Abstract
Rationale: Prone positioning is an appealing therapeutic strategy for nonintubated hypoxic patients with coronavirus disease (COVID-19), but its effectiveness remains to be established in randomized controlled trials.
Objectives: To identify contextual factors relevant to the conduct of a definitive clinical trial evaluating a prone positioning strategy for nonintubated hypoxic patients with COVID-19.
Methods: We conducted a cluster randomized pilot trial at a quaternary care teaching hospital. Five inpatient medical service teams were randomly allocated to two treatment arms: 1) usual care (UC), consisting of current, standard management of hypoxia and COVID-19; or 2) the Awake Prone Positioning Strategy (APPS) plus UC. Included patients had positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing or suspected COVID-19 pneumonia and oxygen saturation less than 93% or new oxygen requirement of 3 L per minute or greater and no contraindications to prone positioning. Oxygenation measures were collected within 48 hours of eligibility and included nadir oxygen saturation to fraction of inspired oxygen (S/F) ratio and time spent with S/F ratio less than 315. Concurrently, we conducted an embedded implementation evaluation using semistructured interviews with clinician and patient participants to determine contextual factors relevant to the successful conduct of a future clinical trial. The primary outcomes were drawn from an implementation science framework including acceptability, adoption, appropriateness, effectiveness, equity, feasibility, fidelity, and penetration.
Results: Forty patients were included in the cluster randomized trial. Patients in the UC group (n = 13) had a median nadir S/F ratio over the 48-hour study period of 216 (95% confidence interval [95% CI], 95–303) versus 253 (95% CI, 197–267) in the APPS group (n = 27). Patients in the UC group spent 42 hours (95% CI, 13–47) of the 48-hour study period with an S/F ratio below 315 versus 20 hours (95% CI, 6–39) for patients in the APPS group. Mixed-methods analyses uncovered several barriers relevant to the conduct of a successful definitive randomized controlled trial, including low adherence to prone positioning, large differences between physician-recommended and patient-tolerated prone durations, and diffusion of prone positioning into usual care.
Conclusions: A definitive trial evaluating the effect of prone positioning in nonintubated patients with COVID-19 is warranted, but several barriers must be addressed to ensure that the results of such a trial are informative and readily translated into practice.
Keywords: COVID-19, hypoxia, prone positioning, implementation science
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus causing the coronavirus disease (COVID-19) pandemic, has rapidly led to significant morbidity and mortality worldwide, primarily through lower respiratory tract involvement progressing from hypoxemia to acute respiratory distress syndrome (1, 2). Novel approaches to improving oxygenation are urgently needed to avoid downstream consequences for patients as well as limit resource scarcity associated with advanced respiratory support.
Prone positioning in mechanically ventilated patients with hypoxic respiratory failure has been associated with improvement in oxygenation and mortality in patients with acute respiratory distress syndrome (3–6). The prone position provides more uniform lung perfusion, shifting ventilation to well-perfused lung segments, and recruits dependent atelectatic regions of lung (7–9). Physiological alterations associated with the prone position would foreseeably also apply to spontaneously breathing patients, and evidence from small observational studies suggests that prone positioning in nonintubated patients is feasible and associated with improved oxygenation (10–13). However, it remains unknown if a prone ventilation strategy is definitively beneficial for nonintubated hypoxic patients with COVID-19, and this question has stimulated interest in the conduct of rigorous randomized controlled trials (RCTs) (14–16).
Because the awake prone strategy is a complex medical intervention, there are multiple implementation nuances such as adoption, feasibility, and tolerability that may affect successful conduct of a definitive RCT. We designed a novel pilot study with embedded implementation evaluation to inform successful future RCTs (17). The aim of the APPS (Awake Prone Positioning Strategy) pilot trial was to assess feasibility and important contextual factors for a large RCT comparing the clinical effectiveness of an Awake Prone Positioning Strategy (APPS) versus usual care (UC) alone for hypoxic adults with COVID-19. The specific objectives of this pilot trial included both process and research objectives. The process objectives focus on the feasibility of implementation and adoption of APPS into routine care and acceptability of study design features for a future large cluster RCT. The research objectives test outcome data collection methods and explore clinical and safety outcomes.
Methods
Design
We conducted a pilot study between June 1 and August 31, 2020, using a convergent mixed-methods design with hybrid effectiveness and implementation focus integrating a pragmatic, two-arm parallel cluster RCT and a qualitative study of patients’ and clinicians’ experiences. The pragmatic trial was designed in close adherence with the PRECIS-2 criteria (18) to test the feasibility of implementing the APPS intervention within the course of UC for hypoxic nonintubated patients with COVID-19. Five inpatient medical service teams were randomly allocated to two treatment arms: 1) UC, consisting of current, standard management of hypoxia and COVID-19; or 2) the APPS plus UC. We compared clinical and safety outcomes between patients in the UC and APPS treatment arms. Alongside the pragmatic trial, we collected qualitative data from semistructured interviews with patients in the APPS treatment arm who did and did not adhere to the prescribed prone strategy and clinicians who provided care to trial participants. The trial was approved by the Atrium Health Institutional Review Board (06-20-03E) with a waiver of individual informed consent.
Setting and Population
The study occurred at a quaternary referral center in Charlotte, North Carolina. Adult patients were eligible if they were admitted to the hospital by one of the study teams, had tested positive for SARS-CoV-2 within 7 days or were suspected to have COVID-19 pneumonia, and experienced 1) room air oxygen saturation <93% or 2) oxygen requirement of 3 L per minute or greater without the need for mechanical ventilation. Patients contraindicated for APPS intervention (e.g., unable to self-turn, spinal instability, facial or pelvic fractures, open chest or abdomen, altered mental status, anticipated difficult airway, signs of respiratory fatigue, or receiving end-of-life care) were ineligible. Patients were only eligible once for trial participation. The full trial protocol is available in the online supplement.
Randomization and Blinding
Five medical admitting teams were randomized using computer-generated random numbers. Teams were randomized in a near 1:1 ratio to deliver UC alone (n = 2 clusters) versus UC plus APPS intervention (n = 3 clusters). Eligible patients followed the care strategy to which their admitting team was randomized. Clinicians were unblinded to treatment allocation, and enrolled patients were considered unblinded. Clinical and safety outcomes were collected from the electronic health record by study investigators blinded to treatment assignment (S.P.T. and M.A.K.).
Study Procedures
The trial adopted a pragmatic approach to testing the APPS. Broadly, the APPS is a strategy of guiding hospitalized patients with COVID-19 to adopt the prone position when hypoxia thresholds are met. Importantly, the prone position applied on awake patients depends on tolerance and adherence. The APPS protocol combined 1) delivery of prone positioning education and explanation of risks and benefits to patients by bedside clinicians; 2) routine monitoring for worsening status; and 3) attempts to improve comfort as needed. Patients were encouraged to sustain the prone position as long as possible but were allowed to return to the supine position as necessary.
Medical teams assigned to the intervention arm received 1-hour educational training on the APPS and trial processes before patient enrollment. Teams randomly assigned to APPS were instructed to commence prone positioning for eligible patients with COVID-19 beginning at the time of eligibility and sustained for at least 48 hours or until intubation, intensive care unit (ICU) transfer, hospital discharge, or death. Because this pilot trial evaluated the addition of APPS within the context of usual care, usual care provided to patients is determined by clinical assessments and need. Prone positioning was neither encouraged nor disallowed. We expected UC to be consistent with routine practice for hospitalized patients with COVID-19–related hypoxia and to be similar for patients enrolled in both groups.
Embedded Implementation Evaluation Procedures
We used qualitative methods to obtain contextual insight and assess implementation outcomes from patients and clinicians involved in the care of hypoxic patients with COVID-19 (physicians, respiratory therapists, and nurses). Patients who participated in the pilot RCT were contacted 48 hours after enrollment to complete a telephone interview. Patients in the APPS arm who did and did not adhere to the APPS were invited to participate in the interviews. Clinicians providing care for patients in the pilot RCT were also contacted to participate in an interview. The interviewers had access to translation services, and there were no exclusion criteria. Informed assent was obtained before the interview.
The semistructured telephone interviews were given in either English or Spanish. Patient interviews sought a subjective, experiential knowledge of prone positioning. Clinician interviewees were queried about their experiences with APPS and their opinions on the feasibility of an RCT and were asked to reflect on the practical and ethical considerations of conducting an APPS trial.
Data Collection
We collected and managed quantitative trial data at the time of eligibility, 48 hours, and hospital discharge using REDCap electronic data capture tools hosted at Atrium Health (19). Patient demographics, clinical characteristics, receipt of intensive care, receipt of adjunctive therapies, adverse events, hospital length of stay, and discharge disposition were abstracted via clinical chart review and directly from the health system’s enterprise data warehouse. Clinicians’ recommendation to prone and patients’ attempt to prone were collected via chart review of physician and nurse progress notes—absence of documentation that prone positioning had been prescribed or that the patient agreed to prone positioning was recorded as “not prescribed” and “not attempted.” Separately, we reviewed progress notes and nursing flow sheets to identify documentation of actual duration of prone positioning.
Outcomes
As a pilot trial, the primary objective was to establish outcomes relative to successful implementation of a future definitive RCT. The study outcomes, shown in Table 1, were guided by an implementation science framework (17, 20). Specific research outcomes included nadir oxygen saturation to fraction of inspired oxygen (S/F) ratio, time spent with S/F ratio less than 315 (a threshold shown to be correlated with P/F ratio [ratio of arterial oxygen partial pressure to fractional inspired oxygen] of 300) (21, 22), receipt of intensive care, greater than 6 L/min oxygen support, intubation, hospital length of stay, and hospital mortality.
Table 1.
Results of convergent mixed-methods analysis presented using implementation outcome framework with associated implications for a future RCT
| Implementation Outcome | Quantitative Results | Qualitative Results with Example Quotes | Implication for Future RCT |
|---|---|---|---|
| Acceptability of an RCT evaluating APPS | 57% of clinicians perceive randomization to control group unacceptable | Clinicians report perceived lack of equipoise for prone positioning, lack of alternatives: “[Avoiding proning] is a little bit more challenging to me because we obviously don’t have a lot of things that work for COVID…I think a lot of the patients do respond to going to a prone position as far as their oxygen saturation levels, so, telling people to not do that, I do have a little hesitation with that.” |
May hinder recruitment or lead to selection bias; consider clinician education strategy or switch to nontraditional (e.g., quasi-experimental) trial design |
| Adoption of an RCT evaluating APPS | 74% of physicians assigned to APPS prescribed the intervention to eligible patients | May require modifying intervention to encourage uptake, anticipate dilution of treatment effect in intention-to-treat analyses | |
| Appropriateness of an RCT evaluating APPS | 71% of clinicians reported that trial intervention has become usual care | Consider organizational education strategy to reinforce equipoise or quasi-experimental design | |
| Effectiveness of an RCT evaluating APPS | Direction of research outcomes favored prone positioning. 100% of respondents endorsed ICU use and/or advanced respiratory support rates to be relevant and patient-centered primary outcome |
Patients subjectively felt that prone positioning improved their breathing: “I mean, [proning] did work.” “Yes. [Proning] actually helps, a lot.” “Thank God, I feel better now.” |
Further investigation of prone positioning for nonintubated patients in larger studies likely warranted; potential patient-centered outcomes might include ICU or advanced respiratory support use |
| Equity of an RCT evaluating APPS | Lower rates of adherence among Black (19%) compared with white (56%) and non-Black Hispanic (71%) patients | Develop culturally tailored approaches to reduce disparities in adherence | |
| Feasibility of an RCT evaluating APPS | 95% enrollment rate (only 2 patients met exclusion criteria). 98% of patients completed the study. Only 2 of 27 patients had documentation of prone position duration. Outcome data collection: no missing data for ICU transfer, advanced respiratory support, or mortality |
Nurses reported adherence to a strict positioning schedule to be challenging owing to complexities of care environment: “Working on the COVID unit is just so unpredictable and everybody can be fine one minute and the next they are not. So, we could say at 2:00 we are going to prone all of our patients for 1 h; however, at 2:00, everything can go awry and we get nobody proned, and then it would be completely off schedule.…So, there would definitely be a lot of challenges to it.” |
Tailor strategies to reduce complexity and increase flexibility of the intervention delivery protocol. Traditional RCTs with active data collection or novel approaches such as smart phone applications and patient-reported measures will be needed if reliable estimates of prone duration are desired. Otherwise, pragmatic trials should not plan specific analyses around these data |
| Fidelity of an RCT evaluating APPS | 50% of patients had protocol violations/crossovers. 0% of patients managed the 12- to 16-h prone target time suggested by clinicians. Patients estimated spending between 10 and 120 min a day in prone position |
Patients perceived prone positioning to be difficult: “Just that at the time, in the condition that my body was in, I could not bear it for too long with my back pain. So, I had to turn a lot.” |
Will require strategies to enhance organizational and individual buy-in and improve comfort/tolerability. Consider more flexible prone time targets than those recommended in mechanically ventilated patients. Anticipate dilution of treatment effect, plan education strategy to clinicians to limit crossovers |
| Penetration of an RCT evaluating APPS | No patients experienced intubation or death during hospitalization | Adapt recruitment strategies, may require inclusion of nonintubated patients admitted to ICU |
Definition of abbreviations: APPS = Awake Prone Positioning Strategy; COVID = coronavirus disease; ICU = intensive care unit; RCT = randomized controlled trial.
Safety Monitoring
The safety of the APPS intervention was explored by the number of adverse events documented. In mechanically ventilated patients, prone positioning can be associated with complications such as pressure wounds, unintentional extubation, loss of intravenous and other catheters, facial edema, corneal ulceration, and pressure neuropathies (23). Owing to patients’ awake, spontaneous breathing status, allowance of terminating the prone positioning per patient request, and the short duration of the study, these complications were not anticipated. We assessed potential serious adverse events (emergent intubation [defined as intubation on medical floor] and new anterior pressure wound) as well as a nonserious adverse event (loss of intravenous catheter).
Statistical Analysis
Process and implementation objectives
Process and implementation outcomes were assessed using mixed-methods data from study records and telephone interviews. We calculated descriptive statistics to measure the proportion of participants who 1) found the randomization approach acceptable (clinician and patient), 2) adhered to the assigned intervention strategy (patient), and 3) perceived the APPS treatment protocol practical to implement within the context of routine care using existing resources (clinician). Qualitative data analysis of the interviews was interpretive and iterative, using a thematic analysis approach. Interview recordings were transcribed for coding and thematic analysis was performed using ATLAS.ti 8 Windows (Scientific Software Development GmbH) to assess contextual influencers for the implementation of a successful future RCT. Data saturation was determined to be reached when thematic redundancy was observed beginning with the 11th interview.
Research objectives
Baseline demographic and clinical data were summarized for the intention-to-treat and as-treated population groups. Continuous variables were summarized using mean, standard deviation, median, and interquartile range estimates, and categorical variables were described using frequency and percentages.
Statistical analyses were conducted on clinical outcomes to describe and explore changes in defined endpoints, using both intention-to-treat and as-treated groups. Separation between the groups was evaluated by nadir S/F ratio and the time spent with S/F ratio <315 in the first 48 hours following randomization. Median S/F ratios were plotted to visualize longitudinal trajectory of patient groups from baseline to 48 hours. We used descriptive statistics to measure the frequency of secondary outcomes and adverse events overall and in each treatment group. For each measure, 95% confidence intervals (95% CIs) were calculated. Analyses of clinical outcomes were conducted using SAS Enterprise Guide 7.1 (SAS Institute Inc.).
Results
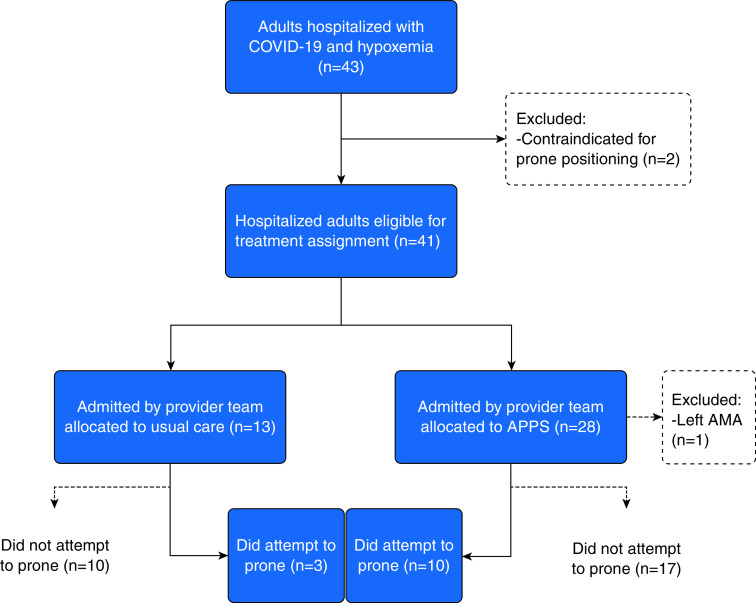
Between June and August 2020, a total of 43 patients met inclusion criteria (Figure 1). Of these, two patients (5%) met one or more exclusion criteria. At the time of eligibility, 23 (53%) patients had positive testing for SARS-CoV-2 and 20 (47%) patients were suspected to have COVID-19. Eight (19%) patients ultimately had negative testing for SARS-CoV-2. Thirteen patients were admitted to the two teams randomly assigned to the UC group and 28 patients were admitted to three teams assigned to the APPS group. One patient randomized to the APPS group left against medical advice before being prescribed prone positioning and was removed from the study, leaving 40 (98%) patients who completed the study. Ten (37%) patients in the APPS group and three (23%) patients in the UC group attempted prone positioning.
Figure 1.
Flow diagram of patients with COVID-19 acute respiratory failure enrolled into the APPS pilot trial. AMA = against medical advice; APPS = Awake Prone Positioning Strategy; COVID-19 = coronavirus disease.
Baseline Patient Characteristics
As expected, there were baseline imbalances between groups (Table 2). In the as-treated population comparisons, patients who did not attempt prone positioning more frequently were male, were Black, had chronic lung disease or heart failure, and had 6 or more pack-years’ smoking history compared with those who did attempt prone positioning.
Table 2.
Baseline demographics and clinical characteristics of patients with COVID-19 acute respiratory failure enrolled into the APPS pilot trial, by assigned treatment and prone positioning status within 48 hours
| Assigned Treatment Group |
Prone Positioning Status |
|||
|---|---|---|---|---|
| Usual Care (n = 13) | APPS (n = 27) | Did Not Attempt (n = 27) | Did Attempt (n = 13) | |
| Median age at admission (IQR), yr | 60 (54–63) | 56 (45–66) | 61 (55–66) | 50 (45–57) |
| Female sex, n (%) | 3 (23) | 10 (37) | 7 (26) | 6 (46) |
| Race | ||||
| Black | 6 (46) | 16 (59) | 19 (70) | 3 (23) |
| White | 5 (38) | 9 (33) | 7 (26) | 7 (54) |
| Other | 2 (16) | 2 (8) | 1 (4) | 3 (23) |
| Hispanic or Latino, n (%) | 3 (23) | 7 (26) | 4 (15) | 6 (46) |
| Comorbid conditions, n (%) | ||||
| None | 5 (19) | 2 (15) | 4 (15) | 3 (23) |
| Chronic lung disease | 3 (23) | 6 (22) | 8 (30) | 1 (8) |
| Chronic renal disease | 2 (15) | 7 (26) | 6 (22) | 3 (23) |
| Diabetes | 5 (38) | 10 (37) | 9 (33) | 6 (46) |
| Heart failure | 4 (31) | 5 (19) | 7 (26) | 2 (15) |
| Median BMI (IQR), kg/m2 | 31 (28–38) | 29 (26–39) | 31 (27–37) | 30 (27–42) |
| BMI >30 kg/m2, n (%) | 6 (46) | 14 (52) | 14 (52) | 6 (46) |
| Smoking history, n (%) | ||||
| Unknown | 0 (0) | 2 (7) | 2 (7) | 0 (0) |
| Never | 7 (54) | 19 (70) | 15 (56) | 11 (85) |
| 1–5 cigarette pack-years | 1 (8) | 3 (11) | 2 (7) | 2 (15) |
| 6 or more cigarette pack-years | 5 (38) | 3 (11) | 8 (30) | 0 (0) |
| Median pneumonia severity index (IQR) | 81 (67–84) | 72 (57–95) | 79 (66–106) | 64 (45–80) |
| Median hours from presentation to eligibility (IQR) | 7 (2–26) | 4 (1–24) | 6 (2–37) | 4 (0–7) |
| Suspected COVID-19 at time of eligibility*, n (%) | 5 (39) | 10 (37) | 11 (41) | 4 (31) |
| Median oxygen saturation at baseline (IQR) | 93 (91–95) | 92 (89–94) | 93 (90–94) | 92 (90–93) |
| Oxygen support at baseline, n (%) | ||||
| Room air | 1 (8) | 0 (0) | 1 (4) | 0 (0) |
| <4 L nasal cannula | 7 (54) | 15 (56) | 18 (67) | 4 (31) |
| 4–6 L nasal cannula | 3 (23) | 11 (41) | 5 (19) | 9 (69) |
| Medium flow nasal cannula | 2 (15) | 0 (0) | 2 (15) | 0 (0) |
| Humidified high-flow nasal cannula | 0 | 0 (0) | 0 (0) | 0 (0) |
| Bilevel positive airway pressure | 0 | 1 (4) | 1 (4) | 0 (0) |
Definition of abbreviations: APPS = Awake Prone Positioning Strategy; BMI = body mass index; COVID-19 = coronavirus disease; IQR = interquartile range.
COVID-19 testing ultimately negative (n = 8): by assigned treatment group (usual care [n = 2] vs. APPS [n = 6]); by prone positioning status (did not attempt [n = 7] vs. did attempt [n = 1]).
Embedded Implementation Evaluation
Nine patients and eight clinicians were contacted for inclusion; one clinician and two patients were not reached, and one patient declined. Six patients and seven clinicians (three physicians, three nurses, and one respiratory therapist) agreed to participate. Patient interviews ranged from 4 to 9 minutes (mean = 6 min), and clinician interviews ranged from 8 to 12 minutes (mean = 10 min). Four of seven clinicians (57%) felt that randomizing patients to a no-prone-positioning control group was unacceptable. Similarly, four of seven clinicians (57%) reported significant logistical barriers to the conduct of an RCT evaluating a prone positioning protocol. None of the patients interviewed reported being hesitant to try prone positioning. However, four of six patient interviewees (67%) found the position uncomfortable or intolerable in practice. Although six of seven clinicians (86%) endorsed 12–16 hours as the daily recommended prone time, patients reported that they were only able to lie prone for between 10 and 120 minutes per day. Nurses also indicated that recommended prone time targets were unfeasible because of competing demands of other care tasks requiring supine or upright positioning. Although tolerability was limited, five of six patients (83%) reported that prone positioning subjectively improved their dyspnea. The results of the convergent mixed-methods analysis using a combination of electronic health record data, study records, and interview data are presented in Table 1, organized by implementation outcome. Additional qualitative findings are provided in Table E1.
Oxygenation outcomes
There were no missing values for oxygen saturation or fraction of inspired oxygen. Figures 2A and 2B show the distribution of the lowest S/F ratios for patients over the first 48 hours of the intervention based on individual patient measurements. Patients in the UC group had a median nadir S/F ratio over the 48-hour study period of 216 (95% CI, 95–303) versus 253 (95% CI, 197–267) in the APPS group (intraclass correlation coefficient, ρ = 0.11; 95% CI, 0.05–0.18). In the as-treated populations, patients who did not attempt prone positioning had a median nadir S/F ratio of 225 (95% CI, 196–258) versus 256 (95% CI, 185–284) in patients who attempted prone positioning. Patients in the UC group spent 42 hours (95% CI, 13–47) of the 48-hour study period with an S/F ratio below 315 versus 20 hours (95% CI, 6–39) for patients in the APPS group. Patients who did not attempt prone positioning spent 30 hours (95% CI, 11–46) of the 48-hour study period with an S/F ratio below 315 versus 20 hours (95% CI, 0–45) for patients who attempted prone positioning. Figures 2C and 2D show longitudinal trends in median S/F ratios for patients in each group over the study period. Table 3 shows hospital outcomes experienced by patients by intention-to-treat and as-treated groups.
Figure 2.
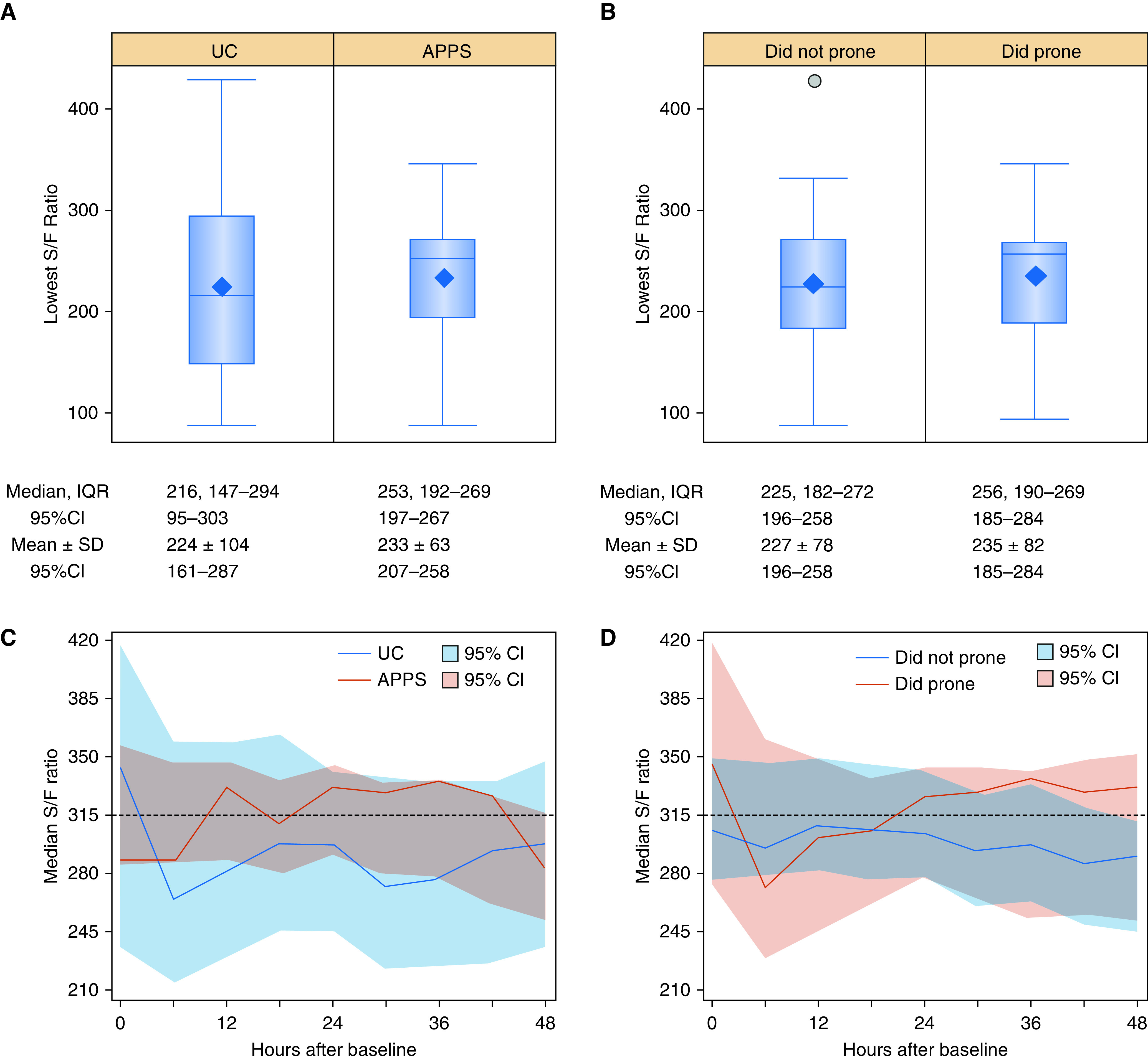
Separation of lowest S/F ratio and trends in median S/F ratio within 48 hours from baseline for patients with coronavirus disease (COVID-19) acute respiratory failure enrolled into the APPS pilot trial. Box and whisker plots depict the lowest S/F ratio for (A) patients randomly assigned to UC and APPS and (B) patients who did not prone and did prone. Median and IQR and mean and SD estimates along with 95% CIs are shown below the plot for each group. Outlier data points are presented with a circle for any estimates located at least 1.5 times the IQR below the first quartile or above the third quartile. The group median S/F ratio is shown plotted longitudinally at 6-hour intervals for (C) patients randomly assigned to UC and APPS and (D) patients who did not prone and did prone. APPS = Awake Prone Positioning Strategy; CI = confidence interval; IQR = interquartile range; SD = standard deviation; S/F = oxygen saturation to fraction of inspired oxygen; UC = usual care.
Table 3.
Hospital outcomes experienced by patients with COVID-19 acute respiratory failure enrolled into the APPS pilot trial, by assigned treatment and prone positioning status within 48 hours
| Assigned Treatment Group |
Prone Positioning Status |
|||
|---|---|---|---|---|
| Usual Care (n = 13) | APPS (n = 27) | Did Not Attempt (n = 27) | Did Attempt (n = 13) | |
| Attempted prone position within 48 h | 3 (23) | 10 (37) | 0 (100) | 13 (100) |
| Oxygen support required 48 h after baseline | ||||
| Room air | 2 (15) | 0 (0) | 2 (7) | 0 (0) |
| <4 L nasal cannula | 3 (23) | 10 (37) | 10 (37) | 3 (23) |
| 4–6 L nasal cannula | 1 (8) | 12 (44) | 5 (19) | 8 (62) |
| Medium-flow nasal cannula | 2 (15) | 2 (7) | 4 (15) | 0 (0) |
| Humidified high-flow nasal cannula | 3 (23) | 2 (7) | 3 (11) | 2 (15) |
| Bilevel positive airway pressure | 2 (15) | 1 (4) | 3 (11) | 0 (0) |
| Intubated | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Adjunctive therapies | ||||
| Corticosteroids | 9 (69) | 19 (70) | 19 (70) | 9 (69) |
| Remdesivir | 5 (38) | 10 (37) | 11 (41) | 4 (31) |
| Convalescent plasma | 2 (15) | 1 (4) | 1 (4) | 2 (15) |
| Adverse events within 48 h | ||||
| Anterior pressure wound | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Loss of intravenous catheter | 0 (0) | 1 (4) | 0 (0) | 1 (8) |
| Emergent intubation outside of the ICU | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Required ICU admission within 48 h | 3 (23) | 8 (30) | 9 (33) | 2 (15) |
| Required ICU admission during hospitalization | 6 (46) | 8 (30) | 11 (41) | 3 (23) |
| Required intubation during hospitalization | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Median hospital length of stay (IQR), d | 5 (2–9) | 6 (3–12) | 9 (3–13) | 5 (3–8) |
| Discharge disposition | ||||
| Expired | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Home | 11 (84) | 24 (89) | 23 (85) | 12 (92) |
| Skilled nursing facility | 1 (8) | 2 (7) | 2 (7) | 1 (8) |
| Acute care hospital | 1 (8) | 1 (4) | 2 (7) | 0 (0) |
Definition of abbreviations: APPS = Awake Prone Positioning Strategy; COVID-19 = coronavirus disease; ICU = intensive care unit; IQR = interquartile range.
Safety monitoring
There were no serious adverse events. One patient in the APPS group experienced loss of an intravenous catheter that was deemed likely to be related to the trial intervention.
Discussion
This study demonstrates a novel approach to pilot study design using an explicit implementation science framework to ensure that key contextual factors are identified and addressed. We prioritized rapid knowledge gain that can be used to make responsive adjustments to a planned RCT.
Table 1 shows how the results of the study can be used to inform a future trial evaluating a prone positioning strategy for nonintubated patients. Key applications of our results to the design, eligibility criteria, intervention, data collection, and outcome selection of a future trial evaluating awake prone positioning are discussed briefly here.
Design
Our findings inform the choice of study design to evaluate an awake prone positioning strategy. Clinician interviews indicated that patient-level randomization could be problematic owing to a perceived lack of equipoise about the intervention, and nurses indicated that applying different prone positioning strategies to patients on the same unit would be operationally difficult. Additionally, we found that patients met eligibility criteria within a few hours from arrival to the hospital, and the cluster-level design may obviate delays in promptly applying prone positioning in the intervention group. However, a disadvantage of the cluster-level randomization is observed diffusion of prone positioning into the UC group, reinforced by interview data whereby many clinicians revealed that prone positioning was already considered UC for nonintubated patients in their setting. The implications of these findings include lack of group separation in pragmatic trials due to treatment contamination in the UC group. Given the challenges identified with both individual- and cluster-level randomized designs, quasi-experimental approaches such as regression discontinuity designs also warrant consideration (24, 25).
Eligibility Criteria
Our pilot study included suspected COVID-19 diagnosis in the eligibility criteria, resulting in the inclusion of patients who ultimately tested negative for SARS-CoV-2. Investigators must consider tradeoffs associated with maintaining fidelity of the target study population versus avoiding delays in intervention assignment while awaiting test results for eligibility determination. Additional considerations for selecting eligibility criteria include enrichment strategies to target patients at increased risk of poor outcomes, as none of the patients in our study experienced intubation or death during hospitalization. However, our clinician interviews suggest that the tradeoff for enrolling more severely ill patients could be less willingness to withhold prone positioning.
Data Collection
Our results indicate that assessing fidelity to prone positioning is a major challenge in a pragmatic study design using data captured for clinical use. Whereas clinical documentation agreed with interview data for whether patients attempted prone positioning, only two patients had chart documentation of time spent in prone position, and nurse interviewees confirmed low accuracy of this data element.
Outcome Selection
Given the small size of the pilot study, we used S/F ratio as a surrogate marker to explore physiologic differences owing to its availability, noninvasive collection, and demonstrated concordance with P/F ratios (21). However, the S/F ratio is highly variable depending on the oxygen support strategy chosen and may be less accurate in dark-skinned individuals (26). Ideally, a future RCT would evaluate clinically relevant endpoints such as requirement for intensive care, advanced respiratory support, or mortality, which were all viewed as important, patient-centered outcomes by our interviewees. However, low event rates and power are tradeoffs to consider.
Intervention
Important findings include low adoption of prone positioning by patients. In our study, using a pragmatic approach to intervention implementation, fewer than half of patients assigned to the APPS group had documentation of attempted prone positioning, with particularly low uptake among Black patients. Our qualitative analysis indicates that reasons for poor uptake of prone positioning included patient comfort, anxiety, and interference with other aspects of care. Broad uptake of prone positioning within the context of a clinical trial will require dedicated efforts to address these barriers to intervention adherence, with emphasis on addressing disparities in uptake. Second, we found a striking disconnect between the daily time clinicians considered necessary for patients to adhere to prone positioning, which ranged from 12 to 16 hours, and the daily time patients reported spending in the prone position, which most patient interviewees estimated at a little over an hour. In addition to addressing barriers to tolerability of prone positioning, future clinical trials may need to decrease the “dose” of prone positioning required to consider nonintubated patients adherent with trial protocols, rather than extrapolate high values from trials in mechanically ventilated patients (3–6).
Our study has notable limitations. First, the study was small and not appropriate to assess estimates of treatment effectiveness—our results should be considered exploratory to inform future investigations. Second, although interview responses typically matched chart documentation for adoption of prone positioning, our assessments of adoption rates based on clinical documentation may be inaccurate. Third, the study occurred at a single site, which may limit generalizability for implementation of an RCT at sites with different operational and clinical structures. Finally, although our interview sampling strategy included multidisciplinary clinicians to obtain a variety of perspectives, the views of respondents may differ from those who chose not to participate.
Conclusions
We describe a novel approach to improving the likelihood of a rigorous, generalizable RCT by first rapidly conducting a pilot study with embedded implementation science evaluation. Our results suggest that a definitive trial evaluating the effect of prone positioning in nonintubated patients with COVID-19 is warranted, but they uncover several considerations for the design and conduct of such a trial.
Footnotes
The trial was funded internally as part of the Atrium Health COVID-19 Research Task Force initiative, with support from the Atrium Health Center for Outcomes Research and Evaluation. S.P.T. and M.A.K. received funding from the National Institutes of Health (NIH).
Author Contributions: S.P.T. and M.A.K. had full access to the data in the study, take responsibility for the integrity of the data and the accuracy of the data analysis, and are the guarantors of the content of this manuscript. S.P.T., B.T., and M.A.K. contributed to study concept and design. H.B., W.M.S., and S.S. contributed to qualitative data collection. H.B. and M.A.K. contributed to data analysis. S.P.T. and M.A.K. contributed to the initial drafting of the manuscript. All authors contributed to the interpretation of the data and critically revised the manuscript for important intellectual content.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abroug F, Ouanes-Besbes L, Elatrous S, Brochard L. The effect of prone positioning in acute respiratory distress syndrome or acute lung injury: a meta-analysis: areas of uncertainty and recommendations for research. Intensive Care Med. 2008;34:1002–1011. doi: 10.1007/s00134-008-1062-3. [DOI] [PubMed] [Google Scholar]
- 4. Sud S, Friedrich JO, Taccone P, Polli F, Adhikari NK, Latini R, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med. 2010;36:585–599. doi: 10.1007/s00134-009-1748-1. [DOI] [PubMed] [Google Scholar]
- 5. Gattinoni L, Carlesso E, Taccone P, Polli F, Guérin C, Mancebo J. Prone positioning improves survival in severe ARDS: a pathophysiologic review and individual patient meta-analysis. Minerva Anestesiol. 2010;76:448–454. [PubMed] [Google Scholar]
- 6. Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 7. Lamm WJE, Graham MM, Albert RK. Mechanism by which the prone position improves oxygenation in acute lung injury. Am J Respir Crit Care Med. 1994;150:184–193. doi: 10.1164/ajrccm.150.1.8025748. [DOI] [PubMed] [Google Scholar]
- 8. Henderson AC, Sá RC, Theilmann RJ, Buxton RB, Prisk GK, Hopkins SR. The gravitational distribution of ventilation-perfusion ratio is more uniform in prone than supine posture in the normal human lung. J Appl Physiol (1985) 2013;115:313–324. doi: 10.1152/japplphysiol.01531.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gattinoni L, Pelosi P, Vitale G, Pesenti A, D’Andrea L, Mascheroni D. Body position changes redistribute lung computed-tomographic density in patients with acute respiratory failure. Anesthesiology. 1991;74:15–23. doi: 10.1097/00000542-199101000-00004. [DOI] [PubMed] [Google Scholar]
- 10. Scaravilli V, Grasselli G, Castagna L, Zanella A, Isgrò S, Lucchini A, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30:1390–1394. doi: 10.1016/j.jcrc.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 11. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24:28. doi: 10.1186/s13054-020-2738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10:33. doi: 10.1186/s13613-020-00650-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taboada M, Rodríguez N, Riveiro V, Abelleira R, Ricoy J, Lama A, et al. Short-term outcomes of 50 patients with acute respiratory distress by COVID-19 where prone positioning was used outside the ICU. J Clin Anesth. 2020;67:110028. doi: 10.1016/j.jclinane.2020.110028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. ClinicalTrials.gov Identifier: NCT04344587. 2020 [accessed 2020 Sep 10]. Available from: https://clinicaltrials.gov/ct2/show/NCT04344587.
- 15. ClinicalTrials.gov Identifier: NCT04383613. 2020 [accessed 2020 Sep 10]. Available from: https://clinicaltrials.gov/ct2/show/NCT04383613.
- 16. ClinicalTrials.gov Identifier: NCT04359797. 2020 [accessed 2020 Sep 10]. Available from: https://clinicaltrials.gov/ct2/show/NCT04359797.
- 17. Taylor SP, Kowalkowski MA. Using implementation science-guided pilot studies to assess and improve the informativeness of clinical trials. J Gen Intern Med. doi: 10.1007/s11606-020-06220-3. [online ahead of print] 11 Sep 2020; DOI: 10.1007/s11606-020-06220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147. doi: 10.1136/bmj.h2147. [DOI] [PubMed] [Google Scholar]
- 19. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. REDCap Consortium. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65–76. doi: 10.1007/s10488-010-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adams JY, Rogers AJ, Schuler A, Marelich GP, Fresco JM, Taylor SL, et al. Association between peripheral blood oxygen saturation (SpO2)/fraction of inspired oxygen (FiO2) ratio time at risk and hospital mortality in mechanically ventilated patients. Perm J. 2020;24:19.114. doi: 10.7812/TPP/19.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rice TW, Wheeler AP, Bernard GR, Hayden DL, Schoenfeld DA, Ware LB. National Institutes of Health, National Heart, Lung, and Blood Institute ARDS Network. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132:410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 23. Bloomfield R, Noble DW, Sudlow A. Prone position for acute respiratory failure in adults. Cochrane Database Syst Rev. 2015;2015:CD008095. doi: 10.1002/14651858.CD008095.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moscoe E, Bor J, Bärnighausen T. Regression discontinuity designs are underutilized in medicine, epidemiology, and public health: a review of current and best practice. J Clin Epidemiol. 2015;68:122–133. doi: 10.1016/j.jclinepi.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 25. Walkey AJ, Drainoni ML, Cordella N, Bor J. Advancing quality improvement with regression discontinuity designs. Ann Am Thorac Soc. 2018;15:523–529. doi: 10.1513/AnnalsATS.201712-942IP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bickler PE, Feiner JR, Severinghaus JW. Effects of skin pigmentation on pulse oximeter accuracy at low saturation. Anesthesiology. 2005;102:715–719. doi: 10.1097/00000542-200504000-00004. [DOI] [PubMed] [Google Scholar]