Abstract
Background: Well-designed clinical research needs to obtain information that is applicable to the general population. However, most current studies fail to include substantial cohorts of racial/ethnic minority populations. Such underrepresentation may lead to delayed diagnosis or misdiagnosis of disease, wide application of approved interventions without appropriate knowledge of their usefulness in certain populations, and development of recommendations that are not broadly applicable.
Goals: To develop best practices for recruitment and retention of racial/ethnic minorities for clinical research in pulmonary, critical care, and sleep medicine.
Methods: The American Thoracic Society convened a workshop in May of 2019. This included an international interprofessional group from academia, industry, the NIH, and the U.S. Food and Drug Administration, with expertise ranging from clinical and biomedical research to community-based participatory research methods and patient advocacy. Workshop participants addressed historical and current mistrust of scientific research, systemic bias, and social and structural barriers to minority participation in clinical research. A literature search of PubMed and Google Scholar was performed to support conclusions. The search was not a systematic review of the literature.
Results: Barriers at the individual, interpersonal, institutional, and federal/policy levels were identified as limiting to minority participation in clinical research. Through the use of a multilevel framework, workshop participants proposed evidence-based solutions to the identified barriers.
Conclusions: To date, minority participation in clinical research is not representative of the U.S. and global populations. This American Thoracic Society research statement identifies potential evidence-based solutions by applying a multilevel framework that is anchored in community engagement methods and patient advocacy.
Keywords: recruitment, retention, clinical research, minorities, health disparities
Contents
-
Overview
Summary of Workshop Consensus Recommendations for Interventions
Introduction
-
Methods
Committee Composition and Meetings
Literature Search and Appraisal of Existing Evidence
Research Recommendations
Document Development
Conceptual Framework
-
Partnering with PAOs
Case Study 1
-
Applying a Community-engaged Perspective to Research
Case Study 2
-
Addressing Individual-Level Barriers
Adding Flexibility and Addressing Resource Constraints
Case Study 3
-
Addressing Interpersonal-/Provider- Level Barriers
Addressing Bias
Building Trust
Case Study 4
Enhancing Recruitment with Sociocultural and Multimedia Outreach
Case Study 5
-
Addressing System-/Institutional- Level Barriers
Diversifying the Research Team
Case Study 6
Aligning Clinical and Research Enterprise Priorities
-
Addressing Federal-/Policy-Level Barriers
Role of the NIH
Retrain the Study Section Reviewer
Role of the FDA
Special Considerations for International Studies
Toolkit: Suggested Enhancements for Research Protocol Implementation
Study Design Considerations
Conclusions
Overview
The rate of inclusion of historically underrepresented racial and ethnic minorities in clinical research remains low and falls short of mirroring the diversity of the U.S. population. The lack of clinical research studies in racial and ethnic communities leads to the misapplication and overgeneralization of findings from non-Hispanic White populations to all other populations. Although other populations, including older, rural, and low-socioeconomic-status groups, are also underrepresented in clinical research, this research statement focuses on strategies to enhance the participation of racial and ethnic minority groups in clinical research. The current status quo for inclusion in research severely limits progress toward precision medicine diagnostic and therapeutic approaches for respiratory disease and contributes to existing disparities in health outcomes.
This research statement attempts to comprehensively examine the existing barriers to minority participation in pulmonary, critical care, and sleep clinical research; however, it is not a systematic review. We present a multilevel conceptual framework (Figure 1) to identify shared barriers to clinical research participation that are common across racial and ethnic minority groups at the individual, interpersonal, institutional, and federal levels. We start by describing the role of patient advocacy groups and community engagement in research and how these efforts are foundational to increasing minority participation by directly and indirectly addressing barriers presented across all levels. We then present suggestions for necessary systemic changes at the community, institutional, and federal-policy levels that would facilitate clinical research that is more representative of the U.S. population and would target those with the greatest disease burden. To propel clinical research toward increasing representation, we end with tangible (i.e., easier-to-implement) recommendations that target individual- and interpersonal-level barriers that research teams may implement more immediately.
Figure 1.
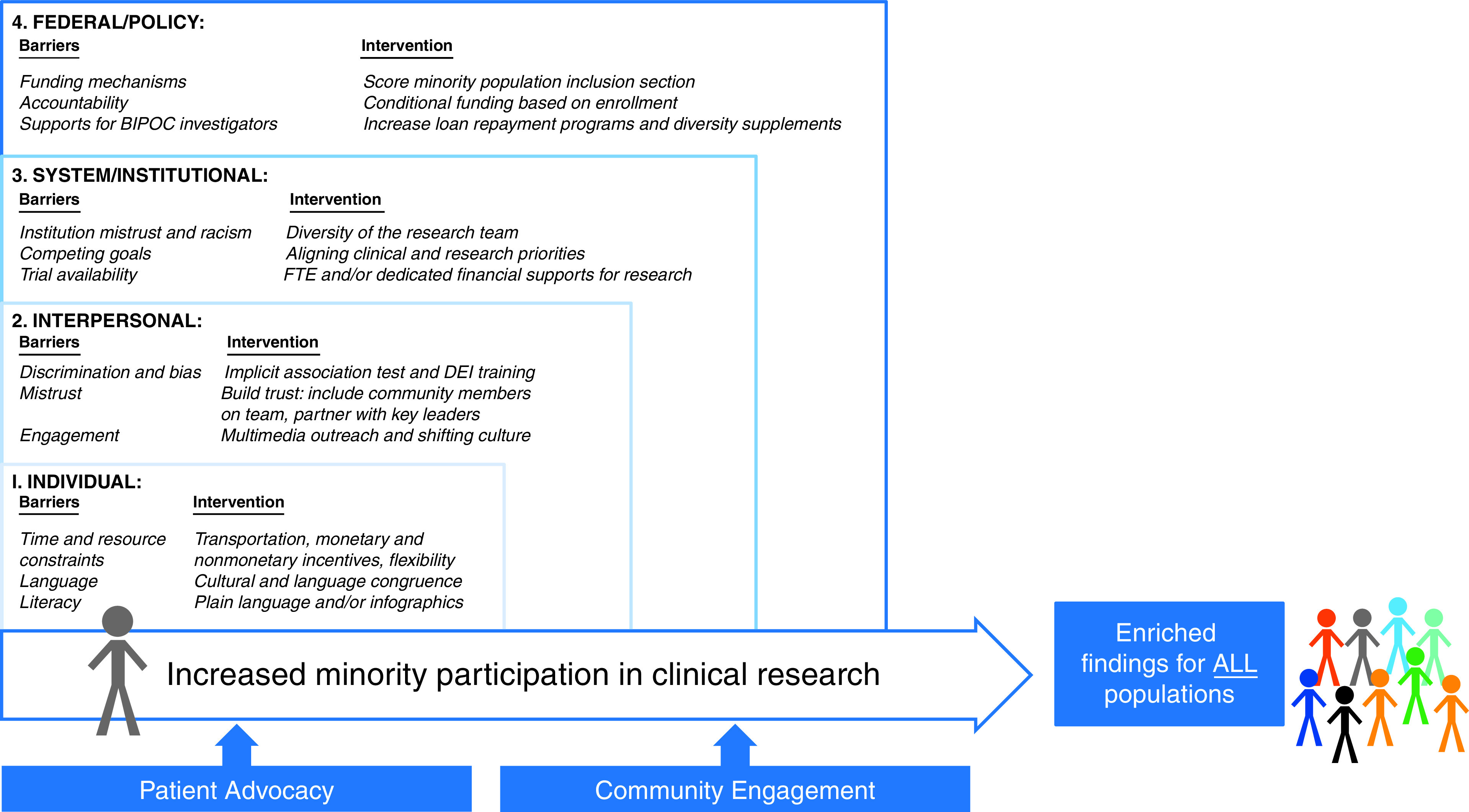
A multilevel framework of shared participation barriers and potential interventions to promote participation in clinical research for historically underrepresented minority groups. BIPOC = Black, indigenous, people of color; DEI = diversity, equity, and inclusion; FTE = full-time equivalent.
Many of the strategies presented in this research statement would likely enhance recruitment and retention across all racial and ethnic groups (including non-Hispanic White populations). Concerted efforts and resources, particularly financial supports, from multiple stakeholders—including educational and healthcare institutions and organizations and the federal government—are needed to purposefully apply these strategies in a way that increases engagement and representation of minority populations in clinical research.
Summary of Workshop Consensus Recommendations for Interventions
Recommendations provided for each level of the framework are based on group consensus, with notations being given for strategies with established evidence versus those with growing evidence but a high potential for achieving effects (established vs. novel) and for strategies that would be implemented with ease versus those that require more investment (short- vs. long-term). Strategies that span levels and address multiple barriers are likely to have the greatest effect.
Foundational strategies.
Foundational strategies provide roadways and a base structure on which other interventions may build as part of a long-term strategy to increase engagement.
-
•
Engage with patient advocacy groups by forming relationships with local, regional, and national groups as equal partners (novel, long-term).
-
•
Lead with community engagement by working with community partners to set priorities and strategies for addressing local needs (established, long-term).
Individual-level strategies.
-
•
Address social and structural barriers by providing fair and usable compensation, providing transportation options, and building flexibility in scheduling and the mode of administration (established, short-term).
-
•
Provide language-congruent materials and use plain language in consent and study information (established, short-term).
Interpersonal-level strategies.
-
•
Address bias through self-assessment and training and developing protocols that promote inclusion in research, such as universal screening (novel, short- and long-term).
-
•
Build trust with community partners who are trusted leaders (i.e., clinicians, religious leaders, etc.) and can facilitate referrals from the target community (novel, long-term).
-
•
Increase engagement through multimedia outreach through outlets, including social media platforms, commonly patronized by the target community (established, short-term).
Institutional-level strategies.
-
•
Diversify the research team to build trust and engagement by having racial, ethnic, and cultural congruence. This includes the incorporation of lay persons from the target community as part of the research team. This strategy addresses key barriers, including increasing engagement with the community, addressing social barriers, and building trust (established, long-term).
-
•
Align clinical and research priorities and funding to build capacity for clinical research availability and address community needs (established, long-term).
Federal-/policy-level strategies.
-
•
Invest in resources to support recruitment of minority populations (incentivize); this can include the addition of supplements to awards provided for focusing on recruitment of minority populations (established, long-term).
-
•
Score the Inclusion of Women and Minorities section. Raising the significance of this section to a scorable section would positively impact recruitment and retention efforts (novel, long-term).
-
•
Retrain the scientific review officers and the study section reviewer to recognize bias in the study section (novel, long-term).
-
•
Provide supports that directly or indirectly remove the financial burden of pursuing clinical research endeavors for Black, Latinx, and indigenous trainees and early-career faculty (established, long-term).
Introduction
In 1993, the U.S. Congress passed the Revitalization Act, making law the NIH policy that mandated that all NIH-funded biomedical and behavioral research (1) include women and racial and ethnic minorities, with no exceptions for cost. Although the number of women included in NIH-funded research has increased over the last several decades, the rate of inclusion of minorities in clinical research remains low (2) and fails to reflect the diversity of the U.S. population; by 2030, minorities will represent nearly 50% of the nation’s population (3).
Between 1993 and 2013, it was estimated that less than 5% of published, NIH-funded, pulmonology-related research studies included members of racial or ethnic minority groups (4). Of all NIH-sponsored published research studies in 2015, race or ethnicity was included in the analysis or presented as a subgroup analysis 13.4% of the time (5). We recognize that these estimates may be falsely low, given the higher ability to publish secondary data analyses in majority populations than in minority populations. Promisingly, ongoing and persistent efforts by the NIH and the Food and Drug Administration (FDA) have had positive effects. In 2018, the NIH reported that minorities represented 31.1% of all participants enrolled in phase III clinical trials (2). Although such increased representation is encouraging, this estimate aggregates all racial and ethnic groups and likely reflects a number of factors. First, the reporting of the mean percentage of minority enrollment in clinical studies is likely to be heavily influenced by clinical trials and research studies that are focused on specific minority populations (falsely driving up estimates). Second, representation estimates from phase III clinical trials may falsely drive up estimates, given recent efforts by the FDA to mandate the inclusion of minority populations (see Federal-/Policy-Level Barriers) (6). Lastly, underrepresentation continues to be a problem. When we look across top pulmonary diagnoses, we see that studies often fail to include adequate representation based on the proportion of the population with a given disease (Table 1). For example, 11% of all patients with lung cancer are Black, with Black individuals having an age-adjusted population rate similar to that of White patients; yet in 2018, only 5% of individuals enrolled in NIH-funded lung cancer research studies were Black.
Table 1.
Disease Prevalence and Composition by Racial and Ethnic Groups for Top Pulmonary Disorders in Comparison with Representation across Clinical Research Studies Funded by the NIH
| NIH RCDC Category* | Race† |
Ethnicity† |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White |
Black |
Asian |
American Indian/ Alaska Native |
Latinx |
|||||||||||
| Population Prevalence/ Frequency‡ | Proportion of Diseased Population§ | Median % Enrolled in NIH Research‖ | Population Prevalence/ Frequency‡ | Proportion of Diseased Population§ | Median % Enrolled in NIH Research‖ | Population Prevalence/ Frequency‡ | Proportion of Diseased Population§ | Median % Enrolled in NIH Research‖ | Population Prevalence/ Frequency‡ | Proportion of Diseased Population§ | Median % Enrolled in NIH Research‖ | Population Prevalence/ Frequency‡ | Proportion of Diseased Population§ | Median % Enrolled in NIH Research‖ | |
| Asthma (161) | 8% | 63% | 56% | 10.7% | 17% | 20% | 4.5% | 3% | <1% | 10.4% | 1% | <1% | 6.5% | 15% | 6% |
| COPD (162) | 3.6% | 82% | 63% | 3.4% | 12% | 27% | 1.1% | 2% | <1% | NA | NA | <1% | 2.7% | 11% | 2% |
| Cystic fibrosis (163) | 1:2,500 | 94% | 97% | 1:17,000 | 5% | <1% | 1:35,100 | NA | <1% | NA | NA | <1% | 1:13,500 | 9% | <1% |
| Lung cancer (164) | 56.1 | 82% | 80% | 55.5 | 11% | 5% | 33.4 | 3% | 2% | 39.1 | <1% | 1% | 27.7 | 4% | 3% |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; NA = not available; RCDC = research disease and condition category.
Categories defined as per Reference 138.
Racial categories are inclusive of persons identified as being of Hispanic/Latinx ethnicity, and Latinx ethnicity includes persons of different races.
Percentage of persons with disease from each racial/ethnic group; for cystic fibrosis, this is presented as the frequency, and for lung cancer, this is presented as the age-adjusted rate per 100,000 persons.
Percentage of persons from each racial/ethnic group among the total population of persons with disease.
The median percent participation in NIH-funded research studies within each demographic group and RCDC category is based on participants enrolled in human-subject studies during Fiscal Year 2018.
Perhaps the most obvious example of minority underrepresentation in clinical research is in the field of genomics. Of the 35 million samples included in genome-wide association studies in 2016, only 5% were collected from minority individuals (7). Such underrepresentation severely limits progress toward precision medicine diagnostic and therapeutic approaches in lung disease (8), perpetuating respiratory health disparities. For example, cystic fibrosis (CF) predominately affects non-Hispanic White individuals; however, Black and Latinx individuals with CF suffer a disproportionate burden of disease morbidity (9). This is partly due to nonidentification of disease-causing CF mutations in non-White individuals (10), leading to delayed diagnosis and treatment for many minorities. In addition, new CFTR (CF transmembrane receptor) modulators essentially correct the genetic disorder in 72% of non-Hispanic White populations but are only effective for 44% of Black individuals with CF and 54% of Latinx individuals with CF (11). This disparity is further exacerbated by underrepresentation of minorities in clinical trials for CF therapeutics (12). CF is not the only example of underrepresentation of minority populations in clinical research (13), particularly in therapeutic trials, as this also occurs in studies of other lung diseases that disproportionately affect minority groups, including asthma (14, 15) and lung cancer (16).
For the purpose of this research statement, we use the term “minority” to refer to racially and ethnically distinct groups who have been historically disadvantaged in the United States, including Black or African Americans, Hispanic or Latinx groups, American Indian or Alaska Native groups, and Native Hawaiians or Pacific Islanders. We have broadly defined “clinical research” as research involving human participants in clinical trials, clinical research, and behavioral health interventions. This research statement attempts to comprehensively examine the existing barriers to minority participation in clinical research but is not meant to be a systematic review. We present a multilevel conceptual framework to increase representation in respiratory clinical research by addressing key barriers to participation at the individual, interpersonal, institutional, and federal/policy levels. We also present suggestions for necessary systemic changes at these levels that would facilitate truly inclusive and representative clinical research in the United States. Lastly, for practical reasons, we provide a toolkit for researchers that includes recruitment protocol enhancements that can be quickly implemented and that would have more immediate, but likely modest, effects boosting minority participation.
Methods
Committee Composition and Meetings
The workshop organizers included representatives from the American Thoracic Society (ATS) Health Equality and Diversity Committee, the ATS Behavioral Science and Health Services Research Assembly, and the National Institute of Minority Health and Health Disparities (NIMHD). Co-chairs invited individuals to participate on the basis of their research expertise or ability to influence best practices for increasing minority participation in clinical research. Potential conflicts of interest were disclosed and managed in accordance with the policies and procedures of the ATS. Participants were assigned to one of four priority areas on the basis of their area of expertise or the group represented by the individual.
Two virtual (planning) meetings and one in-person (workshop) meeting were held. During the planning meetings, participants discussed and refined the objectives for the project and selected topics for discussion under each priority area. This included development of the multilevel framework introduced in this statement. These meetings informed the agenda for the in-person meeting at the 2019 ATS International Conference in Dallas, Texas.
Literature Search and Appraisal of Existing Evidence
Our literature search was not a systematic review. Group leads performed a literature search of PubMed and Google Scholar for each topic. The literature review included topics related to racial/ethnic health disparities in pulmonary, sleep, and critical care medicine; identifying trials designed to increase minority participation in research; and reviewing multilevel frameworks to address minority participation in research. Search results were sent to groups, who reviewed and selected the studies that they deemed relevant to their topic. Group members supplemented the literature search by identifying relevant studies from other sources.
Research Recommendations
Each group appraised and summarized the existing evidence for each level of impact and then identified salient knowledge gaps. Final recommendations were determined via discussion and consensus. Specifically, consensus was used to define strategies as being supported by strong evidence (i.e., established methods for increasing participation) or as being promising; having emerging evidence for impact (i.e., novel); and as having a short-term, more immediate effect versus requiring more long-term investment before seeing an effect on minority participation.
Document Development
Topic expert participants sent drafts to a co-chair (N.T.) who collated and edited the group’s contributions into a single document, which was sent back to all participants for review. Multiple cycles of revision and feedback followed until all participants agreed on the final version.
Conceptual Framework
Lack of participation in clinical research by minority populations stems from several sources, including mistrust of the scientific community (17–19), racial and ethnic bias and racism toward minority populations (20–22), and social and structural barriers to participation (22–24). Social barriers include competing responsibilities that overtax economically disadvantaged populations and unmet social needs, such as financial strain and transportation, that may impede the ability to participate. Structural barriers include institutional policies and infrastructure supports, including clinical research availability, and federal policies that fail to finance and facilitate recruitment from minority populations (1). In addition, individual barriers such as language concordance and health literacy may impede participation in clinical research (22, 23, 25, 26).
To address barriers to participation in research, it is important to understand the historical and current contexts of minority populations and research. The most widely recognized source of mistrust in the Black community is the Tuskegee syphilis study (27), yet systemic abuse and disregard for the protection of human subjects is not limited to Tuskegee and extends to other minority groups (17, 18). Poignant historical examples include efforts to sterilize indigenous populations in the United States (28), the birth control trials in Puerto Rico (29), and the HeLa cell line derived without permission from Henrietta Lacks (30). Although less obvious, and often under the premise of advancing science and improving health for the community in question, modern-day examples of unethical research practices continue to occur. This includes a study of Black boys that manufactured study conditions to provoke aggressive behaviors by purposely withholding medications for chronic health problems, isolating boys from their parents, and giving a medicine believed to promote aggressive behaviors (31). The stated purpose of the study was to identify a gene associated with aggressive behaviors, yet no other racial/ethnic populations were included. Another example was the misuse of blood samples from Havasupai tribal members to explore genetic associations with mental health disorders, alcoholism, and the degree of inbreeding in a study with a stated focus on diabetes, a disease that was common among tribal members, the prevalence of which was the reason the community was willing to participate (32). Such violations of informed consent, combined with repeated discriminatory interactions with the healthcare system when pursuing care (20), are modern-day contributors to the ongoing mistrust of research-oriented institutions in vulnerable communities.
By using a multilevel framework (Figure 1) (33, 34), workshop members identified shared barriers to participation in clinical research for minority groups at the individual, interpersonal, institutional, and federal/policy levels. We then examined the role of patient advocacy organizations (PAOs) and community engagement for removing barriers to research participation across all levels. Lastly, we included established and promising (when there is limited evidence) interventions, identified areas for future research, and suggested funding and policy priorities to facilitate minority participation in respiratory clinical research. In Table 2, we include exemplary studies that have employed strategies with demonstrated success across diverse settings. As a committee, we selected studies that best captured the highlighted strategy (i.e., not bundled with other strategies or bundled only with other strategies that target the same level of intervention). The provided effect sizes are not necessarily generalizable to all settings, and interpretation should be limited to the provided study context.
Table 2.
Summary of Exemplary Studies and/or Programs Addressing Recruitment and Retention Barriers to Inclusion of Minority Populations in Clinical Research
| Challenge | Strategy | Study: Study Focus; Study Type | Population | Intervention | Results |
|---|---|---|---|---|---|
| Individual level | |||||
| Time/competing demands | • Flexible appointment times | Healthy Aging in Neighborhoods of Diversity across the Life Span (165): recruitment and retention; single-arm study | 30- to 64-yr-old urban-dwelling adults in Forest Park, Baltimore, MD (n = 745) | Multicomponent intervention that addressed individual-level and community-level barriers. Transportation barriers were addressed by free transport and mobile examination centers to bring the study to participants; economic and time-constraint barriers were addressed through flexible scheduling and providing adequate compensation. Other barriers (including bias and mistrust) were addressed by being community based, having community input, and having culturally congruent team members.* | At 5 yr, reported 79.2% retention for Forest Hill participants (7.4% decreased, 5.5% dropped, and 7.5% lost to follow-up). |
| • Alternative methods and modes for survey collection | |||||
| Logistics/lost to follow-up | • Multiple modes of contact | ||||
| EVMC Model (64): retention; single-arm study | Low- socioeconomic-status, urban-dwelling youth with asthma (n = 620) | Adaptation of the EVMC Model, which is an evidence-based recruitment strategy (61) with demonstrated success in longitudinal studies with individuals with substance abuse disorder (95% completion rate across seven studies, n = 5,000) (6). Engagement: at enrollment, collecting multiple forms of contact, including family (n = 3) and friend (n = 3) contacts for follow-up; Verification: verify phone contact of at least three individuals on contact form using text and known number; Maintenance and Confirmation: provide flexibility to mode and method of scheduling and survey administration. This included contacting family and friends to reach each participant; scheduling appointments by phone, text, or e-mail; providing a variety of options to complete self-administered survey (in person, online, by mail, or over the phone); and offering an extra incentive and shorter survey for nonresponders. | Completion at 6 mo and 12 mo was 87.6% and 91.0%. | ||
| Cost and resource constraints | • Monetary and nonmonetary incentives | Cancer Care Equity Program (166): recruitment; interrupted times series, comparing with the 2 yr before implementation | Participants in clinical trials for cancer treatment (n = 1,217) | Provided financial assistance for participants describing need. Specifically gave reimbursement for costs of travel and lodging associated with participating in cancer trial. | Greater increase in trial participation over expected trend (19 more participants/mo), but not specifically for minority populations. Notes increases in participation from younger, low-income groups and those living further away. |
| • Provide transportation and/or reimbursement for transportation | |||||
| Language/health literacy | • Cultural congruency | Kulusugan ay Kayamanan (Health is Wealth) Study (89): recruitment and retention; single-arm study | Older Filipino women in L.A. County (n = 530) for a cancer prevention educational strategy | Intervention: Five strategies, informed by target population, were employed: Word of Mouth across community; Presentations by Filipino Project Director at community locations; Church Announcements, Flyers, Sign-up to increase awareness; Female Project Liaisons (to provide personal invitations to potential participants and lead outreach for ongoing engagement with study); and Incentives. | 88% retention at 12 mo and 76% retention at 24 mo. |
| Interpersonal level | |||||
| Mistrust | • Engagement with providers, community leaders, or other trusted entities who predominantly serve minority communities | RECRUIT (87): recruitment; clustered, randomized controlled trial | Increase recruitment of minority populations into cancer clinical trials (n = 50 clinics) | Trust-based continuous quality- improvement intervention to build trust between specialist physician investigators and community minority-serving physicians and ultimately potential trial participants. | Overall, no intervention effect was detected (odds ratio, 1.3; 95% CI, 0.7–2.4). However, heterogeneity across parent trials may have muted the effect, with three of four trials enrolling more minorities in the intervention than in the control group. |
| • CHWs | IMPaCT (167): recruitment and retention; single-arm study compared with historic period | African American adults with cancer referred for consideration for interventional cancer trials at the institution (n = 424) | Trained lay individuals as PNs to provide education on clinical trials; to provide supports, including transportation assistance, reminder calls, and social work referrals; and to serve as liaisons among the study team, clinician, and patient. | Overall, accrual increased from 9% Black participation in 2007 to 16% Black participation in 2014 over the study period; 74.5% of those who received navigational services completed the trial compared with 37.5% of Black participants who did not consent to PN services (P < 0.001). | |
| Reaching target community | • Targeted advertising | Engagement in Weight-loss Intervention (88): recruitment; single-arm study | African American families (n = 528) | Sociocultural outreach through community partnerships, word-of-mouth, sociocultural events, and placing culturally relevant advertisements. | Families recruited through sociocultural outreach were 1.96 times (95% CI: 1.05–3.68) more likely to schedule a baseline visit than families recruited through nonsociocultural avenues. No difference in enrollment or retention. |
| • Social media | Social Media Outreach to Pregnant Women (94): recruitment; nonrandomized comparison trial of two recruitment methods (social media vs. in-person methods) | Pregnant women | Targeted advertisement via Facebook for a 27-d period. | Overall increase in recruitment via social media–based method (1,178 vs. 219 women). An almost threefold increased recruitment of Black pregnant women (29.4% vs. 11.2%; P < 0.001). | |
| Lack of engagement | • Building relationships | Ecological Nurse Case Management Intervention for Hypertension (168): recruitment and retention; single-arm study | Low-income African American adults with hypertension (n = 59) | Multiple strategies employed to ensure engagement: team members committed to entire study period, prior experience with study population, and nonstudy communication with site partners and with participants. Nonengagement strategies included use of incentives and expanding eligibility criteria to capture younger patients with hypertension. | Accrued 97% of target population; retention rates were 91.5%, 88.1%, and 83.1% at 1, 3, and 6 mo, respectively. |
| • Community engagement | CEASE Study (49): retention; single-arm study with comparison of retention rates to prior study phases (phase II and III to phase I) | Retention of low-socioeconomic-status smokers from two underserved urban communities | Informed by stakeholders, moved intervention from the clinic to community venues and employed peer motivators over healthcare providers (phase II and III). For phase III, allowed for more personalization of the intervention to participants’ needs.* | Retention increased from 13.8% (phase I) to 51.9% and 67.9% in phase II and phase III, respectively. Retention of African American participants also increased significantly across phases (phase I: 63.7%; phase II: 82.1%; phase III: 84.6%; P < 0.001). | |
| Institutional level | |||||
| Lack of representation | • Increasing the diversity of research team | Nuevo Amanecer-II Biospecimen Study (169): recruitment and retention | Latina breast cancer survivors (n = 103) | To overcome cultural, linguistic, and health-literacy barriers, the study team was composed of Latina investigators, involved culturally and linguistically concordant CHWs, and partnered with local community groups. Together, the team identified acceptable types and methods for biospecimen collection; addressed mistrust by having community-identifying investigators on the team, including CHWs, and partnering with community groups; developed plain-language, approachable materials that covered the extent of the study. | Investigators were successful in obtaining repeated collections of biospecimens from participants and reported high rates of retention at 6 mo (85%). |
| Lack of trial availability | • Aligning clinical and research priorities | University of AZ–Banner Health All of Us Research Program (170): recruitment | Adults in AZ receiving care through Banner Health (n = 30,000, goal of 50% Latinx and 10% from other non-White race/ethnicities) | Partnership with health system that included aligning goals, expectations, and responsibilities and defining value for each partner; focused on hospitals that served desired demographics (i.e., central and southern AZ) and that had research infrastructure in place, including the ability to embed the study into electronic health records and having space to build on-site enrollment sites; engaged with stakeholder communities to address and build trust; and ensured that presence of provider champions and partners on site. | Accrual of 30,000 participants (surpassing initial goals) and matching goals by race/ethnicity. |
| Competing demands/priorities | |||||
| Federal/policy level | |||||
| Recruitment of minority participants | • Policy mandating enrollment of minorities | NIH Revitalization Act of 1993 (1): recruitment and retention | Minority and female participants across the life span | Congress required the NIH to establish guidelines for inclusion of women and minorities in clinical research. Resulted in mandate that clinical research must include women and minority populations unless justification as to why they cannot be included is provided. | Increased reporting of minority and women included in research. Review of published studies in 2015 vs. 2004 and 2009. Successful in increasing participation of women in research (median of 46% in 2015 vs. 38% in 2009 and 43% in 2004); however, only 26.2% included analysis by sex or included this variable in statistical analysis (vs. 25.0% in 2009 and 13% in 2004). Less able to assess success with increasing minority participation, as many studies still fail to include demographic data for racial and ethnic minority groups (32.4% and 53.5% in 2015 vs. 43% and 52% in 2009 and 33.3% and 36% in 2004 for African American and Hispanic groups, respectively). Furthermore, few studies included analysis by race/ethnicity or included this variable in statistical analysis (13.4% in 2015 vs. 14% in 2009 and 8.7% in 2004) (5). |
| Retention of minority scientists | • Diversity supplements | NIGMS Research Supplements to Promote Diversity in Health-related Research (171): recruitment and retention; longitudinal follow-up of undergraduates, graduate students, and postdoctoral participants | Scholars from groups underrepresented in biomedical science supported between 1986 and 2006 | Supplement (up to $100,000 in 2020) to eligible parent NIH grant that provides support for trainee to engage in research (salary support allowed). | 73% vs. 50–58% national completion rate for STEM-related doctoral programs; 65% of all diversity supplement graduate and postdoctoral recipients achieved research careers. |
| • Loan repayment programs | NIH Intramural Research Loan Repayment Program: retention; comparison between recipients and nonrecipients working in similar settings | Early-stage investigators with significant student debt | Competitive grant for loan repayment (up to $50,000 in 2020). | Increased retention in NIH research positions starting at Year 2 (99% vs. 87%) and persisting for >10 yr after receipt. Increased initial retention in research starting at Year 3 (93% vs. 86%) that reemerges at 10 yr after receipt (74% vs. 52%) (172).† From 2015 to 2019, success rates by race/ethnicity were 53% for non-Hispanic White applicants, 41% for Latinx applicants, and 32% for Black applicants (173). |
Definition of abbreviations: AZ = Arizona; CBPR = community-based participatory research; CEASE = Communities Engaged and Advocating for a Smoke-free Environment; CHW = community health worker; CI = confidence interval; EVMC = Engagement, Verification, Maintenance, and Confirmation; IMPaCT = Increasing Minority Participation in Clinical Trials; L.A. = Los Angeles; NIGMS = National Institute of General Medical Sciences; PN = patient navigator; RECRUIT = Randomized Recruitment Intervention Trial; STEM = science, technology, engineering, and math.
Included CBPR perspective for intervention design.
Data not available by race/ethnicity.
Partnering with PAOs
PAOs, ranging from large, formal organizations like the CF Foundation to small, informal consortiums like the Hermansky-Pudlak Syndrome (HPS) Network, have an important role in providing patients and caregivers with information and resources, while also influencing funding priorities and research agendas. Their unique position makes them a natural partner for increasing research engagement. Indeed, efforts of the Patient-Centered Outcomes Research Institute (35), formed in 2010, and the 2012 FDA Safety and Innovation Act (FDASIA) (6) have formalized the involvement of PAOs as key stakeholders. As we move into precision medicine, contribution from these groups is necessary across the research spectrum, extending from bench science and drug development to health outcome research.
An underrecognized role of PAOs is their ability to gather and empower a cohort of patients, many of whom are interested in directing and participating in research leading to impactful treatments for their disease. When engaging with PAOs, one must consider their funding sources and whether their priorities are aligned with the proposed research goals. Involvement of patients and PAOs can range from directing the research aims to participating in other meaningful engagement activities (see Applying a Community-engaged Perspective to Research).
Engagement with PAOs may increase minority participation in studies across the research spectrum. An example of successful engagement includes the genetic study on primary ciliary dyskinesia (PCD), in which “the PCD Foundation’s research group ‘went on the road’ and took vans outfitted with genetic testing equipment [as well as basic clinical care equipment] to a number of Amish communities, as it was culturally difficult for them to come to us” (Michele Manion, President and Executive Director of the PCD Foundation; written personal communication, January 28, 2020). In another example, the Scleroderma Foundation National Director of Programs and Services, Kerri Connolly, successfully reached the Black community through “faith-based organizations and specific radio stations” (written personal communication, January 28, 2020).
Case Study 1
Our organization, the HPS Network, was so small that our first conferences were hosted in my home. The drive to participate was altruistic, to contribute to our understanding of a rare disease that disproportionately impacted our community in Puerto Rico. Motivation and creativity allowed us to move forward in those early days: our members willingly collected 24-hour urine buckets for two days, overcoming the need for refrigeration by storing samples for the research team in snow.
Due to a genetic founder’s effect, our membership is largely composed of individuals of Puerto Rican descent. As the primary recruiter for two drug trials, we identified many challenges to participation. Our community struggles with language barriers; and, as many with HPS are legally blind and chronically ill, most individuals are not financially stable. A day’s work is missed by both our participants and the caregiver[s] who accompany them. Transportation to a clinical site is also a major barrier because they do not drive. To address these barriers, we provided transportation money and advocated for participants to go to study visits via a buddy system.
In response to our members’ difficulty [with] travel, we proposed a program to bring clinical research to the patient, instead of having them to go to the clinic. ‘We’re Drawn Together’ was started at the 2018 HPS conference, which included both patients and researchers. This initiative invited researchers and academic centers to understand transportation challenges, financial barriers, disability, and chronic illness when enrolling research participants. We used the conference to recruit 98 participants into five separate research protocols, all with different IRBs [institutional review boards], enrolling in two languages with different consents in a two-hour span; an accomplishment that previously took several years.
We also studied the history of clinical research in Puerto Rico to increase our understanding of the deep-seated reservations to joining studies. Prior abusive clinical research in Puerto Rico has caused reservations about research participation that [are] further fueled by the isolation of the rarity of the disease. Realizing how much trust is a part of the formula, we created “Individual Research Plans” and used our vetted tool to dive deep into the barriers and challenges faced by our members. Targeted and personal interventions show support and encourage a more committed engagement.
—Donna Appell, R.N., Executive Director, HPS Network
Applying a Community-engaged Perspective to Research
The principal goal of community-engaged research is to reflect the concerns and questions of communities bearing the burden of disease and/or exposure of interest; this approach can also increase study participation (36–38). The degree to which the local community is involved in the research process differentiates community-placed research from community-based participatory research (CBPR) (Table 3) (39, 40). In CBPR, mutual collaboration predates the research. Community partners lead efforts to identify research needs, which are both integral to and integrated into research planning before the other phases of research (e.g., implementation, evaluation, and dissemination). In CBPR, community–academic partners make all decisions collaboratively, with the opinions of the community advisory board/steering committee being given the same weight as those of the scientists. This is in contrast to an investigator entering a community with a predetermined research agenda and unequal ownership of the research, even when the research is invited and supported by the community. Community-engaged research is not a method but is an orientation to research that is equally effective in quantitative, qualitative, and mixed-method designs (41, 42). For long-term success, community–academic partnerships must build capacity and honor commitments to sustain the relationship between projects (43, 44).
Table 3.
CBPR versus Community-placed Research
| Community-placed Research | CBPR | |
|---|---|---|
| Goals | To generate new knowledge | Research is a vehicle for immediate action |
| To decrease health inequities | ||
| Agenda setting | Academia | Collaborative effort between academia and community |
| Primary emphasis | Advancing science to improve health | Action to improve health |
| Publication | Empowering the community | |
| Sustainability | ||
| Expertise | Academia | Academia and community |
| Level of community participation | Mostly subjects; may aid in recruitment | Engaged partners throughout the research process |
| Dissemination | Primarily through publication to the medical community | To the affected communities, policy makers, health advocacy groups, and the medical community |
| Sustainability | Not a focus | Necessary for success |
| Funding | Grants written by researchers; funds go to researchers | Shared grant writing |
| Equitable compensation | ||
| Added challenges | None | Building trusting relationships |
| Data sharing/management | ||
| Engagement of a vulnerable community | ||
| Time intensive |
Definition of abbreviation: CBPR = community-based participatory research.
Reprinted from Reference 39.
On the spectrum of community-engaged research approaches are action research and participatory action research, which are approaches that seek to improve a problematic (even oppressive) situation. The term “action research” indicates that research is conducted by those in privileged positions for the benefit of those who do not have privilege. “Participatory action research” implies a collective research effort between those who hold power and those who are disempowered (45). The term “critical” is applied to action research that rejects scholarly research as exclusive and exploitative and instead seeks to create social change by privileging nonacademic knowledge for the cocreation of knowledge that brings about lasting local change at the community, institutional, or societal level (46).
Community-engaged research is effective in enhancing minority participation in clinical research (22, 36, 47). A long-term investment in the community–academic alliance is needed for equitable collaborations and for mutual benefit. Such alliances tap into the strengths and resources of both partners while focusing on locally relevant public health problems (48). Successful examples include the CEASE (Communities Engaged and Advocating for a Smoke-free Environment) study (49), the BEAMS (Breathe with Ease: A Unique Approach to Managing Stress) study (50–52), and the WORD (Wholeness, Oneness, Righteousness, Deliverance) weight-loss study (53), which developed intervention components, iteratively changed the intervention delivery method (healthcare provider to lay person), and/or changed the setting (clinical to nonclinical) on the basis of community input, resulting in enhanced recruitment and retention strategies that increased participation two- to threefold from the original study design (36, 49–53).
By inviting the community to identify salient areas for partnership, there is shared enthusiasm and buy-in to codevelop knowledge and build capacity while increasing research participation that creates social change that may reduce health disparities (39, 41). Such community advocacy and research methodologies democratize science, producing culturally tailored and culturally informed interventions in which community values are integrated into the research plan, thus addressing a wide range of recruitment and retention challenges (41).
Case Study 2
In 2014, the investigators were conducting an unrelated clinical trial at an urban, federally qualified health center (FQHC) in the zip code with the highest city rate of asthma morbidity and mortality. During this time, the study’s principal investigator met with FQHC administrators and with Black adults receiving asthma care. From the patients and providers, the investigators heard about the unique challenges to asthma management, including the limited time and resources available to the clinicians and the deeply entrenched health beliefs about asthma and its treatment that undermined patients’ adherence to controller therapies (54). Administrators and patients indicated that better patient–provider communication and partnerships could improve adherence to controller medicine.
The principal investigator invited a patient participant to join a newly forming research team as a coinvestigator to design a patient–provider partnership intervention. In the first phase of the trial, six focus groups with 46 Black adults with asthma and their loved ones designed the intervention, which focused on fostering shared decision-making (55–57). In the group-randomized trial that followed, 10 FQHC clinicians delivered the active or dose-matched attention control intervention to 80 Black adults with uncontrolled asthma. Of 124 potential participants reached to glean enrollment interest, 28 (23%) declined to be screened. Of the 89 who were eligible, 80 were enrolled. Participant retention over the 3-month trial was 95%, 289 (90%) of 320 scheduled follow-up visits were completed, and there was a high degree of satisfaction with trial participation and the intervention components. Taken together, these data suggest that interventions created with or in partnership with the community can overcome challenges to recruitment and retention of minority participants.
Addressing Individual-Level Barriers
Adding Flexibility and Addressing Resource Constraints
Structural and social determinants are significant barriers to participation (23), particularly for economically disadvantaged and racial/ethnic minority communities (51). Commonly cited barriers include transportation, distance, time required, competing caregiving and job responsibilities, and the financial costs associated with these factors (22–24, 51). Low or limited health literacy also hinders recruitment and may pose ethical challenges when ensuring informed consent (25). Study consent processes may be overly complex to meet institutional review board requirements (26) and may place disproportionate demands on Latinx, Black, and indigenous communities, in which English proficiency is often limited and health literacy is lower than that in non-Hispanic White adult communities (58).
Most published recruitment and retention strategies address these individual-level barriers through bundled interventions, making it difficult to discern the independent effects of each component. The largest amount of evidence-based data exists for transportation and for flexibility in scheduling and the mode of survey administration (59–64). However, the majority of transportation-aid studies have had a single arm; thus, the expected effects may vary depending on the baseline/preintervention attrition rates (61, 65, 66). The Engagement, Verification, Maintenance, and Confirmation Model is an evidence-based recruitment strategy (62, 64) that extends flexibility to the mode and method of survey administration during its maintenance phase. This model has been successfully adapted and implemented to increase recruitment and retention of adolescents in an urban asthma study, resulting in a retention rate of 85–91% (64). In the Toolkit: Suggested Enhancements for Research Protocol Implementation section, we outline these and additional strategies that target individual-level barriers, have a lower cost, and can be quickly added to enhance recruitment and retention efforts.
Case Study 3
The Patient-Centered Outcomes Research Institute funded eight comparative-effectiveness studies to address the disparity in asthma control and morbidity in African American and Latinx populations (51). Six of the eight studies were in urban settings, and all had a stakeholder engagement component and involved self-management, care coaches, or community health workers as part of their intervention. Studies varied in their time commitment (7.5–19 h over the study period), outreach efforts for recruitment, travel supports or reimbursements, and incentives (ranging from $50 to $345, with or without nonmonetary incentives).
Sites at the higher end of accrual (range, 3–67%) at the midpoint of enrollment offered higher incentives ($150–345). Querying the site research teams revealed difficulties with reaching populations and competing demands, self-perception and disease understanding, (i.e., eligible participants did not believe they had the disease of interest), and mistrust or aversion to participating in research as barriers. Each site developed protocols to address barriers (see Table 3 in Reference 51). At the conclusion of the trials, five of eight sites reached their enrollment goals (range, 57–105%), and those with available data (seven of eight studies) reported 59–91% retention at study completion (67).
Addressing Interpersonal-/Provider-Level Barriers
Addressing Bias
Despite barriers, acceptance rates for study participation by minority groups are at levels similar to those for non-Hispanic White populations (68, 69). Furthermore, retention rates are similar across racial/ethnic groups when participation barriers are addressed (70). This suggests that overt and covert forms of discrimination are significant barriers to trial participation (20, 22). In a systematic review of 65 studies, a third of studies reported providers’ attitudes as a barrier to minority participation in research (24). In one study, clinicians failed to present active research opportunities to their African American patients 24% of the time compared with 8% of the time for all other groups (71). In a qualitative study of clinical and research professionals, the perception that “minority participants are not perceived to be ideal study candidates” emerged as a main theme regarding barriers to inclusion of minority populations in cancer clinical trials (72). Reasons highlighted in the literature are clinicians’ assumptions regarding the ability to participate, including logistic concerns; belief that they knew their patients’ preferences; and concerns about nonadherence (22, 24).
In health care, bias has significant impact on who receives guideline-based care (73). Schulman and colleagues (74) found that sex and race were independent factors in determining a providers’ recommendations for cardiac catherization for patients presenting with chest pain. In another study, it was shown that oncologists with high implicit bias have shorter interactions and use less patient-centered communication with their African American patients than providers with less implicit bias (75). Implicit bias is insidious, occurs across the healthcare spectrum, and likely has an important role in the decision-making process for determining participant eligibility and whether a research study will be presented at all. This has likely led to systemic underenrollment of minorities in research. To combat the role of implicit bias, those involved in enrollment must first understand their own biases. The Implicit Association Test, developed by Greenwald and colleagues (76, 77), is a tool designed to detect the strength of a person’s automatic associations among mental representations of objects or persons in memory, therefore raising awareness of one’s own actual biases, which often contrast with the perceived biases.
Literature examining implicit bias in health care is limited (73, 78), despite its far-reaching effects on disease management and the development of clinical research studies. Identifying implicit bias through mechanisms such at the Implicit Association Test or through equity, diversity, and inclusion training is a first step toward dismantling racial and ethnic bias (and racism) (76). It is not enough to recognize our own biases; rather, purposeful steps must be taken to reverse them. Actively implementing efforts to address provider bias and attitudes is likely an effective strategy for increasing recruitment and engagement with research (20, 79).
Building Trust
Although the source of mistrust may differ by community, mistrust is a universally identified barrier to participation in clinical research (22). Understanding the unique sources of mistrust is a necessary first step toward building trust, which can then be followed by other approaches to build trust and authentic relationships with key community leaders. Here, we focus on the role of the healthcare provider. Although potential participants report distrust of study investigators and research institutes (22–24), they report a high level of trust in their healthcare providers (80, 81). Patients are more likely to agree to participate in trials if they have had positive interactions with the healthcare system (20), if they trust their healthcare provider, and if their provider recommends that they take part in research (82). Patients trust their healthcare provider to act in their best interests and assume that medical researchers will also act in the patients’ interests and not just in those of the investigators (83).
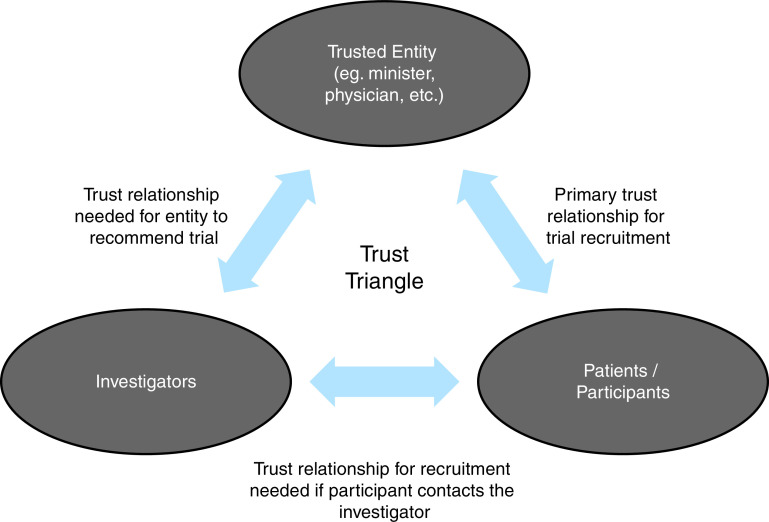
For successful recruitment, trust must be present in many interconnected relationships (see the Trust Triangle in Figure 2) (84). Patients must trust healthcare providers and key (community, opinion, and religious) leaders, and at the same time, these leaders, who are referring minority patients, must trust the researchers.
Figure 2.
Trust Triangle of the investigator, the community-based trusted entity/leader, and the participant. Adapted by permission from Reference 84.
Despite the key role that trust plays in minority recruitment, few published studies have focused on strategies to increase trust between minority patients or their healthcare providers and the process of recruitment into clinical research. To date, trust-based interventions that focused on the relationship between referring physicians and researchers have shown mixed results in terms of recruitment (85). There are several ongoing trust-based interventions that will further contribute to the evidence behind such strategies (84, 86). Other studies on trust and minority recruitment have focused on building trust between the community and researchers by using CBPR rather than randomized trial interventions (37, 38).
Case Study 4
The largest trial focused on increasing minority recruitment using a trust-based intervention (RECRUIT [Randomized Recruitment Intervention Trial]) was a stratified, clustered, randomized-design trial embedded in large, oncology-focused trials (84). Fifty specialty clinics (sites) from four NIH-funded parent trials (PACES [Preventing Adenomas of the Colon with Eflornithine and Sulindac], CABANA [Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation], STEADY-PDIII [Safety, Tolerability, and Efficacy Assessment of Isradipine for Parkinson's Disease], and BMT-CTN [Blood and Marrow Clinical Trials Network]) were randomized to the trust-based intervention or control. There was considerable heterogeneity among parent trials, and when all four parent trials were analyzed together, there was no overall intervention effect (87). However, in three of four parent trials (PACES, CABANA, and STEADY-PDIII), the intervention sites exhibited greater minority enrollment (29.9%) than the control sites (8.2%), suggesting that trust-based interventions are an effective way to increase enrollment from minority communities.
The Trust Triangle highlights the importance of the relationship between the investigator and the patient, as well as the relationship between the investigator and the referring trusted entity/leader. The RECRUIT strategy fails when the investigator does not work toward building trust with the referring trusted entity/leader, neglecting that side of the triangle. If the trusted entity/leader does not have a positive relationship with the investigator, or even the institution, then they will not vouch for the investigator and may in fact openly lobby against participation in the study.
The other factor that fosters success in the RECRUIT strategy is a high prevalence of the disease of interest, which will facilitate a measurable increase in referrals. Building a positive relationship between the investigator and the trusted entity/leader is necessary, but if the provider does not have any patients to refer, the impact of the RECRUIT strategy will be small.
Enhancing Recruitment with Sociocultural and Multimedia Outreach
Communication on social media platforms has become ubiquitous in many communities. Combining the advent of social media with the effectiveness of sociocultural outreach (88–90) has the potential to allow investigators to access large cohorts of people with common characteristics. The term “social media” represents any internet-based platform that allows users to make profiles and interact with other users. According to a 2019 Pew Research Center survey, 70% of Black Americans and 69% of Latinx Americans have used Facebook, whereas 16–51% of Black A and Latinx Americans have used other social media platforms (e.g., Instagram, LinkedIn, Snapchat, and Twitter) (91). Social media platforms have the capacity to capture wide, captive audiences of potential research participants from minority groups in an efficient and cost-effective way.
Both active and passive recruitment strategies have been effective in enrolling minority participants in clinical research, depending on the context of the study (92). The term “active” is here defined as placing the onus for contact on the research team, whereas “passive” is descriptive of the patient having to be the one to initialize contact with the research team. Recruitment through social media represents one form of passive or indirect recruitment in which information about the research study is advertised publicly as opposed to being disseminated through direct, face-to-face communication with study personnel. In a systematic review of 30 studies that used social media as one of several recruitment strategies, 40% of studies reported that such a recruitment approach was more effective than other passive and active recruitment methods (93). One study reported nearly threefold increased recruitment of Black pregnant women through social media as compared with clinic-based recruitment approaches (94). Several studies have identified social media as an effective mode for recruiting vulnerable and difficult-to-access populations, including minority youth (95, 96). Of note, observational studies may benefit more from recruitment through social media platforms than interventional studies (93).
Although recruitment for research participation through social media is a novel and attractive strategy, this recruitment method is in its infancy. Thus, guidelines for the ethical use of social media have not been universally adopted by institutional review boards. Gelinas and colleagues (97) have proposed a set of guidelines regarding recruitment through social media. They note that although social media as a source of communication can be very useful, there may be implications stemming from participants communicating and disclosing information that may jeopardize recruitment, reveal treatment statuses, or influence public perception. Combining this with what we have learned from targeted recruitment through culturally relevant advertising and word-of-mouth campaigns, similar care should be applied to develop social media campaigns in an effort to increase underrepresented minorities in clinical research.
Case Study 5
Compared with traditional passive recruitment strategies, a social media campaign via Facebook improved recruitment for a smoking cessation trial. Investigators placed paid advertisements on Facebook targeting users aged 18 and above who lived near the study site. Advertisements varied throughout the recruitment period on the basis of the number of clicks the advertisement generated (advertisements receiving fewer clicks were removed) and the volume of recruitment generated. When the advertisement was clicked by a user, it led the user to a new webpage that provided general information on the study and allowed the user to enter screening information for recruitment. Users were then contacted by research personnel, and eligibility screening took place over the telephone. Approximately half (52%) of all participants enrolled in the study were recruited through Facebook. Participants who were recruited through Facebook were 6.8 years younger on average than those recruited through traditional, passive recruitment strategies, yet the two groups were similar in terms of gender, ethnicity, education, and income (98).
Addressing System-/Institutional-Level Barriers
Health system– and institutional-level barriers to minority participation in research are far reaching and include limited trial availability in nonacademic centers (99, 100), insufficient infrastructure supports, lack of supports (including funding) for minority investigators and health disparities research, and nonengagement with target communities (24, 99, 100).
Diversifying the Research Team
“Team diversity” refers to differences between individuals on any attribute that may lead to the perception that another person is different from oneself (101, 102). These differences include observable demographic attributes such as race/ethnicity, gender, and age. Other attributes of diversity include functional, educational, or social backgrounds. The benefits of a diverse research team in terms of creativity and impact have been extensively reported (103–105). In routine clinical care, provider–patient race concordance is associated with better communication and satisfaction (106–108). Team diversity increases the novelty and breadth of inquiry (109), as exemplified by a higher number of citations per publication for studies by diverse teams than for studies conducted by less diverse groups (105, 110, 111) and the fact that diverse teams are more likely to address issues relevant to all members of society, including racial and ethnic health inequities (109, 112). To this end, the ATS should make purposeful efforts to ensure diverse representation in all sponsored documents, given their influence on clinical care and research directions. Whenever possible, the ATS should educate members, committees, assemblies, etc. on the importance of diverse representation. In an approach similar to federal efforts, the ATS should review and make publicly available the racial/ethnic, gender, and educational backgrounds of document committee members to track progress and identify gaps that are amendable to intervention.
Although there are no studies that have directly investigated the impact of the diversity of study investigators on minority recruitment, an increase in minority recruitment has been demonstrated in studies in which there is racial, ethnic, or cultural concordance between the project team members and the potential participants (54, 89, 113). Such an approach can minimize participation barriers, including mistrust, lack of information, and nonawareness (22, 89, 114–116). Among Asian Americans, Latinx groups, and Pacific Islanders, language-appropriate materials and research staff have been reported as important facilitators to participation (117–121). Prominent examples of success from study teams that involved diverse team members include the HCHS/SOL (Hispanic Health Study/Study of Latinos), which enrolled 16,000 Latinx participants; the Mano a Mano study, which enrolled 19,000 participants of Mexican descent; and the Black Women’s Health Study, which enrolled 59,000 participants (122–124).
Case Study 6
The New York City Inner-City Asthma Consortium (125) site at the Columbia University Irving Medical Center is consistently one of the most successful sites in terms of recruitment and retention. This site has exceeded target recruitment by up to 54% in five out of the six most recent Inner-City Asthma Consortium pediatric studies. The site also has achieved 89–100% retention rates in randomized control trials and observational studies, as well as achieving 76% retention within a longitudinal birth cohort through 15 years of age. Much of this success is due to the efforts of an extraordinary team of clinical research staff. There has been a long-standing history of diversity within the research team, with previous members coming from various cultures and backgrounds. The current team of research coordinators is led by a native Spanish speaker from Peru and includes four additional research coordinators, three of whom are of Caribbean descent (Dominican, Nicaraguan, and Ecuadorian). The team also includes a research nurse who is a native Spanish speaker from Cuba; two nurse practitioners, including a Black woman of Caribbean descent; and four physicians, including one Black woman of Caribbean descent. The racial and ethnic demographic of the team closely resembles the demographic of the study participants in the Northern Manhattan community surrounding the medical center, including the predominately Dominican neighborhood of Washington Heights and the predominantly Black neighborhood of Harlem. The cultural diversity within the team contributes to improved team dynamics and increased engagement with the diverse research participants.
The importance of the ability of staff to connect with research participants cannot be overstated. All team members, regardless of race or ethnicity, either come from the community served or have lived and worked in similar types of communities for years. This enables the research team to break down cultural barriers, assuage fears, and garner trust from study participants and families. Team members are able to communicate with families in their native language and address cultural needs to ease the communication process and ensure understanding. In addition, the research team serves as a recognizable and trustworthy point of access to the medical system. Because of this familiarity and accessibility, the site successfully engaged participants in research study protocols and facilitated access to a broad array of medical care. These positive experiences often lead to many families recruiting other family members and friends to participate in studies. This experience has also inspired many team members to pursue higher education in public health and medical fields. Building a team with members of the community and people who share similar cultural backgrounds has resulted in relatable role models for young patients and lasting ties with the community.
Aligning Clinical and Research Enterprise Priorities
The silo culture of clinical and research enterprises exacerbates the lack of participation in clinical research among minorities. Although healthcare professionals remain the most trusted source of information (80, 126), most clinical research has failed to engage providers who directly care for minority patients (127).
Aligning clinical and research priorities also has the potential to fulfill the Internal Revenue Service mandate that hospitals conduct community needs assessments to maintain their 501(c) nonprofit status (128). Meaningful partnerships across enterprises would facilitate needs assessments, improve engagement with communities, provide an avenue to study and implement interventions to address the identified needs, and avoid conflict arising from nonaligned priorities. Successful examples of such partnerships exist throughout the United States (129–132). One example is the Rhode Island’s Health Equity Zone initiative, a community-led program to improve the health of the communities in collaboration with diverse partners, including healthcare systems. Since inception, the program has provided mental health and suicide-prevention training to over 1,000 community members, has trained and deployed community health workers to build community–clinical linkage, and has implemented a walking school bus program to boost school attendance. Furthermore, the Health Equity Zone program’s initiatives reduced childhood lead poisoning by 44% and reduced teen pregnancy by 24% while increasing community engagement by 163% (132, 133).
In addition to the inclusion of the clinical enterprise in the programmatic and recruitment efforts of research, other forms of integration across systems can improve efforts to reach and recruit minority participants in research. One example is harnessing electronic health records (EHRs), which can provide an avenue for furthering the understanding of diseases that disproportionately impact certain populations (i.e., assist with needs assessment), identify potential participants, and provide a platform to collect and analyze local data. In addition, natural language processing tools used in concert with conventional EHR data can equitably identify all patients who meet eligibility requirements. Furthermore, as EHRs develop more remote monitoring and communication tools, they offer secure platforms that can be leveraged to communicate with participants. Another example is including members of the clinical team (e.g., nurses and medical assistants) in the development of recruitment protocols and as part of the research team. These support staff members often belong to the same community as potential participants, helping research teams overcome cultural and trust barriers. Lastly, offering enrollment at clinic sites increases awareness about, the availability of, and accessibility to research for populations that do not historically receive care at large academic centers (99, 100).
Addressing Federal-/Policy-Level Barriers
Role of the NIH
In the United States, the NIH is a major source of funding and, consequently, is a driver of respiratory research in all groups, including minority populations. In response to the 1985 report from the Secretary’s Task Force on Black and Minority Health, the NIH recommended the “expansion of biomedical and behavioral research to assure appropriate emphasis on health problems that disproportionately affect U.S. racial/ethnic minority populations” (134). The need for attention to study-design and sample-size concerns when including minority groups was also emphasized. The NIH Revitalization Act of 1993 further expanded recommendations, requiring all NIH-supported human-subject research to include women and members of minority populations and subpopulations and requiring the NIH to regularly report aggregate data on the inclusion of women and minorities across human-subject research to Congress (135). The NIMHD—established by the passage of the Minority Health and Health Disparities Research and Education Act of 2000, Public Law 106-525, as the National Center for Minority Health and Health Disparities and later redesignated as the NIMHD with the passing of the Patient Protection and Affordable Care Act in 2010—has been instrumental in spearheading initiatives for guiding the role of the NIH (136).
To date, policy changes have had suboptimal impact on minority participation in research. In response, the 21st Century Cures Act of 2016 updated the NIH policy and now requires NIH-defined phase III clinical trials to include valid analyses by sex/gender and race/ethnicity in the results reported in clinicaltrials.gov (137). Implementation and enforcement of this policy is expected to occur at multiple points, including at the time of the grant application and during the review process, as well as when research is funded and ongoing. On a regular basis, the advisory council/board for each of the NIH institutes, centers, and offices (ICOs) are to review compiled inclusion data for funded human-subject research to ensure compliance with this policy. The impact of this new policy is to be determined. Furthermore, the policy falls short; compliance with population composition does not ensure the ability to perform meaningful subgroup analysis for the majority of smaller-sized clinical trials. In addition, this policy for the inclusion of valid subanalyses does not extend to other types of clinical research, including device trials, trials at other stages of the FDA process, or observational research.
Another concern is that the strategies implemented by the NIH to enforce this policy vary across NIH ICOs. Efforts should be made to standardized strategies across NIH ICOs and include processes through which to inform and train scientific review officers (who oversee study section reviews) and program officers (who work closely with investigators after an award is made). A laudable step forward is that since May of 2019, annual data on the inclusion of women and minorities by research, condition, and disease categories in NIH-funded research may be found in the NIH research, condition, and disease category inclusion statistics report (138). Making these data publicly available provides a measurable parameter on which to measure the influence of the Cures Act of 2016 and other efforts to increase minority participation in research. A further step would be to have the data stratified by studies that are minority focused and by those that aim to recruit the general public. Increased transparency will provide guidance on where future efforts should be placed (e.g., more funding/special announcements to support minority-focused studies vs. efforts to improve minority recruitment in general).
The NIH has engaged thought leaders and sponsored workshops to identify barriers for the training of a diverse workforce. Several studies have demonstrated that minority investigators are less likely to obtain R01 funding than their White counterparts (139–142). This has important implications, as diversity study team improves minority recruitment and retention. A 2005 effort highlighted several barriers to NIH funding for minority investigators (143): inadequate research infrastructure, training, and development for minority scientists as independent researchers; inadequate mentoring; insensitivity, misperceptions, and miscommunication about the specific needs of investigators involved in research with minority communities; institutional bias in NIH policies; unfair, competitive environments; lack of institutional support; limited attention to topics/methods relevant to research with minority communities; and social, cultural, and environmental barriers. Since these pivotal publications (139, 143), the funding gap for early-career awards through the K mechanism has narrowed significantly for minority investigators. Funding for R01 grants to Black and Hispanic/Latinx applicants increased by 8.4% and 10.9%, respectively, from 2013 to 2018. However, we also saw an increase (9.6%) in funding for non-Hispanic White applicants over this time period; thus, the 10% and 4% funding gaps persist for Black and Hispanic/Latinx applicants, respectively (144). Despite more than a decade having passed, more recent assessments identified barriers similar to those highlighted in 2005. To move forward more effectively, Duncan and colleagues (145) recommended a multipronged approach “to enable the professional development and retention of underrepresented minorities in biomedical research, including addressing individual and social factors and [involving] funding agencies, academic institutions, mentoring teams, professional societies, and peer collaboration.”
The NIH has engaged in purposeful efforts to address deficiencies in the recruitment and retention of minorities in clinical research, as well as in developing a diverse workforce. This includes the recent release of dedicated funding for research on health disparities and/or upstream factors contributing to racial/ethnic disparities (e.g., BUILD [Building Infrastructure Leading to Diversity] [146], FIRST [Faculty Institutional Recruitment for Sustainable Transformation] Cohort [147], DECIPHeR [Disparities Elimination through Coordinated Interventions to Prevent and Control Heart and Lung Disease Risk] [148], NOT-MD-21-008 [149], and NOT-MD-21-016 [150]) However, the full impact of this funding will not be evident for years to come and may not close the observed gaps. Continuing evaluation of the status, implementation of innovative incentives for minority recruitment and retention in clinical trials, and long-term investment in the development of a diverse research community will require continued advocacy, a reprioritization of resources and funding, and a recognition that the goal of equity in respiratory health is unreachable without everyone’s participation—within and outside of the NIH. To accelerate progress, we provide additional recommendations that would more immediately improve the inclusion of minorities in clinical research:
-
1.
Score or raise the importance of the Inclusion of Women and Minorities section of the NIH grant application.
-
2.
Invest in resources to support recruitment of minority populations (e.g., adding supplements to awards to expand recruitment efforts to include minority populations or to further support awards that already focus on minority populations).
-
3.
Revise criteria in funding announcements to emphasize that recruitment should include racial/ethnic minorities, particularly for diseases that disproportionately affect these populations.
-
4.
Ensure that scientific review officers receive training to recognize bias in the study section and provide regular instruction to reviewers to reduce bias.
-
5.
Ensure that clinical research, particularly research on diseases affecting minority populations, is adequately funded when awarded and is sufficiency powered to address differences among underrepresented minority groups.
-
6.
Increase the availability of loan repayment programs or other policies that directly or indirectly remove the financial burden of pursuing clinical research endeavors for Black, Latinx, and indigenous trainees and early-career faculty.
Retrain the Study Section Reviewer
When examining NIH funding mechanisms, one should consider the key role of the study section reviewer in the grant application process. Reviewers must be critical of studies that fail to meet the 1993 mandate and must give favorable consideration to well-designed studies attempting to meet this mandate. After the policy put forth in the 1993 mandate, applications that do not appropriately include women or minorities must be held accountable and provide scientific justification for the proposed inclusion and exclusion criteria.
Reviewers should query whether the proposed study includes a representative sample of the population disproportionately burdened by the disease of interest. For example, asthma disproportionately burdens Black and Puerto Rican populations; thus, studies of asthma should include adequate samples from one or both of these groups. To ensure successful accrual of representative samples, reviewers also need to assess minority inclusion plans and outreach initiatives, taking these into consideration when evaluating the scientific merit of the application and overall scoring. This would help ensure that minority populations are adequately represented in federally funded studies.
Role of the FDA
The FDASIA was signed into law on July 9, 2012 (6). Section 907 of the FDASIA included requirements from the U.S. Congress for the FDA to submit a report on the extent of clinical trial participation and the inclusion of safety and effectiveness data by sex, age, race, and ethnicity.
The 2014 FDA report reviewed the statutes, regulations, and policies currently in place (151). The report concluded that most sponsors describe the demographic profiles of their clinical participants by sex and age, and most applications included subset analysis by sex. However, there were few reports on race/ethnicity, and the inclusion of subgroups did not ensure that enough data were collected to conduct meaningful analysis or detect differences.
In response, the FDA developed an action plan with extensive public and stakeholder engagement. Feedback from patient and health professional groups concluded the following:
-
1.
The proportion of women, minorities, and older individuals in industry-sponsored clinical trials is not consistent with the prevalence of the disease in the underlying population.
-
2.
Health professionals and patients do not have enough demographic information to make well-informed treatment and diagnostic decisions.
-
3.
Inclusion of data from non–U.S.-based studies created a problem in terms of relevance to U.S. racial and ethnic groups.
In 2016, Congress recommended that the FDA work in cooperation with industry to ensure that the representation of women and minorities in industry-sponsored clinical research is comparable with that of that of NIH-funded trials. It was also recommended that meaningful subgroup analyses for safety and efficacy be performed with transparent, public reporting of results.
The FDA action plan addresses four overarching priorities:
-
1.
Improving the completeness and quality of demographic subgroup data. This includes taking steps to strengthen reviewer training with education on demographic inclusion, analysis, and communication of clinical data.
-
2.
Enhancing the systems for collecting, analyzing, and communicating diverse clinical information to optimize safe and effective use of medical products in diverse populations over the life cycle of the product.
-
3.
Promoting and conducting research on specific areas of public health concern related to demographic subgroups. For example, this includes working to identify barriers to subgroup enrollment in clinical trials and employing strategies to encourage greater participation.
-
4.
Making demographic subgroup data more available and transparent. The FDA now posts the demographic composition and analysis of subgroups in pivotal clinical studies for FDA-approved products.
To achieve these priorities, the FDA has applied methods to consistently communicate meaningful information on demographic subgroups in medical product labeling; implemented communication strategies that are sensitive to the needs of underrepresented subpopulations (focusing on language access and health literacy); and, finally, provided oversight through an FDA steering committee that tracks the implementation of the action plan and serves as a planning group for any related workshops. The efforts underway should be lauded for their comprehensiveness and broad reach. The FDA, in its regulatory role, impacts enforcement of the inclusion of diverse populations in the drug-development pipeline and in clinical trials, which could have spillover effects on all of clinical research. To ensure efforts are carried forward, we must closely watch to see if these initiatives have the intended effect and be at the ready to request additional efforts for self-evaluation if they fail to make progress.
Special Considerations for International Studies
Barriers that impact recruitment and retention of minorities in clinical research are often magnified in international studies, particularly those conducted in low- and middle-income countries (LMICs), where scarce resources, language differences, and cultural differences need to be addressed (152–154). Such factors are compounded by the presence of different regulatory rules across countries, as well as by heterogeneity in the application of ethical principles (e.g., informed consent). The local context of poverty and low access to health care increases power imbalances between researchers and participants, putting participants into a vulnerable position. Participants may feel compelled to participate in the hope of receiving free health care, which may interfere with ethical informed consent and result in unmet expectations (153, 154). In addition to planning for translation, investigators should also plan for a culturally sensitive consent process, consider different patterns of decision-making in different societies, simplify medical terms, and address misconceptions and local beliefs. Low levels of literacy in LMICs impact informed consent and reduce the efficacy of printed materials (154). Other barriers amplified in LMIC settings include the perception of disempowerment and distrust in clinical research (153), concerns regarding study complexity (155), lack of time and resources, and difficulties integrating the role of researcher into a busy clinical schedule (152). These concerns are often amplified in the contexts of limited health-system capacity to engage in research or lack of research infrastructure. Research specific to diseases and conditions more prevalent in LMICs, such as HIV/AIDS, tuberculosis, malaria, and neglected tropical diseases, must often focus on participants with risk factors that complicate their participation in clinical research (153). This includes challenges such as mobile populations, fear of discrimination, and fear of stigmatization (153).
Reports from successful research studies conducted in LMICs shed light on innovative solutions (152). A key facilitator is engagement of community leaders and stakeholders (152, 153), as well as recruitment of intermediaries, such as local community workers, with knowledge of local customs (153). Regular meetings with the community, before and during the study implementation, inform the trials’ progress and partial results and offer the opportunity to get feedback from the community and address misconceptions (152). Logistic strategies include mapping and documenting the community before launching the study, using mobile phones to communicate with participants and coordinators, promoting the study in local events (152), managing different expectations, and respecting hierarchical structures in the community (153).
Toolkit: Suggested Enhancements for Research Protocol Implementation
In considering strategies to enhance recruitment and retention of minority populations for clinical research, investigators should first consider the site for recruitment so as to best tailor and implement effective strategies. Clinic-based research may focus on chart reviews or approaching potential participants during clinic appointments. This requires using strategies that differ from those of community-based research, which aims to connect with potential participants where they live or work, an environment that may not be solely health focused. Clinic-based recruitment and retention require careful coordination with the clinical care team to ensure that the clinic flow is not disrupted and ascertain the need for separate space. Community-based research requires attention to selecting locations that are both convenient for the population of interest and provide a safe and appropriate environment for the research-related tasks.
Workshop members agreed that approaches to enhance recruitment and retention can either be accomplished at the individual investigator level or may require institutional and system-wide efforts that result in the culture changing to be more inclusive of minority individuals in clinical research. As described, methods for recruitment and retention are broadly categorized as active and passive methods, with active methods being preferable and having a lower participant burden. Using the example of sending introductory letters for the purpose of recruitment, active recruitment would entail the research team contacting potential participants after a predetermined number of days that would allow the patient to opt out of being approached (156). Passive recruitment would entail potential participants having to opt in and contact the research team directly to learn more, leaving the research team being unable to reach out otherwise.
Table 4 provides a summary of actions that can be swiftly applied to enhance the recruitment and retention of minority populations. If funding permits, the single most effective strategy for increasing minority recruitment and retention in clinical research is including persons who identify with the target community, such as community health workers, lay persons from the target community, or patient navigators (113, 157, 158). Such individuals often serve as a cultural bridge to the target community, provide a continuity link to the research study, and can help address logistic and economic barriers, addressing many identified barriers to participation in clinical research (Figure 1).
Table 4.
Enhancements for Implementation by Investigators into Research Protocols to Enhance Minority Recruitment and Retention in Clinical Research
| Recruitment | Retention | Overall |
|---|---|---|
| ✓ Culturally sensitive visuals on patient-facing materials ✓ Appropriate literacy of written materials ✓ Authentic and careful recruitment language ✓ Obtain feedback on patient-facing materials from target population ✓ Allow opt out rather than opt in for contact by research team when feasible |
✓ Collect multiple methods of contact (e-mail, phone numbers, texting, social media, alternate contact person) ✓ Implement follow-up data collection methods that are as low patient burden as possible (home visit, phone, community location) ✓ Patient research participation appreciation and engagement (certificates, token gift, study gatherings, birthday card, newsletter) ✓ Provide a direct connection to the study team |
✓ Ensure flexible scheduling available (including evenings and weekends) ✓ Transportation assistance ✓ Train staff to use communication and interpersonal skills to establish trust and build rapport ✓ Offer meals for longer study visits ✓ Increase financial compensation if appropriate ✓ Offer childcare if target population is likely to be caring for children/grandchildren ✓ Elicit feedback from participants on their research experience ✓ Provide research in more than one language, when possible ✓ Establish standardized hard-to-reach protocol |
Methods specific to improving recruitment of minority populations broadly include ensuring culturally sensitive and literacy level–appropriate patient-facing materials and active engagement with populations of interest. Investigators should also evaluate potential barriers that may exist for disease populations of interest to ensure that appropriate recruitment strategies are used. For instance, a social media campaign may be effective for engaging with younger adults with asthma but may be less effective for older adults with chronic obstructive pulmonary disease. Universal screening, defined as the review of all potentially eligible participants and approaching all eligible individuals, regardless of perceived interest or past participation in research, is another strategy that may be effective for recruitment.
Retention-specific strategies include collecting multiple methods of contact, establishing regular non–study visit engagement with participants, and ensuring that follow-up data collection is conducted via the lowest-burden modality. For instance, if a participant needs to complete a survey, this could be completed via a phone call or be self-administered through a website (e.g., the Engagement, Verification, Maintenance, and Confirmation Model) (62).
Strategies that can be applicable to both recruitment and retention efforts include flexible scheduling, accommodating participant barriers to research participation (transportation, childcare, language, providing food), ensuring that the type of financial compensation is usable by the participant (e.g., cash vs. gift cards that may require access to online retailers or travel to nonlocal stores) and is commensurate with the participant’s effort, and training staff to use communication skills to build trust and rapport with participants. Standardization across research team members for contacting potential or enrolled research participants can also be effective. In one study targeting low-income families of children with asthma, implementation of a standardized hard-to-reach protocol resulted in a 16% increase in the proportion of potentially eligible participants who consented and an 11% decrease in the proportion of potentially eligible participants who could not be contacted (159). In that study, the hard-to-reach protocol consisted of mailing letters, calling each available number at least 10 times on various days and at various times (including on weekends), sending e-mails and text messages, and connecting with the community partner for updated contact information. Participants had no major issues with the rigor of this hard-to-reach protocol for this study and generally appreciated the research team’s persistence and flexibility in terms of working around competing life demands. Establishing protocols and processes for outreach, including the timing, frequency, and methods of contact, ensures that participants are not excluded simply because of being harder to reach. Finally, recruitment and retention efforts should be recorded and tracked in a systematic manner to allow for periodic review by investigators and research team members to identify successes and barriers. Such regular review of recruitment and retention efforts can allow troubleshooting to occur in real time with the protocol to maximize recruitment and retention within the research project.
Recruitment and retention of minority populations at the institutional level should include regular engagement with community members and patient advocates to bring their voices to research. Institutions should provide resources to investigators around effective recruitment and retention strategies and encourage clinical research that is representative of the disease population. Using diverse strategies to ensure that minority populations are represented in both clinic- and community-based research trials is crucial to ensuring that conclusions derived from data collection are truly generalizable and representative of the targeted population.
Study Design Considerations
We have presented several strategies to increase minority participation in biomedical research, including community-engaged methods, use of PAOs, and more systematic changes at the institutional and federal level. An additional consideration is the study design for interventional studies. Although randomized controlled trials are often thought of as the gold standard for determining intervention effects, there are limitations in strictly adhering to the study protocol, which also may limit participation by minority communities and minority-serving health systems. Given this, quasiexperimental designs are viable alternatives (160). A large range of study designs fall under this category, including those that are capable of retaining some level of randomization (e.g., stepped-wedge, wait-list, crossover, and inverse-rollout designs) and others relying on multiple time points before and after intervention (interrupted time series). By allowing access to the intervention for the entire study population, such designs also address the ethical dilemma of moving forward with interventions found to be efficacious, but there remains a need to study implementation.
Conclusions
Well-designed clinical studies and trials are needed to obtain information that is applicable to the general population. However, recent studies suggest that most clinical studies of respiratory disease fail to include a significant proportion of racial/ethnic minorities. The lack of inclusion of minorities in clinical research may lead to misdiagnosis or misclassification of disease, wide application of approved interventions without appropriate knowledge of their usefulness in certain populations, and the development of recommendations that are not broadly applicable to the general population. This ATS research statement identifies potential solutions to the underrepresentation of minorities in clinical research by applying a multilevel framework that is anchored in community engagement methods and patient advocacy and includes evidence-based interventions that target key social and structural barriers extending from the individual level to the federal level. Because of the multiprong approach most researchers use to increase minority recruitment into research, we are limited in quantifying the independent or additive effect of each specific strategy. This is a crucial area for future research. We also need standardized reporting of research with regard to reporting demographics and when to include stratified analyses, which will provide transparency to identifying who is actually included in research studies.
We recognize that many of the strategies presented would likely enhance recruitment and retention of all racial and ethnic groups (including non-Hispanic White populations). Concerted efforts and resources from multiple stakeholders—including but not limited to educational and healthcare institutions and organizations and the NIH—are needed to apply these strategies and increase representation of minorities in clinical research.
Acknowledgments
This official research statement was prepared by an ad hoc subcommittee of the ATS Health Equality and Diversity Committee and the Assembly on Behavioral Science and Health Services Research.
Members of the subcommittee are as follows:
Neeta Thakur, M.D., M.P.H. (Co-Chair)1
Fernando Holguin, M.D., M.P.H. (Co-Chair)2
Jennifer Alvidrez, Ph.D. (Co-Chair)3*
Raolat Abdulai, M.D., M.S.4*
Donna Appell, R.N.5,6‡
Richardae Araojo, R.Adm.7*
Christian Bime, M.D., M.Sc.8
Esteban Burchard, M.D., M.P.H.1*
Lauren Castro, A.P.N.-B.C.9
Juan C. Celedón, M.D., Dr.P.H.10
Juliana Ferreira, M.D.11
Marvella E. Ford, Ph.D.12*
Maureen George, Ph.D., R.N., A.E.-C.13
LeRoy Graham, M.D.14*
Carolyn Hendrickson, M.D, M.P.H.1*
James P. Kiley, M.S., Ph.D.15*
Stephanie Lovinsky-Desir, M.D.16
Yolanda Mageto, M.D.17
Arch G. Mainous III, Ph.D.18
Smita Pakhale, M.D., M.Sc.19
Rodney Reese20‡
Kristin A. Riekert, Ph.D.21
Jesse Roman, M.D.22,23
Elizabeth Ruvalcaba, M.S.P.H.21
Sunil Sharma, M.D.24
Priya Shete, M.D., M.P.H.1
Ginger Spitzer, B.A.20‡
Juan P. Wisnivesky, M.D., Dr.P.H.25
*Member of the subcommittee who was not part of the writing committee.
‡Patient representative.
1Department of Medicine, University of California, San Francisco, San Francisco, California; 2Department of Medicine, University of Colorado, Denver, Colorado; 3Division of Scientific Programs, National Institute on Minority Health and Health Disparities, Bethesda, Maryland; 4Sanofi U.S., Bridgewater, New Jersey; 5Hermansky-Pudlak Syndrome Network, Oyster Bay, New York; 6Public Advisory Roundtable, American Thoracic Society, New York, New York; 7Office of Minority Health and Health Equity, U.S. Food and Drug Administration, White Oak, Maryland; 8Department of Medicine, University of Arizona, Tucson, Arizona; 9Department of Medicine, University of Illinois, Chicago, Illinois; 10Division of Pulmonary Medicine, Department of Pediatrics, University of Pittsburgh, Pittsburgh, Pennsylvania; 11Divisao de Pneumologia, Instituto do Coracao, Hospital das Clinicas, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, Brazil; 12Department of Public Health Sciences, Medical University of South Carolina, Charleston, South Carolina; 13School of Nursing and 16Department of Pediatrics, Columbia University Irving Medical Center, Columbia University, New York, New York; 14Bridge Atlanta Medical Center, Atlanta, Georgia; 15Division of Lung Diseases, NHLBI, Bethesda, Maryland; 17Center for Advanced Heart and Lung Disease, Baylor University Medical Center, Dallas, Texas; 18Department of Health Services Research, Management and Policy, University of Florida, Gainesville, Florida; 19Department of Medicine, Ottawa Hospital and Ottawa Hospital Research Institute, School of Epidemiology and Public Health, University of Ottawa, Ottawa, Ontario, Canada; 20Foundation for Sarcoidosis Research, Chicago, Illinois; 21Department of Medicine, Johns Hopkins University, Baltimore, Maryland; 22Department of Medicine and 23Jane and Leonard Korman Respiratory Institute, Thomas Jefferson University, Philadelphia, Pennsylvania; 24Department of Medicine, West Virginia University Critical Care & Trauma Institute, School of Medicine, West Virginia University, Morgantown, West Virginia; and 25Department of Medicine, The Icahn School of Medicine at Mount Sinai, New York, New York
Footnotes
An Executive Summary of this document is available at https://www.atsjournals.org/doi/suppl/10.1164/rccm.202105-1210ST.
Author Disclosures: R. Abdulai was an employee of AstraZeneca. R. Araojo was an employee of the Food and Drug Administration. J.C.C. received research support from GlaxoSmithKline, Merck, and Pharmavite; and was president of the American Thoracic Society from 2020 to 2021. J.F. served as a speaker for Medtronic. M.G. served on an advisory committee for Sanofi/Regeneron; served as a consultant for AstraZeneca, Genentech, Mylan, Sanofi/Regeneron, and Teva; and served as a speaker for AstraZeneca, Genentech, and Teva. J.P.K. was an employee of the National Heart, Lung, and Blood Institute. Y.M. served as a speaker for Boehringer Ingelheim and Genentech; and served on a data safety and monitoring board for United Therapeutics. K.A.R. served on an advisory committee for the Cystic Fibrosis Foundation, Gilead, and Spark Healthcare; received research support from the National Institutes of Health and Cystic Fibrosis Foundation; and received royalties from Springer Publishing. J.R. served on an advisory committee for Boehringer Ingelheim; served on a data safety and monitoring board for Genentech; and received research support from the Department of Veteran Affairs, Galapagos, the National Institutes of Health, and Novartis. S.S. served as a consultant for Actelion; and received research support from ResMed. J.P.W. served as a consultant for Atea, Banook Group, GlaxoSmithKline, Quintiles, and Sanofi; received research support from Arnold Consultants, Quorum, and Sanofi; and has ownership or investment interests in Merus. N.T., F.H., J.A., D.A., C.B., E.B., L.C., M.E.F., L.G., C.H., S.L.-D., A.G.M., S.P., R.R., E.R., P.S., and G.S. reported no commercial or relevant noncommercial interests.
Contributor Information
Collaborators: on behalf of the American Thoracic Society Health Equality and Diversity Committee and the Assembly on Behavioral Science and Health Services Research
References
- 1.Kennedy EM. Washington, DC: U.S. Senate; 1993. [Google Scholar]
- 2.Advisory Committee on Research on Women’s Health, NIH Office of Research on Women’s Health. Bethesda, MD: NIH Office of Research on Women’s Health; 2017. [Google Scholar]
- 3.Vespa J, Armstrong DM, Medina L. Washington, DC: U.S. Census Bureau; 2018. [Google Scholar]
- 4. Burchard EG, Oh SS, Foreman MG, Celedón JC. Moving toward true inclusion of racial/ethnic minorities in federally funded studies: a key step for achieving respiratory health equality in the United States. Am J Respir Crit Care Med. 2015;191:514–521. doi: 10.1164/rccm.201410-1944PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Geller SE, Koch AR, Roesch P, Filut A, Hallgren E, Carnes M. The more things change, the more they stay the same: a study to evaluate compliance with inclusion and assessment of women and minorities in randomized controlled trials. Acad Med. 2018;93:630–635. doi: 10.1097/ACM.0000000000002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Food and Drug Administration Safety and Innovation Act (FDASIA). Silver Spring, MD: Food and Drug Administration; 2018 [Google Scholar]
- 7. Popejoy AB, Fullerton SM. Genomics is failing on diversity. Nature. 2016;538:161–164. doi: 10.1038/538161a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu AC, Kiley JP, Noel PJ, Amur S, Burchard EG, Clancy JP, et al. Current status and future opportunities in lung precision medicine research with a focus on biomarkers: an American Thoracic Society/National Heart, Lung, and Blood Institute research statement. Am J Respir Crit Care Med. 2018;198:e116–e136. doi: 10.1164/rccm.201810-1895ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buu MC, Sanders LM, Mayo JA, Milla CE, Wise PH. Assessing differences in mortality rates and risk factors between Hispanic and non-Hispanic patients with cystic fibrosis in California. Chest. 2016;149:380–389. doi: 10.1378/chest.14-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pique L, Graham S, Pearl M, Kharrazi M, Schrijver I. Cystic fibrosis newborn screening programs: implications of the CFTR variant spectrum in nonwhite patients. Genet Med. 2017;19:36–44. doi: 10.1038/gim.2016.48. [DOI] [PubMed] [Google Scholar]
- 11. Pettit RS, Fellner C. CFTR modulators for the treatment of cystic fibrosis. P&T. 2014;39:500–511. [PMC free article] [PubMed] [Google Scholar]
- 12. McGarry ME, McColley SA. Minorities are underrepresented in clinical trials of pharmaceutical agents for cystic fibrosis. Ann Am Thorac Soc. 2016;13:1721–1725. doi: 10.1513/AnnalsATS.201603-192BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lederer DJ, Caplan-Shaw CE, O’Shea MK, Wilt JS, Basner RC, Bartels MN, et al. Racial and ethnic disparities in survival in lung transplant candidates with idiopathic pulmonary fibrosis. Am J Transplant. 2006;6:398–403. doi: 10.1111/j.1600-6143.2005.01205.x. [DOI] [PubMed] [Google Scholar]
- 14. Akenroye A, Keet C. Underrepresentation of Blacks, smokers, and obese patients in studies of monoclonal antibodies for asthma. J Allergy Clin Immunol Pract. 2020;8:739–741, e6. doi: 10.1016/j.jaip.2019.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kesh S, Jones B. Racial/ethnic demographics of participants in clinical trials of biologics used for asthma. Ann Allergy Asthma Immunol. 2018;121:S41. [Google Scholar]
- 16. Pang HH, Wang X, Stinchcombe TE, Wong ML, Cheng P, Ganti AK, et al. Enrollment trends and disparity among patients with lung cancer in national clinical trials, 1990 to 2012. J Clin Oncol. 2016;34:3992–3999. doi: 10.1200/JCO.2016.67.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smirnoff M, Wilets I, Ragin DF, Adams R, Holohan J, Rhodes R, et al. A paradigm for understanding trust and mistrust in medical research: the Community VOICES study. AJOB Empir Bioeth. 2018;9:39–47. doi: 10.1080/23294515.2018.1432718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scharff DP, Mathews KJ, Jackson P, Hoffsuemmer J, Martin E, Edwards D. More than Tuskegee: understanding mistrust about research participation. J Health Care Poor Underserved. 2010;21:879–897. doi: 10.1353/hpu.0.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Assche K, Gutwirth S, Sterckx S. Protecting dignitary interests of Biobank research participants: lessons from Havasupai Tribe v Arizona Board of Regents. Law Innov Technol. 2013;5:54–84. [Google Scholar]
- 20. Devlin A, Gonzalez E, Ramsey F, Esnaola N, Fisher S. The effect of discrimination on likelihood of participation in a clinical trial. J Racial Ethn Heal Disparities. 2020;7:1124–1129. doi: 10.1007/s40615-020-00735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe NR. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine (Baltimore) 2008;87:1–9. doi: 10.1097/MD.0b013e3181625d78. [DOI] [PubMed] [Google Scholar]
- 22. George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health. 2014;104:e16–e31. doi: 10.2105/AJPH.2013.301706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmotzer GL. Barriers and facilitators to participation of minorities in clinical trials. Ethn Dis. 2012;22:226–230. [PubMed] [Google Scholar]
- 24. Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112:228–242. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 25. Tamariz L, Palacio A, Robert M, Marcus EN. Improving the informed consent process for research subjects with low literacy: a systematic review. J Gen Intern Med. 2013;28:121–126. doi: 10.1007/s11606-012-2133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Glickman SW, Ndubuizu A, Weinfurt KP, Hamilton CD, Glickman LT, Schulman KA, et al. Perspective: The case for research justice: inclusion of patients with limited English proficiency in clinical research. Acad Med. 2011;86:389–393. doi: 10.1097/ACM.0b013e318208289a. [DOI] [PubMed] [Google Scholar]
- 27.The U.S. Public Health Service Syphilis Study at Tuskegee. Atlanta, GA: CDC; [Google Scholar]
- 28.Torpy SJ.Native American women and coerced sterilization: on the Trail of Tears in the 1970s Am Indian Cult Res J 2000241–22.. [Google Scholar]
- 29. Roberts WC. Facts and ideas from anywhere. Proc (Bayl Univ Med Cent) 2015;28:421–432. doi: 10.1080/08998280.2015.11929297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Henrietta Lacks: science must right a historical wrong. Nature. 2020;585:7. doi: 10.1038/d41586-020-02494-z. [DOI] [PubMed] [Google Scholar]
- 31.Washington HA. 1st ed. New York, NY: Doubleday; 2006. [Google Scholar]
- 32. Sterling RL. Genetic research among the Havasupai: a cautionary tale. Virtual Mentor. 2011;13:113–117. doi: 10.1001/virtualmentor.2011.13.2.hlaw1-1102. [DOI] [PubMed] [Google Scholar]
- 33. Hamel LM, Penner LA, Albrecht TL, Heath E, Gwede CK, Eggly S. Barriers to clinical trial enrollment in racial and ethnic minority patients with cancer. Cancer Contr. 2016;23:327–337. doi: 10.1177/107327481602300404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paskett ED, Katz ML, DeGraffinreid CR, Tatum CM. Participation in cancer trials: recruitment of underserved populations. Clin Adv Hematol Oncol. 2003;1:607–613. [PubMed] [Google Scholar]
- 35.Patient-Centered Outcomes Research Institute. Washington, DC: Patient-Centered Outcomes Research Institute; 2020 [Google Scholar]
- 36. De D, Nueces L, Hacker K, Digirolamo A, Hicks LS. A systematic review of community-based participatory research to enhance clinical trials in racial and ethnic minority groups. Health Serv Res. 2012;47:1363–1386. doi: 10.1111/j.1475-6773.2012.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Christopher S, Watts V, McCormick AKHG, Young S. Building and maintaining trust in a community-based participatory research partnership. Am J Public Health. 2008;98:1398–1406. doi: 10.2105/AJPH.2007.125757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sankaré IC, Bross R, Brown AF, Del Pino HE, Jones LF, Morris DM, et al. Strategies to build trust and recruit African American and Latino community residents for health research: a cohort study. Clin Transl Sci. 2015;8:412–420. doi: 10.1111/cts.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harris DA, Pensa MA, Redlich CA, Pisani MA, Rosenthal MS. Community-based participatory research is needed to address pulmonary health disparities. Ann Am Thorac Soc. 2016;13:1231–1238. doi: 10.1513/AnnalsATS.201601-054PS. [DOI] [PubMed] [Google Scholar]
- 40. Bordeaux BC, Wiley C, Tandon SD, Horowitz CR, Brown PB, Bass EB. Guidelines for writing manuscripts about community-based participatory research for peer-reviewed journals. Prog Community Health Partnersh. 2007;1:281–288. doi: 10.1353/cpr.2007.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wallerstein N, Duran B. Community-based participatory research contributions to intervention research: the intersection of science and practice to improve health equity. Am J Public Health. 2010;100:S40–S46. doi: 10.2105/AJPH.2009.184036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. D’Alonzo KT. Getting started in CBPR: lessons in building community partnerships for new researchers. Nurs Inq. 2010;17:282–288. doi: 10.1111/j.1440-1800.2010.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brush BL, Mentz G, Jensen M, Jacobs B, Saylor KM, Rowe Z, et al. Success in long-standing community-based participatory research (CBPR) partnerships: a scoping literature review. Health Educ Behav. 2020;47:556–568. doi: 10.1177/1090198119882989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Israel BA, Krieger J, Vlahov D, Ciske S, Foley M, Fortin P, et al. Challenges and facilitating factors in sustaining community-based participatory research partnerships: lessons learned from the Detroit, New York City and Seattle urban research centers. J Urban Health. 2006;83:1022–1040. doi: 10.1007/s11524-006-9110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Adelman C. Kurt Lewin and the origins of action research. Educ Action Res. 1993;1:7–24. [Google Scholar]
- 46.Clark BAM, Given LM. In: Given LM, editor. New York, NY: Sage Publications; 2018. Critical realism; pp. 168–170. [Google Scholar]
- 47. Berry DC, Neal M, Hall EG, McMurray RG, Schwartz TA, Skelly AH. et al. Recruitment and retention strategies for a community-based weight management study for multi-ethnic elementary school children and their parents. Public Health Nurs. 2013;30:80–86. doi: 10.1111/phn.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Burdine JN, McLeroy K, Blakely C, Wendel ML, Felix MRJ. Community-based participatory research and community health development. J Prim Prev. 2010;31:1–7. doi: 10.1007/s10935-010-0205-9. [DOI] [PubMed] [Google Scholar]
- 49. Estreet A, Apata J, Kamangar F, Schutzman C, Buccheri J, O’Keefe AM, et al. Improving participants’ retention in a smoking cessation intervention using a community-based participatory research approach. Int J Prev Med. 2017;8:106. doi: 10.4103/ijpvm.IJPVM_303_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shelef DQ, Rand C, Streisand R, Horn IB, Yadav K, Stewart L, et al. Using stakeholder engagement to develop a patient-centered pediatric asthma intervention. J Allergy Clin Immunol. 2016;138:1512–1517. doi: 10.1016/j.jaci.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 51. Kramer CB, LeRoy L, Donahue S, Apter AJ, Bryant-Stephens T, Elder JP, et al. Enrolling African-American and Latino patients with asthma in comparative effectiveness research: lessons learned from 8 patient-centered studies. J Allergy Clin Immunol. 2016;138:1600–1607. doi: 10.1016/j.jaci.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 52. Teach SJ, Shelef DQ, Fousheé N, Horn IB, Yadav K, Wang Y, et al. Randomized clinical trial of parental psychosocial stress management to improve asthma outcomes. J Asthma. 2021;58:121–132. doi: 10.1080/02770903.2019.1665063. [DOI] [PubMed] [Google Scholar]
- 53. Kim KHC, Linnan L, Campbell MK, Brooks C, Koenig HG, Wiesen C. The WORD (Wholeness, Oneness, Righteousness, Deliverance): a faith-based weight-loss program utilizing a community-based participatory research approach. Health Educ Behav. 2008;35:634–650. doi: 10.1177/1090198106291985. [DOI] [PubMed] [Google Scholar]
- 54. George M, Topaz M, Rand C, Sommers MLS, Glanz K, Pantalon MV, et al. Inhaled corticosteroid beliefs, complementary and alternative medicine, and uncontrolled asthma in urban minority adults. J Allergy Clin Immunol. 2014;134:1252–1259. doi: 10.1016/j.jaci.2014.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. George M, Arcia A, Chung A, Coleman D, Bruzzese JM. African Americans want a focus on shared decision-making in asthma adherence interventions. Patient. 2020;13:71–81. doi: 10.1007/s40271-019-00382-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. George M, Pantalon MV, Sommers MLS, Glanz K, Jia H, Chung A, et al. Shared decision-making in the BREATHE asthma intervention trial: a research protocol. J Adv Nurs. 2019;75:876–887. doi: 10.1111/jan.13916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. George M, Bruzzese JMS, Sommers MLS, Pantalon MV, Jia H, Rhodes J, et al. Group-randomized trial of tailored brief shared decision-making to improve asthma control in urban Black adults. J Adv Nurs. 2021;77:1501–1517. doi: 10.1111/jan.14646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kutner M, Greenberg E, Jin Y, Paulsen C, White S. Washington, DC: U.S. Department of Education; 2003. [Google Scholar]
- 59. Melnikow J, Paliescheskey M, Stewart GK. Effect of a transportation incentive on compliance with the first prenatal appointment: a randomized trial. Obstet Gynecol. 1997;89:1023–1027. doi: 10.1016/s0029-7844(97)00147-6. [DOI] [PubMed] [Google Scholar]
- 60. Vais S, Thomson L, Williams A, Sobota A. Rethinking rideshares: a transportation assistance pilot for pediatric patients with sickle cell disease. J Health Care Poor Underserved. 2020;31:1457–1470. doi: 10.1353/hpu.2020.0105. [DOI] [PubMed] [Google Scholar]
- 61. Chaiyachati KH, Hubbard RA, Yeager A, Mugo B, Shea JA, Rosin R, et al. Rideshare-based medical transportation for Medicaid patients and primary care show rates: a difference-in-difference analysis of a pilot program. J Gen Intern Med. 2018;33:863–868. doi: 10.1007/s11606-018-4306-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Scott CK. A replicable model for achieving over 90% follow-up rates in longitudinal studies of substance abusers. Drug Alcohol Depend. 2004;74:21–36. doi: 10.1016/j.drugalcdep.2003.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McGarry ME, Castellanos E, Thakur N, Oh SS, Eng C, Davis A, et al. Obesity and bronchodilator response in Black and Hispanic children and adolescents with asthma. Chest. 2015;147:1591–1598. doi: 10.1378/chest.14-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Davis E, Demby H, Jenner LW, Gregory A, Broussard M. Adapting an evidence-based model to retain adolescent study participants in longitudinal research. Eval Program Plann. 2016;54:102–111. doi: 10.1016/j.evalprogplan.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 65. Leavens ELS, Stevens EM, Brett EI, Molina N, Leffingwell TR, Wagener TL. Use of rideshare services to increase participant recruitment and retention in research: participant perspectives. J Med Internet Res. 2019;21:e11166. doi: 10.2196/11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Frank S, Berk S, Hernandez L, Hogarth P, Shill HA, Siddiqi B, et al. Transportation innovation to aid Parkinson disease trial recruitment. Contemp Clin Trials Commun. 2019;16:100449. doi: 10.1016/j.conctc.2019.100449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Asthma Evidence to Action Network. Washington, DC: Patient-Centered Outcomes Research Institute; 2015 [Google Scholar]
- 68. Langford AT, Resnicow K, Dimond EP, Denicoff AM, Germain DS, McCaskill-Stevens W, et al. Racial/ethnic differences in clinical trial enrollment, refusal rates, ineligibility, and reasons for decline among patients at sites in the National Cancer Institute’s Community Cancer Centers Program. Cancer. 2014;120:877–884. doi: 10.1002/cncr.28483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Simon MS, Du W, Flaherty L, Philip PA, Lorusso P, Miree C, et al. Factors associated with breast cancer clinical trials participation and enrollment at a large academic medical center. J Clin Oncol. 2004;22:2046–2052. doi: 10.1200/JCO.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 70. Webb DA, Coyne JC, Goldenberg RL, Hogan VK, Elo IT, Bloch JR, et al. Recruitment and retention of women in a large randomized control trial to reduce repeat preterm births: the Philadelphia Collaborative Preterm Prevention Project. BMC Med Res Methodol. 2010;10:88. doi: 10.1186/1471-2288-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. DeFreitas D. Race and HIV clinical trial participation. J Natl Med Assoc. 2010;102:493–499. doi: 10.1016/s0027-9684(15)30558-7. [DOI] [PubMed] [Google Scholar]
- 72. Niranjan SJ, Martin MY, Fouad MN, Vickers SM, Wenzel JA, Cook ED, et al. Bias and stereotyping among research and clinical professionals: perspectives on minority recruitment for oncology clinical trials. Cancer. 2020;126:1958–1968. doi: 10.1002/cncr.32755. [DOI] [PubMed] [Google Scholar]
- 73. Hall WJ, Chapman MV, Lee KM, Merino YM, Thomas TW, Payne BK, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health. 2015;105:e60–e76. doi: 10.2105/AJPH.2015.302903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schulman KA, Berlin JA, Harless W, Kerner JF, Sistrunk S, Gersh BJ, et al. The effect of race and sex on physicians’ recommendations for cardiac catheterization. N Engl J Med. 1999;340:618–626. doi: 10.1056/NEJM199902253400806. [DOI] [PubMed] [Google Scholar]
- 75. Penner LA, Dovidio JF, Gonzalez R, Albrecht TL, Chapman R, Foster T, et al. The effects of oncologist implicit racial bias in racially discordant oncology interactions. J Clin Oncol. 2016;34:2874–2880. doi: 10.1200/JCO.2015.66.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Greenwald AG, Nosek BA, Banaji MR. Understanding and using the implicit association test: I. An improved scoring algorithm. J Pers Soc Psychol. 2003;85:197–216. doi: 10.1037/0022-3514.85.2.197. [DOI] [PubMed] [Google Scholar]
- 77. Greenwald AG, McGhee DE, Schwartz JLK. Measuring individual differences in implicit cognition: the implicit association test. J Pers Soc Psychol. 1998;74:1464–1480. doi: 10.1037//0022-3514.74.6.1464. [DOI] [PubMed] [Google Scholar]
- 78. FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics. 2017;18:19. doi: 10.1186/s12910-017-0179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. O’Brien RL, Kosoko-Lasaki O, Cook CT, Kissell J, Peak F, Williams EH, et al. Self-assessment of cultural attitudes and competence of clinical investigators to enhance recruitment and participation of minority populations in research. J Natl Med Assoc. 2006;98:674–682. [PMC free article] [PubMed] [Google Scholar]
- 80.Findings at a glance: medical doctors. Washington, DC: Pew Research Center; 2019 [Google Scholar]
- 81. Thom DH, Kravitz RL, Bell RA, Krupat E, Azari R. Patient trust in the physician: relationship to patient requests. Fam Pract. 2002;19:476–483. doi: 10.1093/fampra/19.5.476. [DOI] [PubMed] [Google Scholar]
- 82. Sherber NS, Powe NR, Braunstein JB. Personal physicians as study investigators: impact on patients’ willingness to participate in clinical trials. Contemp Clin Trials. 2009;30:227–232. doi: 10.1016/j.cct.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 83. Mainous AG, Smith DW, Geesey ME, Tilley BC. Development of a measure to assess patient trust in medical researchers. Ann Fam Med. 2006;4:247–252. doi: 10.1370/afm.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tilley BC, Mainous AG, III, Smith DW, McKee MD, Amorrortu RP, Alvidrez J, et al. Design of a cluster-randomized minority recruitment trial: RECRUIT. Clin Trials. 2017;14:286–298. doi: 10.1177/1740774517690146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tilley BC, Mainous AG, III, Elm JJ, Pickelsimer E, Soderstrom LH, Ford ME, et al. A randomized recruitment intervention trial in Parkinson’s disease to increase participant diversity: early stopping for lack of efficacy. Clin Trials. 2012;9:188–197. doi: 10.1177/1740774512436881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Harle CA, Golembiewski EH, Rahmanian KP, Brumback B, Krieger JL, Goodman KW, et al. Does an interactive trust-enhanced electronic consent improve patient experiences when asked to share their health records for research? A randomized trial. J Am Med Inform Assoc. 2019;26:620–629. doi: 10.1093/jamia/ocz015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tilley BC, Mainous AG III, Amorrortu RP, McKee MD, Smith DW, Li R, et al. Contemp Clin Trials. doi: 10.1016/j.cct.2021.106519. [DOI] [PMC free article] [PubMed]
- 88. Huffman LE, Wilson DK, Kitzman-Ulrich H, Lyerly JE, Gause HM, Resnicow K. Associations between culturally relevant recruitment strategies and participant interest, enrollment and generalizability in a weight-loss intervention for African American families. Ethn Dis. 2016;26:295–304. doi: 10.18865/ed.26.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Maxwell AE, Bastani R, Vida P, Warda US. Strategies to recruit and retain older Filipino-American immigrants for a cancer screening study. J Community Health. 2005;30:167–179. doi: 10.1007/s10900-004-1956-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Uybico SJ, Pavel S, Gross CP. Recruiting vulnerable populations into research: a systematic review of recruitment interventions. J Gen Intern Med. 2007;22:852–863. doi: 10.1007/s11606-007-0126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Perrin A, Anderson M. Washington, DC: Pew Research Center; 2019. [Google Scholar]
- 92. Ibrahim S, Sidani S. Strategies to recruit minority persons: a systematic review. J Immigr Minor Health. 2014;16:882–888. doi: 10.1007/s10903-013-9783-y. [DOI] [PubMed] [Google Scholar]
- 93. Topolovec-Vranic J, Natarajan K. The use of social media in recruitment for medical research studies: a scoping review. J Med Internet Res. 2016;18:e286. doi: 10.2196/jmir.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Admon L, Haefner JK, Kolenic GE, Chang T, Davis MM, Moniz MH. Recruiting pregnant patients for survey research: a head to head comparison of social media-based versus clinic-based approaches. J Med Internet Res. 2016;18:e326. doi: 10.2196/jmir.6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bull SS, Levine DK, Black SR, Schmiege SJ, Santelli J. Social media-delivered sexual health intervention: a cluster randomized controlled trial. Am J Prev Med. 2012;43:467–474. doi: 10.1016/j.amepre.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Martinez O, Wu E, Shultz AZ, Capote J, López Rios J, Sandfort T, et al. Still a hard-to-reach population? Using social media to recruit Latino gay couples for an HIV intervention adaptation study. J Med Internet Res. 2014;16:e113. doi: 10.2196/jmir.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gelinas L, Pierce R, Winkler S, Cohen IG, Lynch HF, Bierer BE. Using social media as a research recruitment tool: ethical issues and recommendations. Am J Bioeth. 2017;17:3–14. doi: 10.1080/15265161.2016.1276644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Frandsen M, Walters J, Ferguson SG. Exploring the viability of using online social media advertising as a recruitment method for smoking cessation clinical trials. Nicotine Tob Res. 2014;16:247–251. doi: 10.1093/ntr/ntt157. [DOI] [PubMed] [Google Scholar]
- 99. Joseph G, Dohan D. Recruiting minorities where they receive care: institutional barriers to cancer clinical trials recruitment in a safety-net hospital. Contemp Clin Trials. 2009;30:552–559. doi: 10.1016/j.cct.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 100. Wujcik D, Wolff SN. Recruitment of African Americans to national oncology clinical trials through a clinical trial shared resource. J Health Care Poor Underserved. 2010;21:38–50. doi: 10.1353/hpu.0.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. van Knippenberg D, Schippers MC. Work group diversity. Annu Rev Psychol. 2007;58:515–541. doi: 10.1146/annurev.psych.58.110405.085546. [DOI] [PubMed] [Google Scholar]
- 102. Phillips KW, O’Reilly CA. Demography and diversity in organizations: a review of 40 years of research. Res Organ Behav. 1998;20:77–140. [Google Scholar]
- 103.Science benefits from diversity [editorial] Nature 20185585. [DOI] [PubMed] [Google Scholar]
- 104. Nielsen MW, Bloch CW, Schiebinger L. Making gender diversity work for scientific discovery and innovation. Nat Hum Behav. 2018;2:726–734. doi: 10.1038/s41562-018-0433-1. [DOI] [PubMed] [Google Scholar]
- 105.AlShebli BK, Rahwan T, Woon WL.2018. [DOI] [PMC free article] [PubMed]
- 106. Gomez LE, Bernet P. Diversity improves performance and outcomes. J Natl Med Assoc. 2019;111:383–392. doi: 10.1016/j.jnma.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 107. Cooper LA, Roter DL, Johnson RL, Ford DE, Steinwachs DM, Powe NR. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139:907–915. doi: 10.7326/0003-4819-139-11-200312020-00009. [DOI] [PubMed] [Google Scholar]
- 108. Shen MJ, Peterson EB, Costas-Muñiz R, Hernandez MH, Jewell ST, Matsoukas K, et al. The effects of race and racial concordance on patient-physician communication: a systematic review of the literature. J Racial Ethn Health Disparities. 2018;5:117–140. doi: 10.1007/s40615-017-0350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Swartz TH, Palermo AS, Masur SK, Aberg JA. The science and value of diversity: closing the gaps in our understanding of inclusion and diversity. J Infect Dis. 2019;220:S33–S41. doi: 10.1093/infdis/jiz174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Powell K. These labs are remarkably diverse: here’s why they’re winning at science. Nature. 2018;558:19–22. doi: 10.1038/d41586-018-05316-5. [DOI] [PubMed] [Google Scholar]
- 111. Freeman RB, Huang W. Collaboration: strength in diversity. Nature. 2014;513:305. doi: 10.1038/513305a. [DOI] [PubMed] [Google Scholar]
- 112. Sutton MY, Lanier YA, Willis LA, Castellanos T, Dominguez K, Fitzpatrick L, et al. Strengthening the network of mentored, underrepresented minority scientists and leaders to reduce HIV-related health disparities. Am J Public Health. 2013;103:2207–2214. doi: 10.2105/AJPH.2013.301345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Nipp RD, Hong K, Paskett ED. Overcoming barriers to clinical trial enrollment. Am Soc Clin Oncol Educ Book. 2019;39:105–114. doi: 10.1200/EDBK_243729. [DOI] [PubMed] [Google Scholar]
- 114. Calderón JL, Baker RS, Fabrega H, Conde JG, Hays RD, Fleming E, et al. An ethno-medical perspective on research participation: a qualitative pilot study. MedGenMed. 2006;8:23. [PMC free article] [PubMed] [Google Scholar]
- 115.Smith YR, Johnson AM, Newman LA, Greene A, Johnson TRB, Rogers JL.Perceptions of clinical research participation among African American women J Womens Health (Larchmt) 200716423–428.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Johnson VA, Edwards KA, Sherman SL, Stephens LD, Williams W, Adair A, et al. Decisions to participate in fragile X and other genomics-related research: Native American and African American voices. J Cult Divers. 2009;16:127–135. [PubMed] [Google Scholar]
- 117. Sadler GR, Gonzalez J, Mumman M, Cullen L, Lahousse SF, Malcarne V, et al. Adapting a program to inform African American and Hispanic American women about cancer clinical trials. J Cancer Educ. 2010;25:142–145. doi: 10.1007/s13187-009-0032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tu SP, Chen H, Chen A, Lim J, May S, Drescher C. Clinical trials: understanding and perceptions of female Chinese-American cancer patients. Cancer. 2005;104:2999–3005. doi: 10.1002/cncr.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Zúñiga ML, Blanco E, Martínez P, Strathdee SA, Gifford AL. Perceptions of barriers and facilitators to participation in clinical trials in HIV-positive Latinas: a pilot study. J Womens Health (Larchmt) 2007;16:1322–1330. doi: 10.1089/jwh.2006.0234. [DOI] [PubMed] [Google Scholar]
- 120.Gollin LX, Harrigan RC, Calderón JL, Perez J, Easa D.Improving Hawaiian and Filipino involvement in clinical research opportunities: qualitative findings from Hawai’i Ethn Dis 200515S5-111-9. [PMC free article] [PubMed] [Google Scholar]
- 121. Rivera-Goba MV, Dominguez DC, Stoll P, Grady C, Ramos C, Mican JM. Exploring decision-making of HIV-infected Hispanics and African Americans participating in clinical trials. J Assoc Nurses AIDS Care. 2011;22:295–306. doi: 10.1016/j.jana.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hispanic Community Health Study/Study of Latinos. Chapel Hill, NC: University of North Carolina at Chapel Hill [Google Scholar]
- 123.Mexican American Health Study. . Houston, TX: University of Texas MD Anderson Cancer Center; [Google Scholar]
- 124.Black Women’s Health Study. Boston, MA: Boston University [Google Scholar]
- 125.National Institute of Allergy and Infectious Diseases. Bethesda, MD: NIH; 2020. [Google Scholar]
- 126.Davidson P.
- 127.Research America. Public perception of clinical trials. 2017
- 128.Community health needs assessment for charitable hospital organizations: section 501(r)(3) Washington, DC: Internal Revenue Service; 2020. https://www.irs.gov/charities-non-profits/community-health-needs-assessment-for-charitable-hospital-organizations-section-501r3 [Google Scholar]
- 129.Internal revenue bulletin: 2011-30. Washington, DC: Internal Revenue Service; 2011. https://www.irs.gov/irb/2011-30_IRB [Google Scholar]
- 130.What is the All of Us Research Program? Tucson, AZ: University of Arizona; https://www.allofusaz.org/ [Google Scholar]
- 131.California Preterm Birth Initiative. . San Francisco, CA: University of California San Francisco; https://pretermbirthca.ucsf.edu/california-preterm-birth-initiative [Google Scholar]
- 132.Innovation Station, Association of Maternal and Child Health Programs. Washington, DC: Association of Maternal and Child Health Programs; 2019. http://www.amchp.org/programsandtopics/BestPractices/InnovationStation/ISDocs/Health%20Equity%20Zones.pdf [Google Scholar]
- 133.ChangeLab Solutions and Rhode Island Department of Health. Health equity zones: a toolkit for building healthy and resilient communities; 2021. [Google Scholar]
- 134.NIH. Bethesda, MD: NIH; 1987. https://grants.nih.gov/grants/guide/historical/1987_09_25_Vol_16_No_32.pdf [Google Scholar]
- 135.Federal Register, volume 59, issue 59. Washington, DC: Government Publishing Office; 1994. https://www.govinfo.gov/content/pkg/FR-1994-03-28/html/94-5435.htm [Google Scholar]
- 136.About NIMHD. Bethesda, MD: NIH [Google Scholar]
- 137.Amendment: NIH policy and guidelines on the inclusion of women and minorities as subjects in clinical research. Notice number: NOT-OD-18-014. Bethesda, MD: NIH; 2017. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-18-014.html [Google Scholar]
- 138.NIH RCDC inclusion statistics report. . Bethesda, MD: NIH; 2018. https://report.nih.gov/RISR/#/ [Google Scholar]
- 139. Ginther DK. Schaffer WT. Schnell J. Masimore B. Liu F. Haak LL. et al. Race, ethnicity, and NIH research awards. Science. 2011;333:1015–1019. doi: 10.1126/science.1196783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Ginther DK, Kington R, Haak LL, Schaffer WT, Kington R. Are race, ethnicity, and medical school affiliation associated with NIH R01 type 1 award probability for physician investigators? Acad Med. 2012;87:1516–1524. doi: 10.1097/ACM.0b013e31826d726b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ginther DK, Kahn S, Schaffer WT. Gender, race/ethnicity, and National Institutes of Health R01 research awards: is there evidence of a double bind for women of color? Acad Med. 2016;91:1098–1107. doi: 10.1097/ACM.0000000000001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Warner ET, Carapinha R, Weber GM, Hill E V, Reede JY. Gender differences in receipt of National Institutes of Health R01 grants among junior faculty at an academic medical center: the role of connectivity, rank, and research productivity. J Womens Health (Larchmt) 2017;26:1086–1093. doi: 10.1089/jwh.2016.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shavers VL, Fagan P, Lawrence D, McCaskill-Stevens W, McDonald P, Browne D, et al. Barriers to racial/ethnic minority application and competition for NIH research funding J Natl Med Assoc 2005971063–1077.. [PMC free article] [PubMed] [Google Scholar]
- 144.Valantine HA, Wilson MR. Bethesda, MD: NIH; 2019. [Google Scholar]
- 145. Duncan GA, Lockett A, Villegas LR, Almodovar S, Gomez JL, Flores SC, et al. National Heart, Lung, and Blood Institute Workshop summary: enhancing opportunities for training and retention of a diverse biomedical workforce. Ann Am Thorac Soc. 2016;13:562–567. doi: 10.1513/AnnalsATS.201509-624OT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Davidson PL, Maccalla NMG, Afifi AA, Guerrero L, Nakazono TT, Zhong S, et al. A participatory approach to evaluating a national training and institutional change initiative: the BUILD longitudinal evaluation. BMC Proc. 2017;11:15. doi: 10.1186/s12919-017-0082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.FIRST Cohort funding opportunity (U54 clinical trial optional). Bethesda, MD: NIH; 2019 [Google Scholar]
- 148.RFA-HL-20-003: Disparities Elimination through Coordinated Interventions to Prevent and Control Heart and Lung Disease Risk (DECIPHeR) (UG3/UH3 clinical trial optional). Bethesda, MD: NIH; 2019 [Google Scholar]
- 149.NOT-MD-21-008: notice of special interest (NOSI) Bethesda, MD: NIH; 2020. [Google Scholar]
- 150.NOT-MD-20-023: notice of intent to publish a funding opportunity announcement for community interventions to address the consequences of the COVID-19 pandemic for health disparity and vulnerable populations (R01 clinical trial optional). . Bethesda, MD: NIH; 2021. [Google Scholar]
- 151.U.S. Food and Drug Administration. Washington, DC: U.S. Government Printing Office; 2014. [Google Scholar]
- 152. Idoko OT. Owolabi OA. Odutola AA. Ogundare O. Worwui A. Saidu Y. et al. Lessons in participant retention in the course of a randomized controlled clinical trial. BMC Res Notes. 2014;7:706. doi: 10.1186/1756-0500-7-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Joseph PD, Caldwell PHY, Tong A, Hanson CS, Craig JC. Stakeholder views of clinical trials in low- and middle-income countries: a systematic review. Pediatrics. 2016;137:e20152800. doi: 10.1542/peds.2015-2800. [DOI] [PubMed] [Google Scholar]
- 154. Nama N, Swartz L. Ethical and social dilemmas in community-based controlled trials in situations of poverty: a view from a South African project. J Community Appl Soc Psychol. 2002;12:286–297. doi: 10.1002/casp.682. [DOI] [PubMed] [Google Scholar]
- 155. Kadam RA, Borde SU, Madas SA, Salvi SS, Limaye SS. Challenges in recruitment and retention of clinical trial subjects. Perspect Clin Res. 2016;7:137–143. doi: 10.4103/2229-3485.184820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Cardillo L, Cahill F, Wylie H, Williams A, Zylstra J, Davies A, et al. Patients’ perspectives on opt-out consent for observational research: systematic review and focus group. Br J Nurs. 2018;27:1321–1329. doi: 10.12968/bjon.2018.27.22.1321. [DOI] [PubMed] [Google Scholar]
- 157. Ghebre RG, Jones LA, Wenzel JA, Martin MY, Durant RW, Ford JG. State-of-the-science of patient navigation as a strategy for enhancing minority clinical trial accrual. Cancer. 2014;120:1122–1130. doi: 10.1002/cncr.28570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Cottler LB, Striley CW, Elliott AL, Zulich AE, Kwiatkowski E, Nelson DR. Pragmatic trial of a study navigator model (NAU) vs. ambassador model (N+) to increase enrollment to health research among community members who use illicit drugs. Drug Alcohol Depend. 2017;175:146–150. doi: 10.1016/j.drugalcdep.2016.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ruvalcaba E, Green A, Chang S-E, Rand CS, Riekert KA, Eakin MN.
- 160. Handley MA, Lyles CR, McCulloch C, Cattamanchi A. Selecting and improving quasi-experimental designs in effectiveness and implementation research. Annu Rev Public Health. 2018;39:5–25. doi: 10.1146/annurev-publhealth-040617-014128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Most recent national asthma data. Atlanta, GA: CDC; 2019 [Google Scholar]
- 162.Table A-2a. Age-adjusted percentages (with standard errors) of selected respiratory diseases among adults aged 18 and over, by selected characteristics: United States, 2018. . Atlanta, GA: CDC; 2018. [Google Scholar]
- 163.Cystic Fibrosis Foundation 2018 patient registry annual data report. . Bethesda, MD: Cystic Fibrosis Foundation; 2018. [Google Scholar]
- 164.United States cancer statistics: data visualizations. Atlanta, GA: CDC; 2017 [Google Scholar]
- 165. Ejiogu N, Norbeck JH, Mason MA, Cromwell BC, Zonderman AB, Evans MK. Recruitment and retention strategies for minority or poor clinical research participants: lessons from the Healthy Aging in Neighborhoods of Diversity across the Life Span study. Gerontologist. 2011;51:S33–S45. doi: 10.1093/geront/gnr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Nipp RD, Lee H, Powell E, Birrer NE, Poles E, Finkelstein D, et al. Financial burden of cancer clinical trial participation and the impact of a cancer care equity program. Oncologist. 2016;21:467–474. doi: 10.1634/theoncologist.2015-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fouad MN, Acemgil A, Bae S, Forero A, Lisovicz N, Martin MY, et al. Patient navigation as a model to increase participation of African Americans in cancer clinical trials J Oncol Pract 201612556–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Taani MH, Zabler B, Fendrich M, Schiffman R. Lessons learned for recruitment and retention of low-income African Americans. Contemp Clin Trials Commun. 2020;17:100533. doi: 10.1016/j.conctc.2020.100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Samayoa C, Santoyo-Olsson J, Escalera C, Stewart AL, Ortiz C, Márquez-Magaña L, et al. Participant-centered strategies for overcoming barriers to biospecimen collection among Spanish-speaking Latina breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2020;29:606–615. doi: 10.1158/1055-9965.EPI-19-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Ilori TO, Viera E, Wilson J, Moreno F, Menon U, Ehiri J, et al. Approach to high volume enrollment in clinical research: experiences from an All of Us Research Program site. Clin Transl Sci. 2020;13:685–692. doi: 10.1111/cts.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hall A. Bethesda, MD: NIH; 2015. [Google Scholar]
- 172.Glazerman S, Seftor N. Washington, DC: Mathematica Policy Research; 2005. [Google Scholar]
- 173.LRP dashboard. Bethesda, MD: NIH; 2020 [Google Scholar]