Hardly a day goes by in the ICU without the lament arising at some point on rounds that “there are no data” to inform some challenging clinical decision. Daily rounds in the ICU occasion hundreds, if not thousands, of clinical decisions by the interdisciplinary team. Very few of these decisions can be made with explicit reference to a specific clinical trial; too often we can only acknowledge that “we need a trial.” Yet clinical trials are costly, time-consuming, and burdensome affairs. It is easy to say that we need a trial; it is quite another thing to actually make the trial happen. Thus, clinicians are left to make most of their decisions using judgment informed by experience and mechanistic understanding, giving rise to considerable variation in practice (and outcomes) between centers and countries (1).
One approach to address this pressing need for trials is “learning while doing” (2). Advocates of this pragmatic research philosophy envision a “learning health system” that incorporates randomization to various treatments as part of routine clinical care (3), reflecting the genuine clinical equipoise and uncertainty that clinicians have over specific clinical decisions. It is a compelling and lofty vision but one that has, to date, achieved only limited implementation.
In this issue of the Journal, Casey and colleagues (pp. 294–302) report a pragmatic clinical trial (PROPER [Protocolized Post-Extubation Respiratory Support]) that provides an important and instructive exemplar of the learning health system concept in critical care medicine (4). They studied postextubation respiratory support, inquiring whether a strategy of routine postextubation respiratory support by either high-flow nasal cannula (HFNC) or noninvasive ventilation (NIV) was superior in terms of reintubation rate in comparison to usual care (which, in their ICU, meant NIV for high-risk patient groups). These forms of postextubation respiratory support have some proven efficacy to reduce the risk of reintubation (5). The PROPER trial compared a pragmatic strategy of postextubation respiratory support in “all-comers” as compared with the usual strategy of selectively applying postextubation respiratory support according to clinical judgment. The main effect of the routine postextubation respiratory support strategy was to dramatically increase the use of HFNC after extubation (75% vs. 3%); the use of NIV was similar under both strategies (18% vs. 14%). The trial demonstrated a small and “nonsignificant” difference in the risk of reintubation between strategies (16% vs. 13%), with a low posterior probability of any benefit under varying priors.
These findings are of considerable interest to the clinical community. The data suggest that, in a similar medical ICU population, routine use of postextubation respiratory support (especially HFNC) does not improve outcome in comparison to selective application of postextubation respiratory support based on established risk categories (chronic hypercapnia, etc.). But we suggest that clinicians should sit up and especially take notice of the almost breathtakingly simple and cost-effective manner in which the trial was conducted. The investigators divided their ICU in half, treating the individual beds in each half as a cluster. The two strategies under investigation were applied alternately between clusters over time. The strategies were pragmatic and respiratory therapist led. The primary outcome (reintubation) was rapid and easily ascertained. And the success of the approach—more than 700 patients randomized at a single center in less than 2 years to achieve a definitive answer to an important pragmatic clinical question—is undeniable. The PROPER trial convincingly demonstrates the potential of the learning health system concept to resolve simple, pragmatic research questions in a timely fashion. For this the investigators must be congratulated.
In view of the results of the PROPER trial, work remains to be done to improve our understanding of the mechanisms leading to postextubation respiratory distress and need for reintubation—on this point, methods to assess and enhance expiratory muscle function deserve greater attention (6), as also noted by the PROPER investigators. A mechanistic understanding is especially important to accurately identify individual patients who are most likely to benefit from postextubation respiratory support. Just because there was, on average, no improvement in outcome between groups does not entail that none of the individual patients accrued benefit from the intervention.
And here we must address the more general issue of moving from clinical trials to clinical decisions. The reported treatment effect in a trial represents an “average treatment effect” (ATE), the mean of many values for “individual treatment effect” (ITE) for each patient included in the trial (Figure 1). Depending on the heterogeneity of the population, the ITE may differ considerably from the ATE in some patients. Clinical decisions for individual patients are ideally based on an informed estimate of the ITE for the patient at the center of the clinical encounter, not the ATE for the heterogeneous population that happens to include the patient. In the PROPER trial, there were signals of possible variation in treatment effect among patient subgroups, especially based on duration of ventilation before randomization. The possibility that some patients can benefit from routine use of postextubation respiratory support cannot therefore be ruled out. Trials can be designed to adapt sample size requirements as the trial progresses to ensure sufficient information to definitively rule in or rule out treatment benefit in homogeneous subgroups of patients, and advances in analysis of data from trials can move us closer to estimates of ITE for each patient (7, 8). The learning health system approach could facilitate accrual to feasibly achieve the larger sample sizes needed for such designs. Combining the learning health system concept with adaptive designs that account for heterogeneity of treatment effect could substantially inform and improve clinical decision-making for individual patients.
Figure 1.
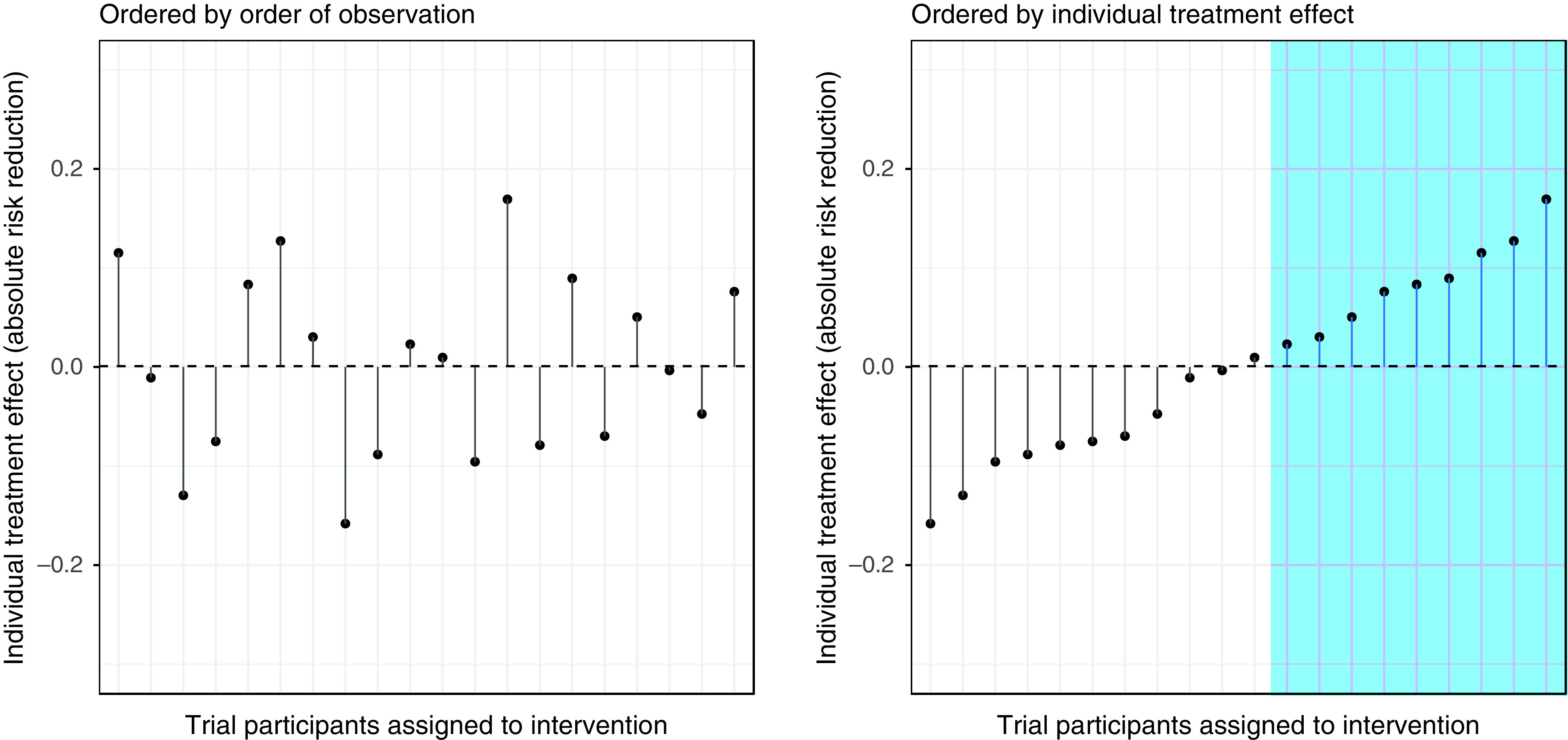
Average treatment effect (dashed line) and individual treatment effect (points) in a theoretical population of patients enrolled in a trial. Treatment is appropriate in patients with a favorable individual treatment effect (blue shaded region). Designing trials with the goal of learning individual treatment effect will maximize the relevance of clinical trials to clinical decisions.
In sum, by making learning and discovery a formal part of patient care, perhaps some decisions on ICU rounds may eventually be a little easier.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202104-0844ED on April 26, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Peñuelas O, Muriel A, Abraira V, Frutos-Vivar F, Mancebo J, Raymondos K, et al. Inter-country variability over time in the mortality of mechanically ventilated patients. Intensive Care Med. 2020;46:444–453. doi: 10.1007/s00134-019-05867-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Angus DC. Optimizing the trade-off between learning and doing in a pandemic. JAMA. 2020;323:1895–1896. doi: 10.1001/jama.2020.4984. [DOI] [PubMed] [Google Scholar]
- 3. Goligher EC, Zampieri F, Calfee CS, Seymour CW. A manifesto for the future of ICU trials. Crit Care. 2020;24:686. doi: 10.1186/s13054-020-03393-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Casey JD, Vaughan EM, Lloyd BD, Billas PA, Jackson KE, Hall EJ, et al. Vanderbilt Learning Healthcare System and the Pragmatic Critical Care Research Group. Protocolized postextubation respiratory support to prevent reintubation: a randomized clinical trial. Am J Respir Crit Care Med. 2021;204 doi: 10.1164/rccm.202009-3561OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Granton D, Chaudhuri D, Wang D, Einav S, Helviz Y, Mauri T, et al. High-flow nasal cannula compared with conventional oxygen therapy or noninvasive ventilation immediately postextubation: a systematic review and meta-analysis. Crit Care Med. 2020;48:e1129–e1136. doi: 10.1097/CCM.0000000000004576. [DOI] [PubMed] [Google Scholar]
- 6. Shi ZH, Jonkman A, de Vries H, Jansen D, Ottenheijm C, Girbes A, et al. Expiratory muscle dysfunction in critically ill patients: towards improved understanding. Intensive Care Med. 2019;45:1061–1071. doi: 10.1007/s00134-019-05664-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pirracchio R, Hubbard A, Sprung CL, Chevret S, Annane D. Rapid Recognition of Corticosteroid Resistant or Sensitive Sepsis (RECORDS) Collaborators. Assessment of machine learning to estimate the individual treatment effect of corticosteroids in septic shock. JAMA Netw Open. 2020;3:e2029050. doi: 10.1001/jamanetworkopen.2020.29050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Athey S, Imbens G. Proc Natl Acad Sci USA. 2016;113:7353–7360. doi: 10.1073/pnas.1510489113. [DOI] [PMC free article] [PubMed] [Google Scholar]


