Abstract
In mammalian embryos, proper zygotic genome activation (ZGA) underlies totipotent development. DUX-family factors participate in ZGA, and mouse Dux is required for forming cultured 2C-like cells. Remarkably, in mouse embryonic stem cells, mouse Dux is activated by the tumor suppressor p53, and Dux expression promotes differentiation into expanded-fate cell types. Long-read sequencing and assembly of the mouse Dux locus reveals its complex chromatin regulation including putative positive and negative feedback loops. We show that the p53-DUX/DUX4 regulatory axis is conserved in humans. Furthermore, we demonstrate that cells derived from facioscapulohumeral muscular dystrophy (FSHD) patients activate human DUX4 during p53 signaling via a p53 binding site within a primate-specific subtelomeric LTR10C element. In summary, our work shows p53 activation convergently evolved to couple p53 to Dux/DUX4 activation in embryonic stem cells, embryos, and FSHD patient cells, potentially uniting the developmental and disease regulation of DUX-family factors, and identifying evidence-based therapeutic opportunities for FSHD.
Fertilization and early embryogenesis involve the transition from specialized unipotent gametes to totipotent embryos. After fertilization, mammalian embryos rely on maternally deposited RNA, but subsequently initiate ZGA during which embryonic transcription begins1. Diverse mechanisms control the timing of ZGA, such as controlling RNA polymerase activity, nuclear:cytoplasmic ratio, or translation of critical ZGA transcription factors (TFs) in Caenorhabditis elegans, Drosophila melanogaster, or Danio rerio, respectively1. Currently, we have an incomplete understanding of how the transcriptional machinery (RNA polymerases) and/or sequence-specific TFs dictate the timing of ZGA in mammals and contribute to developmental potential.
Recent work identified the TF DUX (DUX4 in humans) as a key regulator of ZGA gene expression2–5. When ectopically expressed in cells, DUX and DUX4 activate many ZGA genes, including the earliest wave of ZGA genes in human and mouse2,3. However, the extent to which DUX is required for appropriate ZGA is unclear, as the reported effect of genetic loss of Dux ranges from minor molecular to major transcriptional defects and decreased development in mouse or human embryos4–6.
To study ZGA using a cellular model, we and others have utilized two-cell-embryo-like cells (2CLCs), which are an endogenously fluctuating subpopulation of mouse embryonic stem cells (mESCs) that recapitulate several key features of ZGA7. 2CLCs activate transcripts characteristic of the 2-cell mouse embryo (Dux, Zscan4, MERVL, Kdm4dl, etc.), lack chromocenters, and downregulate pluripotency markers such as OCT42,7,8. Importantly, Dux is required for 2CLC formation, and when expressed in mESCs is sufficient to activate the 2CLC state at the transcriptional and chromatin level2,3. Mouse DUX is encoded by a retrogene array of >28 copies (unassembled in mm10), and a set of repressors are known to coordinate Dux array repression8–10. However, it is currently unclear which TF activators directly activate Dux. Notably, although DPPA2/4 TFs and the pause release factor NELFA are reported to bind the Dux promoter, they lack clear DNA sequence-specific binding and are probably not gene selectivity factors11,12. As Dux transcripts are not maternally inherited and as Dux is activated in early ZGA2, we hypothesized that a maternally deposited (and previously unidentified) TF activates mouse Dux, and serves as the upstream trigger for ZGA and the emergence of 2CLCs in mESC cultures.
Likewise, the activator for the human ortholog, DUX4, remains unknown – either during ZGA or in pathogenic contexts. DUX4 reactivation from the 4qA permissive haplotype containing a poly-adenylation signal causes the human disease FSHD13, characterized by a progressive degeneration of affected muscle groups14. Normally, the DUX4 locus exists as ~11–100 tandem repeats of the D4Z4 repeat unit, but in FSHD1 patients, D4Z4 contraction to <8 repeat units relieves epigenetic silencing of the DUX4 locus and allows for stochastic DUX4 activation14,15. FSHD2 is caused by loss-of-function mutations in the locus encoding heterochromatin protein SMCHD1 (MommeD1 gene in mouse16), and confers DUX4 activation of the wildtype (WT) D4Z4 repeat locus17. DUX4 activation causes PKR- and MYC-dependent cell death in cultured FSHD myoblasts18, and the DUX4 locus is normally silenced by several heterochromatin proteins (SMCHD1, CHD4, etc.)17,19. However, as with mouse Dux locus, it is unclear what transcriptional activator(s) regulate the human DUX4 locus and whether regulation of DUX4 during embryonic genome activation (EGA) is similar to the FSHD disease state.
Here, we use the 2CLC system to identify p53 as a key driver of Dux expression. First, we reveal that Dux activation in mESCs requires the DNA damage response (DDR) pathway20. In contrast to a recent report20, we demonstrate that p53 is required for DNA-damage-mediated DUX induction and 2CLC emergence. Critically, there are multiple sources of endogenous DNA damage present in the early embryo21–24, and we find p53 activated soon after fertilization. Although not strictly required, p53 is important for full/proper DUX activation and DUX target expression during ZGA. By sequencing and assembling the mouse Dux locus, we discover an unusual “poised” chromatin signature, and regulatory features including a p53-dependent Dux promoter, a DUX positive-feedback loop, and ZSCAN4 binding to a (CA)repeat embedded in each Dux repeat unit. Transient DUX expression alters the cellular differentiation of mESCs, biasing them to an expanded fate potential. Importantly, we find the regulatory relationship between p53 and DUX4 conserved in humans, and that cells derived from FSHD patients contain inducible DUX4 alleles, are hypersensitive to DNA damage, and use a primate-specific p53-bound LTR10C element to activate the locus. Surprisingly, our data show that the mouse and human Dux/DUX4 loci likely convergently evolved p53 regulation. Previously, the signal initiating DUX4 expression in FSHD was elusive, and our findings identify a promising disease mechanism for therapeutic intervention. Together, our results uncover a regulatory role for p53 in 2CLCs and DUX4 expression in FSHD.
RESULTS
DNA damage induces Dux expression and 2CLC emergence
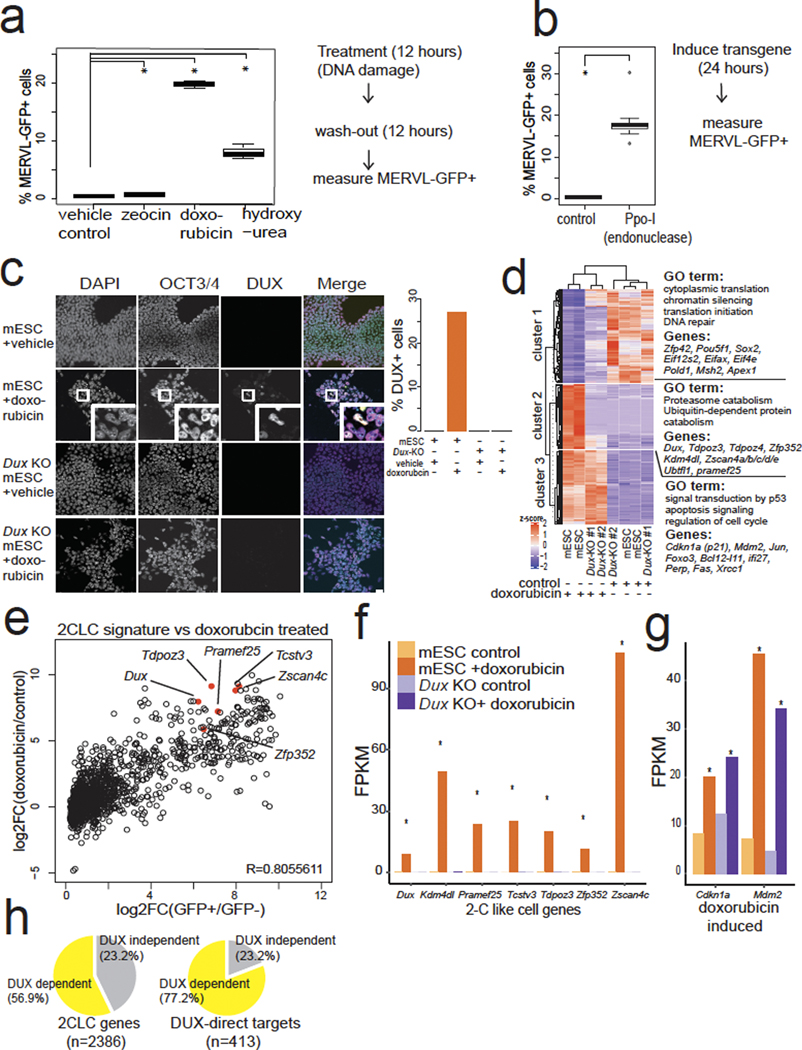
First, we searched for conditions in which downstream DUX targets were activated. DNA damaging agents can induce the expression of Zscan425, which is a direct DUX target2. Here, chemicals that induce double-stranded DNA breaks directly or indirectly (doxorubicin25–27 or hydroxyurea/aphidicolin, respectively) led to a much higher fraction of MERVL-GFP+ (a reporter for DUX activity2) cells in mESC cultures (Fig. 1a, Extended Data Fig. 1a). Additionally, expression of the PpoI endonuclease (which makes double-stranded DNA breaks26) induced the MERVL-GFP reporter (Fig. 1b), indicating that DNA breaks induce 2C reporter activation. To test whether DUX itself was induced by DNA damage upstream of Zscan4 and MERVL activation, we raised a highly specific antibody to mouse DUX (Extended Data Fig. 1b,c,d,e), which stained mESC nuclei treated with doxorubicin, but not Dux knock-out (KO) mESCs (Fig. 1c), showing DUX protein is induced by doxorubicin.
Fig. 1: DNA damage induces a 2CLC signature in mESCs, and requires Dux.
a, Small molecules that cause DNA damage induce MERVL-GFP. mESCs treated with indicated DNA-damage-inducing agents, MERVL-GFP+ cells were quantified by flow cytometry. ** FDR <0.05, One-way ANOVA, one-sided t-test, n = 3 biological replicates. b, Direct formation of DNA double-stranded breaks induce the MERVL-GFP reporter. mESCs expressing Ppo-I endonuclease or control, MERVL-GFP+ cells were quantified 24 hours later. * P <0.05, one-sided t-test, P = 4.361 × 10−6, n = 8 biological replicates. c, Immunostaining of control or Dux KO mESCs treated with vehicle control or doxorubicin 6 hours, washed out for 6 hours. Merge: DAPI = cyan, OCT4 = magenta, DUX = yellow. Quantification on right, representative image shown of 3 fields of view. Representative example from 5 independent experiments. Scale bar = 25 μm. DAPI = 4′,6-diamidino-2-phenylindole. d, Hierarchical clustering of RNA-seq from control or Dux KO mESCs treated with vehicle (control) or doxorubicin for 6 hours, then 12 hours of washout recovery, with DESeq2 FDR <0.01. N = 2 biological replicates for each genotype and condition. e, Doxorubicin treatment in mESCs induces 2CLC transcriptional signature. Endogenously fluctuating 2CLC genes2 (x-axis) were compared to the effect of doxorubicin vs. control (y-axis). N = two biological replicates. 6 hours doxorubicin, 12 hours washout. f, Key 2CLC genes require Dux for their induction after doxorubicin treatment. RNA-seq: N = 2 biological replicates. * FDR < 0.05 DESEeq2. 6 hours doxorubicin, 12 hours washout. FPKM = fragments per kilobase/million reads. g, Dux KO mESCs induce DNA-damage-responsive genes. N = 2 biological replicates, * FDR < 0.05 DESEeq2. 6 hours doxorubicin, 12 hours washout. h, Most 2CLC transcripts show DUX-dependent induction after doxorubicin treatment (left) and 77.2% of direct DUX targets (those bound by DUX via ChIP-seq2 and transcriptionally induced by Dux-transgene expression) require Dux for their induction. Two biological replicates of Dux KO and WT mESCs for each treatment (vehicle vs. doxorubicin), FDR < 0.05 DESEeq2. 6 hours doxorubicin, 12 hours washout. For boxplots in Figure 1a,b, the median is shown as a line in the box, and the outline of the box is depicted at the 25th and 75th percentile. The extended whiskers depict Q1 – 1.5 × IQR and Q3 + 1.5 × IQR. Outliers points are depicted as dots.
To test whether Dux is required for the DNA damage induction of 2C genes, we performed RNA-seq with Dux KO and control mESCs treated with doxorubicin. Hierarchical clustering of the differentially expressed transcripts revealed three main classes of genes: cluster 1 contained genes downregulated after doxorubicin treatment, cluster 2 contained 2C-like genes that fail to activate in Dux KO mESCs, while cluster 3 contained classical DDR genes27 that are activated in both Dux KO and control Dux WT mESCs (Fig. 1d). Surprisingly, the 2C-like network is evidently a subset of a non-canonical DDR in mESCs. Naturally fluctuating MERVL-GFP+ 2CLCs also exhibit a similar 2C gene expression profile to doxorubicin-treated mESCs2, highlighting the similarity between the stochastic occurrence of 2CLCs and the DNA damage stimulated signature (correlation r = 0.8055, Fig. 1e). Doxorubicin treatment induced Dux RNA and 2C gene expression in Dux wildtype control mESCs, but not in Dux KO mESCs, which exhibited negligible induction of classical 2C genes such as Prame25, Zscan4c, Zfp352, Tdpoz3, Kdm4dl and Tsvtv3 (Fig. 1f). Importantly, both Dux KO and wildtype control mESCs activate classical DDR genes27 such as Cdkn1a (p21) and Mdm2 after doxorubicin treatment, indicating that DUX is not required for the canonical DDR typical of somatic cells (Fig. 1g). Most of the 2CLC gene network shows Dux-dependent activation after doxorubicin treatment; thus, DNA damage is coupled to 2CLC induction through DUX (Fig. 1h, Extended Data Fig. 1f, g).
DDR and p53 are required for stimulus-induced 2CLCs
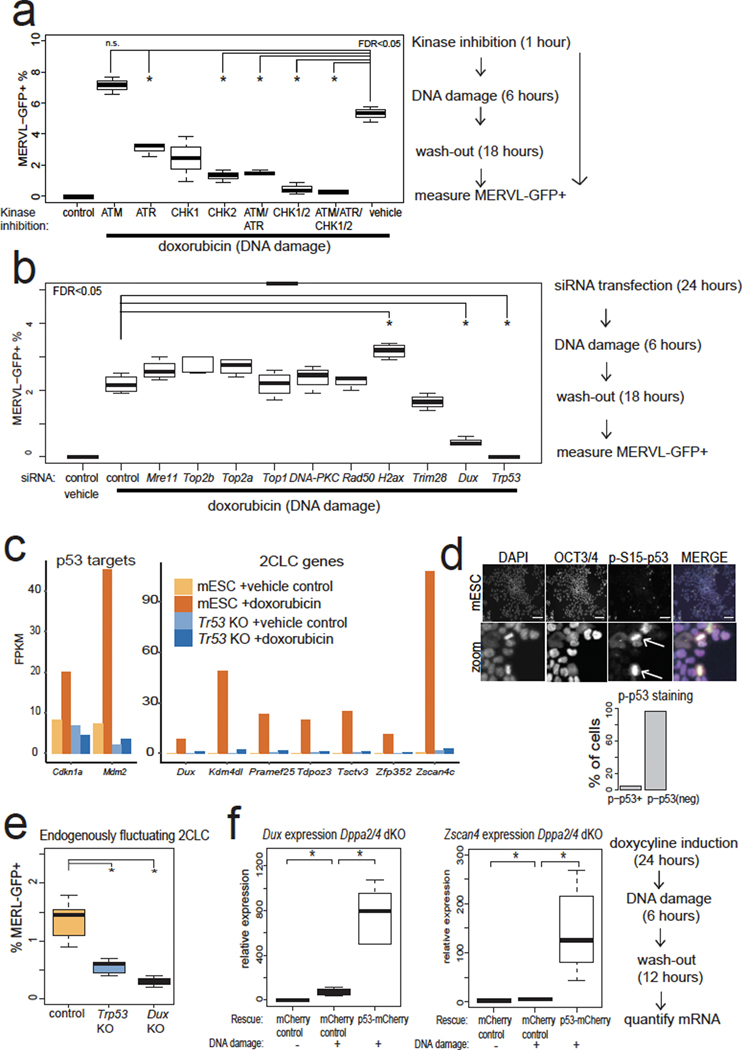
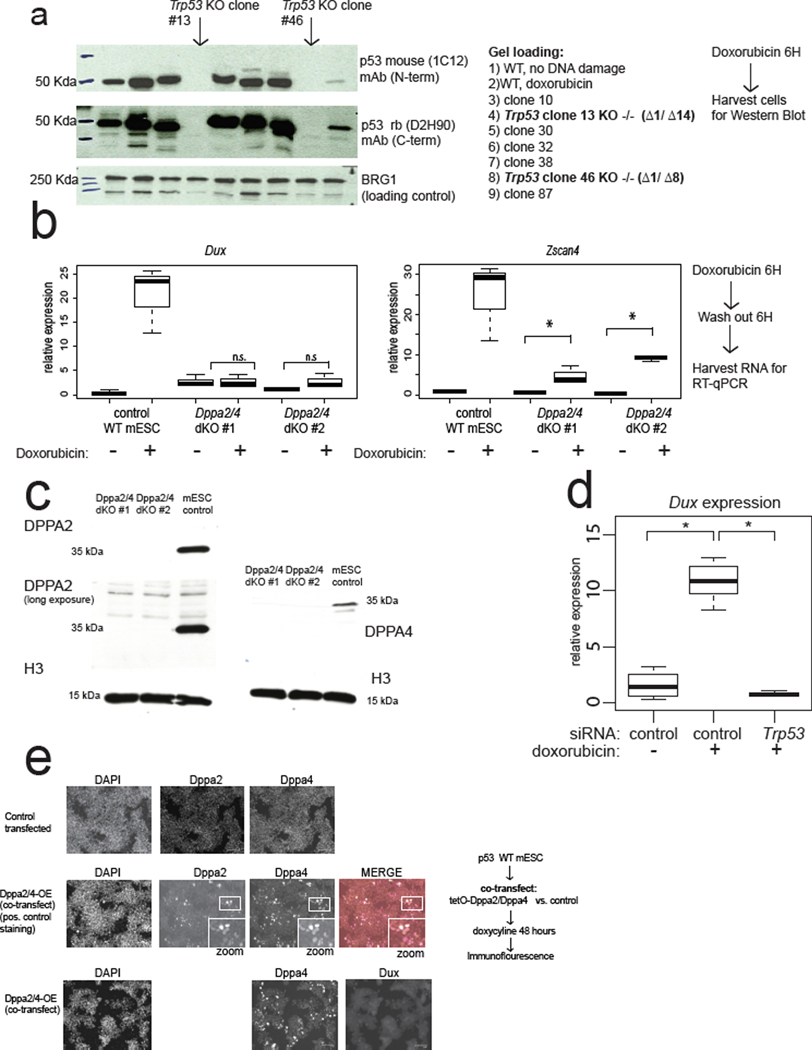
Next, we identified factors downstream of DNA damage and upstream of Dux induction. DDR involves the kinases ATR and ATM that sense DNA damage, and the kinase transducers CHK1/2 28. Treatment of cells with all four small-molecule inhibitors of ATR, ATM, CHK1, and CHK2 together nearly eliminated MERVL-GFP+ cells after doxorubicin treatment (Fig. 2a). Interestingly, siRNA-mediated depletion of p53, the main transcriptional effector of DDR, likewise led to an almost complete loss of MERVL-GFP+ mESCs (Fig. 2b). We confirmed that p53 is required for full 2C gene and Cdkn1a/Mdm2 expression induction after DNA damage through RNA-seq analysis of two independent Trp53 KO mESC clones treated with doxorubicin (Fig. 2c, Extended Data Fig. 2a). Thus, p53 is evidently required for Dux and 2C-gene expression after DNA damage (Fig. 2c).
Fig. 2: 2CLC emergence and Dux expression after doxorubicin treatment require canonical DNA-damage response (DDR) signaling and p53.
a, DDR kinase inhibition in mESCs shows that ATR, CHK1, and CHK2 activity are required for MERVL-GFP induction in mESCs after doxorubicin treatment. MERVL-GFP+ cells were quantified with flow cytometry. N = 3 biological replicates, * FDR <0.05, One-way ANOVA. b, siRNA-mediated depletion in mESCs demonstrates that Trp53 is required for MERVL-GFP induction after doxorubicin treatment. MERVL-GFP+ cells were quantified by flow cytometry. N = 3 biological replicates, * FDR < 0.05, One-way ANOVA. c, RNA-seq analysis of Trp53 KO mESCs after doxorubicin treatment shows that p53 is required for 2CLC gene induction. WT control or Trp53 KO mESC RNA-seq. N = two biological replicates. * FDR < 0.05 DESEeq2. d, phospho-S15-p53-high cells in mESCs. WT mESCs stained with indicated antibodies, showing mitotic phospho-p53+ cells, indicated by arrows. Quantification below, n = 365 cells. Merge: DAPI = cyan, OCT4 = magenta, phospho-p53 = yellow. Scale bar = 125 μm. Results are representative from 3 independent experiments. e, Quantification of endogenously fluctuating MERVL-GFP+ 2CLCs in WT, Dux KO, or Trp53 KO mESCs. N = 8 biological replicates * FDR < 0.05, One-way ANOVA. f, Dppa2/4 dKO mESCs lose high levels of Dux and Zscan4c inducibility after doxorubicin treatment, which can be rescued by p53-overexpression. RT-qPCR quantification of n = 3 biological replicates, normalized to actin RNA. * FDR < 0.05, One-way ANOVA. For boxplots in Figure 2a,b,e,f: the median is shown as a line in the box, and the outline of the box is depicted at the 25th and 75th percentile. Extended whiskers depict Q1 – 1.5 × IQR and Q3 + 1.5 × IQR. Outliers points depicted as dots.
Loss of p53 decreases endogenously fluctuating 2CLCs
We next tested whether endogenously fluctuating 2CLCs require p53 activation for their generation. Staining of mESCs indicates that ~4% are phospho-p53+ (Fig. 2d) and mitotic cells with γH2AX+ chromosomes or phospho-p53+ are often observed, consistent with G2/M enrichment of 2CLCs29 (Fig. 2d). The loss of Dux or Trp53 decreased the proportion of MERVL-GFP+ cells, indicating that DUX and p53 are required for the normal fluctuation of mESCs into the 2CLC state (Fig. 2e). This suggests that DNA-damage-induced 2CLCs and endogenously fluctuating 2CLCs share a similar mechanistic trigger.
p53 expression rescues Dux activation in Dppa2/4 dKO mESCs
We next determined the epistatic relationship between p53 and other genes that influence 2CLC emergence and encode putative Dux activators, such as Dppa2/4. We confirmed that double knockout (dKO) of Dppa2/4 leads to the loss of DNA-damage-dependent Dux and Zscan4 induction (Extended Data Fig. 2b, c). However, over-expression of p53 strongly reactivated Dux and Zscan4 after doxorubicin treatment in Dppa2/4 dKO mESCs, indicating that p53 can rescue the loss of Dppa2/4 and is likely downstream of Dppa2/4 (Fig. 2f). In contrast, over-expression of DPPA2/4 was not sufficient to reactivate Dux, even in Trp53 WT mESCs (Extended Data Fig. 2e). These observations favor a model in which Dppa2/4 helps preserve the inducibility of the Dux locus11,30, but Dppa2/4 loss can be bypassed through over-expression of an activator of Dux such as p53.
Dux locus assembly reveals novel regulatory features
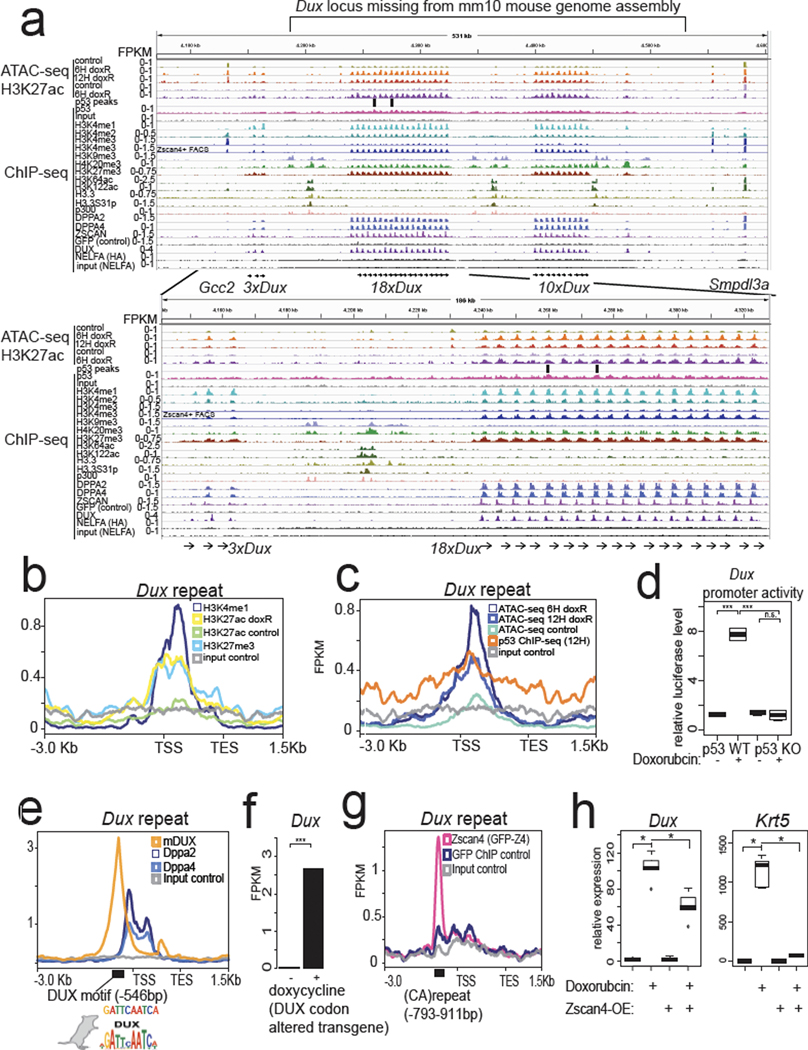
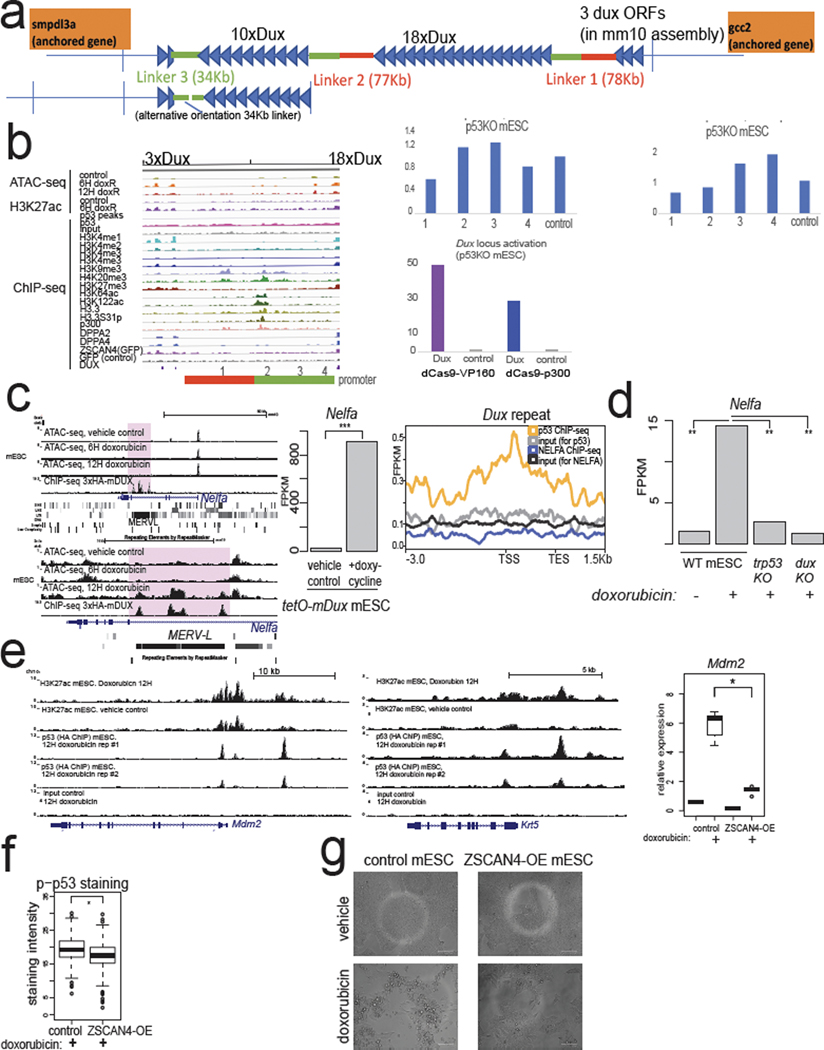
To understand the regulatory landscape of the mouse Dux locus, which is currently in an assembly gap in the mm10 mouse genome build, we performed long-read sequencing (via PacBio) and assembled the locus (Extended Data Fig. 3a). Consistent with prior Southern blotting31, the mouse ~350-kb Dux locus exists as two tandem repeats of 18 or 10 units (which we call 18×Dux or 10×Dux respectively) separated by ~77-kb “linker” sequences (Extended Data Fig. 3a).
We next remapped epigenomic datasets to understand the chromatin regulation of the Dux locus. In mESCs, we detected an unusual chromatin signature at the repeated Dux gene promoters: high H3K4me1/H3K27me3 and low levels of H3K4me3 (Fig. 3a). After doxorubicin treatment, the Dux arrays gain H3K27ac, open chromatin, and are marked by H3K4me3 in Zscan4-reporter+ mESCs (Fig. 3a,b,c, Extended Data Fig. 1f). This signature is similar to “poised” enhancers and is characterized by H3K4me1/H3K27me3 until activation, when these poised enhancers gain H3K27ac32. We also detect broad p53 ChIP-seq signal across the Dux arrays and MACS2 peak-calling identifies p53 ChIP-seq peaks at the promoters of the Dux repeat array (Fig. 3a, 3c), although we cannot rule out an additional indirect effect of p53 on the Dux locus. In reporter assays, a 1.3-kb fragment of the Dux promoter has strong doxorubicin-stimulated activity, and this effect is only seen in Trp53 WT mESCs (Fig. 3d). CRISPRa targeting to the Dux promoter, but not four other regions surrounding the locus, strongly induced Dux expression in Trp53 KO mESCs (Extended Data Fig. 3b). Although we cannot rule out additional distal regulatory elements for the Dux locus, targeting a strong activator to the Dux promoter is sufficient to activate expression and bypass loss of p53. This indicates that de-repression is not required per se for Dux activation in mESCs and underscores the potent inducibility of the locus consistent with its “poised” chromatin status.
Fig. 3: Assembly of the mouse Dux locus reveals chromatin and transcriptional regulatory features.
a, Genome browser snapshots of the newly assembled PacBio-sequenced mouse Dux locus with indicated epigenomic datasets, zoom-in on 18×Dux array below (see methods for all GEO accessions). ATAC-seq = Active Transposition into Active Chromatin, ChIP-seq = chromatin immunoprecipitation followed by sequencing. b, Metagene plot of Dux repeat unit with indicated histone ChIP-seq datasets, TSS = transcription start site, TES = transcription end site. c, Metagene plot of Dux repeat unit with ATAC-seq or p53-ChIP-seq datasets. d, Luciferase assay testing a 1.3-kb fragment of the Dux promoter in WT or Trp53 KO mESCs, with vehicle treatment or doxorubicin treatment. Normalized to co-transfected Renilla luciferase. N = 4 biological replicates, *** FDR < 0.001, One-way ANOVA. e, Metagene plot of the Dux repeat showing enrichment of DPPA2/4 or DUX with the DUX TF motif shown below (Hendrickson et al. Nature Genetics, 2017). f, Doxycycline induction of codon-altered Dux transgene activates the endogenous Dux locus, RNA-seq data reprocessed from Hendrickson et al. Nature Genetics, 2017. N = 2 biological replicates, FDR < 0.01, DESeq2. g, Metagene plot of the Dux array showing enrichment of ZSCAN4 ChIP-seq signal at the (CA)repeat unit, data reprocessed from Srinivasan et al. Science Advances, 2020. h, Clonal ZSCAN4-overexpression mESCs shows lower Dux and Krt5 expression after doxorubicin treatment compared to control. N = 5 biological replicates, * FDR < 0.05, One-way ANOVA. For boxplots in Figure 3d and h, the median is shown as a line in the box, and the outline of the box is depicted at the 25th and 75th percentile. The extended whiskers depict Q1 – 1.5 × IQR and Q3 + 1.5 × IQR. Outliers points are depicted as dots.
Next, we analyzed other TFs and DNA binding proteins implicated in the 2CLC state. We detected strong enrichment of DPPA2/4 binding near the transcriptional start site (TSS) of the Dux repeats, consistent with several recent reports11 (Fig. 3a,e). Surprisingly, we also detected strong enrichment of DUX protein binding to the Dux gene through a perfect match to the DUX motif ~500 bp upstream of the TSS (Fig. 3e). A codon-altered Dux transgene activates the endogenous Dux locus ~100-fold (Fig. 3f) identifying a possible positive feedback loop.
Additionally, NELFA was previously reported to drive the 2CLC state by directly activating Dux12, but our reanalysis of published ChIP-seq shows low enrichment of NELFA at the Dux locus (Fig. 3a, Extended Data Fig. 3c). Instead, we find that Nelfa is a direct target of DUX through a tandem MERVL inserted near the 3’ end of the Nelfa gene (Extended Data Fig. 3c). Importantly, Dux transgene expression activates Nelfa ~900-fold, Nelfa transcription is induced by doxorubicin, and this effect requires p53 and DUX (Extended Data Fig. 3c,d). Taken together, our findings indicate NEFLA expression is a marker rather than a driver of the 2CLC state, but Nelfa knockout mESCs or maternal-zygotic (MZ)-KO Nelfa embryos will help clarify NELFA’s role in 2CLCs or ZGA embryos.
Additionally, we detect strong ZSCAN4 protein binding to a (CA)repeat ~800 bp upstream of the TSS (Fig. 3a,g). Since ZSCAN4 is one of the strongest direct targets of DUX2, it is possible that ZSCAN4 transcriptionally regulates the Dux locus, thus providing a feedback loop affecting Dux expression. In clonal ZSCAN4 over-expression (OE) mESCs, Dux and other direct p53 targets such as Mdm2 and Krt5 were significantly downregulated after doxorubicin treatment compared to control doxorubicin-treated mESCs (Fig. 3h, Extended Data Fig. 3e). Although we cannot exclude a direct transcriptional repression effect or a competitive-inhibition effect of ZSCAN4 on Dux expression, we propose that ZSCAN4 OE prevents DNA damage and/or decreases p53 signaling—consistent with its function in protecting 2-cell-stage mouse embryos from transcription-induced DNA damage24 (Extended Data Fig. 3f). Consistent with this, ZSCAN4-OE mESCs exhibited decreased phospho-p53 levels, lower p53-target gene induction, and less cell death after doxorubicin treatment (Fig. 3h, Extended Data Fig. 3e,f,g).
p53 MZ-KO embryos exhibit lower DUX-target gene activation
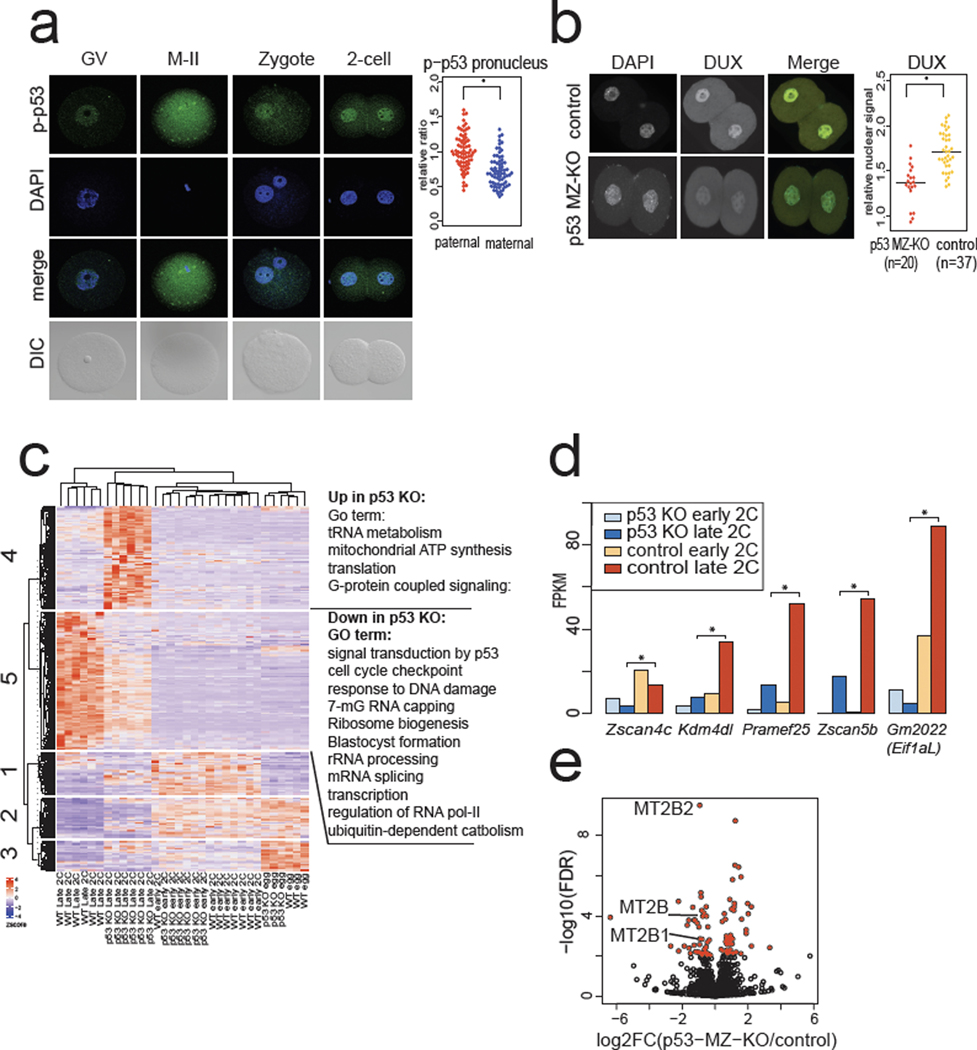
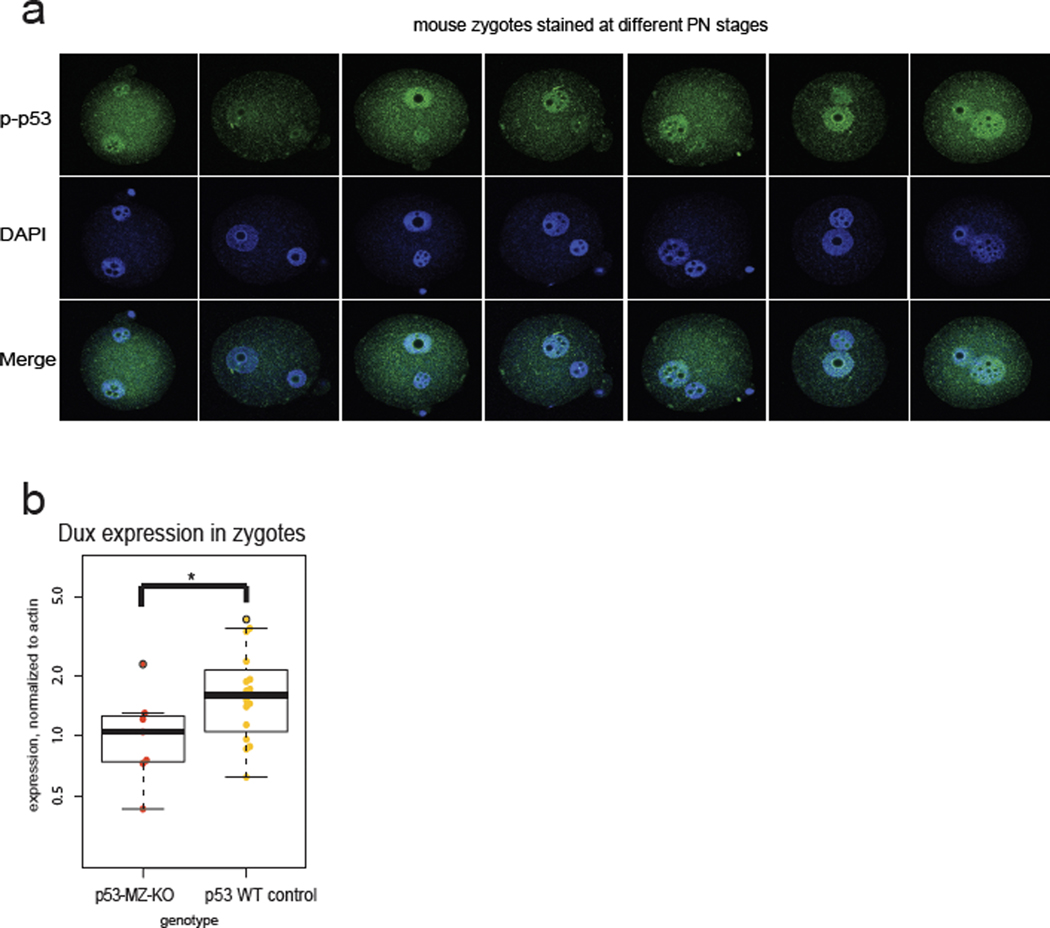
Next, we asked if maternally deposited p53 impacts ZGA in vivo. Although some MZ-KO p53 embryos are viable33, there is a neural tube defect resulting in female-specific midgestation loss of p53 KO embryos34,35, and it is unclear whether loss of maternal p53 confers defects at earlier stages. Notably, we found enriched phospho-p53 in the paternal pronucleus of zygotes and early 2-cell stage mouse embryos (Fig. 4a, Extended Data Fig. 4a), consistent with known high γH2AX in mouse zygotes21,23. p53 MZ-KO embryos had fewer Dux transcripts at the PN5 zygote stage and lower DUX protein expression at the early 2-cell stage, although p53 MZ-KO embryos still stained with the DUX antibody, indicating a potential compensatory or partly redundant mechanism for Dux activation (Fig. 4b, Extended Data Fig. 4b).
Fig. 4: p53 accumulates in mouse zygotes after fertilization and Trp53 MZ-KO embryos have lower DUX and DUX-target expression.
a, Immunostaining of mouse oocytes, eggs, and embryos using a phospho-S15 (S18 in mouse) p53 antibody. Quantification of significantly higher phospho-p53 signal in the paternal pronucleus (PN) is shown on right, n = 69 zygotes, * P < 0.05 one-sided t-test, P = 2.9 × 10−10. Scale bar = 40 μm. GV = germinal vesicle stage oocyte, M-II = meiosis II egg, DIC = differential interference contrast. Data combined from 3 independent experiments. Scale bar = 40 μm. b, Immunostaining of Trp53 (maternal zygotic) MZ-KO early 2-cell stage embryos with anti-DUX antibody. Merge: DAPI = cyan, DUX = yellow. Quantification is shown on right, * P < 0.05 one-sided t-test, P = 5.543 × 10−7. Scale bar = 40 μm. c, RNA-seq analysis of Trp53 MZ-KO eggs and embryos compared to WT controls. Hierarchical clustering of DESeq2 FDR < 0.01 transcripts. N = 6 embryos, n = 3 eggs per genotype per stage. d, RNA-seq from Trp53 MZ-KO embryos versus WT control embryos, Key ZGA genes shown, * FDR < 0.05 DESeq2. N = 6 embryos per genotype per stage. e, Repetitive element expression, RNA-seq from Trp53 MZ-KO embryos versus WT control embryos at late 2-cell stage, red indicates FDR < 0.05 TEtranscipts. N = 6 biological replicates per genotype.
We then tested whether p53 MZ-KO embryos exhibited normal ZGA gene expression. Hierarchical clustering revealed a class of transcripts significantly down-regulated in p53 MZ-KO embryos (n = 1,056 decreased out of n = 1,971 differentially expressed ZGA-specific genes) characterized by GO terms such as p53 signaling, cell cycle checkpoints, and transcription (Fig. 4c). Notably, p53 MZ-KO embryos have significantly lower expression of direct DUX targets Zscan4c, Kdm4dl, Eif1a-Like retrogenes, and MERVL2 (Fig. 4d, 4e). Thus, p53 loss correlates with transcriptional defects of DUX targets alongside indirect maternal mRNA clearance defects (Fig. 4c), consistent with the effects of siRNA-mediated DUX4 depletion in human embryos5.
DUX biases mESCs towards extraembryonic differentiation
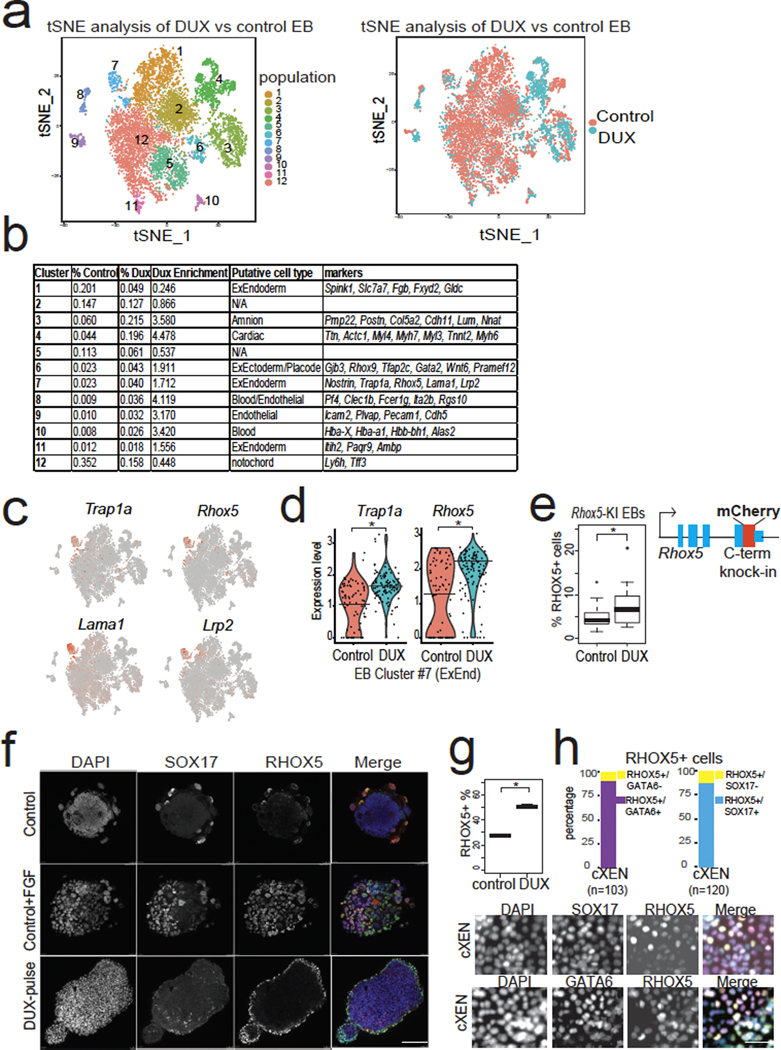
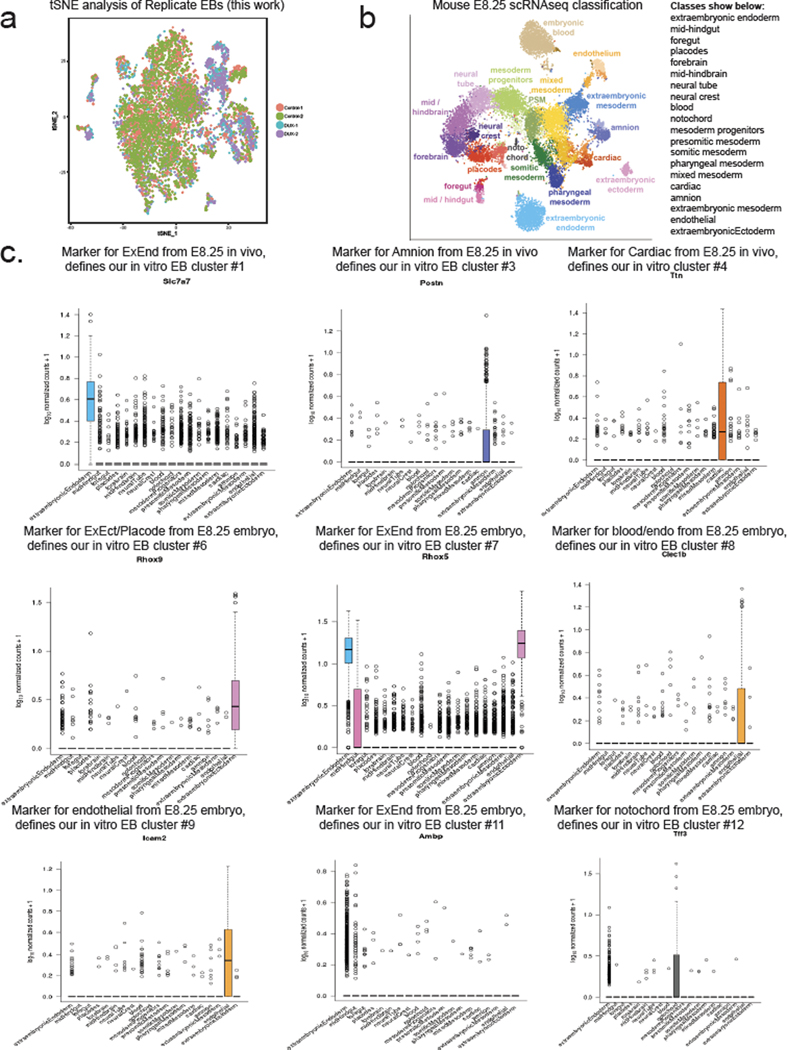
We next tested the developmental outcome of 2CLCs that transiently express DUX. Notably, 2CLCs contribute to both embryonic (post-implantation epiblast-derived) and extraembryonic tissues (primitive endoderm and trophectoderm-derived) in chimeras7, which is often referred to as “expanded potential”, and single-cell injection of 2CLCs into embryos demonstrates their totipotency36. To characterize the downstream cellular fates of DUX-expressing mESCs, we pulsed a low dose of DUX in mESCs cultured in 2iLIF medium, and then withdrew 2iLIF and formed embryoid bodies (EBs), which allows cells to exit pluripotency and differentiate in a non-directed fashion, enabling evaluation of intrinsic developmental potential in an unbiased way37,38. Single-cell RNA-seq (scRNA-seq) of day 9 EBs formed from DUX-pulsed mESCs and control mESCs revealed multiple populations of cells representing endoderm, ectoderm, and mesoderm, with high concordance between biological replicates (Fig. 5a, Extended Data Fig. 5a,b).
Fig. 5: Transient DUX-expression in mESCs confers increased expanded fate potential.
a, scRNA-seq identifies cell populations (clusters #1–12) from differentiated embryoid bodies (EBs) top, and scRNA-seq colored by treatment (bottom). N = 2 biological replicates per condition. tSNE = t-distributed stochastic neighbor embedding. b, Table of scRNA-seq clusters, with putative cell identity using E8.25 mouse embryo scRNA-seq34. N = 2 biological replicates per condition. c, Marker expression for cluster #7 showing canonical extraembryonic endoderm genes (ExEnd). d, Quantification of expression of Trap1a and Rhox5 (ExEnd) markers in cluster #7, showing higher levels in DUX-pulsed cells. * FDR < 0.05, Seurat. e, Quantification of % RHOX5+ cells in day 8 EBs from Rhox5-mCherry knock-in mESC clone. N = 15 biological replicates per condition, * P < 0.05, P = 0.04517, one-sided t-test. f, Immunofluorescence of EBs stained with anti-SOX17 and anti-RHOX5 antibodies. SOX17+/RHOX5+ cells can be induced in WT control EBs treated with FGF. DUX-pulsed EBs show RHOX5+ cells on the outside layer. RHOX5+ outgrowth indicated with white arrow. Representative image from n = 10 imaged EBs from each experimental condition. Merge: DAPI = cyan, RHOX5 = green, SOX17 = red. Scale bar = 150 μm. g, Extraembryonic endoderm cell (XEN) differentiation of DUX-pulsed or control mESCs, and quantification of RHOX5-mCherry-knock-in (KI) cells using flow cytometry. * P < 0.05, P = 0.001301, one-sided t-test, n = 3 biological replicates per condition. h, XEN differentiation of DUX-pulsed mESCs using Rhox5-mCherry KI clone stained with antibodies against the ExEnd marker GATA6 (n = 103 cell) or the endoderm marker SOX17 (n = 120 cells). Immunofluorescent images on bottom, with quantification of RHOX5+ cells on top. Merge: DAPI = cyan, GATA6 or SOX17 = magenta, RHOX5-mCherry = yellow. Scale bar = 50 μm. For boxplots in Figure 5e and h, the median is shown as a line in the box, and the outline of the box is depicted at the 25th and 75th percentile. The extended whiskers depict Q1 – 1.5 × IQR and Q3 + 1.5 × IQR. Outliers points are depicted as dots.
We then identified cell types that were generated preferentially in DUX-pulsed EBs, by quantifying the proportion of cells in each cluster (Fig. 5a,b). Interestingly, cells from DUX-pulsed EBs were enriched in clusters expressing markers from E8.25 mouse embryos39: amnion (cluster #3), cardiac, blood, and endothelial (clusters #4, #8, and #9 respectively) and non-post-implantation epiblast derivatives: extraembryonic ectoderm (cluster #6) and extraembryonic endoderm (cluster #7)(Fig. 5b, Extended Data Fig. 5b, 5c). We further characterized cluster #7, which expressed higher levels of classical extraembryonic endoderm (ExEnd) genes such as Trap1a, Rhox5—distinguishing these cells from embryonic endoderm40 (Fig. 5c, 5d). We confirmed the scRNA-seq data with flow cytometry from EBs using a Rhox5-mCherry knock-in mESC clone (Fig. 5e). We note that p53/p73 activation occurs upon LIF withdrawal during differentiation41, which may contribute to the low percentage of RHOX5+ cells in control non-DUX pulsed EBs. Using co-staining with the pan-endoderm marker SOX17, we detected RHOX5+/SOX17+ ExEnd cells at low frequencies on the surface of EBs – but found the frequency of RHOX5+/SOX17+ cells increased by FGF treatment, which promotes ExEnd differentiation in the inner cell mass (ICM) in vivo42 (Fig. 5f). Strikingly, we found DUX-pulsed EBs were covered in an outer layer of RHOX5+ cells and RHOX5+ outgrowths (Fig. 5f), even in the absence of exogenous FGF.
Under normal conditions, mESCs cultured in 2iLIF rarely generate ExEnd, but mESCs treated with retinoic acid and activin can generate “converted extraembryonic endoderm stem cells” (cXEN)43. In cXEN differentiation, we found that DUX-pulsed mESCs generated a higher percentage of RHOX5-mCherry+ cells, most of which are SOX17+ and GATA6+, markers for endoderm and ExEnd respectively (Fig. 5g,h). In summary, we use two different assays (EB formation or cXEN differentiation) to demonstrate that DUX-pulsed mESCs exhibit “expanded fate” potential, and we speculate that this capacity might impact cell fate after ZGA (see Discussion).
FSHD iPSCs preferentially activate DUX4 via p53 signaling
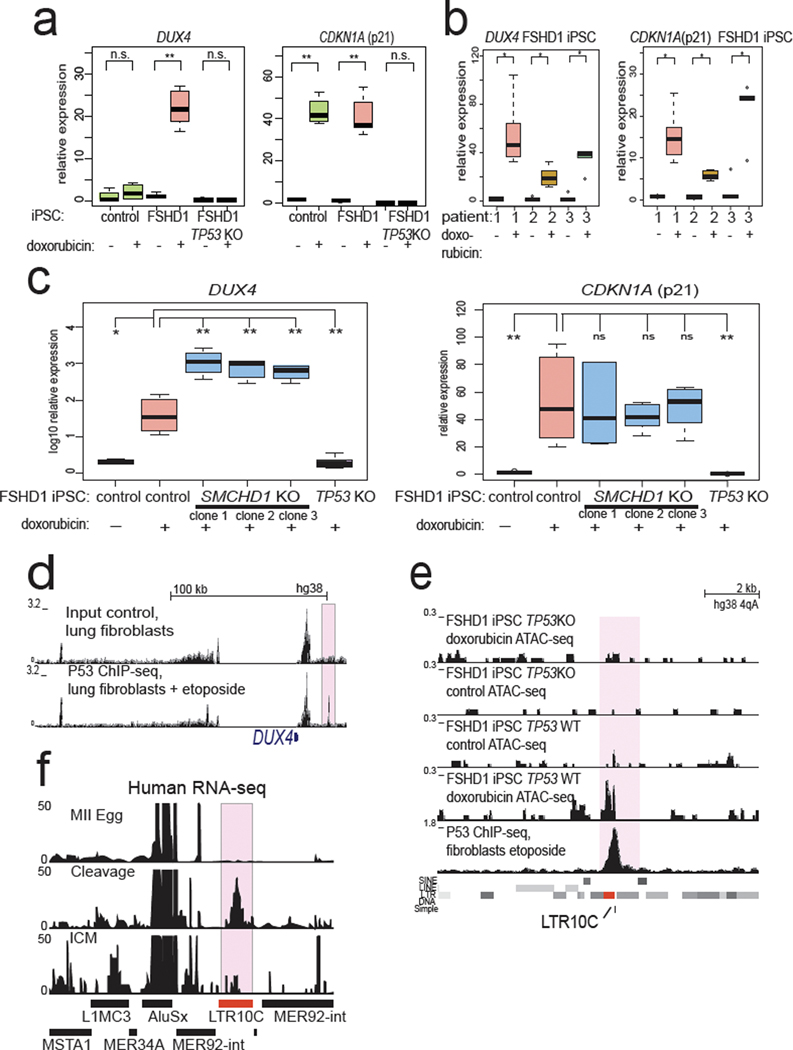
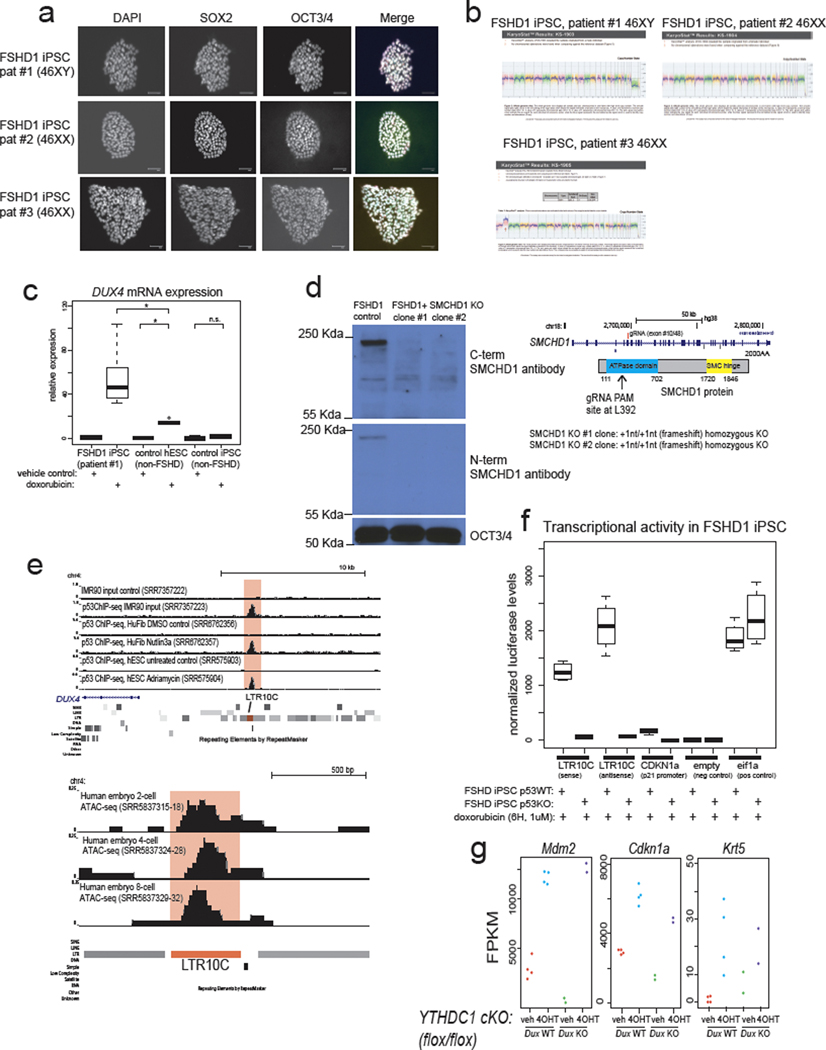
Next, we tested whether the regulatory relationships we found between p53 and mouse Dux were preserved at the human DUX4 locus. Importantly, the mouse Dux and human DUX4 loci are not in syntenic positions and are thought to have been generated through independent retrogene formation31,44, raising the possibility their regulation has also evolved independently. Because D4Z4 contractions of the 4qA permissive haplotype cause FSHD115, we derived independent induced pluripotent stem cell (iPSC) lines from three unrelated FSHD1 patients to model the unusual repeat-contraction involvement in this disease (Extended Data Fig. 6a,b). Surprisingly, doxorubicin treatment elicited DUX4 transcriptional induction in FSHD1 iPSCs, but not in control non-FSHD iPSCs and less potently in an independent non-FSHD human embryonic stem cell (hESC) line (Fig. 6a, Extended Data Fig. 6c) and this induction required p53 (Fig. 6a,b). Furthermore, isogenic FSHD1 male iPSC line #1 in which we knocked out SMCHD1 (which encodes a chromatin protein required for efficient silencing of the DUX4 locus17) in three independent clones, were likewise hyper-responsive to doxorubicin treatment compared to the isogenic SMCHD1 WT parental FSHD1 iPSC line (note log10 scale; Fig. 6c, Extended Data Fig. 6d). These data indicate that loss of epigenetic silencing, through DUX4 repeat contraction or combined SMCHD1 loss potentiates the inducibility of the locus by p53 signaling. Taken together, these data suggest that the human DUX4 locus, like the mouse Dux locus, is induced by DNA damage through p53 and epigenetic derepression of the DUX4 locus to create a hyper-inducible state.
Fig. 6: p53 is required for DUX4 activation after DNA damage in FSHD cells.
a, In FSHD1 iPSCs, DUX4 is hypersensitive to doxorubicin treatment and this effect requires p53. Non-FSHD control iPSCs (male WT33), FSHD1 iPSCs (male patient #1), or isogenic FSHD1 cells with TP53 KO from FSHD1 patient #1 were treated with doxorubicin or vehicle control, DUX4 expression and CDKN1A (p21) expression was quantified with RT-qPCR, normalized to 18S rRNA. N = 5 biological replicates * FDR < 0.05, one-sided t-test. b, Additional FSHD1 iPSC lines also show DUX4 induction after doxorubicin treatment. Line 1 is an additional clone of patient #1 (shown in Fig. 5a) and patients #2 and #3 are from non-related female FSHD1 patients, normalized to 18S rRNA. Line 1 = 6 biological replicates, Lines #2 and #3 = 5 biological replicates. * FDR < 0.05, one-sided t-test. c, Loss of SMCHD1 in male FSHD1 iPSCs leads to non-additive activation of DUX4 expression. FSHD1 iPSC patient #1 line, or isogenic FSHD1+SMCDH1 KO iPSCs from the same FSHD1 patient #1 (3 independent SMCHD1 KO clones), or TP53 KO FSHD iPSCs from the same FSHD1 patient #1 were treated with doxorubicin or vehicle control, and DUX4 or CDKN1a(p21) expression was quantified with RT-qPCR, normalized to 18S rRNA. N = 6 biological replicates * P < 0.05, one-sided t-test. d, A strong, subtelomeric p53 binding site 18 kb from the DUX4 locus. Public data from human lung fibroblasts treated with etoposide were used for p53 ChIP-seq39. e, Comparison of the subtelomeric p53 binding site that overlaps an LTR10C element shows that it gains open chromatin in a doxorubicin treatment- and p53-dependent manner in FSHD1 patient #1 iPSCs (top four tracks of ATAC-seq). N = 2 biological replicates per condition per genotype. f, RNA-seq coverage from human MII-stage eggs, cleavage-stage embryos, or isolated ICM show that LTR10C generates RNA transcripts. Data reanalyzed from Hendrickson et al. Nature Genetics 2017. For Figure 6a, b, c, the median is shown as a line in the box, and the outline of the box is depicted at the 25th and 75th percentile. The extended whiskers depict Q1 – 1.5 × IQR and Q3 + 1.5 × IQR. Outliers points are depicted as dots.
An LTR10C element is required for full p53-responsiveness of DUX4
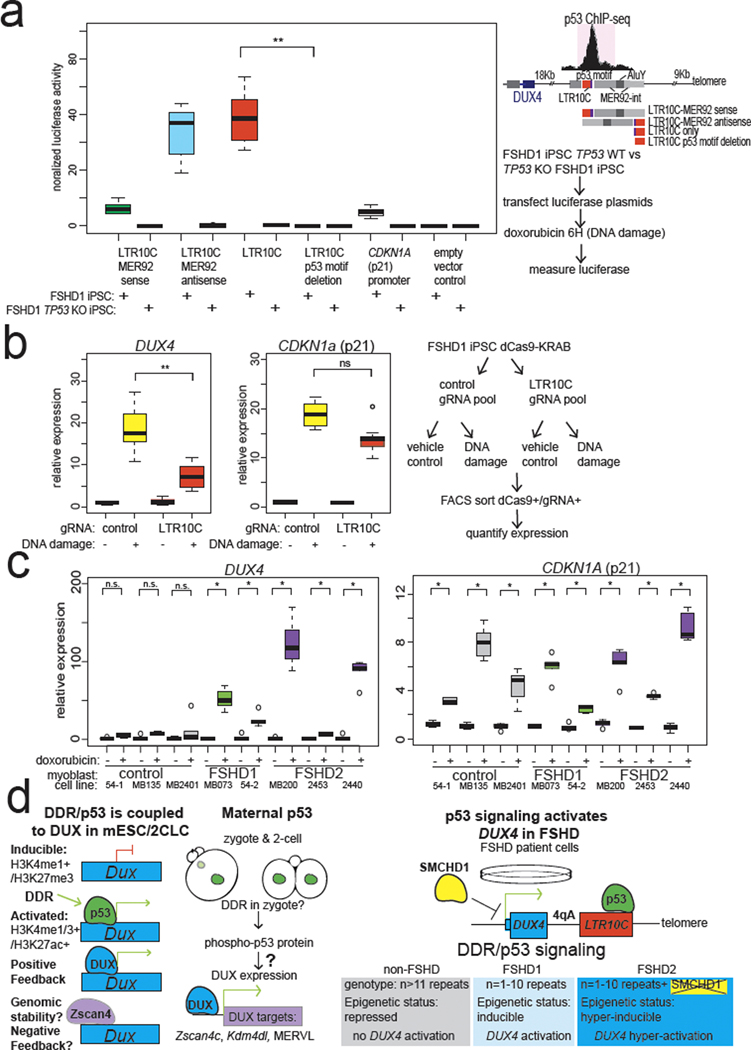
To test whether the DUX4 locus is directly regulated by p53 we searched for nearby p53 binding sites. By reanalyzing published data from etoposide-treated human lung fibroblasts45, nutlin3-treated human foreskin fibroblasts46, or doxorubicin-treated hESCs47, we found a strong p53 binding site between the DUX4 locus and the 4qA telomere45 (Fig. 6d, Extended Data Fig. 6e). This p53 binding site also gains open chromatin in FSHD1 iPSCs after doxorubicin treatment in a p53-dependent manner (Fig. 6e). Remarkably, the p53 binding site resides within an LTR10C element, which is a primate-specific long terminal repeat (LTR) known to be p53 responsive (Fig. 6e)48. Notably, LTR10C generates RNA transcripts at higher levels in human cleavage-stage embryos compared to eggs or blastocysts (Fig. 6f), and shows open chromatin signal in ATAC-seq datasets from human cleavage-stage embryos (Extended Data Fig. 6e), suggesting contemporaneous activation of LTR10c and DUX4 at EGA. To test whether the LTR10C distal to DUX4 is p53 responsive, we cloned a 3.1-kb fragment that includes LTR10C to test its activity in luciferase reporter assays. After doxorubicin treatment, the LTR10C directed high levels of expression, comparable to the relatively strong p53 binding site near CDKN1A (p21) (Fig. 7a, Extended Data Fig. 6f)27. Importantly, LTR10C element activity required p53, as TP53 KO eliminated activity (Fig. 7a). We further mapped the minimal region within the 3.1-kb fragment to the ~500-bp LTR10C itself, which contains the p53 motif (Fig. 7a, Extended Data Fig. 6f)49.
Fig. 7: The subtelomeric p53-binding site at the LTR10C is required for the full activation of the DUX4 locus and myoblasts from FSHD patients are similarly hypersensitive to DNA damage.
a, Luciferase reporter assay on deletion analysis of the ~3-kb LTR10C-mer92 fragment near the DUX4 locus identifies the LTR10C sequence as sufficient for doxorubicin treatment responsive activity in a p53-dependent manner, and deletion of the p53 binding site (motif) within LTR10C abolishes the activity of this element. N = 8 biological replicates of control FSHD1 patient #1 iPSCs or isogenic TP53 KO FSHD1 iPSCs both from FSHD patient #1, * FDR < 0.05, one-sided t-test. Values normalized to co-transfected Renilla luciferase plasmid. b, CRISPRi analysis using dCas9-KRAB-MECP2-mediated repression of the LTR10C in FSHD1 patient #1 iPSCs indicates that it is required for full activation of DUX4 expression. RT-qPCR quantification of indicated expression after doxorubicin- or vehicle-treated in FSHD iPSCs, normalized to 18S rRNA. N = 9 biological replicates, ** P < 0.005, DUX4 expression P = 4.958 × 10−5, p21 expression P = 0.7343. one-sided t-test. c, FSHD1 and FSHD2 myoblasts are hypersensitive to doxorubicin treatment compared to non-FSHD control myoblasts. RT-qPCR analysis of non-FSHD control (54–1, 135, 2401), FSHD1 (073, 54–2), or FSHD2 (MB200, 2453, 2440) myoblasts with doxorubicin or vehicle treatment, normalized to 18S rRNA. N = 5 biological replicates * FDR < 0.02, one-sided t-test. d, Model figure integrating p53 activation of Dux/DUX4 in mouse embryos, 2CLCs and FSHD patient cells. While control cell lines with non-contracted D4Z4 arrays are refractory to activation by p53, D4Z4 contractions in FSHD1 patients confer p53 inducibility on the DUX4 alleles in FSHD1 cells, and loss-of-function mutations in SMCHD1 confer p53-hyper-inducibility on DUX4 alleles in FSHD1 cells. For Figure 7a, b, c, the median is shown as a line in the box, and the outline of the box is depicted at the 25th and 75th percentile. The extended whiskers depict Q1 – 1.5 × IQR and Q3 + 1.5 × IQR. Outliers points are depicted as dots.
To test whether the p53 binding site within the LTR10C distal to DUX4 is required for locus inducibility, we used CRIPSR-interference to repress the LTR10C. By using a catalytically inactive dCas9 fused to a strong transcriptional repressor (KRAB/MeCP2)50 expressed in FSHD1 iPSCs, we found that repressing the LTR10C significantly reduced DUX4 inducibility after doxorubicin treatment (Fig. 7b). Importantly, CDKN1A activation was largely unaffected, indicating that both experimental groups experienced similar DNA damage and/or p53 activation (Fig. 7b)45–47. Thus, we conclude that the p53-bound LTR10C distal to the DUX4 locus is required for its full transcriptional activation by p53. Since the LTR10C becomes occupied by p53 following doxorubicin treatment even in non-FSHD cells (Fig. 6d, Extended Data Fig. 6e), p53 binding is evidently insufficient to strongly activate the DUX4 locus; rather, it likely must be combined with the permissive 4qA haplotype15 and D4Z4 contractions or loss of repressors such as SMCHD116, which relieve chromatin repression and/or may permit looping between LTR10C and DUX4 promoters.
Developmental/disease DUX4 regulation in FSHD myoblasts
It is unknown whether the regulatory sequences that activate Dux/DUX4 loci in the early embryo are the same that activate the DUX4 locus in muscle cells during FSHD pathogenesis. Remarkably, we found that myoblasts from five different FSHD1 or FSHD2 patients19 induced DUX4 expression after doxorubicin treatment, indicating that the DDR-hypersensitivity of the DUX4 locus is not only present in iPSCs, but also in myoblasts, a cell type relevant to the muscle pathology seen in FSHD patients (Fig. 7c).
Discussion
Previous work identified DUX as a driver of the 2CLC state, and of sets of genes and retrogenes during ZGA2–4, but how Dux itself was regulated at the transcriptional level was unknown. In this work, we show a coupling of p53 to Dux/DUX4 activation, potentially uniting the developmental regulation of the Dux locus at ZGA/2CLCs and the regulation of the DUX4 locus in FSHD patients (Fig. 7d).
Our work is in apparent contrast with a recent report20, which claimed that p53 is not required for ATR-dependent replication stress-induced Dux activation. However, their work did not use a clonally derived and validated Trp53 KO mESC line20. Here, our data provide several independent lines of evidence that the majority of the effect on Dux induction and DUX-target activation acts through p53. Loss-of-function assays using Trp53 siRNA, two independent early-passage Trp53 KO clones, and Trp53 KO mESCs derived from floxed/floxed Trp53 KO blastocysts indicate that p53 is required for full Dux activation (Fig. 2b,c, Extended Data Fig. 2d). Our work also establishes Nelfa as a DUX target – and therefore a marker for 2CLC state, rather than a driver. Additionally, p53 over-expression strongly rescues the failure of Dppa2/4 dKO cells to induce Dux and DUX-targets (Fig. 2f, Extended Data Fig. 2b). Although we demonstrate p53 as a clear Dux activator, we cannot rule out a minor p53-independent role in residual Dux expression in mESCs, and emphasize that our data supports both p53-dependent and p53-independent mechanisms for regulating DUX levels in embryos.
What is the benefit of coupling p53 activation to DUX expression? Since DNA damage (from paternal DNA demethylation23, ALT-telomere elongation22, replication stress23, and transcription-mediated DNA damage24) and subsequent p53 activation is an intrinsic process of preimplantation development, we speculate that p53 activation could allow the embryo to ensure that a robust DNA damage response is coupled to ZGA and thus development. Critically, early cleavage-stage embryos are not competent to undergo apoptosis51, which we suggest provides a unique developmental time-window to utilize active p53 signaling without triggering cell death. However, our analysis of p53 MZ-KO embryos indicates that p53 impacts, but is not absolutely required for DUX-target expression, highlighting a key difference between the mouse ZGA embryo and 2CLCs. Interestingly, DNA damage has recently been shown to control the onset of ZGA in C. elegans primordial germ cells52.
Additionally, DUX is a potent transcriptional activator of Zscan4, which prevents excessive transcription-induced DNA damage during ZGA 22,24. We show that ZSCAN4 binds to the mouse Dux array at a (CA)-repeat present in each repeat unit (Fig. 3a,g) and ZSCAN4-OE mESCs have lower Dux expression after doxorubicin-induced DNA damage (Fig. 3h). However, since ZSCAN4-OE mESCs also have lower phospho-p53, p53 target activation, and less cell death after doxorubicin treatment – the decrease of DUX expression we observe in ZSCAN4-OE mESCs is likely an indirect effect on p53 signaling strength (Fig. 3h, Extended Data Fig. 3e,f,g). Instead, we speculate that ZSCAN4 might bind to the (CA)-repeat at each Dux repeat unit and promote locus/genomic stability, perhaps through protecting Z-DNA-prone regions (known to mark sites of chromosomal translocations53), consistent with ZSCAN4 preventing sister-chromatid exchange54. Recombination-mediated expansion underlies gene ‘accordions’55, prompting future work to determine whether and how ZSCAN4 regulates Dux via this process.
A major implication of our work is that embryonic cells with excessive and/or unrepaired DNA damage may have higher and prolonged DUX expression, and therefore adopt a non-postimplantation-epiblast fate – which may serve to protect the soma or the germline from damaged cells. Consistent with a “quality control” function for DUX-conferred expanded fate is the finding that Trp53 KO tetraploid cells contribute to the embryo, while almost all tetraploid cells from Trp53 wildtype embryos are excluded from the embryo proper, and instead contribute to extraembryonic tissues56. Whether DUX reactivation in vivo occurs outside of ZGA and confers a similar “expanded” potential fate will be the focus of future work. Since expanded fate of mESCs is seen after PARP1 inhibition57 or loss of H2AX58 – and both conditions inhibit DNA break repair and prolong DDR/p53 signaling – the coupling of DDR/p53 activation to DUX activation may be a unifying theme for conferring expanded fate potential to mESCs.
We note three other reported links of p53 to 2CLC induction. First, mir34a KO mESCs have a higher percentage of stochastically fluctuating 2CLC36. Since mir34a is a direct target of p5359, loss of the tumor suppressor mir34a may allow p53-high mESCs to survive, thus allowing 2CLC accumulation in culture. Second, depletion of UBC9, an E2 enzyme required for sumoylation, has also been shown to activate 2CLCs60. Interestingly, p53 is also sumoylated by UBC961, and this sumoylation can decrease p53 activity62. Therefore, loss of UBC9 could indirectly activate Dux through p53-sumolyation/activity, in addition to the reported bona fide chromatin targets of sumoylation60. Third, loss of the N6-methyladenosine reader protein YTHDC1 results in Dux activation and increased 2CLCs, which was attributed to LINE-1-mediated Dux induction63. However, our reanalysis of Ythdc1 KO mESC RNA-seq63 data reveals strong induction of direct p53 targets (Mdm2, Cdkn1a (p21) and Krt5) (Extended Data Fig. 6g), suggesting p53 activation of Dux as a likely unifying theme in the biology of 2CLCs.
Although p53 activates both mouse Dux and human DUX4, the molecular mechanism in mouse and human cells is nevertheless quite different. While mouse Dux has a p53-reponsive promoter (Fig. 3d), the human DUX4 locus is regulated by a p53-bound LTR10C distal enhancer (Figs. 6d,e, 7b). Since the LTR10C was acquired in the primate lineage and is absent in rodents, this implies that the mouse Dux and the human DUX4 loci evolved p53 regulation convergently. Characterization of the intron-containing progenitor DUXC array could help clarify whether p53 regulation is an ancestral property that was supplanted by the LTR10C enhancer in primates, or if the rodent and primate lineages independently evolved this regulatory feature.
Our work also has implications for the mechanistic understanding of FSHD etiology. Although there is a model of how DUX4 expression leads to downstream cell death14,18, it was previously unclear which transcriptional activators control DUX4 expression. Here, we discovered that in FSHD1 and FSHD2 cells DUX4 is hypersensitive to p53 activation while DUX4 from non-FSHD cells is not, likely due to the relief of chromatin/epigenetic silencing only in FSHD genotypes (Fig. 7d) – potentially explaining stochastic DUX4 expression in muscle cells from FSHD patients, which are known to exhibit DNA damage64. Importantly, p53 is necessary for regulated myogenesis, as p53 activation during muscle differentiation is required for the balance between quiescence and differentiation65. Future work will be needed to identify the contexts that lead to p53 activation in FSHD patients, and whether effective interventions (such as CHK1 inhibitors66) can be pursued to mitigate DUX4 activation and FSHD pathogenesis in humans.
Methods
This research complies with all relevant ethical regulations. Animal experiments performed at the University of Utah were first granted IACUC and Institutional approval, under protocol #18–07014. Informed consent was obtained from all human participants and overseen by University of Utah IRB approved protocol #40092. No participant compensation was provided. Written informed consent was obtained from all participating subjects under a University of Utah Institutional Review Board approved protocol (IRB registrations: 30923).
Mouse ESC culture
Mycoplasma-free E14 mESCs were cultured on gelatin in 2i+LIF medium containing Gibco KO-DMEM with nonessential amino acids, 2-mercaptoethanol, and dipeptide glutamine, and were supplemented with 15% ESC-grade FBS, leukemia inhibitory factor (LIF) (Thermo Fisher), 1 mM PD0325901 (Sigma-Aldrich), and 3 mM CHIR99021 (Sigma-Aldrich). Stable cell lines were selected using puromycin (Thermo Fisher Scientific A11138–03) at 0.5–1 μg/ml, Geneticin (Life Technologies 10131–035) at 100–200 μg/ml, or Blasticidin (Fisher Scientific B12150–0.1) at 1.5–3 μg/ml.
Transfection of mESCs
mESCs were transfected in opti-MEM medium (Thermo Fisher Scientific 31985070) using RNAiMAX (Thermo Fisher Scientific 13778150) for siRNAs or Lipofectamine 3000 (Thermo Fisher Scientific L3000–015) for DNA transfections.
siRNA transfections for mESCs
siRNA pools were generated with Giardia Dicer. Briefly, primers were designed to amplify two ~100–300-bp fragments of the indicated ORF from genomic mouse DNA and to add T7 handles (Supplementary Table 1). Purified PCR products were then used as a template for in vitro transcription with a MEGAscript T7 Transcription kit (Thermo Fisher, AM1334). Template DNA was then degraded, and the dsRNA was allowed to anneal before dicing. Diced siRNAs were purified with a PureLink Micro-to-Midi Total RNA purification kit (Invitrogen, 12183–018) with modifications. siRNA concentration was measured with a Qubit RNA HS Assay kit (Thermo Fisher, Q32852). mESCs containing the MERVL:GFP reporter were transfected with 20 pmol (10 pmol of each) of total siRNA with RNAiMAX (Life Technologies). 24 hours after siRNA transfection, mESCs were treated with doxorubicin or vehicle control for 6 hours, then washed and medium was replaced with 2iLIF for 18 hours. MERVL-GFP+ was measured as per below.
Human pluripotent stem cell culture
Human iPSCs or ESCs were maintained on Matrigel (BD Corning® Matrigel® Matrix, VWR 356231) coated plates using STEM-MACS IPSC-brew (Miltenyi Biotec, Inc. 130–104-368), passaged using ReLesr (STEMCELL Technologies 05872).
Transfections of human pluripotency stem cells
Cells were transfected in opti-MEM supplemented with Rock-i (Selleck Chem S1049) at 5 μM with Fugene6 (Promega E2691) for 1–2 hours and then hPSC medium was added.
Human fibroblast collection
Dermal fibroblasts were obtained from skin biopsies taken from patients with FSHD under a University of Utah IRB approved protocol #40092. Fibroblasts were expanded in DMEM-high glucose (Thermo Fisher Scientific 11965118) supplemented with nonessential amino acids, 2-mercaptoethanol, and dipeptide glutamine, and were supplemented with 20% ESC-grade FBS. Skin punch biopsies from patients with diagnosed facioscapulohumeral dystrophy were dissociated with sterile forceps and scalpels and placed on gelatin-coated 6-well plates in high-glucose-containing DMEM with 20% FBS. Primary outgrowth was monitored for 4 days, and subsequently medium was changed every 2–3 days until fibroblasts cultures were visible and ~90% confluent. Fibroblasts were then passaged at a ratio of 1:6 onto gelatin-coated 6-well plates. Primary fibroblast cultures were passaged at 70% confluence 4–5 times before iPSC reprogramming.
Human iPSC reprogramming
Patient fibroblasts were grown on gelatin-coated 6-well plates to roughly 50% confluence before reprogramming. Sendai-virus-mediated iPSC reprogramming was performed using the CytoTune-iPS 2.0 Sendai Reprogramming kit from Thermo Fisher (Cat – A16518) according to the manufacturer instructions for reprogramming fibroblasts. Independent colonies were picked to establish clonal cell lines from each patient, which were assessed for pluripotency by immunofluorescence, as well as embryoid body formation. iPSC lines were karyotyped using the GeneChip and KaryoStat assays by Thermo Fisher. All lines tested were karyotypically euploid, other than a 105.274 kb duplication of Chr1:q21.1 in one patient’s iPSCs (Extended Data Fig. 6b). Multiple independently reprogrammed iPSC clones from this patient’s fibroblast cultures exhibited this abnormality, strongly suggesting this alteration is not a reprogramming artifact and instead is a pre-existing chromosomal abnormality in the skin sample from this patient.
Reprogrammed iPSC lines were subsequently grown on Vitronectin (Gibco – A14700) coated 6-well plates in Essential 8 medium (Thermo Fisher – A1517001), with medium changes every day and passaging every 3–4 days, on average and then adapted to IPS-brew medium and matrigel coating. Pluripotency marker staining for OCT4 and SOX2 was performed (Extended Data Fig. 5a).
Human myoblast culture
Cell lines and their D4Z4 repeat contraction status are described elsewhere (Campbell et al. eLife, 2018). Myoblasts were maintained in Ham’s F-10 Nutrient Mix (Gibco, Waltham, MA) supplemented with 20% HyClone Fetal Bovine Serum (GE Healthcare Life Sciences, Pittsburgh, PA), 100 U/100 μg penicillin/streptomycin (Gibco), 10 ng/ml recombinant human basic fibroblast growth factor (Promega Corporation, Madison, WI) and 1 μM dexamethasone (Sigma-Aldrich D4902–25MG).
Drug treatments of cells
Small molecules were dissolved in DMSO or water. Zeocin (InvivoGen ant-zn-05), Doxorubicin (Selleck S1208) used a 1 μM, hydroxyurea (VWR, IC10202325) at 5 mM, aphidicolin (Abcam ab142400) at 6 μM, hydrogen peroxide (Sigma, 216763) 10 μM, ATM-I (KU-55933, Selleck S1092) used at 10 μM, ATR-I (Selleck VE-821, S8007) used at 10 μM, Chk1-I (LY2603618 Selleck No.S2626), Chk2-I (Selleck, MK-8776 SCH 900776) used at 10 μM, doxycycline (CloneTech 631311) used at 0.25–2 μg/ml. For inhibition of ATM, ATR, Chk1, Chk2 in mESCs, cells were pre-treated with inhibitors or vehicle for 1 hour, then treated with doxorubicin 1 μM in combination with ATR/ATM/Chk1/Chk2 inhibitors for 6 hours, and then washed and replaced either with kinase inhibitors or vehicle control for 18 hours.
Anti-DUX antibody production
We used the KLH-conjugated peptide sequence PQEEAGSTGMDTSSPSD to inject rabbits using Covance. Serum was purified using SulfoLink™ Coupling Resin (Thermo Fisher) following manufacturer instructions. Antigen purified fraction was evaluated on SDS-PAGE gel for purity and dialyzed against PBS/0.1% Tween-20.
Flow cytometry
A BD LSRFortessa instrument with lasers for 351 nm, 488 nm, 561 nm, and 640 nm was used to quantify MERVL-GFP+ mESCs. Live vs. dead discrimination was routinely performed using propidium iodide (Invitrogen P1304MP) and samples were gated using forward FSA and side-scatter SSA to isolate cells from debris and then double discrimination was performed using FSH × FSW and SSH × SSW. FACS was performed on a BD-Aria instrument with comparable gating strategies to the flow cytometry. Purity checks were performed post-sort and routinely >97% pure.
DNA damage using PpoI endonuclease
Stable cell lines using the PpoI coding sequence from Addgene Plasmid #46963 or mCherry control Piggyback vectors were created through transfection of E14 mESCs and selection with 3 μg/ml blasticidin for 7 days. Resultant polyclonal cell cultures were induced using doxycycline at 2 μg/ml for 24 hours before quantification using flow cytometry.
dCas9-KRAB-mediated repression of the LTR10C in human iPSCs
Transgenic FSHD1 patient #1 cells were first created using integration of a constitutively expressed GFP and dCas9-KRAB Piggyback plasmid. After antibiotic selection of these cells with 0.5 μg/ml puromycin and expansion for 2 weeks, we transfected cells with Piggyback plasmids containing an mCherry cassette and encoding gRNAs targeting the LTR10C locus or control gRNA. After selection with blastocidin at 1.2 μg/ml, and 1 week of expansion in culture, we obtained a mixed population of cells. This mixed population of cells was used for the doxorubicin treatment, and after 6 hours of 1 μM doxorubicin treatment, we FACS-sorted the double positive GFP+/mCherry+ cells from either the LTR10C gRNA+/dCas9-KRAB+ cells or the gRNA control+/dCas-KRAB+ cells. RNA was extracted using TRIzol (Thermo Fisher Life Sciences) and RT-qPCR was performed as described for other human cells in the paper measuring DUX4 transcripts and normalized to 18S rRNA.
CRISPR-activation using dCas9-p300HAT or dCas9-VP160
Extended Data Figure 2b: E14 mESCs were transfected with Piggyback transgenes on plasmids encoding eif1a-GFP and eif1a-dCas9-p300 or eif1a-GFP/eif1a-dCas9-VP160. After selection with 3 μg/ml blasticidin for 7 days, cells were expanded and transfected with a plasmid encoding either a control gRNA or gRNAs (2–3 gRNAs per regions #1–4 in the Dux locus linker or 3 gRNAs targeting the Dux promoter). 36 hours after transfection, cells were harvested with TRIzol (Thermo Fisher) and RNA was purified, subjected to reverse-transcription as described above, and qPCR was performed (as described above) to quantify the levels of Dux RNA normalized to Actin (as described above).
CRISPR knock-out, knock-in clone screening
Please see Supplementary Methods section.
PCR
PCR primers for genotyping Trp53 KO mice or amplifying CRIPSR/Cas9 edited alleles are found in Supplementary Table 1. PCR was performed using Phusion High Fidelity PCR mastermix (New England Biolabs M0531S).
Rescue of Dppa2/4 dKO mESCs with p53 expression
Dppa2/4 dKO mESCs were transfected with mCherry control or mouse Trp53-mCherry piggyback plasmids. Rescue clones were screened for p53-mCherry expression using fluorescence monitoring for high p53 expression. Dppa2/4 dKO + p53-mCherry or mCherry control rescue were doxycycline-induced for 24 hours, then DNA-damaged with 1 μM doxorubicin for 6 hours, followed by medium washout for 12 hours. RNA was then harvested in TRIzol (Life Sciences).
DPPA2/4-OE experiments in WT mESCs
Doxycycline-inducible piggyback vectors that encode DPPA2-IRES-GFP or DPPA4-IRES-GFP were delivered into E14 mESCs by cotransfection (as above). 48 hours after doxycycline induction, cells were fixed with PFA and immunofluorescence was performed as above using DPPA2/DPPA4 and DUX antibodies.
Western blotting
RIPA buffer (10 mM Tris-HCl pH = 8.0, 150 mM NaCl, 1% NP-40, 0.1% Na-deoxycholate) with protease inhibitors was used to lyse cells. Lysates were further sonicated for 15 min with 30 sec on/off in a Diagenode Biorupter Pico to solubilize chromatin-bound proteins. Protein lysates were quantified with BioRad (500–0001EDU) Bradford reagent and loaded on SDS-PAGE gels. After transfer to nitrocellulose membranes (VWR 95040–108), the membranes were blocked with TBST (Tris buffered saline +0.1% Tween-20) and 5% milk and then incubated with antibodies overnight (see Supplementary Table 3) and detected using HRP-conjugated secondary antibodies and Western Lightning® Plus-ECL, Enhanced Chemiluminescence (PerkinElmer Health Sciences, Inc., nel 105001EA).
RT-qPCR
Isolation of RNA with TRIzol extraction. Unless indicated all RNA samples were treated with DNase to eliminate genomic DNA contamination. RT was performed with SuperScript IV (Invitrogen) with random hexamer primer (Invitrogen), and qPCR (see Supplementary Table 1 for qPCR primer pairs) was performed with iTaq Universal SYBR Green Supermix (Bio-Rad) using a BioRad qPCR CFX connect instrument. Experiments were performed in biological replicates as indicated in figure legends. Mouse RT-qPCR used actin RNA for normalization and human RT-qPCR experiments were normalized to 18S rRNA.
Immunofluorescence of culture cells
Cells were fixed in 4% paraformaldehyde at room temperature for 15 min and extensively washed with PBS. Cells were permeabilized using PBS/5% BSA/0.3% Triton-X at room temperature for 1 hour. Primary antibodies (see Supplementary Table 3) were diluted in PBS/1% BSA/0.1% Triton-X and incubated overnight at 4°C, washed 3× in PBS/0.1% Triton, and incubated with secondary antibodies (see Supplementary Table 3 for details) diluted in PBS/1% BSA/0.1% Triton-X and incubated for 1 hour at room temperature. Samples were washed in PBS/0.1% Triton-X and mounted with Prolong Gold Anti-fade with DAPI mounting medium (FISHER SCI CO P-36931).
Immunofluorescence of embryos
Zonae pellucidae were removed immediately prior to fixation using acid Tyrode’s solution (137 mM NaCl, 2.68 mM KCl, 1.63 mM CaCl2, 0.49 mM MgCl2, 5.55 mM glucose, 0.01 mM polyvinyl pyrrolidone, pH 2.5). Embryos were fixed for 30 min in 2.5% paraformaldehyde in PBS and then permeabilized for 20 min in 0.1% Triton X-100 in PBS. Embryos were then incubated at 4°C overnight in primary antibody, washed, then incubated in secondary antibody (Thermo Fisher; 1:500) for 1 h at room temperature. All embryos were mounted in Vectashield containing 1.5 μg/ml DAPI (Vector Laboratories, Burlingame, CA) and slides were scanned using a Zeiss LSM 510 UV confocal microscope (p53 staining) or Leica SP8 Confocal (DUX stained embryos). All immunofluorescence experiments were repeated a minimum of three different times using at least 10 embryos per group.
Microscopy of cultured cells and embryos
Positive cells were counted using Image-J plug-in “Cell Counter”. Fluorescence signal for mouse eggs or embryos was quantified using Image-J.
RNA-seq
For mESC RNA-seq, RNA was extracted using TRIzol, and libraries generated according to manufacturer instructions: poly-A selected RNA using NEB-next kit (New England Biolabs e7500s). For embryo RNA-seq, zona pellucida was removed using acid Tyrode’s and lysed directly in lysis buffer from SMART-Seq® v4 Ultra® Low Input RNA Kit for Sequencing (Takara Bio 634890). 15 cycles of pre-amp were performed before following manufacturer instructions for Tagmentation into ds-cDNA using Nextera XT DNA Library Preparation Kit (Illumina FC-131–1024). Purified libraries were quantified on an Agilent Technologies 2200 TapeStation with a D1000 ScreenTape assay. The molarity of adaptor-modified molecules was defined by quantitative PCR with a Kapa Library Quant Kit (Kapa Biosystems). Individual libraries were normalized to 10 nM, and equal volumes were pooled in preparation for Illumina sequence analysis. Sequencing libraries (25 pM) were chemically denatured and applied to an Illumina HiSeq paired-end flow cell with an Illumina cBot. Flow cells were then transferred to an Illumina HiSeq 2000 instrument and sequenced in 125-bp paired-end mode.
RNA-seq analysis
RNA-seq reads were trimmed and filtered for quality using BBduk and FASTQC (v 0.10.1). Processed reads were aligned using Tophat2 v2.1.0 (--t --q --N1 --L 25 --X 2000 --no-mixed --no-discordant), and counts for each transcript were generated using “Get datasets” (https://metacpan.org/pod/distribution/Bio-ToolBox/scripts/get_datasets.pl). Differential expression analysis was performed using DESEq2 (v3.11). Hierarchical clustering was performed using R package “ComplexHeatmap” with inputs being differentially expressed transcripts (DESEq FDR<0.05) Z-score normalized from MARSS R package: (v3.10.12).
Repetitive element analysis
TEtranscripts (Version: 2.1.4) was used to identify differentially expressed repetitive elements in mouse embryo SMART-seq libraries.
GO term analysis
GO terms were identified using Gene Ontology Resource (http://geneontology.org/).
2CLC and DUX direct target identification
2CLC-enriched transcripts were defined by comparison of MERVL-GFP+-sorted mESCs and GFP− mESCs, n = 2,385 (Ishiuchi et al. Nat Struct Mol Biol. 2015). DUX direct targets were defined as genes bound by DUX-3×HA ChIP-seq (Hendrickson et al. Nat Genet. 2017) and upregulated after Dux transgene expression (Hendrickson et al. Nat Genet. 2017).
Embryoid body differentiation
Please see Supplementary Methods.
Single-cell RNA-seq and analysis
Please see Supplementary Methods.
XEN differentiation
Differentiation was performed as described in Niakan et al. Nature Protocols 2013. Briefly, Rhox5-mCherry knock-in mESCs either pulsed with doxycycline or vehicle-treated in 2iLIF medium and then transferred to XEN medium (RPMI 1640 supplemented with 15% (vol/vol) FBS and 0.1 mM β-mercaptoethanol, 1% (vol/vol) penicillin-streptomycin. Standard XEN medium supplemented with 0.01 μM all-trans retinoic acid (Sigma-Aldrich, cat. no. R2625) dissolved in DMSO plus activin A (R & D Systems, cat. no. 338-AC-010) 10 ng/ml.
ATAC-seq
Please see Supplementary Methods.
Luciferase assay
Please see Supplementary Methods.
Myoblast DNA damage
Human myoblasts were treated with 0.25 μM doxorubicin or vehicle control for 24 hours before RNA harvesting in TRIzol and processing for RT-qPCR.
PacBio sequencing and Dux locus assembly
DNA was extracted from mixed background Bl6 mESCs using the Genomic-tip 100/G kit (Qiagen) and DNA integrity was assessed with the Femto Pulse system (Agilent). Shearing, SMRT library preparation and Blue Pippin size-selection was done following the PacBio Procedure & Checklist – Preparing HiFi SMRTbell® Libraries using SMRTbell Express Template Prep Kit 2.0 (PN 101–853-100 Version 01, September 2019).
DNA was sheared with a g-TUBE (Covaris), RNase-treated and purified with AMPure XP beads. After assessment of the fragmented DNA on Femto Pulse, 10 μg of sheared DNA was converted into a SMRT library. The library was size-selected using the Blue Pippin (Sage Science), collecting 9–13-kb and >15-kb size fractions. The >15-kb fraction was loaded at a 50 pM concentration on 2 Sequel®II cells using Binding kit 2.0, Sequencing reagent 2.0, sequencing primer V2 and a movie collection time of 30 h with a pre-extension of 2 h.
Consensus reads (CCS reads) were generated using ccs software v.4.0.0 in smrtlink v8 with --minPasses 3 --minPredictedAccuracy 0.99 --maxLength 50000. Total CCS read yield was 50 Gb.
The mouse genome was assembled using HiCanu v2 with default parameters and a genome size of 2.7 Gb. Starting from a total of 2,997,947 HiFi reads, (~18.5-fold coverage of the genome), the final assembly had a total length of 2.79 Gb resolved in 1,551 contigs with N50 of 5.7 Mb.
Contigs from the primary assembly were aligned to the mm10 genome using minimap2 and the Dux locus was visually inspected on the IGV browser to find the best matching contigs.
ChIP-seq
Please see Supplementary Methods.
HTPS data availability
The data from this study are deposited at GEO under accession number GSE149267 and summarized in Supplementary Table 4.
Public datasets reanalyzed/remapped to the new Dux locus contig:
H3K4me1, H3K4me2, H3K4me3, H3K27me3 and p300 ChIP-seq in mESCs: GSE98063; H4K20me3 ChIP-seq in mESCs: GSE130721; H3K122ac and H3K64ac ChIP-seq in mESCs: GSE66023; H3.3S31ph ChIP-seq in mESCs: SRR8310851; H3.3 ChIP-seq in mESCs: GSE42152; DPPA2 and DPPA4 ChIP-seq in mESCs: GSE117173; ZSCAN4 ChIP-seq in mESCs: GSE140621; DUX ChIP-seq in mESCs: GSE95519; NELFA ChIP-seq in mESCs: GSE113671; H3K4me3 ChIP-seq in Zscan4+ in mESCs: GSE164486; p53 ChIP-seq in human fibroblasts: GSE115940; p53 ChIP-seq in human fibroblasts: GSE111009; p53 ChIP-seq in hESCs: GSE39912.
The Dux locus contig sequence/assembly was deposited in GENbank as submission # BankIt2443327 tig00005009 MW810794.
Biological replicate definition
Biological replicates are samples analyzed from distinct samples, and where possible, were different independent clones (for example, KO clones isolated separately to control for clonal derivation technical variation).
Adjustment for multiple comparisons
Where appropriate, multiple comparisons were reported as statistically different if the Bonferroni-corrected P value (stated as FDR in the figure legends) met indicated significance threshold. DESeq2 analysis was reported using adjusted P value (FDR) which is Benjamini-Hochberg-corrected.
Mycoplasma testing
Cells were routinely tested for mycoplasma using the Lonza MycoAltert test kit and the results were negative.
Boxplot conventions
The median is shown as a line in the box, and the outline of the box is depicted at the 25th and 75th percentile. The extended whiskers depict Q1 – 1.5 × IQR and Q3 + 1.5 × IQR. Outliers points are depicted as dots.
Data Availability
All data, cell lines, reagents, and unique materials are available upon request. ChIP-seq, ATAC-seq, scRNA-seq, and RNA-seq are deposited under GSE149267 and are detailed in Supplementary Table 4.
Code Availability
No custom code or algorithms were generated or used. All software used is described in the Methods.
Extended Data
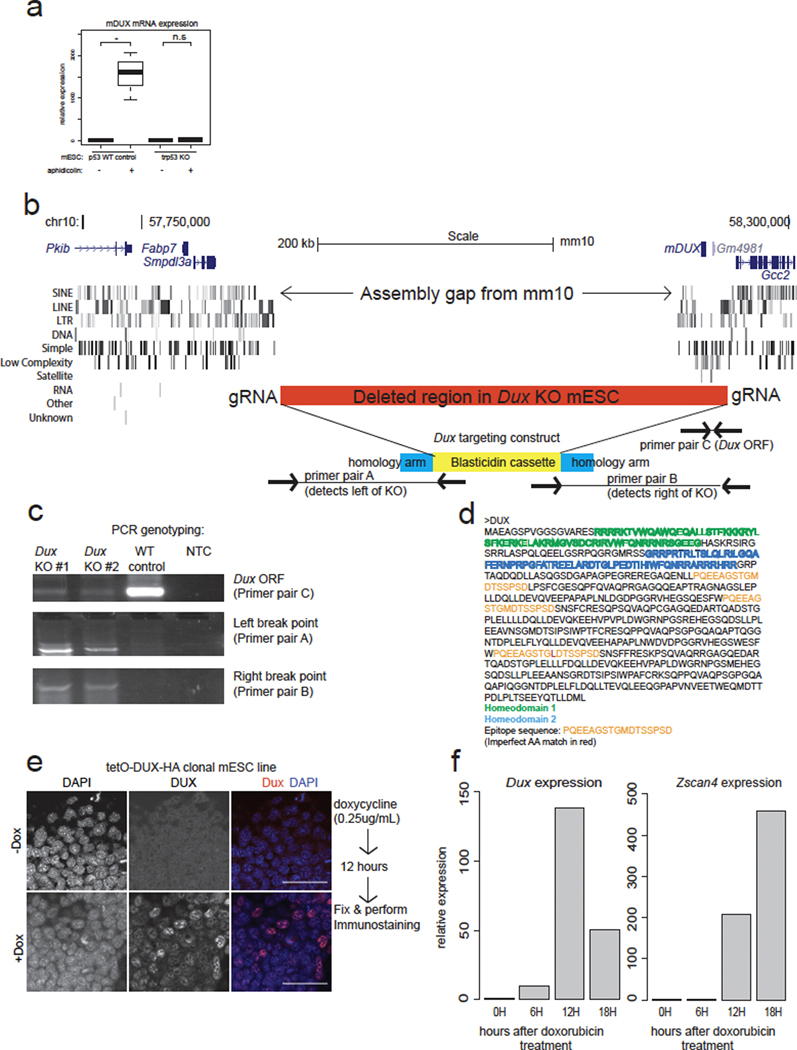
Extended Data Figure 1:
a) Dux expression in aphidicolin or vehicle control treatment of Trp53 WT mESC or Trp53 KO mESC. RT-qPCR, n=3 biological replicates, * pvalue<0.05, t-test, one sided.
b) Schematic of Dux locus in mm10 genome assembly, the design of the targeting construct, location of gRNAs for Dux KO mESC line generation. Shown below are locations of genotyping primers.
c) PCR genotyping results of Dux KO mESC clones #1 and #2, which were used for experiments in Figure 1.
d) Design of DUX peptide antigen for antibody creation.
e) immunofluorescence with the rabbit polyclonal anti-DUX antibody using mESC with a tetracycline inducible DUX-3xHA transgene.
f) Kinetic analysis of Dux and Zscan4 transcript induction in WT mESC treated with 1μM of doxorubicin for indicated times. Note earlier induction of Dux compared to Zscan4, the RNAseq from Fig1 and Fig2 is using the later time point 18H.
Extended Data Figure 2:
a) Western blot analysis and KO deletion allele Sanger sequencing results for two independent Trp53 KO mESC clones (used for Fig 2).
b) RT-qPCR measure of Dux or Zscan4 expression levels in two independent Dppa2/4 dKO mESC clones. Dppa2/4 dKO clone #1 was used for Fig 2E with p53 rescue experiments.
c) Western blot confirmation of dKO Dppa2/4 mESC clones #1, 2.
d) Dux expression in Trp53 WT mESC after control or Trp53 siRNA knockdown, with vehicle or doxorubicin treatment. RT-qPCR, N=6 biological replicates, * p-value <0.05, one-sided t-test.
e) Co-overexpression of DPPA2/4 in Trp53 WT mESC does not activate Dux expression. Representative image from 3 independent experiments.
Extended Data Figure 3:
a) Schematic of long-read (PacBio) sequenced and assembled mouse Dux locus.
b) Browser snapshot showing the location of the different gRNAs for the CRISPR-A experiment using dCas9-VP160 or dCas9-p300 fusions performed in Trp53 KO mESC. Only performing CRIPSR-A with the Dux promoter-targeted gRNAs strongly activates Dux expression. RT-qPCR, n=3 biological replicates.
c) Genome browser snapshots of the mouse Nelfa locus showing strong enrichment of DUX binding at the 3’ end of the gene at an intronic MERVL element. Bottom panel is zoomed in view showing doxorubicin-induced open chromatin at this MERVL element (ATAC-seq data from this paper, n=2 biological replicates for each condition). Barplot (middle panel) of doxycycline induced Dux transgenic mESC showing strong induction of Nelfa transcripts (RNA-seq, n=2 biological replicates, * FDR <0.05, DESeq2, data reprocessed from Hendrickson, et al. Nature Genetics 2017). Right-most panel: Metagene plot of the 28xDux repeat units showing p53-ChIP-seq and input control (from this paper) and NELFA ChIP-seq and input control (blue and black lines respectively reprocessed from Hu, et al. Nature Cell Biology, 2020—please note lower NELFA ChIP-seq signal compared to the matched-control input).
d) Nelfa is transcriptionally induced by doxorubicin treatment, and this requires both p53 and DUX. RNA-seq from this paper, n=2 biological replicates, * FDR<0.05 DESeq2.
e) Mdm2 and Krt5 are direct p53 targets. Genome browser snap shots of p53 ChIP-seq and H3K27ac ChIP-seq (n=2 biological replicates for each condition, data produced in this paper). RT-qPCR measuring Mdm2 expression in control mESC or ZSCAN4-OE mESC, treated with vehicle control or doxorubicin; n=5 biological replicates, *p-value <0.05, one-sides t-test.
f) Immunoflourescence staining quantification of control mESC (n=135 cells) or clonal ZSCAN4-OE mESC (n=487 cells) using phospho-p53 antibodies after doxorubicin treatment, n. *<0.001, Wilcox test.
g) Brightfield image showing decreased cell death after doxorubicin treatment in ZSCAN4-OE mESC compared to control mESC. Image is representative from 3 independent experiments.
Extended Data Figure 4:
a) Immunofluorescence of pronuclei (PN)-stage zygotes showing nuclear phospho-S15 p53 staining (quantified in Fig 4A).
b) Single mouse zygote RT-qPCR measuring Dux expression in PN5 stage zygotes (n=7 p53 MZ-KO, n=16 p53 WT), * p-value <0.05, t-test.
Extended Data Figure 5:
a) Comparison of two biological replicates for EB scRNAseq (control vs Dux-pulsed) showing high concordance between samples.
b) Ibarra-Soria, et al. Nature Cell Biology “Defining murine organogenesis at single-cell resolution reveals a role for the leukotriene pathway in regulating blood progenitor formation” depicting different cell types defined in E8.25 mouse embryos, (data retrieved from https://marionilab.cruk.cam.ac.uk/organogenesis/ February 2020).
c) Analysis of Ibarra-Soria, et al. data compared to our EB scRNAseq data with indicated markers identifying cell types (see Fig 4C table). Each plot shows the marker identified in our Seurat analysis of EB scRNAseq as discriminating between other cell type clusters, and the data shows the distribution of that marker in E8.25 mouse in vivo cell types.
Extended Data Figure 6:
a) FSHD1 iPSC (patient #1, 2, 3) immunostaining for pluripotency markers SOX2 and OCT3/4.
b) Thermo-Fisher Karyostat report for FSHD1 iPSC clones patients #1,
c) RT-qPCR after vehicle control or doxorubicin treatment measuring DUX4 levels in FSHD1 iPSC patient #1, non-FSHD hESC female “LSJ2”, and non-FSHD iPSC “WT33”. N=6 biological replicates, * p-value <0.05 one-sided t-test.
d) Western blot with N- and C-term SMCHD1 antibodies that the 2 independent clones show in Fig. 6C are KO, isogenically created in FSHD1 patient #1. CRISPR/Cas9 deletion strategy shown on right top, with the Sanger sequencing of KO clones shown on bottom right.
e) Genome browser snap-shot of ATAC-seq performed in human embryos showing open chromatin signal at the 4qA LTR10C element.
f) Luciferase assay testing directionality of the LTR10C element in FSHD1 patient #1 iPSC (p53 WT or isogenic p53 KO). N=4 biological replicates.
g) RNA-seq analysis from Liu, et al. Nature 2021 showing reactivation of direct p53 targets (Mdm2, Cdkn1a (p21), and Krt5) in Ythdc1 conditional knockout (cKO) mESC +/− Dux KO, treated with vehicle or 4-OHT (tamoxifen) to eliminate the YTHDC1 protein.
Supplementary Material
Acknowledgements
We thank the members of the Cairns laboratory and Mahesh B. Chandrasekharan for fruitful discussions. We are grateful to the patients that made this work possible. We thank Trudy Oliver for the p53 flox/flox mouse, Stephen J. Tapscott for FSHD1/FSHD2 myoblasts, Feng Zhang for the px330-Cas9 plasmid, Steven Jackson for the pICE-HA-NLS-I-PpoI plasmid, Alejandro Chavez & George Church for the dCas9-KRAB-MeCP2 plasmid, Charles Gersbach for the pcDNA-dCas9-p300(HAT) plasmid. We also thank Brian Dalley in the HCI High-Throughput Genomics and Bioinformatic Analysis Shared Resource (NCI grant P30CA042014), The CCTS Stem Cell Facility (NIH UL1TR002538), James Marvin and the University of Utah Flow Cytometry Facility (NIH 1S10RR026802-01, NCI 5P30CA042014-24), and the University of Utah Cell Imaging Core. This work supported C.J.W. in part by the Intramural Research Program of the National Institutes of Health (NIEHS 1ZIAES102985), NCI (P30 CA015704-45S6) and W.OP.14-01 from the Prinses Beatrix Spierfonds to S.V.D.M., Wellstone Center from UMass (NICHD P50HD060848) to R.B., NIH F30HD098000 to B.W., NICHD F32HD104442 to S.C.S., NICHD F32HD094500 and Lalor Foundation Fellowship #10041116 to E.J.G., Howard Hughes Medical Institute (HHMI) and NICHD 1R01HD095833 to B.R.C. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing Interests statement: The authors declare no competing interests.
References:
- 1.Jukam D, Shariati SAM & Skotheim JM Zygotic Genome Activation in Vertebrates. Dev. Cell 42, 316–332 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hendrickson PG et al. Conserved roles of mouse DUX and human DUX4 in activating cleavage-stage genes and MERVL/HERVL retrotransposons. Nat. Genet 49, 925–934 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Iaco A. et al. DUX-family transcription factors regulate zygotic genome activation in placental mammals. Nat. Genet 49, 941–945 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iaco AD, Verp S, Offner S, Grun D. & Trono D. DUX is a non-essential synchronizer of zygotic genome activation. Development 147, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vuoristo S. et al. DUX4 regulates oocyte to embryo transition in human. bioRxiv 732289 (2019) doi: 10.1101/732289. [DOI] [Google Scholar]
- 6.Chen Z. & Zhang Y. Loss of DUX causes minor defects in zygotic genome activation and is compatible with mouse development. Nat. Genet 51, 947–951 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macfarlan TS et al. Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature 487, 57–63 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishiuchi T. et al. Early embryonic-like cells are induced by downregulating replication-dependent chromatin assembly. Nat. Struct. Mol. Biol 22, 662–671 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Percharde M. et al. A LINE1-Nucleolin Partnership Regulates Early Development and ESC Identity. Cell 174, 391–405.e19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez-Terrones D. et al. A molecular roadmap for the emergence of early-embryonic-like cells in culture. Nat. Genet 50, 106–119 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckersley-Maslin M. et al. Dppa2 and Dppa4 directly regulate the Dux-driven zygotic transcriptional program. Genes Dev. 33, 194–208 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu Z. et al. Maternal factor NELFA drives a 2C-like state in mouse embryonic stem cells. Nat. Cell Biol 22, 175–186 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Dixit M. et al. DUX4, a candidate gene of facioscapulohumeral muscular dystrophy, encodes a transcriptional activator of PITX1. Proc. Natl. Acad. Sci 104, 18157–18162 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Himeda CL & Jones PL The Genetics and Epigenetics of Facioscapulohumeral Muscular Dystrophy. Annu. Rev. Genomics Hum. Genet 20, 265–291 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Lemmers RJLF et al. A Unifying Genetic Model for Facioscapulohumeral Muscular Dystrophy. Science 329, 1650–1653 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blewitt ME et al. SmcHD1, containing a structural-maintenance-of-chromosomes hinge domain, has a critical role in X inactivation. Nat. Genet 40, 663–669 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Lemmers RJLF et al. Digenic inheritance of an SMCHD1 mutation and an FSHD-permissive D4Z4 allele causes facioscapulohumeral muscular dystrophy type 2. Nat. Genet 44, 1370–1374 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shadle SC et al. DUX4-induced dsRNA and MYC mRNA stabilization activate apoptotic pathways in human cell models of facioscapulohumeral dystrophy. PLOS Genet. 13, e1006658 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell AE et al. NuRD and CAF-1-mediated silencing of the D4Z4 array is modulated by DUX4-induced MBD3L proteins. eLife 7, e31023 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atashpaz S. et al. ATR expands embryonic stem cell fate potential in response to replication stress. eLife https://elifesciences.org/articles/54756/figures (2020) doi: 10.7554/eLife.54756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziegler-Birling C, Helmrich A, Tora L. & Torres-Padilla M-E Distribution of p53 binding protein 1 (53BP1) and phosphorylated H2A.X during mouse preimplantation development in the absence of DNA damage. Int. J. Dev. Biol 53, 1003–1011 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Liu L. et al. Telomere lengthening early in development. Nat. Cell Biol 9, 1436–1441 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Wossidlo M. et al. Dynamic link of DNA demethylation, DNA strand breaks and repair in mouse zygotes. EMBO J. 29, 1877–1888 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srinivasan R. et al. Zscan4 binds nucleosomal microsatellite DNA and protects mouse two-cell embryos from DNA damage. Sci. Adv 6, eaaz9115 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Storm MP et al. Zscan4 Is Regulated by PI3-Kinase and DNA-Damaging Agents and Directly Interacts with the Transcriptional Repressors LSD1 and CtBP2 in Mouse Embryonic Stem Cells. PLoS ONE 9, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Britton S, Coates J. & Jackson SP A new method for high-resolution imaging of Ku foci to decipher mechanisms of DNA double-strand break repair. J. Cell Biol 202, 579–595 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Census and evaluation of p53 target genes | Oncogene. https://www.nature.com/articles/onc2016502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blackford AN & Jackson SP ATM, ATR, and DNA-PK: The Trinity at the Heart of the DNA Damage Response. Mol. Cell 66, 801–817 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Eckersley-Maslin MA et al. MERVL/Zscan4 Network Activation Results in Transient Genome-wide DNA Demethylation of mESCs. Cell Rep. 17, 179–192 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eckersley-Maslin MA et al. Epigenetic priming by Dppa2 and 4 in pluripotency facilitates multi-lineage commitment. Nat Struct Mol Biol. 27, 696–705 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clapp J. et al. Evolutionary Conservation of a Coding Function for D4Z4, the Tandem DNA Repeat Mutated in Facioscapulohumeral Muscular Dystrophy. Am. J. Hum. Genet 81, 264–279 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rada-Iglesias A. et al. A unique chromatin signature uncovers early developmental enhancers in humans. Nature 470, 279–283 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu W, Feng Z, Teresky AK & Levine AJ p53 regulates maternal reproduction through LIF. Nature 450, 721–724 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Sah VP et al. A subset of p53 -deficient embryos exhibit exencephaly. Nat. Genet 10, 175–180 (1995). [DOI] [PubMed] [Google Scholar]
- 35.Delbridge ARD et al. Loss of p53 Causes Stochastic Aberrant X-Chromosome Inactivation and Female-Specific Neural Tube Defects. Cell Rep. 27, 442–454.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 36.hoi YJ et al. Deficiency of microRNA miR-34a expands cell fate potential in pluripotent stem cells. Science aag1927 (2017) doi: 10.1126/science.aag1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Los Angeles A. et al. Hallmarks of pluripotency. Nature 525, 469–478 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Behringer R, Gertsenstein M, Nagy KV & Nagy A. Differentiating Mouse Embryonic Stem Cells into Embryoid Bodies by Hanging-Drop Cultures . Cold Spring Harb. Protoc 2016, pdb.prot092429 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Ibarra-Soria X. et al. Defining murine organogenesis at single-cell resolution reveals a role for the leukotriene pathway in regulating blood progenitor formation. Nat. Cell Biol 20, 127–134 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan MM et al. Molecular recording of mammalian embryogenesis. Nature 570, 77–82 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He H. et al. p53 and p73 Regulate Apoptosis but Not Cell-Cycle Progression in Mouse Embryonic Stem Cells upon DNA Damage and Differentiation. Stem Cell Rep. 7, 1087–1098 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamanaka Y, Lanner F. & Rossant J. FGF signal-dependent segregation of primitive endoderm and epiblast in the mouse blastocyst. Development 137, 715–724 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Niakan KK, Schrode N, Cho LTY & Hadjantonakis A-K Derivation of extraembryonic endoderm stem (XEN) cells from mouse embryos and embryonic stem cells. Nat. Protoc 8, 1028–1041 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leidenroth A. et al. Evolution of DUX gene macrosatellites in placental mammals. Chromosoma 121, 489–497 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Catizone AN, Good CR, Alexander KA, Berger SL & Sammons MA Comparison of genotoxic versus nongenotoxic stabilization of p53 provides insight into parallel stress-responsive transcriptional networks. Cell Cycle Georget. Tex 18, 809–823 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karsli Uzunbas G, Ahmed F. & Sammons MA Control of p53-dependent transcription and enhancer activity by the p53 family member p63. J. Biol. Chem 294, 10720–10736 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akdemir KC et al. Genome-wide profiling reveals stimulus-specific functions of p53 during differentiation and DNA damage of human embryonic stem cells. Nucleic Acids Res. 42, 205–223 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang T. et al. Species-specific endogenous retroviruses shape the transcriptional network of the human tumor suppressor protein p53. Proc. Natl. Acad. Sci 104, 18613–18618 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tutton S. et al. Subtelomeric p53 binding prevents accumulation of DNA damage at human telomeres. EMBO J. 35, 193–207 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yeo NC et al. An enhanced CRISPR repressor for targeted mammalian gene regulation. Nat. Methods 15, 611–616 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hardy K. Apoptosis in the human embryo. 10. [DOI] [PubMed] [Google Scholar]
- 52.Butuči M, Williams AB, Wong MM, Kramer B. & Michael WM Zygotic Genome Activation Triggers Chromosome Damage and Checkpoint Signaling in C. elegans Primordial Germ Cells. Dev. Cell 34, 85–95 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Wang G, Christensen LA & Vasquez KM Z-DNA-forming sequences generate large-scale deletions in mammalian cells. Proc. Natl. Acad. Sci 103, 2677–2682 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zalzman M. et al. Zscan4 regulates telomere elongation and genomic stability in ES cells. Nature 464, 858–863 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elde NC et al. Poxviruses Deploy Genomic Accordions to Adapt Rapidly against Host Antiviral Defenses. Cell 150, 831–841 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horii T. et al. p53 Suppresses Tetraploid Development in Mice. Sci. Rep 5, 1–9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang Y. et al. Derivation of Pluripotent Stem Cells with In Vivo Embryonic and Extraembryonic Potency. Cell 169, 243–257.e25 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu T. et al. Histone Variant H2A.X Deposition Pattern Serves as a Functional Epigenetic Mark for Distinguishing the Developmental Potentials of iPSCs. Cell Stem Cell 15, 281–294 (2014). [DOI] [PubMed] [Google Scholar]
- 59.Rokavec M, Li H, Jiang L. & Hermeking H. The p53/miR-34 axis in development and disease. J. Mol. Cell Biol 6, 214–230 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Cossec J-C et al. SUMO Safeguards Somatic and Pluripotent Cell Identities by Enforcing Distinct Chromatin States. Cell Stem Cell 23, 742–757.e8 (2018). [DOI] [PubMed] [Google Scholar]
- 61.Kahyo T, Nishida T. & Yasuda H. Involvement of PIAS1 in the Sumoylation of Tumor Suppressor p53. Mol. Cell 8, 713–718 (2001). [DOI] [PubMed] [Google Scholar]
- 62.Association of Ubc9, an E2 ligase for SUMO conjugation, with p53 is regulated by phosphorylation of p53 - Lin - 2004 - FEBS Letters - Wiley Online Library. 10.1016/j.febslet.2004.07.059. [DOI] [PubMed]
- 63.Liu J. et al. The RNA m6A reader YTHDC1 silences retrotransposons and guards ES cell identity. Nature (2021) doi: 10.1038/s41586-021-03313-9. [DOI] [PubMed] [Google Scholar]
- 64.Sasaki-Honda M. et al. A patient-derived iPSC model revealed oxidative stress increases facioscapulohumeral muscular dystrophy-causative DUX4. Hum. Mol. Genet 27, 4024–4035 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Le Roux I, Konge J, Le Cam L, Flamant P. & Tajbakhsh S. Numb is required to prevent p53-dependent senescence following skeletal muscle injury. Nat. Commun 6, 1–11 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dent P. et al. CHK1 Inhibitors in Combination Chemotherapy. Mol. Interv 11, 133–140 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data, cell lines, reagents, and unique materials are available upon request. ChIP-seq, ATAC-seq, scRNA-seq, and RNA-seq are deposited under GSE149267 and are detailed in Supplementary Table 4.