Abstract
Rationale: Respiratory support (noninvasive ventilation or high-flow nasal cannula) applied at the time of extubation has been reported to reduce reintubation rates, but concerns regarding effectiveness have limited uptake into practice.
Objectives: To determine if providing postextubation respiratory support to all patients undergoing extubation in a medical ICU would decrease the incidence of reintubation.
Methods: We conducted a pragmatic, two-armed, cluster–crossover trial of adults undergoing extubation from invasive mechanical ventilation between October 1, 2017, and March 31, 2019, in the medical ICU of an academic medical center. Patients were assigned to either protocolized postextubation respiratory support (a respiratory therapist–driven protocol in which patients with suspected hypercapnia received noninvasive ventilation and patients without suspected hypercapnia received high-flow nasal cannula) or usual care (postextubation management at the discretion of treating clinicians). The primary outcome was reintubation within 96 hours of extubation.
Measurements and Main Results: A total of 751 patients were enrolled. Of the 359 patients assigned to protocolized support, 331 (92.2%) received postextubation respiratory support compared with 66 of 392 patients (16.8%) assigned to usual care, a difference driven by differential use of high-flow nasal cannula (74.7% vs. 2.8%). A total of 57 patients (15.9%) in the protocolized support group experienced reintubation compared with 52 patients (13.3%) in the usual care group (odds ratio, 1.23; 95% confidence interval, 0.82 to 1.84; P = 0.32).
Conclusions: Among a broad population of critically ill adults undergoing extubation from invasive mechanical ventilation at an academic medical center, protocolized postextubation respiratory support, primarily characterized by an increase in the use of high-flow nasal cannula, did not prevent reintubation compared with usual care.
Clinical trial registered with www.clinicaltrials.gov (NCT0328831).
Keywords: reintubation, invasive mechanical ventilation, noninvasive respiratory support
At a Glance Commentary
Scientific Knowledge on the Subject
Postextubation respiratory support, in which noninvasive ventilation or high-flow nasal cannula is administered immediately after extubation, has been proposed as a method to prevent reintubation of critically ill adults based on prior trials among patients with hypercapnia, nonhypercapnic patients at high risk of reintubation, and nonhypercapnic patients at low risk of reintubation. Recent guidelines have broadly recommended postextubation respiratory support, but uncertainty regarding the generalizability of the findings from prior trials persists.
What This Study Adds to the Field
In the PROPER (Protocolized Post-Extubation Respiratory Support) trial, protocolized postextubation respiratory support, primarily characterized by the use of high-flow nasal cannula after extubation, did not prevent reintubation compared with usual care among a broad population of critically ill adults undergoing extubation from invasive mechanical ventilation at an academic medical center.
Protocols for low-Vt ventilation (1), daily spontaneous awakening trials (2), and daily spontaneous breathing trials (3, 4) have improved survival for critically ill adults receiving invasive mechanical ventilation. The period immediately after extubation, however, remains high risk for morbidity. Approximately 15% of patients experience reintubation within 96 hours (5–9), and reintubation is associated with increased risk of nosocomial infection (10) and death (8, 11, 12).
Postextubation respiratory support, in which noninvasive ventilation (NIV) or high-flow nasal cannula (HFNC) is administered immediately after extubation, has been proposed as a method to prevent reintubation of critically ill adults (13). Randomized trials have demonstrated that provision of NIV to patients with hypercapnia (14, 15), provision of NIV or HFNC to nonhypercapnic patients at high risk of reintubation (6, 15, 16), and provision of HFNC to nonhypercapnic patients at low risk of reintubation (17) reduce the incidence of postextubation respiratory failure or reintubation. Together, these groups represent nearly all patients undergoing extubation in the ICU. Recent guidelines have broadly recommended postextubation respiratory support (13). However, uncertainty regarding the generalizability of the findings from prior trials persists (18). A recent study reported that only half of ICU providers believe postextubation respiratory support reduces the risk of reintubation among patients at high risk (19).
The PROPER (Protocolized Post-Extubation Respiratory Support) Trial examined the effect of routinely providing postextubation respiratory support with NIV or HFNC (“protocolized support”) to all patients undergoing extubation in an ICU to test the hypothesis that protocolized support would decrease the incidence of reintubation within 96 hours compared with usual care. Some of the results of this study have been previously reported in the form of an abstract (20).
Methods
Study Design and Oversight
The PROPER trial was a prospective, unblinded, pragmatic, cluster–crossover trial comparing protocolized support (a respiratory therapis–driven protocol that advised the provision of NIV or HFNC based on patient characteristics) to usual care (postextubation management at the discretion of treating clinicians). The trial was conducted between October 1, 2017, and March 31, 2019, in the medical ICU of Vanderbilt University Medical Center in Nashville, Tennessee (see online supplement for site characteristics).
The trial was approved with a waiver of informed consent by the Institutional Review Board at Vanderbilt University Medical Center (see online supplement). The trial was registered online before initiation (NCT03288311), and the study protocol and statistical analysis plan were published before the conclusion of enrollment (21).
Study Sites and Patient Population
All adults (age ⩾18 yr) undergoing extubation from invasive mechanical ventilation in the study ICU were enrolled at the time of extubation, except those who had received less than 12 hours of invasive mechanical ventilation, had directives not to be reintubated after extubation, or had been reintubated previously during the hospitalization (see online supplement for complete inclusion and exclusion criteria).
Randomization and Treatment Allocation
The study ICU was divided into two geographic clusters (front hallway and back hallway), each staffed by a separate respiratory therapist. During each 3-month block of the study, all eligible patients extubated in one cluster received protocolized support delivered by one respiratory therapist, whereas all patients extubated in the other cluster received usual care delivered by the other respiratory therapist. The clusters alternated treatment group assignment every 3 months so that each cluster experienced an equal number of months assigned to protocolized support and usual care (see Figure E1 in the online supplement). Simple randomization was used to determine which of the two clusters would receive protocolized support during the first 3-month block. Patients being admitted to the study ICU were assigned to a bed by administrative hospital personnel unaware of treatment group assignment. All beds in the study ICU care for patients of the same acuity without selection by patient characteristics. Patients, clinicians, and investigators were not blinded to group assignment.
Study Interventions
Study protocol determined only the approach to postextubation respiratory support. Patients in the protocolized support group received postextubation respiratory support beginning at the time of extubation. The respiratory therapist selected between NIV and HFNC using a standardized protocol (Figure E2). The protocol recommended NIV for patients intubated for an acute exacerbation of chronic obstructive pulmonary disease (22) and patients with chronic hypercapnic respiratory failure, obesity hypoventilation syndrome, or a Pco2 greater than 45 mm Hg on an arterial blood gas during a spontaneous breathing trial (see Methods in the online supplement) (14, 15). Preextubation arterial blood gases were not routinely collected in the study unit. HFNC was recommended for all patients without an indication for NIV and for patients for whom NIV was contraindicated (e.g., facial trauma or active emesis). Patients who were extubated to NIV but were unable to tolerate it could be transitioned to HFNC. For patients receiving NIV or HFNC in either group, study protocols specified initial settings, titration, and weaning (Figures E3 and E4), including an initial FiO2 of 0.4 for all patients, an inspiratory airway pressure of 14 cm H2O and expiratory airway pressure of 8 cm H2O for patients receiving NIV, and an initial flow rate of 40 L/min for patients receiving HFNC. Institutional practice included setting the initial gas flow temperature for HFNC at 31°C (23).
Protocolized support was provided from extubation until at least 5:00 a.m. the following day, at which time the respiratory therapist assessed for readiness to transition to conventional oxygen (Figures E3 and E4). Postextubation respiratory support could be continued beyond 5:00 a.m. for patients with a respiratory rate greater than 25 breaths per minute, an FiO2 greater than 0.4, an oxygen saturation less than 90%, or at the discretion of treating clinicians. Postextubation respiratory support could be discontinued earlier than 5:00 a.m. on the day after extubation if the patient was transferred out of the ICU, the patient declined further postextubation respiratory support, or the treating clinicians determined that discontinuation was needed for the optimal care of the patient.
In the usual care group, all aspects of postextubation management were determined by treating clinicians. Treating clinicians could administer conventional oxygen therapy or could administer NIV or HFNC to patients they believed would benefit from postextubation respiratory support.
Cointerventions
Patients in both groups received established clinical protocols for analgesia (24), sedation (25, 26), delirium assessment (27, 28), daily spontaneous awakening and breathing trials (4), and early mobility (29). Spontaneous breathing trials in the study unit were conducted via pressure-support ventilation with an inspiratory pressure of 5 cm H2O and a positive end-expiratory pressure of 5 cm H2O for at least 30 minutes (13, 30). Because the goal of the trial was to evaluate the performance of protocolized postextubation respiratory support in “real-world” practice, decisions regarding timing of extubation, provision of rescue support, reintubation, and all other aspects of postextubation management were made by treating clinicians. Rescue therapy was defined as the treatment of respiratory failure with NIV or HFNC for a patient not currently receiving postextubation respiratory support.
Data Collection
Data were collected by trained study personnel. Data included demographics, diagnoses, indication for intubation, duration of mechanical ventilation before extubation, Acute Physiology and Chronic Health Evaluation II score (31), failure of more than one spontaneous breathing trial, respiratory rate during the preextubation spontaneous breathing trial, receipt of HFNC and NIV as postextubation respiratory support or rescue therapy, reintubation, ICU and hospital length of stay, and in-hospital mortality.
Study Outcomes
The primary outcome was reintubation in the 96 hours after extubation. Reintubation was defined as the placement of an endotracheal tube or tracheostomy tube in the trachea for any reason, including intubation for a procedure. To account for the competing risk of death, patients who died in the 96 hours after extubation without experiencing reintubation were classified as having experienced the primary outcome in the primary analysis.
The single, prespecified, secondary outcome was the number of ICU-free days from extubation to 28 days after extubation. Exploratory outcomes included all-cause in-hospital mortality, ventilator-free days from extubation to 28 days after extubation, time from extubation to reintubation, indication for reintubation, and the highest respiratory rate in the 24 hours after extubation.
Statistical Analysis
Complete details of the sample size estimation have been published previously and are provided in the online supplement (21). The planned study duration of 18 months was anticipated to result in the enrollment of 630 patients. Assuming a reintubation rate of 12.1% in the usual care group, enrolling 630 patients would provide 80% power at a type 1 error rate of 0.05 to detect a 55% relative risk reduction (absolute risk reduction of 6.7%) for reintubation with protocolized support (6, 15, 17, 32, 33).
Analyses were conducted at the level of each patient’s hospitalization in an intention-to-treat fashion. Continuous variables were reported as medians and interquartile ranges (IQRs); categorical variables were reported as frequencies and proportions.
The primary analysis was a comparison of the primary outcome between the protocolized support group and the usual care group using generalized estimating equations adjusting for location. The model took into account the correlation of clustering by location-by-period (see online supplement) (34, 35). In addition to the primary analysis, which provides marginal effect estimates, conditional effect estimates were calculated using a generalized linear mixed-effects model with a logit link function (see online supplement).
Prespecified secondary analyses used a similar approach and included an adjusted comparison accounting for prespecified baseline characteristics (age, Acute Physiology and Chronic Health Evaluation II score, duration of invasive mechanical ventilation, indication for intubation, chronic hypercapnia, chronic pulmonary disease, and respiratory rate on a spontaneous breathing trial), a sensitivity analysis classifying patients who died without reintubation as not meeting the primary outcome, a modified intention-to-treat analysis excluding patients who received less than 5 hours of postextubation respiratory support in the protocolized support group, and a proportional subdistribution hazards regression model to compare time to reintubation between groups with death as the competing risks (36). Post hoc sensitivity analyses included alternative definitions of reintubation as tracheal intubation within 48 hours, 72 hours, or 7 days of extubation. To augment the interpretation of the results, a post hoc Bayesian analysis was performed using skeptical, optimistic, and pessimistic priors (see online supplement).
All continuous and ordinal outcomes were analyzed using a proportional odds model adjusting for location. The Huber-White method was used to correct for heteroscedasticity and for correlated responses from clustering of location-by-period. For the sole prespecified secondary outcome of ICU-free days in the 28 days after enrollment, comparisons were calculated both without and with adjustment for prespecified baseline characteristics using the same approach as that for the primary outcome.
A two-sided P < 0.05 indicated statistical significance for the primary and secondary outcome. Between-group differences in exploratory outcomes are reported using point estimates and 95% confidence intervals (CIs). The widths of the CIs have not been adjusted for multiplicity and should not be used to infer statistical significance of differences in treatment effects between groups. No data for any outcome were missing. Missing data for baseline variables were imputed using multiple imputations for adjusted analyses. All analyses were performed using the statistical software R, version 3.3.0 (R Foundation for Statistical Computing).
Results
Baseline Characteristics
Of the 1,517 patients who received invasive mechanical ventilation during the study period, 952 survived and underwent extubation in the study ICU, of whom 751 (78.9%) met no exclusion criteria and were enrolled (Figure E5). The median age was 57 years, and the median duration of invasive mechanical ventilation was 3 days; nearly half of the patients were intubated for respiratory failure, and approximately 90% of patients had at least one risk factor for reintubation (Table 1, and Tables E1–E5) (17, 21, 33). The 359 patients (47.8%) assigned to the protocolized support group and the 392 patients (52.2%) assigned to the usual care group were similar at baseline (Table 1).
Table 1.
Patient Characteristics at Baseline
| Patient Characteristic | Protocolized Support (n = 359) |
Usual Care (n = 392) |
|---|---|---|
| Age, median (IQR), yr | 56 (43–66) | 57 (42–66) |
| Sex, M, n (%) | 204 (56.8) | 205 (52.3) |
| White race*, n (%) | 287 (81.3) | 317 (81.7) |
| Body mass index†, median (IQR), kg/m2 | 26.6 (23.0–31.7) | 28.0 (23.2–33.8) |
| Chronic respiratory comorbidity‡, n (%) | 138 (38.4) | 163 (41.6) |
| Chronic obstructive pulmonary disease | 58 (16.2) | 87 (22.2) |
| Asthma | 21 (5.8) | 18 (4.6) |
| Obstructive sleep apnea | 39 (10.9) | 47 (12.0) |
| Pulmonary malignancy | 14 (3.9) | 16 (4.1) |
| Indications for intubation‡, n (%) | ||
| Airway protection for decreased level of consciousness | 180 (50.1) | 168 (42.9) |
| Hypoxemic respiratory failure | 141 (39.3) | 175 (44.6) |
| Hypercarbic respiratory failure | 42 (11.7) | 65 (16.6) |
| Preprocedural | 45 (12.5) | 36 (9.2) |
| Duration of invasive mechanical ventilation before extubation, d, median (IQR) | 3.0 (2.0–5.0) | 3.0 (2.0–4.0) |
| Unplanned extubation | 17 (4.7%) | 27 (6.9%) |
| APACHE II score at ICU admission§, median (IQR) | 19 (12–24) | 18 (13–24) |
| APACHE II score at extubation§, median (IQR) | 17 (13–22) | 17 (12–22) |
| Active medical conditions‡, n (%) | ||
| Sepsis or septic shock | 136 (37.9) | 146 (37.2) |
| Pneumonia | 135 (37.6) | 135 (34.4) |
| Acute respiratory distress syndrome | 23 (6.4) | 21 (5.4) |
| Aspiration | 44 (12.3) | 57 (14.5) |
| Gastrointestinal bleeding | 45 (12.5) | 42 (10.7) |
| Altered mental status | 190 (52.9) | 204 (52.0) |
| Vasopressors in the 6 h before extubation, n (%) | 47 (13.1) | 48 (12.2) |
| Failed one or more spontaneous breathing trial, n (%) | 83 (23.1) | 80 (20.4) |
| Highest respiratory rate on a spontaneous breathing trial‖ | 22 (17–28) | 22 (18–28) |
| At least one risk factor for reintubation¶ | 316 (88.0) | 350 (89.3) |
Definition of abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation; IQR = interquartile range.
Race was reported by patients or their surrogates and recorded in the electronic health record as a part of routine clinical care. Race was missing for 10 patients (1.3%): 6 patients in the protocolized support group and 4 patients in the usual care group.
Information on body mass index at enrollment was missing for 21 patients (2.8% patients): 13 from the protocolized support group and 8 from the usual care group.
Patients could have more than one.
APACHE II score ranges from 0 to 71, with higher scores indicating higher severity of illness.
Respiratory rate on spontaneous breathing trial was missing for 41 patients (5.4% patients): 14 from the protocolized support group and 27 from the usual care group.
Risk factors for reintubation, as defined by Hernández and colleagues (17, 33), included age >65 years, heart failure, moderate to severe chronic obstructive pulmonary disease, APACHE II score at extubation >12, body mass index >30 kg/m2, failure of >1 spontaneous breathing trial, and duration of mechanical ventilation ⩾7 days.
Postextubation Management
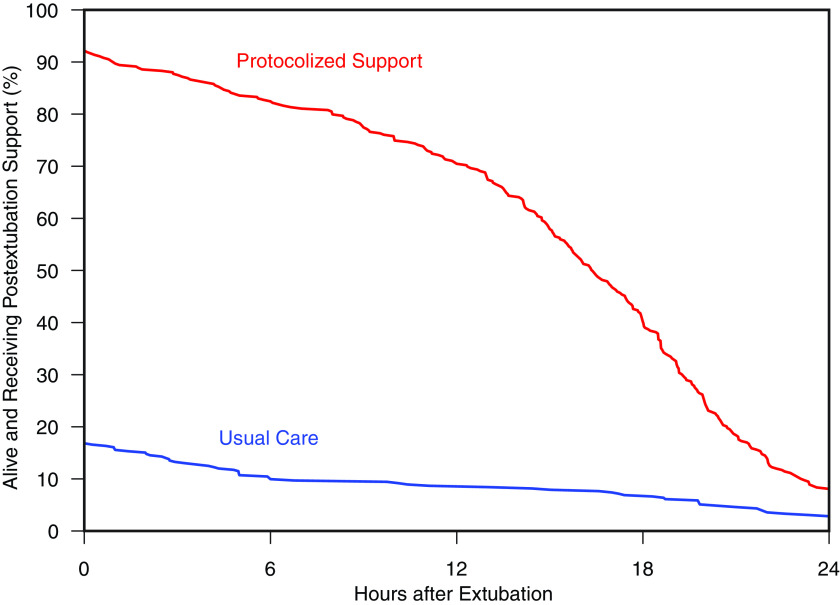
A total of 331 patients (92.2%) in the protocolized support group received postextubation respiratory support starting at extubation compared with 66 patients (16.8%) in the usual care group. HFNC was initiated at extubation for 268 patients (74.7%) in the protocolized support group and 11 patients (2.8%) in the usual care group, whereas NIV was initiated at extubation for 63 patients (17.5%) in the protocolized support group and 55 patients (14.0%) in the usual care group (Table E6). Among the 331 patients who received postextubation support in the protocolized support group, the median duration of support was 16 hours (IQR, 11–20). For the 66 patients who received postextubation support in the usual care group, the median duration of support was 13 hours (IQR, 5–22) (Figure 1).
Figure 1.
Postextubation respiratory support by study group. The figure displays the proportion of patients alive and receiving postextubation respiratory support (y-axis) between the time of extubation and 24 hours after extubation (x-axis) for patients in the protocolized support group (red) and usual care group (blue).
In the protocolized support group, 213 patients (59.3%) received support for the entire intervention period, 43 patients (12.0%) discontinued support early because of intolerance, 12 patients (3.3%) did not tolerate any duration of support, and 63 patients (17.5%) experienced early termination without a provided rationale (Table E7). For patients receiving HFNC, the median flow rate in the 6 hours after extubation was 40 L/min (IQR, 40–40 L/min). For patients receiving NIV, the median inspiratory positive airway pressure in the 6 hours after extubation was 10 cm H2O (IQR, 10–12 cm H2O), and the median expiratory positive airway pressure was 5 cm H2O (IQR, 5–6 cm H2O) (Table E9).
In the usual care group, treating clinicians elected to administer postextubation respiratory support predominantly to patients with chronic respiratory disease, obesity, or respiratory failure as the indication for intubation (Table E8); only one patient (0.3%) without one of these conditions received support.
HFNC or NIV was administered as rescue therapy to 36 patients (10.0%) in the protocolized support group and 55 patients (14.0%) in the usual care group (Figure E6, Table E6).
Physiologic Measures
Patients’ highest respiratory rate in the 6 hours after extubation was lower in the protocolized support group (median, 23 breaths per minute; IQR, 20–28) compared with the usual care group (median, 24 breaths per minute; IQR, 21–28) (odds ratio [OR], 0.77; 95% CI, 0.60–0.98) (Table E10). Patients’ lowest oxygen saturation in the 6 hours after extubation was higher in the protocolized support group (median, 94%; IQR, 91–97%) compared with the usual care group (median, 93%; IQR, 90–95%; OR, 1.85; 95% CI, 1.44–2.39). The highest FiO2 in the 6 hours after extubation was higher in the protocolized support group (median, 0.40; IQR, 0.36–0.45) than in the usual care group (median, 0.33; IQR, 0.27–0.41; OR, 2.61; 95% CI, 1.67–4.07).
Primary Outcome
Reintubation in the 96 hours after extubation occurred in 57 patients (15.9%) in the protocolized support group and 52 patients (13.3%) in the usual care group (OR, 1.23; 95% CI, 0.82–1.84; P = 0.32) (Table 2, Figure 2). The results were similar in multivariable analyses adjusting for prespecified covariates, sensitivity analyses using alternative study populations and alternative definitions of the primary outcome (e.g., reintubation within 48 hours or reintubation within 72 hours), and modified intention-to-treat analysis (Tables E11 and E12). Indications for reintubation are presented in Table E13. On post hoc Bayesian analysis, protocolized postextubation respiratory support was associated with a low probability of clinically significant benefit or harm under all assumptions (skeptical, optimistic, or pessimistic prior; Table E14).
Table 2.
Clinical Outcomes
| Outcomes | Protocolized Support (n = 359) |
Usual Care (n = 392) |
Odds Ratio (95% Confidence Intervals) |
|---|---|---|---|
| Primary outcome* | |||
| Reintubation in the 96 h after extubation, n (%) | 57 (15.9) | 52 (13.3) | 1.23 (0.82–1.84) |
| Indication for reintubation†, n (%) | |||
| Hypoxemic respiratory failure | 25 (43.9) | 24 (46.2) | — |
| Hypercapnic respiratory failure | 5 (8.8) | 2 (3.8) | — |
| Hypercapnic, hypoxemic respiratory failure | 5 (8.8) | 7 (13.5) | — |
| Altered mental status | 6 (10.5) | 5 (9.6) | — |
| Procedure | 7 (12.3) | 8 (15.4) | — |
| Other | 3 (5.3) | 6 (11.5) | — |
| Death without reintubation | 6 (10.5) | 0 (0.0) | — |
| Secondary outcome | |||
| ICU-free days, median (IQR) | 26 (23–26) | 26 (22–26) | 0.96 (0.81–1.13) |
| Exploratory outcomes‡ | |||
| Highest respiratory rate within 6 h after extubation§, median (IQR), breaths per minute | 23 (20–28) | 24 (21–28) | 0.77 (0.60–0.98) |
| Lowest SaO2 within 6 h after extubation‖, median (IQR), % | 94 (91–97) | 93 (90–95) | 1.85 (1.44–2.39) |
| Highest FiO2 within 6 h after extubation¶, median (IQR) | 0.40 (0.36–0.45) | 0.33 (0.27–0.41) | 2.61 (1.67–4.07) |
| Reintubation within 28 d¶, n (%) | 80 (22.3) | 82 (20.9) | 1.07 (0.79–1.45) |
| Time from extubation to reintubation, median (IQR), h | 56 (21–147) | 47 (18–163) | — |
| Ventilator-free days, median (IQR) | 28 (28–28) | 28 (28–28) | 1.21 (0.91–1.62) |
| Died before hospital discharge, n (%) | 29 (8.1) | 41 (10.5) | 0.76 (0.53–1.08) |
Definition of abbreviation: IQR = interquartile range.
For the purpose of the primary outcome, patients who died within 96 hours without experiencing reintubation were classified as reintubated. A total of 15 patients (2.0%) died within 96 hours of extubation: 9 patients in the protocolized support group (3 of whom had experienced reintubation) and 6 patients in the usual care group (all of whom had experienced reintubation). See Table E16 for additional details on patients who died within 96 hours without experiencing reintubation.
A list of indications for reintubation within 28 days is available in Table E13.
Oxygen saturation, FiO2, and positive end-expiratory pressure were measured from 0 to 6 hours, 6 to 12 hours, and 12 to 24 hours postextubation. Values for all time points are available in Table E10.
Highest respiratory rate in the first 6 hours postextubation was missing for six patients (0.8%): three in the protocolized support group and three in the usual support group.
Lowest oxygen saturation in the first 6 hours postextubation was missing for three patients (0.4%): one in the protocolized support group and two in the usual support group. Outcome was added post hoc.
Outcome was added post hoc.
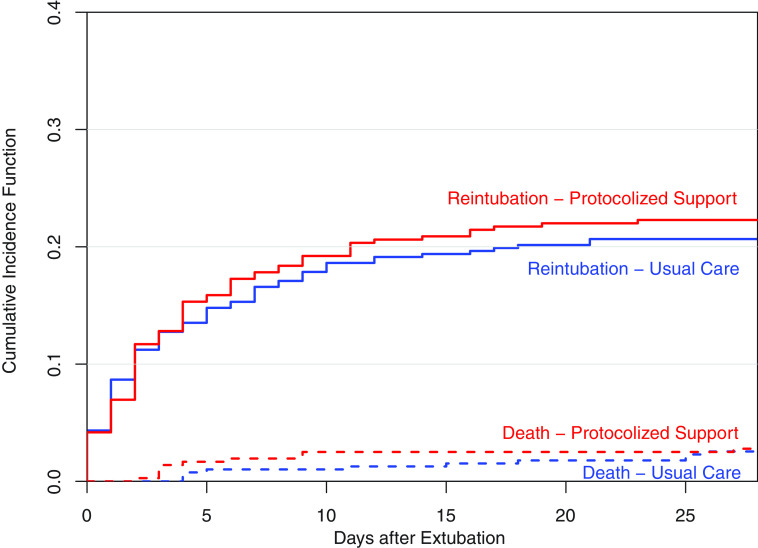
Figure 2.
Competing risk analysis of reintubation or death. In a proportional subdistribution hazards regression model of reintubation with death as the competing risks (36), the time to reintubation within 28 days of extubation did not differ significantly between the protocolized support group and the usual care group (hazard ratio, 1.07; 95% confidence interval, 0.78–1.46).
In the prespecified subgroup analyses, protocolized support did not appear to be more effective than usual care among patients at higher risk of reintubation, patients with higher severity of illness at extubation, patients who had previously failed more than one spontaneous breathing trial, or patients with higher body mass index (Figure 3, Figures E7 and E8). The duration of protocolized support did not appear to modify its effect on reintubation (Figure 3). The duration of mechanical ventilation before extubation appeared to potentially modify the effect of protocolized support on reintubation, with protocolized support associated with numerically lower rates of reintubation among patients who had received at least 7 days of invasive mechanical ventilation (P value for interaction = 0.08) (Figure E7).
Figure 3.
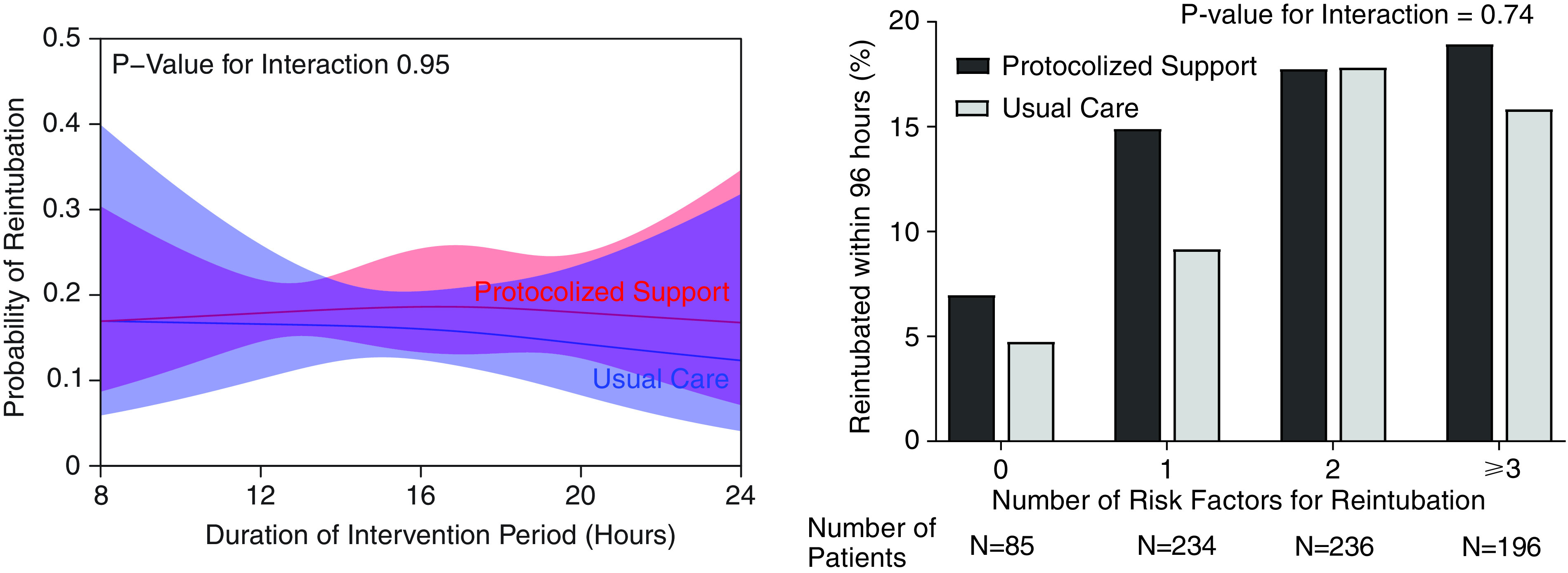
Effect modification by risk of reintubation and duration of ventilation. The left panel displays the probability of reintubation within 96 hours (mean and 95% confidence interval) for patients assigned to protocolized support (red) and usual care (blue) relative to the duration of the intervention period. The duration of the intervention period was based on the time of extubation. Because postextubation respiratory support was removed at 5:00 a.m. on the day after extubation, the duration of the intervention period (x-axis) ranged from a minimum of 5 hours for patients extubated just before midnight to a maximum of 29 hours for patients extubated just before 5:00 a.m. The P value for the interaction between study group assignment and the duration of the intervention period was 0.96, suggesting that longer duration of support did not appear to make protocolized support more effective. The right panel displays the observed incidence of reintubation within 96 hours in each group relative to the number of risk factors for reintubation (17, 33). Protocolized support did not appear to reduce the risk of reintubation compared with usual care among patients with lower or higher risk of reintubation.
Compared with usual care, protocolized support did not appear to reduce the incidence of reintubation among patients with hypercapnia or a chronic obstructive pulmonary disease exacerbation. However, more than 60% of patients with these conditions in the usual care group received NIV or HFNC in the 96 hours after extubation (Table E15).
Secondary Outcome
The number of ICU-free days from extubation to Day 28 was 26 (IQR, 23–26) in the protocolized support group and 26 (IQR, 22–26) in the usual care group (OR, 0.96; 95% CI, 0.81–1.13; P = 0.61) (Table 2). The results were similar in analyses adjusting for prespecified covariates (Table E11).
Additional Outcomes
Reintubation within 28 days of extubation occurred in 80 patients (22.3%) in the protocolized support group and 82 patients (20.9%) in the usual care group (OR, 1.07; 95% CI, 0.79–1.45). Time to reintubation, ventilator-free days, and in-hospital mortality did not significantly differ between groups (Figure 2, Table 2).
Discussion
This pragmatic trial found that, among critically ill adults undergoing extubation in an academic medical ICU, protocolized support with NIV or HFNC did not prevent reintubation compared with usual care.
Prior trials among hypercapnic patients (14), nonhypercapnic high-risk patients (6, 15, 16, 32, 37), and nonhypercapnic low-risk patients (17) suggested that postextubation respiratory support might prevent reintubation for the majority of ICU patients treated in clinical practice. Our trial evaluated the implementation of protocolized postextubation respiratory support for a broad population of ICU patients and found that protocolized support did not appear to prevent reintubation compared with usual care. Several factors may explain this difference in findings.
Compared with prior trials, which provided a fixed duration of postextubation support of 24–48 hours, postextubation support in the PROPER trial was provided from extubation to at least 5:00 a.m. the following day, resulting in a median of 16 hours of support in the protocolized support group (6, 14, 15, 17, 32, 33). Longer duration of support, however, did not appear to increase the effectiveness of the intervention.
Although protocolized support failed to prevent reintubation, on average, in the PROPER trial, it remains possible that specific patients or groups may benefit from postextubation respiratory support. Reintubation rates in the control groups of prior trials (12% among low-risk patients [17] and 19–24% among high-risk patients [6, 14–16, 33]) were higher than in large observational registries (9), suggesting that even prior trials targeting low-risk patients may have been prognostically enriched for higher risk patients than cared for in routine clinical practice. However, almost 90% of patients in our trial had at least one risk factor for reintubation, and protocolized support did not appear to be more effective among patients at higher risk. The duration of mechanical ventilation before extubation may modify the effect of protocolized support on reintubation. In our trial, patients who had received more than 7 days of mechanical ventilation before extubation appeared to have a numerically lower rate of reintubation with protocolized support. This subgroup comprised up to half of patients in prior trials (6, 15, 16, 33) but represented less than 10% of patients in our trial. Finally, in prior trials, the control group was required to receive conventional oxygen therapy, whereas in our trial, treatment of the control group was determined by treating clinicians, with 16.8% of patients receiving postextubation support in the usual care group. Allowing treating clinicians to identify patients likely to benefit from postextubation respiratory support may be as effective as a protocolized approach to postextubation respiratory support.
Our study has several strengths. The cluster–crossover design enrolled a study population representative of the patients cared for in current clinical practice and achieved balance in baseline characteristics between groups. Cluster-level group assignment and delivery of the protocolized support by clinical respiratory therapists achieved adequate compliance and reflected the delivery of these interventions in clinical practice. Lower respiratory rate in the 6 hours after extubation in the protocolized support group suggests that the protocolized support successfully decreased work of breathing after extubation, demonstrating separation between groups in a physiologic measure of intermediate efficacy. Finally, as the largest trial of postextubation respiratory support to date, important patient subgroups were well represented.
Our study has several limitations. Conducting it at a single center limits generalizability, as usual care in the study ICU may not reflect usual care at other centers. Arterial blood gases were not routinely collected during spontaneous breathing trials, limiting the ability to identify patients with hypercapnia. Most patients with suspected hypercapnia received postextubation NIV in both groups, limiting conclusions about the effect of protocolized support in this important subgroup. Cough strength and secretion volume, proposed risk factors for reintubation, were not collected because they are not objectively measured during routine clinical care. The nature of the study intervention did not allow blinding, which could have affected postextubation management, particularly regarding the timing of extubation and the decision to reintubate. We did not find evidence of this, however, as baseline characteristics such as duration of mechanical ventilation were similar between groups at extubation and indications for reintubation were similar between groups. Finally, the trial intervention, protocolized support, included both NIV and HFNC, but the difference between groups in postextubation respiratory support was driven almost exclusively by a difference in HFNC. The results of the trial could have been different had all patients in the intervention group received NIV, had the duration of postextubation support been fixed or longer, or had higher flow rates been provided with HFNC (16).
Conclusions
Among critically ill adults undergoing extubation from invasive mechanical ventilation in an academic medical ICU, protocolized postextubation respiratory support, primarily characterized by an increase in the use of HFNC, did not prevent reintubation compared with usual care.
Footnotes
Supported in part by NIH grants T32HL087738-12 and K12HL133117 (to J.D.C.), NIH grant UL1 RR024975 (to T.W.R.), NHLBI grants K12HL133117 and K23HL143053 (to M.W.S), and the VICTR Learning Healthcare System Platform under CTSA Award No. UL1 TR002243 from the National Center for Advancing Translational Sciences. The contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the NIH.
Author Contributions: Study concept and design: J.D.C., G.R.B., T.W.R., E.W.E., and M.W.S. Acquisition of data: J.D.C., E.M.V., B.D.L., P.A.B., K.E.J., E.J.H., A.H.T., K.G.B., R.M.B., R.K.R., J.C.R., R.B.B., and M.W.S. Analysis and interpretation of data: J.D.C., L.W., C.J.L., and M.W.S. Drafting of the manuscript: J.D.C. and M.W.S. Critical revision of the manuscript for important intellectual content: J.D.C., E.M.V., B.D.L., P.A.B., K.E.J., E.J.H., A.H.T., K.G.B., R.M.B., R.K.R., J.C.R., L.W., C.J.L., E.W.E., W.H.S., G.R.B., T.W.R., and M.W.S.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202009-3561OC on April 1, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: for the Vanderbilt Learning Healthcare System and the Pragmatic Critical Care Research Group
References
- 1. Acute Respiratory Distress Syndrome Network; Brower RG. Matthay MA. Morris A. Schoenfeld D. Thompson BT. Wheeler A.. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 2. Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 3.Ely EW, Baker AM, Dunagan DP, Burke HL, Smith AC, Kelly PT, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996;335:1864–1869. doi: 10.1056/NEJM199612193352502. [DOI] [PubMed] [Google Scholar]
- 4. Girard TD, Kress JP, Fuchs BD, Thomason JWW, Schweickert WD, Pun BT, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 5. Thille AW, Boissier F, Ben-Ghezala H, Razazi K, Mekontso-Dessap A, Brun-Buisson C, et al. Easily identified at-risk patients for extubation failure may benefit from noninvasive ventilation: a prospective before-after study. Crit Care. 2016;20:48. doi: 10.1186/s13054-016-1228-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nava S, Gregoretti C, Fanfulla F, Squadrone E, Grassi M, Carlucci A, et al. Noninvasive ventilation to prevent respiratory failure after extubation in high-risk patients. Crit Care Med. 2005;33:2465–2470. doi: 10.1097/01.ccm.0000186416.44752.72. [DOI] [PubMed] [Google Scholar]
- 7.Thille AW, Richard J-CM, Brochard L. The decision to extubate in the intensive care unit. Am J Respir Crit Care Med. 2013;187:1294–1302. doi: 10.1164/rccm.201208-1523CI. [DOI] [PubMed] [Google Scholar]
- 8. Thille AW, Harrois A, Schortgen F, Brun-Buisson C, Brochard L. Outcomes of extubation failure in medical intensive care unit patients. Crit Care Med. 2011;39:2612–2618. doi: 10.1097/CCM.0b013e3182282a5a. [DOI] [PubMed] [Google Scholar]
- 9. Miltiades AN, Gershengorn HB, Hua M, Kramer AA, Li G, Wunsch H. Cumulative probability and time to reintubation in U.S. ICUs. Crit Care Med. 2017;45:835–842. doi: 10.1097/CCM.0000000000002327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Torres A, Gatell JM, Aznar E, el-Ebiary M, Puig de la Bellacasa J, González J, et al. Re-intubation increases the risk of nosocomial pneumonia in patients needing mechanical ventilation. Am J Respir Crit Care Med. 1995;152:137–141. doi: 10.1164/ajrccm.152.1.7599812. [DOI] [PubMed] [Google Scholar]
- 11. Frutos-Vivar F, Esteban A, Apezteguia C, González M, Arabi Y, Restrepo MI, et al. Outcome of reintubated patients after scheduled extubation. J Crit Care. 2011;26:502–509. doi: 10.1016/j.jcrc.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 12. Sellares J, Ferrer M, Cano E, Loureiro H, Valencia M, Torres A. Predictors of prolonged weaning and survival during ventilator weaning in a respiratory ICU. Intensive Care Med. 2011;37:775–784. doi: 10.1007/s00134-011-2179-3. [DOI] [PubMed] [Google Scholar]
- 13. Ouellette DR, Patel S, Girard TD, Morris PE, Schmidt GA, Truwit JD, et al. Liberation from mechanical ventilation: an official American College of Chest Physicians/American Thoracic Society Clinical Practice Guideline: inspiratory pressure augmentation during spontaneous breathing trials, protocols minimizing sedation, and noninvasive ventilation immediately after extubation. Chest. 2016;151:166–180. doi: 10.1016/j.chest.2016.10.036. [DOI] [PubMed] [Google Scholar]
- 14. Ferrer M, Sellarés J, Valencia M, Carrillo A, Gonzalez G, Badia JR, et al. Non-invasive ventilation after extubation in hypercapnic patients with chronic respiratory disorders: randomised controlled trial. Lancet. 2009;374:1082–1088. doi: 10.1016/S0140-6736(09)61038-2. [DOI] [PubMed] [Google Scholar]
- 15. Ferrer M, Valencia M, Nicolas JM, Bernadich O, Badia JR, Torres A. Early noninvasive ventilation averts extubation failure in patients at risk: a randomized trial. Am J Respir Crit Care Med. 2006;173:164–170. doi: 10.1164/rccm.200505-718OC. [DOI] [PubMed] [Google Scholar]
- 16. Thille AW, Muller G, Gacouin A, Coudroy R, Decavèle M, Sonneville R, et al. HIGH-WEAN Study Group and the REVA Research Network. Effect of postextubation high-flow nasal oxygen with noninvasive ventilation vs high-flow nasal oxygen alone on reintubation among patients at high risk of extubation failure: a randomized clinical trial. JAMA. 2019;322:1465–1475. doi: 10.1001/jama.2019.14901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hernández G, Vaquero C, González P, Subira C, Frutos-Vivar F, Rialp G, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA. 2016;315:1354–1361. doi: 10.1001/jama.2016.2711. [DOI] [PubMed] [Google Scholar]
- 18. Telias I, Ferguson ND. Added benefit of noninvasive ventilation to high-flow nasal oxygen to prevent reintubation in higher-risk patients. JAMA. 2019;322:1455–1457. doi: 10.1001/jama.2019.14609. [DOI] [PubMed] [Google Scholar]
- 19. Nuzzo EA, Kahn JM, Girard TD. Provider perspectives on preventive postextubation noninvasive ventilation for high-risk intensive care unit patients. Ann Am Thorac Soc. 2020;17:246–249. doi: 10.1513/AnnalsATS.201904-295RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casey JD., Vaughn EM, Lloyd BD, Bilas PA, Jackson KE, Hall EJ, et al. Pragmatic Critical Care Research Group Effect of protocolized post-extubation respiratory support on reintubation: a randomized clinical trial [abstract] Am J Respir Crit Care Med 2020201A5964. [Google Scholar]
- 21. Casey JD, Vaughan ER, Lloyd BD, Bilas PA, Hall EJ, Toporek AH, et al. Pragmatic Critical Care Research Group. Protocolized post-extubation respiratory support to prevent reintubation: protocol and statistical analysis plan for a clinical trial. BMJ Open. 2019;9:e030476. doi: 10.1136/bmjopen-2019-030476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khilnani GC, Galle AD, Hadda V, Sharma SK. Non-invasive ventilation after extubation in patients with chronic obstructive airways disease: a randomised controlled trial. Anaesth Intensive Care. 2011;39:217–223. doi: 10.1177/0310057X1103900210. [DOI] [PubMed] [Google Scholar]
- 23. Mauri T, Galazzi A, Binda F, Masciopinto L, Corcione N, Carlesso E, et al. Impact of flow and temperature on patient comfort during respiratory support by high-flow nasal cannula. Crit Care. 2018;22:120. doi: 10.1186/s13054-018-2039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanji S, MacPhee H, Singh A, Johanson C, Fairbairn J, Lloyd T, et al. Validation of the critical care pain observation tool in critically ill patients with delirium: a prospective cohort study. Crit Care Med. 2016;44:943–947. doi: 10.1097/CCM.0000000000001522. [DOI] [PubMed] [Google Scholar]
- 25.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 26. Ely EW, Truman B, Shintani A, Thomason JWW, Wheeler AP, Gordon S, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 27. Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 28. Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: validity and reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 29. Needham DM. Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA. 2008;300:1685–1690. doi: 10.1001/jama.300.14.1685. [DOI] [PubMed] [Google Scholar]
- 30. Subirà C, Hernández G, Vázquez A, Rodríguez-García R, González-Castro A, García C, et al. Effect of pressure support vs T-piece ventilation strategies during spontaneous breathing trials on successful extubation among patients receiving mechanical ventilation: a randomized clinical trial. JAMA. 2019;321:2175–2182. doi: 10.1001/jama.2019.7234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 32. Maggiore SM, Idone FA, Vaschetto R, Festa R, Cataldo A, Antonicelli F, et al. Nasal high-flow versus Venturi mask oxygen therapy after extubation. Effects on oxygenation, comfort, and clinical outcome. Am J Respir Crit Care Med. 2014;190:282–288. doi: 10.1164/rccm.201402-0364OC. [DOI] [PubMed] [Google Scholar]
- 33. Hernández G, Vaquero C, Colinas L, Cuena R, González P, Canabal A, et al. Effect of postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA. 2016;316: 1565–1574. doi: 10.1001/jama.2016.14194. [DOI] [PubMed] [Google Scholar]
- 34. Turner RM, White IR, Croudace T. PIP Study Group. Analysis of cluster randomized cross-over trial data: a comparison of methods. Stat Med. 2007;26:274–289. doi: 10.1002/sim.2537. [DOI] [PubMed] [Google Scholar]
- 35. Parienti J-J, Kuss O. Cluster-crossover design: a method for limiting clusters level effect in community-intervention studies. Contemp Clin Trials. 2007;28:316–323. doi: 10.1016/j.cct.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 36. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 37. Ornico SR, Lobo SM, Sanches HS, Deberaldini M, Tófoli LT, Vidal AM, et al. Noninvasive ventilation immediately after extubation improves weaning outcome after acute respiratory failure: a randomized controlled trial. Crit Care. 2013;17:R39. doi: 10.1186/cc12549. [DOI] [PMC free article] [PubMed] [Google Scholar]