Abstract
Background:
Health care workers have a critical role in the pandemic response to COVID-19 and may be at increased risk of infection. The objective of this study was to assess the seroprevalence of SARS-CoV-2 immunoglobulin G (IgG) antibodies among health care workers during and after the first wave of the pandemic.
Methods:
We conducted a prospective multicentre cohort study involving health care workers in Ontario, Canada, to detect IgG antibodies against SARS-CoV-2. Blood samples and self-reported questionnaires were obtained at enrolment, at 6 weeks and at 12 weeks. A community hospital, tertiary care pediatric hospital and a combined adult–pediatric academic health centre enrolled participants from Apr. 1 to Nov. 13, 2020. Predictors of seropositivity were evaluated using a multivariable logistic regression, adjusted for clustering by hospital site.
Results:
Among the 1062 health care workers participating, the median age was 40 years, and 834 (78.5%) were female. Overall, 57 (5.4%) were seropositive at any time point (2.5% when participants with prior infection confirmed by polymerase chain reaction testing were excluded). Seroprevalence was higher among those who had a known unprotected exposure to a patient with COVID-19 (p < 0.001) and those who had been contacted by public health because of a nonhospital exposure (p = 0.003). Providing direct care to patients with COVID-19 or working on a unit with a COVID-19 outbreak was not associated with higher seroprevalence. In multivariable logistic regression, presence of symptomatic contacts in the household was the strongest predictor of seropositivity (adjusted odds ratio 7.15, 95% confidence interval 5.42–9.41).
Interpretation:
Health care workers exposed to household risk factors were more likely to be seropositive than those not exposed, highlighting the need to emphasize the importance of public health measures both inside and outside of the hospital.
Health care workers have a critical role in the pandemic response to COVID-19, potentially increasing their risk for infection as a consequence.1–3 It is important to understand risk factors that may predispose health care workers to SARS-CoV-2 infection and guide targeted interventions and improved direct health and safety measures. Understanding risk and effective preventive measures is important to both ensure a healthy essential workforce and protect patients and health care workers from potential nosocomial transmission.
Estimates of SARS-CoV-2 infection using only molecular diagnostic tests can lead to substantial testing bias and may underestimate the prevalence of infection.4 In contrast to molecular tests, which primarily detect acute infection, serologic testing can assist in assessing prior infection and identifying cases that may not have had acute diagnostic testing. As such, the use of serologic assays targeting SARS-CoV-2 antibodies is a useful tool to understand the epidemiology of COVID-19 within a population and the burden of previous mild or asymptomatic infection.5 Serology tests typically have a high sensitivity for previous SARS-CoV-2 infection when testing occurs more than 14 days after the onset of symptoms.6,7
Some studies assessing whether SARS-CoV-2 seropositivity in health care workers is elevated compared with the general population have reported higher seroprevalence.8–10 In addition to risk factors shared with the general population, such as age, ethnicity, household exposure to SARS-CoV-2 and burden of COVID-19 in the residing communities, there are potential risk factors specific to the hospital, including general inpatient care, direct care of patients with COVID-19 and working on a COVID-19 ward.8,11–15 It is therefore critical to place the risk of health care workers acquiring COVID-19 in a local clinical context, which addresses hospital safety practices and also community disease prevalence.
The purpose of this study was to assess the overall seroprevalence of SARS-CoV-2 immunoglobulin G (IgG) antibodies in a population of health care workers within Ontario during and immediately after the first wave of the pandemic, and to explore factors associated with seropositivity. We also sought to explore the durability of antibodies specific to SARS-CoV-2 over time.
Methods
Study design
We conducted a prospective multicentre cohort study involving health care workers in Ontario, Canada, to detect IgG antibodies against SARS-CoV-2. The study was proposed to hospitals across Ontario through an infection prevention and control community of practice with representation from more than 30 hospitals. After review and approval of the protocol, interested sites obtained research ethics and legal approvals, leading to variable start dates. The sites that completed recruitment during and immediately after the first wave (Apr. 1 to Nov. 13, 2020) were included in this analysis.
Setting
Three hospitals from 3 Ontario regions16 participated during the study period: The Hospital for Sick Children (SickKids), a tertiary care pediatric hospital in Toronto, Ontario (Toronto Region); London Health Sciences Centre, an academic centre in London, Ontario, consisting of 2 hospitals including a combined pediatric–adult hospital (South West Region); and Markham Stouffville Hospital, a community hospital in Markham, Ontario (Central East Region). Infection prevention and control guidelines were the same across the hospitals and aligned with provincial guidelines, including use of droplet and contact precautions for routine care of patients with suspected or confirmed COVID-19, with N95 respirators used for aerosol-generating medical procedures.17 Information on the number of patients with COVID-19 receiving treatment during the study period was collected from each hospital.
Participants
Health care workers invited to participate included health care professionals, defined as physicians, nurses and nurse practitioners; allied health workers, defined as phlebotomists, respiratory therapists, social workers, dieticians, diagnostic imaging staff, physiotherapists, occupational therapists and dentistry staff; and auxiliary health care workers (as defined by the World Health Organization as workers who may have had contact with patients, their body fluids or their environments18), including environmental services, patient transport and laboratory personnel, and ward clerical workers.
Recruitment tools included posters, all-staff emails from leadership, computer screen savers and a website (http://cancovid19plasma.ca/healthcare-worker-serology/) that provided general information about the study and contact information for the study coordinators. In addition, we specifically recruited health care workers who worked in emergency departments, COVID-19 wards or units and intensive care units, and those involved with aerosol-generating medical procedures (e.g., anesthesia and respiratory therapy) through directed communication at departmental meetings or emails by clinical directors, as these groups may have had a higher risk of exposure to SARS-CoV-2.
Procedures
Blood samples and self-reported questionnaires were obtained from all enrolled participants at baseline (i.e., enrolment), at 6 weeks and at 12 weeks. Blood samples were separated by centrifugation, and serum was stored frozen at −80°C. Questionnaires asked about potential risk factors for SARS-CoV-2 exposure and mitigation strategies, including travel history, care of patients with COVID-19, known exposure (occupational or otherwise) to a confirmed case of COVID-19, perceived adherence to physical distancing measures and the type of personal protective equipment (PPE) used during patient encounters (all patients and patients with suspected or confirmed COVID-19) (Appendix 1, available at www.cmajopen.ca/content/9/4/E929/suppl/DC1). In addition, all participants were emailed weekly to request that they report any new symptoms.
Our proposed sample size of at least 1000 health care workers would allow us to determine seropositivity at baseline with an 80% probability that the confidence interval (CI) has a precision of ± 1.5%, assuming a seroprevalence of 5% (80% power, α of 0.05).
Outcome
The EUROIMMUN Anti-SARS-CoV-2 IgG enzyme-linked immunoassay (ELISA)19 was used for testing in accordance with the manufacturer’s directions on the EUROIMMUN Analyzer I. This Health Canada–approved semiquantitative assay detects a recombinant S1 protein of SARS-CoV-2. Interpretation was based on the index values (signal to cut-off ratios) of less than 0.8 reported as negative, 0.8 or greater to less than 1.1 as borderline, and 1.1 or greater as positive.19 This assay has a reported sensitivity of greater than 90% and specificity of greater than 98% in patients 15 days or more post–symptom onset.20 All testing was performed at the Microbiology Laboratory at SickKids.
Statistical analysis
We reported continuous variables using the mean and standard deviation or median and interquartile range as appropriate. We reported numbers and percentages for dichotomous outcomes. Proportion of samples seropositive at each time point (baseline, 6 weeks and 12 weeks) was calculated overall and stratified by whether participants had a known SARS-CoV-2 infection before enrolment. The proportions with seropositive results at each time point were compared between sites using χ2 tests. Spaghetti plots were used to display antibody responses over time.
Detailed information on several potential predictors will be studied in a larger longitudinal study that is ongoing. Given the small number of seropositive participants, we focused this analysis on potential hospital risk factors and household exposure, and included only 5 predictors in the multivariable model using the 10 events per variable rule of thumb. We targeted the univariable analyses to hospital risk factors (working on a unit with a COVID-19 outbreak, providing care for patients with COVID-19, having had an unprotected COVID-19 exposure) and nonhospital risk factors (symptomatic household contacts as defined by participant, contacted by public health about exposure) and evaluated the relation with seropositivity using the χ2 or Fisher exact test. All analyses were based on the baseline questionnaire responses. The multivariable logistic regression model included predictors identified a priori including age, sex, race or ancestry, a non-hospital risk factor (symptomatic contacts in the household) and a hospital risk factor (care of patients with COVID-19). We used generalized estimating equations with an exchangeable correlation structure to adjust for clustering at the site. A sensitivity analysis was conducted removing patients with known infection at baseline.
All estimates are presented with 95% CIs. A p value less than 0.05 was considered significant. All analyses were conducted using R (R Core Team, 2020).
Ethics approval
Research ethics approval was obtained by the Clinical Trials Ontario Research Ethics Board (Project ID 3182), with local site approvals as required. All participants provided informed consent.
Results
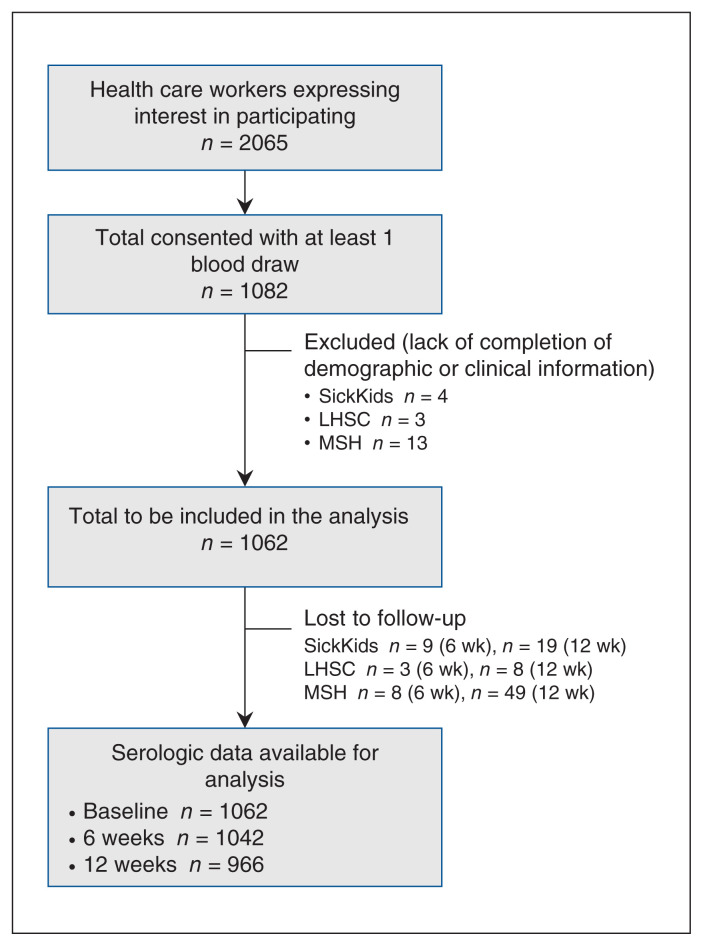
This analysis includes data from the first 3 hospitals recruited to participate in our study. A total of 2065 health care workers contacted the study team to learn more about the study, and 1082 consented to participate. Of those who consented, 1062 health care workers had baseline information available and bloodwork completed, and were included in the study from SickKids (n = 376), London Health Sciences Centre (n = 349) and Markham Stouffville Hospital (n = 337). This resulted in a total of 1062 baseline tests, 1042 six-week samples and 966 twelve-week samples (Figure 1). Over the study period, each hospital saw more than 100 patients with COVID-19. The range of timing of recruitment and sample collection at each site is shown in Figure 2.
Figure 1:
Participant inclusion flow diagram. Note: LHSC = London Health Sciences Centre, MSH = Markham Stouffville Hospital, Sick-Kids = The Hospital for Sick Children.
Figure 2:
Percentage of participants with positive serology for SARS-CoV-2 by month and by site. Horizontal lines represent the mean percent positivity at enrolment, 6-week or 12-week collection period. Note: LHSC = London Health Sciences Centre, MSH = Markham Stouffville Hospital, SickKids = The Hospital for Sick Children.
The median age of health care workers was 40 (interquartile range 32–51) years, and 834 (78.5%) were female (Table 1). Participants were predominantly nurses from inpatient units, critical care and the emergency department. Most participants racially self-identified as White, followed by Asian, with less than 2% self-identifying for each of Black, Hispanic, and Inuit, First Nations and Métis.
Table 1:
Baseline participant characteristics and potential risk factors for SARS-CoV-2 infection
| Characteristic | No. (%) of participants* | |||
|---|---|---|---|---|
| Total n = 1062 |
SickKids n = 376 |
London Health Sciences n = 349 |
Markham Stouffville n = 337 |
|
| Age, yr, median (IQR) | 40 (32–51) | 38 (31–49) | 39 (31–52) | 42 (33–51) |
| Sex, female | 834 (78.5) | 272 (72.3) | 283 (81.1) | 279 (82.8) |
| Role | ||||
| Physician | 237 (22.3) | 121 (32.2) | 66 (18.9) | 50 (14.8) |
| Nurse practitioner | 15 (1.4) | 5 (1.3) | 3 (0.9) | 7 (2.1) |
| Nurse | 446 (42.0) | 135 (35.9) | 195 (55.9) | 116 (34.4) |
| Allied health worker | 159 (15.0) | 34 (9.0) | 47 (13.5) | 78 (23.1) |
| Respiratory therapy | 52 (4.9) | 15 (4.0) | 20 (5.7) | 17 (5.0) |
| Auxiliary health worker | 76 (7.2) | 41 (10.9) | 14 (4.0) | 21 (6.2) |
| Other† | 115 (10.8) | 39 (10.4) | 16 (4.6) | 60 (17.8) |
| Workplace | ||||
| Emergency department | 306 (28.8) | 102 (27.1) | 129 (37.0) | 75 (22.3) |
| Critical care | 245 (23.1) | 70 (18.6) | 125 (35.8) | 50 (14.8) |
| Hospital ward | 373 (35.1) | 121 (32.2) | 128 (36.7) | 124 (36.8) |
| Perioperative services or surgical ward | 157 (14.8) | 60 (16.0) | 49 (14.0) | 48 (14.2) |
| COVID-19 assessment centre | 37 (3.5) | 8 (2.1) | 5 (1.4) | 24 (7.1) |
| Other‡ | 257 (24.2) | 99 (26.3) | 51 (14.6) | 107 (31.8) |
| No. of individuals in household, median (IQR) | 3 (2–4) | 3 (2–4) | 3 (2–4) | 4 (2–4) |
| No. with ≥ 3 individuals in household (including participant) | 602/1043 (57.7) | 178/359 (49.6) | 182/349 (52.1) | 242/335 (72.2) |
| No. with children (< 18 yr) in the household | 401 (37.8) | 122 (32.4) | 121 (34.7) | 158 (46.9) |
| Underlying medical conditions | 386 (36.3) | 124 (33.0) | 135 (38.7) | 127 (37.7) |
| Race or ancestry | ||||
| Inuit, First Nations, Métis | 3 (0.3) | 0 | 1 (0.3) | 2 (0.6) |
| White | 734 (69.1) | 243 64.6 | 296 (84.8) | 195 (57.9) |
| Black | 16 (1.5) | 9 (2.4) | 3 (0.9) | 4 (1.2) |
| Hispanic | 14 (1.3) | 10 (2.7) | 3 (0.9) | 1 (0.3) |
| Asian | 172 (16.2) | 52 (13.8) | 25 (7.2) | 95 (28.2) |
| Middle Eastern | 31 (2.9) | 8 (2.1) | 12 (3.4) | 11 (3.3) |
| Other | 55 (5.2) | 21 (5.6) | 7 (2.0) | 27 (8.0) |
| Unknown/unspecified | 40 (3.8) | 33 (8.8) | 3 (0.9) | 4 (1.2) |
| Travel since Jan. 1, 2020 | 402 (37.9) | 159 (42.3) | 138 (39.5) | 105 (31.2) |
| Worked on a unit with a COVID-19 outbreak | 120 (11.3) | 3 (0.8) | 93 (26.6) | 24 (7.1) |
| Provided direct care to patient with COVID-19 | 439 (41.3) | 29 (7.7) | 230 (65.9) | 180 (53.4) |
| Known unprotected occupational exposure with direct patient care | 41/439 (9.3) | 4/29 (13.8) | 24/230 (10.4) | 13/180 (7.2) |
| Known SARS-CoV-2 positive by PCR before enrolment | 40 (3.8) | 17 (4.5) | 7 (2.0) | 16 (4.7) |
| Positivity proportion (to SARS-CoV-2 immunoglobulin G antibodies) | ||||
| Overall (at any time point) | 57/1062 (5.4) | 24/376 (6.4) | 12/349 (3.4) | 21/337 (6.2) |
| Baseline (at enrolment) | 48/1062 (4.5) | 19/376 (5.1) | 10/349 (2.9) | 19/337 (5.6) |
| 6 weeks | 53/1042 (5.1) | 22/367 (6.0) | 10/346 (2.9) | 19/329 (5.8) |
| 12 weeks | 48/966 (5.0) | 18/348 (5.2) | 11/338 (3.3) | 19/280 (6.8) |
Note: IQR = interquartile range, PCR = polymerase chain reaction, SickKids = The Hospital for Sick Children.
Unless stated otherwise.
Included roles such as midwife, child life specialist, research coordinator, paramedic or transport personnel, speech and language therapists, and counselors.
Included workplaces such as diagnostic imaging, intravenous or phlebotomy, labour and delivery or midwifery, infection prevention and control, and research.
Overall seropositivity
Overall, 57/1062 (5.4%) of health care workers were seropositive at any time point, of which 31 (54.4%) had a history of confirmed SARS-CoV-2 infection by polymerase chain reaction (PCR) testing before enrolment. An additional 9 participants had previous confirmed SARS-CoV-2 infection but were seronegative. Of the 1022 health care workers with no confirmed SARS-CoV-2 infection before enrolment (i.e., excluding those with known recruitment bias), 26 (2.5%) were seropositive at any time point over the study (Table 2). Seroprevalence varied minimally by time point (Figure 2), and there was no significant difference in seroprevalence by site (p = 0.1).
Table 2:
Seroprevalence at collection time points overall and by SARS-CoV-2 infection confirmed by polymerase chain reaction testing
| Serology status | Prior PCR status, no. | Total no. (%) of samples positive and negative at each time point | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | |||
| Baseline | 30/40 | 18/1022 | 48/1062 (4.5) |
| 6 weeks | 30/39 | 21/1003 | 51/1042 (4.9) |
| 12 weeks | 29/38 | 19/928 | 48/966 (6.0) |
| At any point | 31/40 | 26/1022 | 57/1062 (5.4) |
| Negative | |||
| Baseline | 10/40 | 1004/1022 | 1014/1062 (95.5) |
| 6 weeks | 9/39 | 982/1003 | 991/1042 (95.1) |
| 12 weeks | 9/38 | 909/928 | 918/966 (95.0) |
| At any point | 9/40 | 996/1022 | 1005/1062 (94.6) |
| Total | 40 | 1022 | 1062 |
Note: PCR = polymerase chain reaction.
Antibody responses
Of the 57 health care workers with positive serology at any time over the course of the study, 48 (84.2%) were positive at baseline testing, and 9 (15.8%) seroconverted during the study. Of the 9 who seroconverted, 1 had a confirmed SARS-CoV-2 infection and had baseline testing before 15 days. Of the remaining 8 without previous confirmed infection, 3 were only transiently positive at the 6-week collection, 1 had more than 1 positive result but at a relatively low antibody index value, and the remaining 4 were positive only on the 12-week sample with a low antibody index value; none of these participants had confirmed infection over the course of the study.
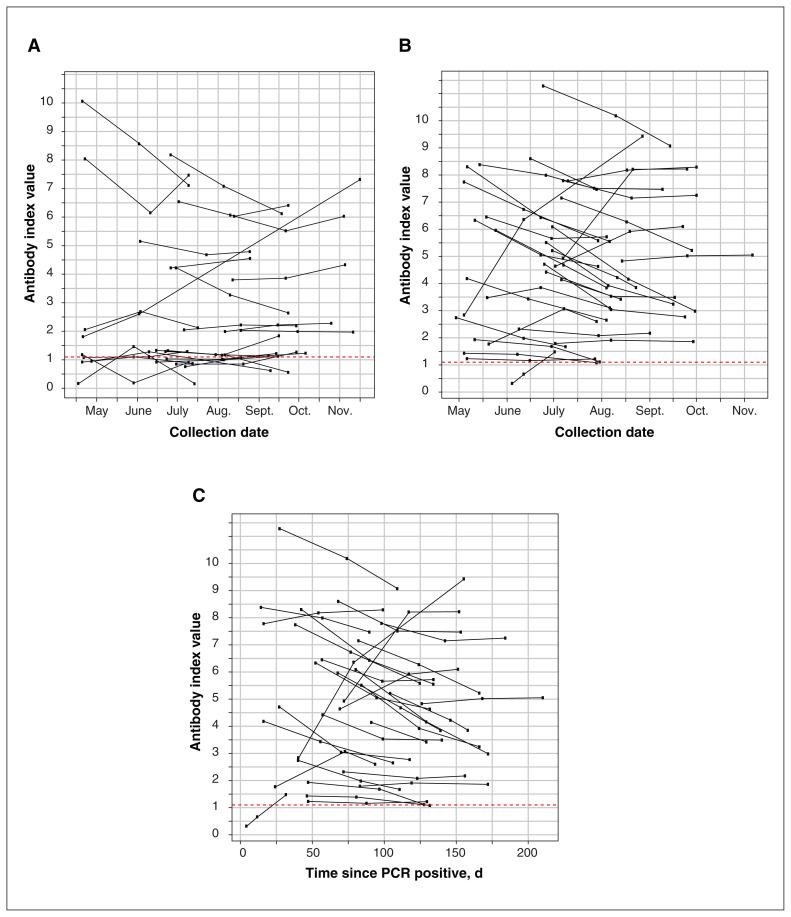
Figure 3A shows the antibody responses in the 26 participants who were antibody positive but had no history of confirmed SARS-CoV-2 infection by PCR testing. Antibody responses of the 31 participants with positive serology and history of previous PCR-confirmed infection are shown in Figure 3B (by month) and Figure 3C (days since positive PCR test).
Figure 3:
(A) Antibody responses of the 26 participants who had no history of confirmed SARS-CoV-2 infection and tested positive for SARS-CoV-2 antibodies at any time point during the study. Points above the dashed red line represent a positive antibody result. (B) Antibody responses of the 31 participants who had confirmed SARS-CoV-2 infection by polymerase chain reaction (PCR) testing and tested positive for SARS-CoV-2 antibodies at any time point during the study by collection time. Points above the dashed red line represent a positive antibody result. (C) Antibody responses of the 31 participants who had confirmed SARS-CoV-2 infection by molecular testing and tested positive for SARS-CoV-2 antibodies at any time point during the study expressed as a time from their positive PCR result. Points above the dashed red line represent a positive antibody result.
Predictors of seropositivity
A comparison of clinical and other possible exposures by detectable antibody status is summarized in Table 3 (additional factors are described in Appendix 2 available at www.cmajopen.ca/content/9/4/E929/suppl/DC1, Supplementary Table 1). Seroprevalence was higher among those who had a known unprotected exposure to a patient with COVID-19 (29.6% v. 8.0%, p < 0.001), those who had been contacted by public health because of a nonhospital exposure (15.8% v. 5.5%, p = 0.003) and those with confirmed infection before enrolment (54.4% v. 0.9%, p < 0.001). Working on a unit with a COVID-19 outbreak was not associated with higher seroprevalence (8.8% v. 11.5%, p = 0.7).
Table 3:
Factors associated with having detectable SARS-CoV-2 antibodies (univariable comparisons)
| Variable | No. (%) of participants | p value | |
|---|---|---|---|
| SARS-CoV-2 serology positive n = 57* |
SARS-CoV-2 serology negative n = 1005* |
||
| Symptomatic contacts in the household | 7/53 (13.2) | 25/971 (2.6) | < 0.001 |
| Provided direct care to patients with COVID-19 | 27 (47.4) | 412/995 (41.4) | 0.5 |
| Unprotected occupational exposure to a patient with COVID-19† | 8/27 (29.6) | 33/411 (8.0) | < 0.001 |
| Worked on a unit with a COVID-19 outbreak | 5 (8.8) | 115/996 (11.5) | 0.7 |
| Contacted by public health to indicate exposure | 9 (15.8) | 54/985 (5.5) | 0.003 |
| Known positive PCR test at baseline | 31 (54.4) | 9 (0.9) | < 0.001 |
Note: PCR = polymerase chain reaction.
The denominator of each outcome variable is the total in the column heading unless stated otherwise.
Only those health care workers who indicated they had direct patient contact were asked this question.
In the multivariable model (Table 4), presence of symptomatic contacts in the household was the strongest predictor of seropositivity (adjusted odds ratio [OR] 7.15, 95% CI 5.42–9.41). When health care workers with known infection at baseline were removed, several other predictors were identified. Presence of symptomatic contacts in the household remained a strong predictor (adjusted OR 7.22, 95% CI 3.65–14.3). Younger age by year (adjusted OR 0.94, 95% CI 0.91–0.98) and non-White race (adjusted OR 2.85, 95% CI 1.36–5.98) were also found to be significant. Providing direct care to patients with COVID-19 was found to be associated with a lower odds of infection (adjusted OR 0.50, 95% CI 0.36–0.70).
Table 4:
Multivariable model for predictors of having SARS-CoV-2 antibodies
| Variable | Adjusted odds ratio (95% CI)* | |
|---|---|---|
| All participants n = 1008 |
Participants excluding those with previously confirmed SARS-CoV-2 infection n = 971 |
|
| Age by year | 0.98 (0.96–0.99) | 0.94 (0.91–0.98) |
| Female sex | 1.86 (0.72–4.78) | 1.60 (0.48–5.35) |
| Non-White race† | 1.26 (0.46–3.52) | 2.85 (1.36–5.98) |
| Symptomatic household exposure | 7.15 (5.42–9.41) | 7.22 (3.65–14.3) |
| Direct care of patients with COVID-19 | 1.33 (0.72–2.47) | 0.50 (0.36–0.70) |
Note: CI = confidence interval.
Adjusted for all variables in table and for clustering by hospital site.
Participants indicating unknown ancestry were excluded.
Symptom history
A total of 47.9% (n = 23) of health care workers with positive serology at baseline reported a history of symptomatic illness (52.1% asymptomatic). The most reported symptoms included cough (n = 17, 35.4%) and fatigue (n = 17, 35.4%) (Appendix 2, Supplementary Table 2). Those with symptoms documented at least 2 symptoms (n = 22), with 1 health care worker reporting isolated anosmia.
Interpretation
Among the health care workers sampled across several Ontario hospital sites, including a community hospital, tertiary care pediatric hospital and a combined adult–pediatric academic health centre, seroprevalence of SARS-CoV-2 antibodies was 5.4%. The prevalence was even lower, at 2.5%, when we took into account recruitment bias of prior infection before enrolment. Among health care workers, the main risk factors identified for seroprevalence were outside of the hospital (household or community exposure), unless the worker had a known unprotected health care exposure.
Our finding of 2%–5% prevalence of seropositivity depending on prior infection is consistent with most findings of other seroprevalence studies involving health care workers, in which seroprevalence ranged from 0% to 44%, depending on the jurisdiction.8,9,11,12,21–33 Since the start of the pandemic, given the experience with severe acute respiratory syndrome (SARS)34–36 and studies of SARS-CoV-2 showing environmental contamination37 and occasionally (though not consistently) presence in air samples, there was a concern of higher prevalence of infection among health care workers.38,39 Not surprisingly, we found higher seroprevalence among health care workers from jurisdictions with higher community rates. Overall, seroprevalence in the 2 hospitals from the Greater Toronto Area (where community rates and seroprevalence were higher16,40) was 6.4% (2.5% excluding known positives) and 6.2% (3.1% excluding known positives), whereas in southwestern Ontario (a community where incidence and seroprevalence were lower) it was 3.4% (2.0% excluding known positives).
In addition to variation in COVID-19 burden by region,9,12,28,31 studies showing higher seroprevalence among health care workers attributed these estimates to availability of PPE27,32 and delayed implementation of public health measures in the hospital (i.e., universal masking).28,29 Shortages of PPE and episodes of lacking facial coverings while caring for patients with COVID-19 (defined as lack of surgical mask, N95 respirator or powered air purifying respirator [PAPR]), were associated with seropositivity in a multicentre US-based serosurvey.24 This is in line with our finding of a higher odds of infection among health care workers who had unprotected exposures with patients with COVID-19. Across our hospitals, as across Canada, medical masks are used as part of droplet and contact precautions for routine care of patients, with N95 respirator or PAPRs recommended for use only in aerosol-generating medical procedures.17 This approach differs from that of the United States, where an N95 respirator or PAPR is recommended for all encounters with patients with COVID-19, while acknowledging that medical masks are an acceptable alternative.41 Although further studies are needed, our results suggest a lack of substantially different seroprevalence in our health care workers compared with either rates in the local communities or other health care workers involved in seroprevalence studies in other countries. This is reassuring that our current infection prevention and control practices appear to be effective.
We found that exposure to a symptomatic household member was the strongest predictor of positive serology, and providing direct care to patients with COVID-19 or working on a unit with a COVID-19 outbreak was not a significant predictor. Evidence supporting household exposure as potentially contributing more to infection risk than the health care environment has been previously described. Wilkins and colleagues found that exposure outside of the hospital was strongly associated with seropositivity in a large seroprevalence study involving health care workers in Chicago,13 and Steensels and colleagues found that having a household contact with suspected COVID-19 was strongly associated with seropositivity.11 Additionally, younger age and non-White race were significant predictors of seropositivity, a finding described in other studies42,43 and consistent with community risk factors.44
Only about half of the health care workers with antibodies in our study reported signs or symptoms of COVID-19. Similar prevalence findings among asymptomatic or paucisymptomatic health care workers with positive serology were documented in other studies.9,23–28,45 This highlights the need for a low threshold for testing among health care workers as well as ensuring that health and safety measures are followed consistently in hospitals and the community.
The longitudinal collection of samples allowed for the evaluation of the durability of the antibody response. Present evidence suggests that measurable antibody responses may decrease over time.46–48 This decline has been observed in assays using the SARS-CoV-2 S protein, including the one used for this study.49 It was surprising that a decline in antibody levels that resulted in a change of serostatus from positive to negative was rare, occurring in only 3 health care workers who had positive baseline serology, in contrast to the significant decline of more than 50% seen over a 60-day period among health care workers in a study by Patel and colleagues.46
Limitations
Limitations of this study include the convenience sampling of health care workers and modest sample size. Owing to logistical difficulties in bringing on study sites midpandemic, only 3 sites were included in this analysis, which focuses on the first wave. In addition, given the passive and broad nature of recruitment, it is difficult to know the exact number of health care workers notified about the study at each site to obtain an accurate recruitment rate. Ongoing recruitment at additional hospital sites has also focused on increasing the number of high-risk workers.
Our study had low power to detect differences between seropositive and seronegative groups. Furthermore, as commonly seen in studies assessing seroprevalence, there may have been a recruitment bias toward health care workers who suspected previous infection and were interested in their antibody response (e.g., history of undiagnosed respiratory symptoms or previous confirmed infection). In terms of the risk-factor assessment, questionnaires were self-completed. However, antibody testing was batched, and questionnaires were completed before results were available, so the results should not have biased the responses.
The serologic response to SARS-CoV-2 can cross-react with antibodies after infections with SARS, Middle East respiratory syndrome coronavirus (MERS-CoV) and other seasonal coronaviruses in circulation.50 Two individuals with self-reported previous infection with SARS or MERS-CoV were tested, with 1 being seropositive. Although we did not perform orthogonal testing with an alternative target antigen, we followed patient status over time as a mitigation strategy, with 87% of participants with positive testing remaining positive on more than 1 blood collection. False-negative results may have occurred because the assay may have failed to detect a measurable antibody response from a limitation in its sensitivity. 51–53 False-negative results may also occur if a participant did not mount a robust antibody response or if the antibody response waned before recruitment.54,55 Additionally, the assay used was not quantitative, and instead signal to cut-off ratios were used as a surrogate for antibody titres.
Conclusion
We found that health care workers with community risk factors such as household or community exposure were more likely to be seropositive with SARS-CoV-2 antibodies, and direct care of patients with COVID-19 was not associated with increased seropositivity. Our results highlight the importance of public health measures both inside and outside of the hospital.
Supplementary Material
Acknowledgements
The authors extend their appreciation to Supriya Parikh for her support with project and data management, and James Wright for his support with the manuscript figures.
Footnotes
Competing interests: Shelly Bolotin has received grants for COVID-19 work, unrelated to this manuscript, from the Canadian Institutes of Health Research (CIHR), the Public Health Agency of Canada (PHAC), the COVID-19 Immunity Task Force (CITF) and the Canadian Immunity Research Network; has participated on the executive management committee for the MOSAIC COVID-19 vaccine clinical trial (CITF funded); and is a member of the PHAC Vaccine Surveillance Reference Group and the Canadian Immunization Research Network Management Committee. Michael Silverman reports an unrestricted grant paid to Western University from the Academic Medical Organization of Southwestern Ontario. Allison McGeer reports research grants to her institution from Sanofi Pasteur, Pfizer, Merck, CIHR, CITF, University of Toronto and Appili Therapeutics; funding from AstraZeneca for the preparation and delivery of webinars about vaccination; and participation on a data safety monitoring board or advisory board regarding COVID-19 therapy and vaccines for AstraZeneca, Janssen, Moderna, Pfizer, GlaxoSmithKline, Sanofi Pasteur, Merck, Medicago and Novavax. Upton Allen reports an honorarium provided to SickKids from the St. Jude Pediatric Infectious Diseases course. Jerome Leis reports a grant payment from the University of Toronto COVID-19 Action Initiative to his organization, Sunnybrook Health Sciences Centre, as a participant site; and payment for expert testimony regarding COVID-19 infection prevention and control management for the Ontario Hospital Association and the Ontario Ministry of the Attorney General. Jeffrey Pernica reports grant funding from bioMérieux to his institution for a clinical trial and from MedImmune to his institution for a prospective cohort study; and payment for an educational event from the Canadian Paediatric Review. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Shelly Bolotin, Aaron Campigotto, Rulan Parekh, Michelle Science and Michael Silverman conceived and designed the work; contributed to the acquisition, analysis and interpretation of data; and drafted the manuscript. Jeya Nadarajah contributed to the acquisition, analysis and interpretation of data. Bryan Maguire performed the statistical analysis. Allison McGeer, Kevin Schwartz, Laura Alexander, Upton Allen, Archchun Ariyarajah, Lucas Castellani, Ronald Cohn, Mark Downing, Kevin Katz, Kescha Kazmi, Jerome Leis, Derek Liu, Jeffrey Pernica, Jane Schneiderman and Maya Sumaida contributed to the interpretation of the data for the work. All of the authors revised the manuscript critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: The Hospital for Sick Children Foundation, University of Toronto COVID-19 Action Initiative, Ontario COVID-19 Rapid Research Fund and Academic Medical Organization of Southwestern Ontario provided funding.
Data sharing: The data set from this study are held securely in coded form at The Hospital for Sick Children. As subsequent analyses will be conducted after the completion of the larger study, an anonymized subset of data may be made available beginning 3 months and ending 5 years after publication to researchers who provide a methodologically sound proposal. Proposals should be directed to Michelle Science (michelle.science@sickkids.ca) or Aaron Campigotto (aaron.campigotto@sickkids.ca). Research ethics board approval and a data access agreement will need to be signed to gain access to the data.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/9/4/E929/suppl/DC1.
References
- 1.Kampf G, Bruggemann Y, Kaba HEJ, et al. Potential sources, modes of transmission and effectiveness of prevention measures against SARS-CoV-2. J Hosp Infect. 2020;106:678–97. doi: 10.1016/j.jhin.2020.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reynolds MG, Anh BH, Thu VH, et al. Factors associated with nosocomial SARS-CoV transmission among healthcare workers in Hanoi, Vietnam, 2003. BMC Public Health. 2006;6:207. doi: 10.1186/1471-2458-6-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz J, King CC, Yen MY. Protecting healthcare workers during the coronavirus disease 2019 (COVID-19) outbreak: lessons from Taiwan’s severe acute respiratory syndrome response. Clin Infect Dis. 2020;71:858–60. doi: 10.1093/cid/ciaa255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Havers FP, Reed C, Lim T, et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23–May 12, 2020. JAMA Intern Med. 2020 Jul 21; doi: 10.1001/jamainternmed.2020.4130. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Loeffelholz MJ, Tang YW. Laboratory diagnosis of emerging human coronavirus infections — the state of the art. Emerg Microbes Infect. 2020;9:747–56. doi: 10.1080/22221751.2020.1745095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiang F, Wang X, He X, et al. Antibody detection and dynamic characteristics in patients with coronavirus disease 2019. Clin Infect Dis. 2020;71:1930–4. doi: 10.1093/cid/ciaa461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis. 2020;71:2027–34. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iversen K, Bundgaard H, Hasselbalch RB, et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis. 2020;20:1401–8. doi: 10.1016/S1473-3099(20)30589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houlihan CF, Vora N, Byrne T, et al. Pandemic peak SARS-CoV-2 infection and seroconversion rates in London frontline health-care workers. Lancet. 2020;396:e6–7. doi: 10.1016/S0140-6736(20)31484-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai X, Wang M, Qin C, et al. Coronavirus disease 2019 (COVID-2019) infection among health care workers and implications for prevention measures in a tertiary hospital in Wuhan, China. JAMA Netw Open. 2020;3:e209666. doi: 10.1001/jamanetworkopen.2020.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steensels D, Oris E, Coninx L, et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324:195–7. doi: 10.1001/jama.2020.11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moscola J, Sembajwe G, Jarrett M, et al. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City area. JAMA. 2020;324:893–5. doi: 10.1001/jama.2020.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkins JT, Gray EL, Wallia A, et al. Seroprevalence and correlates of SARS-CoV-2 antibodies in health care workers in Chicago. Open Forum Infect Dis. 2020;8:ofaa582. doi: 10.1093/ofid/ofaa582.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen LH, Drew DA, Graham MS, et al. Risk of COVID-19 among frontline health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5:e475–e483. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lidstrom AK, Sund F, Albinsson B, et al. Work at inpatient care units is associated with an increased risk of SARS-CoV-2 infection; a cross-sectional study of 8679 healthcare workers in Sweden. Ups J Med Sci. 2020;125:305–10. doi: 10.1080/03009734.2020.1793039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epidemiologic summary: COVID-19 in Ontario — January 15, 2020 to December 20, 2020. Toronto: Ontario Agency for Health Protection and Promotion (Public Health Ontario); 2020. [accessed 2021 Apr. 30]. Available: https://www.publichealthontario.ca/-/media/documents/ncov/epi/2020/covid-19-daily-epi-summary-report.pdf?la=en. [Google Scholar]
- 17.IPAC recommendations for use of personal protective equipment for care of individuals with suspect or confirmed COVID-19. Toronto: Ontario Agency for Health Protection and Promotion (Public Health Ontario); 2020. [accessed 2020 Dec.19]. Available: https://www.publichealthontario.ca/-/media/documents/ncov/updated-ipac-measures-covid-19.pdf?la=en. [Google Scholar]
- 18.Protocol for assessment of potential risk factors for 2019-novel coronavirus (COVID-19) infection among health care workers in a health care setting. Geneva: World Health Organization; 2020. [accessed 2020 Dec. 19]. Available https://who.int/publications-detail/protocol-for-assessment-of-potential-risk-factors-for-2019-novel-coronavirus-(2019-ncov)-infection-among-health-care-workers-in-a-health-care-setting. [Google Scholar]
- 19.SARS-CoV-2 ELISA test systems from EUROIMMUN. Luebeck (Germany): EUROIMMUN Medizinische Labordiagnostika AG; [accessed 2020 Dec. 19]. Available: https://www.coronavirus-diagnostics.com/antibody-detection-tests-for-covid-19.html. [Google Scholar]
- 20.Theel ES, Harring J, Hilgart H, et al. Performance characteristics of four high-throughput immunoassays for detection of IgG antibodies against SARS-CoV-2. J Clin Microbiol. 2020;58:e01243–20. doi: 10.1128/JCM.01243-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeremias A, Nguyen J, Levine J, et al. Prevalence of SARS-CoV-2 infection among health care workers in a tertiary community hospital. JAMA Intern Med. 2020;180:1707–9. doi: 10.1001/jamainternmed.2020.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrett ES, Horton DB, Roy J, et al. Prevalence of SARS-CoV-2 infection in previously undiagnosed health care workers in New Jersey, at the onset of the U.S. COVID-19 pandemic. BMC Infect Dis. 2020;20:853. doi: 10.1186/s12879-020-05587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stubblefield WB, Talbot HK, Feldstein L, et al. Seroprevalence of SARS-CoV-2 among frontline healthcare personnel during the first month of caring for COVID-19 patients — Nashville, Tennessee. Clin Infect Dis. 2020;72:1645–48. doi: 10.1093/cid/ciaa936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Self WH, Tenforde MW, Stubblefield WB, et al. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network — 13 academic medical centers, April–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1221–6. doi: 10.15585/mmwr.mm6935e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korth J, Wilde B, Dolff S, et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128:104437. doi: 10.1016/j.jcv.2020.104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Godbout EJ, Pryor R, Harmon M, et al. COVID-19 seroprevalence among healthcare workers in a low prevalence region. Infect Control Hosp Epidemiol. 2020 Dec 14; doi: 10.1017/ice.2020.1374.. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansour M, Leven E, Muellers K, et al. Prevalence of SARS-CoV-2 antibodies among healthcare workers at a tertiary academic hospital in New York City. J Gen Intern Med. 2020;35:2485–6. doi: 10.1007/s11606-020-05926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grant JJ, Wilmore SMS, McCann NS, et al. Seroprevalence of SARS-CoV-2 antibodies in healthcare workers at a London NHS Trust. Infect Control Hosp Epidemiol. 2021;42:212–4. doi: 10.1017/ice.2020.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudberg AS, Havervall S, Manberg A, et al. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun. 2020;11:5064. doi: 10.1038/s41467-020-18848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eyre DW, Lumley SF, O’Donnell D, et al. Differential occupational risks to healthcare workers from SARS-CoV-2 observed during a prospective observational study. eLife. 2020;9:e60675. doi: 10.7554/eLife.60675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galan MI, Velasco M, Casas ML, et al. Hospital-wide SARS-CoV-2 seroprevalence in health care workers in a Spanish teaching hospital. Enferm Infecc Microbiol Clin (Engl Ed) 2020 Dec 18; doi: 10.1016/j.eimc.2020.11.015.. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Tong X, Wang J, et al. High SARS-CoV-2 antibody prevalence among healthcare workers exposed to COVID-19 patients. J Infect. 2020;81:420–6. doi: 10.1016/j.jinf.2020.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Insua C, Stedile G, Figueroa V, et al. Seroprevalence of SARS-CoV-2 antibodies among physicians from a children’s hospital. Arch Argent Pediatr. 2020;118:381–5. doi: 10.5546/aap.2020.eng.381. [DOI] [PubMed] [Google Scholar]
- 34.Varia M, Wilson S, Sarwal S, et al. Investigation of a nosocomial outbreak of severe acute respiratory syndrome (SARS) in Toronto, Canada. CMAJ. 2003;169:285–92. [PMC free article] [PubMed] [Google Scholar]
- 35.Masur H, Emanuel E, Lane HC. Severe acute respiratory syndrome: providing care in the face of uncertainty. JAMA. 2003;289:2861–3. doi: 10.1001/jama.289.21.JED30036. [DOI] [PubMed] [Google Scholar]
- 36.Fowler RA, Guest CB, Lapinsky SE, et al. Transmission of severe acute respiratory syndrome during intubation and mechanical ventilation. Am J Respir Crit Care Med. 2004;169:1198–202. doi: 10.1164/rccm.200305-715OC. [DOI] [PubMed] [Google Scholar]
- 37.Ye G, Lin H, Chen S, et al. Environmental contamination of SARS-CoV-2 in healthcare premises. J Infect. 2020;81:e1–5. doi: 10.1016/j.jinf.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo ZD, Wang ZY, Zhang SF, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. 2020;26:1583–91. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li YH, Fan YZ, Jiang L, et al. Aerosol and environmental surface monitoring for SARS-CoV-2 RNA in a designated hospital for severe COVID-19 patients. Epidemiol Infect. 2020;148:e154. doi: 10.1017/S0950268820001570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.COVID-19 seroprevalence in Ontario: March 27, 2020 to June 30, 2020. Toronto: Ontario Agency for Health Protection and Promotion (Public Health Ontario); 2020. [accessed 2021 Apr. 30]. Available: https://www.publichealthontario.ca/-/media/documents/ncov/epi/2020/07/covid-19-epi-seroprevalence-in-ontario.pdf?la=en. [Google Scholar]
- 41.Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic. Atlanta: Centers for Disease Control and Prevention; [accessed 2020 Dec. 19]. updated 2020 Dec 14. Available https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. [Google Scholar]
- 42.Rosser JI, Roltgen K, Dymock M, et al. SARS-CoV-2 seroprevalence in healthcare personnel in Northern California early in the COVID-19 pandemic. Infect Control Hosp Epidemiol. 2020 Dec 9; doi: 10.1017/ice.2020.1358.. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebinger JE, Botwin GJ, Albert CM, et al. Seroprevalence of antibodies to SARS-CoV-2 in healthcare workers: a cross-sectional study. BMJ Open. 2021;11(2):e043584. doi: 10.1136/bmjopen-2020-043584.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.COVID-19 in Ontario — a focus on diversity: January 15, 2020 to May 14, 2020. Toronto: Ontario Agency for Health Protection and Promotion (Public Health Ontario); 2020. [accessed 2020 Dec. 19]. Available: https://www.publichealthontario.ca/-/media/documents/ncov/epi/2020/06/covid-19-epi-diversity.pdf?la=en. [Google Scholar]
- 45.Garcia-Basteiro AL, Moncunill G, Tortajada M, et al. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun. 2020;11:3500. doi: 10.1038/s41467-020-17318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel MM, Thornburg NJ, Stubblefield WB, et al. Change in antibodies to SARS-CoV-2 Over 60 days among health care personnel in Nashville, Tennessee. JAMA. 2020;324:1781–2. doi: 10.1001/jama.2020.18796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5:1598–607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roltgen K, Powell AE, Wirz OF, et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci Immunol. 2020;5:eabe0240. doi: 10.1126/sciimmunol.abe0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choe PG, Kang CK, Suh HJ, et al. Waning antibody responses in asymptomatic and symptomatic SARS-CoV-2 infection. Emerg Infect Dis. 2021;27:327–9. doi: 10.3201/eid2701.203515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lisboa Bastos M, Tavaziva G, Abidi SK, et al. Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis. BMJ. 2020;370:m2516. doi: 10.1136/bmj.m2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Elslande J, Houben E, Depypere M, et al. Diagnostic performance of seven rapid IgG/IgM antibody tests and the Euroimmun IgA/IgG ELISA in COVID-19 patients. Clin Microbiol Infect. 2020;26:1082–7. doi: 10.1016/j.cmi.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manthei DM, Whalen JF, Schroeder LF, et al. Differences in performance characteristics among four high-throughput assays for the detection of antibodies against SARS-CoV-2 using a common set of patient samples. Am J Clin Pathol. 2021;155:267–79. doi: 10.1093/ajcp/aqaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beavis KG, Matushek SM, Abeleda APF, et al. Evaluation of the EUROIMMUN anti-SARS-CoV-2 ELISA assay for detection of IgA and IgG antibodies. J Clin Virol. 2020;129:104468. doi: 10.1016/j.jcv.2020.104468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Plebani M. Antibody responses in mild COVID-19 hospital staff. EBioMedicine. 2020;59:102940. doi: 10.1016/j.ebiom.2020.102940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ibarrondo FJ, Fulcher JA, Goodman-Meza D, et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild COVID-19. N Engl J Med. 2020;383:1085–7. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.