Abstract
Introduction
Enteric fever (caused by Salmonella enterica serovars Typhi and Paratyphi) frequently presents as an acute, undifferentiated febrile illness in returning travellers, requiring timely empirical antibiotics.
Gap statement
Determining which empirical antibiotics to prescribe for enteric fever requires up-to-date knowledge of susceptibility patterns.
Aim
By characterising factors associated with antimicrobial resistance in cases of S. Typhi and S. Paratyphi imported to England, we aim to guide effective empirical treatment.
Methodology
All English isolates of S. Typhi and S. Paratyphi 2014–2019 underwent antimicrobial susceptibility testing; results were compared to a previous survey in London 2005–2012. Risk factors for antimicrobial resistance were analysed with logistic regression models to predict adjusted odds ratios (aOR) for resistance to individual antibiotics and multi-drug resistance.
Results
We identified 1088 cases of S. Typhi, 729 S. Paratyphi A, 93 S. Paratyphi B, and one S. Paratyphi C. In total, 93 % were imported. Overall, 90 % of S. Typhi and 97 % of S. Paratyphi A isolates were resistant to ciprofloxacin; 26 % of S. Typhi were multidrug resistant to ciprofloxacin, amoxicillin, co-trimoxazole, and chloramphenicol (MDR+FQ). Of the isolates, 4 % of S. Typhi showed an extended drug resistance (XDR) phenotype of MDR+FQ plus resistance to third-generation cephalosporins, with cases of XDR rising sharply in recent years (none before 2017, one in 2017, six in 2018, 32 in 2019). For S. Typhi isolates, resistance to ciprofloxacin was associated with travel to Pakistan (aOR=32.0, 95 % CI: 15.4–66.4), India (aOR=21.8, 95 % CI: 11.6–41.2), and Bangladesh (aOR=6.2, 95 % CI: 2.8–13.6) compared to travel elsewhere, after adjusting for rising prevalence of resistance over time. MDR+FQ resistance in S. Typhi isolates was associated with travel to Pakistan (aOR=3.5, 95 % CI: 2.4–5.2) and less likely with travel to India (aOR=0.07, 95 % CI 0.04–0.15) compared to travel elsewhere. All XDR cases were imported from Pakistan. No isolate was resistant to azithromycin. Comparison with the 2005–2012 London survey indicates substantial increases in the prevalence of resistance of S. Typhi isolates to ciprofloxacin associated with travel to Pakistan (from 79–98 %) and Africa (from 12–60 %).
Conclusion
Third-generation cephalosporins and azithromycin remain appropriate choices for empirical treatment of enteric fever in most returning travellers to the UK from endemic countries, except from Pakistan, where XDR represents a significant risk.
Keywords: Antimicrobial resistance, Enteric fever, Paratyphoid, Travel medicine, Typhoid
Introduction
With a global burden estimated at 14 million cases and 136 000 deaths per year, enteric fever (caused by Salmonella enterica subspecies enterica serovars Typhi and Paratyphi A, B, and C) remains a major health concern for people living in or travelling to and from endemic regions [1, 2]. However, in many endemic settings, the risk to residents and travellers is difficult to ascertain: limited capacity for microbiological diagnostics and surveillance impedes estimates of the local risk of infection, and the susceptibility to antimicrobial drugs. Moreover, timely data on outbreaks in endemic settings are limited, and there is some evidence that outbreaks caused by multidrug resistant strains are more prolonged [3].
In settings with high standards of sanitation, almost all cases occur either in returning travellers or close contacts of travellers [4]. The clinical presentation is non-specific, and preventing complications requires the timely initiation of effective antimicrobial therapy. Therefore, antibiotics are often initiated empirically when a clinical suspicion of enteric fever is raised, or with preliminary microbiological results. To ensure effective treatment, clinicians and microbiologists must know which antibiotics are likely to be useful for a given traveller, before a full susceptibility profile is obtained.
Salmonella Typhi and S. Paratyphi A have rapidly developed resistance to antimicrobial agents used to treat them [5]. Multi-drug resistance (MDR)–traditionally defined as resistance to amoxicillin (or ampicillin), co-trimoxazole (sulphamethoxazole-trimethoprim), and chloramphenicol–has been recognised widely in S. Typhi isolates from Asia and Africa [6, 7]. This has led to widespread changes in prescribing practices, favouring fluoroquinolones and cephalosporins. In some areas, this change has been associated with a re-emergence of non-MDR strains, but in others, the problem has been compounded by increasing resistance to newly introduced drugs [5]. Resistance to ciprofloxacin and other fluoroquinolones is well characterised in South and South East Asia. Meanwhile, in Africa, ciprofloxacin is still widely perceived as an effective agent, though localised instances of fluoroquinolone resistance have been observed [8–10]. While third generation cephalosporins, the mainstay of parenteral therapy, remain an effective choice in most settings, there have been reports of extended spectrum beta-lactamase (ESBL)-producing organisms in South Asia and South America [11, 12]. From 2016 to 2018, an outbreak of extensively drug resistant (XDR) S. Typhi was identified in Pakistan, affecting over 5000 people, with resistance to ampicillin, co-trimoxazole, chloramphenicol, fluoroquinolones, and third-generation cephalosporins, limiting treatment options still further [13].
In this investigation, we will characterise the burden and epidemiology of typhoidal salmonellas in England between 2014 and 2019 and and assess the changing profiles of antimicrobial resistance in relation to earlier surveys. In doing so, we seek to identify risk factors associated with antimicrobial resistance among travel-associated isolates, with the aim of informing clinical decision-making and public health action.
Methods
Context and specimen flow
Isolates of S. Typhi and S. Paratyphi from all diagnostic laboratories in England are sent to the national reference laboratory–Public Health England Salmonella Reference Service (PHE SRS) within the Gastrointestinal Bacteria Reference Unit (GBRU)–for confirmation of species and further characterisation. Enteric fever records were collated from all SRS isolates of any specimen type between April 2014 and December 2019.
Microbiological confirmation and characterization
All presumptive enteric fever isolates were processed at containment level three and plated out onto MacConkey agar for purity checking and processing. DNA was extracted from each isolate using the QIAsymphony automated DNA extraction machine (QIAGEN, UK) and sequenced using the Illumina HiSeq 2500 platform in rapid run mode (2×100 base pair reads). Identification of Salmonella isolates using sequence data was performed using multiple programmes and pipelines as previously described [14]. Serovar determination was predicted using Salmonella eBURST group or Sequence Type and checked against a validated PHE database [14, 15].
Laboratory susceptibility testing conducted by PHE SRS was performed retrospectively on all isolates recovered from the PHE archive based on the EU protocol for monitoring of antimicrobial resistance [16]. Minimal Inhibitory Concentrations (MICs) were determined by agar dilution using Mueller–Hinton agar for the following antimicrobials: amoxicillin, azithromycin, cefoxitin, ceftazidime, ceftriaxone, chloramphenicol, ciprofloxacin, co-trimoxazole (sulphamethoxazole-trimethoprim), colistin (from January 2016), ertapenem, tetracycline, and trimethoprim. Standard protocols of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) to define in clinical breakpoints and susceptibility to a given antimicrobial agent were used [17, 18]. Where multiple isolates were obtained from the same individual, duplicate records were removed. MDR was defined as resistance to amoxicillin, chloramphenicol, and co-trimoxazole, and MDR+FQ resistance was defined as resistance to these three drugs plus ciprofloxacin, and XDR was defined as resistance to MDR agents, ciprofloxacin, and third generation cephalosporins, in keeping with WHO terminology [19].
Enhanced public health surveillance of enteric fever
Enteric fever is a notifiable disease in England, and enhanced surveillance has been undertaken by PHE since 2006. Laboratory-confirmed cases (or guardians of paediatric cases) were interviewed using a standardised enhanced surveillance questionnaire to ascertain demographic details, symptoms, vaccination and travel histories, and–for cases in which a travel history is not evident–food history and history of contact with travellers. Where the antimicrobials used to treat the case could be determined, these data were obtained.
While PHE’s public health guidance recommends that cases be regarded as travel-associated where symptom onset occurs within 28 days of travel [20], for the purposes of this investigation, we regarded travel within 60 days as pertinent to ascertaining the likely geographical source of an isolate. Where cases had no direct travel history but had recent, close contact with a traveller, the case was regarded as associated with that region of travel as a propagated infection. Travel to each country or region was recorded as a separate dichotomous variable to allow capture of travel to multiple endemic settings by the same individual.
Socio-economic deprivation was calculated from postcode using the 2019 English Indices of Deprivation score from the Ministry of Housing, Communities and Local Government, which incorporates measures of deprivation related to income, employment, education, health and disability, crime barriers to housing and services, and living environment [21]. The population was divided into quintiles based on this score.
Univariable analysis
Statistical analysis was undertaken using STATA/IC 14.2. Continuous variables were described using medians, interquartile ranges (IQR) and ranges, and compared using the Mann-Witney U test. Categorical variables were described using numbers and percentages in each category and compared by chi-squared test. Prevalence of antimicrobial resistance among isolates from each location was calculated compared to earlier resistance prevalence records in travellers returning to London from 2005 to 2012, as reported by Dave et al. [22]. Comparable methodologies were used for both studies (though the ciprofloxacin breakpoint has been revised by one dilution since the earlier report by Dave et al. from 0.125mg l−1 to 0.06 mg l−1, in line with updated EUCAST recommendations) [17, 18].
For a given location, prevalence of ciprofloxacin resistance, MDR, and combined resistance to MDR agents and ciprofloxacin (MDR+FQ) were calculated and compared to the earlier survey by Fisher’s exact test. To ascertain changes in antimicrobial resistance over the course of the present survey, resistance over the first 2 years (2014–2015) was compared to the final 2 years (2018–2019) by chi-squared test, overall and for individual regions.
Logistic regression models
A multivariable logistic regression model was constructed for S. Typhi to characterise factors associated with resistance to ciprofloxacin, and MDR+FQ resistance, and for S. Paratyphi A to characterise factors associated with ciprofloxacin resistance. Source locations assessed as exposure variables were Pakistan, India, Bangladesh, other Asian countries, and Africa–each scored dichotomously with the possibility of travel to multiple locations. Potential confounders assessed for the model were age, gender, index of deprivation for the UK address (poorest quintile vs. others), ethnicity, birth in the UK (versus elsewhere in the world), travel to visit friends and relatives (VFR, versus other or unknown purpose of travel), and direct vs. propagated infection. Specimen year was treated as a continuous variable. Final models were constructed using a stepwise, subtractive approach to identify variables independently associated with resistance (considering for inclusion those with a P-value of less than 0.4) to predict adjusted odds ratios (aOR) for resistance to individual antibiotics and multi-drug resistance, favouring a parsimonious model guided by Akaike’s information criteria (AIC) and Bayesian information criteria (BIC) to appraise the effects of removing variables, with likelihood ratio tests employed to address ambiguous results.
Results
Characteristics of isolates and cases
During the study period, we analysed laboratory and questionnaire data from 1088 cases with S. Typhi, 729 with S. Paratyphi A, 93 with S. Paratyphi B, and one with S. Paratyphi C. Of 1645 isolates with a recorded specimen type, 1213 (74 %) were from blood, 407 (25 %) from faeces, and 25 (2 %) from other specimen types (such as urine or abdominal fluid). Of all 1910 cases of S. Typhi and S. Paratyphi A and B, 1781 (93 %) had a clear history of recent travel, and a further 19 (1 %) had not travelled themselves but had a clear link to a recent traveller, with presumed transmission of the infection in England.
Table 1 shows the population characteristics of cases included in the study presenting with typhoid and paratyphoid A and B. Cases were disproportionately resident in more deprived areas of England. Most cases of typhoid and paratyphoid A occurred among travellers to South Asia (86 and 90% respectively having visited Pakistan, India, or Bangladesh). Most reported visiting friends and relatives (VFR) as the purpose of travel, with ethnicity closely matching destination of travel. A higher proportion of cases of paratyphoid B were associated with travel to South East Asia, the Middle East, or the Americas, VFR and non-VFR travel were more equally represented, and a greater proportion of cases reported white British ethnicity. At the time of interview, among 1785 cases for whom admission status could be ascertained, 1493 (83 %) had been admitted to hospital, and 302 (17 %) had not. Symptoms at the time of interview are described in Table S1 (available in the online version of this article) online: 92 % reported fever, and 79 % reported at least one gastrointestinal symptom.
Table 1.
Population characteristics of cases identified with typhoid and paratyphoid A and B
|
Typhoid |
Paratyphoid A |
Paratyphoid B |
|||||
|---|---|---|---|---|---|---|---|
|
Characteristic |
Group |
N (1088) |
(%) |
N (729) |
(%) |
N (93) |
(%) |
|
Age group |
0–14 years |
345 |
(32 %) |
116 |
(16 %) |
28 |
(30 %) |
|
|
15–29 years |
319 |
(29 %) |
240 |
(33 %) |
36 |
(39 %) |
|
|
30–44 years |
260 |
(24 %) |
216 |
(30 %) |
15 |
(16 %) |
|
|
45–59 years |
117 |
(11 %) |
92 |
(13 %) |
6 |
(6 %) |
|
|
60+ years |
47 |
(4 %) |
65 |
(9 %) |
8 |
(9 %) |
|
Gender |
Female |
525 |
(48 %) |
360 |
(49 %) |
50 |
(54 %) |
|
|
Male |
563 |
(52 %) |
369 |
(51 %) |
43 |
(46 %) |
|
Deprivation index |
1 most deprived |
334 |
(33 %) |
226 |
(32 %) |
23 |
(26 %) |
|
(by UK postcode) |
2 |
291 |
(29 %) |
202 |
(29 %) |
20 |
(23 %) |
|
|
3 |
172 |
(17 %) |
120 |
(17 %) |
13 |
(15 %) |
|
|
4 |
117 |
(12 %) |
72 |
(10 %) |
11 |
(13 %) |
|
|
5 least deprived |
97 |
(10 %) |
79 |
(11 %) |
21 |
(24 %) |
|
|
missing |
77 |
. |
30 |
. |
5 |
. |
|
Ethnicity |
Indian |
315 |
(33 %) |
253 |
(39 %) |
1 |
(1 %) |
|
|
Pakistani |
382 |
(41 %) |
204 |
(32 %) |
9 |
(13 %) |
|
|
Bangladeshi |
78 |
(8 %) |
65 |
(10 %) |
. |
. |
|
|
White British |
57 |
(6 %) |
77 |
(12 %) |
39 |
(56 %) |
|
|
Other Asian |
30 |
(3 %) |
22 |
(3 %) |
5 |
(7 %) |
|
|
Other Mixed |
31 |
(3 %) |
19 |
(3 %) |
16 |
(7 %) |
|
|
Black African/Caribbean |
48 |
(5 %) |
6 |
(1 %) |
. |
. |
|
|
not stated |
147 |
. |
83 |
. |
23 |
. |
|
Birthplace |
UK born |
411 |
(59 %) |
295 |
(44 %) |
57 |
(76 %) |
|
|
Non-UK born |
598 |
(41 %) |
382 |
(56 %) |
18 |
(24 %) |
|
|
missing |
79 |
. |
52 |
. |
18 |
. |
|
Travel related |
Yes-direct |
1013 |
(99 %) |
701 |
(99 %) |
77 |
(99 %) |
|
|
Yes-propagated |
13 |
(1 %) |
5 |
(1 %) |
1 |
(1 %) |
|
|
No known travel |
62 |
. |
23 |
. |
15 |
. |
|
Purpose of travel |
VFR* |
770 |
(81 %) |
503 |
(76 %) |
35 |
(49 %) |
|
|
Non-VFR |
186 |
(19 %) |
159 |
(24 %) |
36 |
(51 %) |
|
|
no travel/missing |
132 |
. |
67 |
. |
22 |
. |
|
Source of infection† |
India |
384 |
(37 %) |
323 |
(45 %) |
2 |
(3 %) |
|
|
Pakistan |
417 |
(41 %) |
225 |
(32 %) |
6 |
(8 %) |
|
|
Bangladesh |
73 |
(7 %) |
70 |
(10 %) |
. |
. |
|
|
Other Asia |
37 |
(4 %) |
50 |
(7 %) |
32 |
(41 %) |
|
|
Africa |
57 |
(6 %) |
5 |
(1 %) |
. |
. |
|
|
Americas |
12 |
(1 %) |
1 |
(0.1 %) |
36 |
(46 %) |
|
|
Europe |
6 |
(1 %) |
1 |
(0.1 %) |
. |
. |
|
|
Pacific |
2 |
(0.2 %) |
. |
. |
. |
. |
|
|
Multiple possible |
38 |
(4 %) |
29 |
(4 %) |
2 |
(3 %) |
|
|
not determined |
29 |
. |
15 |
. |
8 |
. |
|
|
missing |
33 |
. |
10 |
. |
7 |
. |
*VFR, visiting friends and relatives.
†Includes the sources of both imported cases and cases with infections propagated from an imported case. Cases associated with travel to 19 other countries in Asia, 18 countries in Africa, 18 in the Americas, four in Europe, and two in the Pacific.
Among 1088 isolates of S. Typhi, 980 (90 %) were resistant to ciprofloxacin, 349 (32 %) to co-trimoxazole, 330 (30 %) to chloramphenicol, and 310 (28 %) to amoxicillin. Of these, 42 isolates (4 %) were ESBL producers, displaying resistance to ceftriaxone and ceftazidime, imported from Pakistan (n=39), Iraq (n=2), and India (n=1). No isolates were resistant to azithromycin, ertapenem, or colistin. In total, 293 isolates (27 %) were multi-drug resistant (MDR), with resistance to amoxicillin, chloramphenicol, and co-trimoxazole. We found 285 isolates (26 %) showed MDR+FQ resistance, while 39 isolates (4 %) were XDR (MDR+FQ+resistance to third generation cephalosporin), all associated with travel from Pakistan. Three isolates (two imported from Iraq and one from India) exhibited ceftriaxone and ciprofloxacin resistance in the absence of a full XDR phenotype (being susceptible to co-trimoxazole and chloramphenicol).
Among 729 isolates of S. Paratyphi A, 710 (97 %) were resistant to ciprofloxacin, with relative preservation of susceptibility to other drugs. One isolate from an imported case from Bangladesh was an ESBL producer (resistant to ceftriaxone and ceftazidime) and also resistant to ciprofloxacin. No resistance was identified to azithromycin, chloramphenicol, co-trimoxazole, colistin, or ertapenem. S. Paratyphi B isolates (n=93) showed relative preservation of antimicrobial susceptibility: 12 isolates (13 %) were resistant to ciprofloxacin, with no resistance detected to other classes of antibiotics tested. The single isolate of S. Paratyphi C that was available was susceptible to all antibiotics tested.
Distribution of S. Typhi antimicrobial resistance
S. Typhi isolates linked to travel to Pakistan (n=427) showed the highest prevalence of antimicrobial resistance, with 98 % resistant to ciprofloxacin, 52 % MDR, 52 % MDR+FQ, and 9 % XDR. Among isolates linked to India (n=407), 96 % were resistant to ciprofloxacin, with lower rates of resistance to amoxicillin (3 %), chloramphenicol (3 %) and co-trimoxazole (5 %), such that 2 % had MDR and 2 % MDR+FQ profiles. Among isolates linked to Bangladesh (n=77), 88 % were ciprofloxacin-resistant, 26 % were MDR, and 25 % were MDR+FQ-resistant. Isolates linked to other Asian countries (n=68) were ciprofloxacin resistant in 67 % of cases; 24 % were MDR and 24 % were MDR+FQ resistant. Isolates linked to Africa (n=58) were ciprofloxacin-resistant in 60 % of cases, 40 % MDR, and 30 % MDR+FQ.
Univariable analysis of risk factors for S. Typhi resistance to ciprofloxacin, and MDR+FQ resistance profiles are presented online in Tables S2 and S3, and multivariable logistic regression models are presented in Table 2. Resistance to ciprofloxacin was most strongly associated with travel to Pakistan (aOR 32.0, 95 % CI 15.4–66.4, P < 0.001), and was also associated with travel to India (aOR 21.8, 95 % CI 11.6–41.2, P < 0.001) and Bangladesh (aOR 6.2, 95 % CI 2.8–13.6), P < 0.001), compared to travel elsewhere, after adjusting for an overall increase in prevalence of resistance over time (with an increase in aOR of 1.2 for each year). Incorporation of other potential confounders (age, sex, birth in the UK, UK deprivation index, and purpose of travel) did not improve the strength of the model. MDR+FQ resistance was associated with travel to Pakistan (aOR 2.5, 95 % CI 2.4–5.2, P < 0.001), and was less likely with travel to India (aOR 0.07, 95 % CI 0.04–0.15, P < 0.001).
Table 2.
Characteristics associated with ciprofloxacin and MDR+FQ resistance* among cases with typhoid, 2014–2019 in multivariable logistic regression models
|
Characteristic |
Crude OR |
Adjusted OR |
(95 % CI) |
P-value |
|---|---|---|---|---|
|
Ciprofloxacin resistance model:† |
|
|
|
|
|
Travel to Pakistan |
6.1 |
32.0 |
(15.4–66.4) |
< 0.001 |
|
Travel to India |
3.4 |
21.9 |
(11.6–41.2) |
< 0.001 |
|
Travel to Bangladesh |
0.7 |
6.2 |
(2.8–13.6) |
< 0.001 |
|
Adjustment for Time (per year) |
1.3 |
1.2 |
(1.0–1.4) |
0.025 |
|
MDR+FQ resistance model:‡ |
|
|
|
|
|
Travel to Pakistan |
10.9 |
3.5 |
(2.4–5.2) |
< 0.001 |
|
Travel to India |
0.03 |
0.07 |
(0.04–0.15) |
< 0.001 |
*MDR+FQ resistance=multi drug resistance (to amoxicillin, chloramphenicol, and co-trimoxazole) plus fluoroquinolone resistance (to ciprofloxacin).
†Among 1025 cases with known travel history or close link to a known traveller tested for ciprofloxacin resistance.
‡Among 1024 with known travel history or close link to a known traveller tested for resistance all four antimicrobials.
All 39 cases of XDR S. Typhi were associated with travel to Pakistan (with three cases travelling by way of the United Arab Emirates). Two cases of S. Typhi with resistance to third generation cephalosporins, amoxicillin, and ciprofloxacin, but preserved susceptibility to co-trimoxazole and chloramphenicol (and therefore not XDR) were associated with travel to Iraq; further details of these cases are provided elsewhere [23].
Distribution of S. Paratyphi antimicrobial resistance
Resistance profiles for S. Paratyphi serovars were more homogeneous than those of S. Typhi. For S. Paratyphi A, resistance to ciprofloxacin was widespread (429 of 442 isolates; 97 %), but susceptibility to other antimicrobials tested was preserved (with the exception of a single isolate showing resistance to beta-lactam agents). Univariable analysis of associations with ciprofloxacin resistance among S. Paratyphi A cases is presented in Table S4, and multivariable logistic regression in Table 3. With only 19 ciprofloxacin-sensitive isolates, the logistic model was strengthened by combining links to Pakistan, India, and Bangladesh into a single variable; links to any of these countries was associated greater odds of resistance, and links to Africa with lower odds, with the caveat that only seven cases with links to Africa were identified (three of them ciprofloxacin resistant) during this survey.
Table 3.
Characteristics associated with ciprofloxacin resistance among cases with paratyphoid A, 2014–2019 in a multivariable logistic regression model
|
Characteristic* |
Crude OR |
Adjusted OR |
(95 % CI) |
P-value |
|---|---|---|---|---|
|
Travel to Pakistan, India, or Bangladesh |
45.3 |
33.4 |
(10.0–112.0) |
< 0.001 |
|
Travel to Africa |
0.01 |
0.11 |
(0.02–0.64) |
0.015 |
*Among 704 cases with known travel history or close link to a known traveller tested for ciprofloxacin resistance.
Isolates of S. Paratyphi B differed markedly from those of other typhoidal salmonellas in their geography: of 93 isolates, 37 arose from South America, 25 from Iraq, seven from Turkey, six from Pakistan, two from India; one case had recently travelled to both Pakistan and Saudi Arabia; for 15, no travel link was established. The number of cases with antimicrobial resistance (12 for ciprofloxacin, none for other drugs) was insufficient to characterize geographical associations, but ciprofloxacin resistant and sensitive cases were imported from South Asia and the Americas; all isolates from the Middle East were ciprofloxacin-sensitive.
Antibiotics used to treat infections
Among 258 isolates of S. Typhi for which the antibiotics used to treat the infection could be ascertained from surveillance interviews, 179 (69 %) were treated with ceftriaxone, 44 (17 %) with azithromycin, 31 (12 %) with ciprofloxacin, 18 with amoxicillin (7 %), 11 (4 %) with co-amoxiclav, five (2 %) with meropenem, three (1 %) with co-trimoxazole (no cases reported use of chloramphenicol). Of the 31 cases reporting treatment with ciprofloxacin, 20 (65 %) had isolates that were found to be ciprofloxacin resistant; in 12 of these cases, it was reported to be the only antibiotic given.
Changes in antimicrobial resistance over time
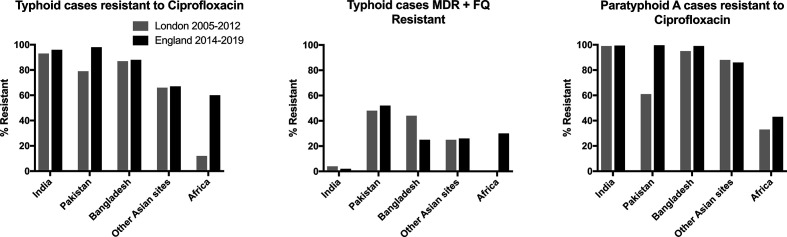
In comparison to a survey of Salmonella isolates in returning travellers in London 2005–2012 (Fig. 1), current observations of S. Typhi show an increase in ciprofloxacin resistance among travellers from Africa from 12–60 % (P < 0.001), and an increase in MDR+FQ resistance profile from 0–30 % (P=0.005), with no clear evidence of a shift in resistance to MDR first-line agents (amoxicillin, chloramphenicol, and co-trimoxazole) [22]. Among travellers from Pakistan, prevalence of ciprofloxacin resistance increased from 79–98 % (P < 0.001). No cases of the XDR phenotype were noted in the previous London survey, nor in this survey prior to 2017. In the present survey, one XDR case in 2017 was followed by six in 2018, and 32 in 2019.
Fig. 1.
Comparison between antimicrobial resistance patterns of isolates from imported cases of typhoid and paratyphoid A presenting in England 2014–2019 and London 2005–2012. MDR+FQ=multi-drug resistance (to amoxicillin, chloramphenicol, co-trimoxazole) plus fluoroquinolone resistance (to ciprofloxacin).
Since the 2005–2012 London survey, ciprofloxacin resistance of S. Paratyphi A isolates in returning travellers from Pakistan has increased from 61–99 % (P < 0.001). S. Paratyphi A ciprofloxacin resistance rates in travellers from India, Bangladesh, and elsewhere in Asia remain high. The number of S. Paratyphi A isolates in travellers from Africa was low in both studies, making the comparison under-powered to detect a change (two of six isolates were resistant in the previous survey, compared to three of seven in this survey).
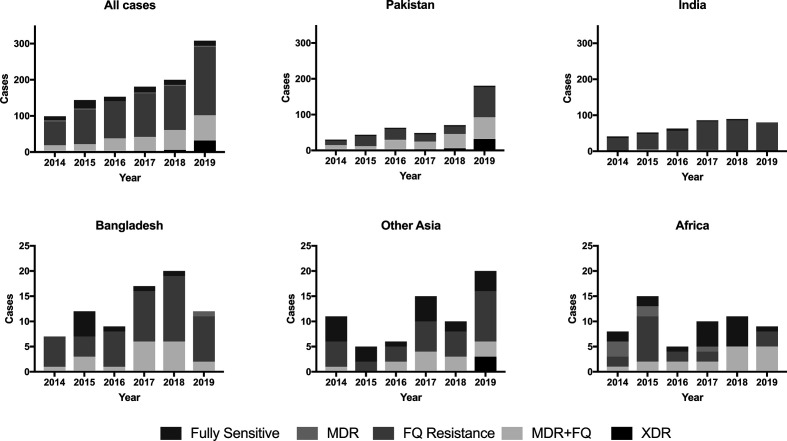
Comparing the first 2 years of this survey to the final 2 years, we found evidence of increased resistance of S. Typhi to ciprofloxacin (OR 2.9, 95 % CI 1.8–4.7, P < 0.001) and of MDR+FQ resistance (OR 2.3, 95 % CI 1.5–3.3, P < 0.001). The magnitude of this increase was greatest among isolates from India (OR 5.9, 95 % CI 1.1–30.3, P=0.017), Bangladesh (OR 5.4, 95 % CI 0.8–34.1, P=0.046), and other Asian countries (OR 4.0, 95 % CI 1.0–16.4, P=0.038), with no evidence of an increase in Pakistan (where resistance was nearly universal throughout) or Africa (where the number of isolates each year was small). S. Typhi MDR+FQ resistance increased in Pakistan (OR 2.0, 95 % CI 1.1–3.4, P=0.014) and Africa (OR 6.7, 95 % CI 1.3–34.8, P=0.009). Changing patterns of S. Typhi AMR for individual regions are illustrated in Fig. 2. Over the duration of this survey, we did not identify a change in S. Paratyphi A’s AMR profile, overall or at a regional level.
Fig. 2.
Comparison of antimicrobial resistance patterns of S. Typhi over time by region of travel. Note different y-axis scales for upper and lower panels. MDR=multi drug resistance (to amoxicillin, chloramphenicol, co-trimoxazole); FQ Resistance=fluoroquinolone resistance (to ciprofloxacin); MDR+FQ=MDR plus fluoroquinolone resistance; XDR=extended drug resistance: MDR plus FQ resistance plus resistance to third-generation cephalosporins (ceftriaxone and ceftazidime).
Discussion
Our findings demonstrate the increasing limits imposed by antimicrobial resistance on the effective treatment options for enteric fever in returning travellers. Reports from reference laboratories in the UK have demonstrated consistent S. Typhi resistance to fluoroquinolones since the late 1990s among travellers returning from South Asia [24]. Until recently, however, fluoroquinolones remained a viable empirical treatment for cases returning from regions such as Africa, where resistance was lower [25]. In this investigation, we report further increases in the prevalence and distribution of antimicrobial resistance among imported cases. S. Typhi infections imported from Pakistan, India, and Bangladesh had greater odds of ciprofloxacin resistance, but susceptibility can no longer be anticipated in travellers returning from other Asian countries (with 67 % ciprofloxacin resistance) or Africa (with 60 % resistance). S. Paratyphi A also shows increasing resistance to ciprofloxacin in travellers returning from Pakistan, such that it is now almost universal among travellers to Asia; the organism is less commonly imported from Africa, but there, too, we find increasing evidence of resistance.
Multi-drug resistance is also growing concern: the risk of S. Typhi resistance to ciprofloxacin, amoxicillin, chloramphenicol, and co-trimoxazole was highest among travellers returning from Pakistan, but has risen in Africa to 30 %. XDR S. Typhi shows emergence over recent years, with the first imported case identified in 2017, but 12 % of cases linked to Pakistan showing this phenotype in 2019. Imported infections caused by the XDR S. Typhi strain from Pakistan reflect the ongoing outbreak which was first reported in 2017, and has expanded since to include several regions within the country [26]. One isolate of S. Paratyphi A also showed extended resistance to beta-lactam antibiotics including ceftriaxone [27].
For most imported isolates, sensitivity to third generation cephalosporins is preserved. We also found no evidence of azithromycin resistance. These agents remain appropriate first-line therapies for cases returning from all regions with suspected enteric fever while awaiting microbiological confirmation and drug susceptibility profiles. However, this study highlights that travellers returning from Pakistan may be at risk of infection from XDR S. Typhi: when such cases present as complicated enteric fever, a carbapenem may be the empirical antibiotic of choice until culture and sensitivity results become available.
Our findings are consistent with trends observed in endemic settings globally, where MDR and fluoroquinolone resistance among S. Typhi isolates, and fluoroquinolone resistance among S. Paratyphi A isolates, are observed with increasing prevalence in all regions [5]. Similar findings have been reported among imported cases in other resource-rich countries [13].
Surveillance data of this kind do not permit us to comment on risk factors for acquiring enteric fever, nor the relative risk of infection associated with different travel destinations–only on the likelihood of antimicrobial resistance in infected individuals. In practice, empirical prescribing decisions are based upon the provisional diagnosis, severity of infection, travel history, and the risk of AMR. Much of the information gathered by health protection teams in the enhanced surveillance of enteric fever focusses on risk factors for infection itself, rather than risk factors for drug resistance. Gathering additional information concerning exposure to urban and rural areas, healthcare settings, and use of antibiotics while travelling may help us characterise the risk factors for AMR with greater precision.
Because our findings are limited to returning travellers, they may not reflect the patterns of antimicrobial resistance among longer-term residents in endemic areas. Observing patterns of infection in returning travellers can add to a global perspective on antimicrobial resistance, serving as sentinel surveillance and permitting the use of a single reference laboratory’s procedures for organisms acquired from multiple settings, but they will be subject to demographic, geographical and behavioural biases of international travel [28]. Alongside such observations, then, there is an urgent need to strengthen diagnostic capacity and surveillance within endemic countries, and to facilitate international communication and comparison through AMR surveillance networks [29].
Conclusions
The rise in resistance of both S. Typhi and S. Paratyphi A to ciprofloxacin should guide prescribing recommendations for returning travellers from both Asia and Africa. Clinical and epidemiological alertness to the growing threat of XDR S. Typhi should also inform empirical prescribing recommendations for cases returning from areas of known XDR outbreaks. The changing landscape of AMR underlines the importance of timely surveillance and international health communication.
Supplementary Data
Funding information
The authors received no specific grant from any funding agency.
Acknowledgements
We gratefully acknowledge Martin Day and Amy Gentle from GBRU for performing antimicrobial susceptibility testing, health protection teams and environmental health officers in completing the enhanced surveillance questionnaires for enteric fever, the Travel Health Team for collating and coordinating enhanced surveillance data, colleagues in the National Health Services diagnostic laboratories for referral of isolates to reference laboratory, and cases for participating in enhanced surveillance.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical statement
Ethical approval or specific consent was not required from the cases whose data were used in this analysis as PHE has authority to handle patient data for public health monitoring and infection control under section 251 of the UK National Health Service Act of 2006.
Footnotes
Abbreviations: AIC, Akaike's information criteria; aOR, adjusted odds ratio; BIC, Bayesian information criteria; EUCAST, European Committee on Antimicrobial Susceptibility Testing; FQ, fluoroquinolone; GBRU, Gastrointestinal Bacteria Reference Laboratory; IQR, interquartile range; MDR, multidrug resistance; MIC, minimal inhibitory concentration; PHE SRS, Public Health England Salmonella Reference Service; VFR, visiting friends and relatives; XDR, extended drug resistance.
Four supplementary tables are available with the online version of this article.
References
- 1.Buckle GC, Walker CL, Black RE. Typhoid fever and paratyphoid fever: Systematic review to estimate global morbidity and mortality for 2010. J Glob Health. 2012;2:010401. doi: 10.7189/jogh.02.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Typhoid GBD, Paratyphoid C. The global burden of typhoid and paratyphoid fevers: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis. 2019;19:369–381. doi: 10.1016/S1473-3099(18)30685-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appiah GD, Chung A, Bentsi-Enchill AD, Kim S, Crump JA, et al. Typhoid outbreaks, 1989-2018: Implications for prevention and control. Am J Trop Med Hyg. 2020;102:1296–1305. doi: 10.4269/ajtmh.19-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensenius M, Han PV, Schlagenhauf P, Schwartz E, Parola P. Acute and potentially life-threatening tropical diseases in western travelers--a GeoSentinel multicenter study, 1996-2011. Am J Trop Med Hyg. 2013;88:397–404. doi: 10.4269/ajtmh.12-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Browne AJ, Kashef Hamadani BH, Kumaran EAP, Rao P, Longbottom J. Drug-resistant enteric fever worldwide, 1990 to 2018: A systematic review and meta-analysis. BMC Med. 2020;18(1) doi: 10.1186/s12916-019-1443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barkume C, Date K, Saha SK, Qamar FN, Sur D, et al. Phase I of the surveillance for enteric fever in Asia Project (SEAP): An overview and lessons learned. J Infect Dis. 2018;218:S188–S194. doi: 10.1093/infdis/jiy522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crump JA, Mintz ED. Global trends in typhoid and paratyphoid Fever. Clin Infect Dis. 2010;50:241–246. doi: 10.1086/649541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.International Typhoid C, Wong VK, Holt KE, Okoro C, Baker S, et al. Molecular surveillance identifies multiple transmissions of typhoid in West Africa. PLOS Negl Trop Dis. 2016;10:e0004781. doi: 10.1371/journal.pntd.0004781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Britto CD, Wong VK, Dougan G, Pollard AJ. A systematic review of antimicrobial resistance in Salmonella enterica serovar Typhi, the etiological agent of typhoid. PLoS Negl Trop Dis. 2018;12:e0006779. doi: 10.1371/journal.pntd.0006779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Emran HM, Eibach D, Krumkamp R, Ali M, Baker S, et al. A multicountry molecular analysis of Salmonella enterica serovar typhi with reduced susceptibility to ciprofloxacin in sub-saharan Africa. Clin Infect Dis. 2016;62:S42–46. doi: 10.1093/cid/civ788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Lopez JJ, Piedra-Carrasco N, Salvador F, Rodriguez V, Sanchez-Montalva A. ESBL-producing Salmonella enterica serovar Typhi in traveler returning from Guatemala to Spain. Emerg Infect Dis. 2014;20:1918–1920. doi: 10.3201/eid2011.140525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pokharel BM, Koirala J, Dahal RK, Mishra SK, Khadga PK. Multidrug-resistant and extended-spectrum beta-lactamase (ESBL)-producing Salmonella enterica (serotypes Typhi and Paratyphi A) from blood isolates in Nepal: surveillance of resistance and a search for newer alternatives. Int J Infect Dis. 2006;10:434–438. doi: 10.1016/j.ijid.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Chatham-Stephens K, Medalla F, Hughes M, Appiah GD, Aubert RD, et al. Emergence of extensively Drug-resistant Salmonella typhi infections among travelers to or from Pakistan - United States, 2016-2018. MMWR Morb Mortal Wkly Rep. 2019;68:11–13. doi: 10.15585/mmwr.mm6801a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Achtman M, Wain J, Weill FX, Nair S, Zhou Z. Multilocus sequence typing as a replacement for serotyping in Salmonella enterica . PLoS Pathog. 2012;8:e1002776. doi: 10.1371/journal.ppat.1002776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chattaway MA, Dallman TJ, Larkin L, Nair S, McCormick J, et al. The transformation of reference microbiology methods and surveillance for salmonella with the use of whole genome sequencing in England and Wales. Front Public Health. 2019;7:317. doi: 10.3389/fpubh.2019.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ECDC European Centre for Disease Prevention and Control. Eu Protocol for Harmonised Monitoring of Antimicrobial Resistance in Human Salmonella and Campylobacter Isolates – June 2016. Stockholm: ECDC; 2016. [Google Scholar]
- 17.Howe RA, Andrews JM, BWPoS T. BSAC standardized disc susceptibility testing method (version 11. J Antimicrob Chemother. 2012;67:2783–2784. doi: 10.1093/jac/dks391. [DOI] [PubMed] [Google Scholar]
- 18.European Committee on Antimicrobial Susceptibility Testing Breakpoint tables for interpretation of mics and zone diameters version . 2019;9 [Google Scholar]
- 19.World Health Organization Background document: the diagnosis, treatment and prevention of typhoid fever. 2003 https://www.glowm.com/pdf/WHO-diagnosis%20treatment%20prevention%20of%20typhoid%20fever-2003-CustomLicense.pdf
- 20.Freedman J, Lighton L, Jones J. Defining travel-associated cases of enteric fever. J Infect Public Health. 2014;7:377–385. doi: 10.1016/j.jiph.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Ministry of Housing CLG The English Indices of Deprivation 2019: Technical Report. London: 2019. [Google Scholar]
- 22.Dave J, Warburton F, Freedman J, de Pinna E, Grant K. What were the risk factors and trends in antimicrobial resistance for enteric fever in London 2005-2012? J Med Microbiol. 2017;66:698–705. doi: 10.1099/jmm.0.000484. [DOI] [PubMed] [Google Scholar]
- 23.Godbole G, McCann N, Jones SM, Dallman TJ, Brown M. Ceftriaxone-resistant Salmonella typhi in a traveller returning from a mass gathering in Iraq. The Lancet Infectious Diseases. 2019;19:467. doi: 10.1016/S1473-3099(19)30176-8. [DOI] [PubMed] [Google Scholar]
- 24.Threlfall EJ, Ward LR, Skinner JA, Smith HR, Lacey S. Ciprofloxacin-resistant Salmonella typhi and treatment failure. Lancet. 1999;353:1590–1591. doi: 10.1016/s0140-6736(99)01001-6. [DOI] [PubMed] [Google Scholar]
- 25.Cooke FJ, Wain J, Threlfall EJ. Fluoroquinolone resistance in Salmonella typhi . BMJ. 2006;333:353–354. doi: 10.1136/bmj.333.7563.353-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasheed F, Saeed M, Alikhan NF, Baker D, Khurshid M, et al. Emergence of resistance to fluoroquinolones and third-generation cephalosporins in Salmonella typhi in Lahore, Pakistan. medRxiv. 2020 doi: 10.3390/microorganisms8091336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nair S, Day M, Godbole G, Saluja T, Langridge GC, et al. Genomic surveillance detects Salmonella enterica serovar Paratyphi A harbouring blaCTX-M-15 from a traveller returning from Bangladesh. PLoS One. 2020;15:e0228250. doi: 10.1371/journal.pone.0228250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leder K, Torresi J, Libman MD, Cramer JP, Castelli F. GeoSentinel surveillance of illness in returned travelers, 2007-2011. Ann Intern Med. 2013;158:456–468. doi: 10.7326/0003-4819-158-6-201303190-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashley EA, Recht J, Chua A, Dance D, Dhorda M. An inventory of supranational antimicrobial resistance surveillance networks involving low- and middle-income countries since 2000. J Antimicrob Chemother. 2018;73:1737–1749. doi: 10.1093/jac/dky026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.