A sex disparity in asthma incidence and severity is supported by epidemiological studies that demonstrate males having a higher prevalence of asthma in childhood compared with females and women having the higher prevalence as adults (1). This switch occurs during puberty when androgen levels increase in males (1). Asthma prevalence converges in late adulthood, when androgen levels decline in males and estrogen, progesterone, and androgen levels decline in females, inferring a modulatory role for sex hormones in asthma pathogenesis.
Animal models of asthma have provided insight into the effects of individual sex hormones on lung inflammation through interventions that are otherwise not possible in human studies. Using these models, estrogen signaling through estrogen receptor (ER)-α increases ovalbumin-induced eosinophilic inflammation and methacholine responsiveness, with mice deficient in ER-α having diminished allergen-induced responses compared with wild-type mice (2). Progesterone treatment exacerbates these responses, whereas androgens, dehydroepiandrosterone (DHEA), and testosterone have an opposite effect (2). Women with asthma have more type-2–polarized alveolar macrophages, with the number in the airway corresponding to asthma severity, whereas androgen receptor (AR) deficiency in monocytes/macrophages results in reduced lung inflammation in male mice, suggesting other factors may be important, particularly in females (3).
Group 2 innate lymphoid cells (ILC2s) are a significant source of IL-5 and IL-13, and activation of these cells is now considered a key early event in type-2 inflammatory diseases (4). In the lower airways, ILC2s are detected in greater numbers in the sputum of individuals with severe eosinophilic asthma compared with mild asthma despite high-dose inhaled corticosteroid therapy (5), with females with moderate to severe asthma having increased circulating ILC2s compared with males (6), whereas no sex disparity was seen in healthy control subjects (6). In individuals with mild allergic asthma, airway levels of ILC2 are greater in females, indicating that sex hormones regulate proliferation of lung ILC2s (7). This is supported by murine studies in which testosterone reduces allergen-induced expression of IL-33 and TSLP (thymic stromal lymphopoietin) in the lungs, type 2 cytokine production by ILC2s (Figure 1) (6), and the development of mature ILC2s from precursors that express AR, in which AR signaling reduces differentiation to mature ILC2s (8). Therefore, androgens and AR signaling play a crucial protective role in type-2 airway inflammation, perhaps most importantly within the airways. In addition, the importance of assessing ovarian hormone receptor levels within the airways is highlighted in a recent study showing that signaling through ER-α expressed on human bronchial epithelial cells induced increased IL-33 production in vitro and that, in mice, this signaling indirectly triggered increased allergen-induced airway IL-5 and IL-13 production by ILC2s and eosinophilia compared with wild-type mice (9).
Figure 1.
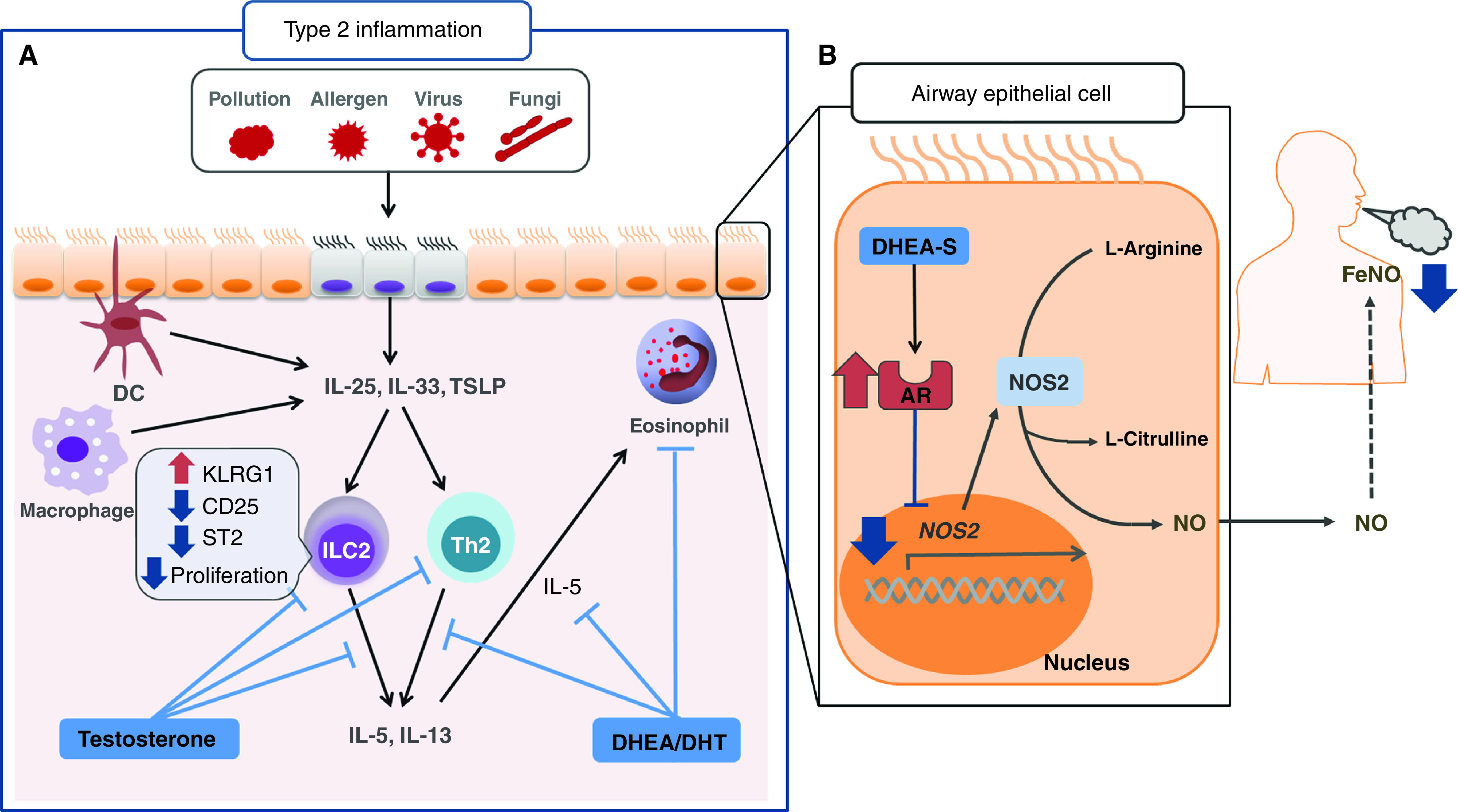
Airway androgen and androgen receptor (AR) axis—putative modulation of asthma pathogenesis. (A) In mice, testosterone and DHEA attenuate type 2 inflammatory airway responses by inhibiting type 2 cytokine (IL-5 and IL-13) generation by ILC2 and Th2 cell populations, thereby attenuating eosinophilia (6). (B) Zein and colleagues report that AR gene and protein ligand expression levels are negatively associated with inducible nitric oxide synthase (NOS2) gene expression and fractional exhaled nitric oxide (FeNO) levels (10). The signaling from AR is postulated to cause suppression of gene expression of NOS2, an enzyme that catalyzes the production of nitric oxide from l-arginine with consequent reduction in FeNO levels. The beneficial role of androgens in the pathophysiology of asthma is partly mediated through AR modulation of the nitrosative capacity of epithelial cells. DC = dendritic cells; DHEA = dehydroepiandrosterone; DHEA-S = DHEA sulfate; DHT = 5α-dihydrotestosterone; ILC2 = group 2 innate lymphoid cell; Th2 = T-helper cell type 2; TSLP = thymic stromal lymphopoietin.
In this issue of the Journal, Zein and colleagues (pp. 285–293) report the expression of AR at the gene and protein level in human airways from a cohort of individuals with severe asthma, and using two additional cohorts (CCHS [Cleveland Clinic Health System] and NHANES [National Health and Nutrition Examination Survey]), they compare the presence of AR expression in bronchial epithelial cells on asthma outcomes (10). This cross-sectional analysis of 1,659 adults enrolled in SARP (Severe Asthma Research Program), 32,527 adults in CCHS, and 2,629 adults in NHANES shows that women had more asthma exacerbations and emergency department visits than men. The authors did a subgroup analysis of 128 patients in the SARP study, after excluding women receiving exogenous hormone treatments, to compare the presence of AR and its ligands with asthma outcomes. The study showed AR gene expression was positively associated with percent predicted FEV1 (FEV1PP), asthma quality of life questionnaire, whereas AR gene expression was negatively associated with fractional exhaled nitric oxide and inducible nitric oxide synthase (Figure 1). Interestingly, AR gene expression did not vary by sex or correlate with asthma exacerbations in the year before SARP enrollment. Given the significant interaction of AR expression on FEV1PP, the authors showed that FEV1PP correlated positively with both DHEA sulfate and testosterone in men; however, in women, there was a positive correlation between FEV1PP and DHEA sulfate but not with free testosterone. The lack of a significant difference in AR gene expression between sexes contrasts a previous study of airway smooth muscle cells in which AR gene expression is lower in females with asthma compared with males with asthma (11). Because AR expression is not limited to the epithelial cells, the presence of AR expression on other cells within the airways may confound the authors’ results. Although these results are novel, support the protective nature of androgens on the pathogenesis of asthma, and further our understanding of sex differences in severe asthma outcomes, they should be interpreted with caution. A small sample size (n = 664) had androgen hormone levels measured, which resulted in the authors being unable to stratify patients by obesity; Han and colleagues recently showed obesity modifies the effects of sex hormones in adults (12). This study did not consider menopause or menstrual cycle phases in women. Androgen levels can fluctuate significantly in premenopausal women, and in men, testosterone levels can have a significant diurnal variation (13), which may result in inaccurate correlations. Importantly, this study did not include estradiol or progesterone levels, which vary in menstruating women and could explain sex-specific differences in asthma. The authors did not exclude patients with a history of polycystic ovarian syndrome, which results not only in an increase in circulating androgen levels (14) but also increases endometrial expression of AR compared with normal ovulating women (15) and could possibly be increased in other organs, such as the lung.
In summary, although the report by Zein and colleagues represents a major advancement in the study of androgens and asthma, additional longitudinal and interventional studies are required to assess 1) the cyclical effects of sex hormones in menstruating females, 2) changes in sex hormones during both menopause and in older males, and 3) the effect these changes have on asthma outcomes. Therefore, given the cross-sectional nature of this study and the absence of female sex hormone measurements, the findings should be interpreted with some caution.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202104-0869ED on May 5, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Zhang G-Q, Bossios A, Rådinger M, Nwaru BI. Sex steroid hormones and asthma in women: state-of-the-art and future research perspectives. Expert Rev Respir Med. 2020;14:543–545. doi: 10.1080/17476348.2020.1741351. [DOI] [PubMed] [Google Scholar]
- 2. Yung JA, Fuseini H, Newcomb DC. Hormones, sex, and asthma. Ann Allergy Asthma Immunol. 2018;120:488–494. doi: 10.1016/j.anai.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Becerra-Díaz M, Strickland AB, Keselman A, Heller NM. Androgen and androgen receptor as enhancers of M2 macrophage polarization in allergic lung inflammation. J Immunol. 2018;201:2923–2933. doi: 10.4049/jimmunol.1800352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dahlgren MW, Molofsky AB. All along the watchtower: group 2 innate lymphoid cells in allergic responses. Curr Opin Immunol. 2018;54:13–19. doi: 10.1016/j.coi.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith SG, Chen R, Kjarsgaard M, Huang C, Oliveria J-P, O'Byrne PM, et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2016;137:75–86.e8. doi: 10.1016/j.jaci.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 6. Cephus J-Y, Stier MT, Fuseini H, Yung JA, Toki S, Bloodworth MH, et al. Testosterone attenuates group 2 innate lymphoid cell-mediated airway inflammation. Cell Rep. 2017;21:2487–2499. doi: 10.1016/j.celrep.2017.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aw M, Nair P, Salter B, Chen R, Machida K, Inman M, et al. Effect of sex on group 2 innate lymphoid cells in the airways of mild and severe asthmatics. Allergy. 2019;74:1397–1400. doi: 10.1111/all.13742. [DOI] [PubMed] [Google Scholar]
- 8. Laffont S, Blanquart E, Savignac M, Cénac C, Laverny G, Metzger D, et al. Androgen signaling negatively controls group 2 innate lymphoid cells. J Exp Med. 2017;214:1581–1592. doi: 10.1084/jem.20161807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cephus JY, Gandhi VD, Shah R, Brooke Davis J, Fuseini H, Yung JA, et al. Estrogen receptor-α signaling increases allergen-induced IL-33 release and airway inflammation. Allergy. 2021;76:255–268. doi: 10.1111/all.14491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zein JG, McManus JM, Sharifi N, Erzurum SC, Marozkina N, Lahm T, et al. NHLBI Severe Asthma Research Program. Benefits of airway androgen receptor expression in human asthma. Am J Respir Crit Care Med. 2021 doi: 10.1164/rccm.202009-3720OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kalidhindi RSR, Katragadda R, Beauchamp KL, Pabelick CM, Prakash Y, Sathish V. Androgen receptor-mediated regulation of intracellular calcium in human airway smooth muscle cells. Cell Physiol Biochem. 2019;53:215–228. doi: 10.33594/000000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Han Y-Y, Forno E, Celedón JC. Sex steroid hormones and asthma in a nationwide study of US adults. Am J Respir Crit Care Med. 2020;201:158–166. doi: 10.1164/rccm.201905-0996OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Kerkhof LW, Van Dycke KC, Jansen EH, Beekhof PK, van Oostrom CT, Ruskovska T, et al. Diurnal variation of hormonal and lipid biomarkers in a molecular epidemiology-like setting. PLoS One. 2015;10:e0135652. doi: 10.1371/journal.pone.0135652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adams JM, Taylor AE, Crowley WF, Jr, Hall JE. Polycystic ovarian morphology with regular ovulatory cycles: insights into the pathophysiology of polycystic ovarian syndrome. J Clin Endocrinol Metab. 2004;89:4343–4350. doi: 10.1210/jc.2003-031600. [DOI] [PubMed] [Google Scholar]
- 15. Apparao KB, Lovely LP, Gui Y, Lininger RA, Lessey BA. Elevated endometrial androgen receptor expression in women with polycystic ovarian syndrome. Biol Reprod. 2002;66:297–304. doi: 10.1095/biolreprod66.2.297. [DOI] [PubMed] [Google Scholar]


