | 1.411 (0.979, 2.035), 0.0650 |
| Q5—job role | ||
| Doctor excluding GP | Reference category | Reference category |
| Allied health professional | 1.063 (0.561, 2.016), 0.8510 | 0.766 (0.427, 1.376), 0.3720 |
| Not specified | 1.535 (0.734, 3.207), 0.2550 | 1.267 (0.620, 2.591), 0.5160 |
| GP | 1.775 (0.813, 3.875), 0.1490 | 1.786 (1.041, 3.064), 0.0350 |
| Research/healthcare scientist | 0.912 (0.340, 2.448), 0.8560 | 0.805 (0.315, 2.057), 0.6510 |
| Management | 0.725 (0.305, 1.724), 0.4660 | 0.641 (0.278, 1.475), 0.2950 |
| Nursing | 1.358 (0.827, 2.231), 0.2270 | 1.122 (0.710, 1.771), 0.6220 |
| Pharmacy | 1.633 (0.380, 7.027), 0.5090 | 1.755 (0.431, 7.140), 0.4320 |
| Clinical psychology professional | 1.374 (0.762, 2.478), 0.2900 | 1.012 (0.605, 1.693), 0.9640 |
| Student | 1.235 (0.251, 6.070), 0.7950 | 1.285 (0.278, 5.946), 0.7480 |
| Wider healthcare team | 1.395 (0.717, 2.716), 0.3270 | 1.138 (0.616, 2.105), 0.6790 |
| Q6—setting | ||
| Primary care | Reference category | Reference category |
| Secondary care—hospital inpatient | 1.037 (0.495, 2.173), 0.9240 | 0.788 (0.441, 1.408), 0.4220 |
| Secondary care—hospital outpatient | 0.891 (0.422, 1.880), 0.7610 | 0.678 (0.384, 1.199), 0.1820 |
| Secondary care—MH inpatient | 0.954 (0.482, 1.885), 0.8910 | 0.767 (0.461, 1.277), 0.3080 |
| Secondary care—MH outpatient | 0.860 (0.478, 1.547), 0.6150 | 0.613 (0.401, 0.937), 0.0240 |
| Specialist tertiary care | 1.424 (0.626, 3.237), 0.3990 | 0.941 (0.483, 1.833), 0.8570 |
| Community care | 0.725 (0.385, 1.366), 0.3190 | 0.551 (0.348, 0.871), 0.0110 |
| Other | 0.514 (0.175, 1.511), 0.2260 | 0.370 (0.144, 0.951), 0.0390 |
| Q8—uses apps in daily clinical practice | ||
| No | Reference category | Reference category |
| Yes | 1.760 (1.265, 2.449), 0.0010 | 1.781 (1.311, 2.418), 0.0000 |
Results from logistic regression with multiple imputation. Multiple imputation was used to impute data for this question item for 303 missing entries out of total 1006. Bold font indicates significant result
Table 6.
Question 10.4—Does data from patients' RMT impact patients' awareness of their own health?
| Question content/variable/category | Multivariate model | Univariate model |
|---|---|---|
| Impact of data on patients' awareness of their own health | OR (95% CI), p | OR (95% CI), p |
| Q3—age | ||
| 18–30 years | 0.861 (0.502, 1.476), 0.5850 | 0.914 (0.569, 1.467), 0.7080 |
| 31–40 years | 1.237 (0.790, 1.937), 0.3530 | 1.204 (0.790, 1.837), 0.3880 |
| 41–50 years | Reference category | Reference category |
| 51–60 years | 1.147 (0.752, 1.750), 0.5250 | 1.113 (0.745, 1.660), 0.6010 |
| Over 60 years | 1.423 (0.591, 3.426), 0.4310 | 1.450 (0.645, 3.262), 0.3680 |
| Q4—specialism | ||
| Mental health | Reference category | Reference category |
| Neurology | 0.805 (0.500, 1.297), 0.3720 | 0.831 (0.531, 1.298), 0.4150 |
| Not specified | 0.910 (0.609, 1.360), 0.6460 | 0.958 (0.656, 1.398), 0.8240 |
| Q5—job role | ||
| Doctor excluding GP | Reference category | Reference category |
| Allied health professional | 1.102 (0.566, 2.143), 0.7750 | 0.814 (0.448, 1.476), 0.4970 |
| Not specified | 0.955 (0.432, 2.112), 0.9090 | 0.835 (0.391, 1.783), 0.6410 |
| GP | 0.834 (0.297, 2.345), 0.7310 | 2.501 (1.280, 4.887), 0.0070 |
| Research/healthcare scientist | 0.859 (0.306, 2.408), 0.7720 | 0.688 (0.257, 1.840), 0.4560 |
| Management | 0.645 (0.279, 1.489), 0.3040 | 0.587 (0.262, 1.312), 0.1940 |
| Nursing | 1.089 (0.640, 1.854), 0.7520 | 0.994 (0.610, 1.620), 0.9820 |
| Pharmacy | 0.429 (0.108, 1.700), 0.2280 | 0.457 (0.125, 1.665), 0.2350 |
| Clinical psychology professional | 1.744 (0.884, 3.440), 0.1090 | 1.558 (0.838, 2.899), 0.1610 |
| Student | 1.507 (0.272, 8.346), 0.6380 | 1.023 (0.197, 5.308), 0.9780 |
| Wider healthcare team | 0.771 (0.378, 1.574), 0.4740 | 0.649 (0.339, 1.242), 0.1920 |
| Q6—setting | ||
| Primary care | Reference category | Reference category |
| Secondary care—hospital inpatient | 0.252 (0.101, 0.627), 0.0030 | 0.251 (0.130, 0.486), 0.0000 |
| Secondary care—hospital outpatient | 0.403 (0.153, 1.063), 0.0660 | 0.422 (0.207, 0.859), 0.0170 |
| Secondary care—MH inpatient | 0.272 (0.118, 0.631), 0.0020 | 0.261 (0.146, 0.465), 0.0000 |
| Secondary Care—MH outpatient | 0.291 (0.130, 0.651), 0.0030 | 0.309 (0.184, 0.517), 0.0000 |
| Specialist tertiary care | 0.414 (0.140, 1.217), 0.1090 | 0.437 (0.191, 1.002), 0.0500 |
| Community care | 0.240 (0.103, 0.559), 0.0010 | 0.251 (0.142, 0.442), 0.0000 |
| Other | 0.222 (0.069, 0.709), 0.0110 | 0.210 (0.084, 0.525), 0.0010 |
| Q8—uses apps in daily clinical practice | ||
| No | Reference category | Reference category |
| Yes | 1.678 (1.178, 2.390), 0.0040 | 1.496 (1.091, 2.051), 0.0120 |
Results from logistic regression with multiple imputation. Multiple imputation was used to impute data for this question item for 282 missing entries out of total 1006. Bold font indicates significant result
Table 7.
Question 10.5—Does data from patients' RMT impact your decision-making processes during consultation?
| Question content/variable/category | Multivariate model | Univariate model |
|---|---|---|
| Impact of data on decision-making during consultation | OR (95% CI), p | OR (95% CI), p |
| Q3—age | ||
| 18–30 years | 0.593 (0.358, 0.983), 0.0430 | 0.610 (0.382, 0.976), 0.0390 |
| 31–40 years | 0.724 (0.474, 1.105), 0.1340 | 0.695 (0.461, 1.048), 0.0830 |
| 41–50 years | Reference category | Reference category |
| 51–60 years | 0.835 (0.555, 1.257), 0.3870 | 0.816 (0.548, 1.214), 0.3150 |
| Over 60 years | 0.696 (0.334, 1.448), 0.3310 | 0.767 (0.383, 1.533), 0.4520 |
| Q4—specialism | ||
| Mental health | Reference category | Reference category |
| Neurology | 0.784 (0.514, 1.197), 0.2600 | 0.779 (0.520, 1.168), 0.2270 |
| Not specified | 0.990 (0.695, 1.411), 0.9560 | 0.977 (0.696, 1.371), 0.8920 |
| Q5—job role | ||
| Doctor excluding GP | Reference category | Reference category |
| Allied health professional | 1.014 (0.552, 1.865), 0.9640 | 0.736 (0.419, 1.292), 0.2850 |
| Not specified | 0.948 (0.438, 2.053), 0.8930 | 0.806 (0.385, 1.688), 0.5670 |
| GP | 0.494 (0.221, 1.101), 0.0850 | 0.904 (0.519, 1.574), 0.7210 |
| Research/healthcare scientist | 0.504 (0.169, 1.506), 0.2200 | 0.382 (0.133, 1.097), 0.0740 |
| Management | 0.661 (0.273, 1.602), 0.3590 | 0.620 (0.268, 1.431), 0.2620 |
| Nursing | 1.090 (0.648, 1.834), 0.7450 | 0.938 (0.580, 1.515), 0.7920 |
| Pharmacy | 0.975 (0.247, 3.856), 0.9720 | 1.182 (0.314, 4.457), 0.8040 |
| Clinical psychology professional | 0.772 (0.414, 1.439), 0.4140 | 0.627 (0.362, 1.086), 0.0960 |
| Student | 0.789 (0.133, 4.662), 0.7930 | 0.521 (0.098, 2.776), 0.4450 |
| Wider healthcare team | 0.854 (0.437, 1.670), 0.6450 | 0.689 (0.367, 1.293), 0.2460 |
| Q6—setting | ||
| Primary care | Reference category | Reference category |
| Secondary care—hospital inpatient | 0.485 (0.226, 1.040), 0.0630 | 0.703 (0.392, 1.260), 0.2360 |
| Secondary care—hospital outpatient | 0.532 (0.247, 1.146), 0.1070 | 0.830 (0.468, 1.473), 0.5240 |
| Secondary care—MH inpatient | 0.571 (0.287, 1.136), 0.1100 | 0.823 (0.498, 1.359), 0.4470 |
| Secondary care—MH outpatient | 0.426 (0.236, 0.769), 0.0050 | 0.576 (0.376, 0.882), 0.0110 |
| Specialist tertiary care | 0.885 (0.369, 2.120), 0.7830 | 1.233 (0.606, 2.510), 0.5630 |
| Community care | 0.415 (0.219, 0.789), 0.0070 | 0.629 (0.398, 0.994), 0.0470 |
| Other | 0.266 (0.091, 0.782), 0.0160 | 0.384 (0.150, 0.982), 0.0460 |
| Q8—uses apps in daily clinical practice | ||
| No | Reference category | Reference category |
| Yes | 1.600 (1.129, 2.268), 0.0080 | 1.666 (1.195, 2.323), 0.0030 |
Results from logistic regression with multiple imputation. Multiple imputation was used to impute data for this question item for 306 missing entries out of total 1006. Bold font indicates significant result
Across all five sub-questions in question 10, the independent variable on HCP use of smartphone apps (question 8), gave a significant result in the multivariate logistic regression models, indicating that those respondents who reported using apps were more likely to respond with ‘definitely’ or ‘sometimes’ to the items discussing the impact of data from RMT on their work.
In relation to clinical setting, the reference category across all regression analyses in sub-questions 10.1 to 10.5 was ‘primary care’. The univariate analyses showed that in all 5 sub-questions, respondents from at least one category of setting gave responses which were significantly different to those in primary care. The odds ratios in these cases were below one, indicating that respondents working in multiple settings outside of primary care were less likely than those in primary care to respond positively (choose ‘definitely’ or ‘sometimes’ rather than ‘unsure’, ‘hardly’ or ‘never’) concerning the impact of RMT on their work. However, in the multivariate regression analyses, only 4 out of 5 sub-questions (10.1, 10.2, 10.4 and 10.5) showed significant differences between primary care and other settings, when other variables were controlled for.
With regard to job role, the reference category was ‘doctors (excluding GPs)’. In 3 out of the 5 question items, the general practitioner job role was significantly different to the reference category (doctors excluding GPs) in the univariate regression analysis, however this was not sustained in the multivariate models for these sub-questions where other variables were controlled for.
In relation to age, only sub-question 10.5, concerning the impact of RMT data on decision-making processes in consultations, showed any difference between age groups. Here, according to both univariate and multivariate models, respondents in the 18–30 age category were less likely than those in the reference category (ages 41–50) to indicate that patients’ RMT data had an impact on their decision-making processes during consultation. There was no significant difference between any specialism and the reference category (mental health) in the regression models for any of the question items.
Discussion
This study achieved its aim of eliciting views from a large sample of healthcare staff working in the care of patients with epilepsy, MS or depression. The results from this study have added to prior work in this area [19, 20] by showing that smartphone apps are used by more than half (56%) of HCPs and that more than three quarters (78%) report that their patients use RMTs for health-related purposes. These figures are greater than in previous research studies that surveyed the use of apps in treatment by healthcare professionals working in epilepsy, where 45% of respondents previously reported using apps with their patients [28], and in depression, where 21.2% of respondents in a prior study stated they used apps in treatment for depression [25]. Our findings thus may indicate their growing use in clinical practice (no prior evidence is available on clinicians’ use of apps in MS).
HCPs reported that their patients use smartphone apps for the purposes of: managing weight; setting personal health goals; and monitoring sleep, activity and mood. Wearable sensing devices are used by their patients for sleep and activity monitoring, and a smaller percentage report patient use of these devices for weight management and monitoring a specific condition (Fig. 5). The majority of HCPs (mean average 62%) indicated that the data from these RMTs impacts their clinical practice and also indicated that RMT data has an impact on their patients’ awareness of their own health (see Fig. 7). Specifically, RMT data was found to impact on communication with patients, on HCPs’ understanding of patient health state, and on HCPs’ decision-making processes. Regression analyses show that HCP views on the impact of RMT data vary according to whether respondents use apps themselves. This adds to recent research in the area of depression care indicating an association between level of technology experience and consideration of use of apps in clinical practice [25]. Views on the impact of RMT data were also found to vary according to clinical setting, job role and age.
Our results suggest RMT is currently having a greater impact in primary care than in other healthcare sectors. A higher proportion of general practitioner respondents reported their patients used devices to monitor activity and sleep and manage weight than in other job roles. This suggests a greater awareness among GPs than among other health care professionals concerning the devices patients use, and the purposes for which they may use them. In addition, GPs were significantly more likely than other kinds of doctor to indicate that RMT data had an impact on their work, on 3 out of 5 questions on this topic (see Tables 3, 4, 5, 6 and 7). Furthermore, those working in primary care were significantly more likely than those in secondary care (mental health outpatient), community care and ‘other’ settings to report that RMT data impacted their work, on all five questions on this topic. This may be because patients are more likely to discuss the data from devices they have bought themselves with primary care practitioners, who they see more regularly, and for whom relational aspects of their work are considered more important. While prior work in both Denmark [29] and the UK [20] has shown that general practitioners recognise benefits to patient use of wearables and apps, the present study has demonstrated more precisely how the attitudes of primary care professionals differ from those in other areas of care.
Our sub-group analysis of job roles that use apps in their clinical practice revealed that a larger proportion of pharmacists (100%) and medical students (90%) use apps in their clinical practice than those in other job categories. The most commonly used app types by these groups were prescribing/dosing apps and guidelines apps, respectively. However, it is important to note that these job roles were only represented by a small number of respondents relative to other job roles (15 pharmacists and 10 medical students), meaning it is possible that only those very most interested in the application of technology chose to take part in this study.
Our data on the type of RMT device used by patients for each health-related purpose (Fig. 6) showed that use of wearables was less prevalent than use of smartphones for certain purposes (weight management, setting personal health goals, monitoring mood). Recalling that this survey was conducted with HCPs rather than patients, it is possible that HCPs are unaware that their patients use wearables in these ways where patients do not deem it relevant to inform clinicians of this, and thus their use may be under-represented in these survey results. Wearables are not as ubiquitous as smartphones, and this may also explain the lower reported use of these for health-related purposes. Another possible explanation is that wearable devices have smaller screens and lack the space to incorporate an easy method for manual data entry and are thus better suited to ‘passive’ means of data collection than ‘active’ means.
In comparison to other health conditions, this survey, targeting HCPs working in the care of people with MS, epilepsy or depression, demonstrated a higher proportion of respondents reporting patients using RMT for health related purposes (78%) than was the case in studies on diabetes (type 1: < 60%, type 2: ~ 33%) [1] and hypertension (< 50%) [2]. Given the recency of these prior studies, it is unlikely that this is simply a result of technologies becoming more widespread, and may reflect a difference in how these conditions are managed, with both blood pressure and blood sugar being measured using bespoke (non-wearable) devices, and therefore not requiring a smartphone app to be able to measure the symptoms of these conditions.
Our work is novel in exploring views on the application of these technologies in the care of people with epilepsy, MS, or depression, from a large sample. This evidence adds to a growing body of work understanding the relationships between healthcare technology use, competence and adoption by healthcare professionals. Recent work by Vallo et al. [30] indicates that adoption and use of digital technology are “related to perceived performance, social influence and organisational context” and the role of ICT [Information and Communication Technology] in clinical professional development. This work highlights the need for a holistic service design approach [31] to reduce external and internal barriers to adoption and optimise the benefits and professional competence of use of these technologies in practice [32].
Recommendations for future work
While HCPs reported using apps, some of the app names they reported appear to be patient-facing, and it is not clear from our data whether they are using these apps themselves or are recommending these to their patients. We cannot tell from our study the extent to which apps and wearables are prescribed or recommended to patients by healthcare professionals. In the UK, some smartphone apps are now highlighted in the NHS apps library [33], and the National Institute for Health and Care Excellence (NICE) has issued guidance that HCPs consider digital mobile health interventions, including smartphone apps, for behaviour change [34]. However, the present study has not been able to ascertain how many HCPs actively recommend these to their patients and this is an interesting question for future work. In other European countries including Germany and Sweden, governments incentivise HCPs to recommend digital solutions to their patients [35]. Further work could also usefully compare use of treatment pathways involving apps and wearables in countries with different incentivisation policies to understand the benefits of these approaches. There are also questions around the regulation of apps and the effect of regulation on their adoption that could be usefully explored.
Recently, NHS guidance has been aimed at primary and secondary care services reducing face-to-face appointments during the COVID-19 pandemic [23, 36] and there is some suggestion that healthcare professionals may have become more favourably disposed to the use of digital technologies to support their clinical practice during this period [37]. However, this evidence relates largely to the use of video conferencing software and telephone consultations rather than the use of wearables and apps to generate data on behavioural and physiological aspects of health, although these are beginning to be evaluated in practice. Given that clinicians would be required to review the data from such RMT on a digital device (e.g. computer) even if patients were present at a face-to-face appointment, it is not clear whether the recent reduction in face-to-face appointments would cause a change of attitudes towards the use of this data in practice. Further work would be required to understand precisely how views on these specific areas may have changed or not because of the reorganisation of care for non-COVID-19 patients during the pandemic.
Strengths and limitations
A key strength of this study is the large sample size, with over 1000 respondents. Another is the exploration of the impact that RMT data has on patient care. The application of regression techniques has enabled us to control for demographic factors when exploring the variation in these views. Despite a coordinated effort to facilitate responses from clinicians working outside of the UK (translation into multiple European languages, advertisement via social media, network connections), the proportion of those working in other countries among respondents to the survey was small. However, we were able to reach a representative sample of respondents from different age groups, job roles and clinical settings, which has enabled sub-group analyses of the views and experiences presented. There is some risk that the self-selecting sample for this study were those most interested in the use of technology and this may mean that views expressed are more positive than is the case across all healthcare professionals. A small percentage of respondents (6%) were from management or healthcare science roles where their contact with patients, and therefore their understanding of factors relevant to the application of RMT, may be more limited. As an exploratory study, the data were analysed without multiplicity adjustment and results were mainly interpreted for exploratory and not confirmatory purposes [38].
Conclusions
This survey study achieved its aim of eliciting opinions and experiences from a large sample of HCPs working in the care of patients with epilepsy, MS or depression. Our main objectives in the present work were: (i) to determine the extent of smartphone app usage among health care professionals; (ii) to determine the extent of HCP experience of managing patients’ use of RMT; and (iii) to understand the impact of patient RMT usage on HCPs’ work, including how this may vary by demographic factors. The results show that prior to the COVID-19 pandemic, smartphone apps were already widely used by more than half of HCPs (objective i), and RMT was used by patients of more than three quarters of respondents (objective ii). HCPs widely believed that the data from RMT impacted their clinical practice, although there is some evidence that their views vary according to demographic and technology usage factors (objective iii). We consider it unlikely that views on the usefulness of the data from RMT will be substantially different after the pandemic, given similar practical requirements for its application in both face-to-face and remote consultations, although further research would be needed to test this hypothesis. We do expect that more HCPs will become more familiar with RMT and that adoption of mobile platforms will become more prevalent in routine health and social care settings in the aftermath of COVID-19.
Supplementary Information
Additional file 1. Survey instrument used in the study, including participant consent form.
Acknowledgements
We would like to acknowledge the support of all members of the RADAR-CNS consortium, who are named on the RADAR-CNS webpage (http://www.radar-cns.org/). The RADAR-CNS project has received funding from the Innovative Medicines Initiative (IMI) 2 Joint Undertaking under Grant Agreement No 115902 (http://www.imi.europa.eu/). This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation program and the European Federation of Pharmaceutical Industries and Associations (EFPIA). This communication reflects the views of the RADAR-CNS consortium, and neither IMI nor the European Union and EFPIA are liable for any use that may be made of the information contained herein. The funding body has not been involved in the design of the study, the collection or analysis of data, or the interpretation of data. This research was supported by the NIHR MindTech MedTech Co-operative, NIHR Nottingham Biomedical Research Centre, and NIHR Applied Research Collaboration East Midlands. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Abbreviations
- COVID-19
Coronavirus Disease 2019
- CCG
Clinical Commissioning Group
- FMI
Fraction of Missing Information
- ICT
Information Communication Technology
- GP
General Practitioner
- GPS
Global Positioning System
- HCP
Healthcare Professional
- MS
Multiple Sclerosis
- NHS
National Health Service
- NICE
National Institute for Health and Care Excellence
- NIHR
National Institute for Health Research
- RADAR-CNS
Remote Assessment of Disease and Relapse – Central Nervous System
- RMT
Remote Measurement Technology
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- UK
United Kingdom
Authors' contributions
The survey was designed by AL, MC, RM and CH. The survey was disseminated by AL, JA and MC. The results were analysed by JA and BG. The manuscript was drafted by JA. Comments on the manuscript were provided by MC, AL, BG and CH. All authors read and approved the final manuscript.
Funding
The RADAR-CNS project has received funding from the Innovative Medicines Initiative (IMI) 2 Joint Undertaking under Grant Agreement No 115902. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation program and the European Federation of Pharmaceutical Industries and Associations (EFPIA). This research was supported by the NIHR MindTech MedTech Co-operative, NIHR Nottingham Biomedical Research Centre, and NIHR Applied Research Collaboration East Midlands. The funding bodies did not take a role in the design of the study or collection, analysis or interpretation of the data or in the writing of the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and received a favourable review from the University of Nottingham Research Ethics Committee (ref 277-1802) and from the Health Research Authority (Ref 19/HRA/5041). Respondents provided informed consent to take part in the study by reading the information provided on the first page of the study and indicating consent using the questions listed on the first page of the online questionnaire.
Consent for publication
Survey respondents remained anonymous and as such this section is not applicable.
Competing interests
There are no competing interests to declare.
Footnotes
The original version of this article was revised: “the statements in the Abstract (Result) section and Conclusion have been revised.”
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
12/11/2023
A Correction to this paper has been published: 10.1186/s12911-023-02390-2
References
- 1.Kebede MM, Schuett C, Pischke CR. The role of continuous glucose monitoring, diabetes smartphone applications, and self-care behavior in glycemic control: results of a multi-national online survey. J Clin Med. 2019;8(1):66. doi: 10.3390/jcm8010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langford AT, Solid CA, Scott E, Lad M, Maayan E, Williams SK, et al. Mobile phone ownership, health apps, and tablet use in US adults with a self-reported history of hypertension: cross-sectional study. JMIR Mhealth Uhealth. 2019;7(1):e12228. doi: 10.2196/12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McIntyre RS, Best MW, Bowie CR, Carmona NE, Cha DS, Lee Y, et al. The THINC-Integrated Tool (THINC-it) Screening Assessment for cognitive dysfunction: validation in patients with major depressive disorder. J Clin Psychiatry. 2017;78(7):873–881. doi: 10.4088/JCP.16m11329. [DOI] [PubMed] [Google Scholar]
- 4.Goodday SM, Atkinson L, Goodwin G, Saunders K, South M, Mackay C, et al. The true colours remote symptom monitoring system: a decade of evolution. J Med Internet Res. 2020;22(1):e15188. doi: 10.2196/15188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brick TR, Mundie J, Weaver J, Fraleigh R, Oravecz Z. Low-burden mobile monitoring, intervention, and real-time analysis using the wear-IT framework: example and usability study. JMIR Form Res. 2020;4(6):e16072. doi: 10.2196/16072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qu C, Sas C, Dauden Roquet C, Doherty G. Functionality of top-rated mobile apps for depression: systematic search and evaluation. JMIR Ment Health. 2020;7(1):e15321. doi: 10.2196/15321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen N, Levitan MJ, Johnson A, Bender JL, Hamilton-Page M, Jadad AA, et al. Finding a depression app: a review and content analysis of the depression app marketplace. JMIR Mhealth Uhealth. 2015;3(1):e16. doi: 10.2196/mhealth.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Escoffery C, McGee R, Bidwell J, Sims C, Thropp EK, Frazier C, et al. A review of mobile apps for epilepsy self-management. Epilepsy Behav. 2018;81:62–69. doi: 10.1016/j.yebeh.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Stoll S, Litchman T, Wesley S, Litchman C. Multiple sclerosis apps: the dawn of a new era: a comprehensive review (P3.2–021) Neurology. 2019;6:66. [Google Scholar]
- 10.Mosa ASM, Yoo I, Sheets L. A systematic review of healthcare applications for smartphones. BMC Med Inform Decis Mak. 2012;12(1):67. doi: 10.1186/1472-6947-12-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holcroft J. Clinical Guidelines App. http://guidelinesapp.com/. Accessed 29 Sept 2020.
- 12.Bierbrier R, Lo V, Wu RC. Evaluation of the accuracy of smartphone medical calculation apps. J Med Internet Res. 2014;16(2):e32. doi: 10.2196/jmir.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haffey F, Brady RR, Maxwell S. Smartphone apps to support hospital prescribing and pharmacology education: a review of current provision. Br J Clin Pharmacol. 2014;77(1):31–38. doi: 10.1111/bcp.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon WJ, Landman A, Zhang H, Bates DW. Beyond validation: getting health apps into clinical practice. NPJ Digit Med. 2020;3:14. doi: 10.1038/s41746-019-0212-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ranjan Y, Rashid Z, Stewart C, Conde P, Begale M, Verbeeck D, et al. RADAR-base: open source mobile health platform for collecting, monitoring, and analyzing data using sensors, wearables, and mobile devices. JMIR Mhealth Uhealth. 2019;7(8):e11734. doi: 10.2196/11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marra C, Chen JL, Coravos A, Stern AD. Quantifying the use of connected digital products in clinical research. NPJ Digit Med. 2020;3(1):50. doi: 10.1038/s41746-020-0259-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simblett S, Greer B, Matcham F, Curtis H, Polhemus A, Ferrao J, et al. Barriers to and facilitators of engagement with remote measurement technology for managing health: systematic review and content analysis of findings. J Med Internet Res. 2018;20(7):e10480. doi: 10.2196/10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simblett S, Matcham F, Siddi S, Bulgari V, di San Barattieri, Pietro C, HortasLopez J, et al. Barriers to and facilitators of engagement with mHealth technology for remote measurement and management of depression: qualitative analysis. JMIR Mhealth Uhealth. 2019;7(1):e11325. doi: 10.2196/11325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craven MP, Andrews JA, Lang AR, Simblett SK, Bruce S, Thorpe S, et al. Informing the development of a digital health platform through universal points of care: qualitative survey study. JMIR Form Res. 2020;4(11):e22756. doi: 10.2196/22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrews JA, Craven MP, Jamnadas-Khoda J, Lang AR, Morriss R, Hollis C, et al. Health care professionals' views on using remote measurement technology in managing central nervous system disorders: qualitative interview study. J Med Internet Res. 2020;22(7):e17414. doi: 10.2196/17414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eysenbach G, Wyatt J. Using the Internet for surveys and health research. J Med Internet Res. 2002;4(2):E13. doi: 10.2196/jmir.4.2.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Majeed A, Maile EJ, Bindman AB. The primary care response to COVID-19 in England's National Health Service. J RSoc Med. 2020;113(6):208–10. doi: 10.1177/0141076820931452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NHS England, NHS Improvement. Clinical guide for the management of remote consultations and remote working in secondary care during the coronavirus pandemic. 2020. Accessed 27 March 2020.
- 24.Kuek A, Hakkennes S. Healthcare staff digital literacy levels and their attitudes towards information systems. Health Inform J. 2020;26(1):592–612. doi: 10.1177/1460458219839613. [DOI] [PubMed] [Google Scholar]
- 25.Kerst A, Zielasek J, Gaebel W. Smartphone applications for depression: a systematic literature review and a survey of health care professionals' attitudes towards their use in clinical practice. Eur Arch Psychiatry Clin Neurosci. 2020;270(2):139–152. doi: 10.1007/s00406-018-0974-3. [DOI] [PubMed] [Google Scholar]
- 26.JISC. The online survey tool designed for academic research, education and public sector organisations. www.onlinesurveys.ac.uk.
- 27.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30(4):377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 28.Bruno E, Simblett S, Lang A, Biondi A, Odoi C, Schulze-Bonhage A, et al. Wearable technology in epilepsy: the views of patients, caregivers, and healthcare professionals. Epilepsy Behav. 2018;85:141–9. doi: 10.1016/j.yebeh.2018.05.044. [DOI] [PubMed] [Google Scholar]
- 29.Brandt CJ, Sogaard GI, Clemensen J, Sndergaard J, Nielsen JB. General practitioners' perspective on ehealth and lifestyle change: qualitative interview study. JMIR Mhealth Uhealth. 2018;6(4):e88. doi: 10.2196/mhealth.8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vallo Hult H, Hansson A, Gellerstedt M. Digitalization and physician learning: individual practice, organizational context, and social norm. J Contin Educ Health Prof. 2020;40(4):220–227. doi: 10.1097/CEH.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw J, Agarwal P, Desveaux L, Palma DC, Stamenova V, Jamieson T, et al. Beyond "implementation": digital health innovation and service design. NPJ Digit Med. 2018;1:48. doi: 10.1038/s41746-018-0059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mesko B, Gyorffy Z. The rise of the empowered physician in the digital health era: viewpoint. J Med Internet Res. 2019;21(3):e12490. doi: 10.2196/12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.NHS. NHS Apps Library. 2021. https://www.nhs.uk/apps-library/. Accessed 19 Feb 2021.
- 34.National Institute for Health and Care Excellence (NICE). NICE Guideline [NG183] Behaviour change: digital and mobile health interventions. 2020.
- 35.Brinkmann-Sass C, Richter L, Silberzahn T, Somauroo A. The European path to reimbursement for digital health solutions. McKinsey & Company, 2020. Accessed 17 Sept 2020.
- 36.NHS England, NHS Improvement. Coronavirus guidance for clinicians and NHS managers. 2020.
- 37.Intelligence DH. NHS IT leadership Survey 2020. 2020.
- 38.Bender R, Lange S. Adjusting for multiple testing–when and how? J Clin Epidemiol. 2001;54(4):343–349. doi: 10.1016/s0895-4356(00)00314s-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Survey instrument used in the study, including participant consent form.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.