Abstract
Extracorporeal boiling histotripsy (BH), a noninvasive method for mechanical tissue disintegration, is getting closer to clinical applications. However, motion of the targeted organs, mostly resulting from the respiratory motion, reduces the efficiency of the treatment. Here, a practical and affordable unidirectional respiratory motion compensation method for BH is proposed and evaluated in ex vivo tissues. The BH transducer is fixed on a robotic arm following the motion of the skin, which is tracked using an inline ultrasound imaging probe. In order to compensate for system lags and obtain a more accurate compensation, an autoregressive motion prediction model is implemented. BH pulse gating is also implemented to ensure targeting accuracy. The system is then evaluated with ex vivo BH treatments of tissue samples undergoing motion simulating breathing with movement of amplitudes between 5 to 10 mm, frequency between 16 to 18 breaths per minute, and a maximum speed of 14.2 mm/s. Results show a reduction of at least 89% of the value of the targeting error during treatment, while only increasing the treatment time by no more than 1%. The lesions obtained by treating with the motion compensation were close in size and affected area to the no-motion case, whereas lesions obtained without the compensation were often incomplete and had larger affected area. This approach to motion compensation could benefit extracorporeal BH and other histotripsy methods in clinical translation.
Keywords: Histotripsy, motion compensation
I. Introduction
Extracorporeal high intensity focused ultrasound (HIFU) treatments have been shown to be a promising noninvasive alternative for tumor ablation in the liver [1], [2], breast [3], [4], pancreas [5], [6] and kidney [2], [7]. Targeting of the extracorporeal HIFU treatment is usually performed with ultrasound imaging or magnetic resonance imaging (MRI) [8]. In ultrasound imaging guidance, the diagnostic probe is usually located at the center of the transducer and is axially aligned with its focus, referred to as inline imaging probe.
Using HIFU, different methods of tissue ablation are possible, such as thermal ablation, where long - in the range of hundreds of milliseconds to seconds - ultrasound pulses delivered at high duty cycle elevate the temperature through absorption at the focus up to 70°C [9]. Recently, several methods of mechanical tissue ablation were developed where short (microseconds to milliseconds) high amplitude ultrasound pulses delivered at low duty cycle generate intense bubble activity at the focus resulting in tissue fractionation [10], [11]. In particular, boiling histotripsy (BH) uses millisecond-long pulses of highly nonlinear HIFU waves to heat the focus up to boiling temperature, forming a mm-sized vapor bubble that interacts with the remaining pulse resulting in tissue liquefaction [12], [13].
However, the physiological motion of the organs, mainly resulting from respiration, can cause a time-varying offset between the targeted tissue and the transducer focus, and thus have a negative impact on the precision and safety of HIFU treatment. For thermal ablation, this means a reduction in the treatment efficiency [14], and in both mechanical modalities of ablation this motion has been shown in vivo to cause larger, irregularly shaped lesions [15]–[18]. The possibility of damage to adjacent healthy tissues is a safety concern with all treatment modalities.
Multiple ways of mitigating the physiological motion during HIFU treatment have been proposed over the years. The simplest method was to maintain breath hold during treatment, either self induced [19] or under general anaesthesia and mechanical ventilation [20], however as a consequence the treatment time was substantially increased [21]. Treatments can be also performed during continuous breathing but require motion tracking of the targeted tissue. This tracking can either be implemented using visual tracking of the skin surface [22], [23], MRI [24], [25], maintaining isoforce contact with the skin [26], or ultrasound imaging [27]–[29]. Once the motion is properly estimated, it can be accounted for passively by respiratory gating [23], i.e. the HIFU is only turned on when the focus is aligned within a certain margin to the targeted tissue. However, this method increases the treatment time and reduces its efficiency. Another method is to implement active motion compensation using robots that follow the tracked target, effectively canceling most of the motion [22], [26], [28], [29]. Alternatively, active compensation using a multi-element HIFU transducer in pulse-echo mode for the motion tracking and correcting for it by steering the focus electronically was also proposed [30]. However, this method requires a transducer with a large number of elements for proper tracking and steering.
All of the methods of motion compensation described above were originally designed for thermal HIFU treatments, in which long HIFU pulses interfere with ultrasound imaging, such that interleaved ultrasound imaging and HIFU sonication sequences had to be developed [28], [29]. Unlike thermal HIFU, histotripsy uses infrequent short pulses, therefore the problem of synchronizing HIFU bursts and imaging is not as critical.
Some of the prior studies on using ultrasound imaging for motion estimation employed real-time speckle-tracking algorithms in the region of HIFU focus for precise displacement estimation [28], [29]. Implementation of these algorithms for extracorporeal histotripsy in vivo is complicated by several factors that are challenging to overcome. First, due to the large water-filled standoff typical for extracorporeal HIFU setups, the distance from the imaging probe to the HIFU focus is usually quite large (120 mm in present case). This large distance combined with relatively small imaging array aperture often restricted by the size of the central opening (40 mm in our setup), usually results in low signal-to-noise ratio (SNR), thus complicating real-time speckle tracking algorithms. Furthermore, out of plane motion results in decorrelation of speckles [31], which makes them more prone to generating false motion estimates. These issues were overcome by Seo et al. by implementing motion tracking in 3D, but required the addition of a second imaging system and probe to the setup and a sophisticated algorithm [32], [33]. Another successful method to enhance speckle tracking capability during thermal HIFU treatments was based on tracking the hyperechoic region that gradually appears at the HIFU focus with tissue coagulation [33]. However, this approach is unlikely to be effective in histotripsy because changes in tissue echogenicity at the focus are rapid (on the order of milliseconds) and transient due to high speed and vorticity of residual bubble motion through liquefied tissue [18].
Other previous works on active motion compensation tracked and compensated for the movement of skin closest to the organ of interest [22], [26]. In the case of extracorporeal HIFU setups, the interface between coupling water (completely anechoic) and skin represents a steep contrast change on the B-mode imaging, making it an easy and reliable reference to track. However, the motion of the liver due to respiration in porcine model and human is complex and dependent of the part of the organ targeted [34], [35], and as such, tracking the skin would only allow for partial, unidirectional compensation of its motion. In both humans and pigs, generally the dominant liver motion components are cranio-caudal and anterior-posterior. Both motion components are dependent on the specific lobe and the subject’s position, and the exact reported displacement values vary widely, within 10–25 mm (humans) and 2–5 mm (pigs) for cranio-caudal direction and 2.5–12 mm (humans) and 1–10 mm (pigs) for anterior-posterior direction [34], [35]. The former can not be compensated with the unidirectional method proposed here, only the latter.
In this paper, we present an active unidirectional respiratory motion compensation method using a robotic arm that is adapted to histotripsy, specifically BH. The objective was to develop a method that would be affordable and relatively generic in its implementation for use in ongoing in vivo BH treatments of the liver in porcine models, and humans in the future. The capabilities of the method were evaluated by performing BH ablations of ex vivo tissue undergoing quasiperiodic motion replicating pig respiration: the amplitude of the motion ranged between 5 to 10 mm, frequency of around 17 breaths per minute, and speeds within 5–14 mm/s.
II. Materials and Methods
A. HIFU Transducer, Imaging Probe and Robotic Arm
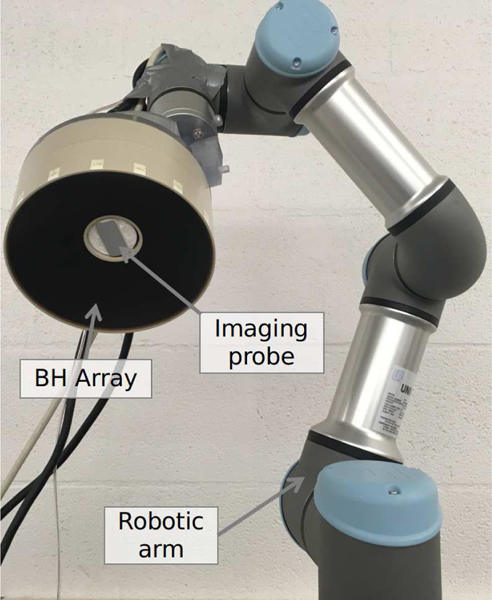
A 1.5 MHz, 256-element array designed to deliver abdominal BH treatments [36]–[38] made of piezoelectric composite material (Imasonic, Voray sur l’Ognon, France) was used in this study and is shown in Fig. 1. The elements diameter was 7 mm; and transducer had a nominal focal distance of 120 mm, an outer diameter of 144 mm, and a central opening of 40 mm diameter where a coaxial ultrasound imaging probe (ATL P6–3) was set. The transducer was designed for extracorporeal BH treatments and as such could generate the required high amplitude shock fronts (up to 100 MPa) at the focus at the peak acoustic power of 275 W in water. The transducer was driven by a 4-boards V1 Verasonics research system (V-1 Ultrasound Acquisition platform, Verasonics Inc, Kirkland, WA, USA) with HIFU option.
Fig. 1.
Photograph of the BH transducer array with inline imaging probe mounted on the robotic arm.
The 128-element phased array imaging probe was connected to a separate 2-board V1 Verasonics system. The B-mode ultrasound imaging acquisition was made using a transmit at 4.5 MHz and a sampling frequency Fs = 18 MHz, and the reconstruction was made using 36 scan lines focused at the same depth as the transducer, 120 mm, and distributed in a rectangular area directly in front of the imaging probe array of 34 mm width and 135 mm depth, with a frame rate of 27.5 Hz.
The HIFU array with the inline imaging probe was then attached to a robotic arm (UR3e, Universal Robots, Odense, Denmark) using a custom 3D-printed holder as seen in Fig. 1. The robotic arm was a six degree-of-freedom cobot, i.e. specifically designed for human interaction, had a 500 mm reach, a maximum payload weight of 3 kg, a repeatability of movement of ± 0.03 mm, and maximum speed of 1 m/s. The arm was mounted on a tripod support with retractable wheels.
B. Motion Tracking
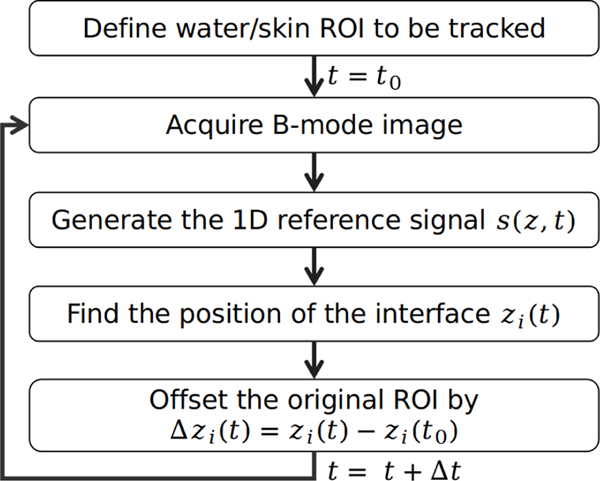
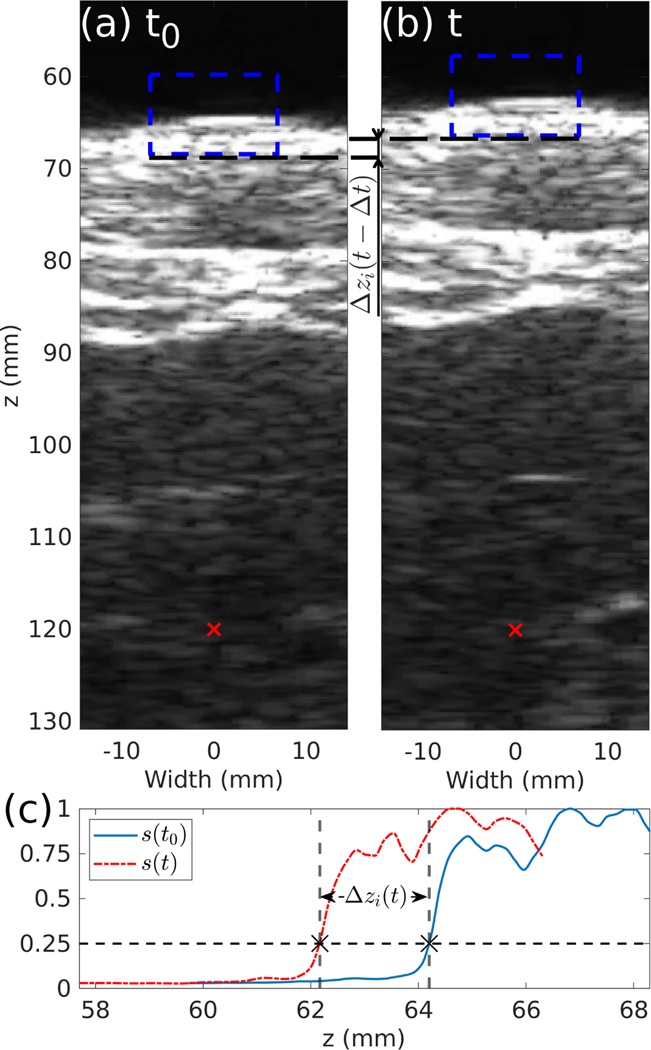
The approach to track the motion of the water/skin interface along the axis of propagation of the HIFU transducer and imaging probe, referenced as z, is described in this section. The ultrasound imaging-derived signal used in the tracking algorithm was similar to that presented by Chanel et al. [28], and is illustrated with a diagram in Fig. 2. An example of the implementation of the tracking algorithm on a free breathing pig during an independent prior in vivo BH liver treatment is shown in Fig. 3. A region of interest (ROI) including the waterskin interface was selected on the B-mode image (illustrated as dashed blue boxes in Fig. 3(a-b)). The brightness values of the pixels along the transverse dimension were then summed and normalized to generate a 1D signal, shown in Fig. 3(c), that was used for the tracking. Here, the ROI enclosed the interface between the water and the skin, with its width and height chosen such that the interface contrast change on the 1D signal could be easily tracked with a certain threshold value over the entire respiratory cycle. In some cases where the contrast change was not steep enough, the 1D signal was also squared to improve the signal contrast. This 1D signal was then interpolated by a factor of 16 with a piecewise cubic Hermite interpolation to increase its sampling rate to 288 MHz in order to improve the resolution of the tracking. This resulting signal will be referred to as , where z is the imaging depth relative to the imaging probe and t is the time.
Fig. 2.
Flow diagram of the water/skin interface tracking algorithm.
Fig. 3.
(a-b) B-mode images taken with the inline imaging probe during an in vivo BH treatment of the liver of a pig in free breathing condition, where the boxes in  represent the ROI to be tracked and the red cross is the focus of the transducer. Here t−t0 = 1 s. (c) Normalized 1D signals from the ROI in (a)
represent the ROI to be tracked and the red cross is the focus of the transducer. Here t−t0 = 1 s. (c) Normalized 1D signals from the ROI in (a)  and (b)
and (b)  used in the tracking algorithm. The threshold value T is represented by
used in the tracking algorithm. The threshold value T is represented by  .
.
Once a suitable signal had been generated from the B-mode image, tracking of the interface position was performed by choosing an adequate threshold T that allows to track the surface smoothly and using a zero crossing detection on the signal to find the position zi(t) corresponding to distance of the water/skin interface relative to the imaging probe, as shown in Fig. 3(c). The value of T was chosen as slightly above the maximum amplitude of the noise in front of the water/skin interface. The displacement of the interface relative to its initial value, where t0 is the time that the tracking algorithm was initialized, was defined. Finally, in order to ensure the same interface was tracked continuously over a long time, the ROI of the tracking algorithm was translated by , therefore making the ROI follow the interface as illustrated between Fig. 3(a) and Fig. 3(b).
C. Motion Prediction
The output of the displacement estimation described above could theoretically serve as direct input to the robotic arm for motion compensation. However, the lags stemming from limited B-mode imaging frame rate (27.5 Hz in present case), execution time of motion tracking algorithm, communication between the different modules (ultrasound imaging, computers and the robot), and limited acceleration of the robotic arm (the safest, and therefore most restrictive setting for acceleration was used here) would accumulate (over 100 ms in present case) and could lead to less accurate and thus less efficient motion compensation, even with an appropriately tuned controller, as demonstrated by another group [39]. One solution to this problem is the use of motion prediction algorithms in tandem with motion estimation. Motion prediction methods rely on a priori knowledge of some of the motion characteristics. For example, the free breathing motion in both anesthetized pigs and humans is known to be almost periodic, with only small variations of the motion period and amplitude between each breath [40], [41]. In this study, a time-varying seasonal autoregressive (TVSAR) prediction model, originally developed for the motion prediction of lung tumors during radiotherapy [42] was used.
The TVSAR model is based on the seasonal autoregressive (SAR) model used to model time series with periodical variations. The SAR model is as follows:
| (1) |
where , are the SAR coefficients, n the order of the SAR model, is a Gaussian white noise with zero mean and variance σ2, and p is the period. To obtain future prediction for a h-time ahead, the prediction model becomes:
| (2) |
where is the predicted value of at the instant t, and are the estimated values of . Equation (2) allows for predicting future values of as a weighted sum of its previous values for n periods back.
The TVSAR model allows for small variations in period by replacing the term δp in Eqs. (1) and (2) by the reference intervals rδ(t), and thus the prediction model becomes:
| (3) |
The values of the reference intervals represent the local period of the δth breath. At the initialization of the algorithm, a recording of length Ti of the respiration motion containing a known number of N ≥ n breath periods is obtained, resulting in an estimated period of . The following correlation function CF is then defined as:
| (4) |
where k is a lag value, μ is the mean of over the interval . The values of the reference interval were then determined by looking at the peak of CF:
| (5) |
where Kδ is the search range for the δth interval which is set as:
| (6) |
Following each time the values of rδ(t) were calculated, the value of p was replaced such as its value for the next update of the values of rδ(t) was the mean of their preceding values:
| (7) |
Finally, the coefficient of the TVSAR model in Eq. 3 were estimated with a recursive least square (RLS) filter with forgetting factor [43]. The input and coefficient vectors, x(t) and respectively, were set as:
| (8) |
| (9) |
where the initial values of the coefficient vector were set as null:
| (10) |
The update of the values of was introduced as follows:
| (11) |
where e(t) is the error between the filter output and the desired values zi(t) and R(t) is the inverse of the autocorrelation matrix, and are calculated as follow:
| (12) |
| (13) |
where λ is the forgetting factor that should be in range from 0 to 1, and with , where I is the identity matrix and γ is a large value (e.g. [43]).
D. Implementation
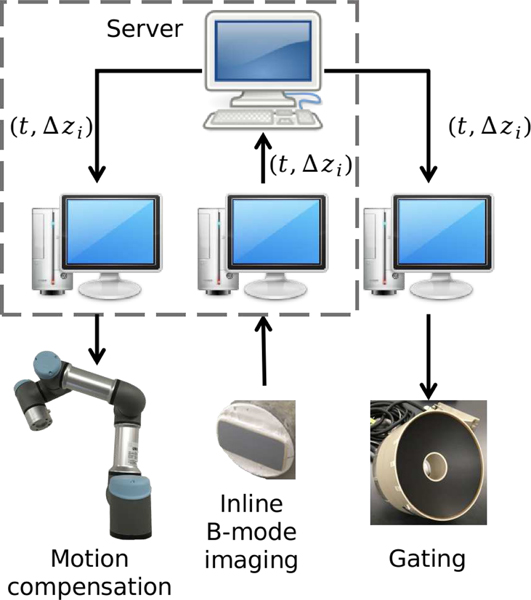
The purpose of the setup was to deliver extracorporeal BH treatment while compensating for the respiratory motion, and treatment gating in case the compensation was not sufficient. The diagram in Fig. 4 illustrates the communication between the three modules involved in the system: B-mode imaging, the robot controller and the HIFU transducer array driving module. The data communicated between the modules were: 1) the displacement of water/skin interface, , calculated by the B-mode imaging system as described above and used in the feedback control loop of the robotic arm and, if needed, gating of the HIFU transducer, and 2) the time t of the B-mode image acquisition. The data from the imaging system was sent by TCP/IP to a server hosted locally on the same computer. This data could be then queried by both the robot controller, also hosted on the same computer, and the HIFU transducer control system hosted on a separate computer.
Fig. 4.
Diagram of the communications between parts of the respiratory motion compensation and gating system. The  frame represents a localhost communication.
frame represents a localhost communication.
The displacement estimation was built into the MatLab Verasonics imaging script as an external process event immediately following the reconstruction event and before the image display event as the display event generated a non negligible lag of around 5 to 10 ms. As soon as the value of was obtained, it was sent to the server along with its acquisition time t. Note that in the current implementation of the system the 10 ms long BH pulse emissions were not synchronized with B-mode imaging and could interfere with image acquisition during a pulse. However, given the low pulse repetition frequency (PRF) of 1 Hz, only one B-mode frame per second would potentially be affected. To avoid an arbitrary displacement value resulting from the obstruction of the B-mode by the BH pulse, a check was implemented whether the B-mode image was blocked by the HIFU interference by detecting large sudden changes of the value of zi, i.e. . Here ξ was set to 1 mm. The value of zi(t) was then ignored and, instead, its previous value was sent to the server. The refresh rate of the imaging (27.5 Hz) was fast enough compared to the respiratory motion (<0.4 Hz) that this did not impact the motion compensation.
The gating was also implemented in the MatLab HIFU Verasonics script as an external process event located just before the BH pulse emission event. The process would query the server and check if the value of was lower than an arbitrary chosen maximum allowed displacement value ε. If , the process would freeze the BH treatment script and query the value of with a refresh rate of 100 Hz until it became lower than ε or reached a timeout, which was set here as 10 seconds. Reaching the timeout would close the treatment program as a protection measure for both the HIFU transducer and the patient.
The server was programmed in Python and received the value of as a character string that it would then be able to broadcast to other programs when queried with a specific command. The robot controller and the motion prediction algorithm were also programmed in Python and communicated to the robotic arm via its proprietary script language (URScript). A control loop, with a refresh rate of 110 Hz, was implemented and was controlling the speed of the robot along the axis of the BH array and inline imaging probe for the motion compensation. Because the B-mode imaging rate of 27.5 Hz and therefore the sampling rate of was significantly lower, they were first resampled using cubic interpolation at 60 Hz and then filtered with a low-pass filter with a cutoff frequency of 5 Hz. The values of outside the sampling rate were obtained by cubic interpolation between the two closest samples available.
The values of the coefficient of the TVSAR model in Eq. 3 were calculated using the implementation of the RLS filter available in the Python Adaptive Signal Processing library [44] and the value of the forgetting factor was set as λ = 0.1. The speed command vr of the robot was calculated using a discrete proportional derivative controller such that its value was:
| (14) |
where KP and KD are the proportional and derivative gain of the discrete controller, respectively. Once the motion prediction program was initialized and the robotic arm was using its output to compensate for the respiratory motion, the value of the displacement fed to the motion prediction algorithm was changed from to , where was the displacement of the robotic arm relative to its initial position.
Finally, a software safety was introduced to avoid potential harm to the equipment and personnel. As such, the transducer displacement was limited to a spherical space of radius Rs, and if the robot left this space it would stop and await a new order. This safety measure was redundant with the hardware safety settings that were already set at the most restrictive level.
The optimal parameters used for the motion compensation controller were found experimentally as KP = 10 and KD = 0.1. The motion prediction parameter n, corresponding to the number of previous movement periods to be considered for the prediction, should be chosen between 2 and 5 to avoid long calculation of the AR coefficients by the RLS filter. Here this parameter was set as n = 3. The remaining parameter of the motion prediction module, the look ahead time h, is chosen experimentally as to minimize the error between the predicted and actual movement, and was set here as h = 126 ms. The maximum displacement allowed for the gating of the transducer was determined experimentally as the value that would yield a lesion similar to the no-motion case, and was set here as ε = 1 mm. The safety radius was set as Rs = 20 mm.
Execution time on an Intel i9–9900k processor was less than 1 ms for the motion tracking algorithm and around 12 ms for the motion prediction. All the scripts implemented here are available on request, and are compatible with any URx e-series cobot model from Universal Robots.
E. Ex vivo Experiments
The efficiency of the motion compensation approach presented here was tested in ex vivo BH treatments implemented in bovine heart tissue. Note that although the BH system is intended for applications primarily in liver and kidney, the heart tissue was chosen for testing to isolate only the influence of motion compensation on the BH lesion size and quality. This is due to its relative macroscopic homogeneity resulting in consistency of size and shape of BH lesions, as well as the ease of gross visual evaluation of the lesions [45]. Fresh bovine heart was obtained from a local abattoir on the day of the experiments and all testing occurred within 36 hours from its receipt. The tissue was kept on ice during transportation and before the experiments. Prior to BH exposure, the heart was cut into cuboids of about 40 mm x 80 mm x 40 mm size and pericardial fat, if any, was removed. The samples were then placed into cold degassed saline and degassed in a desiccant chamber for at least 1 hour. Separately, an agarose gel was prepared by mixing agarose powder (UltraPure Agarose, Invitrogen) into de-ionized water (1.5% wt./vol. agarose/water). The resulting solution was then boiled for 10 minutes in a microwave oven to displace any dissolved gases present. The solution was then poured into a plastic mold of 80 mm x 50 mm x 50 mm size and cooled by placing the mold in a reservoir filled with cold water. Once the temperature of the gel reached 40°C, the heart sample was placed into the mold and the gel was left to polymerize for about 15 minutes. Once polymerized, the embedded sample was transferred into a custom-made sample holder with acoustic windows on its four rectangular sides.
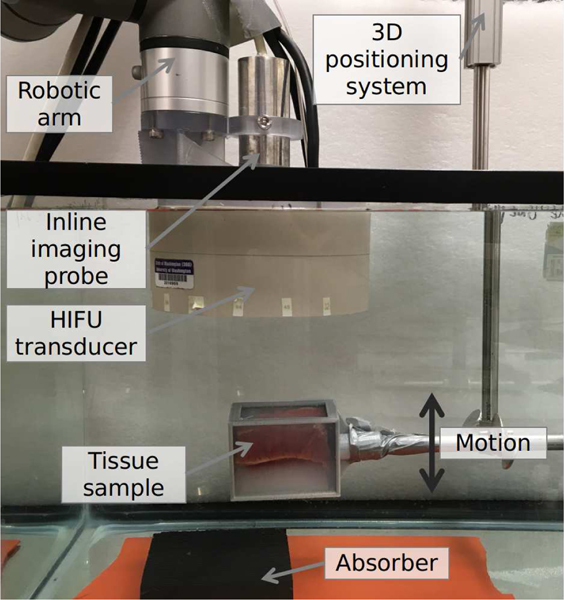
The position of the sample holder was controlled by a 3D positioning system (Velmex Inc, Bloomfield, NY, USA) within a tank filled with de-ionized water at room temperature and degassed to below 10% oxygen saturation. A polyurethane rubber acoustic absorber was placed about 6 cm behind the sample to avoid reverberation of HIFU and imaging waves. A photograph of the experimental setup is shown Fig. 5.
Fig. 5.
Photograph of the experimental setup
The experiments consisted of producing small volumetric BH lesions in the sample in 3 different scenarios: immobile sample, and moving sample with or without motion compensation activated. Volumetric BH treatment was performed by electronically steering the HIFU focus through a rectangular grid of 13×5 foci in a plane orthogonal to the axis of the HIFU array, centered around its geometric focus, with 1 mm spacing between the foci, which resulted in a total of 65 points treated. This specific pattern was selected following previous experiments with this transducer [38] and should result in a total ablated volume of approximately 15 mm x 7 mm x 6 mm. All the treatments were performed with the geometric focus of the HIFU transducer placed about 8 mm inside the tissue sample. The parameters of the BH treatments were: pulses of 10 ms duration, delivered at a PRF of 1 Hz, and each sonication point in the treatment plane received a single BH pulse before repeating the sonication, for a total of 8 BH pulses per point. The voltage on the transducer elements was chosen before the treatment by delivering isolated BH pulses at escalating voltage to a different part of the tissue until a hyperechoic region was clearly visible on the B-mode imaging. The transducer voltage was then adjusted for every steered point according to previous methodology [38].
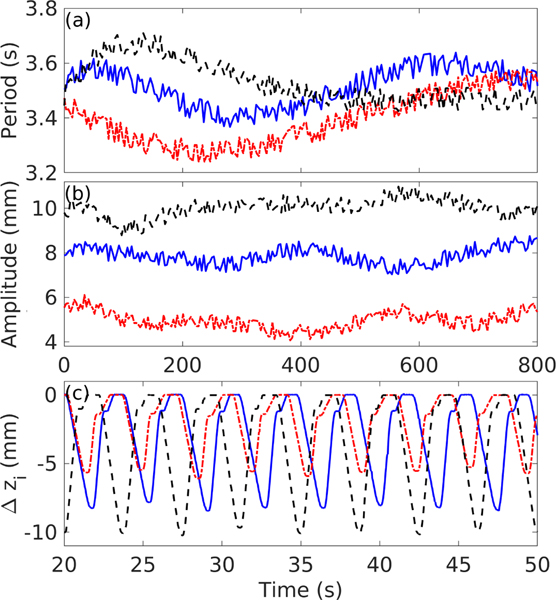
For the scenarios where the tissue sample was moving, the motion was introduced by controlling the 3D positioning system along the same direction as the HIFU array axis. Three different motions, referenced as motion A, B, and C, were implemented, and their shape, period, and amplitude were based on previous observations during in vivo liver BH treatments in free breathing porcine model from our group [18] and published data [35], [40], [41]. All the motions periods and amplitudes had continuous as well as discontinuous variations over time, as shown in Fig. 6 and detailed in Table. I.
Fig. 6.
Characteristics of the three modes of respiratory motions induced, with  representing motion A,
representing motion A,  representing motion B, and
representing motion B, and  representing motion C. (a) Period and (b) amplitude of the motion as a function of time. (c) A 30 second long excerpt of sample displacement as measured by the inline ultrasound imaging probe during treatments without motion compensation.
representing motion C. (a) Period and (b) amplitude of the motion as a function of time. (c) A 30 second long excerpt of sample displacement as measured by the inline ultrasound imaging probe during treatments without motion compensation.
TABLE I.
Motion parameters.
| Motion | Period (s) | Amplitude (mm) | Peak inspiration speed (mm/s) | Peak expiration speed (mm/s) |
|---|---|---|---|---|
| A | 3.48 ± 0.08 | 8 ± 0.43 | 5.74 | 11.7 |
| B | 3.41 ± 0.1 | 5.18 ± 0.48 | 5.59 | 9.9 |
| C | 3.51 ± 0.07 | 10 ± 0.38 | 7.82 | 14.2 |
III. Results
In total, two BH treatments were performed without motion, four with and without motion compensation using motion A, and two with and without motion compensation using motions B and C each.
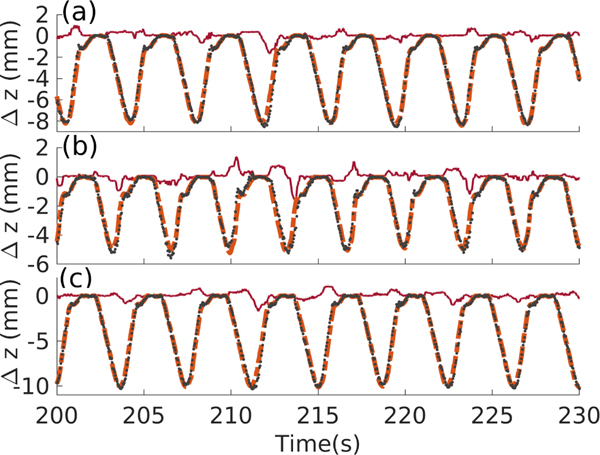
During the treatments with motion compensation, the values of the offset between the desired position of the water/sample interface and its actual position , the motion of the interface and its predicted motion at instant were recorded and a representative excerpt is presented in Fig. 7. The value of while the motion compensation system was running can be interpreted as the compensation error, and its statistics for the three types of motion are compiled in Table II.
Fig. 7.
30 second long excerpt of the sample surface displacement illustrating motion compensation for each mode of motion, where  represents ,
represents ,  represents , and
represents , and  represents . (a) Motion A; (b) motion B; (c) motion C.
represents . (a) Motion A; (b) motion B; (c) motion C.
TABLE II.
Motion compensation error statistics.
| Motion | RMS() (mm) | Maximum (mm) |
|---|---|---|
| A (n=4) | 0.39 | 4 |
| B (n=2) | 0.32 | 2.3 |
| C (n=2) | 0.42 | 3.1 |
The results of the motion compensation error statistics are presented in Table. II and show that the root mean square (RMS) values of the HIFU array focus position relative to the target was lower than half a millimeter for almost periodic movements with amplitudes within 5 to 10 mm range, indicating that the motion compensation was efficient. However, the variability, and thus unpredictability, of the amplitude and period of the movement resulted in short spikes of error, clearly visible in Fig. 7, that can reach values of up to 4 mm here. This demonstrates that gating of the BH pulses from the HIFU array, employed when the error was 1 mm or more, is complementary to the motion compensation to ensure the safety and accuracy of the treatment.
For every treatment, each time a BH pulse was emitted, the time and the offset of the interface were recorded. The resulting treatment statistics are presented in Table III, with root mean square average and the maximum of , the total treatment time, and affected tissue length along the HIFU array axis z. The affected tissue length corresponding to the maximum length where gross tissue damage (whether mechanical or thermal) was visible; and was measured on the gross histological cut using ImageJ [46]. The total treatment time increased for the cases with motion compensation as a result of gating the BH pulses. The benefit of the motion compensation and gating system on the ex vivo treatment efficiency was a reduction of the targeting error (root mean square average of ) by 92%, 89%, and 93% for the motion A, B, and C, respectively; while only making the treatment no more than 1% longer in all cases.
TABLE III.
Treatments statistics.
| Treatment | RMS (mm) | max (mm) | Total time (s) | Affected tissue length along z (mm) |
|---|---|---|---|---|
| Without motion (n=2) | 0 | 0 | 520 | 6.5±0.5 |
| A without compensation (n=4) | 4.1 | 9.2 | 520 | 12.6±0.9 |
| B without compensation (n=2) | 2.5 | 5.8 | 520 | 9.9±0.5 |
| C without compensation (n=2) | 5.2 | 11 | 520 | 13.8±2.3 |
| A with compensation (n=4) | 0.33 | 1 | 525.7±1 | 7.1±0.7 |
| B with compensation (n=2) | 0.27 | 0.97 | 524±0.1 | 7.5±1.3 |
| C with compensation (n=2) | 0.35 | 0.98 | 525.2±1 | 8.1±0.2 |
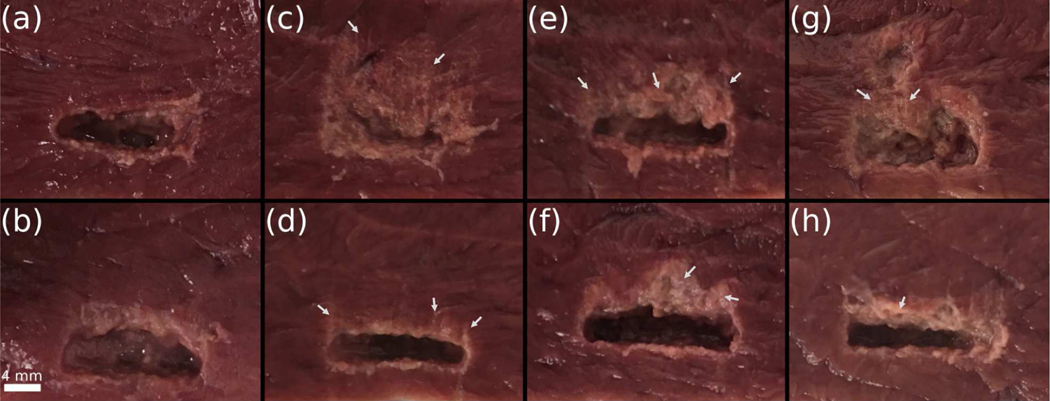
Photographs of the bisected BH lesions produced during treatments without any motion, treatments with motion and treatments with motion and the motion compensation system activated are presented in Fig. 8. In all cases, the liquefied tissue content inside the lesions could be easily washed out using saline.
Fig. 8.
Gross evaluation of the volumetric BH lesions under different motion conditions. The cut was made perpendicular to the imaging/treatment plane and along the largest width of the treatment plane. The liquefied tissues present were delicately washed out prior to the photographs were taken. BH treatment was from the bottom of the frames. (a-b) No motion during the treatment; (c-d) treatments under motion A (c) without motion compensation and (d) with motion compensation; (e-f) treatments under motion B (e) without motion compensation and (f) with motion compensation; (g-h) treatments under motion C (g) without motion compensation and (h) with motion compensation. The white arrows designate examples of thermal damage.
The lesions produced in the immobile samples had sharp and smooth boundaries, with a blanched rim of width ranging within 1–2 mm around the edges, which was observed previously in BH treatments of bovine heart. The rim most probably represented partially fractionated tissue resulting from incomplete merging of the distal ”tails” of the BH lesions. The lesions produced in moving samples without motion compensation had lumps of untreated tissues mixed in with the liquefied debris, and an overall larger affected region compared to the immobile case - 94%, 52%, and 112% larger for motion A, B, and C, respectively. With motion compensation and compared to the no motion case, the affected tissue length were only 9%, 15% and 25% larger for motion A, B, and C, respectively. The treatments with motion compensation yielded lesions of similar size and quality as those produced in immobile samples albeit with a wider (by 1–3 mm) blanched rim along the distal side of the lesion, with occasional narrow areas characteristic of either thermal damage or changes in heme iron associated with lower temperature increases, as observed in BH lesions previously [45]. This thermal effect on the distal side could be the result of occasional positioning of the focus behind the lesion within the margin allowed by the motion compensation and gating algorithm (1 mm maximum for the motion compensation case here with the gating setting), and accentuated attenuation caused by prefocal cavitation inside the liquefied tissue - caused by bubble nuclei from previous treatment point - preventing the formation of a boiling bubble at that point [18], [47].
Videos documenting treatments under motion C are available in the supplementary multimedia file. One video shows the treatment without motion compensation (no_compensation.mp4), and another one shows the treatment with motion compensation (motion_compensation.mp4). The boiling bubbles resulting from the BH treatment are visible on the B-mode images as 1 mm diameter hyperechoic regions [15].
IV. Discussion and Conclusions
In this paper, a practical way of active compensation for the respiratory motion during BH treatment was described and implemented. While it only offers a partial compensation of the respiratory motion during treatment due to the fact that the skin is used as a reference for tracking, its implementation does not require any changes to the HIFU system and is fast and simple to implement. The robotic arm used in this work is relatively affordable and performs well in matching the speed of respiratory motion and in terms of precision and accuracy of the movement. Being a cobot, it also offers hardware safety settings that allow it to maneuver safely around the staff and animal/patient treated.
As the B-mode imaging frame rate was 27.5 Hz here, similar results could be obtained by using a commercial ultrasound imaging system with a similar frame rate and a frame grabber instead of the Verasonics setup used here. As the tracking is interpolated in time in the motion prediction module, even lower values of B-mode frame rate would suffice, as long as the motion of the skin is spatially resolved decently; and the simplicity of the tracking algorithm allows for tracking the water/tissue interface even with sub-optimal imaging quality at the focal area of the transducer.
A SAR motion prediction algorithm was used here in order to compensate for any response time between the ultrasound imaging acquisition and the robot. This also allows for flexibility in the implementation as even longer response time than presented here, for example, resulting from a lower B-mode frame rate in the case of using an image grabber with a clinical ultrasound imaging system, should be efficiently compensated as well. Unfortunately, being an autoregressive algorithm, it does not deal well with sudden pattern changes, as it could happen for in vivo cases with an unexpected movement from the patient. However, the transducer gating implemented here would make sure that the treatment is paused in that case, both ensuring the safety of the patient and the transducer. The sudden error increase from this event could also be easily detected and dealt with by resetting the motion prediction algorithm.
The HIFU transducer could also be of any type, e.g. single element transducer or a phased array as used here, as long as the transducer allows for inline ultrasound imaging. While not implemented here, changing the position of the transducer to mechanically ablate a volume - instead of electronically steering as in this work - while keeping the motion compensation and gating operational is a possibility.
The implemented motion tracking uses the skin/water rather than the targeted area at the focus of the HIFU transducer. This was done to avoid tracking issues at the region of interest as a consequence of the low SNR of B-mode imaging, out of plane motion, and transient and chaotic echogenicity changes due to bubble motion within the liquefied area following each BH pulse. As such, a comparison of the algorithm’s performance characteristics with other methods designed for thermal HIFU present in the literature would be difficult. Ex vivo experiments showed a net improvement of the treatment precision and completeness under simulated, unidirectional respiratory motion when the developed motion compensation system was used by creating lesions similar in dimensions and quality to a no-motion case. In the present case, the gating ensured that the compensation error was always less than a millimeter when emitting a BH pulse. Another important point here is that the actual precision of the motion compensation system can be easily customized to the need of the user by changing the gating value. However, using a gating limit close or even inferior to the RMS of the compensation error would result in more significant increase of the treatment time than observed in this work.
The movement compensation presented here was implemented only in one direction - along the HIFU transducer axis - which means that the respiratory motion would only be compensated partially. Depending on the organ targeted, the subject positioning, the position of the transducer and even the region in the organ targeted, the efficiency of this aprroach may vary. As such, in vivo testing of this system on free breathing anesthetized pigs will follow in order to obtain realistic values of the total respiratory compensation resulting from this approach, and its benefit on BH ablation precision.
Finally, while the system was conceived with BH treatments in mind, it could be easily adapted to shock scattering histotripsy as it also consists of short pulses emitted at low duty cycle, allowing for ultrasonic imaging. Application to thermal HIFU is also a possibility, but will require the use of an interleaved HIFU/imaging scheme, similar to the ones proposed in [28], [29].
Supplementary Material
Contributor Information
Gilles P.L. Thomas, Center for Industrial and Medical Ultrasound, Applied Physics Laboratory, University of Washington, Seattle, WA.
Tatiana D. Khokhlova, University of Washington School of Medicine, Division of Gastroenterology, Seattle, WA.
Vera A. Khokhlova, Center for Industrial and Medical Ultrasound, Applied Physics Laboratory, University of Washington, Seattle, WA. Physics Faculty, Moscow State University, Moscow, Russia.
References
- [1].Wu F, Wang Z-B, Chen W-Z, Zhu H, Bai J, Zou J-Z, Li K-Q, Jin CB, Xie F-L, and Su H-B, “Extracorporeal high intensity focused ultrasound ablation in the treatment of patients with large hepatocellular carcinoma,” Annals of surgical oncology, vol. 11, no. 12, p. 1061, 2004. [DOI] [PubMed] [Google Scholar]
- [2].Illing R, Kennedy J, Wu F, Ter Haar G, Protheroe A, Friend P, Gleeson F, Cranston D, Phillips R, and Middleton M, “The safety and feasibility of extracorporeal high-intensity focused ultrasound (hifu) for the treatment of liver and kidney tumours in a western population,” British journal of cancer, vol. 93, no. 8, pp. 890–895, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wu F, Ter Haar G, and Chen WR, “High-intensity focused ultrasound ablation of breast cancer,” Expert review of anticancer therapy, vol. 7, no. 6, pp. 823–831, 2007. [DOI] [PubMed] [Google Scholar]
- [4].Merckel LG, Knuttel FM, Deckers R, van Dalen T, Schubert G, Peters NH, Weits T, van Diest PJ, Willem PTM, Vaessen PH et al. , “First clinical experience with a dedicated mri-guided high-intensity focused ultrasound system for breast cancer ablation,” European radiology, vol. 26, no. 11, pp. 4037–4046, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hwang JH, Wang Y-N, Warren C, Upton MP, Starr F, Zhou Y, and Mitchell SB, “Preclinical in vivo evaluation of an extracorporeal hifu device for ablation of pancreatic tumors,” Ultrasound in medicine & biology, vol. 35, no. 6, pp. 967–975, 2009. [DOI] [PubMed] [Google Scholar]
- [6].Chang W, Lee JY, Lee JH, Bae JS, Cho YJ, Kang KJ, Son K, Chung YR, Lee KB, and Han JK, “A portable high-intensity focused ultrasound system for the pancreas with 3d electronic steering: a preclinical study in a swine model,” Ultrasonography, vol. 37, no. 4, p. 298, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ritchie RW, Leslie T, Phillips R, Wu F, Illing R, Ter Haar G, Protheroe A, and Cranston D, “Extracorporeal high intensity focused ultrasound for renal tumours: a 3-year follow-up,” BJU international, vol. 106, no. 7, pp. 1004–1009, 2010. [DOI] [PubMed] [Google Scholar]
- [8].Yiallouras C. and Damianou C, “Review of mri positioning devices for guiding focused ultrasound systems,” The International Journal of Medical Robotics and Computer Assisted Surgery, vol. 11, no. 2, pp. 247–255, 2015. [DOI] [PubMed] [Google Scholar]
- [9].ter Haar G. and Coussios C, “High intensity focused ultrasound: physical principles and devices,” International journal of hyperthermia, vol. 23, no. 2, pp. 89–104, 2007. [DOI] [PubMed] [Google Scholar]
- [10].Xu Z, Ludomirsky A, Eun LY, Hall TL, Tran BC, Fowlkes JB, and Cain CA, “Controlled ultrasound tissue erosion,” IEEE transactions on ultrasonics, ferroelectrics, and frequency control, vol. 51, no. 6, pp. 726–736, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Khokhlova TD, Canney MS, Khokhlova VA, Sapozhnikov OA, Crum LA, and Bailey MR, “Controlled tissue emulsification produced by high intensity focused ultrasound shock waves and millisecond boiling,” The Journal of the Acoustical Society of America, vol. 130, no. 5, pp. 3498–3510, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Simon JC, Sapozhnikov OA, Khokhlova VA, Wang Y-N, Crum LA, and Bailey MR, “Ultrasonic atomization of tissue and its role in tissue fractionation by high intensity focused ultrasound,” Physics in Medicine & Biology, vol. 57, no. 23, p. 8061, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pahk KJ, Gelat P, Sinden D, Dhar DK, and Saffari N, “Numericaĺ and experimental study of mechanisms involved in boiling histotripsy,” Ultrasound in Medicine & Biology, vol. 43, no. 12, pp. 2848–2861, 2017. [DOI] [PubMed] [Google Scholar]
- [14].Melodelima D, N’Djin WA, Miller NR, Bamber JC, and Chapelon JY, “Comparative study of the effects of respiratory motion on in-vivo hifu treatments in the liver,” in 2009 IEEE International Ultrasonics Symposium. IEEE, 2009, pp. 1314–1317.
- [15].Khokhlova TD, Wang Y-N, Simon JC, Cunitz BW, Starr F, Paun M, Crum LA, Bailey MR, and Khokhlova VA, “Ultrasoundguided tissue fractionation by high intensity focused ultrasound in an in vivo porcine liver model,” Proceedings of the National Academy of Sciences, vol. 111, no. 22, pp. 8161–8166, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kim Y, Vlaisavljevich E, Owens G, Allen S, Cain C, and Xu Z, “In vivo transcostal histotripsy therapy without aberration correction,” Physics in Medicine & Biology, vol. 59, no. 11, p. 2553, 2014. [DOI] [PubMed] [Google Scholar]
- [17].Vlaisavljevich E, Owens G, Lundt J, Teofilovic D, Ives K, Duryea A, Bertolina J, Welling TH, and Xu Z, “Non-invasive liver ablation using histotripsy: preclinical safety study in an in vivo porcine model,” Ultrasound in Medicine & Biology, vol. 43, no. 6, pp. 1237–1251, 2017. [DOI] [PubMed] [Google Scholar]
- [18].Khokhlova TD, Schade GR, Wang Y-N, Buravkov SV, Chernikov VP, Simon JC, Starr F, Maxwell AD, Bailey MR, Kreider W. et al. , “Pilot in vivo studies on transcutaneous boiling histotripsy in porcine liver and kidney,” Scientific reports, vol. 9, no. 1, pp. 1–12, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Okada A, Murakami T, Mikami K, Onishi H, Tanigawa N, Marukawa T, and Nakamura H, “A case of hepatocellular carcinoma treated by mr-guided focused ultrasound ablation with respiratory gating,” Magnetic Resonance in Medical Sciences, vol. 5, no. 3, pp. 167–171, 2006. [DOI] [PubMed] [Google Scholar]
- [20].Wu F, Wang Z-B, Chen W-Z, Zou J-Z, Bai J, Zhu H, Li K-Q, Jin C-B, Xie F-L, and Su H-B, “Advanced hepatocellular carcinoma: treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization,” Radiology, vol. 235, no. 2, pp. 659–667, 2005. [DOI] [PubMed] [Google Scholar]
- [21].Muller A, Petrusca L, Auboiroux V, Valette P, Salomir R, and Cotton F, “Management of respiratory motion in extracorporeal high-intensity focused ultrasound treatment in upper abdominal organs: current status and perspectives,” Cardiovascular and interventional radiology, vol. 36, no. 6, pp. 1464–1476, 2013. [DOI] [PubMed] [Google Scholar]
- [22].Ginhoux R, Gangloff J, de Mathelin M, Soler L, Sanchez MMA, and Marescaux J, “Active filtering of physiological motion in robotized surgery using predictive control,” IEEE Transactions on Robotics, vol. 21, no. 1, pp. 67–79, 2005. [Google Scholar]
- [23].Auboiroux V, Petrusca L, Viallon M, Muller A, Terraz S, Breguet R, Montet X, Becker CD, and Salomir R, “Respiratory-gated mrghifu in upper abdomen using an mr-compatible in-bore digital camera,” BioMed Research International, vol. 2014, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].De Senneville BD, Mougenot C, and Moonen CT, “Real-time adaptive methods for treatment of mobile organs by mri-controlled high-intensity focused ultrasound,” Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, vol. 57, no. 2, pp. 319–330, 2007. [DOI] [PubMed] [Google Scholar]
- [25].Stemkens B, Tijssen RH, De Senneville BD, Lagendijk JJ, and Van Den Berg CA, “Image-driven, model-based 3d abdominal motion estimation for mr-guided radiotherapy,” Physics in Medicine & Biology, vol. 61, no. 14, p. 5335, 2016. [DOI] [PubMed] [Google Scholar]
- [26].Diodato A, Cafarelli A, Schiappacasse A, Tognarelli S, Ciuti G, and Menciassi A, “Motion compensation with skin contact control for high intensity focused ultrasound surgery in moving organs,” Physics in Medicine & Biology, vol. 63, no. 3, p. 035017, 2018. [DOI] [PubMed] [Google Scholar]
- [27].Krupa A, Fichtinger G, and Hager GD, “Real-time motion stabilization with b-mode ultrasound using image speckle information and visual servoing,” The International Journal of Robotics Research, vol. 28, no. 10, pp. 1334–1354, 2009. [Google Scholar]
- [28].Chanel L-A, Nageotte F, Vappou J, Luo J, Cuvillon L, and de Mathelin M, “Robotized high intensity focused ultrasound (hifu) system for treatment of mobile organs using motion tracking by ultrasound imaging: An in vitro study,” in 2015. 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE, 2015, pp. 2571–2575. [DOI] [PubMed] [Google Scholar]
- [29].Seo J, Koizumi N, Mitsuishi M, and Sugita N, “Ultrasound image based visual servoing for moving target ablation by high intensity focused ultrasound,” The International Journal of Medical Robotics and Computer Assisted Surgery, vol. 13, no. 4, p. e1793, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pernot M, Tanter M, and Fink M, “3-d real-time motion correction in high-intensity focused ultrasound therapy,” Ultrasound in medicine & biology, vol. 30, no. 9, pp. 1239–1249, 2004. [DOI] [PubMed] [Google Scholar]
- [31].Gee AH, Housden RJ, Hassenpflug P, Treece GM, and Prager RW, “Sensorless freehand 3d ultrasound in real tissue: speckle decorrelation without fully developed speckle,” Medical image analysis, vol. 10, no. 2, pp. 137–149, 2006. [DOI] [PubMed] [Google Scholar]
- [32].Seo J, Koizumi N, Funamoto T, Sugita N, Yoshinaka K, Nomiya A, Homma Y, Matsumoto Y, and Mitsuishi M, “Biplane us-guided real-time volumetric target pose estimation method for theragnostic hifu system.” J. Robotics Mechatronics, vol. 23, no. 3, pp. 400–407, 2011. [Google Scholar]
- [33].Seo J, Koizumi N, Funamoto T, Sugita N, Yoshinaka K, Nomiya A, Homma Y, Matsumoto Y, and Mitsuishi M, “Visual servoing for a us-guided therapeutic hifu system by coagulated lesion tracking: a phantom study,” The International Journal of Medical Robotics and Computer Assisted Surgery, vol. 7, no. 2, pp. 237–247, 2011. [DOI] [PubMed] [Google Scholar]
- [34].Clifford MA, Banovac F, Levy E, and Cleary K, “Assessment of hepatic motion secondary to respiration for computer assisted interventions,” Computer Aided Surgery, vol. 7, no. 5, pp. 291–299, 2002. [DOI] [PubMed] [Google Scholar]
- [35].Srimathveeravalli G, Leger J, Ezell P, Maybody M, Gutta N, and Solomon S, “A study of porcine liver motion during respiration for improving targeting in image-guided needle placements,” International journal of computer assisted radiology and surgery, vol. 8, no. 1, pp. 15–27, 2013. [DOI] [PubMed] [Google Scholar]
- [36].Khokhlova VA, Yuldashev PV, Rosnitskiy PB, Maxwell AD, Kreider W, Bailey MR, and Sapozhnikov OA, “Design of hifu transducers to generate specific nonlinear ultrasound fields,” Physics Procedia, vol. 87, pp. 132–138, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ghanem MA, Maxwell AD, Kreider W, Cunitz BW, Khokhlova VA, Sapozhnikov OA, and Bailey MR, “Field characterization and compensation of vibrational nonuniformity for a 256-element focused ultrasound phased array,” IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, vol. 65, no. 9, pp. 1618–1630, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bawiec CR, Khokhlova TD, Sapozhnikov OA, Rosnitskiy PB, Cunitz BW, Ghanem MA, Hunter C, Kreider W, Schade GR, Yuldashev PV et al. , “A prototype therapy system for boiling histotripsy in abdominal targets based on a 256-element spiral array,” IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, 2020. [DOI] [PMC free article] [PubMed]
- [39].Fujii T, Koizumi N, Kayasuga A, Lee D, Tsukihara H, Fukuda H, Yoshinaka K, Azuma T, Miyazaki H, Sugita N. et al. , “Servoing performance enhancement via a respiratory organ motion prediction model for a non-invasive ultrasound theragnostic system,” Journal of Robotics and Mechatronics, vol. 29, no. 2, pp. 434–446, 2017. [Google Scholar]
- [40].Maier-Hein L, Tekbas A, Franz AM, Tetzlaff R, Müller SA, Pianka F, Wolf I, Kauczor H-U, Schmied BM, and Meinzer H-P, “On combining internal and external fiducials for liver motion compensation,” Computer Aided Surgery, vol. 13, no. 6, pp. 369–376, 2008. [DOI] [PubMed] [Google Scholar]
- [41].Fehrenbach J, Masmoudi M, and Melodelima D, “Low dimensional optimization for in vivo real-time porcine liver motion estimation using ultrasound imaging,” Ultrasonics, vol. 50, no. 1, pp. 44–51, 2010. [DOI] [PubMed] [Google Scholar]
- [42].Ichiji K, Homma N, Sakai M, Narita Y, Takai Y, Zhang X, Abe M, Sugita N, and Yoshizawa M, “A time-varying seasonal autoregressive model-based prediction of respiratory motion for tumor following radiotherapy,” Computational and mathematical methods in medicine, vol. 2013, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hatada K, Hirata K, and Sato T, “Power assisting method for almost-periodic motions based on motion prediction,” in 2015. IEEE International Conference on Industrial Technology (ICIT). IEEE, 2015, pp. 417–422. [Google Scholar]
- [44].Cejnek M, “Python adaptive signal processing,” http://matousc89.github.io/padasip/.
- [45].Wang Y-N, Khokhlova T, Bailey M, Hwang JH, and Khokhlova V, “Histological and biochemical analysis of mechanical and thermal bioeffects in boiling histotripsy lesions induced by high intensity focused ultrasound,” Ultrasound in medicine & biology, vol. 39, no. 3, pp. 424–438, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Schneider CA, Rasband WS, and Eliceiri KW, “Nih image to imagej: 25 years of image analysis,” Nature methods, vol. 9, no. 7, pp. 671–675, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Khokhlova TD, Haider YA, Maxwell AD, Kreider W, Bailey MR, and Khokhlova VA, “Dependence of boiling histotripsy treatment efficiency on hifu frequency and focal pressure levels,” Ultrasound in medicine & biology, vol. 43, no. 9, pp. 1975–1985, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.