Abstract
Despite ongoing advancements in the field of medicine, glioblastoma multiforme (GBM) is presently incurable, making this advanced brain tumor the deadliest tumor type in the central nervous system. The primary treatment strategies for GBM (i.e. surgical resection, radiation therapy, chemotherapy, and newly incorporated targeted therapies) fail to overcome the challenging characteristics of highly aggressive GBM tumors and are presently given with the goal of increasing the quality of life for patients. With the aim of creating effective treatment solutions, research has shifted toward utilizing injectable biomaterial adjuncts to minimize invasiveness of treatment, provide spatiotemporal control of therapeutic delivery, and engage with cells through material-cell interfaces. This review aims to summarize the limitations of the current standard of care for GBM, discuss how these limitations can be addressed by local employment of injectable biomaterial systems, and highlight developments in the field of biomaterials for these applications.
Keywords: glioblastoma, biomaterials, tumor therapies, injectable
Graphical Abstract
Glioblastoma is the most aggressive type of brain tumor and is presently uncurable. Numerous limitations of treating brain tumors can be mitigated by incorporating injectable biomaterials for delivery of therapeutic payloads. Injectable biomaterials in combination with chemotherapy, radiation therapy, and targeted therapy strategies are being explored to overcome the limitations of treatment and increase therapeutic efficacy.
1. Introduction
Glioblastoma multiforme (GBM) is the most aggressive form of malignant brain cancer. There are an estimated 17,000 new patients diagnosed with GBM each year, with an incidence rate of 3.19 out of every 100,000 people.[1] Unfortunately, this grade IV astrocytoma remains incurable with a median survival of merely fifteen months.[2] Despite recent advances in technology and tumor treatment, the current standard of care for GBM has been unchanged since established in 2005: maximally safe surgical resection followed by radiation therapy and concurrent Temozolomide (TMZ) chemotherapy.[3] Patients with inoperable tumors are unable to undergo surgical tumor resection and are faced with worse prognoses. However, after first-line treatment, patients with resected tumors observe high incidence (>99%) of local tumor recurrence.[4] In addition to the standard of care strategies, targeted therapies (i.e. treatments targeting genes or proteins specific to tumor tissue) have gained traction as a second-line treatment option for GBM. Even so, there is currently no cure for this highly aggressive form of cancer. The bleak outcomes for GBM patients are a result of the numerous challenges associated with treating this tumor type using the current standard of care. Because GBM is a brain tumor, tumor resection is conducted using ‘maximally safe’ surgical margins with the goal of removing all cancerous cells while leaving as much of the healthy parenchyma intact as possible.[5] This strategy is particularly important for GBM because damaging or removing the highly specialized healthy brain tissue will result in language, motor, or neurocognitive deficits. Drug delivery is limited in the brain by the blood-brain barrier (BBB) as well as the abnormal tumor vasculature in GBM tumors which causes inhomogeneous delivery to the entire tumor.[6] In addition to abnormal vasculature, other microenvironmental factors such as tumor hypoxia and the resultant interstitial pressure also support immune evasion and reduce the efficacy of GBM immunotherapies.[7] While the molecular drivers of GBM are similar to other aggressive tumor types, the effectiveness of chemotherapy and radiation therapy against GBM is impaired by robust DNA-repair mechanisms which enable intrinsic resistance of the GBM cells.[8] In combination, these challenging characteristics of highly aggressive GBM tumors have limited the progress in creating effective treatment solutions. As a result, current GBM therapies are presently given with the goal of slowing tumor growth and increasing the quality of life for patients.
One notable fault with the traditional approach to GBM treatment is the unilateral focus on killing GBM cells and lack of consideration for microenvironmental contributors. The role of the extracellular matrix (ECM) is now understood to be a key driver of tumor development and progression but controlling this facet of the tumor microenvironment (TME) has yet to be exploited for clinical translation in GBM treatment.[9] However, varieties of 3D hydrogel formulations mimicking the ECM have been engineered to develop in vitro tumor models.[10] Material properties such as stiffness[11], degradability[12], topography[13], and bioactive ligand presentation[14] have been tuned to modulate tumorigenic responses. In vitro models are commonly designed to support pro-tumorigenic responses for the purpose of evaluating anti-tumor therapies for cost effective, higher through-put screening than animal tumor models. Dissecting ECM characteristics in 3D have enabled a better understanding of the role of the ECM in driving tumor progression. While optimizing these models for tumor growth and progression, these works have conversely demonstrated that the surrounding matrix can be manipulated to provide anti-tumorigenic responses. In vitro studies have demonstrated that matrices mimicking blood vessels and white matter tracks encourage tumor cell migration; as a result, Jain, et al. exploited this migratory characteristic of GBM tumors for anti-tumor strategies by implanting polycaprolactone (PCL)-based nanofibers to encourage cell migration away from the primary tumor site to an extracortical location.[13b] In the case of matrix stiffness, rigid 2D hydrogel substrates support increased glioma cell proliferation and spreading compared to soft substrates with modulus of brain tissue.[15] However in 3D, polyethylene glycol (PEG) and hyaluronic acid matrices with moduli values comparable to normal brain tissue (1 kPa) and GBM tumor tissue (26 kPa) altered U87 cell morphology, proliferation, and migration differentially.[16] Unlike in 2D, with the additional factor of dimensionality U87 cells proliferate and form spheroids less readily in the stiff 3D matrix but form denser spheroids with deeper protrusions. To this end, harnessing the potential for biomaterials to modulate the tumor cell-ECM interface is of interest for creating innovative tumor treatments.
With the focus on advancing the gold standard for GBM treatment, research has shifted toward utilizing biomaterials as adjuncts to the classic pillars of GBM therapy (i.e. surgical resection, chemotherapy, radiation therapy, and the newly included targeted therapies). Biomaterials provide a means for spatial and temporal control of therapeutic delivery while synergistically interacting with local cell populations and influencing signal transduction. By harnessing these capabilities, different formulations including nano- [17] and microparticles [18], liposomes [19], implantable scaffolds [20], and injectable bulk hydrogels [21] have been studied for diagnosing and treating various cancer types. Biomaterials can be engineered for unique cancer applications by modulating material composition (i.e. natural protein or polysaccharide polymers, synthetic polymers, nucleic acids, and/or self-assembling peptides), mechanical properties, topography, payload delivery, and presentation of bioactive cues.
Specific to GBM, the versatility of biomaterials enables their application as A) scaffolds for filling the tissue void following tumor resection, B) reservoirs for housing radioactive materials at the tumor site, C) depots for sustained, local chemotherapeutic delivery, and D) vehicles for targeted therapies (Figure 1). Biomaterials can also serve to minimize the invasiveness of tumor treatment strategies by including properties of injectability. For example, nanoparticle formulations suspended in solution can be easily delivered through a syringe, as can bulk hydrogels exhibiting shear-thinning behavior or in situ crosslinking mechanisms with low-viscosity precursor solutions.[21a, 22] Injectable biomaterials enhanced with therapeutic benefits for GBM minimize the invasiveness of treatment and also create opportunities for both reaching and treating inoperable tumors. This review summarizes the transition to biomaterial approaches as adjuncts for each pillar of GBM treatment whilst highlighting advances using injectable biomaterials, rather than implants, to revolutionize GBM standard of care. The scope of this review is limited to biomaterials injected locally at the tumor site: either intratumorally, peritumorally, or within the tumor cavity after resection surgery and excludes those material formulations delivered systemically.
Figure 1.

To increase the efficacy of GBM treatment, anti-tumor agents such as chemotherapy, radiation, and targeted therapies can be combined with biomaterial formulations for intratumoral or peritumoral injection, as well as within the surgical cavity after tumor resection.
2. Pillars of GBM treatment improved by injectable biomaterials
2.1. Surgical Resection
Radical removal of tumors through surgical resection was the first approach to treating patients with solid tumors and can be traced back to the early 1900s.[23] Tumor resection surgeries have since become more precise with the invention of better diagnostic and intraoperative technology and techniques (i.e. advanced imaging modalities, functional mapping, as well as fluorescence-guided surgery for locating the tumor and determining safe resection margins).[24] GBM is predominantly found within the central nervous system (CNS), rarely metastasizing more than a few centimeters from the primary tumor making resection of the primary tumor an attractive solution for first-line treatment.[25] Not only does resection of the tumor reduce the number of cancer cells, but it also alleviates compression and injury caused by mass effect.[26] Physical removal of the hypoxic core of the tumor eliminates the more radiation resistant GBM cells which are also more difficult to reach with chemotherapy.[26] After tumor resection, patients receive high doses of corticosteroids to reduce tumor-associated edema, but the remaining cavity is left untouched allowing the parenchyma to scar.[5] Tumor recurrence after resection is a result of remaining, unresected tumor cells proliferating to form new tumor tissue. Often, regimented therapies like chemotherapy and radiation are given post-operatively in an attempt to eradicate any lingering tumor cells.[3b] Despite this aggressive treatment approach, nearly all tumors recur within ten months of resection surgery, and 90% are found within 2 cm of the primary tumor location.[4, 27] With such high rates of tumor recurrence, there is a demonstrated need for improving the method of eradicating residual GBM cells after tumor resection. The importance of the microenvironment in promoting tumor growth is evident in the proximity of the recurring tumor and primary tumor site. Introducing an alternative matrix-cell interface through a biomaterial is one strategy for changing the microenvironmental cues dictating tumor cell growth. Taken together, injectable biomaterials provide an avenue for filling the void left after tumor resection, delivering and retaining anti-tumor treatment locally, as well as preventing tumor recurrence.
GBM tumors can be found anywhere within the brain, brainstem, or cerebellum, but most commonly arise in the subcortical white matter of the frontal, temporal, and parietal lobes.[28] Surgical brain injury is inevitable during a procedure like GBM tumor resection, causing an array of neurological repercussions depending on the tumor location. After tumor resection surgery, hyperintense lesions surrounding the resection cavity can be observed through magnetic resonance-diffusion weight imaging.[29] The indication of acute ischemic cerebral injury in the peritumoral region is a result of blood vessel injury, brain tissue retraction, and high temperatures from electrocautery during tumor resection surgery.[30] Tissue injury and its associated inflammation have long been linked to both angiogenesis and tumorigenesis.[31] Particularly in the CNS, cellular responses to injury (i.e. cytokine release profile, clotting activation, and neovascularization) can all have repercussions on tumorigenesis for the residual GBM cells.[32] It is hypothesized that controlling inflammation in the tumor cavity and promoting anti-tumor processes while inhibiting pro-tumor processes could aid in preventing tumor recurrence.[33] In addition to cell and drug delivery, injectable hydrogels have been employed in the brain to control inflammation and promote wound healing.[34] In combination with anti-tumor therapeutics, there is potential for these pro-repair biomaterials to create innovative solutions for tumor treatment. With the established use of biomaterials for tissue engineering and regenerative medicine, the opportunity to use biomaterials to complement tumor resection surgery becomes evident for 1) filling the void space left after resection, 2) preventing tumor recurrence, and 3) encouraging tissue regeneration of the surrounding parenchyma.
The first FDA-approved adjunct material to resection surgery was Gliadel® wafers. These biodegradable polyanhydride copolymer wafers contain carmustine (1, 3-bis (2-chloroethyl)-1-nitrosourea, or BCNU), an alkylating chemotherapeutic agent intended to eradicate residual tumor cells after resection.[35] In total, eight Gliadel® wafers are needed to deliver a sufficient dosage of BCNU and require placement by the surgeon as an intracranial implant. As a result of their fixed shape, the wafers fail to fill the entirety of the resection cavity thus limiting the biomaterial-tissue interface for BCNU delivery. Other implantable materials loaded with anti-tumor agents have also yielded promising results for prolonging tumor recurrence, but these materials require surgical implantation as well. Examples of these implantable devices include poly (lactic-co-glycolic acid) (PLGA) nanofibers [36] or polymer sheets [37] and dextran scaffolds [38].
2.1.1. Material Adjuncts to Surgical Resection
Injectable bulk biomaterials (whether nonporous, porous, or granular) provide an alternative avenue for delivering anti-tumor agents while providing the advantages of minimal invasiveness and completely filling the resection cavity. For these purposes, hydrogels that undergo a sol-gel transition in situ can be engineered to deliver anti-tumor payloads to eradicate residual tumor cells. These hydrogels are injected as a liquid and transition to a solid through either chemical (covalent bonding) and/or physical (sensitive to changes in pH, ionic strength, or temperature) crosslinking.[39] An example of a chemical crosslinking strategy is a photopolymerizable PEG-dimethacrylate (PEG-DMA) hydrogel mixed with Paclitaxel (PTX)-loaded PLGA nanoparticles (Figure 2a). Following resection surgery in a GBM orthotopic model, the hydrogel was injected into the tumor cavity then photopolymerized (Figure 2b–c). Even without drug loading, the hydrogel with PLGA nanoparticles slowed down tumor recurrence and improved the length of mouse survival (Figure 2d).[40] Crosslinking mechanism and density are commonly modulated to tune the material’s mechanical properties which provide mechanosensitive cues to the interfacing cells. The material properties must be tailored to the tissue at the injection site, as the resulting downstream signaling from the mechanical stimuli modulates cell response.[16]
Figure 2.

a) Schematic of GBM tumor resection followed by either tumor recurrence or injection of PTX PLGA-NPs loaded in a PEG-dimethacrylate (PEG-DMA) hydrogel. b) (Top) Photographs depicting mouse fixed on stereotactic frame for tumomr cell injection (left), biopsy punch resection of tumor (middle), and photopolymerization of nanoparticle-loaded PEG-DMA hydrogels in situ (right). c) Axial (T2-weighted) images of mouse brain: brain tumor before resection (day 12, left), treatment with PTX PLGA-NPs/PEG-DMA hydrogel day 62 (middle) and day 146 (right) post-tumor inoculation. d) Kaplan-Meier survival curves for mice after treatment in the resection cavity (n=7–9 for all groups), **p< 0.01, ***p< 0.001).
Reproduced with permission. 2018, Elsevier.[40]
In addition to antitumoral benefits, exploration has just begun on the idea of delivering an ECM-mimetic material to provide regenerative cues to encourage healthy parenchyma growth after tumor resection. A pro-reparative biomaterial in the TME was first introduced with injectable particles of decellularized urinary bladder matrix (UBM).[41] In the presence of multiple non-CNS syngeneic tumor cell lines, UBM particles (injected by suspension in saline solution) hindered tumor growth while eliciting a type 2 wound-healing immune signature.[41] This work was the first to demonstrate in vivo that employing pro-repair scaffolds alone in the tumor cavity post-resection has the potential to enhance wound healing while preventing tumor re-growth and can be a promising design for GBM treatment strategies. Using decellularized biological scaffolds does, however, limit the control of understanding the cues it presents to elicit such a response and for tuning cell-material interactions. Employing more defined biological scaffolds or ECM materials in the TME has been particularly under-studied, but these biocompatible materials hold promise as a more controllable platform for providing both regenerative and anti-tumor cues in the resection cavity.
2.2. Radiation therapy
Surgical resection was the only form of tumor treatment until the advent of radiation therapy in the early 1900s.[42] Radiation therapy is still an integral part of cancer treatment today. Based on the guidelines established by Stupp et al., GBM patients receive a total of 60 Gy (absorbed energy per unit mass of tissue with 1 Gy = 1 Joule/kilogram), generally with 2 Gy of radiation given 5 days per week for a total of 30 days.[3b] A megavoltage energy-capable linear accelerator is used to deliver radiation either through 3D conformal- or intensity modulated- radiotherapy.[28] Typically, the brain volume receiving radiation is the tumor (as determined by magnetic resonance imaging (MRI) plus 1–2.5 cm) to minimize radiation of non-tumor tissue.[28] Alternatives to external beam radiation include brachytherapies which utilize radioisotopes with short tissue penetration depth that can be placed near the tumor site for local tumor ablation either as radioactive seeds or as a liquid within a balloon catheter.[18b] While the current standard of care for radiation is delivered externally, α-emitting isotopes (i.e. 225Actinium (Ac), 213Bismuth (Bi), 211Astatine (At)) and β−-emitting isotopes (i.e. 131Iodine (I), 90Yttrium (Y), 177Lutetium (Lu), 192Iridium (Ir)) must be administered locally to reach the tumor with therapeutically relevant radiation strength.[43] The α-particle emitters provide a shorter range of penetration than β-particle emitters, while also delivering a higher radiation energy.[43] However, if the radiation energy is too high, brain tissue necrosis can occur. When using α- and β-particle emitters, one must balance the characteristics of penetration depth and emission energy to provide the safest, most effective treatment for the patient. Particularly for GBM, 125I and 192Ir are the most relevant isotopes for brachytherapy.[44]
In an attempt to minimize off-target radiation, internal radiotherapy implants are being implemented for GBM treatment. To determine the clinical utility of brachytherapy for GBM treatment, Barabrite, et al. reviewed 32 studies comparing outcomes and adverse events attributed to brachytherapy treatment in 1571 GBM patients (both primary and recurrent tumors). Overall, brachytherapy resulted in extended median overall survival compared to external beam radiotherapy but 27% of patients reported adverse events associated with the treatment.[44] An example of an internal radiotherapy implant is a balloon catheter filled with an aqueous radiation source (GliaSite®) that obtained FDA approval for brain tumor treatment in 2001.[45] Though this balloon catheter originally required surgical implantation (at the time of tumor resection) and subsequent removal, it can now be implanted through a catheter. Limitations of this technology include its fixed spherical shape and limited efficacy in treating large, heterogenous tumors due to the penetration depth of its aqueous radiation source.[44] Another type of brachytherapy, now termed, microbrachytherapy, began as holmium acetylacetonate crystals for radioablation of liver malignancies.[46] This technology was advanced by incorporating beta-emitting nucleotides and loading the constituent parts in poylmeric microspheres to create microbrachytherapy (brachytherapy using microspheres).[47] Taking this approach to the nanoscale for preclinical GBM treatment, liposomes loaded with rhenium-186 (a β−-emitting isotope) were injected intracranially and delivered passively through the enhanced permeability and retention (EPR) effect which prolonged survival by more than 50% in an orthotopic GBM rat model.[48] Without a means of targeting the tumor or containing the spread upon administration, injection or intravenous (IV) administration of these radioactive particles alone for passive delivery can cause widespread off-target radiation and negative side effects for the patient. Liposomes loaded with 225Ac (an α-emitting isotope) targeted the αvβ3 integrins expressed on angiogenic endothelial cells and invading GBM cells to effectively increase the permeability of the BBB with the aim of increasing the uptake of circulating chemotherapeutics.[49] In addition to micro- or nanomaterial carriers, radionuclides can also be delivered using radioimmunotherapy which relies on monoclonal antibodies to effectively target the tumor.[50] Radioimmunotherapies must be delivered intracranially because the BBB prevents antibodies from accessing the tumor.
While radiation is given routinely as part of the GBM standard of care, many patients exhibit resistance to radiation therapy. As such, radiosensitizing agents have been implemented preclinically to decrease the level of ionizing radiation patients can withstand and synergize with radiation therapy to increase cell death.[51] Types of radiosensitizing agents include high atomic number nanoparticles (i.e. gold (Au) or silver (Ag)) or coated superparamagnetic ions (Fe3O4, Fe2O3).[52] A hybrid variation of these radiosensitizing nanoparticles (Fe3O4@Ag) demonstrated increased rates of calcium-dependent apoptosis in GBM cell lines in vitro when subjected to beam radiation.[44] For more effective delivery, locally administered biomaterials can serve as reservoirs for housing the radioactive particles and/or radiosensitizing agents in proximity to the tumor site. Immobilizing these particles, whether through encapsulation or conjugation, prevents systemic distribution and off-target radiation of healthy tissues. Incorporating properties of slow degradation in the biomaterial design eliminates the need for implant removal while ensuring the particles will not be released until sufficiently decayed and deemed non-radioactive. Additionally, injectable materials with properties of degradability mitigate the need for removal while also providing a therapy capable of filling the cavity with minimal compression on the parenchyma.
2.2.1. Material Adjuncts to Radiation therapy
Combining the aforementioned characteristics for material adjuncts to GBM radiation therapy, De la Puente, at al. designed a preclinical injectable, degradable chitosan hydrogel capable of delivering the radioactive isotope 131I in encapsulated alginate microparticles (Figure 2a–b). 131I is capable of emitting low penetrating β-particles to provide local radiotherapy and minimize off-tumor radiation and is characterized by a short half-life of only 8 days. The products of its decay are non-radioactive 131Xe and iodine, making 131I an ideal radiotherapeutic to be retained in a degradable bulk material reservoir. Using this local biomaterial depot, negligible amounts of 131I released from the hydrogel which decreased side effects (Figure 2c). However, TMZ was still effectively released from the gel Figure 2d) which resulted in decreased tumor size and prolonged survival in a subcutaneous GBM model (Figure 2e).[53]
Locally injected biomaterials for the delivery of radionuclides mitigate the need for using targeted antibodies and ensure localization in the tumor site more so than passively delivered liposomes. The use of biocompatible hydrogels for this application is advantageous for containing radioactive particles in controllably degradable matrix. For clinical translation, there are no addition steps required by the physician for this treatment as compared to other catheter-style brachytherapies. Additionally, without having to cross the BBB, a greater variety of anti-tumor therapies can be incorporated into the material design.
2.3. Chemotherapy
Chemotherapeutic agents work to arrest the mitotic activity of tumor cells. A handful of chemotherapeutics are FDA-approved for GBM treatment, including nitrosoureas (i.e. lormustine (CCNU), carmustine (BCNU)) or antineoplastic alkylating agents (i.e. Temozolomide).[54] Since its approval in 2005, Temozolomide has been the most commonly prescribed alkylating agent for GBM due to the systemic toxicity of nitrosoureas.[55] Temozolomide is administered orally and once reaching the bloodstream is capable of crossing the BBB to exert anti-cancer effects through DNA methylation of glioma cells. A downside to oral administration is that systemic delivery results in off-target effects; to reduce the toxicity of systemic drug delivery, a lower concentration than ideal must be administered. To account for this, localized delivery has been investigated as a means for limiting off-target effects and enabling higher strength dosages.
Another limitation of alkylating agents like Temozolomide is the intrinsic chemoresistance of GBM cells to DNA methylation.[56] In fact, approximately two thirds of GBM patients are resistant to alkylating agents which limits their success in treating GBM. Methylation of the O6 site of guanine is responsible for the cytotoxic effect of Temozolomide.[57] Chemoresistance can be identified in patients by high expression of the active O6-methylguanine-DNA methyltransferase (MGMT).[56] Because of this, alternative drug targets are being investigated for efficacy of GBM treatment while circumventing GBM chemoresistance. Anthracyclines (i.e. Doxorubicin (Dox), Epirubucin) are responsible for inducing cell death through multiple mechanisms – one of which being intercalation of DNA strands thereby preventing DNA and RNA synthesis.[58] The potency of these drugs has been utilized to disrupt vascular mimicry (VM) channels which GBM tumor cells form to deliver nutrients in addition to the typical neovasculature with tumor endothelial cells.[59] Consequently, these VM channels support tumor growth and recurrence which make them another target for tumor therapy. Other drugs demonstrate anti-tumor effects experimentally, but off-target effects or inability to cross the BBB (such as PTX) prevent their use clinically for GBM treatment. Harnessing the controllable payload release of materials, as well as the stealth characteristics of PEGylated nanoscale technologies, make biomaterials an attractive solution to many of these limitations of chemotherapy.
2.3.1. Material Adjuncts to Chemotherapy
Biomaterials can serve as delivery vehicles for chemotherapeutic agents to provide a means for targeted or local delivery to reduce off-tumor effects. Biomaterials encapsulate drugs through liposome or micelle formulation, as well as during bulk hydrogel crosslinking.[60] These materials can serve as drug depots or reservoirs which can be engineered to sustainably release drugs. Degradable linkers can be incorporated into the design of crosslinked scaffolds to increase the matrix mesh size and facilitates drug diffusion upon cleavage. Additionally, degradable scaffolds encourage cells to infiltrate upon remodeling and mitigates the need for surgical removal of the material.[60] Direct injection of bulk materials in the TME is one such approach which bypasses the highly regulated BBB and can be injected in physical proximity to the tumor for effective drug delivery. Oftentimes, IV or oral delivery of chemotherapy drugs requires repeated administration to achieve sufficient dosage. The dual-functional chitosan hydrogels engineered by de la Puente et al. (Figure 2) were loaded with Temozolomide, injected in the GBM tumor bed, and effectively delivered drug at 10-fold greater concentration than systemic delivery.[53]
Biomaterials also provide safe strategies for implementing innately toxic compounds to provide anti-tumor effects while preventing off-target tissue damage. For example, antineoplastic drug SN-38 demonstrates potent chemotherapeutic effects but elicits severe side effects which has prevented its translation to the clinic. Additionally, poor solubility of the drug requires encapsulation in a carrier for delivery. Loading SN-38 into an injectable, self-solidifying polymeric (PLEC) depot for intra-tumoral delivery in a GBM xenograft model provided localized delivery and mitigated both systemic and neurological toxicity caused by free SN-38.[61] Another intra-tumoral drug delivery system, described by Bastianchich, et al., consists of lipid nanocapsule (LNC) hydrogels comprised of the chemotherapy drug Germcitabine (GemC12) with Lauroyl surfactant (GemC12-LNCs) (Figure 4a).[62] This anti-tumor strategy significantly increased mouse survival compared to untreated controls (Figure 4b–c). Additionally, when injected in the surgical cavity post-resection, GemC12-LNC hydrogel delayed tumor recurrence (Figure 4d). This injectable system demonstrated efficacy as a GBM treatment solution for both intra-tumoral and perisurgical applications.
Figure 4.
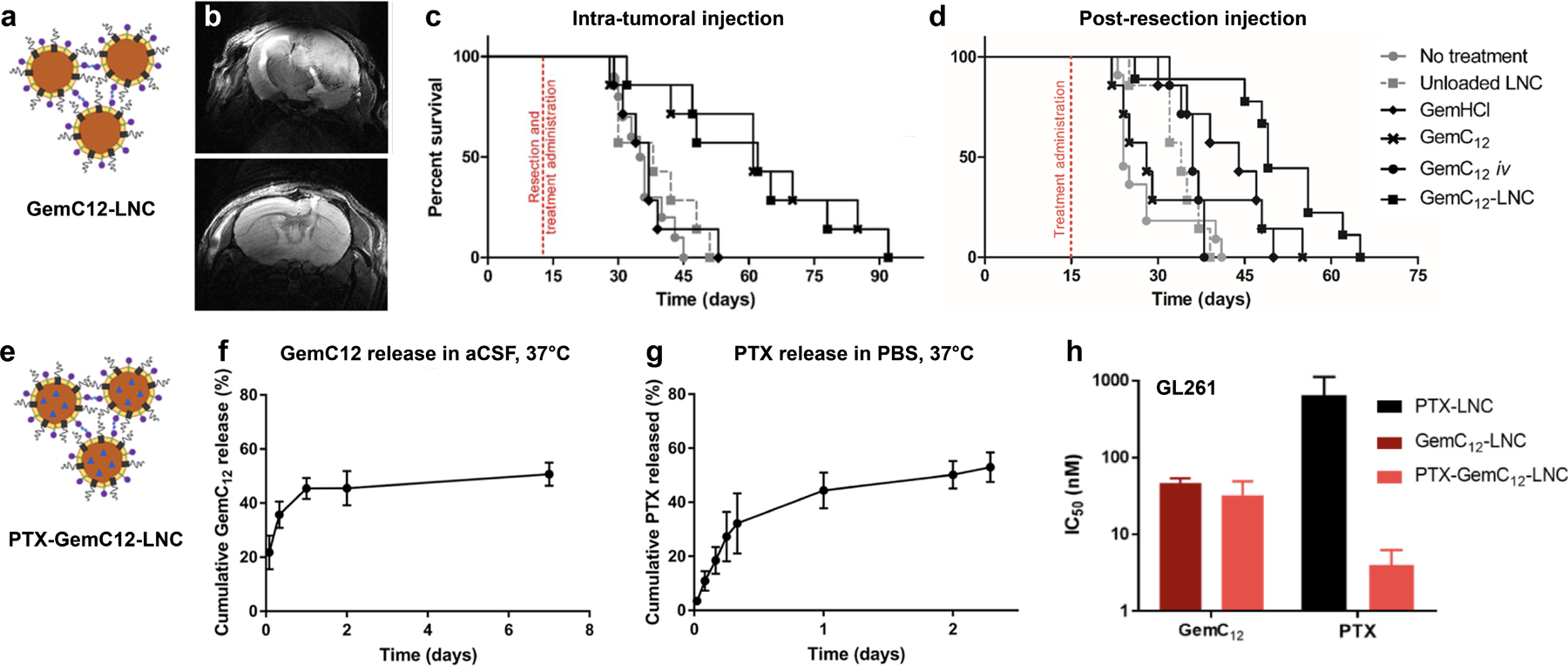
a) Schematic of Germcitabine (GemC12)-loaded LNCs prior to hydrogel formation. b) Axial (T2-weighted) MRI of mouse brain: untreated (day 31 post-resection, top) and GemC12-LNC treated (day 61 post-resection, bottom). Kaplan-Meier survival curves for c) intratumoral injection and d) post-resection injection in orthotopic U87 mouse models. E) Schematic of dual GemC12- and PTX-loaded LNCs (PTX-GemC12-LNC) prior to hydrogel formation. f) Cumulative release of GemC12 and g) PTX from the LNCs over time. h) IC50 values of GemC12 and PTX alone and dually loaded in LNCs for GL261 cell line.
Reproduced with permission.[62–63] 2017, Elsevier. 2019, Elsevier.
In addition to dual functionality for combining treatment types (i.e. radiotherapy and chemotherapy), biomaterial vehicles are also capable of delivering drugs in different combinations for more personalized tumor treatment. Bastianchich, et al. altered the GemC12-LNC technology for this approach by incorporating PTX (as a model therapeutic) for co-delivery (Figure 4e–h).[63] This combinatorial approach increased survival in several orthotopic GBM models compared to either treatment in the hydrogel alone.[62–63] With the aim of desensitizing GBM to Temozolomide therapy, PEGylated nanoparticles were loaded with both Temozolomide and a bromodomain inhibitor. In combination, this therapy leads to increased markers of apoptosis and DNA damage.[64] Hydrogels loaded with combinations of drugs or anticancer agents of varying hydrophobicity can work to release the unique payloads in different timeframes for optimal tumor treatment. For example, self-assembling peptides capable of forming hydrogels in situ were loaded with Dox and curcurmin and released Dox within 4 days while prolonging the release of curcurmin (more hydrophobic) over 20 days.[65] Curcurmin is a compound isolated from the root of the Curcuma longa plant that exhibits anti-inflammatory and anti-cancer activity; specifically for GBM, curcumin targets pathways implicated in drug-resistance.[66] The delivery of both Dox and curcurmin from the peptide nanofiber hydrogel demonstrated increased cellular uptake and cytotoxicity in vitro than either agent in the hydrogel alone.[65]
Due to the limitations of intrinsic chemoresistance, combinatorial therapies are of interest for eliciting anti-tumoral effects through multiple mechanisms. Zhao, et al. recently developed an injectable, enzyme-responsive hydrogel loaded with both TMZ and MGMT inhibitor (O6-benzylamine or BG) for post-resection treatment (Figure 5a). This material composed of triglycerol monostearate (Tm) releases the loaded cargo upon entering a region with high matrix metalloproteinase (MMP) activity, such as the tumor resection site (Figure 5b). Ultimately, this dual-acting biomaterial depot increased the efficacy of TMZ for inhibiting tumor growth by combatting chemoresistance with delivery of the MGMT inhibitor (Figure 5c–f).[67]
Figure 5.
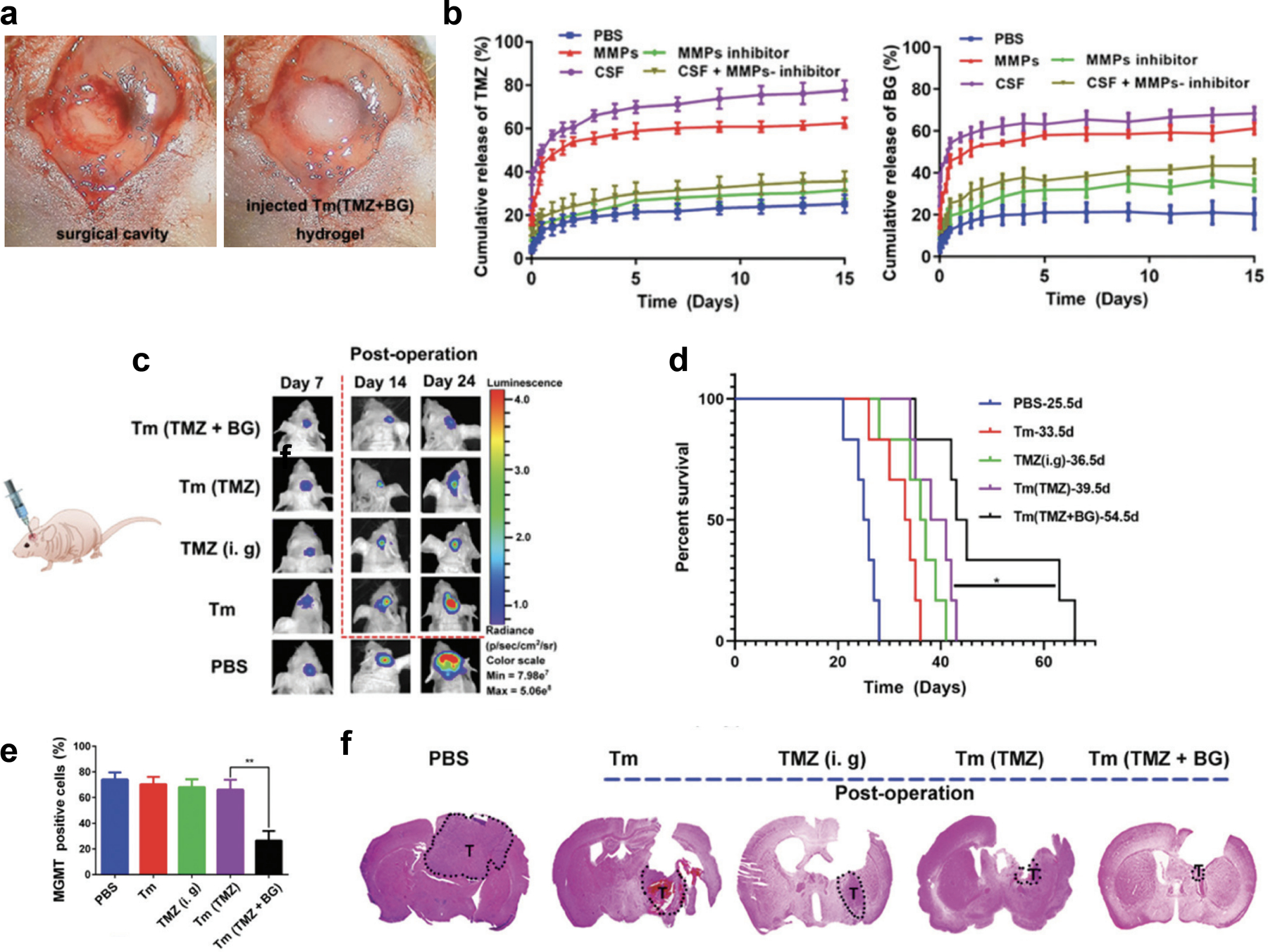
a) Photograph of the surgical cavity made by creating a cranial window through an incision in the midline to expose the brain for cutting the brain-tumor tissue (left) followed by injection of the therapeutic hydrogel (right) before sealing the cranial window. b) Cumulative release profiles of both TMZ (left) and BG (right) from Tm (TMZ + BG) hydrogel in PBS at 37 °C with gentle stirring under MMPs, MMPs + inhibitor, CSF and CSF + inhibitor conditions (data are presented as mean ± SEM, n = 4). c) Bioluminescence images of glioma-bearing mice 7, 14, and 24 days after post-operative treatment with PBS, Tm, TMZ (i. g.), Tm (TMZ), and Tm (TMZ + BG) hydrogel. d) Survival rate of the glioma-bearing mice after treatment. e) Percentage of MGMT positive cells as determined by immunohistochemistry staining of the tumor tissues dissected at day 26 after tumor implantation and treatment. f) H&E-stained brain sections where the dashed shape labeled T denotes tumor tissue.
Reproduced with permission. 2020, Royal Society of Chemistry.[67]
In addition to providing anti-tumoral effects, chemotherapeutic biomaterials can also be engineered with pro-regenerative cues to encourage the growth of healthy brain parenchyma as tumor tissue is eliminated. Similar to the aim of implementing pro-repair decellularized biological matrices (Wolf, et al.) after tumor resection, Huang et al. developed a synthetic nanofiber membrane to enable explicit control of pro-regenerative cues as well as deliver chemotherapy drugs. These PCL nanofiber membranes are capable of concurrently delivering Temozolomide (to prevent tumor recurrence) and neuron growth factor (to improve the reconstruction of local neural tissue). In vitro assessment demonstrated effective inhibition of glioma cell growth while inducing neuronal differentiation.[68] The strategy of including growth factors to promote tissue repair after tumor resection is complicated by the fact that a variety of growth factors implicated in tissue repair (e.g. transforming growth factor (TGF), vascular endothelial growth factor (VEGF), or fibroblastic growth factor (FGF)) are upregulated in the TME and contribute to tumorigenesis.[69] To this end, incorporating pro-regenerative cues within the matrix requires a precise balance; tumor toxicity must be maintained while encouraging selective regeneration of the healthy brain tissue.
As adjuncts to chemotherapy, biomaterials enable sustained, local drug delivery at therapeutically relevant concentrations without repeated administration. Nanoscale biomaterial formulations are capable of crossing the BBB for delivery of drugs otherwise incapable of reaching brain tumors. Drug loading can be tailored for patients with varying levels of chemoresistance and can allow for combinatorial drug loading to deliver potent chemotherapeutic cocktails.
2.4. Targeted Therapies
Due to the atypical state of tumorigenic tissue, many signaling cascades and molecular players expressed by tumor cells are often dysregulated compared to cells of healthy tissue. These markers can vary across tumor types or between cell types comprising the same tumor, but overexpressed molecules can be exploited as targets for anti-tumor therapy. While some diseases are characterized by a single pathway, GBM is characterized by dysregulation of multiple pathways which increases the challenge of using a single targeted therapy to treat GBM. These pathways include phosphoinositide 3-kinase (PI3K), protein kinase B (AKT), mammalian target of rapamycin (mTOR), the p53 and the retinoblastoma (RB) pathways, or epidermal growth factor receptor (EGFR).[70] Examples of therapies targeting these pathways include sorafenib which inhibits the activation of key kinases in tumor cells (RAF/MEK/ERK)[71] and temsirolimus which capitalizes on the hallmark mammalian target of rapamycin (mTOR) signaling by inhibiting mTOR-dependent signaling and its tumor-promoting cascade.[72] Other molecular targets for therapies, such as epidermal growth factor receptor (EGFR) tyrosine kinase, originally gained traction for success in experimental studies but ultimately failed to translate during clinical trials.[73] Targeted therapies for these pathways (small molecule inhibitors, antibodies, or antibody-drug conjugates) have been well studied but fail to improve survival outcomes; instead, well-tolerated targeted therapies are often given as a salvage treatment to alleviate GBM symptoms.[74] Examples of targeted therapeutics for GBM include small molecules [70], proteins [75], antibodies [76], and exogenously engineered or activated cells [77]. Because EGFR-targeting therapies (e.g. erlotinib) have been successful in other (non-CNS) malignancies with EGFR amplification, Xiao, et al. hypothesized that the brain microenvironment contributes to this therapeutic resistance.[78] To demonstrate the role of matrix-cell interactions in acquired drug resistance, patient-derived GBM cells were cultured in 3D hydrogels in which HA content, integrin-binding peptide concentration and compressive modulus mimicked GBM tumors. Erlotinib resistance was shown to increase in soft (1kPa compressive modulus), HA-rich gels with integrin-engaging peptides. By understanding the role of the ECM in acquired drug resistance, injectable scaffolds can be designed to alter these cell-matrix interactions.
In addition to GBM cell targets, microenvironmental players such as blood vessels and immune cells are of interest as alternative targets. To date, the only targeted therapy approved by the FDA for GBM is Bevacizumab. Bevacizumab is a monoclonal antibody against humanized vascular endothelial growth factor-A (VEGF-A), a growth factor whose overexpression causes tumor progression and sustained angiogenesis.[79] Bevacizumab works by binding circulating VEGF-A to prevent its interaction with the VEGF receptor-2 (VEGFR2) on tumor endothelial cells.[80] While the therapeutic benefits of this treatment are controversial, several studies have shown Bevacizumab works for transiently normalizing the tumor vasculature and increases median survival by approximately one month. Monoclonal antibodies like Bevacizumab are too large to freely cross the BBB and therefore rely on nanocarrier vehicles for delivery.[74]
Currently, nanoscale materials are the key focus of delivering targeted therapies due to their ability to cross the BBB with anti-tumor therapeutics. Nanomaterials delivered intravenously (IV) can passively collect in the tumor site as a result of the EPR effect of the tumor vasculature; additionally, inclusion of tumor-targeting moieties on the material surface increases their accumulation. For example, PEG-PCL nanoparticles can be conjugated with Angiopep, a peptide which targets low density lipoprotein receptor related protein (LRP) receptor upregulated on both the BBB and GBM cells.[81] Another preclinical targeted approach using PEG-PCL nanoparticles incorporates low molecular weight protamine on the surface which is activated by the overexpressed MMP-2/9 in glioma tumors.[82] Targeting gene transcription is another approach for targeted therapy. In a patient-derived xenograft model of GBM, infusion of lipopolymeric nanoparticles encapsulating multiplexed RNAi knocking down four transcription factors implicated in tumorigenesis delayed GBM progression.[83] Material formulations can also be engineered for targeting immune cells to promote anti-tumor immunity. For example, in an orthotopic GBM xenograft model, Dox-loaded polyglycerol-nanodiamond composites (Nano-Dox) elicited an enhanced, dendritic cell (DC)-driven anti-tumor immune response.[84] This composite nanoscale delivery vehicle works to increase GBM cell death by promoting antigen donation from GBM cells to DCs.[84]Another nanoscale formulation promoting anti-tumor immunity is mesoporous silica nanoparticles that target the BBB/tumor and deliver immune checkpoint inhibitor 1-methyltryptophan to orthotopic GBM tumors.[85] While IV administration of these technologies has its benefits, limitations include biodistribution in non-target tissues possibly attributed to inter- and intra-tumoral variability of vascular permeability and expression of the target ligand.[86] Nanoparticle circulation also alters the drug dose delivery as nanoparticles are subject to clearance (both renal and immune).[86] Design considerations for adjunct materials for targeted therapy delivery of anti-tumor nanoparticle formulations include their within a bulk, locally injected scaffold. Bulk materials enable diffusion of anti-tumor agents through the mesh network or can release bound agents upon degradation to provide controlled, sustained drug release over time and eliminate the need for repeated IV treatment.
2.4.1. Material Adjuncts to Targeted Therapies
For targeted immunotherapies, such as checkpoint blockade inhibitors, cancer vaccines, and chimeric antigen receptor (CAR) T-cells, biomaterials serve to limit off-tumor effects, direct cancer-specific responses, and amplify immunomodulation. Injectable bulk hydrogels implemented for biomaterials-based immunomodulation have yet to be fully realized for their potential in GBM treatment. The biological scaffold shown by Wolf, et al. to elicit a type-2 immune signature and delay tumor growth also demonstrated synergy with immune checkpoint blockade immunotherapy (anti-PD-1) for treating melanoma.[41] Also in a melanoma model, Song et al. developed a polypeptide (PEG-b-poly(L-alanine)) hydrogel for co-delivery of immune checkpoint inhibitors (anti-CTLA-4/PD-1 antibody) and a tumor vaccine. This combinatorial design effectively released melanoma tumor antigens, recruited/activated DCs, and induced a robust T-cell response.[87] The sustained delivery and immune modulation demonstrated by this formulation can be tailored for applications in other tumors, such as GBM. For CAR T-cell therapies targeting GBM, targets of IL-13 Rα2, HER2, and EGFRvIII are included as the surface-exposed tumor antigen specified on the CAR’s extracellular targeting moiety.[77a] In other tumor types, injectable hydrogel scaffolds loaded with CAR T-cells are being introduced to increase localization and persistence of the adoptive T-cell therapy which has prospects for application in GBM as well.[88] There are significant opportunities for these approaches though they currently have been poorly established for GBM treatment.
3. Conclusion
The dismal survival rate for patients suffering from GBM is motivating a breadth of research aimed at improving the current standard of care for GBM treatment. Despite advances in surgical techniques, chemotherapeutic drug discovery, radiation treatment strategies, design of targeted therapies, and understanding GBM pathology, the pillars of GBM treatment (i.e. surgical resection, radiation therapy, chemotherapy, and newly implemented targeted therapies) are still failing to treat this aggressive tumor type. Many of the limitations for treating GBM (i.e. crossing the BBB, administering therapeutically relevant drug dosages, minimizing off-tumor effects) can be mitigated by locally applying injectable biomaterials. Injectable biomaterial adjuncts to surgical resection, radiation therapy, chemotherapy, and targeted therapies are understudied and while some have demonstrated experimental success in improving the treatment of GBM, further advancements are still required to make the transition from prolonging survival to curing GBM.
Figure 3.
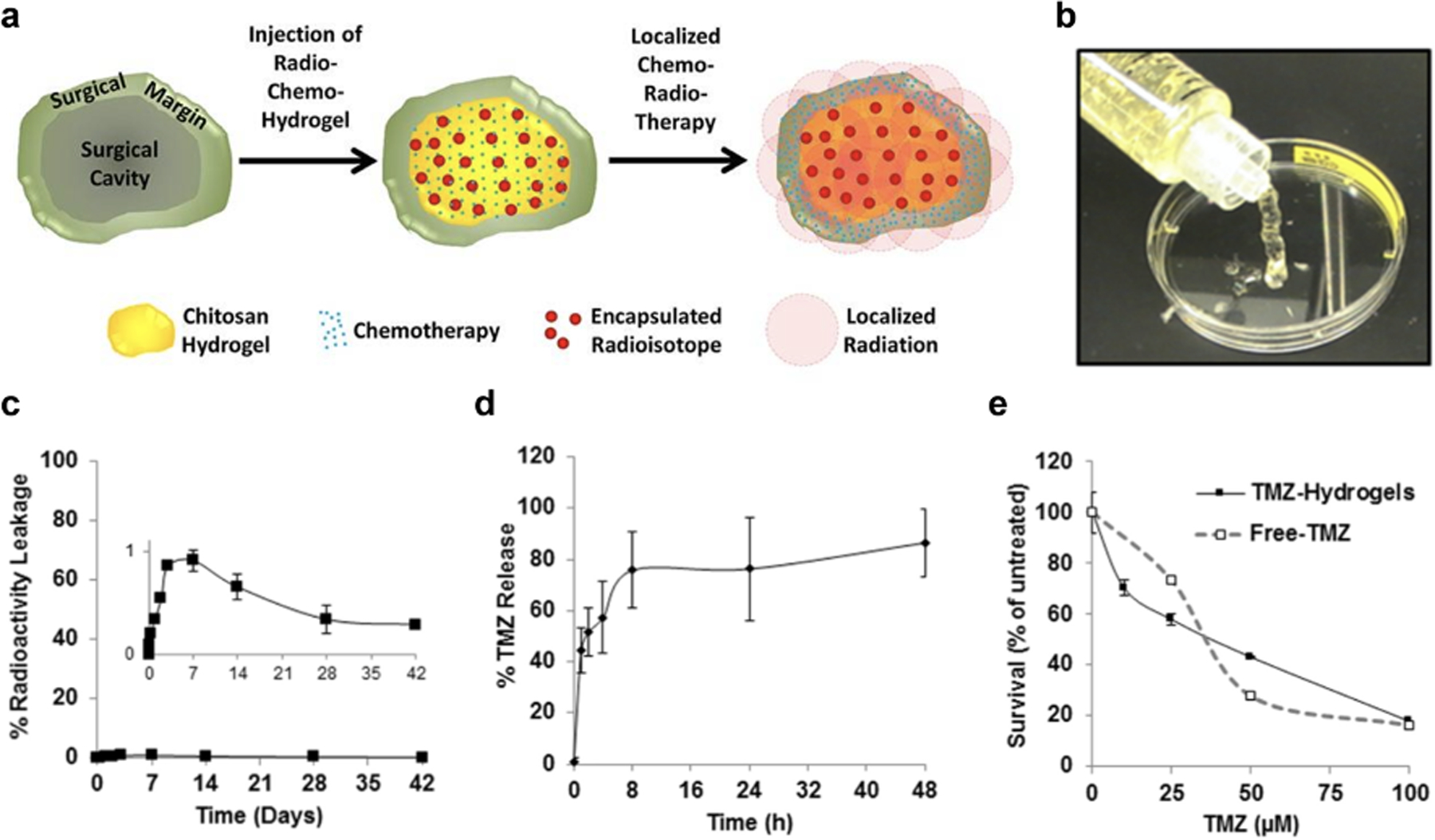
a) Design schematic of combinatorial radio- and chemotherapy chitosan hydrogel b) delivered via injection. c) Radioactive leakage from alginate microparticles in chitosan matrix over 42 days demonstrates minimal (<1%) leakage (see insert). d) TMZ release from the chitosan matrix over 48 hours as well as e) survival of human GBM (D54) cell line during TMZ concentration study with TMZ-loaded hydrogels versus free TMZ.
Reproduced with permission.[53] 2018, Elsevier.
Acknowledgements
The authors acknowledge the support from the National Institutes of Health for funding (grant number R01NS112940).
Biography

Alexa R. Anderson is currently a PhD student at Duke University in the Biomedical Engineering Department. She received her BS in Biomedical Engineering from West Virginia University in 2018 before joining Prof. Tatiana Segura’s group. Her current research focuses on engineering porous, pro-repair scaffolds to promote the formation of normal vasculature. She aims to use her work with injectable biomaterials for augmenting glioblastoma therapy.

Tatiana Segura is a Professor at Duke University, where is she has split appointments between Pratt School of Engineering (Biomedical Engineering) and the School of Medicine (Neurology and Dermatology). Professor Segura’s Laboratory studies the design and use of materials to help our body heal itself. She has been recognized with the Outstanding Young Investigator Award from the American Society of Gene and Cell Therapy, the American Heart Association National Scientist Development Grant, and the CAREER award from National Science Foundation and has been Elected to the College of Fellows at the American Institute for Medical and Biological Engineers (AIMBE).
References
- [1].a) Dolecek T, Propp JM, Stroup NE, Kruchko C, Neuro Oncology 2012, 14; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Malik A. F. J. Tamimi, in Glioblastoma, Vol. Codon Publications; (Ed: D. V. S), Brisbane, AU: 2017. [Google Scholar]
- [2].Ostrom Q, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, Barnholtz-Sloan JS, Neuro Oncology 2019, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].a) Jingjing D, Li Zhang, Chi Chongwei, Xiao Xiong, Wang Junmei, Lang Lixin, Ali Iqbal, Niu Gang, Zhang Liwei, Tian Jie, Ji Nan, Zhu Zhaohui, Chen Xiaoyuan, Theranostics 2018, 8, 2508; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, New England Journal of Medicine 2005, 352, 987. [DOI] [PubMed] [Google Scholar]
- [4].Beauchesne P, Bernier Valerie, Carnin Charlotte, Taillandier Luc, Djabri Mohamed, Martin Laurent, Michel Xavier, Maire Jean-Philippe, Khalil Toufic, Kerr Christine, Gorlia Thierry, Stupp Roger, Pedeux Remy, Neuro-oncology 2010, 12, 595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Stupp R, Brada M, van den Bent MJ, Tonn JC, Pentheroudakis G, Ann Oncol 2014, 25 Suppl 3, iii93.24782454 [Google Scholar]
- [6].a) Arvanitis CDF, Gino B; Jain Rakesh K. , Nature Reviews Cancer 2020, 20, 26; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Harder BG, Blomquist MR, Wang J, Kim AJ, Woodworth GF, Winkles JA, Loftus JC, Tran NL, Front Oncol 2018, 8; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Parodi A, Rudzińska M, Deviatkin AA, Soond SM, Baldin AV, Zamyatnin AA, in Pharmaceutics, Vol. 11 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Monteiro AR, Hill R, Pilkington GJ, Madureira PA, in Cells, Vol. 6 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].a) Osuka S, Van Meir EG, in J Clin Invest, Vol. 127 2017, p. 415; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Pingrong J. Y. Tang; He Junquan; Zhang Yang, Scientific Reports 2018, 8; [Google Scholar]; c) Verhaak RGWH, Katherine A; Purdom Elizabeth; Wang Victoria; Qi Yuan; Wilkerson Matthew D.; Miller C. Ryan; Ding Li; Golub Todd; Mesirov Jill P.; Alexe Gabriele; Lawrence Michelle; O’Kelly Michael; Tamayo Pablo; Weir Barabara A.; Gabriel Stacey; Winckler Wendy; Gupta Supriya; Jakkula Lakshmi; Feiler Heidi S.; Hodgson Graeme; James David; Sarkaria Jann N.; Brennan Cameron; Kahn Ari; Spellman Paul T.; Wilson Richard K.; Speed Terence P.; Gray Joe W.; Meyerson Matthew; Getz Gad; Perou Charles M.; Hayes Neil, 2010, 17; [Google Scholar]; d) Qiulian S. W. Bao; McLendon Roger E.; Hao Yueling; Shi Qing; Hjelmeland Anita B.; Dewhirst Mark W.; Bigner Darell D.; Rich Jeremy N., Nature 2006, 444, 756. [DOI] [PubMed] [Google Scholar]
- [9].a) Mercier F, Cell Mol Life Sci. 2016, 73, 4661; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Miroshnikova YA, Mouw JK, Barnes JM, Pickup MW, Lakins JN, Kim Y, Lobo K, Persson AI, Reis GF, McKnight TR, Holland EC, Phillips JJ, Weaver VM, Nat Cell Biol 2016, 18, 1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].a) Rao SSL, John J; Viapiano Mariano S.; Sarkar Atom; Winter Jessica, Tissue eng Part B: Reviews 2014, 20; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Badriprasad A. A. Rape; Kumar Sanjay, Advanced Drug Delivery Reviews 2014, 79–80, 172; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Alireza W. S. Xiao; Seidlits Stephanie K., Future Science OA 2017, 3; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Joseph K. J. C. Wolf; Coombes Jason D.; Aghi Manish K.; Kumar Sanjay, Nature Reviews Materials 2019, 4, 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xinming C. T. Wang; Yang Fan, Molecular Pharmaceutics 2014, 11, 2115. [DOI] [PubMed] [Google Scholar]
- [12].Xinming C. T. Wang; Xinyi Jiang; Yang Fan, Journal of Biomedical Materials Research 2017, 105, 770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].a) Gawain A. T. Beliveau; Gong Jiaxin; Wen Qi; Jain Anjana Scientific Reports 2016, 6; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Martha A. B. Jain; Patel Gaurangkumar D.; Valmikinathan Chandra M.; Mukhatyar Vivek J.; Vakharia Ajit; Pai S. Balakrishna; Brahma Barunashish; MacDonald Tobey J.; Bellamkonda Ravi V., Nature Materials 2014, 13, 308. [DOI] [PubMed] [Google Scholar]
- [14].a) Mikhail A. Z. Rape; Murthy Niren; umar Sanjay, Nature Communications 2015, 6; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Smritee P. S. D. Zustiak; Medina Carlos; Steczina Sonette; Chehreghanianzabi Yasaman; Ashraf Anisa; Asuri Prashanth, Biotechnology & Bioengineering 2016, 113. [DOI] [PubMed] [Google Scholar]
- [15].Ulrich TA, de Juan Pardo EM, Kumar S, Cancer Res 2009, 69, 4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xinming C. T. Wang; Yang Fan, Mol Pharm 2014, 11, 2115. [DOI] [PubMed] [Google Scholar]
- [17].a) Cheng YM, Ramin A; Tobias Alex L.; Lesniak Maciej S., Advanced Drug Delivery Reviews 2014, 66, 42; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Santosh C.-M. J. A. Hu; Zhang Liangfang, Therapeutic Delivery 2010, 1; [Google Scholar]; c) Yuan C. S. Kang; Zhu Jing; Li Wen; Zhang Aili; Kuang Tairong; Xie Jing; Yang Zhaogang, Current Drug Metabolism 2016, 17, 745; [DOI] [PubMed] [Google Scholar]; d) Joao Jose M. S. Mendes; Pais Alberto; Vitorino Carla, Pharmaceutics 2018, 10; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Mahsa F. H. Pourgholi; Farhad Jadidi-Niaragh; Kafil Hossein Samadi; Yousefi Mehdi, Biomedicine & Pharmacotherapy 2016, 77, 98; [DOI] [PubMed] [Google Scholar]; f) Robert A. Z. L. Wang; Farokhzad Omid C., Annual Review of Medicine 2012, 63, 185. [DOI] [PubMed] [Google Scholar]
- [18].a) Mohamed H. M. G. Abdelaziz; Abd-Elwakil Mahmoud M.; Mabrouk Moustafa T.; Elgohary Mayada M.; Kamel Nayra M.; Kabary Dalia M.; Freag May S.; Samaha Magda W.; Mortada Sana M.; Elkhodairy Kadria A.; Fang Jia-You; Elzogbhy Ahmed O., Journal of Controlled Release 2018, 269, 374; [DOI] [PubMed] [Google Scholar]; b) Bakker RCL, Marnix GEH; van Nimwegen Sebastian A.; Rosenberg Antione J.W.P.; van Es Robert J.J.; Njisen J. Frank W., Journal of Radiation Oncology 2017, 6, 323; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Jaiswal RS, Lisa M, Frontiers in oncology 2019.
- [19].a) Sindhu U. D. Bulbake; Kommineni Nagavendra; Khan Wahid, Pharmaceutics 2017, 9; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Jain AJ, S.K., Current Molecular Medicine 2018, 18, 44; [DOI] [PubMed] [Google Scholar]; c) Rui-Jun L.-M. J. Mu; Liu Rui; Bu Ying-Zi; Zhang Jing-Ying; Li Xue-Qi; Zeng Fan; Lu Wan-Liang, Advanced Drug Delivery Reviews 2017, 115, 46; [DOI] [PubMed] [Google Scholar]; d) Salkho NMT, R.Z.; Guessoum O; Martins AM; Vitor RF; Husseini GA, Current Molecular Medicine 2017, 17, 668; [DOI] [PubMed] [Google Scholar]; e) Roya S. Z. S. Vahed; Davaran Soodabeh; Sharifi Simin, Materials Science and Engineering: C 2017, 71, 1327. [DOI] [PubMed] [Google Scholar]
- [20].a) Asma N. A. Hassanein, Advances in Modern Oncology Research 2018, 4; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Simon D. G. Y. Leach; Hartgerink Jeffrey D., Acta Biomaterialia 2019, 15; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Gijung H. K. Kim; Kim Kwangmeyung; Yoon Hong Yeol; Kwon Ick Chan, Biomaterials 2019, 213; [DOI] [PubMed] [Google Scholar]; d) Junjie L.-L. Y. Bu; Wang Zejun; Ruan Huitong; Chen Qian; Gunadhi Vivienne; Bell R. Bryan; Gu Zhen, Biomaterials 2019, 219. [DOI] [PubMed] [Google Scholar]
- [21].a) Cirillo GS, U.G.; Curcio M; Nicoletta FP; Iemma F, Pharmaceutics 2019, 11; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chengjun Z. S. Sun; Wang Chao; Hu Yiqiao; Wu Jinhui, Molecular Pharmaceutics 2020, 17, 373; [DOI] [PubMed] [Google Scholar]; c) Yamei R. L. Xing; Yan Xuehai, Nanoscale 2019;; d) Nastaran M. T. Sirousazar; Roufegari-Nejad Ehsan, in Materials for Biomedical Engineering, Elsevier, 2019, 287. [Google Scholar]
- [22].Guvendiren ML, Hoang D; Burdick Jason A., Soft Matter 2012.
- [23].a) Miles WE, The Lancet 1908, 172, 1812; [Google Scholar]; b) Davies HM, British Journal of Surgery 1913, 1, 228; [Google Scholar]; c) Naef AP, The Annals of Thoracic Surgery 1993, 56, 988. [DOI] [PubMed] [Google Scholar]
- [24].a) Rajiv E. D. M. Bander; Ramakrishna Rohan, World Neurosurgery 2018, 116, 529; [DOI] [PubMed] [Google Scholar]; De Witt Hamer PC, Robles SG, Zwinderman AH, Duffau H, Berger MS, J Clin Oncol 2012, 30, 2559; [DOI] [PubMed] [Google Scholar]; c) Ishizuka M, Abe F, Sano Y, Takahashi K, Inoue K, Nakajima M, Kohda T, Komatsu N, Ogura S, Tanaka T, Int Immunopharmacol 2011, 11, 358; [DOI] [PubMed] [Google Scholar]; d) Alexandra D. A. G. Orringer; Jolesz Ferenc, Expert review of medical devices 2012, 9, 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pasquier B, Pasquier D, N’Golet A, Panh MH, Couderc P, Cancer 1980, 45, 112. [DOI] [PubMed] [Google Scholar]
- [26].Grossman SAB, Julette F, Seminars in Oncology 2004, 31. [Google Scholar]
- [27].Hung AL, Garzon-Muvdi T, Lim M, World Neurosurg 2017, 102, 494. [DOI] [PubMed] [Google Scholar]
- [28].Becker KPY, James, The Cancer Journal 2012, 18, 12.22290252 [Google Scholar]
- [29].Gempt J, Gerhardt J, Toth V, Huttinger S, Ryang YM, Wostrack M, Krieg SM, Meyer B, Forschler A, Ringel F, J Neurosurg 2013, 119, 1395. [DOI] [PubMed] [Google Scholar]
- [30].Jadhav V, Zhang JH, Front Biosci 2008, 13, 3793. [DOI] [PubMed] [Google Scholar]
- [31].a) Aguilar-Cazares D, Chavez-Dominguez R, Carlos-Reyes A, Lopez-Camarillo C, Hernadez ON de la Cruz J. S. Lopez-Gonzalez, Front Oncol 2019, 9; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Rakoff-Nahoum S, Yale J Biol Med 2006, 79, 123. [PMC free article] [PubMed] [Google Scholar]
- [32].Tyagi V, Theobald J, Barger J, Bustoros M, Bayin NS, Modrek AS, Kader M, Anderer EG, Donahue B, Fatterpekar G, Placantonakis DG, in Surg Neurol Int, Vol. 7 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].a) Sergei A. T. Belousov; Shved Nikita; Garbuz Mikhail; Malykin Grigori; Gualaia Valeriia; Kagansky Alexander; Kumeiko Vadim, Frontiers in Bioengineering and Biotechnology 2019; [DOI] [PMC free article] [PubMed]; b) Laurent L. S. Hamard; Berger Francois; van der Sander Boudewjin; Wion Dider, Journal of Neuro-Oncology 2016, 128, 1. [DOI] [PubMed] [Google Scholar]
- [34].a) Kornev VA, Grebenik EA, Solovieva AB, Dmitriev RI, Timashev PS, in Comput Struct Biotechnol J, Vol. 16 2018, p. 488; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Nih LR, Carmichael ST, Segura T, Curr Opin Biotechnol 2016, 40, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].a) Brem H, Mahaley MS Jr., Vick NA, Black KL, Schold SC Jr., Burger PC, Friedman AH, Ciric IS, Eller TW, Cozzens JW, et al. , J Neurosurg 1991, 74, 441; [DOI] [PubMed] [Google Scholar]; b) Xing W, Shao C, Qi Z, Yang C, Wang Z, in Drug Des Devel Ther, Vol. 9 2015, p. 3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].a) Yilong S. H. F. Ranganath; Arifin Davis Y.; Kee Irene; Zheng Lin; Lee How-Sung; Chow Pierce K.H.; Wang Chi-Hwa, Biomaterials 2010, 31, 5199; [DOI] [PubMed] [Google Scholar]; b) Zahra M. A. Norouzi; Liu Song; Miller Donald W., Scientific Reports 2018, 8. [Google Scholar]
- [37].Manome Y, Kobayashi T, Mori M, Suzuki R, Funamizu N, Akiyama N, Inoue S, Tabata Y, Watanabe M, Anticancer Res 2006, 26, 3317. [PubMed] [Google Scholar]
- [38].a) Graham-Gurysh EGM, Kathryn M; Schorzman Allison N.; Lee Taek; Zamboni William C.; Hingtgen Shawn D.; Bachelder Eric M.; Ainslie Kristy M., ACS Applied Material Interfaces 2020, 12, 19345; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Graham-Gurysh EM, Kathryn M; Satterlee Andrew B.; Sheets Kevin T.; Lin Feng-Chang; Bachelder Eric M.; Miller C. Ryan; Hingtgen Shawn D.; Ainslie Kristy M., Molecular Pharmaceutics 2018, 15, 1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Aiye B. L. Xiangdong, Emerging Concepts in Analysis and Applications of Hydrogels 2016, 86, 541. [Google Scholar]
- [40].Fabienne M. D. Zhao; Bastiancich Chiara; Joudiou Nicolas; Pallavi Ganipineni Lakshmi; Tsakiris Nikolaos; Gallez Bernard; des Rieux Anne; Jankovski Aleksandar; Bianco John; Préat Véronique, International Journal of Pharmaceutics 2018, 548, 522. [DOI] [PubMed] [Google Scholar]
- [41].Wolf MT, Ganguly Sudipto, Wang Tony L., Anderson Christopher W., Sadtler Kaitlyn, Narain Radhika, Cherry Christopher, Parrillo Alexis J., Park Benjamin V., Wang Guannan, Pan Fan, Sukumar Saraswati, Pardoll Drew M., Elisseeff Jennifer H., Science Translational Medicine 2019, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gianfaldoni S, Gianfaldoni R, Wollina U, Lotti J, Tchernev G, Lotti T, in Open Access Maced J Med Sci, Vol. 5 2017, p. 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].T. A. T. W. Group, JAMA Oncology 2018, 4, 1765. [DOI] [PubMed] [Google Scholar]
- [44].Barbarite ES, Justin T; Berchmans Emmanuel; Bregy Amade; Shah Ashish H.; Elsayyad Nagy; Komotar Ricardo J., Neurosurgical Review 2017, 40, 195. [DOI] [PubMed] [Google Scholar]
- [45].Tatter SBS, Edward G; Roseblum Mark L.; Karvelis Kastytis C.; Kleinberg Lawrence; Weingart Jon; Olson Jeffrey J.; Crocker Ian R.; Brem Steven; Pearlman James L.; Fisher Joy D.; Carson Kathryn A.; Grossman Stuart A., Journal of Neurosurgery 2003, 99. [DOI] [PubMed] [Google Scholar]
- [46].Bult WS, P. R.; Krijger GC; Visser T; Kroon-Batenburg LMJ; Bakker CJG; Hennink WE; van het Schip AD; Nijsen JFW, Pharmaceutical Research 2009, 26, 1371. [DOI] [PubMed] [Google Scholar]
- [47].Zielhuis SWN, J. F. W.; Krijger GC; van Rijk PP; Hennink WE; van het Schip AD, International Journal of Pharmaceutics 2006, 311, 69. [DOI] [PubMed] [Google Scholar]
- [48].Phillips WT, Goins B, Bao A, Vargas D, Guttierez JE, Trevino A, Miller JR, Henry J, Zuniga R, Vecil G, Brenner AJ, in Neuro Oncol, Vol. 14 2012, p. 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Xiaobing A. X. Sattiraju; Pandya Darpan N.; Wadas Thaddeus J.; Xuan Ang; Sun Yaao; Jung Youngkyoo; Sai Kiran K. S.; Dorsey Jay F.; Li King C.; Mintz Akiva, Molecular Cancer Therapeutics 2017, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bailly C, Vidal A, Bonnemaire C, Kraeber-Bodéré F, Chérel M, Pallardy A, Rousseau C, Garcion E, Lacoeuille F, Hindré F, Valable S, Bernaudin M, Bodet-Milin C, Bourgeois M, Front Pharmacol 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bergs JWW, Matthias G; Hehlgans Stephanie; Piiper Albrecht; Multhoff Gabriele; Rodel Claus; Rodel Franz, Biocheimica et Biophysica Acta 2015, 1856, 130. [DOI] [PubMed] [Google Scholar]
- [52].Zhujun X. L. Zhang; Lou Zhichao; Chen Feng; Chang Shuquan; Miao Yuji; Zhou Zhuo; Hu Xiaodan; Feng Jundong; Ding Qi; Liu Peidang; Gu Ning; Zhang Haiqian, Artificial Cells, Nanomedicine, and Biotechnology 2018, 46, 975. [DOI] [PubMed] [Google Scholar]
- [53].Nicole P. F. de la Puente; Luderer Micah J.; Jin Abbey; Shah Shruti; Muz Barbara; Kapoor Vaishali; Goddu Sreekrishna M.; Salama Noha Nabil; Tsien Christina; Tholata Dinesh; Shoghi Kooresh; Rogers Buck; Azab Abdel Kareem, Journal of Pharmaceutical Sciences 2018, 107, 922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Dobson JMH, Ann E; Peaston Anne E. , in Small Animal Clinical Pharmacology 2nd edition, Vol. 2, Saunders Elsevier, Edinburgh: 2008, 330. [Google Scholar]
- [55].Cohen MHJ, John R; Pazdur Richard, Clinical Cancer Research 2005, 11. [Google Scholar]
- [56].Silber JR, Bobola MS, Blank A, Chamberlain MC, Biochim Biophys Acta 2012, 1826, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tisdale MJ, Biochem Pharmacol 1987, 36, 457. [DOI] [PubMed] [Google Scholar]
- [58].da Ros M, Iorio AL, Lucchesi M, Stival A, de Martino M, Sardi I, in Anticancer Agents Med Chem, Vol. 15 2015, p. 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].a) Chen YS, Chen ZP, Chin J Cancer 2014, 33, 74; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liu XM, Zhang QP, Mu YG, Zhang XH, Sai K, Pang JC, Ng HK, Chen ZP, J Neurooncol 2011, 105, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Li J, Mooney DJ, Nat Rev Mater 2016, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Morased C. N. Manaspon; Chaimongkolnukul Khuanjit; Nittayacharn Pinunta; Vejjasilpa Ketpat; Kengkoom Kanchana; Boongird Atthaporn; Hongeng Suradej, Pharmaceutical Research 2016, 33, 2891. [DOI] [PubMed] [Google Scholar]
- [62].Bastiancich CD, P.; Preat V; Danhier F, Journal of Controlled Release 2017, 243, 29. [DOI] [PubMed] [Google Scholar]
- [63].Elia C. B. Bastiancich; Luyten Urszula; Danhier Fabienne; Bastiat Guillaume; Préat Véronique, International Journal of Pharmaceutics 2019, 559, 220. [DOI] [PubMed] [Google Scholar]
- [64].Lam FCM, Stephen W; Wyckoff Jeffrey; Vu Han Tu-Lan; Hwang Mun Kyung; Maffa Amanda; Balkanska-Sinclair Elena; Yaffe Michael B.; Floyd Scott R.; Hammond Paula T., Nature Communications 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Emmanuel C. P. Karavasili; Vizirianakis Ioannis S.; Koutsopoulos Sotirios; Fatouros Dimitrios G., Pharmaceutical Research 2018, 35. [DOI] [PubMed] [Google Scholar]
- [66].a) Mahtab S. H. Z. Shahcheraghi; Lotfi Marzieh; Ghayour-Mobarhan Majid; Ghorbani Ahmad; Jaliani Hossein Zarei; Sadeghnia Hamid Reza; Sahebkar Amirhossein, Current Pharmaceutical Design 2019, 25, 333; [DOI] [PubMed] [Google Scholar]; b) Wang X, Deng J, Yuan J, Tang X, Wang Y, Chen H, Liu Y, Zhou L, in Int J Oncol, Vol. 51 2017, p. 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Jiawei Z. S. Zhao; Zhang Long; Wang Lansheng; Xu Haoyue; Han Yuhan; Jia Jun; Lu Yang; Yu Rutong; Liu Hongmei, Biomaterials Science 2020.
- [68].Huang D, Lin C, Wen X, Gu S, Zhao P, in PLoS One, Vol. 11 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].a) Yun T. D. Wu, Cancer Letters 2017, 387, 61; [DOI] [PubMed] [Google Scholar]; b) Maryam F. S. Zarei, Artificial Cells, Nanomedicine, and Biotechnology 2018, 46, 906. [DOI] [PubMed] [Google Scholar]
- [70].Matthias E. P. Le Rhun; Roth Patrick; Reardon David A.; van den Bent Martin; Wen Patrick; Reifenberger Guido; Weller Michael, New Drugs 2019, 80. [DOI] [PubMed] [Google Scholar]
- [71].Adnane LT, Pamela A; Taylor Ian; Wilhelm Scott M., Methods Enzymol. 2006, 407, 597. [DOI] [PubMed] [Google Scholar]
- [72].Rini BI, Clinical Cancer Research 2008, 14. [Google Scholar]
- [73].Karpel-Massler GW, Andrew M; Kast Richard E; Wirtz Christian Rainer; Halatsch Marc-Eric, Anticancer Agents Med Chem 2011, 11, 748. [DOI] [PubMed] [Google Scholar]
- [74].Jain KK, 2018, Frontiers in Oncology.
- [75].a) Majed C. A. R. Dufes; Somani Sukrut, Therapeutic Delivery 2013, 4, 629; [DOI] [PubMed] [Google Scholar]; b) Iglesia RP, Fernandes CL, Coelho BP, Prado MB, Melo Escobar MI, Almeida G, Lopes MH, in Int J Mol Sci, Vol. 20 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Sousa FM, Rui P; Moreira Elias; Martins Claudia; Sarmento Bruno, Adv Protein Chem Struct Biol 2018, 112, 61. [DOI] [PubMed] [Google Scholar]
- [77].a) Bagley SJD, Arati S; Linette Gerald P.; June Carl H.; O’Rourke Donald M., Neuro Oncology 2018, 20, 1429; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Burger MC, Zhang C, Harter PN, Romanski A, Strassheimer F, Senft C, Tonn T, Steinbach JP, Wels WS, Front Immunol 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Rongyu W. Z. Xiao; Sohrabi Alireza; Ehsanipour Arshia; Sun Songping; Liang Jesse; Walthers Christopher M.; Ta Lisa; Nathanson David A.; Seidlits Stephanie K., Cancer Research 2018, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Omuro A, and Lisa M DeAngelis, JAMA 2013, 310, 1842. [DOI] [PubMed] [Google Scholar]
- [80].Ferrara NH, Kenneth J; Novotny William, Biochemical and Biophysical Research Communications 2005, 333, 328. [DOI] [PubMed] [Google Scholar]
- [81].Xianya H. S. Xin; Jiang Xinyi; Zhang Wei; Chen Liangcen; Fang Xialong, Biomaterials 2012, 33, 8167. [DOI] [PubMed] [Google Scholar]
- [82].Huimin G. X. Gu; Hu Quanyin; Liu Zhongyang; Jiang Mengyin; Kang Ting; Miao Deyu; Tu Yifan; Pang Zhiqing; Song Qingxiang; Yao Lei; Chen Hongzhan; Gao Xiaoling; Chen Jun, Biomaterials 2013, 34, 196. [DOI] [PubMed] [Google Scholar]
- [83].Yu DK, Omar F; Suva Mario L.; Dong Biqin; Panek Wojciech K.; Xiao Ting; Wu Meijing; Han Yu; Ahmed Atique U.; Balyasnikova Irina V.; Zhang Hao F.; Sun Cheng; Langer Robert; Anderson Daniel G.; Lesniak Maciej S., Proc Natl Acad Sci USA 2017, 114, E6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ke T.-F. L. Li; Zhang Quan; Wang Chao; Yue Yuan; Chen Zhuo; Yuan Shen-Jun; Liu Xin; Wen Yu; Han Min; Komatsu Naoki; Xu Yong-Hong; Zhao Li; Chen Xiao, Biomaterials 2018, 181, 35. [DOI] [PubMed] [Google Scholar]
- [85].Wen J. S. Kuang; Yin Jun; Zeng Xuan; Han Song; Zhao Yi-Peng; Tao Jun; Liu Chuan-Jun; He Xiao-Hua; Zhang Xian-Zheng, Advanced Functional Materials 2018, 28. [Google Scholar]
- [86].Manzoor AAL, Lars H; Landon Chealsea D.; Park Ji-Young; Simnick Andrew J.; Dreher Matthew R.; Das Shiva; Hanna Gabi; Park Won; Chilkoti Ashutosh; Koning Gerven A.; ten Hagen Timo L.M.; Needham David; Dewhirst Mark W., Cancer Research 2012, 72, 5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Song H, Yang P, Huang P, Zhang C, Kong D, Wang W, in Theranostics, Vol. 9 2019, p. 2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Weiden J, Voerman D, Dölen Y, Das RK, van Duffelen A, Hammink R, Eggermont LJ, Rowan AE, Tel J, Figdor CG, Front Immunol 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]


