Abstract
Background
Symptomatic peripheral arterial disease (PAD) has several treatment options, including angioplasty, stenting, exercise therapy, and bypass surgery. Atherectomy is an alternative procedure, in which atheroma is cut or ground away within the artery. This is the first update of a Cochrane Review published in 2014.
Objectives
To evaluate the effectiveness of atherectomy for peripheral arterial disease compared to other established treatments.
Search methods
The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, Cumulative Index to Nursing and Allied Health Literature (CINAHL) and Allied and Complementary Medicine (AMED) databases, and the World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov trials registers to 12 August 2019.
Selection criteria
We included all randomised controlled trials that compared atherectomy with other established treatments. All participants had symptomatic PAD with either claudication or critical limb ischaemia and evidence of lower limb arterial disease.
Data collection and analysis
Two review authors screened studies for inclusion, extracted data, assessed risk of bias and used GRADE criteria to assess the certainty of the evidence. We resolved any disagreements through discussion. Outcomes of interest were: primary patency (at six and 12 months), all‐cause mortality, fatal and non‐fatal cardiovascular events, initial technical failure rates, target vessel revascularisation rates (TVR; at six and 12 months); and complications.
Main results
We included seven studies, with a total of 527 participants and 581 treated lesions. We found two comparisons: atherectomy versus balloon angioplasty (BA) and atherectomy versus BA with primary stenting. No studies compared atherectomy with bypass surgery. Overall, the evidence from this review was of very low certainty, due to a high risk of bias, imprecision and inconsistency.
Six studies (372 participants, 427 treated lesions) compared atherectomy versus BA. We found no clear difference between atherectomy and BA for the primary outcomes: six‐month primary patency rates (risk ratio (RR) 1.06, 95% confidence interval (CI) 0.94 to 1.20; 3 studies, 186 participants; very low‐certainty evidence); 12‐month primary patency rates (RR 1.20, 95% CI 0.78 to 1.84; 2 studies, 149 participants; very low‐certainty evidence) or mortality rates (RR 0.50, 95% CI 0.10 to 2.66, 3 studies, 210 participants, very low‐certainty evidence). One study reported cardiac failure and acute coronary syndrome as causes of death at 24 months but it was unclear which arm the participants belonged to, and one study reported no cardiovascular events.
There was no clear difference when examining: initial technical failure rates (RR 0.48, 95% CI 0.22 to 1.08; 6 studies, 425 treated vessels; very low‐certainty evidence), six‐month TVR (RR 0.51, 95% CI 0.06 to 4.42; 2 studies, 136 treated vessels; very low‐certainty evidence) or 12‐month TVR (RR 0.59, 95% CI 0.25 to 1.42; 3 studies, 176 treated vessels; very low‐certainty evidence). All six studies reported complication rates (RR 0.69, 95% CI 0.28 to 1.68; 6 studies, 387 participants; very low‐certainty evidence) and embolisation events (RR 2.51, 95% CI 0.64 to 9.80; 6 studies, 387 participants; very low‐certainty evidence). Atherectomy may be less likely to cause dissection (RR 0.28, 95% CI 0.14 to 0.54; 4 studies, 290 participants; very low‐certainty evidence) and may be associated with a reduction in bailout stenting (RR 0.26, 95% CI 0.09 to 0.74; 4 studies, 315 treated vessels; very low‐certainty evidence). Four studies reported amputation rates, with only one amputation event recorded in a BA participant. We used subgroup analysis to compare the effect of plain balloons/stents and drug‐eluting balloons/stents, but did not detect any differences between the subgroups.
One study (155 participants, 155 treated lesions) compared atherectomy versus BA and primary stenting, so comparison was extremely limited and subject to imprecision. This study did not report primary patency. The study reported one death (RR 0.38, 95% CI 0.04 to 3.23; 155 participants; very low‐certainty evidence) and three complication events (RR 7.04, 95% CI 0.80 to 62.23; 155 participants; very low‐certainty evidence) in a very small data set, making conclusions unreliable. We found no clear difference between the treatment arms in cardiovascular events (RR 0.38, 95% CI 0.04 to 3.23; 155 participants; very low‐certainty evidence). This study found no initial technical failure events, and TVR rates at six and 24 months showed little difference between treatment arms (RR 2.27, 95% CI 0.95 to 5.46; 155 participants; very low‐certainty evidence and RR 2.05, 95% CI 0.96 to 4.37; 155 participants; very low‐certainty evidence, respectively).
Authors' conclusions
This review update shows that the evidence is very uncertain about the effect of atherectomy on patency, mortality and cardiovascular event rates compared to plain balloon angioplasty, with or without stenting. We detected no clear differences in initial technical failure rates or TVR, but there may be reduced dissection and bailout stenting after atherectomy although this is uncertain. Included studies were small, heterogenous and at high risk of bias. Larger studies powered to detect clinically meaningful, patient‐centred outcomes are required.
Plain language summary
Atherectomy for peripheral arterial disease
Background
Peripheral arterial disease is a narrowing or blockage of the arteries in the legs. People with this condition can experience pain on walking, pain at rest, or leg ulceration due to poor blood supply. Treatment options are: surgery, using a blood vessel or graft to bypass the narrowed or blocked section of the artery; balloon angioplasty, when a deflated balloon is passed into the narrowing at the end of a wire, then blown up to stretch the artery; and stenting (used in addition to balloon angioplasty), which holds open the balloon‐stretched section for extra support. A final option, less commonly used, is a technique called atherectomy. This treatment cuts or grinds away the fatty deposition (atheroma) within the artery that is causing the narrowing or occlusion.
Key results
In this review, we compared atherectomy with the other treatment options described above. We also looked within the two groups to assess whether using drug‐releasing balloons or stents impacted on participants' outcomes. We identified seven studies with a total of 527 participants.
Six trials compared atherectomy against balloon angioplasty (372 participants, 427 treated lesions). We found no clear difference between the procedures when examining artery patency at six and 12 months, risk of death, initial procedure failure rates, need to re‐treat the artery, risk of forming clots (embolisation), complication rates or risk of amputation. We found that atherectomy was associated with lower rates of emergency stenting during the procedure and lower balloon inflation pressures when compared with balloon angioplasty alone. We found no difference in results depending on whether the balloons were drug‐releasing or not.
One study compared atherectomy against balloon angioplasty and primary stenting (155 participants and 155 treated lesions). This study did not report primary patency. We found no clear difference between the treatment arms in risk of death, complication rates, cardiovascular events and the need to re‐treat the artery. This study found no initial procedure failure events,
We did not find any studies that compared bypass surgery against atherectomy.
Certainty of the evidence
Overall, our certainty in the evidence is very low, which means we do not have confidence that our results show the true effect of the treatments. We downgraded our certainty in the evidence because the studies were at high risk of bias (lack of blinding of participants or assessors, several outcomes were not reported and a number of the participants did not complete the studies); the trials were all small; and their results were inconsistent.
Conclusions
In conclusion, we have found no clear difference in effect on patency, mortality or cardiovascular event rates when comparing atherectomy against balloon angioplasty with or without stenting. The limited evidence available does not support a significant advantage of atherectomy over conventional balloon angioplasty or stenting.
Summary of findings
Background
Description of the condition
Symptomatic peripheral arterial disease (PAD) may be treated by a number of options, including exercise therapy, angioplasty, stenting and bypass surgery (Fowkes 1998; Fowkes 2008; Watson 2008). Atherectomy is a competing technique that uses a rotating cutting blade to excise the atheroma (Garcia 2009). Due to the risk of vessel perforation, atherectomy tends to be performed only in the superficial femoral and popliteal arteries, though it may be used in infrapopliteal vessels. While established treatments have a strong evidence base and guidelines for their use (TASC II 2007), the outcomes for atherectomy are less well understood. The National Institute for Health and Care Excellence (NICE) in the UK published guidelines in 2011, stating that there was inadequate evidence, especially given the risk of embolisation, and therefore they would not support the use of atherectomy outside of clinical trials (NICE 2011). This guideline is still in place.
Description of the intervention
Atherectomy is an endovascular procedure for revascularisation. Pieces of atherosclerotic plaque are removed in order to increase the luminal diameter of the vessel (Schwarzwälder 2010). The procedure is normally performed percutaneously through a 7‐French (F) or 8‐F sheath, unless vessel access is difficult, in which case an arterial cut‐down is required. The mechanism used to remove pieces of plaque can involve a variety of techniques, but usually involves some kind of rotating cutting blade, often with a chamber to store the cut pieces.
Why it is important to do this review
This is an update of a Cochrane Review first published in 2014, which included four trials with small numbers of participants (Ambler 2014). The low number of included studies and participants made it difficult for the review authors to draw conclusions. This update is important to ensure that all current evidence from randomised trials that compare atherectomy with any established treatment for PAD is identified, in order to aid decision making.
Objectives
To evaluate the effectiveness of atherectomy for peripheral arterial disease compared to other established treatments.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that compared atherectomy with other established treatments, including angioplasty, stenting and bypass surgery.
Types of participants
We included participants with symptomatic peripheral arterial disease (PAD) with either claudication or critical limb ischaemia and evidence of lower limb arterial disease. We considered arterial disease in any peripheral territory. We excluded studies with participants who had previously had bypass, percutaneous transluminal angioplasty (PTA) or stents in the target lesion, as these treatments might affect the primary patency rates.
Types of interventions
We included RCTs that compared atherectomy against any established treatment for PAD, in order to evaluate the effectiveness of atherectomy. We identified the following comparisons for the inclusion criteria:
atherectomy versus balloon angioplasty, with or without stenting;
atherectomy plus adjunctive balloon angioplasty versus balloon angioplasty; and
atherectomy versus surgical bypass procedures.
Types of outcome measures
Primary outcomes
Primary vessel patency, as assessed by ankle brachial index (ABI), arterial doppler ultrasound or angiography at six months and one year, and as data available in the studies
All‐cause mortality at six months and one year, and as data available in the studies
Fatal and non‐fatal cardiovascular events at six months and one year, and as data available in the studies
Secondary outcomes
Immediate procedural and angiographic outcomes (technical failure rates)
Target vessel revascularisation rates (TVR)
Complication rates, including thrombus, embolus, perforation and aneurysm
-
Morbidity assessment, including:
tissue healing;
avoidance of any amputation; and
performance of less extensive amputation
Quality of life (QoL) outcomes, as measured in the included studies
Clinical and symptomatic outcomes, e.g. improved walking distance, symptom relief
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases for randomised controlled trials and controlled clinical trials without language, publication year or publication status restrictions:
Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web) (searched on 12 August 2019);
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 8) via the Cochrane Register of Studies Online (CRSO)
MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE) 1946 to Present (searched from 1 January 2017 to 12 August 2019);
Embase Ovid (searched from 1 January 2017 to 12 August 2019);
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; searched from 1 January 2017 to 12 August 2019);
AMED Ovid (Allied and Complementary Medicine Database; searched from 1 January 2017 to 12 August 2019).
The Information Specialist modelled search strategies for other databases on the search strategy designed for MEDLINE or CENTRAL. Where appropriate, the Information Specialist combined these with adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6, Lefebvre 2011). Search strategies for major databases are provided in Appendix 1.
The Information Specialist searched the following trials registries on 12 August 2019:
the World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch);
ClinicalTrials.gov (clinicaltrials.gov).
Searching other resources
We did not search any other resources.
Data collection and analysis
Selection of studies
Two review authors (BW and GA) independently selected trials for inclusion in the review. They resolved any disagreements through discussion. The section 'Criteria for considering studies for this review' details the inclusion criteria used for the selection process.
Data extraction and management
BW extracted the data, and GA cross‐checked them. They resolved any disagreements through discussion. BW extracted the following information for each trial.
Trial methods: method of randomisation, method of allocation
Participants: country of origin, age, sex distribution, severity of disease, as measured by the ABI and using the European Consensus definition of critical ischaemia (European Consensus Document 1989), inclusion and exclusion criteria
Interventions: type of procedure (atherectomy, angioplasty or bypass)
Outcomes: primary and secondary outcomes, as listed in ''Types of outcome measures'
We extracted data directly from the published papers using data extraction forms, and did not make any attempt to obtain additional unpublished data. We based all analyses on endpoint data from the individual clinical trials, which all provided intention‐to‐treat results. We synthesised the data by comparing group results and did not amalgamate individual participant data from different trials.
Assessment of risk of bias in included studies
Two review authors (BW, GA) assessed the included studies' risk of bias independently, using Cochrane's 'Risk of bias' tool, according to the guidelines given in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 (Higgins 2011).
The review authors assessed the following domains as 'low risk of bias', 'unclear risk of bias' or 'high risk of bias':
sequence generation;
allocation concealment;
blinding of personnel and participants;
blinding of outcome assessors;
incomplete outcome data;
selective outcome reporting; and
other risk of bias.
The Characteristics of included studies table reports the assessments for each individual study.
Measures of treatment effect
We measured the treatment effects for dichotomous outcomes using risk ratios (RR) with 95% confidence intervals (CI). For continuous outcomes, we measured treatment effects as the mean difference (MD) with 95% CI.
Unit of analysis issues
For the outcomes of mortality, fatal and non‐fatal cardiovascular events, complications, quality of life, and clinical and symptomatic outcomes, the unit of analysis was the individual participant rather than the treated vessel. Three trials included multiple treated vessels per participant in some cases (Dattilo 2014; Shammas 2011; Shammas 2012). This means that the observations from these trials will not be totally independent, and therefore should have less emphasis placed on them in the meta‐analysis. However, as the majority of participants in these trials had only one treated vessel, and very few (16%) had more than one treated vessel, it is not likely that this will have a large impact on the results presented below. We therefore did not feel it was necessary to introduce more sophisticated statistical methods such as meta‐regression to take account of these differences. We could not re‐examine the data at an individual participant level.
Dealing with missing data
We performed analysis on a complete case basis, and it was not necessary to contact authors for additional data.
Assessment of heterogeneity
We looked for clinical heterogeneity by examination of the study details, and used Chi2 tests to assess heterogeneity between trials, using P values less than 0.1 to indicate the possible presence of significant heterogeneity. Since trials contained low participant numbers, the power of this test is likely to be low if a small P value is used (Higgins 2011).
Assessment of reporting biases
We planned to assess the likelihood of potential publication bias using funnel plots, but we identified insufficient studies to create a funnel plot (Higgins 2011).
Data synthesis
We intended to pool data from all studies when the clinical procedures followed were comparable. Where possible, we used inverse‐variance random‐effects models for data synthesis because the included studies used different devices for atherectomy (clinical heterogeneity) (DerSimonian 1986). We used Review Manager 5.3 software to synthesise the data (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
We had planned to carry out subgroup analyses where the studies reported the presence or absence of concomitant illness such as diabetes, hypertension, hyperlipidaemia, or chronic kidney disease. We had also planned to conduct subgroup analyses if the studies reported data on smoking, gender of participants, lesion location, length and percentage of stenosis, including whether any studies classified lesion length and percentage of stenosis according to the Trans‐Atlantic Inter‐Society Consensus Document on Management of Peripheral Arterial Disease (TASC II) (TASC II 2007). However, the included studies did not report data on these subgroups.
We performed subgroup analysis to investigate the differences between:
atherectomy versus plain balloon angioplasty or drug‐eluting angioplasty;
atherectomy versus drug‐eluting stent plus angioplasty or plain stenting plus angioplasty.
We performed this subgroup analysis given the recent concerns regarding paclitaxel‐eluting devices and their potential correlation with increased risk of mortality, which arose from a systematic review and meta‐analysis by Katsanos 2018. There was significant heterogeneity between the subgroups for plain balloon angioplasty/stenting versus drug‐eluting balloon angioplasty/stenting in both groups, so we used random‐effect models to calculate the risk ratios.
Sensitivity analysis
Many participants in the atherectomy arm of the included studies underwent additional angioplasty. Not all studies specified details of this exactly, so we were unable to analyse these participants separately. The result of atherectomy is still considered successful even with additional angioplasty, so we included these participants in the atherectomy arm for analysis. Only one trial did not perform routine angioplasty with atherectomy (Vroegindeweij 1995). We performed sensitivity analysis to assess the effect of including this study in the overall meta‐analyses of the primary outcomes.
Summary of findings and assessment of the certainty of the evidence
We included 'Summary of findings' tables in this update to present the most important findings and the certainty of the evidence for the most clinically relevant outcomes. The seven outcomes in the 'Summary of findings' tables are: primary patency (six and 12 months); mortality; fatal and non‐fatal cardiovascular events; TVR (six and 12 months); and complications.
We included one 'Summary of findings' table for the comparison 'Atherectomy compared to balloon angioplasty for peripheral arterial disease' (Table 1) and one for 'Atherectomy compared to balloon angioplasty with primary stenting for peripheral arterial disease' (Table 2). We determined the certainty of the evidence for each outcome using the GRADE approach, which considers the overall risk of bias of the included studies, the directness of the evidence, inconsistency within the results, precision of the estimate and risk of publication bias (Guyatt 2008). We created the 'Summary of findings' tables using GRADEpro GDT 2015 software.
Summary of findings 1. Atherectomy compared to balloon angioplasty for peripheral arterial disease.
| Atherectomy compared to BA for PAD | |||||
| Patient or population: people with PAD Setting: hospital Intervention: atherectomy Comparison: BA | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with BA | Risk with atherectomy | ||||
|
Primary patency (follow‐up: 6 months) |
186 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWa | RR 1.06 (0.94 to 1.20) | Study population | |
| 575 per 1000 | 609 per 1000 (540 to 690) | ||||
|
Primary patency (follow‐up: 12 months) |
149 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWa | RR 1.20 (0.78 to 1.84) | Study population | |
| 671 per 1000 | 805 per 1000 (524 to 1000) | ||||
|
Mortality (follow‐up: 12 months) |
210 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWa | RR 0.50 (0.10 to 2.66) | Study population | |
| 102 per 1000 | 51 per 1000 (10 to 271) | ||||
|
Fatal and non‐fatal cardiovascular events (follow‐up: 24 months) |
160 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWa | — | Zeller 2017 reported cardiac failure and acute coronary syndrome as causes of death at 24 months, but it was unclear for which participants in which arms this was accountable for. Shammas 2011 declared embolic stroke and myocardial infarction to be secondary outcomes, but no events were recorded in either arm | |
|
TVR (follow‐up: 6 months) |
136 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWa | RR 0.51 (0.06 to 4.42) | Study population | |
| 70 per 1000 | 36 per 1000 (4 to 311) | ||||
|
TVR (follow‐up: 12 months) |
176 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWa | RR 0.59 (0.25 to 1.42) | Study population | |
| 140 per 1000 | 82 per 1000 (35 to 198) | ||||
|
Complication rates (follow‐up: 12 months) |
387 (6 RCTs) | ⊕⊝⊝⊝ VERY LOWa | RR 0.69 (0.28 to 1.68) |
Study population | |
| 219 per 1000 | 151 per 1000 (61 to 367) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BA: balloon angioplasty; CI: confidence interval; PAD: peripheral arterial disease; RR: risk ratio; TVR: target vessel revascularisation | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | |||||
a We downgraded by three steps due to risk of bias (lack of blinding and high rates of attrition); imprecision (small trials with few participants and events); and inconsistency (heterogeneity).
Summary of findings 2. Atherectomy compared to balloon angioplasty with primary stenting for peripheral arterial disease.
| Atherectomy compared to BA and primary stenting for PAD | |||||
| Patient or population: people with PAD Setting: hospital Intervention: atherectomy Comparison: BA with primary stenting | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with BA with primary stenting | Risk with atherectomy | ||||
|
Primary patency (follow‐up 6 months) |
Not reported for this comparison | ||||
|
Primary patency (follow‐up 12 months) |
Not reported for this comparison | ||||
|
Mortality (follow‐up: 24 months) |
155 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa | RR 0.38 (0.04 to 3.23) | Study population | |
| 40 per 1000 | 15 per 1000 (2 to 129) | ||||
|
Fatal and non‐fatal cardiovascular events (follow‐up: 24 months) |
155 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa | RR 0.38 (0.04, 3.23) | Ott 2017 reported 4 deaths at 24 months (3 deaths in the drug‐eluting balloon and stent arm and 1 death in the plain balloon and stent arm) which they attributed to underlying cardiovascular disease, but no specific causes were stated. | |
|
TVR (follow‐up: 6 months) |
155 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa | RR 2.27 (0.95 to 5.46) | Study population | |
| 80 per 1000 | 182 per 1000 (76 to 437) | ||||
|
TVR (follow‐up: 24 months) |
155 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa | RR 2.05 (0.96 to 4.37) | Study population | |
| 240 per 1000 | 492 per 1000 (230 to 1000) | ||||
|
Complication rates (follow‐up: 24 months) |
155 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa | RR 7.04 (0.80 to 62.23) | Ott 2017 reported 3 complications, all 3 of which were in the atherectomy arm: 2 vessel perforations and 1 flow‐limiting dissection. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BA: balloon angioplasty CI: confidence interval; PAD: peripheral arterial disease; RR: risk ratio; TVR: target vessel revascularisation | |||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
a We downgraded by three steps due to risk of bias (inadequate blinding and high rates of attrition); and imprecision (small trial size, few participants and events, and wide confidence intervals).
Results
Description of studies
Results of the search
See Figure 1.
1.
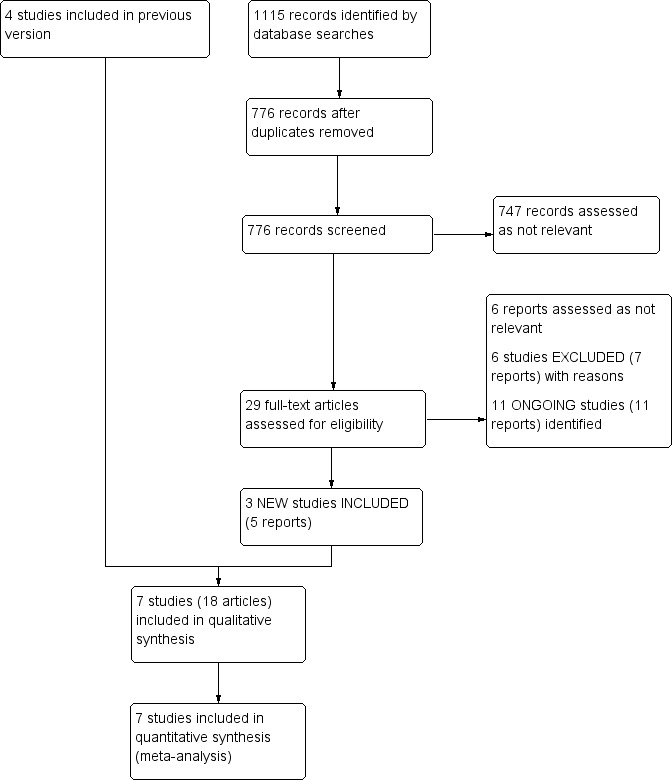
Study flow diagram.
Included studies
The 'Characteristics of included studies' table summarises the details of the included studies.
We identified three new studies for this update (Dattilo 2014; Ott 2017; Zeller 2017). Overall, seven studies involving 527 participants and 581 treated lesions met the selection criteria (Dattilo 2014; Nakamura 1995; Ott 2017; Shammas 2011; Shammas 2012; Vroegindeweij 1995; Zeller 2017). Six studies compared atherectomy to balloon angioplasty (BA) (Dattilo 2014; Nakamura 1995; Shammas 2011; Shammas 2012; Vroegindeweij 1995; Zeller 2017), and one study compared atherectomy to BA and primary stenting (Ott 2017). None of the studies used atherectomy followed by stenting as a primary intervention; they only used bailout stenting.
Four of the six BA studies reported on primary patency (Dattilo 2014; Nakamura 1995; Vroegindeweij 1995; Zeller 2017). Vroegindeweij 1995 reported follow‐up at three‐month intervals for two years. The study defined loss of patency as increased flow velocities (peak systolic velocity ratio (PSVR) ≥ 2.5) or absence of flow in occluded arterial segments on duplex ultrasound scan. Nakamura 1995 reported patencies at six months' follow‐up only, and assessed patency via arterial duplex. Zeller 2017 reported follow‐up patencies at six months and 12 months using duplex ultrasound (defined as PSVR ≤ 2.4). Dattilo 2014 reported clinical follow‐up along with ABIs and Rutherford classifications at 30 days, six months and 12 months. This trial also reported duplex ultrasound at six and 12 months, with primary patency defined as freedom from target lesion revascularisation or restenosis (PSV ≥ 2.5). Ott 2017, the primary stenting trial, did not report primary patency as one of their outcomes. Instead, the study reported target vessel revascularisation data. Shammas 2011 reported follow‐up target lesion revascularisation and target vessel revascularisation at 12 months. Shammas 2012 reported follow‐up target lesion revascularisation/target vessel revascularisation at three, six and 12 months.
There were also differences in modality of follow‐up between studies: Ott 2017 used angiogram, Dattilo 2014 and Vroegindeweij 1995 used duplex ultrasound, Zeller 2017 used duplex ultrasound at six months and plain ultrasound at 12 months, and Nakamura 1995 used doppler pressures. Neither Shammas 2011 nor Shammas 2012 used imaging at follow‐up, instead using ABI and clinical correlation.
Overall, there were a lot of differences in the clinical design and atherectomy devices used in each study.
Nakamura 1995 compared balloon angioplasty to transluminal extraction catheter (TEC) atherectomy (Stack 1988), followed by adjunctive balloon angioplasty in 39 participants with intermittent claudication (IC). TEC atherectomy utilises an over‐the‐wire device with a conical motorised cutting head with triangular blades, which rotate at 700 rpm, with a proximal suction apparatus that removes excised plaque. The study did not specify a medication protocol.
Vroegindeweij 1995 compared balloon angioplasty to Simpson atherectomy (Simpson 1988), in 73 participants with IC. The Simpson atherectomy device consists of cylindrical housing with a longitudinal opening down one side and a balloon on the other side. The balloon is inflated in order to both fix the device in place and press the longitudinal opening up against the wall of the vessel. A rotating cutting blade (2000 rpm) is then advanced through the cylinder so that any part of the vessel wall projecting through the longitudinal window will be cut away. The day before the procedure, all participants commenced low‐dose aspirin therapy.
Shammas 2011 compared balloon angioplasty to Silverhawk atherectomy followed by adjunctive balloon angioplasty in 58 participants with claudication, rest pain or minor tissue loss. The Silverhawk atherectomy device is similar to the Simpson device, described above, except the cylindrical housing is hinged in the region of the window, with the device flexing away from the window causing the tip and tail of the device to press up against one side of the vessel wall while the window is pressed up against the other side. In this trial, a distal embolism filter was used in approximately half of the participants. If participants were not already established on dual antiplatelet therapy (aspirin and clopidogrel), they were given loading doses of aspirin and clopidogrel immediately prior to the procedure. Participants on established therapy continued on their regular doses.
Both Shammas 2012 and Dattilo 2014 compared balloon angioplasty to Diamondback atherectomy (Heuser 2008), followed by adjunctive balloon angioplasty. The Diamondback atherectomy device files away plaque, as opposed to cutting it away, via an eccentrically mounted abrasive crown on a catheter that rotates at high speed (100,000 rpm). This results in extremely small pieces of plaque, so no system for removing resulting debris is required. The Shammas 2012 trial included 50 participants with rest pain or tissue loss and stenosed, calcified vessels. The trial did not specify a medication protocol. The Dattilo 2014 trial included 50 participants (with 65 lesions) with symptomatic femoropopliteal (FP) disease. The participants had to have Rutherford class 2 to 4 symptoms (moderate claudication/Ischaemic rest pain), and de novo FP stenosis > 70% with fluoroscopically visible calcium. Participants were recommended to be on an antiplatelet agent preprocedure (preferably clopidogrel), and then aspirin and clopidogrel for a minimum of four to six weeks postprocedure.
Zeller 2017 compared paclitaxel‐eluting balloon angioplasty and SilverHawk (described above) or TurboHawk atherectomy devices. The TurboHawk device is a cutting or grinding rotational atherectomy device, with the different attachments to be chosen depending upon how calcified the lesions are. It is recommended to be used in conjunction with the SpiderFX embolic protection device if using the larger cutter. This study included 102 participants with claudication or rest pain with a target lesion of ≥ 70% stenosis in the superficial femoral or popliteal artery. A uniform antiplatelet protocol was in place for both arms, requiring dual antiplatelets preprocedure, clopidogrel for four weeks postprocedure, and aspirin indefinitely.
Ott 2017 compared paclitaxel‐eluting balloon angioplasty and stenting, balloon angioplasty and stenting, and SilverHawk atherectomy (described above) with distal protection (spider filter) and bailout stenting. The SpiderFX embolic protection device captures debris from the atherectomy procedure using a braided nitinol basket, and is placed downstream to stop distal trashing or embolisation. This study included 155 participants with symptomatic peripheral vascular disease and angiographic de novo stenosis > 70% or occlusion of the superficial femoral artery. Participants were given 500 mg aspirin intravenously immediately after the procedure, then 100 mg aspirin once daily indefinitely, with 75 mg clopidogrel once daily for six months or more.
Excluded studies
See Characteristics of excluded studies.
We excluded six studies following the most recent search (Del Giudice 2014; Dippel 2015; Gandini 2013; NCT02730234; NCT02832024; Schwindt 2017), bringing the total number of excluded studies to nine (Brodmann 2013; Del Giudice 2014; Dippel 2015; Gabrielli 2012; Gandini 2013; Gisbertz 2009; NCT02730234; NCT02832024; Schwindt 2017). Gabrielli 2012 and Gisbertz 2009 performed remote endarterectomy rather than atherectomy. Brodmann 2013, Del Giudice 2014, Dippel 2015, Gandini 2013 and NCT02832024 included participants with an in‐stent restenosis. We excluded NCT02730234 and Schwindt 2017 as they were non‐randomised single arm trials. We reassessed one previously excluded study as ongoing (NCT01579123).
Ongoing studies
See Characteristics of ongoing studies.
We identified 11 new studies that compared drug‐coated balloon angioplasty with atherectomy, and listed these as ongoing (ChiCTR‐IOR‐17012486; Martinsen 2015; NCT01579123; NCT01763476; NCT02514460; NCT02517827; NCT02561299; NCT02840786; NCT03206762; NCT03380650; NCT03495453). Two studies previously assessed as ongoing are now included studies (Ott 2017; Zeller 2017).
Risk of bias in included studies
The 'Risk of bias' assessments are presented in the 'Characteristics of included studies' table and summarised in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged four of the studies to be at low risk for randomisation methods: Nakamura 1995 used a random numbers table; Ott 2017 used a computer‐generated sequence; Vroegindeweij 1995 used numbered envelopes opened sequentially and Zeller 2017 used block randomisation by centre. Three studies were of unclear risk for randomisation methods: Dattilo 2014 did not state the method of randomisation; Shammas 2011 performed simple randomisation on a 1:1 basis, but did not describe the method of sequence generation; and Shammas 2012 stated that sealed envelopes were provided to all centres for randomisation, but did not report the randomisation method.
We deemed five studies to be at low risk for allocation bias. Dattilo 2014, Shammas 2012, Vroegindeweij 1995 and Ott 2017 performed randomisation only after passing the guidewire and assessing inclusion and exclusion criteria, and used sealed envelopes to conceal allocation. Shammas 2011 used sealed envelopes for allocation concealment. Zeller 2017 assigned participants to treatment groups after successful passage of the guidewire across the target lesion, so we judged this to be at unclear risk. Nakamura 1995 did not report any method of allocation concealment, so we judged this to be at high risk.
Blinding
It is not possible to blind operators for this procedure, so we assessed all trials to be at high risk for performance bias (Dattilo 2014; Nakamura 1995; Ott 2017; Shammas 2011; Shammas 2012; Vroegindeweij 1995; Zeller 2017). We also deemed bailout stenting to be at high risk of bias, given that the decision is made at the time of intervention by non‐blinded technicians, who could therefore influence results. Blinding for postprocedure follow‐up is possible, but Ott 2017 appears to have been the only study to implement this fully, as the independent core laboratory was blinded to the treatment assignment, We therefore considered Ott 2017 to be at low risk of detection bias. Zeller 2017 blinded the duplex ultrasound core laboratory staff and clinical events committee, but none of the other outcome assessors, giving an unclear risk of detection bias. We judged all of the remaining studies to be at high risk of detection bias (Dattilo 2014; Nakamura 1995; Shammas 2011; Shammas 2012; Vroegindeweij 1995). There was, therefore, an overall risk of both performance and detection bias in all seven trials.
Incomplete outcome data
Five of the seven studies had high risk of attrition bias due to significant numbers of participants not being followed up to both six and 12 months (Dattilo 2014; Ott 2017; Shammas 2011; Shammas 2012; Vroegindeweij 1995). We judged Zeller 2017 to be at unclear risk of bias. Although participants in the study were lost to follow‐up, only 15/102 failed to provide primary outcome data. We deemed Nakamura 1995 to be at low risk because there was a complete data set up to six months. Overall, we had serious concerns about the presence of attrition bias.
Selective reporting
All studies reported the primary outcomes fully, but two studies failed to completely report all secondary outcomes. Nakamura 1995 reported initial and six‐month patencies, but only reported ABIs for participants whose vessels remained patent. We therefore judged this study to be at unclear risk of selection bias. Shammas 2011 also failed to completely report follow‐up ABIs and did not fully report major adverse events, so we considered this to be at high risk of selection bias. The remaining studies reported all outcomes fully, so we rated them to be at low risk of selective reporting (Dattilo 2014; Ott 2017; Shammas 2012; Vroegindeweij 1995; Zeller 2017) .
Other potential sources of bias
Antiplatelet protocols were clear and uniform in four studies, reducing the risk of confounding by medication differences and so were at low risk of other bias (Ott 2017; Shammas 2011; Vroegindeweij 1995; Zeller 2017). Shammas 2012 and Nakamura 1995 did not address antiplatelet protocols, which may have impacted outcomes between participant groups. We judged these to be at high risk as no antiplatelet protocol was in place. We considered Dattilo 2014 to be 'unclear' for risk of other bias, as the trial randomised vessels rather than participants, meaning a participant could be enrolled more than once and therefore could confound results.
Effects of interventions
See Table 1 for the comparison 'Atherectomy compared to balloon angioplasty for peripheral arterial disease'.
See Table 2 for the comparison 'Atherectomy compared to balloon angioplasty with primary stenting for peripheral arterial disease'.
We performed meta‐analyses using a random‐effects model as there was clinical heterogeneity between the studies due to the different devices used.
Primary outcomes
Primary vessel patency
Three of the six atherectomy versus BA studies reported primary patency at six months (Nakamura 1995; Vroegindeweij 1995; Zeller 2017). Pooled analysis did not show any clear benefit of atherectomy primary patency at six months (RR 1.06, 95% CI 0.94 to 1.20; 3 studies, 186 participants; very low‐certainty evidence Analysis 1.1) or at 12 months (RR 1.20, 95% CI 0.78 to 1.84; 2 studies, 149 participants; very low‐certainty evidence; Analysis 1.2). We downgraded the certainty of the evidence from high to very low due to risk of bias (inadequate blinding and high rates of attrition); imprecision (small trial sizes); and inconsistency (from heterogeneity).
1.1. Analysis.

Comparison 1: Atherectomy versus balloon angioplasty, Outcome 1: 6‐month primary patency
1.2. Analysis.

Comparison 1: Atherectomy versus balloon angioplasty, Outcome 2: 12‐month primary patency
In the atherectomy versus primary stenting study, Ott 2017 did not report primary patency.
All‐cause mortality
In the atherectomy versus BA comparison, three studies reported mortality rates at one year (Shammas 2011; Shammas 2012; Zeller 2017). In Shammas 2012, there were an unexpectedly high number of deaths in the BA arm (6/25 (24%) participants), with no deaths in the atherectomy arm, though the trialists could find no good explanation for this. Shammas 2011 reported 4/29 (14%) deaths in the BA arm and 2/29 (7%) deaths in the atherectomy arm. Zeller 2017 reported one‐year mortality as 2/48 (4%) deaths in the atherectomy arm compared to 1/54 (2%) deaths in the BA arm. These deaths were not attributed to the procedure, however, with the causes listed as heart failure/stroke, acute coronary syndrome, respiratory failure and neoplastic disorder. Meta‐analysis of this endpoint showed no difference in mortality between the two arms (RR 0.50, 95% CI 0.10 to 2.66; 3 studies; 210 participants; very low‐certainty evidence; Analysis 1.3). We downgraded the certainty of the evidence from high to very low due to risk of bias (inadequate blinding and high rates of attrition); imprecision (small trial sizes); and inconsistency (from heterogeneity).
1.3. Analysis.

Comparison 1: Atherectomy versus balloon angioplasty, Outcome 3: Mortality
In the atherectomy versus primary stenting comparison, Ott 2017 reported mortality at six and 24 months as a secondary outcome. Ott 2017 reported one postprocedural death caused by haemorrhagic shock secondary to retroperitoneal bleeding in the stenting and drug‐eluting balloon arm. At 24 months, they reported three deaths in the drug‐eluting balloon and stent arm, one death in the plain balloon and stent arm and no deaths in the atherectomy arm. All of these deaths were attributed to underlying cardiovascular disease (RR 0.38, 95% CI 0.04 to 3.23; 1 study, 155 participants; very low‐certainty evidence; Analysis 2.1). We downgraded the certainty of the evidence from high to very low due to risk of bias (inadequate blinding and high rates of attrition) and imprecision (small trial size).
2.1. Analysis.

Comparison 2: Atherectomy +/‐ bailout stenting versus primary stenting, Outcome 1: Mortality
Fatal and non‐fatal cardiovascular events
Three studies reported cardiovascular outcomes (Ott 2017; Shammas 2011; Zeller 2017).
In the atherectomy versus BA comparison, Zeller 2017 reported cardiac failure and acute coronary syndrome as causes of death at 24 months, but it was unclear which participants in which arms this related to. Shammas 2011 declared embolic stroke and myocardial infarction to be secondary outcomes, but recorded no events in either arm. We downgraded the certainty of the evidence from high to very low due to risk of bias (inadequate blinding and high rates of attrition); imprecision (small trial sizes); and inconsistency (from heterogeneity).
In the atherectomy versus primary stenting comparison, Ott 2017 reported four deaths at 24 months (three deaths in the drug‐eluting balloon and stent arm and one death in the plain balloon and stent arm). The trialists attributed these to underlying cardiovascular disease, but did not state any specific causes (RR 0.38, 95% CI 0.04 to 3.23; 1 study, 155 participants; very low‐certainty evidence; Analysis 2.2).
2.2. Analysis.

Comparison 2: Atherectomy +/‐ bailout stenting versus primary stenting, Outcome 2: Cardiovascular events
Secondary outcomes
Immediate procedural and angiographic outcomes
All seven trials reported on initial technical failure rates (Dattilo 2014; Nakamura 1995; Ott 2017; Shammas 2011; Shammas 2012; Vroegindeweij 1995; Zeller 2017). We were able to pool the six trials that compared atherectomy to BA. There was no clear improved technical success when using atherectomy compared to BA alone (RR 0.48, 95% CI 0.22 to 1.08; 6 studies; 425 treated vessels; very low‐certainty evidence; Analysis 1.4). In the drug‐eluting balloon angioplasty subgroup, there was an apparent benefit from atherectomy (RR 0.29, 9% CI 0.12 to 0.72, 1 study; 101 treated vessels; very low‐certainty evidence). However, the test for subgroup differences did not demonstrate a difference between the balloon angioplasty and drug‐eluting balloon angioplasty groups (P = 0.32).
1.4. Analysis.

Comparison 1: Atherectomy versus balloon angioplasty, Outcome 4: Initial technical failure rates
In the atherectomy versus primary stenting trial (Ott 2017), there were no initial technical failures in either arm (Analysis 2.3).
2.3. Analysis.

Comparison 2: Atherectomy +/‐ bailout stenting versus primary stenting, Outcome 3: Initial technical failure rates
We downgraded the certainty of the evidence from high to very low across both comparisons due to risk of bias (inadequate blinding and high rates of attrition); imprecision (small trial sizes); and inconsistency (from heterogeneity).
Four studies that compared atherectomy with BA reported rates of bailout stenting, with similar indications (presence of severe dissection or > 30% residual stenosis) in both arms (Dattilo 2014; Shammas 2011; Shammas 2012; Zeller 2017). Shammas 2012 also reported perforation or significant vessel recoil as a reason for bailout stenting. There were higher incidences of bailout stenting in the BA participants (RR 0.26, 95% CI 0.09 to 0.74; 4 studies, 315 treated vessels; very low‐certainty evidence; Analysis 1.5). We downgraded the certainty of the evidence from high to very low due to risk of bias (inadequate blinding and high rates of attrition); imprecision (small trial sizes); and inconsistency (from heterogeneity).
1.5. Analysis.

Comparison 1: Atherectomy versus balloon angioplasty, Outcome 5: Bailout stenting
The atherectomy versus primary stenting trial by Ott 2017 reported that 14/55 (25%) participants in the atherectomy group received bailout stenting due to flow‐limiting dissections, one of whom developed thrombus requiring thrombus aspiration. Two participants had perforations, one treated with a covered stent, the other by prolonged balloon inflation and protamine administration. We downgraded the certainty of the evidence from high to very low due to risk of bias (inadequate blinding and high rates of attrition); imprecision (small trial sizes); and inconsistency (from heterogeneity).
Three studies that compared atherectomy with BA reported balloon inflation pressures (Dattilo 2014; Shammas 2011; Shammas 2012). Meta‐analysis showed a reduction in balloon pressures needed to inflate the angioplasty balloons (MD ‐3.68 mmHg, 95% CI ‐5.36 to ‐2.01; 3 studies, 213 treated vessels; very low‐certainty evidence; Analysis 1.6). We downgraded the certainty of the evidence from high to very low due to risk of bias (inadequate blinding and high rates of attrition); imprecision (small trial sizes); and inconsistency (from heterogeneity). The atherectomy versus primary stenting trial did not report balloon inflation pressures (Ott 2017).
1.6. Analysis.

Comparison 1: Atherectomy versus balloon angioplasty, Outcome 6: Balloon inflation pressure
Target vessel revascularisation rates
There was some variation between trials as to whether they collected target lesion revascularisation data or target vessel revascularisation data, or both. Upon discussion, we made the decision to collect target vessel revascularisation data, as we felt this allowed us to perform the fairest comparison between all studies, as only a small minority of participants had more than one lesion treated and only Dattilo 2014 reported target lesion revascularisation rates. For this reason, we have not included Dattilo 2014 in the meta‐analysis.
Three atherectomy versus BA studies reported TVR as one of their outcomes (Shammas 2011; Shammas 2012; Zeller 2017). Two of the studies reported six‐month TVR. Shammas 2012 reported 0/22 (0%) in the atherectomy arm and 3/20 (15%) in the angioplasty arm; and Zeller 2017 reported 2/43 (5%) in the atherectomy arm and 2/51 (4%) in the angioplasty arm. On pooling the study data, we found no clear differences between the two arms at six months (RR 0.51, 95% CI 0.06 to 4.42; 2 studies, 136 treated vessels; very low‐certainty evidence; Analysis 1.7). We downgraded the certainty of the evidence from high to very low due to risk of bias (inadequate blinding and high rates of attrition); imprecision (small trial sizes); and inconsistency (from heterogeneity). Dattilo 2014 reported that 6/35 (17%) participants in the atherectomy arm and 2/26 (8%) participants in the angioplasty arm required revascularisation by six months.
1.7. Analysis.

Comparison 1: Atherectomy versus balloon angioplasty, Outcome 7: Target vessel revascularisation at 6 months
Shammas 2011, Shammas 2012 and Zeller 2017 all reported 12‐month TVR outcomes. The pooled analysis showed no clear benefit when using atherectomy (RR 0.59, 95% CI 0.25 to 1.42; 3 studies, 176 treated vessels; very low‐certainty evidence; Analysis 1.8). We downgraded the certainty of the evidence from high to very low due to risk of bias (inadequate blinding and high rates of attrition); imprecision (small trial sizes); and inconsistency (from heterogeneity).
1.8. Analysis.

Comparison 1: Atherectomy versus balloon angioplasty, Outcome 8: Target vessel revascularisation at 12 months
For the comparison of atherectomy versus primary stenting with or without drug eluting balloon, Ott 2017 reported TVR at six months and 24 months. Results did not show a clear difference between the treatment arms for either six‐month TVR (RR 2.27, 95% CI 0.95 to 5.46; 1 study, 155 participants; very low‐certainty evidence; Analysis 2.4) or 24‐month TVR (RR 2.05, 95% CI 0.96 to 4.37; 1 study, 155 participants; very low‐certainty evidence; Analysis 2.5). We downgraded the certainty of the evidence from high to very low due to risk of bias (inadequate blinding and high rates of attrition); and imprecision (small trial size and wide confidence intervals).
2.4. Analysis.

Comparison 2: Atherectomy +/‐ bailout stenting versus primary stenting, Outcome 4: Target vessel revascularisation at 6 months
2.5. Analysis.

Comparison 2: Atherectomy +/‐ bailout stenting versus primary stenting, Outcome 5: Target vessel revascularisation at 24 months
Complication rates
All six studies in the atherectomy versus BA comparison reported complication events, with no clear difference detected (RR 0.69, 95% CI 0.28 to 1.68; 6 studies, 387 participants; very low‐certainty evidence; Analysis 1.9) (Dattilo 2014; Nakamura 1995; Shammas 2011; Shammas 2012; Vroegindeweij 1995; Zeller 2017). Atherectomy showed no clear difference in the incidence of embolisation (RR 2.51, 95% CI 0.64 to 9.80; 6 studies, 387 participants; very low‐certainty evidence; Analysis 1.10). We detected lower incidences of dissection following atherectomy (RR 0.28, 95% CI 0.14 to 0.54; 4 studies, 290 participants; very low‐certainty evidence; Analysis 1.11). We downgraded the certainty of the evidence from high to very low due to risk of bias (inadequate blinding and high rates of attrition); imprecision (small trial sizes); and inconsistency (from heterogeneity).
1.9. Analysis.

Comparison 1: Atherectomy versus balloon angioplasty, Outcome 9: Complication rate
1.10. Analysis.

Comparison 1: Atherectomy versus balloon angioplasty, Outcome 10: Embolisation
1.11. Analysis.

Comparison 1: Atherectomy versus balloon angioplasty, Outcome 11: Dissections
Dattilo 2014 reported that 0/25 (0%) participants in the atherectomy arm and 1/25 (4%) participants in the angioplasty arm had a perforation during the procedures. Six participants in the atherectomy arm and 13 participants in the angioplasty arm had a dissection, but the trialists did not report whether any of these participants required further intervention as a result. They did not report any other complications.
Nakamura 1995 reported that 3/13 (23%) participants in the balloon angioplasty group had perforations due to guidewire manipulation, all of which were treated conservatively. In the atherectomy group, 1/26 (4%) participants had a perforation, 4/26 (15%) participants had distal embolisation and two of the atherectomy devices broke intraprocedure. They also reported that one participant had an acute myocardial infarction during the operation, but did not state which intervention arm the participant belonged to.
Shammas 2012 reported that 1/25 (4%) participants in the atherectomy arm and 6/25 (24%) participants in the angioplasty arm experienced vessel dissection. Five of these were treated by stent placement, and two (both in the angioplasty arm) were treated with dilatation. One of the 25 participants (4%) in the atherectomy arm received a stent for slow flow, and 1/25 (4%) participants in the angioplasty arm received a stent for vessel recoil. One of the 25 participants (4%) in the angioplasty arm experienced vessel perforation (treated by balloon dilatation), and 1/25 (4%) participants in the angioplasty arm experienced distal embolisation.
Shammas 2011 reported that one of the 29 participants (3%) in the atherectomy arm, who was not treated with a distal embolisation filter, had a clinically significant distal embolisation that required mechanical and pharmacological therapy. In the atherectomy arm, 17/29 (58%) participants were treated with a distal embolisation filter, of whom 11/17 (65%) had macroembolisation with debris larger than 2 mm captured in the filter. None of the 10 participants in the angioplasty group who were treated with a filter had significant debris caught in it. No participants treated with a filter had clinically significant embolisation distal to the filter, and all filters were removed without further complications.
Vroegindeweij 1995 reported one large dissection that caused superficial femoral occlusion after three months, and one small dissection in the atherectomy arm (38 participants). The study also reported one thrombosis event in the atherectomy arm during the procedure, which was treated with streptokinase, and one case of failure to pass the guidewire. In the balloon angioplasty arm, the trialists reported that there were five small dissections among the 35 participants.
Zeller 2017 reported that there were two clinically significant distal embolisation events that required endovascular intervention, and one distal embolisation event that was not clinically significant in the atherectomy cohort (48 participants). Additionally, two perforations occurred in this group, which were successfully treated with prolonged percutaneous transluminal angioplasty. No embolisations and no perforations occurred in the angioplasty group (54 participants). In the angioplasty arm, 10 grade C or higher dissections occurred, with only one in the atherectomy arm.
Ott 2017 (atherectomy versus primary stenting) reported three complications, all of which were in the atherectomy arm (55 participants): two vessel perforations and one flow‐limiting dissection (Analysis 2.6).
2.6. Analysis.

Comparison 2: Atherectomy +/‐ bailout stenting versus primary stenting, Outcome 6: Complication rate
Morbidity assessment
Four studies reported rates of amputation, three of which compared atherectomy versus BA (Shammas 2011; Shammas 2012; Zeller 2017), with only one event across all trials (in the angioplasty arm of Shammas 2011) (RR 0.33, 95% CI 0.01 to 7.80; 3 studies, 178 participants; very low‐certainty evidence; Analysis 1.12). We downgraded the certainty of the evidence from high to very low due to risk of bias (inadequate blinding and high rates of attrition); imprecision (small trial sizes); and inconsistency (from heterogeneity).
1.12. Analysis.

Comparison 1: Atherectomy versus balloon angioplasty, Outcome 12: Amputation
There were no amputation events in the study that compared atherectomy versus BA and primary stenting (Analysis 2.7; Ott 2017).
2.7. Analysis.

Comparison 2: Atherectomy +/‐ bailout stenting versus primary stenting, Outcome 7: Amputation
Quality of life outcomes
None of the included studies reported on quality of life outcomes.
Clinical and symptomatic outcomes
Two atherectomy versus BA trials reported clinical and symptomatic outcomes. Zeller 2017 reported functional outcomes in their study, including pain score, walking distance score, walking speed score and stair climbing score for baseline, six months and one year. However, they did not report any benefit for any of the outcomes at either time period. None of the other studies reported clinical or symptomatic outcomes. Shammas 2011 reported 30‐day and 12‐month ABI and Rutherford class, and stated that there was no difference between any of these outcomes in the two treatment arms.
The atherectomy versus primary stenting trial did not report clinical and symptomatic outcomes (Ott 2017).
Sensitivity analysis
Three studies treated more than one vessel per participant or limb (Dattilo 2014; Shammas 2011; Shammas 2012). It is possible, therefore, that the outcomes of these trials received greater weight in the meta‐analysis than is appropriate in the analysis of six‐month and 12‐month patency and TVR. Ideally, we would have carried out sensitivity analysis to assess the impact this had upon the results. However, given the low number of studies, this was not possible.
Only Vroegindeweij 1995 did not perform routine angioplasty with atherectomy. We performed sensitivity analysis to assess the effect of including this study in the overall meta‐analyses of the primary outcomes (primary vessel patency and all‐cause mortality), and did not observe any difference between including or excluding this study in the analysis.
Discussion
Summary of main results
The main findings from this update involving seven RCTs (527 participants and 581 treated lesions) show that the evidence is very uncertain about the effect of atherectomy on primary patency compared to balloon angioplasty (BA) or primary stenting at either six or 12 months (Analysis 1.1; Analysis 1.2). There was also no clear difference in mortality between atherectomy and BA or primary stenting (very low‐certainty evidence; Analysis 1.3; Analysis 2.1). Although cardiac events were reported in two of the atherectomy versus BA trials, in one study it was unclear which arm the participants belonged to and the second study reported no events. Cardiac event rates showed no clear difference between atherectomy and primary stenting (very low‐certainty evidence; Analysis 2.2).
Initial technical failure rates showed no clear difference when using atherectomy compared with BA (very low‐certainty evidence; Analysis 1.4), and there were no events available for comparison between atherectomy and BA with primary stenting (very low‐certainty evidence; Analysis 2.3).
There was a reduction in the need for bailout stenting associated with a reduction in the inflation pressure necessary to achieve an optimal balloon inflation in the atherectomy arm compared to BA (very low‐certainty evidence; Analysis 1.5 and Analysis 1.6, respectively).
When comparing atherectomy with BA, TVR was not reduced at either six or 12 months (very low‐certainty evidence; Analysis 1.7 and Analysis 1.8). In the atherectomy versus primary stenting arms, analysis did not show any clear benefit of primary stenting on TVR at either six or 24 months (very low‐certainty evidence; Analysis 2.4; Analysis 2.5).
This review showed there was no overall reduction in complications when using atherectomy compared with BA (very low‐certainty evidence; Analysis 1.9). The atherectomy versus BA trials reported embolisation and dissection events. Embolisation events were fewer in the BA arm, although results are subject to very low certainty; Analysis 1.10). Dissection events were fewer in the atherectomy arm (very low‐certainty evidence; Analysis 1.11).
There was only one amputation event in the three trials (178 participants) which compared atherectomy with angioplasty (very low‐certainty evidence; Analysis 1.12), and there were no events in the atherectomy versus primary stenting trial.
Zeller 2017 was the only trial to report clinical and symptomatic outcomes, such as walking distance or symptom relief, with no reported benefit. Similarily, Shammas 2011 reported no differences between groups in terms of ABI and Rutherford classification; outcomes reported only by Shammas 2011.
We performed subgroup analysis in this review because the included trials used both drug‐eluting and plain balloon angioplasty devices as control arms. However, we found no clear difference between these two groups for any outcome in either comparison.
One concern with atherectomy devices is the risk of distal embolisation, as the devices physically cut or grind plaques (Briguori 2003). One of the included studies found this to be a particular issue, and deployed a distal embolic filter in 17/29 (59%) of participants, which caught macroembolic debris (defined as debris greater than 2 mm in the longest axis) in 11/17 (65%) cases (Shammas 2011). The filter was deployed in 10/29 (34%) of the participants in the BA arm, but did not catch macroemboli in any cases. In addition, one participant in the atherectomy arm who was treated without a filter had a clinically significant distal embolic event. In Shammas 2012, one participant out of 20 (5%) in the BA arm had a clinically significant embolic event. Zeller 2017 reported two clinically significant distal embolic events that required endovascular intervention in the atherectomy cohort. However, the device used comes with a recommendation to use a SpiderFX embolic filter if using the larger atherectomy device.
Overall completeness and applicability of evidence
This update includes all information from RCTs identified by the searches, and we have presented an up‐to‐date meta‐analysis of atherectomy versus any other therapy for peripheral arterial disease (PAD). We found comparisons for atherectomy versus BA and atherectomy versus primary stenting plus angioplasty; but not for atherectomy versus bypass surgery.
The indication for intervention was claudication in two studies (Nakamura 1995; Vroegindeweij 1995); claudication or rest pain in Dattilo 2014; and claudication, rest pain or tissue loss in three studies (Ott 2017; Shammas 2011; Shammas 2012). Results of angioplasty and bypass surgery are known to vary between people with these indications (TASC II 2007), so may bias the results from the different studies. Some of the included studies did not state the severity of claudication, which may mean that the participants would have been managed conservatively in many centres (Frans 2012), so the results should be interpreted with a degree of caution. Unfortunately, we were not able to separate results by symptoms (claudication or critical ischaemia) because of the way the studies reported results. In addition, the majority of included studies did not report on all of this review's prespecified outcomes. Therefore, the findings of this review are based in most cases on results from only one or two studies.
Amputation‐free survival is an important endpoint in trials for chronic limb threatening ischaemia, but the included trials did not report this. One reason may be the reliance on including participants with less severe form of peripheral arterial disease (claudication) into the trials.
Mortality is commonly reported in trials of lower limb revascularisation, which is why we considered it a primary outcome measure. However, mortality rates from angioplasty are much lower than primary patency or limb loss rates (Laird 2010; Schillinger 2006), so trials would not be expected to show a difference if powered to detect primary patency. The results presented may be a consequence of random error due to few events in small sample sizes. There was little difference in all‐cause mortality rates between interventions, but mortality was only reported in four of the seven included trials, and rates of death were low (16 deaths out of 365 participants (4%)).
In addition, there were differences in modality of follow‐up between studies, which could introduce potential bias: Ott 2017 used angiogram; Dattilo 2014 and Vroegindeweij 1995 used duplex ultrasound; Zeller 2017 used duplex ultrasound at six months and plain ultrasound at 12 months; and Nakamura 1995 used doppler pressures. Neither Shammas 2011 nor Shammas 2012 used imaging at follow‐up; instead they used ABI and clinical correlation. These differences in outcome collection should be considered when interpreting the results.
Quality of the evidence
All seven included studies were of poor methodological quality, with a high risk of overall bias due to a lack of blinding and high attrition, meaning the conclusions that can be drawn from the analyses are severely limited. There was significant statistical heterogeneity between studies, due to the small participant numbers. In addition, there was heterogeneity due to clinical differences in participant groups, trial protocols and target vessels. Only one included trial compared stenting versus atherectomy (Ott 2017). We interpreted the results for any outcome with only one study with caution, given the small trial sizes and lack of information. Using the GRADE approach, which considers the overall risk of bias of the included studies, the directness of the evidence, inconsistency within the results, precision of the estimate and risk of publication bias (Guyatt 2008), we judged all outcomes to have very low‐certainty evidence. Table 1 and Table 2 show that there is currently no clear evidence to support the use of atherectomy as a treatment for peripheral vascular disease. We downgraded all the outcomes from high to very low‐certainty due to risk of bias (inadequate blinding and high rates of attrition); imprecision (small trial sizes); and inconsistency (from heterogeneity).
Only Ott 2017 and Shammas 2011 utilised power calculations to assess the required number of participants. Overall study numbers were very low, and meta‐analysis of very low participant numbers in randomised trials can be unreliable (Rerkasem 2010). As a result, the observed lack of difference in primary patency could easily be a type II error. Two of the trials did not state medication protocols (Nakamura 1995; Shammas 2012). This may be important as it is known that the use of antiplatelet agents, cilostazol, and heparin are all associated with lower restenosis rates after angioplasty (Robertson 2012). The included trials did not include important clinical endpoints such as secondary patency, limb survival, and complication rates between techniques in sufficient detail.
One included study compared atherectomy alone with BA (Vroegindeweij 1995), whereas five trials compared atherectomy plus adjunctive BA with BA alone (Dattilo 2014; Nakamura 1995; Shammas 2011; Shammas 2012; Zeller 2017), creating concerns about heterogeneity. Three participants in the atherectomy arm of Vroegindeweij 1995 crossed over and had subsequent BA after failure of atherectomy alone.
Potential biases in the review process
Despite carrying out a thorough unrestricted search, our review process identified only seven trials of varying size, so it is difficult to assess the impact of reporting bias.
Three trials treated more than one vessel per participant or limb (Dattilo 2014; Shammas 2011; Shammas 2012). Failure of patency of any of the treated vessels increases the chances that other treated vessels will cease to be patent, so these observations will be correlated. It is possible, therefore, that the outcomes of these trials are given greater weight in the meta‐analysis than is appropriate in the analysis of six‐month and 12‐month patency. As both the angioplasty and atherectomy arms of these trials included multiple vessels per participant, it is unlikely that the magnitude of the observed effect has been affected significantly, though our degree of confidence in this effect may be overstated.
There was some variation in whether trials collected target lesion revascularisation data or target vessel revascularisation data, or both. Upon discussion, we made the decision to collect target vessel revascularisation, as we felt this allowed us to perform the fairest comparison between all studies. Similarly, there was variation between trials in outcome definitions, with studies collecting primary patency rates or occlusion rates. Given the inter‐trial variation for the definitions between the two, we only included studies that collected 'primary patency' in the meta‐analysis.
Agreements and disagreements with other studies or reviews
This is an update of a previous Cochrane Review of atherectomy for PAD (Ambler 2014). Very little further evidence exists in the literature for the use of atherectomy in peripheral vascular disease since the last review. This may in part be due to NICE guidelines recommending against the use of atherectomy devices unless in clinical trials (NICE 2011).
Akkus 2014 performed a review of atherectomy devices, and argued that different types of atherectomy devices should be chosen to treat the most appropriate types of lesion in order to get the best possible clinical outcomes. The review looked at several studies, including the TALON Registry 2006, ERBAC 1997, and McKinsey 2008. Kim 2018 examined dissection rates in atherectomy after BA, and similarly found dissection rates were reduced compared to angioplasty alone. However, this benefit alone does not justify the use of atherectomy over BA. Ramkumar 2019 studied five‐year clinical outcomes of atherectomy compared to other endovascular interventions using the Medicare‐linked VQI (Vascular Quality Initiative) registry for endovascular interventions from 2010 to 2015. They found an increased risk of any amputation in people treated with atherectomy compared to BA, and found that people who had atherectomy had a higher risk of major amputation, any amputation and major adverse limb event compared to stenting.
Atherectomy has been more thoroughly investigated in the coronary arteries. The ORBIT II trial looked at the three‐year outcomes of de novo, severely calcified coronary lesions treated with a coronary orbital atherectomy system prior to stenting (Lee 2017). This study found a lower rate of adverse ischaemic events compared to historical controls. However the ROTAXUS trial, which examined paclitaxel‐eluting stents with or without atherectomy, found no difference in primary patency at nine months between the two arms (Abdel‐Wahab 2013).
Balloon angioplasty for peripheral vascular disease is widely practised, has a clear evidence base and is constantly evolving, with the use of covered stents and drug‐eluting devices (Schroeder 2017; TASC II 2007). As the technique has evolved, so has the evidence base for its place compared to exercise therapy and bypass surgery (Fu 2015; Kayssi 2016). Fu 2015 demonstrated equivalent results to surgical bypass procedures for treating critical limb ischaemia at five‐year follow‐up for amputation‐free survival, target vessel revascularisation, leg amputation and overall mortality. A cohort study examining subintimal angioplasty versus atherectomy for the treatment of occlusive lesions in lower limbs found that angioplasty appeared superior for both patency and limb salvage at 24 months (Indes 2010). Vroegindeweij 1995 performed a post hoc analysis to assess the effect of lesion length on patency. Using life‐table analysis, they showed that atherectomy was equivalent to BA for short lesions (< 2 cm), but for longer lesions, long‐term patency was significantly better following BA (P = 0.007).
Stenting in PAD has been the focus of significant attention. Several randomised trials have compared stenting to angioplasty alone, the majority favouring stenting (Dake 2011; Spreen 2017). Spreen 2017 found that drug‐eluting stents were associated with significantly lower amputation and event‐free rates at five years compared with percutaneous transluminal angioplasty. Murphy 2015 compared supervised exercise, primary stenting and optimal medical care, and found that stenting and exercise had either superior or equivalent outcomes for both walking distance and quality of life at 18 months.
Authors' conclusions
Implications for practice.
This review update shows that the evidence is very uncertain about the effect of atherectomy on patency, mortality and cardiovascular event rates compared to plain balloon angioplasty, with or without stenting. We did not detect any clear differences in initial technical failure rates or target vessel revascularisation rates, but there may be reduced dissection and bailout stenting after atherectomy (very low‐certainty evidence). Included studies were small, heterogenous and at high risk of bias. Larger studies powered to detect clinically meaningful, patient‐centred outcomes are required. With the exception of bailout stenting, dissection and lower inflation pressures, there was no clear difference between atherectomy and angioplasty for any outcome. There was no evidence for atherectomy versus bypass surgery. The findings of this review agree with current widespread practice and established guidelines for balloon angioplasty in the routine treatment of people with peripheral arterial disease who are amenable to standard angioplasty.
Implications for research.
Current evidence in this area is still limited. Larger and better designed trials in selected subgroups of participants are needed to increase our confidence in the evidence. However, performing a larger trial of atherectomy versus balloon angioplasty with greater power to detect differences in primary patency or limb survival may be inappropriate, considering the lack of difference in this analysis, increased technical difficulty, complication rates and the existing 'gold standard' practice of angioplasty. The exception to this may be in people with Trans‐Atlantic Inter‐Society Consensus Document on Management of Peripheral Arterial Disease (TASC) C or D lesions who are not fit for bypass surgery.
Future trials should address the following factors.
Larger studies to detect smaller differences
Studies examining surgery versus atherectomy, as we found no randomised controlled trials for this comparison. Participants should be stratified according to whether they suffer from intermittent claudication or critical ischaemia.
More rigorous follow‐up. It is important that future studies have both longer follow‐up and blinded outcome assessment. The procedures are sometimes performed by interventional radiologists, but followed up by vascular surgeons, so this aim is achievable.
Study designs should include outcomes which are more participant‐centred, for example: quality of life; pain score; and psychosocial measures.
We could not include cost in this Cochrane review, but this is a significant factor when considering results from atherectomy as it is more expensive than BA angioplasty. Future research should explore this.
What's new
| Date | Event | Description |
|---|---|---|
| 1 October 2019 | New search has been performed | New search run. Three new studies included. Six new studies were excluded and 11 new ongoing studies were identified. |
| 1 October 2019 | New citation required but conclusions have not changed | New search run. Three new studies included. Six new studies were excluded and 11 new ongoing studies were identified. Review text updated in keeping with current Cochrane standards including addition of 'Summary of findings' tables. No change to conclusions. |
History
Protocol first published: Issue 3, 2007 Review first published: Issue 3, 2014
| Date | Event | Description |
|---|---|---|
| 29 October 2008 | Amended | Converted to new review format. |
Acknowledgements
The authors, and the Cochrane Vascular Editorial base, are grateful to the following peer reviewers for their time and comments: Piergiorgio Cao MD, Senior Professor of Vascular Surgery, University of Perugia, Consultant Mater Dei Hospital, Rome, Italy; Dr Sosei Kuma, Department of Vascular Surgery, Fukuoka Higashi Medical Center, Japan; Dr Alok Tiwari, The Queen Elizabeth Hospital, Birmingham, UK.
The authors are also grateful to Paul Hayes, for his contribution to the previous review, and Cathryn Broderick for her invaluable help and expertise in the preparation of this review.
Appendices
Appendix 1. Database search strategy
| Source | Search strategy | Hits retrieved |
| CENTRAL via CRSO | #1 MESH DESCRIPTOR Arteriosclerosis 946 #2 MESH DESCRIPTOR Arteriolosclerosis 0 #3 MESH DESCRIPTOR Arteriosclerosis Obliterans 78 #4 MESH DESCRIPTOR Atherosclerosis 1061 #5 MESH DESCRIPTOR Arterial Occlusive Diseases 819 #6 MESH DESCRIPTOR Intermittent Claudication 825 #7 MESH DESCRIPTOR Ischemia 1542 #8 MESH DESCRIPTOR Peripheral Vascular Diseases EXPLODE ALL TREES 2783 #9 MESH DESCRIPTOR Vascular Diseases 645 #10 MESH DESCRIPTOR Leg EXPLODE ALL TREES 2801 #11 MESH DESCRIPTOR Femoral Artery EXPLODE ALL TREES 904 #12 MESH DESCRIPTOR Popliteal Artery EXPLODE ALL TREES 304 #13 MESH DESCRIPTOR Iliac Artery EXPLODE ALL TREES 159 #14 MESH DESCRIPTOR Tibial Arteries EXPLODE ALL TREES 38 #15 (atherosclero* or arteriosclero* or PVD or PAOD or PAD):TI,AB,KY 12285 #16 ((arter*) near (*occlus* or steno* or obstuct* or lesio* or block* or obliter*) ):TI,AB,KY 6367 #17 ((vascular) near (*occlus* or steno* or obstuct* or lesio* or block* or obliter*) ):TI,AB,KY 842 #18 ((veno*) near (*occlus* or steno* or obstuct* or lesio* or block* or obliter*) ):TI,AB,KY 1151 #19 ((vein*) near (*occlus* or steno* or obstuct* or lesio* or block* or obliter*) ):TI,AB,KY 1346 #20 ((peripher*) near (*occlus* or steno* or obstuct* or lesio* or block* or obliter*) ):TI,AB,KY 2358 #21 (peripheral near/3 dis* ):TI,AB,KY 0 #22 arteriopathic:TI,AB,KY 7 #23 (claudic* or hinken*):TI,AB,KY 1880 #24 (isch* or CLI):TI,AB,KY 32430 #25 dysvascular*:TI,AB,KY 23 #26 (leg near4 (obstruct* or occlus* or steno* or block* or obliter*) ):TI,AB,KY 155 #27 (limb near4 (obstruct* or occlus* or steno* or block* or obliter*) ):TI,AB,KY 239 #28 ((lower near3 extrem*) near4 (obstruct* or occlus* or steno* or block* or obliter*) ):TI,AB,KY 103 #29 ((aort* or iliac or femoral or popliteal or femoro* or fempop* or crural) near3 (obstruct* or occlus*) ):TI,AB,KY 454 #30 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 56041 #31 MESH DESCRIPTOR Atherectomy 24 #32 atherect*:TI,AB,KY 333 #33 (SilverHawk or "Silver Hawk" ):TI,AB,KY 12 #34 Jetstream:TI,AB,KY 2 #35 (plaque near3 excis*):TI,AB,KY 12 #36 (atheroablation or rotational or orbital):TI,AB,KY 1514 #37 (angle near3 blade*):TI,AB,KY 10 #38 (cut near3 blade*):TI,AB,KY 1 #39 (blade near3 cathet*):TI,AB,KY 1 #40 EV3:TI,AB,KY 10 #41 #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 1735 #42 #30 AND #41 201 #43 01/11/2013 TO 20/08/2018:CD 584598 #44 #42 AND #43 110 |
20 August 2018: 110 12 August 2019: 57 |
| Clinicaltrials.gov | peripheral arterial disease OR artery OR vascular OR Arteriosclerosis OR Ischemia | Atherectomy OR atheroablation OR rotational OR orbital OR EV3 | Start date on or after 01/01/2013 | Last update posted on or before 08/08/2019 | 20 August 2018: 76 12 August 2019: 36 |
| ICTRP Search Portal | peripheral arterial disease OR artery OR vascular OR Arteriosclerosis OR Ischemia | Atherectomy OR atheroablation OR rotational OR orbital OR EV3 | Start date on or after 01/01/2013 | Last update posted on or before 08/08/2019 | 20 August 2018: 41 12 August 2019: 14 |
| Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE® | 1 ARTERIOSCLEROSIS/ 56458 2 ARTERIOLOSCLEROSIS/ 150 3 Arteriosclerosis Obliterans/ 3977 4 ATHEROSCLEROSIS/ 31372 5 Arterial Occlusive Diseases/ 26551 6 Intermittent Claudication/ 7623 7 ISCHEMIA/ 47700 8 exp Peripheral Vascular Diseases/ 50278 9 Vascular Diseases/ 35016 10 exp LEG/bs [Blood Supply] 25052 11 exp Femoral Artery/ 27179 12 exp Popliteal Artery/ 9025 13 exp Iliac Artery/ 13402 14 exp Tibial Arteries/ 1501 15 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. 172197 16 (arter* adj (occlus* or steno* or obstuct* or lesio* or block* or obliter*)).ti,ab. 63236 17 (vascular adj (occlus* or steno* or obstuct* or lesio* or block* or obliter*)).ti,ab. 15842 18 (vein* adj (occlus* or steno* or obstuct* or lesio* or block* or obliter*)).ti,ab. 7223 19 (veno* adj (occlus* or steno* or obstuct* or lesio* or block* or obliter*)).ti,ab. 7897 20 (peripher* adj (occlus* or steno* or obstuct* or lesio* or block* or obliter*)).ti,ab. 1931 21 (peripheral adj3 dis*).ti,ab. 38014 22 arteriopathic.ti,ab. 162 23 (claudic* or hinken*).ti,ab. 9857 24 (isch* or CLI).ti,ab. 347912 25 (leg adj4 (obstruct* or occlus* or steno* or block* or obliter*)).ti,ab. 591 26 (limb adj4 (obstruct* or occlus* or steno* or block* or obliter*)).ti,ab. 1841 27 (lower adj3 extrem* adj4 (obstruct* or occlus* or steno* or block* or obliter*)).ti,ab. 1541 28 ((aort* or iliac or femoral or popliteal or femoro* or fempop* or crural) adj3 (obstruct* or occlus*)).ti,ab. 8681 29 dysvascular*.ti,ab. 219 30 or/1‐29 732181 31 ATHERECTOMY/ 667 32 atherect*.ti,ab. 2759 33 (SilverHawk or "Silver Hawk").ti,ab. 66 34 Jetstream.ti,ab. 31 35 (plaque adj3 excis*).ti,ab. 209 36 (atheroablation or rotational or orbital).ti,ab. 90480 37 (angle adj3 blade*).ti,ab. 112 38 (cut adj3 blade*).ti,ab. 80 39 (blade adj3 cathet*).ti,ab. 10 40 EV3.ti,ab. 238 41 or/31‐40 93104 42 30 and 41 3188 43 randomized controlled trial.pt. 467015 44 controlled clinical trial.pt. 92591 45 randomized.ab. 419437 46 placebo.ab. 191087 47 drug therapy.fs. 2041052 48 randomly.ab. 295632 49 trial.ab. 436703 50 groups.ab. 1824460 51 or/43‐50 4263409 52 exp animals/ not humans.sh. 4488176 53 51 not 52 3685450 54 42 and 53 555 55 (2017* or 2018*).ed. 1598344 56 54 and 55 59 57 from 56 keep 1‐59 59 |
20 August 2018: 59 12 August 2019: 59 |
| EMBASE | 1 arteriosclerosis/ 23302 2 arteriolosclerosis/ 539 3 peripheral occlusive artery disease/ 29354 4 atherosclerosis/ 130224 5 peripheral occlusive artery disease/ 29354 6 intermittent claudication/ 8546 7 ischemia/ 70896 8 exp peripheral vascular disease/ 1521794 9 vascular disease/ 50928 10 exp femoral artery/ 28864 11 exp popliteal artery/ 7120 12 exp iliac artery/ 15142 13 exp tibial artery/ 2504 14 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. 221645 15 (arter* adj (*occlus*/ or steno* or obstuct* or lesio* or block* or obliter*)).ti,ab. 81145 16 (vascular adj (*occlus*/ or steno* or obstuct* or lesio* or block* or obliter*)).ti,ab. 19845 17 (vein* adj (*occlus*/ or steno* or obstuct* or lesio* or block* or obliter*)).ti,ab. 9432 18 (veno* adj (*occlus*/ or steno* or obstuct* or lesio* or block* or obliter*)).ti,ab. 10130 19 (peripher* adj (*occlus*/ or steno* or obstuct* or lesio* or block* or obliter*)).ti,ab. 2816 20 (peripheral adj3 dis*).ti,ab. 51397 21 arteriopathic.ti,ab. 179 22 (claudic* or hinken*).ti,ab. 12594 23 (isch* or CLI).ti,ab. 478952 24 dysvascular*.ti,ab. 229 25 (leg adj4 (obstruct* or occlus* or steno* or block* or obliter*)).ti,ab. 731 26 (limb adj4 (obstruct* or occlus* or steno* or block* or obliter*)).ti,ab. 2595 27 (lower dj3 extrem* adj4 (obstruct* or occlus* or steno* or block* or obliter*)).ti,ab. 0 28 ((aort* or iliac or femoral or popliteal or femoro* or fempop* or crural) adj3 (obstruct* or occlus*)).ti,ab. 10343 29 or/1‐28 1820971 30 atherectomy/ 3475 31 atherect*.ti,ab. 3818 32 (SilverHawk or "Silver Hawk").ti,ab. 118 33 Jetstream.ti,ab. 65 34 (plaque adj3 excis*:).ti,ab. 355 35 (atheroablation or rotational or orbital).ti,ab. 82052 36 (angle adj3 blade*).ti,ab. 158 37 (cut adj3 blade*:).ti,ab. 97 38 (cut adj3 blade*).ti,ab. 97 39 (blade adj3 cathet*).ti,ab. 12 40 EV3.ti,ab. 443 41 or/30‐40 86520 42 29 and 41 9677 43 randomized controlled trial/ 485173 44 controlled clinical trial/ 453439 45 random$.ti,ab. 1255139 46 randomization/ 78361 47 intermethod comparison/ 224177 48 placebo.ti,ab. 263135 49 (compare or compared or comparison).ti. 439907 50 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 1683247 51 (open adj label).ti,ab. 61750 52 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 201070 53 double blind procedure/ 144969 54 parallel group$1.ti,ab. 20900 55 (crossover or cross over).ti,ab. 89795 56 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 271905 57 (assigned or allocated).ti,ab. 320231 58 (controlled adj7 (study or design or trial)).ti,ab. 281124 59 (volunteer or volunteers).ti,ab. 217649 60 trial.ti. 234748 61 or/43‐60 3866425 62 42 and 61 1797 63 (2017* or 2018*).em. 2796615 64 62 and 63 278 65 from 64 keep 1‐278 278 |
20 August 2018: 278 12 August 2019: 361 |
| CINAHL | S53 S51 AND S52 9 S52 EM 2016 OR EM 2017 334,593 S51 S40 AND S50 72 S50 S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 267,359 S49 MH "Single‐Blind Studies" or MH "Double‐Blind Studies" or MH "Triple‐Blind Studies" 32,871 S48 MH "Factorial Design" 922 S47 MH "Placebos" 8,375 S46 MH "Clinical Trials" 93,030 S45 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" 4,542 S44 TX crossover OR "cross‐over" 14,660 S43 AB placebo* 28,580 S42 TX trial* 252,485 S41 TX "latin square" 143 S40 S28 AND S39 293 S39 S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 4,240 S38 TX EV3 15 S37 TX blade N3 cathet* 0 S36 TX cut N3 blade* 0 S35 TX angle N3 blade* 0 S34 TX atheroablation or rotational or orbital 4,010 S33 TX plaque N3 excis* 0 S32 TX Jetstream 3 S31 TX SilverHawk or "Silver Hawk" 10 S30 TX atherect* 317 S29 (MH "Atherectomy") 82 S28 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 75,396 S27 TX dysvascular* 172 S26 TX aort* or iliac or femoral or popliteal or femoro* or fempop* or crural) N3 (obstruct* or occlus*) 583 S25 TX lower N3 extrem* N4 (obstruct* or occlus* or steno* or block* or obliter*) 82 S24 TX limb N4 (obstruct* or occlus* or steno* or block* or obliter*) 198 S23 TX leg N4 (obstruct* or occlus* or steno* or block* or obliter*) 92 S22 TX isch* or CLI 39,707 S21 TX claudic* or hinken* 1,389 S20 TX arteriopathic 10 S19 TX peripheral N3 dis* 9,270 S18 TX peripher* N (occlus* or steno* or obstuct* or lesio* or block* or obliter*) 10 S17 TX veno* N (occlus* or steno* or obstuct* or lesio* or block* or obliter*) 11 S16 TX vein* N (occlus* or steno* or obstuct* or lesio* or block* or obliter*) 7 S15 TX vascular N (occlus* or steno* or obstuct* or lesio* or block* or obliter*) 13 S14 TX arter* N (occlus* or steno* or obstuct* or lesio* or block* or obliter* 167 S13 TX atherosclero* or arteriosclero* or PVD or PAOD or PAD 26,503 S12 (MH "Tibial Arteries") 145 S11 (MH "Iliac Artery") 453 S10 (MH "Popliteal Artery") 359 S9 (MH "Femoral Artery") 1,188 S8 (MH "Leg/BS") 450 S7 (MH "Vascular Diseases") 2,381 S6 (MH "Peripheral Vascular Diseases") 0 S5 (MH "Ischemia") 3,387 S4 (MH "Intermittent Claudication") 841 S3 (MH "Arterial Occlusive Diseases") 1,602 S2 (MH "Atherosclerosis") 3,372 S1 (MH "Arteriosclerosis") 4,820 |
20 August 2018: 9 12 August 2019: 31 |
| AMED | 1 ARTERIOSCLEROSIS/ 78 2 ATHEROSCLEROSIS/ 223 3 Intermittent Claudication/ 75 4 ISCHEMIA/ 266 5 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. 810 6 (arter* adj (occlus* or steno* or obstuct* or lesio* or block* or obliter*)).ti,ab. 179 7 (vascular adj (occlus* or steno* or obstuct* or lesio* or block* or obliter*)).ti,ab. 44 8 (vein* adj (occlus* or steno* or obstuct* or lesio* or block* or obliter*)).ti,ab. 4 9 (veno* adj (occlus* or steno* or obstuct* or lesio* or block* or obliter*)).ti,ab. 29 10 (peripher* adj (occlus* or steno* or obstuct* or lesio* or block* or obliter*)).ti,ab. 11 11 (peripheral adj3 dis*).ti,ab. 439 12 arteriopathic.ti,ab. 1 13 (claudic* or hinken*).ti,ab. 150 14 (isch* or CLI).ti,ab. 1687 15 (leg adj4 (obstruct* or occlus* or steno* or block* or obliter*)).ti,ab. 19 16 (limb adj4 (obstruct* or occlus* or steno* or block* or obliter*)).ti,ab. 22 17 (lower adj3 extrem* adj4 (obstruct* or occlus* or steno* or block* or obliter*)).ti,ab. 12 18 ((aort* or iliac or femoral or popliteal or femoro* or fempop* or crural) adj3 (obstruct* or occlus*)).ti,ab. 11 19 dysvascular*.ti,ab. 58 20 or/1‐19 3220 21 (atheroablation or rotational or orbital).ti,ab. 573 22 (angle adj3 blade*).ti,ab. 4 23 or/21‐22 577 24 20 and 23 4 25 exp CLINICAL TRIALS/ 3788 26 RANDOM ALLOCATION/ 314 27 DOUBLE BLIND METHOD/ 667 28 Clinical trial.pt. 1212 29 (clinic* adj trial*).tw. 5438 30 ((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).tw. 2866 31 PLACEBOS/ 591 32 placebo*.tw. 3132 33 random*.tw. 17749 34 PROSPECTIVE STUDIES/ 1119 35 or/25‐34 22789 36 24 and 35 0 |
20 August 2018: 0 12 August 2019: 0 |
Data and analyses
Comparison 1. Atherectomy versus balloon angioplasty.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 6‐month primary patency | 3 | 186 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.94, 1.20] |
| 1.1.1 Balloon angioplasty | 2 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.51, 1.81] |
| 1.1.2 Drug‐eluting balloon angioplasty | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.94, 1.21] |
| 1.2 12‐month primary patency | 2 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.78, 1.84] |
| 1.2.1 Balloon angioplasty | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.90, 2.67] |
| 1.2.2 Drug‐eluting balloon angioplasty | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.86, 1.26] |
| 1.3 Mortality | 3 | 210 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.10, 2.66] |
| 1.3.1 Balloon angioplasty | 2 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.04, 1.68] |
| 1.3.2 Drug‐eluting balloon angioplasty | 1 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 2.25 [0.21, 24.04] |
| 1.4 Initial technical failure rates | 6 | 425 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.22, 1.08] |
| 1.4.1 Balloon angioplasty | 5 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.20, 1.73] |
| 1.4.2 Drug‐eluting balloon angioplasty | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.72] |
| 1.5 Bailout stenting | 4 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.09, 0.74] |
| 1.5.1 Balloon angioplasty | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.07, 0.89] |
| 1.5.2 Drug‐eluting balloon angioplasty | 1 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.01, 4.56] |
| 1.6 Balloon inflation pressure | 3 | 213 | Mean Difference (IV, Random, 95% CI) | ‐3.68 [‐5.36, ‐2.01] |
| 1.7 Target vessel revascularisation at 6 months | 2 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.06, 4.42] |
| 1.7.1 Balloon angioplasty | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.01, 2.38] |
| 1.7.2 Drug‐eluting balloon angioplasty | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.17, 8.07] |
| 1.8 Target vessel revascularisation at 12 months | 3 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.25, 1.42] |
| 1.8.1 Balloon angioplasty | 2 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.15, 1.39] |
| 1.8.2 Drug‐eluting balloon angioplasty | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.22, 3.86] |
| 1.9 Complication rate | 6 | 387 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.28, 1.68] |
| 1.9.1 Balloon angioplasty | 5 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.22, 2.42] |
| 1.9.2 Drug‐eluting balloon angioplasty | 1 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.27, 1.72] |
| 1.10 Embolisation | 6 | 387 | Risk Ratio (M‐H, Random, 95% CI) | 2.51 [0.64, 9.80] |
| 1.10.1 Plain balloon angioplasty | 5 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [0.39, 8.55] |
| 1.10.2 Drug‐eluting balloon angioplasty | 1 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 7.86 [0.42, 148.34] |
| 1.11 Dissections | 4 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.14, 0.54] |
| 1.11.1 Plain balloon angioplasty | 3 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.16, 0.62] |
| 1.11.2 Drug‐eluting balloon angioplasty | 1 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 0.85] |
| 1.12 Amputation | 3 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.80] |
| 1.12.1 Balloon angioplasty | 2 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.80] |
| 1.12.2 Drug‐eluting balloon angioplasty | 1 | 102 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
Comparison 2. Atherectomy +/‐ bailout stenting versus primary stenting.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Mortality | 1 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.04, 3.23] |
| 2.1.1 Plain balloon plus stent | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.03, 14.99] |
| 2.1.2 Drug‐eluting balloon plus stent | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.01, 4.51] |
| 2.2 Cardiovascular events | 1 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.04, 3.23] |
| 2.2.1 Plain balloon plus stent | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.03, 14.99] |
| 2.2.2 Drug‐eluting balloon plus stent | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.01, 4.51] |
| 2.3 Initial technical failure rates | 1 | 155 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.3.1 Plain balloon plus stent | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.3.2 Drug‐eluting balloon plus stent | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.4 Target vessel revascularisation at 6 months | 1 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 2.27 [0.95, 5.46] |
| 2.4.1 Plain balloon plus stent | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 1.93 [0.61, 6.08] |
| 2.4.2 Drug‐eluting balloon plus stent | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 2.86 [0.74, 11.06] |
| 2.5 Target vessel revascularisation at 24 months | 1 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 2.05 [0.96, 4.37] |
| 2.5.1 Plain balloon plus stent | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.85, 2.56] |
| 2.5.2 Drug‐eluting balloon plus stent | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 3.18 [1.44, 7.03] |
| 2.6 Complication rate | 1 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 7.04 [0.80, 62.23] |
| 2.6.1 Plain balloon plus stent | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 9.46 [0.47, 190.41] |
| 2.6.2 Drug‐eluting balloon plus stent | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 5.07 [0.21, 120.38] |
| 2.7 Amputation | 1 | 155 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.7.1 Plain balloon plus stent | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.7.2 Drug‐eluting balloon plus stent | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dattilo 2014.
| Study characteristics | ||
| Methods | Randomisation method: details of sequence generation not stated Allocation: sealed envelopes ‐ not stated if opaque Intervention model: parallel Blinding: not stated |
|
| Participants | Country: United States of America No. of participants: 50 OA + BA: 25 BA alone: 25 Age (mean (years) ± SD): OA + BA: 68.0 ± 11.0 BA alone: 71.3 ± 10.5 Inclusion criteria: eligible participants were 18 years or older; PAD with Rutherford class 2 to 4 symptoms and de novo FP lesions of ≥ 70% stenosis with fluoroscopically visible calcium; gave informed consent; all participants had to have at least 1 patent run‐off vessel Exclusion criteria: anticipated life span of less than 1 year; known allergy to heparin, aspirin, and clopidogrel, or sensitivity to contrast media; chronic renal failure; cardiac arrhythmias; congestive heart failure exacerbation; myocardial infarction |
|
| Interventions | OA + BA vs BA alone | |
| Outcomes | Primary: freedom from TLR, including the need for adjunctive stenting or restenosis (PSVR ≥ 2.5 on duplex ultrasound) per lesion at 6 months. Secondary: changes in ABI and Rutherford Class from baseline to 30 days and 6 and 12 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given about method of random sequence generation. |
| Allocation concealment (selection bias) | Low risk | Randomisation performed only after guidewire passed and inclusion and exclusion criteria assessed. Sealed envelopes, not stated if opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated, but impractical in trials of this type. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Incomplete data set at 6 and 12 months, reasons for attrition not stated. Data only published for 45/50 (90%) at 6 months and 37/50 (74%) at 12 months. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes reported. |
| Other bias | Unclear risk | Clear antiplatelet protocol specified, but unit of analysis issue as the trial randomised treated vessels |
Nakamura 1995.
| Study characteristics | ||
| Methods | Randomisation method: random number table Allocation: not stated Intervention model: parallel Blinding: none |
|
| Participants | Country: United States of America No. of participants: 39: 2.7 mm TEC atherectomy plus BA: 13 4.0 mm TEC atherectomy plus BA: 13 BA: 13 Age (mean (years) ± SD): 2.7 mm TEC: 64 ± 6 4.0 mm TEC: 70 ± 6 BA: 61 ± 4.1 Inclusion criteria: occluded SFA with 1 to 2 block claudication Exclusion criteria: those with previous femoropopliteal graft or "insufficient run‐off vessels" |
|
| Interventions | BA versus 2.7 mm TEC atherectomy plus BA versus 4.0 mm TEC atherectomy plus BA | |
| Outcomes | Initial and 6‐month vessel patency Preprocedure and 6‐month ABI |
|
| Notes | 6‐month ABI only reported for participants with primary patency at 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used for randomisation. |
| Allocation concealment (selection bias) | High risk | Not specifically stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither participants nor personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete data available to 6 months. |
| Selective reporting (reporting bias) | Unclear risk | Initial and 6 month patencies reported. ABI only reported for participants whose vessels remained patent at 6 months. |
| Other bias | High risk | No mention of antiplatelet protocol pre‐ or postprocedure. |
Ott 2017.
| Study characteristics | ||
| Methods | Randomisation method: computer‐generated sequence Allocation: sealed, opaque envelopes Intervention model: parallel assignment Blinding: single (outcomes assessor) |
|
| Participants | Country: Germany No. of participants: 155 Plain BA followed by PEB angioplasty and stenting: 48 BA and stenting: 52 Atherectomy with distal protection and bailout stenting: 55 Age (mean (years) ± SD): PEB + stent: 69.7 ± 9.4 BA + stent: 69.2 ± 8 Atherectomy: 68.8 ± 10 Inclusion criteria: de novo stenosis > 70% or occlusion of the SFA Exclusion criteria: acute ischaemia or acute thrombosis of the SFA; untreated ipsilateral iliac artery stenosis > 70%; previous stenting of the SFA; popliteal stenosis > 70%; severe renal insufficiency (estimated glomerular filtration rate < 30 mL/minute/1.73m2); life expectancy of < 1 year; and contraindication to required medications. |
|
| Interventions | Plain BA followed by PEB angioplasty and stenting versus BA and stenting versus atherectomy with distal protection and bailout stenting. | |
| Outcomes | Primary outcome: percentage diameter stenosis after 6 months, measured by angiography. Secondary outcomes: TLR; thrombosis; ipsilateral amputation; binary restenosis; and all‐cause mortality at 6 and 24 months. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes opened after decision to proceed with the intervention. Randomisation performed only after guidewire passed and inclusion and exclusion criteria assessed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent core laboratory was blinded to the treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 39/155 (25%) participants did not provide primary outcome data. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes addressed. |
| Other bias | Low risk | Clear antiplatelet protocol was the same in all arms. |
Shammas 2011.
| Study characteristics | ||
| Methods | Randomisation method: simple randomisation was performed on a 1:1 basis, no method of sequence generation described Allocation: sealed envelopes, not stated if opaque Intervention model: parallel assignment Blinding: not stated |
|
| Participants | Country: United States of America No. of participants: participants: 58; vessels: 84 Silverhawk atherectomy plus BA: participants: 29; vessels: 36 BA: participants: 29; vessels: 48 Age (mean (years) ± SD): atherectomy: 67.4 ± 9.1 BA: 70.9 ± 13.9 Inclusion criteria: adults with claudication, rest pain or minor tissue loss Exclusion criteria:
|
|
| Interventions | BA versus Silverhawk atherectomy with adjunctive BA | |
| Outcomes | Primary: TLR at 1 year Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Simple randomisation was performed on a 1:1 basis, no method of sequence generation described. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes, not stated if opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated, but impractical in trials of this type. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not stated, probably not done. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Primary outcome only reported for 51/84 (61%) vessels. |
| Selective reporting (reporting bias) | High risk | Secondary outcomes major adverse events and change in ABI incompletely reported. |
| Other bias | Low risk | Clear antiplatelet protocol. |
Shammas 2012.
| Study characteristics | ||
| Methods | Randomisation method: sealed envelopes provided to all centres for randomisation, randomisation method for distribution not stated. Randomisation performed only after inclusion and exclusion criteria assessed. Allocation: sealed envelopes, not stated if opaque Intervention model: parallel assignment Blinding: not stated, probably not done |
|
| Participants | Country: United States of America No. of participants: participants: 50; vessels: 64 Diamondback atherectomy plus BA: participants: 25; vessels: 29 BA: participants: 25; vessels: 35 Age (mean (years) ± SD): atherectomy: 70.7 ± 13.4 BA: 71.8 ± 10.9 Inclusion criteria: adults with rest pain or tissue loss (Rutherford class 4 to 6); angiographic stenosis > 50%; fluoroscopically‐visible calcium > 25% of the treated segment; atherectomy wire must cross all lesions with no subintimal wire passage; main target vessel reference diameter > 1.5 mm; more than one patent distal runoff vessel with brisk flow for any treated popliteal segment; distal portion of anterior tibial or posterior tibial target vessel must reconstitute to the ankle or foot and only proximal one third of the peroneal artery to be treated; distal two thirds must reconstitute. Exclusion criteria:
|
|
| Interventions | BA versus Diamondback atherectomy with adjunctive BA | |
| Outcomes | Primary: ability to achieve adequate lumen diameter, defined as < 30% residual stenosis with no bailout stenting or dissection Secondary:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sealed envelopes provided to all centres for randomisation, randomisation method for distribution not stated. Randomisation performed only after inclusion and exclusion criteria assessed. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes, not stated if opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated, but impractical in trials of this type. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not stated, probably not done. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Secondary outcomes reported for only 33/50 (66%) participants. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported, though significant attrition present. |
| Other bias | High risk | No antiplatelet protocol |
Vroegindeweij 1995.
| Study characteristics | ||
| Methods | Randomisation method: numbered envelopes opened sequentially Allocation: sealed envelopes, not stated if opaque Intervention model: parallel assignment Blinding: not stated, probably not done | |
| Participants | Country: Netherlands No. of participants: 73 Simpson atherectomy: 38 BA: 35 Age (mean (years) range): Atherectomy: 64 (range 49 ‐ 77) BA: 64 (range 46 ‐ 80) Inclusion criteria: intermittent claudication of at least 3 months duration and obstructive lesions of the femoropopliteal arteries with a maximum length of 5 cm or complete occlusions shorter than 2 cm. Exclusion criteria: any previous ipsilateral femoropopliteal endovascular or operative intervention; participant unable to comply with the frequent follow‐up visits required by the protocol. |
|
| Interventions | BA versus Simpson atherectomy | |
| Outcomes | Primary patency during follow‐up Restenosis as determined by duplex ultrasound |
|
| Notes | Four participants crossed over to the other treatment group: three participants had angioplasty following atherectomy, one participant had atherectomy in addition to angioplasty. Results were presented in an intention‐to‐treat format | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Numbered envelopes opened sequentially. Randomisation not performed until after inclusion and exclusion criteria evaluated. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes, not stated if opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated, but impractical in trials of this type. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not stated, probably not done. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Three participants in the BA group were not followed up to 6 months. One participant in the atherectomy group and 10 in the BA group were not followed up to one year. |
| Selective reporting (reporting bias) | Low risk | Primary patency reported fully in life‐table format; restenosis presented graphically. |
| Other bias | Low risk | Clear antiplatelet protocol. |
Zeller 2017.
| Study characteristics | ||
| Methods | Randomisation method: block randomisation by centre Allocation: assigned to treatment groups after successful passage of the guidewire across the target lesion Intervention model: parallel assignment Blinding: duplex outcomes assessor blinded | |
| Participants | Country: Belgium, Germany, Poland, and Switzerland No. of participants: 102 DA + DCB (n = 48) DCB alone (n = 54) non‐randomised DA + DCB (n = 19) Age (mean (years) ± SD): DA + DCB: 70.1 ± 9.7 DCB alone: 69.0 ± 8.2 non‐randomised DA + DCB: 69.7 ± 8.9 Inclusion criteria: Rutherford Clinical Category 2 to 4; at least 18 years of age; is able and willing to provide written informed consent prior to study specific procedures. Exclusion criteria: Has a life expectancy of less than 24 months; is pregnant, of childbearing potential not taking adequate contraceptive measures, or nursing; has one or more of the contraindications listed in the SilverHawk/ TurboHawk or Cotavance IFUs; surgical or endovascular procedure of the target vessel within 14 days before the index procedure; planned intervention within 30 days of the index procedure; had ≥ 2 lesions that required treatment in the target limb (not including the iliac arteries); had a target lesion with an occluded segment ≥ 5cm in length; had in‐stent restenosis of target lesion or restenosis of the target lesion after previous treatment with DCB; had an acute intraluminal thrombus in the target lesion; had an aneurysmal target vessel; participants with severe calcification in the target lesion, defined as fluoroscopic dense circumferential calcification extending > 5 continuous centimetres, were excluded from the randomisation but were eligible for the non‐randomised (NR) treatment arm after meeting all other inclusion criteria and no other exclusion criteria. |
|
| Interventions | Arm 1: randomised ‐ drug‐eluting balloon. Arm 2. randomised ‐ TurboHawk/SilverHawk Device followed by a Cotavance drug‐eluting balloon Arm 3: non‐randomised ‐ TurboHawk/SilverHawk Device followed by a Cotavance drug‐eluting balloon |
|
| Outcomes | Primary outcome: target lesion percentage diameter stenosis at 1 year postprocedure, defined as the narrowest point of the target lesion divided by the estimated native vessel diameter at that location as determined by the angiographic core laboratory. Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation by centre. |
| Allocation concealment (selection bias) | Unclear risk | Assigned to treatment groups after successful passage of the guidewire across the target lesion, concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind investigators, participants, and the angiographic core laboratory to the treatment assignment, however the duplex ultrasound core laboratory staff and the clinical events committee were blinded to the treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not possible to blind investigators, participants, and the angiographic core laboratory to the treatment assignment, however the duplex ultrasound core laboratory staff and the clinical events committee were blinded to the treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 15/102 (15%) failed to provide primary outcome data. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. Primary outcomes reported in table format. |
| Other bias | Low risk | Antiplatelet protocol clear and uniform for both arms. |
ABI: ankle brachial index
BA: balloon angioplasty
DA: directional atherectomy
DCB: drug coated balloon
FP: femoropopliteal
MAE: major adverse event
OA: orbital atherectomy
PAD: peripheral arterial disease
PEB: paclitaxel‐eluting balloon
PSVR: peak systolic velocity ratio
SD: standard deviation
SFA: superficial femoral artery
TEC: transluminal extraction catheter
TLR: target lesion revascularisation
TVR: target vessel revascularisation
WIQ: Walking Impairment Questionnaire
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brodmann 2013 | Participants with a first in‐stent reobstruction |
| Del Giudice 2014 | In‐stent restenosis |
| Dippel 2015 | In‐stent restenosis |
| Gabrielli 2012 | Remote endarterectomy rather than atherectomy |
| Gandini 2013 | In‐stent occlusion |
| Gisbertz 2009 | Remote endarterectomy rather than atherectomy |
| NCT02730234 | Single group assignment |
| NCT02832024 | In‐stent restenosis |
| Schwindt 2017 | Non‐randomised single arm trial |
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐IOR‐17012486.
| Study name | Drug‐coated balloon versus drug‐coated balloon with atherectomy for the treatment of femoral‐popliteal calcified occlusive disease: a randomized, controlled and prospective study |
| Methods | Randomised, parallel controlled trial |
| Participants | 100 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions | Arm 1: drug‐coated balloon combined with atherectomy Arm 2: drug‐coated balloon |
| Outcomes | Primary:
Secondary:
|
| Starting date | 28 August 2017 |
| Contact information | Applicant: Jia Senhao Study leader: Guo Wei |
| Notes |
Martinsen 2015.
| Study name | Economic study design for the optimise study on orbital atherectomy and drug‐coated balloon devices for the treatment of below‐the‐knee peripheral arterial disease |
| Methods | Prospective, multicentre, randomised, postmarket pilot study |
| Participants | Country: Germany, Switzerland and Austria No of participants: 50 |
| Interventions |
|
| Outcomes | Health economic outcomes will be measured at the index procedure, at 30 days, three months, six months, 12 months, and 24 months postprocedure for the treatment of PAD and its complications (repeat procedures, amputations, etc.). Health‐related quality of life will be measured using the EQ‐5D instrument. Resource utilization will be collected from case report forms and hospital accounting systems, using site‐specific procedure code information of relevant German, Swiss, and Austrian sites. Analyses from the third‐party payer perspective will be informed by country‐specific reimbursement amounts, using Germany as the initial reference case. Resulting cost difference and incremental cost‐effectiveness are the main economic outcomes targeted in this analysis |
| Starting date | Unknown |
| Contact information | Unknown |
| Notes | Published need for paper but no results available |
NCT01579123.
| Study name | Laser atherectomy versus angioplasty for the treatment of critical limb ischaemia |
| Methods | Randomised, single blind (participant), parallel controlled trial |
| Participants | 200 participants Inclusion criteria: 18 years of age and older; male or female (non‐pregnant women); participants with PAD that has progressed to CLI; participants undergoing angiography with possible intervention for Rutherford Class 4 to 6 limb ischaemia that may benefit from revascularisation. |
| Interventions | Angioplasty versus laser atherectomy |
| Outcomes | Primary outcome measure: difference in patency rates (one year) |
| Starting date | February 2012 |
| Contact information | William Shutze, MD Baylor Jack and Jane Hamilton Heart Hospital |
| Notes |
NCT01763476.
| Study name | Atherectomy and drug‐coated balloon angioplasty in treatment of long infrapopliteal lesions (ADCAT) |
| Methods | Prospective, open, randomised, parallel assignment, single‐centre clinical trial |
| Participants | Estimated enrolment: 80 participants Ages eligible for study: 50 years to 85 years Inclusion criteria:
Exclusion criteria:
|
| Interventions | Arm 1: paclitaxel‐coated balloon angioplasty Arm 2: atherectomy + paclitaxel‐balloon |
| Outcomes | Primary outcome measure: primary patency of the target lesion 6 months after index procedure measured by duplex ultrasound (PVR > 2.4) and angiography (core lab analysis). Secondary outcome measure: need for TLR from baseline to 6 months after index procedure. Other outcome measures: change in Rutherford‐Becker Class from baseline to 6 and 12 months after index procedure. |
| Starting date | January 2013 |
| Contact information | Aljoscha Rastan, M.D., Herz‐Zentrums Bad Krozingen |
| Notes |
NCT02514460.
| Study name | Clinical study of stent versus direct atherectomy to treat lower limb ischemia |
| Methods | Randomised, single blind (participant), parallel controlled trial |
| Participants | Estimated enrolment: 120 participants Ages eligible for study: 18 years and older Sexes eligible for study: all Inclusion criteria: provides written informed consent; willing to comply with follow‐up evaluations at specified times; has claudication or rest pain due to PAD; disease located within the femoropopliteal artery; participant has a de novo or restenotic lesion(s) with > 50% stenosis documented angiographically and no prior stent in the target lesion; participant has symptoms of PAD classified as Rutherford Category 2 or more. Exclusion criteria: previously implanted stent(s) or stent graft(s) in target leg; life expectancy less than 12 months; has any planned surgical or endovascular intervention of target vessel 30 days before or after index procedure; thrombophlebitis, uremia, or deep venous thrombus, within past 30 days; receiving dialysis or immunosuppressant therapy; recent stroke within past 90 days; known allergies to the following: aspirin, clopidogrel bisulphate (Plavix) or ticlopidine (Ticlid), heparin, Nitinol (nickel titanium), contrast agent, that cannot be medically managed; tissue loss due to ischaemic disease (Rutherford/Becker category 5 or 6); serum creatinine level ≥ 2.5 mg/dL at time of screening visit; known or suspected active infection at the time of the procedure; bleeding diathesis; participant is unwilling or unable to comply with procedures specified in the protocol or has difficulty or inability to return for follow‐up visits as specified by the protocol; participant is known to be pregnant, incarcerated, mentally incompetent, alcohol or drug abuser; participant is currently participating in any other investigational drug or medical device study that has not completed primary endpoint(s) evaluation or clinically interferes with the endpoints from this study or future participation in such studies prior to the completion of this study. |
| Interventions | Arm 1: stents Arm 2: direct atherectomy group |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | January 2014 |
| Contact information | Xuanwu Hospital, Beijing |
| Notes |
NCT02517827.
| Study name | Percutaneous intervention versus surgery in the treatment of common femoral artery lesions (PESTO‐AFC) |
| Methods | Prospective, open, randomised, parallel assignment, multi‐centre clinical trial |
| Participants | Estimated enrolment; 306 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions | Arm Intervention/treatment Active comparator: endovascular procedure Common femoral artery (target lesion) to be treated with directional atherectomy and paclitaxel‐coated balloon angioplasty. Optional: stent implantation. Device: Atherectomy and paclitaxel‐coated balloon angioplasty Directional atherectomy and paclitaxel‐coated balloon angioplasty (optional with stent implantation) of the CFA Active comparator: surgery CFA (target lesion) to be treated with open, surgical endarterectomy Procedure: open, surgical endarterectomy of the CFA |
| Outcomes | Primary outcome measure:
Primary patency (12 months): primary patency of the CFA defined as freedom from target lesion restenosis (luminal narrowing of ≥ 50%) detected with duplex‐ultrasound. The definition of a 50% restenosis is based on the peak systolic velocity ratio > 2.4. Secondary outcome measures:
Other outcome measures: Rutherford‐Becker class (6, 12, and 24 months): change in Rutherford‐Becker class |
| Starting date | 1 August 2017 |
| Contact information | Contact: Aljoscha Rastan, MD Contact: Thomas Zeller, MD |
| Notes |
NCT02561299.
| Study name | Orbital vessel preparation to maximIze DCB efficacy in calcified below the knee (BTK) lesions ‐ a pilot study (OPTIMIZE BTK) |
| Methods | Prospective, open, randomised, parallel assignment clinical trial |
| Participants | Estimated enrolment: 66 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions | Arm 1: lesion preparation with peripheral orbital atherectomy system followed by drug‐coated balloon angioplasty Arm 2: DCB angioplasty |
| Outcomes | Primary outcome measure: device success (per each DCB used during the index procedure), defined as the ability to achieve successful delivery and deployment of the DCB to the target lesion as described per IFU within 3 minutes of insertion without removal and use of an additional device Secondary outcome measures:
|
| Starting date | September 2015 |
| Contact information | Cardiovascular Systems Inc |
| Notes |
NCT02840786.
| Study name | Clinical study of stent versus direct atherectomy to treat arteriosclerosis occlusive disease of lower extremity |
| Methods | Open, randomised, parallel assignment clinical trial |
| Participants | Estimated enrolment: 221 participants Inclusion criteria: Patients were included if they were de novo stenosis > 70% or occlusion of the femoropopliteal at least 18 years of age and referred for claudication (Rutherford‐Becker class II‐III) or critical limb ischaemia (Rutherford‐Becker class IV‐V). Exclusion criteria:
|
| Interventions | Arm 1: stent Arm 2: direct atherectomy |
| Outcomes | Primary outcome measure: 12‐month primary patency rate (12 months) (systolic velocity ratio > 2.4 as measured by Duplex ultrasound) Secondary outcome measures:
|
| Starting date | 21 July 2016 |
| Contact information | |
| Notes |
NCT03206762.
| Study name | JET‐RANGER Trial ‐ JETStream atherectomy with adjunctive paclitaxel‐coated balloon angioplasty versus plain old balloon angioplasty followed by paclitaxel‐coated balloon |
| Methods | Prospective, single blind (participant), randomised, parallel assignment, multi‐centre study |
| Participants | Estimated enrolment: 255 participants Ages eligible for study: 18 years and older Sexes eligible for study: all General inclusion criteria: has a Rutherford Clinical Category of 2 to 4; is willing and capable of complying with all follow‐up evaluations at the specified times (including an angiogram at the 1‐year follow‐up visit); is able and willing to provide written informed consent prior to study specific procedures Angiographic inclusion criteria: Participant must meet all of the following angiographic inclusion criteria. Unless otherwise specified, the Investigator performing the procedure bases all angiographic inclusion criteria on visual determination at the time of the procedure:
General exclusion criteria:
Angiographic exclusion criteria: the Investigator performing the procedure bases all angiographic exclusion criteria on visual determination at the time of the procedure;
|
| Interventions | Arm 1: Jetstream atherectomy used in conjunction with the Ranger DCB or Medtronic IN.PACT D Arm 2: POBA and then DCB treatment (Ranger or IN.PACT) |
| Outcomes | Primary outcome measures:
|
| Starting date | 28 March 2018 |
| Contact information | Midwest Cardiovascular Research Foundation |
| Notes |
NCT03380650.
| Study name | Study of combined use of directional atherectomy and local drug delivery with balloon catheter system in the treatment of femoropopliteal occlusive disease |
| Methods | Single blind (participant), randomised, parallel assignment clinical trial |
| Participants | Estimated enrolment: 40 participants Ages eligible for study: 18 years to 80 years (adult, older adult) Sexes eligible for study: all Inclusion criteria:
Exclusion criteria:
|
| Interventions | Device: directional atherectomy and local drug delivery Device: drug‐coated balloon dilation |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | 1 January 2018 |
| Contact information | Shuofei Yang, MD, PhD |
| Notes |
NCT03495453.
| Study name | Directional versus orbital atherectomy plaque modification and luminal area assessment of the femoropopliteal artery via intravascular ultrasound |
| Methods | Prospective, open, randomised, parallel assignment, single‐centre study |
| Participants | Estimated enrolment: 60 participants
Inclusion criteria:
Exclusion criteria: People who have:
|
| Interventions | Arm Intervention/treatment Active comparator: CSI's DIAMONDBACK 360 Peripheral OAS OAS (using CSI device) followed by Inpact Admiral DCB Device: percutaneous revascularisation of the femoropopliteal arteries using an OAS device HawkOne DAS is a small catheter with cutting device. The doctor slowly and smoothly advances it across the blockage in the artery and shaves the plaque from the vessel wall and collects it in the reservoir. Active Comparator: Medtronic's Hawkone DAS DAS (using the Hawkone device) followed by DCB Device: percutaneous revascularisation of the femoropopliteal arteries using a DAS Diamondback 360 Peripheral OAS is a small catheter with a diamond crown. The doctor inserts it at the groin and advances into the leg.The OAS works by spinning around inside the artery to "sand down" the buildup of material along the artery walls while leaving the healthy vessel behind. |
| Outcomes | Primary outcome measure:
Measure of luminal area measured via IVUS at pretreatment and postatherectomy (12 months). All participants will undergo IVUS at pretreatment run to assess the severity and morphology of the plaque composition and postatherectomy run to assess changes postatherectomy treatment. Secondary outcome measure: Plaque burden reduction (12 months): the amount of removed plaque will be analysed via IVUS pretreatment and postatherectomy. |
| Starting date | 12 April 2018 |
| Contact information | Zulfiya Bakirova |
| Notes |
ABI: ankle brachial index
CFA: common femoral artery
CT: computed tomography
CTA: computed tomography angiogram
CLI: critical limb ischaemia
DAS: directional atherectomy system
DCB: drug coated balloon
DUS: duplex ultrasound
DVT: deep vein thrombosis
EQ‐5D: EuroQoL Quality of Life Questionnaire
IFU: instructions for use
IVUS: intravascular ultrasound
LLL: late lumen loss
MAE: major adverse event
OA: orbital atherectomy
OAS: orbital atherectomy system
PA: popliteal artery
PAD; peripheral arterial disease
POBA: plain old balloon angioplasty
PTA: percutaneous transluminal angioplasty
PVR: peak velocity ratio
QoL: quality of life
QVA: quantitative vascular angiography
RCT: randomised controlled trial
SFA: superficial femoral artery
TASC: Trans‐Atlantic Inter‐Society Consensus Document on Management of Peripheral Arterial Disease
TLR: target lesion revascularisation
TBI: toe brachial index
Differences between protocol and review
We assessed the quality of the trials using Cochrane's 'Risk of bias' tool (Higgins 2011). We have added 'Summary of findings' tables as part of this update, and used the GRADE approach to assess the certainty of the evidence, as recommended by Cochrane.
We have included additional subgroup analyses between drug‐eluting and plain balloon angioplasty' as several RCTs have shown that paclitaxel‐coated balloons and stents reduce the rates of TVR and vessel restenosis after lower extremity interventions. A systematic review and meta‐analysis by Katsanos 2018 examining these studies has shown that the risk of death after a year was increased in participants treated with paclitaxel‐eluting balloons and stents, so we performed subgroup analysis to see if this factor impacted on participants' outcomes.
Contributions of authors
BW: study selection, assessed trial quality, extracted data, updated the review text GA: study selection, assessed trial quality, extracted data, updated the review text RR: reviewed and edited update RH: reviewed and edited update CT: reviewed and edited update
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
BW: none known GA: has declared that he has received money from NIHR for an academic clinical fellowship, but this does not cause any conflict of interest with this review. RR: none known RH: none known CT: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Dattilo 2014 {published data only}
- Dattilo R, Himmelstein SI, Cuff RF. The COMPLIANCE 360 trial: a randomized, prospective, multicenter, pilot study comparing acute and long-term results of orbital atherectomy to balloon angioplasty for calcified femoropopliteal disease. Journal of Invasive Cardiology 2014;26(8):355-60. [PubMed] [Google Scholar]
Nakamura 1995 {published data only}
- Nakamura S, Conroy RM, Gordon IL, Deutsch LS, Maheswaran B, Antone CS, et al. A randomized trial of transcutaneous extraction atherectomy in femoral arteries: intravascular ultrasound observations. Journal of Clinical Ultrasound 1995;23(8):461-71. [DOI] [PubMed] [Google Scholar]
Ott 2017 {published data only}
- NCT00986752. Efficacy study of stenting, paclitaxel eluting balloon or atherectomy to treat peripheral artery disease (ISAR-STATH). clinicaltrials.gov/show/NCT00986752 (first posted 30 September 2009).
- Ott I, Cassese S, Groha P, Steppich B, Hadamitzky M, Ibrahim T, et al. Randomized comparison of Paclitaxel-eluting balloon and stenting versus plain balloon plus stenting versus directional atherectomy for femoral artery disease (ISAR-STATH). Circulation 2017;135(23):2218-26. [DOI] [PubMed] [Google Scholar]
Shammas 2011 {published data only}
- Coiner D, Dippel E, Jerin M, Shammas G, Shammas N. Percutaneous lower extremity arterial interventions using balloon angioplasty versus silverhawk atherectomy: results of the SMARTHAWK randomized trial. Catheterization and Cardiovascular Interventions 2009;73:S21. [Google Scholar]
- Coiner D, Dippel E, Jerin M, Shammas G, Shammas N. Walking impairment questionnaire, ankle-brachial index and Rutherford-Baker class in patients undergoing angioplasty versus Silverhawk atherectomy: results from the smarthawk randomized trial. Catheterization and Cardiovascular Interventions 2009;73:S42. [Google Scholar]
- Shammas NW, Coiner D, Shammas G, Jerin M. Predictors of provisional stenting in patients undergoing lower extremity arterial interventions. International Journal of Angiology 2011;20(2):95-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shammas NW, Coiner D, Shammas GA, Dippel EJ, Christensen L, Jerin M. Percutaneous lower-extremity arterial interventions with primary balloon angioplasty versus Silverhawk atherectomy and adjunctive balloon angioplasty: randomized trial. Journal of Vascular and Interventional Radiology 2011;22(9):1223-8. [DOI] [PubMed] [Google Scholar]
Shammas 2012 {published data only}
- Shammas NW, Lam R, Mustapha J, Ellichman J, Aggarwala G, Rivera E, et al. Comparison of orbital atherectomy plus balloon angioplasty vs. balloon angioplasty alone in patients with critical limb ischemia: results of the calcium 360 randomized pilot trial. Journal of Endovascular Therapy 2012;19(4):480-8. [DOI] [PubMed] [Google Scholar]
- Shammas NW, Lam R, Mustapha J, Ellichman J, Aggarwala G, Rivera E. Orbital atherectomy and balloon angioplasty vs. balloon angioplasty alone in critical limb ischemia: results of the CALCIUM 360 trial. Journal of Vascular and Interventional Radiology 2013;24(1):145.e2-3. [Google Scholar]
- Shammas NWM. Six-month outcomes of prospective, randomized CALCIUM 360 study demonstrate the advantages of plaque modification with the orbital technology versus treatment with balloon angioplasty in patients with critical limb ischemia. Journal of the American College of Cardiology 2011;58(20 Suppl 1):B154. [Google Scholar]
Vroegindeweij 1995 {published data only}
- Tielbeek AV, Vroegindeweij D, Buth J, Landman GH. Comparison of balloon angioplasty and Simpson atherectomy for lesions in the femoropopliteal artery: angiographic and clinical results of a prospective randomized trial. Journal of Vascular and Interventional Radiology 1996;7(6):837-44. [DOI] [PubMed] [Google Scholar]
- Tielbeek AV, Vroegindeweij D, Buth J, Schol FP, Mali WP. Comparison of intravascular ultrasonography and intraarterial digital subtraction angiography after directional atherectomy of short lesions in femoropopliteal arteries. Journal of Vascular Surgery 1996;23(3):436-45. [DOI] [PubMed] [Google Scholar]
- Vroegindeweij D, Kemper FJ, Tielbeek AV, Buth J, Landman G. Recurrence of stenoses following balloon angioplasty and Simpson atherectomy of the femoro-popliteal segment. A randomised comparative 1-year follow-up study using colour flow duplex. European Journal of Vascular Surgery 1992;6(2):164-71. [DOI] [PubMed] [Google Scholar]
- Vroegindeweij D, Kemper FJ, Tielbeek AV, Landman G, Buth J. Recurrence of stenoses following balloon angioplasty and atherectomy of the femoropopliteal segment. A randomized comparative one year follow-up study using color flow duplex. In: European Society for Vascular Surgery 5th Annual Meeting. Warsaw, Poland, 1991:72.
- Vroegindeweij D, Tielbeek AV, Buth J, Schol FP, Hop WC, Landman GH. Directional atherectomy versus balloon angioplasty in segmental femoropopliteal artery disease: two-year follow-up with color-flow duplex scanning. Journal of Vascular Surgery 1995;21(2):255-68; discussion 268-9. [DOI] [PubMed] [Google Scholar]
Zeller 2017 {published data only}
- NCT01366482. Atherectomy followed by a drug coated balloon to treat peripheral arterial disease (DEFINITIVE AR). clinicaltrials.gov/show/NCT01366482 (first posted 6 June 2011).
- Zeller T, Langhoff R, Rocha-Singh KJ, Jaff MR, Blessing E, Amann-Vesti B, et al. Directional atherectomy followed by a Paclitaxel-coated balloon to inhibit restenosis and maintain vessel patency: twelve-month results of the DEFINITIVE AR Study. Circulation: Cardiovascular Interventions 2017;10(9):e004848. [DOI: 10.1161/circinterventions.116.004848] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Brodmann 2013 {published data only}
- Brodmann M, Rief P, Froehlich H, Dorr A, Gary T, Eller P, et al. Neointimal hyperplasia after Silverhawk atherectomy versus percutaneous transluminal angioplasty (PTA) in femoropopliteal stent reobstructions: a controlled, randomized pilot trial. Cardiovascular and Interventional Radiology 2013;36(1):69-74. [DOI] [PubMed] [Google Scholar]
Del Giudice 2014 {published data only}
- Del Giudice C. Synergistic strategy of laser atherectomy and drug-eluting balloon angioplasty for treatment of in-stent restenosis in the superficial femoral artery: a randomised study. In: EuroPCR Abstracts and Posters. 2014. [DOI: 10.1007/978-1-4471-5220-0_14] [DOI]
Dippel 2015 {published data only}
- Dippel EJ, Makam P, Kovach R, George JC, Patlola R, Metzger DC, et al. Randomized controlled study of excimer laser atherectomy for treatment of femoropopliteal in-stent restenosis: Initial results from the EXCITE ISR Trial (EXCImer laser randomized controlled study for treatment of FemoropopliTEal in-stent restenosis). JACC: Cardiovascular Interventions 2015;8(1):95-101. [DOI] [PubMed] [Google Scholar]
- Dippel EJ, Makam P, Kovach RC, George JC, Patlola RR, Metzger DC, et al. Excite ISR: a prospective, randomized controlled trial of excimer laser atherectomy vs balloon angioplasty for the treatment of femoropopliteal in-stent restenosis. Journal of the American College of Cardiology 2014;64(11 suppl 1):B155. [Google Scholar]
Gabrielli 2012 {published data only}
- Gabrielli R, Rosati MS, Vitale S, Baciarello G, Siani A, et al. Randomized controlled trial of remote endarterectomy versus endovascular intervention for TransAtlantic Inter-Society Consensus II D femoropopliteal lesions. Journal of Vascular Surgery 2012;56(6):1598-605. [DOI] [PubMed] [Google Scholar]
Gandini 2013 {published data only}
- Gandini R, Del Giudice C, Merolla S, Morosetti D, Pampana E, Simonetti G. Treatment of chronic SFA in-stent occlusion with combined laser atherectomy and drug-eluting balloon angioplasty in patients with critical limb ischemia: a single-center, prospective, randomized study. Journal of Endovascular Therapy 2013;20:805-14. [DOI] [PubMed] [Google Scholar]
Gisbertz 2009 {published data only}
- Gisbertz SS, Ramzan M, Tutein Nolthenius RP, Laan L, Overtoom TT, Moll FL, et al. Short-term results of a randomized trial comparing remote endarterectomy and supragenicular bypass surgery for long occlusions of the superficial femoral artery (the REVAS Trial). European Journal of Vascular and Endovascular Surgery 2009;37(1):68-76. [DOI] [PubMed] [Google Scholar]
- Gisbertz SS, Tutein Nolthenius RP, Borst GJ, Laan L, Overtoom TT, Moll FL, et al. Remote endarterectomy versus supragenicular bypass surgery for long occlusions of the superficial femoral artery: medium-term results of a randomized controlled trial (the REVAS trial). Annals of Vascular Surgery 2010;24(8):1015-23. [DOI] [PubMed] [Google Scholar]
NCT02730234 {published data only}
- NCT02730234. JetStream atherectomy for the treatment of in-stent restenosis (JET-ISR). clinicaltrials.gov/show/NCT02730234 (first received 6 April 2016).
NCT02832024 {published data only}
- NCT02832024. Clinical study of stent versus direct atherectomy versus angioplasty to treat lower limb in-stent restenosis. clinicaltrials.gov/ct2/show/NCT02832024 (first received 13 July 2016).
Schwindt 2017 {published data only}
- Schwindt AG, Bennett JG Jr, Crowder WH, Dohad S, Janzer SF, George JC, et al. Lower extremity revascularization using optical coherence tomography-guided directional atherectomy: final results of the evaluatIon of the pantheris optIcal coherence tomography imaging atherectomy system for use in the peripheral vasculature (VISION) Study. Journal of Endovascular Therapy 2017;24(3):355-66. [DOI] [PubMed]
References to ongoing studies
ChiCTR‐IOR‐17012486 {published data only}
- ChiCTR-IOR-17012486x. Drug-coated balloon versus drug-coated balloon with atherectomy for the treatment of femoral-popliteal calcified occlusive disease: a randomized, controlled and prospective study. www.chictr.org.cn/showprojen.aspx?proj=21256 (first received 28 August 2017).
Martinsen 2015 {published data only}
- Martinsen BJ, Weber SA, Pietzsch ML, Behrens A, Weatherspoon M, Igyarto Z, et al. Economic study design for the optimize study on orbital atherectomy and drug-coated balloon devices for the treatment of below-the-knee peripheral arterial disease. Value in Health 2015;18(7):A723. [Google Scholar]
NCT01579123 {published data only}
- NCT01579123. Laser atherectomy versus angioplasty for the treatment of critical limb ischemia. clinicaltrials.gov/ct2/show/NCT01579123 (first received 17 April 2012).
NCT01763476 {published data only}
- NCT01763476. Atherectomy and drug-coated balloon angioplasty in treatment of long infrapopliteal lesions. clinicaltrials.gov/ct2/show/NCT01763476 (first received 9 January 2013).
NCT02514460 {published data only}
- NCT02514460. Clinical study of stent versus direct atherectomy to treat lower limb ischemia. clinicaltrials.gov/ct2/show/NCT02514460 (first received 3 August 2015).
NCT02517827 {published data only}
- NCT02517827. Percutaneous intervention versus surgery in the treatment of common femoral artery lesions. clinicaltrials.gov/ct2/show/NCT02517827 (first received 7 August 2015).
NCT02561299 {published data only}
- NCT02561299. Orbital vessel preparation to maximize dcb efficacy in calcified below the knee (BTK) lesions - a pilot study. clinicaltrials.gov/ct2/show/NCT02561299 (first received 28 September 2015).
NCT02840786 {published data only}
- NCT02840786. Clinical study of stent versus direct atherectomy to treat arteriosclerosis occlusive disease of lower extremity. clinicaltrials.gov/ct2/show/NCT02840786 (first received 26 July 2016).
NCT03206762 {published data only}
- NCT03206762. JET-RANGER Trial - JETStream atherectomy with adjunctive Paclitaxel-coated balloon angioplasty vs plain old balloon angioplasty followed by Paclitaxel-coated balloon. clinicaltrials.gov/ct2/show/NCT03206762 (first received 2 July 2017).
NCT03380650 {published data only}
- NCT03380650. Study of DA+LDD in the treatment of femoropopliteal occlusive disease. clinicaltrials.gov/ct2/show/NCT03380650 (first received 21 December 2017).
NCT03495453 {published data only}
- NCT03495453. Directional versus orbital atherectomy plaque modification and luminal area assessment of the femoro-popliteal artery via intravascular ultrasound. clinicaltrials.gov/ct2/show/NCT03495453 (first received 12 April 2018).
Additional references
Abdel‐Wahab 2013
- Abdel-Wahab M, Richardt G, Joachim Buttner H, Toelg R, Geist V, Meinertz T, et al. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: the randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial. ACC Cardiovascular Intervention January 2013;6:10-9. [DOI] [PubMed] [Google Scholar]
Akkus 2014
- Akkus NI, Abdulbaki A, Jimenez E, Tandon N. Atherectomy devices: technology update. Med Devices (Auckl) 2014;8:1-10. [DOI: 10.2147/MDER.S50594] [DOI] [PMC free article] [PubMed] [Google Scholar]
Briguori 2003
- Briguori C, DiMario C, Colombo A. Distal embolization during directional coronary atherectomy. Journal of Invasive Cardiology 2003;15(8):448-50. [PubMed] [Google Scholar]
Dake 2011
- Dake MD, Ansel GM, Jaff MR, Ohki T, Saxon RR, Smouse HB, et al, Zilver PTX Investigators. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. Circulation. Cardiovascular Interventions 2011;4(5):495-504. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI] [PubMed] [Google Scholar]
ERBAC 1997
- Reifart N, Vandormael M, Krajcar M, Göhring S, Preusler W, Schwarz F, et al. Randomized comparison of angioplasty of complex coronary lesions at a single center. Excimer Laser, Rotational Atherectomy, and Balloon Angioplasty Comparison (ERBAC) Study. Circulation 1997;96(1):91-8. [DOI] [PubMed] [Google Scholar]
European Consensus Document 1989
- European Consensus. European consensus on critical limb ischaemia. Lancet 1989;333(8640):737-8. [Google Scholar]
Fowkes 1998
- Fowkes G, Gillespie IN. Angioplasty (versus non surgical management) for intermittent claudication. Cochrane Database of Systematic Reviews 1998, Issue 2. Art. No: CD000017. [DOI: 10.1002/14651858.CD000017] [DOI] [PubMed] [Google Scholar]
Fowkes 2008
- Fowkes F, Leng GC. Bypass surgery for chronic lower limb ischaemia. Cochrane Database of Systematic Reviews 2008, Issue 2. Art. No: CD002000. [DOI: 10.1002/14651858.CD002000.pub2] [DOI] [PubMed] [Google Scholar]
Frans 2012
- Frans FA, Bipat S, Reekers JA, Legemate DA, Koelemay MJ. Systematic review of exercise training or percutaneous transluminal angioplasty for intermittent claudication. British Journal of Surgery 2012;99(1):16-28. [DOI] [PubMed] [Google Scholar]
Fu 2015
- Fu X, Zhang Z, Liang K, Shi S, Wang G, Zhang K, et al. Angioplasty versus bypass surgery in patients with critical limb ischemia: a meta-analysis. International Journal of Clinical and Experimental Medicine 2015;8:10595-602. [PMC free article] [PubMed] [Google Scholar]
Garcia 2009
- Garcia LA, Lyden SP. Atherectomy for infrainguinal peripheral artery disease. Journal of Endovascular Therapy 2009;16(2 Suppl 2):II105-15. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians? BMJ 2008;336:995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heuser 2008
- Heuser RR. Treatment of lower extremity vascular disease: the Diamondback 360 degrees Orbital Atherectomy System. Expert Review of Medical Devices 2008;5(3):279-86. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Indes 2010
- Indes JE, Shah HJ, Jonker FH, Ohki T, Veith FJ, Lipsitz EC. Subintimal angioplasty is superior to SilverHawk atherectomy for the treatment of occlusive lesions of the lower extremities. Journal of Endovascular Therapy 2010;17(2):243-50. [DOI] [PubMed] [Google Scholar]
Katsanos 2018
- Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. Journal of American Heart Association 2018;7(24):e011245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kayssi 2016
- Kayssi A, Al-Atassi T, Oreopoulos G, Roche-Nagle G, Tan KT, Rajan DK. Drug-eluting balloon angioplasty versus uncoated balloon angioplasty for peripheral arterial disease of the lower limbs. Cochrane Database of Systematic Reviews 2016, Issue 8. Art. No: CD011319. [DOI: 10.1002/14651858.CD011319.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2018
- Kim TH, Katsetos M, Dahal K, Azrin M, Lee J. Use of rotational atherectomy for reducing significant dissection in treating de novo femoropopliteal steno-occlusive disease after balloon angioplasty. Journal of Geriatric Cardiology 2018;15:254-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laird 2010
- Laird JR, Katzen BT, Scheinert D, Lammer J, Carpenter J, Buchbinder M, et al, on behalf of the RESILIENT Investigators. Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: twelve-month results from the RESILIENT randomized trial. Circulation. Cardiovascular Interventions 2010;3(3):267-76. [DOI] [PubMed] [Google Scholar]
Lee 2017
- Lee M, Genereux P, Shlofmitz R, Phillipson D, Anose BM, Martinsen BJ, et al. Orbital atherectomy for treating de novo, severely calcified coronary lesions: 3-year results of the pivotal ORBIT II trial. Cardiovascular Revascularization Medicine 2017;18:261-4. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from handbook.cochrane.org.
McKinsey 2008
- McKinsey JF, Goldstein L, Khan HU, Graham A, Rezeyat C, Morrissey NJ, et al. Novel treatment of patients with lower extremity ischemia: use of percutaneous atherectomy in 579 lesions. Annals of Surgery 2008;248(4):519-28. [DOI] [PubMed] [Google Scholar]
Murphy 2015
- Murphy TP, Cutlip DE, Regensteiner JG, Mohler ER, Cohen DJ, Reynolds MR, et al. Supervised exercise, stent revascularization, or medical therapy for claudication due to aortoiliac peripheral artery disease: a randomized clinical trial. Journal of the American College of Cardiology 2015;65:999-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2011
- National Institute for Health and Care Excellence. Percutaneous atherectomy of femoropopliteal arterial lesions with plaque excision devices [IPG380]. Available at: www.nice.org.uk/guidance/IPG380 2011.
Ramkumar 2019
- Ramkumar N, Martinez-Camblor P, Columbo JA, Osborne NH, Goodney PP, O'Malley AJ. Adverse events after atherectomy: analyzing long-term outcomes of endovascular lower extremity revascularization techniques. Journal of the American Heart Association 2019;8(12):e012081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rerkasem 2010
- Rerkasem K, Rothwell PM. Meta-analysis of small randomised controlled trials in surgery may be unreliable. British Journal of Surgery 2010;97(4):466-9. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robertson 2012
- Robertson L, Ghouri MA, Kovacs F. Antiplatelet and anticoagulant drugs for prevention of restenosis/reocclusion following peripheral endovascular treatment. Cochrane Database of Systematic Reviews 2012, Issue 8. Art. No: CD002071. [DOI: 10.1002/14651858.CD002071.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schillinger 2006
- Schillinger M, Sabeti S, Loewe C, Dick P, Amighi J, Mlekusch W, et al. Balloon angioplasty versus implantation of Nitinol stents in the superficial femoral artery. New England Journal of Medicine 2006;354(18):1879-88. [DOI] [PubMed] [Google Scholar]
Schroeder 2017
- Schroeder H, Werner M, Meyer DR, Reimer P, Kruger K, Jaff MR, et al. Low-dose Paclitaxel-coated versus uncoated percutaneous transluminal balloon angioplasty for femoropopliteal peripheral artery disease: one-year results of the ILLUMENATE European randomized clinical trial (randomized trial of a novel paclitaxel-coated percutaneous angioplasty balloon). Circulation 2017;135:2227-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwarzwälder 2010
- Schwarzwälder U, Zeller T. Debulking procedures: potential device specific indications. Techniques in Vascular Interventional Radiology 2010;13(1):43-53. [DOI] [PubMed] [Google Scholar]
Simpson 1988
- Simpson JB, Selmon MR, Robertson GC, Cipriano PR, Hayden WG, Johnson DE, et al. Transluminal atherectomy for occlusive peripheral vascular disease. American Journal of Cardiology 1988;16(14):96G-101G. [DOI] [PubMed] [Google Scholar]
Spreen 2017
- Spreen MI, Martens JM, Knippenberg B, Dijk LC, Vries JPM, Vos JA, et al. Long-term follow-up of the PADI trial: percutaneous transluminal angioplasty versus drug-eluting stents for infrapopliteal lesions in critical limb ischemia. Journal of the American Heart Association 2017;6(4):e004877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stack 1988
- Stack RS, Califf RM, Phillips HR, Pryor DB, Quigley PJ, Bauman RP, et al. Interventional Cardiac Catheterization at Duke Medical Center. American Journal of Cardiology 1988;62(10 Pt2):3F-24F. [PubMed] [Google Scholar]
TALON Registry 2006
- Ramaiah V, Gammon R, Kiesz S, Cardenas J, Runyon JP, Fail P, et al, on behalf of the TALON Registry. Midterm outcomes from the TALON Registry: treating peripherals with SilverHawk: outcomes collection. Journal of Endovascular Therapy 2006;13(5):592-602. [DOI] [PubMed] [Google Scholar]
TASC II 2007
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al, on behalf of the TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease. International Angiology 2007;26(2):81-157. [PubMed] [Google Scholar]
Watson 2008
- Watson L, Ellis B, Leng GC. Exercise for intermittent claudication. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD000990. [DOI: 10.1002/14651858.CD000990.pub2] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ambler 2014
- Ambler GK, Radwan R, Hayes PD, Twine CP. Atherectomy for peripheral arterial disease. Cochrane Database of Systematic Reviews 2014, Issue 3. Art. No: CD006680. [DOI: 10.1002/14651858.CD006680.pub2] [DOI] [PubMed] [Google Scholar]
Viswanathan 2007
- Viswanathan G, Navaneethan SD. Atherectomy for peripheral arterial disease. Cochrane Database of Systematic Reviews 2007, Issue 3. Art. No: CD006680. [DOI: 10.1002/14651858.CD006680] [DOI] [Google Scholar]


