Introduction
Comparison of the benefits from medical interventions across multiple diseases is possible if gains in health are measured in a common metric. Cost per quality-adjusted life-year saved (QALY) is the standard metric recommended by the U.S. Panel on Cost-Effectiveness in Health and Medicine.(1) The theoretical basis for QALYs is the utility, which measures preferences (a term used interchangeably for ‘utilities’ in this paper) for health states on a 0 (dead) to 1 (perfect health) scale. Health status measures such as the SF-36 include only a psychometric-based rank-ordering of health states, not equivalent to a utility scale.(2) Despite the importance of the QALY in cost-utility analyses, utilities for lung cancer have been derived by combining multiple studies with dissimilar patient types and a variety of methodologies for transforming health status measures into proxies for utilities.(3-5)
The U.S. National Cancer Institute and the Department of Veterans Affairs co-funded the Cancer Care Outcomes Research and Surveillance Consortium (CanCORS) to investigate the quality of cancer care and patient outcomes. Beginning in September of 2003, a cohort of 5,015 patients with newly diagnosed lung cancer was recruited from patients receiving care at one of the participating health care systems, which included 5 health maintenance organizations, Veterans Administration medical centers, California counties and the states of Iowa and Alabama.(6) Patients were initially surveyed at approximately 4-6 months post-diagnosis, and medical records were collected to capture details of treatment and disease stage. A follow-up survey was administered to patients at 11-13 months post-diagnosis.
CanCORS provides a unique opportunity to characterize utilities among population-based samples of well-characterized, newly-diagnosed patients, specific to stage at diagnosis and treatment. An advantage of the CanCORS cohort is the large size and standardized data collection, and the inclusion of quality of life indexes, the EQ-5D and SF-6D (extracted from Sf-12v2), in the baseline and follow-up surveys completed by patients. The EQ-5D measures health-related quality of life in five domains, or attributes (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression).(7) In this analysis EQ-5D health states were those experienced by patients shortly after lung cancer diagnosis and 11-13 months after diagnosis. Each combination of attribute levels from the EQ-5D and SF-6D can be assigned preexisting community utilities, yielding utilities for disease states from a societal perspective.(8) The EQ-5D also includes a feeling thermometer visual analog scale for individual current health status (EQ-VAS). The SF-6D uses seven questions from six dimensions (physical functioning, role limitation, social functioning, pain, mental health, and vitality) in the SF-12v2 to calculate health utility.
In this paper, we report on the relationship between disease severity, initial treatment, and utilities for health states in patients with lung cancer. Additionally, a catalog of utilities and VAS health status values is provided for use in comparative effectiveness (or cost-utility) analyses of lung cancer control interventions.
Methods
This study was approved by the institutional review boards of the participating institutions.
Description of patients and data
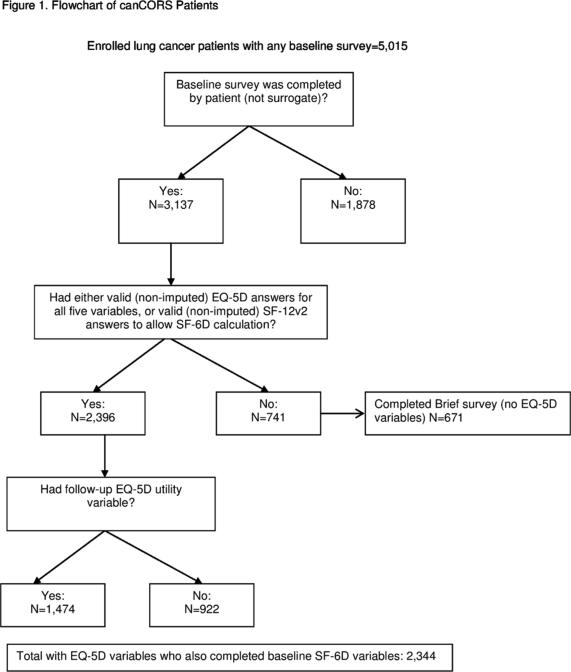
Data for this study were collected as part of a national study of variations in care and outcomes of care for patients with lung cancer undertaken by the CanCORS Consortium.(6) CanCORS assessed the medical care and outcomes of population-based cohorts totaling more than 10,000 patients initially diagnosed with lung cancer in 2003-2005 in Northern California, Los Angeles County, North Carolina, Iowa, or Alabama, or who received care in one of 5 large health maintenance organizations or 15 Veterans Administration Health Care System (VA) study sites. Trained interviewers used computer-assisted interviewing software to survey patients approximately 4-6 months after diagnosis about their experiences with their cancer and their health care. Survey instruments were translated into Spanish and Chinese and administered by bilingual interviewers for patients who preferred these languages. The American Association for Public Opinion Research response rate was 51.0% and the cooperation rate was 59.9%. The patient survey (available at http://www.cancors.org/public) obtained information about a patient's symptoms, quality of life, decision making process, and treatment.(9) We restricted this analysis to the 2,396 patients who were alive and completed the full baseline interview themselves (because the brief and surrogate versions of the survey did not include the EQ-5D questions or the seven SF-6D questions we extracted from the SF-12v2), and who had no missing data on these items. Patients (n=1,474) who participated in a follow-up survey approximately one year after diagnosis were included in an analysis that explored the change in EQ-5D scores over time. Data regarding treatments and cancer stage were obtained by abstracting patients’ medical records (available for 87% of patients). When medical record data were missing, information on treatments received from the survey and staging data from cancer registries were used.
Definition of disease and treatment states
For this analysis, we established mutually exclusive categories of disease and treatment states for lung cancer patients. Based on information available from the medical records collected as part of CanCORS, we mapped all patients included in the analysis to the appropriate disease and treatment states. Patients with medical record data were categorized according their appropriate AJCC stage; patients with only cancer registry staging data identified as local (n=23), regional (n=19), and extensive (n=28) were grouped with stage II, stage III, and stage IV patients, respectively. Within each stage, patients were then categorized into one of the following treatment categories: surgery alone, surgery with adjuvant chemotherapy, surgery with adjuvant chemoradiotherapy, radiotherapy alone, chemoradiotherapy, chemotherapy alone, and no active treatment. Sub-categories of types of surgery and class of chemotherapy were also defined.
To investigate whether the timing of the survey in relation to completing treatment resulted in a difference of utilities, we calculated mean (SD) utility for patients who were still on active treatment or who had undergone surgery within thirty days prior to completion of the CanCORS survey and compared it to the mean (SD) utility for patients who completed treatment (or had no treatment) more than 30 days prior to survey completion. Thirty days was chosen because it was the median time between baseline survey completion and treatment.
Calculating utilities from EQ-5D scores
We calculated community-weight utilities for each of the 243 possible health states using the patient-provided scores for each of the 5 EQ-5D attributes and the algorithm provided by U.S. Agency for Healthcare Research and Quality (AHRQ).(10) Each EQ-5D-3L version attribute (mobility, self-care, usual activities, pain/discomfort, anxiety/depression) is scored as 1, 2, or 3 to indicate the level of severity on that attribute; for example, a score of ‘22222’ would indicate some problems in each attribute. The AHRQ-provided algorithm was based on a random effects model (specifically, the ‘D1 model’) fit to time-tradeoff values provided in 2002 by a probability sample of U.S. adults (civilian, non-institutionalized).(8, 11) The range of utilities possible from this model is -0.11 to 1.00 (i.e., includes states worse than dead). The EQ-5D asks respondents about health “today”.
We also calculated the mean scores for the EQ-5D instrument's accompanying visual analog scale (EQVAS) (range 0 to 100 with scale anchors of worst and best health state imaginable, respectively), for the item “rate your own health today”.
Calculating utilities from SF-12v2 scores
We calculated community-weighted utilities for each state using the algorithm created by and licensed from Brazier et al. which utilizes seven of the SF-12v2 items.(12) Recall time was four weeks. The six dimensions of the SF-6D (physical functioning, role limitation, social functioning, pain, mental health, and vitality) are scored on 3-5 levels to indicate level of severity. Brazier's algorithm is based on preference weights obtained using the standard gamble technique in a sample of the U.K. population. The range of utilities possible from this model is 0.34 to 1.00.
Summated EORTC L13 scores
Eight questions from the EORTC LC-13 were included as part of the baseline survey, pertaining to documenting cough, hemoptysis, trouble swallowing, pain or tingling in hands or feet, sore mouth or tongue, and shortness of breath while resting, walking, and climbing stairs over 4 weeks.(13) The scores were added to create a summated score,(14) with a maximum of 32. Higher scores indicated worse health.
Longitudinal changes in utilities
A subset (n=1,474) of patients completed follow-up EQ-5D questionnaires between 11 and 13 months after diagnosis. We compared the mean baseline EQ-5D utilities of these patients with responses at both time points to their mean utilities on the follow-up survey, stratified by patient characteristics, stage at diagnosis, and treatment.
Statistical analysis
We used χ2 tests to compare characteristics of patients included in this analysis with those excluded for missing data. Utilities can be skewed due to ceiling or floor effects, so we tested for normality (Kolmogorov-Smirnov) and report medians and interquartile ranges of utilities stratified by patient characteristics, in addition to means and standard deviations. To compare utilities/scores across disease/treatment states, non-parametric Kruskal-Wallis one-way analysis of variance and Mann-Whitney U rank-sum tests were performed. For subgroups with statistically significant differences, pairwise comparisons were also performed. Categories based on scores from fewer than 10 patients were not reported. For follow-up utilities, we also reported means (SD) and medians (range) and assessed changes in utility by patient characteristic for participants surveyed twice (paired t-test).
We estimated the degree of correlation of utilities to patient-reported VAS scores using non-parametric Spearman correlations. Tests of agreement between EQ-5D and SF-6D were performed using non-parametric Spearman correlation and the fixed effect model intra-class correlation coefficient (ICC).
We also determined mean and median utilities by disease stage and detailed treatment categories such as pneumonectomy, lobectomy, and wedge resection for surgery and platinum versus non-platinum chemotherapy. We compared utility values for surgical patients with respect to timing of survey completion and surgery (two sample t-test and Wilcoxon Two-sample test).
All statistical tests were performed using SAS 9.2 (Cary, NC, USA). A p-value of <0.05 was considered statistically significant where adjustments were not indicated.
Results
Patient Demographics
Of the 2,396 lung cancer patients included in our analysis, 52% were male; 75% were white; and 58% were aged 65 or older (Table 1). Compared to patients included in our analysis, patients in the CanCORS cohort who did not have EQ-5D data were more likely to be male (p<0.001), non-white (p<0.001) older (p<0.001), and to have been diagnosed at a later stage of disease (p<0.001), a reflection of the fact that the patients with more serious illness or greater disability had surrogates complete the survey on their behalf or were only able to participate in the brief survey.
Table 1.
Patient Characteristics
| Demographic | Patients with EQ-5D N (%) | Patients without EQ-5D N (%) | p-value† |
|---|---|---|---|
| All | 2396 (100%) | 2619 (100%) | |
| Sex | |||
| Male | 1242 (51.84%) | 1628 (62.16%) | <0.0001 |
| Female | 1154 (48.16%) | 991 (37.84%) | |
| Race/Ethnicity | |||
| Caucasian | 1788 (74.62%) | 1826 (69.72%) | <0.0001 |
| Latino | 107 (4.47%) | 176 (6.72%) | |
| African American | 286 (11.94%) | 321 (12.26%) | |
| Asian | 80 (3.34%) | 144 (5.50%) | |
| Other | 135 (5.63%) | 124 (4.73%) | |
| Missing | 0 (0%) | 28 (1.07%) | |
| Age | |||
| <=54 | 353 (14.73%) | 213 (8.14%) | <0.0001 |
| 55-59 | 305 (12.73%) | 250 (9.55%) | |
| 60-64 | 359 (14.98%) | 295 (11.27%) | |
| 65-69 | 410 (17.11%) | 416 (15.90%) | |
| 70-74 | 403 (16.82%) | 483 (18.46%) | |
| 75-79 | 323 (13.48%) | 468 (17.88%) | |
| 80+ | 243 (10.14%) | 492 (18.80%) | |
| Missing | 0 (0%) | 2 (0.08%) | |
| Stage at Diagnosis | |||
| I | 750 (31.30%) | 394 (15.04%) | <0.0001 |
| II | 232 (9.68%) | 143 (5.46%) | |
| III | 649 (27.09%) | 665 (25.39%) | |
| IV | 628 (26.21%) | 1249 (47.69%) | |
| Unknown/Missing | 137 (5.72%) | 168 (6.41%) | |
| Histology | |||
| NSCLC | 2025 (84.52%) | 2077 (79.31%) | <0.0001 |
| SCLC | 264 (11.02%) | 349 (13.33%) | |
| Missing | 107 (4.53%) | 193 (7.37%) | |
| Treatment* | |||
| Surgery | 563 (23.50%) | ||
| Chemotherapy | 360 (15.03%) | ||
| Radiotherapy | 126 (5.26%) | ||
| Surgery w/Chemotherapy | 271 (11.31 %) | ||
| Surgery w/Radiotherapy | 63 (2.63%) | ||
| Chemoradiotherapy | 679 (28.34%) | ||
| Surgery w/Chemo/Rad | 207 (8.64%) | ||
| No Treatment | 127 (5.30%) | ||
| Comorbidity | |||
| None | 413 (17.24%) | 368 (14.05%) | <0.0001 |
| Mild | 744 (31.05%) | 714 (27.26%) | |
| Moderate | 412 (17.20%) | 475 (18.14%) | |
| Severe | 375 (15.65%) | 545 (20.81%) | |
| Unknown/Missing | 452 (18.86%) | 517 (19.74%) |
Treatment category was not determined for patients with no reported EQ-5D scores. Since treatment is often based on stage, similar differences between EQ-5D and no EQ-5D groups are expected.
χ2 test
Comparison of EQ-5D-derived Utilities, SF-6D-derived Utilities, EORTC L13 scores, and EQ-VAS Ratings
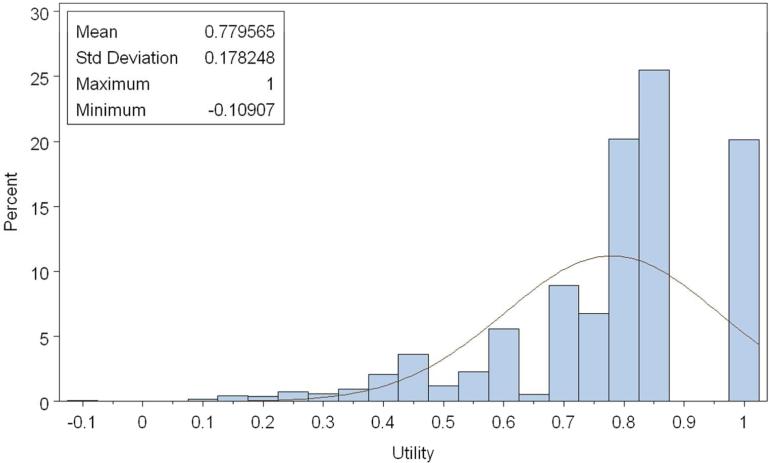
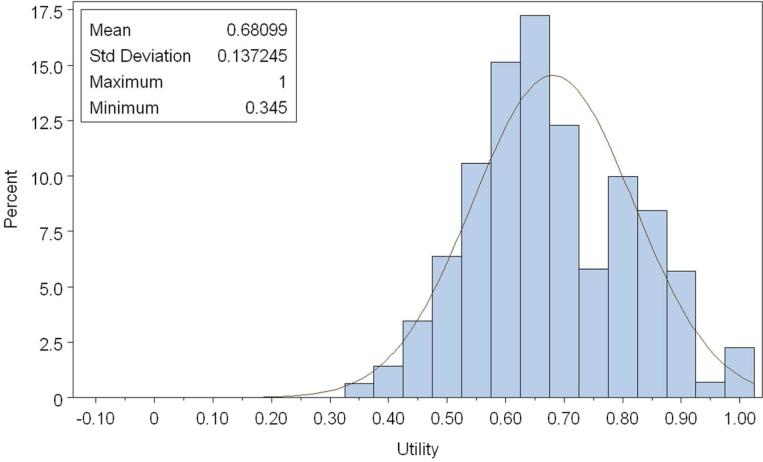
The overall utility based on EQ-5D (Figure 2a and Table 2a) was 0.78 (standard deviation [SD], 0.18, median 0.82, range, -0.11 to 1.00). The overall utility based on SF-6D (Figure 2b and Table 2b) was 0.68 (SD 0.14, median 0.66, range, 0.34 to 1.00). The overall patient-reported health rating mean based on the EQ-VAS was 65.39 (SD 21.30) (see Appendix).
Figure 2a.
Distribution of Overall utility (EQ-5D). Normal curve fit is superimposed. Kolmogorov-Smirnov test for goodness of fit indicates a statistically significant (p<0.01) departure from normality. 298×198mm (100 × 100 DPI)
Table 2a.
Variation in Utilities based on EQ-5D at Baseline Survey
| Demographic | Patients with EQ-5D N (%) | EQ-5D Utilities Mean (SD) | EQ-5D Utilities Median (IQR) |
|---|---|---|---|
| All | 2396 (100%) | 0.78 (0.18) | 0.82 (0.15) |
| Sex | |||
| Male | 1242 (51.84%) | 0.78 (0.17) | 0.82 (0.15) |
| Female | 1154 (48.16%) | 0.77 (0.18) | 0.81 (0.15) |
| p-value* | n.s | ||
| Race/Ethnicity | |||
| Caucasian | 1788 (74.62%) | 0.79 (0.17) | 0.82 (0.15) |
| Latino | 107 (4.47%) | 0.76 (0.19) | 0.80 (0.26) |
| African American | 286 (11.94%) | 0.75 (0.19) | 0.80 (0.17) |
| Asian | 80 (3.34%) | 0.83 (0.17) | 0.81 (0.23) |
| Other | 135 (5.63%) | 0.74 (0.22) | 0.80 (0.15) |
| Missing | 0 (0%) | ||
| p-value† | p=0.0012 | ||
| Age | |||
| <=54 | 353 (14.73%) | 0.72 (0.21) | 0.78 (0.25) |
| 55-59 | 305 (12.73%) | 0.77 (0.17) | 0.81 (0.14) |
| 60-64 | 359 (14.98%) | 0.78 (0.17) | 0.81 (0.14) |
| 65-69 | 410 (17.11%) | 0.80 (0.18) | 0.82 (0.29) |
| 70-74 | 403 (16.82%) | 0.79 (0.18) | 0.83 (0.11) |
| 75-79 | 323 (13.48%) | 0.79 (0.16) | 0.82 (0.15) |
| 80+ | 243 (10.14%) | 0.82 (0.15) | 0.84 (0.08) |
| Missing | 0 (0%) | ||
| p-value† | <0.0001 | ||
| Stage at Diagnosis | |||
| I | 750 (31.30%) | 0.81 (0.17) | 0.83 (0.23) |
| II | 232 (9.68%) | 0.77 (0.17) | 0.81 (0.14) |
| III | 649 (27.09%) | 0.77 (0.18) | 0.81 (0.15) |
| IV | 628 (26.21%) | 0.76 (0.19) | 0.81 (0.15) |
| Unknown/Missing | 137 (5.72%) | 0.76 (0.19) | 0.81 (0.17) |
| p-value† | <0.0001 | ||
| Histology | |||
| NSCLC | 2025 (84.52%) | 0.78 (0.17) | 0.82 (0.15) |
| SCLC | 264 (11.02%) | 0.76 (0.19) | 0.82 (0.15) |
| Missing | 107 (4.53%) | 0.74 (0.22) | 0.81 (0.25) |
| p-value† | n.s. | ||
| Treatment | |||
| Surgery | 563 (23.50%) | 0.81 (0.16) | 0.83 (0.24) |
| Chemotherapy | 360 (15.03%) | 0.79 (0.19) | 0.82 (0.15) |
| Radiotherapy | 126 (5.26%) | 0.75 (0.19) | 0.81 (0.16) |
| Surgery w/Chemotherapy | 271 (11.31 %) | 0.79 (0.16) | 0.82 (0.08) |
| Surgery w/Radiotherapy | 63 (2.63%) | 0.72 (0.20) | 0.78 (0.27) |
| Chemoradiotherapy | 679 (28.34%) | 0.77 (0.18) | 0.81 (0.15) |
| Surgery w/Chemo/Rad | 207 (8.64%) | 0.76 (0.19) | 0.80 (0.14) |
| No Treatment | 127 (5.30%) | 0.79 (0.17) | 0.82 (0.11) |
| p-value† | <0.0001 | ||
| Comorbidity | |||
| None | 413 (17.24%) | 0.79 (0.17) | 0.82 (0.09) |
| Mild | 744 (31.05%) | 0.78 (0.18) | 0.82 (0.15) |
| Moderate | 412 (17.20%) | 0.77 (0.18) | 0.81 (0.14) |
| Severe | 375 (15.65%) | 0.78 (0.18) | 0.82 (0.15) |
| Unknown/Missing | 452 (18.86%) | 0.78 (0.18) | 0.82 (0.15) |
| p-value† | n.s. |
Mann-Whitney U test
Kruskal-Wallis test
Figure 2b.
Distribution of Overall utility (SF-6D) Normal curve fit is superimposed. Kolmogorov-Smirnov test for goodness of fit indicates statistically a significant (p<0.01) departure from normality. 298×198mm (100 × 100 DPI)
Table 2b.
Variation in Utilities based on SF-6D at Baseline Survey
| Demographic | Patients with SF-6D N (%) | SF-6D Utilities Mean (SD) | SF-6D Utilities Median (IQR) |
|---|---|---|---|
|
| |||
| All | 2344 (100%) | 0.68 (0.14) | 0.66 (0.20) |
|
| |||
| Sex | |||
| Male | 1211 (51.66%) | 0.69 (0.13) | 0.66 (0.15) |
| Female | 1154 (48.34%) | 0.68 (0.14) | 0.66 (0.20) |
| p-value* | n.s | ||
|
| |||
| Race/Ethnicity | |||
| Caucasian | 1750 (74.62%) | 0.68 (0.14) | 0.66 (0.20) |
| Latino | 104 (4.44%) | 0.69 (0.15) | 0.67 (0.20) |
| African American | 277 (11.82%) | 0.68 (0.14) | 0.66 (0.20) |
| Asian | 80 (3.41 %) | 0.69 (0.12) | 0.66 (0.19) |
| Other | 133 (5.67%) | 0.66 (0.14) | 0.66 (0.19) |
| p-value** | n.s | ||
|
| |||
| Age | |||
| <=54 | 353 (15.06%) | 0.63 (0.14) | 0.62 (0.18) |
| 55-59 | 299 (12.76%) | 0.66 (0.13) | 0.64 (0.15) |
| 60-64 | 352 (15.02%) | 0.67 (0.14) | 0.66 (0.20) |
| 65-69 | 402 (17.15%) | 0.69 (0.14) | 0.66 (0.20) |
| 70-74 | 393 (16.77%) | 0.70 (0.14) | 0.68 (0.20) |
| 75-79 | 313 (13.35%) | 0.69 (0.13) | 0.67 (0.20) |
| 80+ | 232 (9.90%) | 0.72 (0.13) | 0.72 (0.24) |
| p-value** | <0.0001 | ||
|
| |||
| Stage at Diagnosis | |||
| I | 730 (31.30%) | 0.71 (0.14) | 0.70 (0.22) |
| II | 230 (9.68%) | 0.68 (0.13) | 0.66 (0.21) |
| III | 638 (27.09%) | 0.67 (0.13) | 0.66 (0.17) |
| IV | 612 (26.21%) | 0.66 (0.13) | 0.66 (0.17) |
| Unknown/Missing | 134 (5.72%) | 0.68 (0.14) | 0.67 (0.20) |
| p-value** | <0.0001 | ||
|
| |||
| Histology | |||
| NSCLC | 1979 (84.44%) | 0.68 (0.14) | 0.66 (0.23) |
| SCLC | 258 (11.01 %) | 0.68 (0.14) | 0.66 (0.20) |
| Missing | 107 (4.56%) | 0.66 (0.15) | 0.64 (0.23) |
| p-value** | n.s | ||
|
| |||
| Treatment | |||
| Surgery | 550 (23.46%) | 0.72 (0.15) | 0.72 (0.26) |
| Chemotherapy | 350 (14.93%) | 0.68 (0.14) | 0.66 (0.20) |
| Radiotherapy | 121 (5.16%) | 0.67 (0.12) | 0.66 (0.15) |
| Surgery w/Chemotherapy | 264 (11.26%) | 0.67 (0.12) | 0.66 (0.17) |
| Surgery w/Radiotherapy | 60 (2.56%) | 0.69 (0.15) | 0.68 (0.18) |
| Chemoradiotherapy | 669 (28.54%) | 0.66 (0.13) | 0.66 (0.16) |
| Surgery w/Chemo/Rad | 206 (8.79%) | 0.65 (0.14) | 0.63 (0.22) |
| No Treatment | 124 (5.29%) | 0.70 (0.13) | 0.67 (0.17) |
| p-value** | <0.0001 | ||
|
| |||
| Comorbidity | |||
| None | 409 (17.44%) | 0.69 (0.13) | 0.67 (0.21) |
| Mild | 731 (31.19%) | 0.68 (0.13) | 0.66 (0.20) |
| Moderate | 402 (17.15%) | 0.67 (0.14) | 0.66 (0.21) |
| Severe | 364 (15.53%) | 0.67 (0.14) | 0.66 (0.21) |
| Unknown/Missing | 438 (18.69%) | 0.68 (0.14) | 0.66 (0.19) |
| p-value** | n.s | ||
Mann-Whitney U test
Kruskal-Wallis test
A ceiling effect was observed in EQ-5D utilities, with 20% of lung cancer patients reporting perfect scores (for a utility of 1.0); the overall utilities were not normally distributed (p <0.01, Kolmogorov-Smirnov test)and were skewed towards perfect scores (Figure 2a). Ceiling effects for the individual domains were 60%, 87.7%, 42.3%, 45.1%, and 61.7% for mobility, self-care, usual activities, pain, and anxiety, respectively. Perfect SF-6D utilities (ceiling effect) were found in 2.3% of lung cancer patients; the overall utilities were centered around 0.66 (Figure 2b) but were not normally distributed (p <0.01, Kolmogorov-Smirnov test). Stage I patients had the highest ceiling effect (25.5%, EQ-5D; 3.4% SF-6D). SF-6D utilities were consistently lower than EQ-5D utilities for all subgroups, but showed similar trends. There was no substantial floor effect found in SF-6D utilities, with only 1% of patients reporting a utility of 0.40 or less (Figure 2b). The floor effects of individual domains were 37.9%, 50.3%, 7.5%, 5.3%, 2.6%, and 18.1% for physical, role function, social, pain, mental, and vital domains, respectively. Among patients with an EQ-5D in the lowest quintile, 4.5% had a SF-6D utility of 0.4 or less. The mean SF-6D utility among these patients was 0.56, with a range of 0.34-0.86.
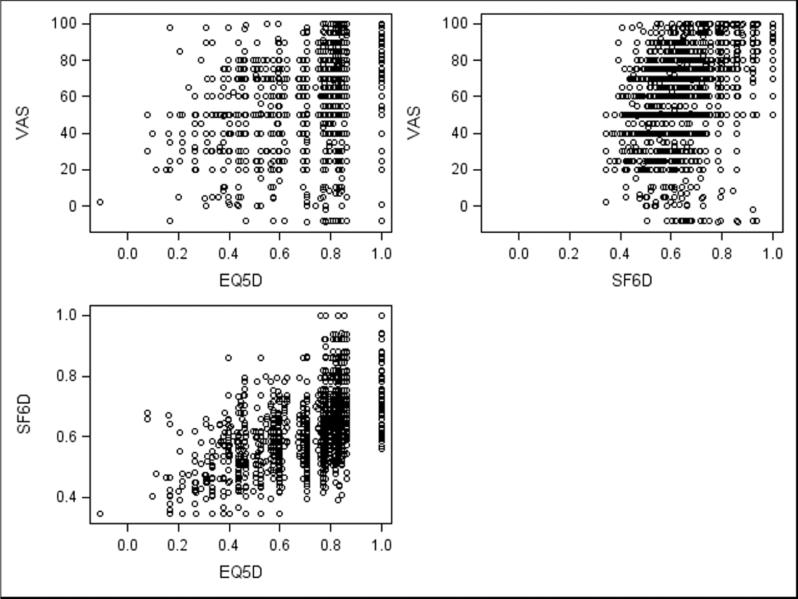
The mean and median scores from the EQ-5D and SF-6D had a statistically significant difference (mean 0.10, 95% CI 0.09-0.14, median 0.12, p<0.0001). This statistically significant difference was also demonstrated in paired analyses of all subgroups. Overall, there was a strong correlation between utilities based on EQ-5D and utilities based on SF-6D (Spearman r=0.67, p<0.0001, Figure 3). The level of agreement between the EQ-5D and SF-6D was moderate (fixed model ICC=0.48). EQ-VAS health ratings showed similar patterns as utilities and were moderately correlated with EQ-5D-derived utilities (Spearman r=0.48, p<0.0001, Figure 3) and slightly more strongly correlated with SF-6D-derived utilities (Spearman r=0.51, p<0.0001, Figure 3).
Figure 3.
Correlations between measures. VAS and EQ-5D: r=0.48 VAS and SF-6D: r=0.51 SF-6D and EQ-5D: r=0.67 260×195mm (100 × 100 DPI)
2,379 of the 2,396 patients also completed the eight questions from the EORTC LC-13(13). Mean EORTC summated scores were 13.68 out of a maximum possible total of 32 (worse health), with a standard deviation of 4.84 (Appendix Table 3). The mean score was 13.68 and the median score was 13.0. When stratified by stage, patients with stage I disease had the lowest EORTC score (better health), with a score of 12.75 (SD 4.71). The EORTC scores of patients with later disease were 13.86 (SD 4.60), 14.31 (SD 4.89), 14.00 (SD 4.85), and 13.93 (SD 4.96) for stages II, III, IV and unknown, respectively. Scores among the stage subgroups were shown to be statistically significant (p-value <0.0001). Pairwise comparisons showed that the significance was due to a statistical difference between stage I and each of the later stages. In addition, pairwise comparisons showed that the statistically significant difference in treatment were driven by differences between the surgery only treatment group (mean 12.44, SD 4.62; median 12.0) and other treatments, which had a mean score of 13.15 or greater, For age, the younger age groups (less than 60) were statistically significant vs older age groups (greater than 60) in a pairwise comparison.
Variation in Utilities and Health Ratings
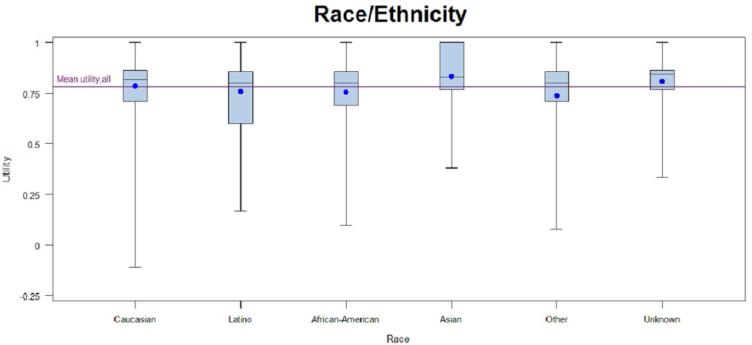
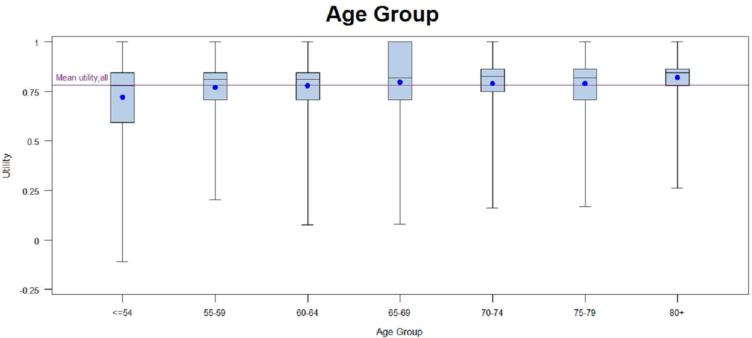
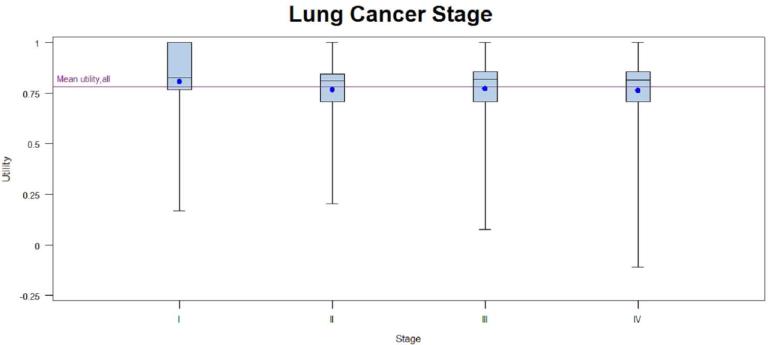
Patients with early-stage disease were associated with higher utilities regardless of scoring algorithm (Tables 2a and 2b). Patients who had surgery only, chemotherapy only, or surgery and chemotherapy had higher EQ-5D scores when compared to patients with other treatment regimens, or with no treatment. Patients with any radiation treatment were more likely (p<0.0001) to report lower EQ-5D scores compared to patients receiving no treatment as well as patients with any combination of surgery and/or chemotherapy. Asian race and older age(80 years and older) were associated with higher utilities (based on EQ-5D) than other races and age groups, and ages 80 years and older were also associated with higher utilities based on SF-6D. The small number of patients with no treatment (n=127) also had higher utilities; however; many patients who never receive treatment did not complete the full survey as they were too ill for treatment and had surrogates complete the survey on their behalf. Patients with no comorbidities had insignificantly higher mean utilities than patients with any comorbidity when using either scoring algorithm but no variation was observed across comorbidity subgroups. There were statistically significant (p-value <0.05) differences in utilities based on EQ-5D across the racial, age, stage, and treatment subgroups but not sex or histology. There were statistically significant (p-value<0.05) differences in utilities based on SF-6D across the age, stage, and treatment subgroups. Utilities based on SF-6D were not significantly different across the sex, race, histology, or comorbidity subgroups. When statistically significant differences across subgroups were seen, they appeared to be primarily driven by one specific group with high utilities (Asian, <= 54, stage I, and surgery; see Figures in Appendix). EQ-5D VAS scores stratified by subgroups are provided in Table 1 in the Appendix.
Utility Catalogs by Stage and Treatment
Mean EQ-5D scores stratified by stage and treatment modality ranged from 0.69 to 0.86 (Table 3). As the stage of disease increased, a trend of lower utility following surgical treatment was observed (0.82 for stage I, 0.80 stage II, 0.79 stage III, 0.69 stage IV). However, standard deviations surrounding the utilities were wide. Eighty percent of patients who received only surgery had stage I lung cancer, and compared to other treatment-stage combinations these patients had the highest mean utility score.
Table 3.
Catalog of EQ-5D Utilities by Stage and Treatment Category
| Stage at Diagnosis /Treatment Category | N | Mean EQ-5D Utility (SD) | Median EQ-5D Utility (IQR) |
|---|---|---|---|
|
| |||
| Stage I | 750 | 0.81 (0.17) | 0.83 (0.23) |
| Surgery | 449 | 0.82 (0.16) | 0.83 (0.23) |
| Lobectomy | 244 | 0.83 (0.16) | 0.83 (0.22) |
| Wedge Resection | 48 | 0.79 (0.15) | 0.80 (0.15) |
| Pneumonectomy | 40 | 0.75 (0.18) | 0.80 (0.25) |
| Other Primary | 42 | 0.84 (0.13) | 0.82 (0.22) |
| Chemotherapy | 17 | 0.75 (0.23) | 0.82 (0.28) |
| Surgery w/Chemotherapy | 113 | 0.81 (0.14) | 0.82 (0.09) |
| Platinum | 41 | 0.79 (0.18) | 0.82 (0.11) |
| Non-Platinum* | 52 | 0.81 (0.11) | 0.82 (0.08) |
| Surgery w/Chemoradiotherapy | 30 | 0.84 (0.13) | 0.83 (0.23) |
| Platinum | 18 | 0.86 (0.12) | 0.84 (0.22) |
| Chest Radiation | 37 | 0.76 (0.20) | 0.82 (0.17) |
| Chemoradiotherapy | 32 | 0.76 (0.23) | 0.82 (0.22) |
| Platinum | 17 | 0.77 (0.26) | 0.82 (0.29) |
| No Treatment | 37 | 0.81 (0.19) | 0.82 (0.24) |
|
| |||
| Stage II | 232 | 0.77 (0.17) | 0.81 (0.14) |
| Surgery | 56 | 0.80 (0.15) | 0.83 (0.08) |
| Lobectomy | 29 | 0.80 (0.16) | 0.82 (0.06) |
| Surgery w/Chemotherapy | 61 | 0.76 (0.18) | 0.81 (0.14) |
| Platinum | 26 | 0.75 (0.18) | 0.81 (0.12) |
| Non-Platinum | 21 | 0.78 (0.20) | 0.81 (0.09) |
| Surgery w/Chemoradiotherapy | 42 | 0.76 (0.18) | 0.79 (0.15) |
| Platinum* | 27 | 0.74 (0.21) | 0.80 (0.28) |
| Non-Platinum* | 12 | 0.79 (0.14) | 0.81 (0.09) |
| Radiotherapy | 13 | 0.72 (0.19) | 0.80 (0.25) |
| Chemoradiotherapy | 28 | 0.78 (0.18) | 0.77 (0.41) |
| Platinum | 16 | 0.81 (0.16) | 0.82 (0.29) |
| No Treatment | 15 | 0.73 (0.14) | 0.75 (0.25) |
|
| |||
| Stage III | 649 | 0.77 (0.18) | 0.82 (0.15) |
| Surgery | 31 | 0.79 (0.17) | 0.82 (0.29) |
| Lobectomy | 14 | 0.78 (0.18) | 0.84 (0.18) |
| Chemotherapy | 93 | 0.78 (0.17) | 0.83 (0.15) |
| Platinum‡ | 29 | 0.78 (0.20) | 0.79 (0.24) |
| Non-platinum§ | 23 | 0.82 (0.14) | 0.83 (0.29) |
| Surgery w/Chemotherapy | 48 | 0.76 (0.15) | 0.80 (0.12) |
| Platinum | 13 | 0.72 (0.19) | 0.79 (0.14) |
| Non-Platinum* | 19 | 0.77 (0.15) | 0.82 (0.14) |
| Surgery w/Chemoradiotherapy | 94 | 0.75 (0.18) | 0.80 (0.14) |
| Platinum† | 41 | 0.72 (0.19) | 0.82 (0.15) |
| Non-Platinum* | 34 | 0.76 (0.18) | 0.77 (0.14) |
| Radiotherapy | 34 | 0.80 (0.14) | 0.83 (0.15) |
| Chemoradiotherapy | 308 | 0.78 (0.18) | 0.82 (0.15) |
| Platinum† | 175 | 0.77 (0.20) | 0.82 (0.09) |
| Non-Platinum† | 95 | 0.79 (0.16) | 0.83 (0.08) |
| No Treatment | 26 | 0.81 (0.17) | 0.82 (0.08) |
|
| |||
| Stage IV | 628 | 0.76 (0.19) | 0.82 (0.15) |
| Surgery | 17 | 0.69 (0.25) | 0.71 (0.41) |
| Chemotherapy | 214 | 0.79 (0.19) | 0.82 (0.09) |
| Platinum† | 78 | 0.79 (0.17) | 0.83 (0.08) |
| Non-platinum‡ | 67 | 0.77 (0.21) | 0.82 (0.10) |
| Surgery w/Chemotherapy | 33 | 0.80 (0.19) | 0.83 (0.23) |
| Platinum‡ | 19 | 0.84 (0.16) | 0.83 (0.19) |
| Surgery w/Chemoradiotherapy | 29 | 0.74 (0.22) | 0.82 (0.25) |
| Non-Platinum† | 13 | 0.80 (0.13) | 0.82 (0.08) |
| Chest Radiation | 29 | 0.72 (0.20) | 0.80 (0.14) |
| Chemoradiotherapy | 270 | 0.75 (0.18) | 0.79 (0.16) |
| Platinum* | 149 | 0.76 (0.17) | 0.80 (0.15) |
| Non-Platinum† | 82 | 0.75 (0.19) | 0.78 (0.14) |
| No Treatment | 33 | 0.79 (0.17) | 0.83 (0.10) |
5-10% patients also received targeted therapy
10-15% patients also received targeted therapy
20-25% patients also received targeted therapy
35% patients also received targeted therapy
A statistically significant difference in the SF-6D based utility (p<0.0001) was observed for patients who were being actively treated (within 30 days) as compared with patients who completed treatment more than 30 days prior to the survey (Table 4). Despite having the same median, the EQ-5D based utility was statistically significant (p=0.0209) using the Mann-Whitney test due to the differences in rank sums between the two groups. (15) However, after using a Bonferroni adjustment this difference was not statistically significant. When stratified by stage no differences were observed after using a Bonferroni adjustment.
Table 4.
Impact of Active as Compared with Treatment >30 days prior to Survey Administration on Utilities by Stage at Diagnosis and Treatment
| Demographic | % Patients with Utility Scores | Mean (SD) EQ-5D Utility N=2396 | Median (IQR) EQ-5D Utility N=2396 | Mean (SD) SF-6D Utility N=2344 | Median (IQR) SF-6D Utility N=2344 |
|---|---|---|---|---|---|
| All | 100 | 0.78 (0.18) | 0.82 (0.15) | 0.68 (0.14) | 0.66 (0.20) |
| Active Treatment <=30 Days | 45% | 0.77 (0.14) | 0.82 (0.15) | 0.66 (0.13) | 0.65 (0.16) |
| Active Treatment >30 Days | 55% | 0.79 (0.18) | 0.82 (0.15) | 0.70 (0.14) | 0.68 (0.20) |
| p-value* | 0.0209* | 0.0001 | |||
| Stage I Treatment | |||||
| Active <=30 Days | 21% | 0.81 (0.14) | 0.82 (0.10) | 0.68 (0.12) | 0.66 (0.20) |
| Active >30 Days | 79% | 0.81 (0.17) | 0.83 (0.23) | 0.72 (0.15) | 0.72 (0.25) |
| p-value* | n.s. | 0.0033** | |||
| Stage II Treatment | |||||
| Active <=30 Days | 43% | 0.75 (0.19) | 0.78 (0.25) | 0.67 (0.14) | 0.65 (0.20) |
| Active >30 Days | 57% | 0.78 (0.16) | 0.82 (0.12) | 0.69 (0.13) | 0.66 (0.20) |
| p-value* | n.s. | n.s. | |||
| Stage III Treatment | |||||
| Active <=30 Days | 59% | 0.78 (0.17) | 0.82 (0.15) | 0.66 (0.13) | 0.65 (0.16) |
| Active >30 Days | 41% | 0.76 (0.18) | 0.82 (0.14) | 0.69 (0.14) | 0.66 (0.19) |
| p-value* | n.s. | n.s. | |||
| Stage IV Treatment | |||||
| Active <=30 Days | 62% | 0.77 (0.19) | 0.82 (0.15) | 0.66 (0.12) | 0.66 (0.16) |
| Active >30 Days | 38% | 0.76 (0.20) | 0.80 (0.17) | 0.67 (0.14) | 0.66 (0.17) |
| p-value* | n.s. | n.s. |
p-value is significant due to differences in rank sum when using the Mann-Whitney test,(13) non-significant after Bonferroni adjustment
non-significant after Bonferroni adjustment
Comparison of Baseline and Follow-Up EQ-5D-derived Utilities
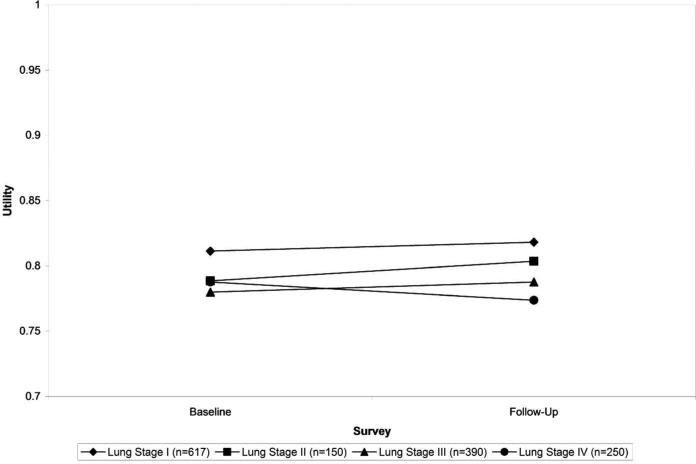
A sub-set of patients (1,474) completed follow-up surveys. Compared to patients who completed a follow-up survey, patients who did not have follow-up data were more likely to be non-white (p=0.02), older (p=0.002), have chemotherapy or chemoradiotherapy (p<0.0001), and to have been diagnosed at a later stage of disease (p<0.0001), a reflection of the fact that patients with more serious illness were more likely to either have died or been unable to complete a follow-up survey (Appendix Table 2a). Similar trends can be seen when comparing the follow-up subset to the baseline patient cohort. Among participants with survey results at both baseline and follow up, a non-statistically significant decrease was observed in their mean utility for stage IV while increases were observed for stages I, II, and III (Figure 4, Appendix Table 2b). Patients who had severe comorbidities had a decrease in utility whereas patients with mild, moderate, or no comorbidities saw an increase in utility. Patients who had no treatment and patients who had chemotherapy only had decreased utility whereas patients in all other treatment categories had increased utility. None of these trends, however, were statistically significant. Among women there was a statistically significant increase in utility (p=0.0340). In addition, we also assessed change in utility on follow-up for surgery patients who completed their baseline survey within 30 days after surgery. Our findings revealed an average increase in EQ-5D utility of 0.0917 (p=0.0807) whereas surgery patients who completed their survey more than 30 days after surgery had an average increase in utility of 0.0086 (p=0.1272).
Figure 4.
Comparison of Baseline vs. follow-up EQ-5D-derived utilities, by stage, among participants with both a baseline and follow-up survey 1521×1040mm (96 × 96 DPI)
Discussion
The primary purpose of our analysis was to provide an off-the shelf catalog of population-based utilities for use in cost-utility analyses of lung cancer interventions conducted from the societal perspective and a comparison of the utilities. Utilities are categorized by patient demographics and disease state (severity of comorbidity, stage at diagnosis, and treatment modality). The detailed categories included in this analysis include all stages at diagnosis and further stratification by treatment modality. Patient-reported EQ-5D and SF-6D scores from a large cohort of patients enrolled at a range of institutions across the U.S. were mapped to standardized, community-weighted preferences.
Not surprisingly, we found that the patients diagnosed at the earliest stages of lung cancer have a higher utility score than later stages. Patients receiving surgery report a higher utility score than other treatment states; however, since 80% of surgical patients were stage I lung cancer patients, this likely reflects the both the early stage of disease (which is generally asymptomatic) as well as the less detrimental impact of surgery compared with other treatments on health status.
Interestingly, younger patients (less than 55 years old) reported a lower utility score than older age groups suggesting that the impact of disease and treatment may have a greater impact on perceived health in younger patients. However, given that among many cancers, development reflects the accumulation of exposure over time, these scores may instead demonstrate that those diagnosed with lung cancer at a younger age are a less healthy group overall.
Patient-reported health ratings (as measured by the EQ-VAS) had a moderate positive association with utilities. VAS scores tended to be lower than utilities because the rating health does not require making tradeoffs, and avoids biases due to time preference or risk aversion that are reflected in utilities. The VAS scores reported here might be useful for comparative effectiveness research studies that do not consider cost per quality-adjusted life-year.(16)
A high ceiling effect obtained from the EQ-5D has been observed in previous studies by Luo et al. (48.6%)(17) and Palta et al., (36%).(18) The Palta study also reported a lower ceiling effect from the SF-6D (4.2%). The possibility of the ceiling effect in the EQ-5D influencing the data cannot be ignored. Additionally, unmeasured patient characteristics might have been stronger predictors of utility. A newer version of the EQ-5D with five levels for each attribute was recently developed in part to reduce the ceiling effect, and definitive valuation studies are underway.(19-22)
A review published in 2000 by Earle, et al.(3) concluded that utility information in lung cancer patients was very limited. A recent (2010) Dutch study (23) described the collection of utilities using the EQ-5D in lung cancer patients (n=260) that differed substantially from the CanCORS cohort. In the Dutch study, patients were surveyed a median of 2.6 years after diagnosis. The short overall survival for lung cancer resulted in few patients (1%) who had been diagnosed in stage IV. In our analysis, the mean lag between diagnosis and completion of the EQ-5D was approximately 4-6 months, and 25.3% of lung cancer patients included were diagnosed in stage IV. Most patients (>60% in both cohorts) had been treated with surgical resection alone or in combination with other modalities. Our catalog includes community (U.S. for EQ-5D and U.K. for SF-6D) preferences, while the Dutch study reported patient preferences.
In all sub-groups, the SF-6D scores were consistently lower than the EQ-5D scores, similar to the findings of Fryback et al. (20) The SF-6D also had a much lower ceiling effect (2.3%) than EQ-5D, a less-skewed distribution, and may be a stronger instrument for detecting changes. However, with some exceptions, the SF-6D did not demonstrate notable sensitivity in detecting differences across subgroups. While the overall floor effect of the SF-6D was 1%, floor effects were seen in the individual domains at a range of 2.6%-50.3%. Floor effects are considered substantial if above 15%;(24) when this criterion is adopted with the SF-6D domains, the floor effects for the physical function, role function, and vital domains are considered substantial. This result suggests that generic quality of life instruments are weaker in these situations, and a preference-based lung cancer-specific instrument may be a useful option to accompany lung-cancer specific psychometric instruments already in existence.(25, 26) Our conclusions on the usefulness of a disease-specific measure, however, must be qualified by the fact that neither the Health Utilities Index nor QWB-SA instruments were included in the CanCors survey. That they were not included is not surprising, given that the QWB-SA is a relatively lengthy instrument, and the HUI instruments are not as brief as the EQ-5D as well as relatively expensive. However, both of these instruments have relatively lower ceiling and floor effects than the EQ-5D and SF-6D. It would be of substantial interest to see how these 2 instruments would perform in the setting of lung cancer.
When considering the differences in utilities between subgroups, it is important to consider whether the differences are clinically important. Pickard et al. demonstrated a Minimally Important Difference (MID) of 0.07 for the EQ-5D,(27) while Walters et al. showed a MID of 0.03 for the SF-6D.(28) Adopting these standards for our mean results, we demonstrate that several subgroups have differences that could be deemed clinically important. For example, EQ-5D utilities for patients aged 65 or older have a utility that is 0.07 or higher than the younger population (54 or less), suggesting that there may be clinically relevant reasons for this difference. In addition, a score of 0.83 for the Asian population is a clinically important difference when compared to the other racial subgroups, except for White. When comparing treatment strategies, the surgery plus radiotherapy group had the lowest EQ-5D score (0.72), with a MID of 0.07 or more when compared to the surgery only, surgery and chemotherapy, chemotherapy only, and no treatment groups. However, the Asian and surgery/radiotherapy subgroups are small, so caution should be taken when considering whether these findings are clinically relevant.
When comparing SF-6D utility scores within subgroups, this difference was not seen with the Asian subgroup, with the exception of the “other” category. Stage I patients had a SF-6D utility that was 0.03 or higher than the other stages, suggesting a clinically important difference among stages. Patients 54 years old or younger had a SF-6D utility score that was worse than all others, and differences were noted among other age groups. MIDs could also be seen within the treatment groups, in particular surgery only, which had a utility at least 0.03 higher than all other treatment groups except no treatment, and surgery and chemoradiotherapy group, which had the lowest score (0.65), and a MID of 0.03 or more when compared to the surgery only, chemotherapy only, surgery plus radiotherapy, and no treatment groups. However, overall there was a lack of substantial differences within the different groups for both the EQ-5D and SF-6D. The ceiling effects with EQ-5D and the floor effects with individual domains of the SF-6D can help account for the lack of a wider range in scores.
The EORTC LC13 summated score showed similar trends to the SF-6D and EQ-5D utilities. For example, patients at earlier stages of disease had lower scores than those at higher stages, indicating better health. Patients receiving surgery as their only treatment reported a lower summated score than other treatment states, including no treatment. Patients less than 55 years old had a higher score than older patients, as seen in the SF-6D and EQ-5D scores. While statistically significant differences were seen in several subgroups, the quantitative differences in actual scores were small, ranging from 12-15. This lack of substantial differences in the EORTC LC13 summated score may be driven by the lack of inclusion of important domains for lung cancer patients. An analysis of the National Health Measurement Study (29) determined that there were three main dimensions in generic quality of life instruments: psychosocial, physical and pain. A review by Chen determined that cough, shortness of breath, anxiety, and fatigue are important symptoms to consider in the quality of life of lung cancer patients.(30) Iyer et al. looked at symptom scores from the Lung Cancer Symptom Scale as predictors of FACT-L scores and found that cough, pain, shortness of breath, and appetite loss significantly predicted quality of life.(31) While the summated EORTC LC13 score covers shortness of breath and cough well, it does not cover these other important dimensions of quality of life among cancer patients.
Clearly, there are important domains of lung cancer-related quality of life that are not well covered by the generic indexes, such as cough, shortness of breath, appetite loss, and fatigue.(32) However, it should be noted that instead of using a disease-specific instrument, the generic utility indexes may indirectly cover some of these areas that they do not explicitly cover by showing generic reductions in physical mobility and increases in emotional distress. Given the substantial cost-effectiveness implications and patient-reported burdens of lung cancer, some investigators may require more specific information about what is really at the root of reductions in more general aspects of quality of life. This need would be particularly relevant in comparing lung cancer treatments.
There has been recent interest in condition-specific indexes in general, and some work has been done by others in lung cancer as noted below. A criticism of condition-specific indexes in general is the possibility of undue focus upon symptoms rather than quality of life, which may not provide QALYs comparable to a generic index. Brazier has contested the significance of this issue.(33) In lung cancer, two groups have developed lung cancer indexes based upon the same subset of FACT-L items. However, neither of these groups provide US societal preferences, and health preferences are known to vary by country.(34) In condition-specific measures, the patient perspective is preferred in the latest instruments, such as the prostate cancer utility scale(35) and the diabetes utility index.(36) In addition, there are other concerns with the FACT-L instruments. The UK version in particular does not clearly provide utilities and the scale of 0.1-0.7 is very constricted. With this scale, a lung cancer patient with no current limitations can never have a utility above 0.7. Pickard calls this a “labelling effect” for having cancer. There are also likely mutual utility independence issues between the FACT-L domains used. Therefore, preference-based measurement in lung cancer awaits further improvement.(37)
Limitations
Some additional limitations of our study are worth noting. The CanCORS cohort had some geographical diversity yet limited representation of different U.S. regions. Therefore, this population is not necessarily representative of lung cancer patients in the United States. Questions pertaining to the EQ-5D and SF-12 were not included on proxy questionnaires, which limited our analysis to patients able to complete the detailed self-survey. Patients who were too ill to complete a detailed survey were not included so our mean utilities likely underestimate the full impact of advanced disease and the heterogeneity in utilities for lung cancer patients. Compared to patients excluded due to non-response on the EQ-5D, it is clear from Table 1a that the patients included in our analysis are younger and healthier and more likely to be female and white. EORTC items suggest that symptom issues were not substantial. In addition, data collection occurred at 4-6 months post-diagnosis, with follow-up at 11-13 months post-diagnosis. These scores may not accurately reflect the scores of long-term lung cancer survivors. Data collection for this study occurred prior to widespread use of targeted therapies for lung cancer, including oral agents, and thus may not represent the utility associated with these therapies. Our EQ-5D catalog provides U.S. preferences only, and results are subject to any inherent limitations of the original methodology used to select the community respondents.(8) The SF-6D scoring system is based on UK preferences, rather than US preferences. Additionally, although the overall sample size was large, the number of patients was small in some subgroups, limiting precision. Although we do report patient-supplied health status (EQ-VAS) scores (best to worst imaginable health state scale), patients in CanCORS were not asked time tradeoff or standard gamble questions so no patient preferences (patient-provided utilities) are available. Patients were not given the full set of questions from the EORTC QLC-30 or L13, so only summated scores of the eight questions available could be calculated, and important domains from these questionnaires, such as fatigue and depression, were not included. There are also limitations of the EQ-5D, SF-6D, and QALYs themselves, reviewed in previous studies.(19)
Conclusion
This study provides a catalog and comparison of utility scores based on EQ-5D, SF-6D, and EQ-VAS that can be considered for cost-effectiveness analyses of lung cancer. However, potential users of these scores need to be aware of the limitations and think carefully about their use in specific studies, taking into consideration the characteristics of the patients. The major strength of these utilities is that they are specific to stage of disease as well as to treatment type. The SF-6D has advantages over EQ-5D due to its lack of strong ceiling effect and its less-skewed distribution. However, the utility scores calculated from both indexes showed similar trends. Researchers should consider the methods used when calculating QALYs for cost-effectiveness studies. A preference-based lung cancer-specific index may be more optimal for detecting differences in utility scores among patients. As we note above, others have been interested in better measurement of lung cancer specific morbidity in developing FACT-L related instruments, but with the substantial limitations we noted at this writing.
Figure 1.
Flowchart of canCORS Patients 215×279mm (300 × 300 DPI)
Acknowledgments
Nancy Keating for helpful comments and suggestions.
Financial support for this study was provided in part by the American Cancer Society (2008A060554, ACT, PMM) and by the National Cancer Institute's Cancer Intervention and Surveillance Modeling Network (CISNET) Lung Group (1U01CA152956, PMM, MM, ACT) The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
Appendix
Appendix Table 1.
Catalog of Scores Based on VAS
| Rate Your Health Today: Lung Cancer Patients | |||
|---|---|---|---|
| Category | N | Mean VAS Score | SD |
|
| |||
| All | 2370* | 65.39 | 21.30 |
| Gender | |||
| Male | 1229 | 64.19 | 21.48 |
| Female | 1141 | 66.69 | 21.03 |
| Race | |||
| Caucasian | 1773 | 65.16 | 21.10 |
| Latino | 105 | 69.37 | 22.30 |
| African American | 281 | 64.67 | 21.66 |
| Asian | 80 | 71.25 | 17.40 |
| Other | 131 | 63.34 | 23.82 |
| Age | |||
| <54 | 350 | 61.46 | 22.12 |
| 55-59 | 303 | 61.81 | 22.41 |
| 60-64 | 355 | 66.47 | 20.33 |
| 65-69 | 407 | 67.72 | 20.35 |
| 70-74 | 397 | 65.95 | 21.81 |
| 75-79 | 317 | 65.65 | 21.11 |
| 80+ | 241 | 68.83 | 19.73 |
| Stage at Diagnosis | |||
| I | 743 | 71.01 | 19.99 |
| II | 230 | 67.07 | 20.28 |
| III | 641 | 63.54 | 20.95 |
| IV | 622 | 60.20 | 22.03 |
| Missing | 134 | 64.27 | 20.92 |
| Histology | |||
| NSCLC | 2005 | 66.33 | 21.08 |
| SCLC | 263 | 61.94 | 23.03 |
| Missing | 103 | 61.67 | 21.32 |
| Treatment | |||
| Surgery | 559 | 71.28 | 19.23 |
| Chemotherapy | 357 | 63.30 | 22.07 |
| Radiation | 123 | 59.54 | 22.74 |
| Surgery w/Chemotherapy | 269 | 69.15 | 19.32 |
| Surgery w/Radiation | 62 | 68.19 | 23.82 |
| Chemoradiotherapy | 670 | 60.92 | 21.68 |
| Surgery w/Chemoradiotherapy | 204 | 65.54 | 20.27 |
| No Treatment | 126 | 65.02 | 21.17 |
| Comorbidity | |||
| None | 412 | 66.99 | 21.65 |
| Mild | 735 | 66.28 | 21.40 |
| Moderate | 408 | 64.67 | 20.38 |
| Severe | 373 | 61.55 | 22.42 |
| Missing | 442 | 66.33 | 20.31 |
26 patients answered “refused” or “don't know.”
Appendix Table 2a.
Baseline Demographics Based on Availability of Follow-Up
| Demographic | With Follow-Up Questionnaire | Without Follow-Up questionnaire | p-value* |
|---|---|---|---|
| All | 1474 | 922 | |
| Sex | |||
| Male | 766 (51.97%) | 476 (51.63%) | NS |
| Female | 708 (48.03%) | 446 (48.37%) | |
| Race/Ethnicity | |||
| Caucasian | 1130 (76.66%) | 658 (71.37%) | 0.02 |
| Latino | 60 (4.07%) | 47 (5.10%) | |
| African American | 171 (11.60%) | 115 (12.47%) | |
| Asian | 39 (2.65%) | 41 (4.45%) | |
| Other | 74 (5.02%) | 61 (6.62%) | |
| Age | |||
| <=54 | 204 (13.84%) | 149 (16.16%) | 0.002 |
| 55-59 | 169 (11.47%) | 136 (14.75%) | |
| 60-64 | 237 (16.08%) | 122 (13.23%) | |
| 65-69 | 276 (18.72%) | 134 (14.53%) | |
| 70-74 | 242 (16.42%) | 161 (17.46%) | |
| 75-79 | 210 (14.25%) | 113 (12.26%) | |
| 80+ | 136 (9.23%) | 107 (11.61%) | |
| Stage | |||
| I | 617 (41.86%) | 133 (14.43%) | <0.0001 |
| II | 150 (10.18%) | 82 (8.89%) | |
| III | 390 (26.46%) | 259 (28.09%) | |
| IV | 250 (16.96%) | 378 (41.00%) | |
| Unknown/Missing | 67 (4.55%) | 70 (7.59%) | |
| Histology | |||
| NSCLC | 1296 (87.92%) | 729 (79.07%) | NS |
| SCLC | 115 (7.80%) | 149 (16.16%) | |
| Missing | 63 (4.27%) | 44 (4.77%) | |
| Treatment | <0.0001 | ||
| Surgery | 470 (31.87%) | 93 (10.09%) | |
| Chemotherapy | 159 (10.79%) | 199 (21.58%) | |
| Radiotherapy | 46 (3.12%) | 80 (8.68%) | |
| Surgery w/Chemotherapy | 211 (14.31%) | 60 (6.51%) | |
| Surgery w/Radiotherapy | 46 (3.12%) | 17 (1.84%) | |
| Chemoradiotherapy | 331 (22.46%) | 348 (37.74%) | |
| Surgery w/Chemo/Rad | 141 (9.57%) | 66 (7.16%) | |
| No Treatment | 70 (4.75%) | 59 (6.40%) | |
| Comorbidity | NS | ||
| None | 263 (17.84%) | 150 (16.27%) | |
| Mild | 487 (33.04%) | 257 (27.87%) | |
| Moderate | 268 (18.18%) | 144 (15.61%) | |
| Severe | 222 (15.06%) | 153 (16.59%) | |
| Unknown/Missing | 234 (15.88%) | 218 (23.64%) |
χ2 test. NS, not significant.
Appendix Table 2b.
EQ-5D-based Scores of Lung Cancer Population at Follow-up
| Demographic | Mean EQ-5D-derived utility(SD) | Median EQ-5D-derived utility (IQR) | |
|---|---|---|---|
| All | 0.80 (0.17) | 0.83 (0.24) | |
| Sex | |||
| Male | 0.80 (0.17) | 0.83 (0.10) | |
| Female | 0.80 (0.18) | 0.82 (0.24) | |
| Race/Ethnicity | |||
| Caucasian | 0.80 (0.17) | 0.83 (0.23) | |
| Latino | 0.81 (0.17) | 0.77 | 0.83 (0.08) |
| African American | (0.20) | 0.81 (0.15) | |
| Asian | 0.86 (0.13) | 0.83 (0.20) | |
| Other | 0.77 (0.19) | 0.81 (0.14) | |
| Age | |||
| <=54 | 0.76 (0.19) | 0.80 (0.13) | |
| 55-59 | 0.80 (0.17) | 0.82 (0.09) | |
| 60-64 | 0.80 (0.17) | 0.83 (0.09) | |
| 65-69 | 0.81 (0.18) | 0.83 (0.23) | |
| 70-74 | 0.79 (0.18) | 0.82 (0.29) | |
| 75-79 | 0.82 (0.16) | 0.83 (0.22) | |
| 80+ | 0.82 (0.17) | 0.83 (0.22) | |
| Stage | |||
| I | 0.82 (0.17) | 0.83 (0.23) | |
| II | 0.80 (0.17) | 0.82 (0.08) | |
| III | 0.79 (0.19) | 0.82 (0.15) | |
| IV | 0.77 (0.19) | 0.81 (0.14) | |
| Unknown/Missing | 0.80 (0.15) | 0.82 (0.09) | |
| Histology | |||
| NSCLC | 0.80 (0.17) | 0.83 (0.24) | |
| SCLC | 0.80 (0.17) | 0.82 (0.10) | |
| Missing | 0.76 (0.21) | 0.81 (0.15) | |
| Treatment | |||
| Surgery | 0.82 (0.17) | 0.83 (0.23) | |
| Chemotherapy | 0.80 (0.16) | 0.82 (0.12) | |
| Radiotherapy | 0.78 (0.18) | 0.80 (0.15) | |
| Surgery w/Chemotherapy | 0.81 (0.16) | 0.83 (0.08) | |
| Surgery w/Radiotherapy | 0.74 (0.23) | 0.82 (0.15) | |
| Chemoradiotherapy | 0.79 (0.17) | 0.82 (0.15) | |
| Surgery w/Chemo/Rad | 0.79 (0.19) | 0.82 (0.29) | |
| No Treatment | 0.80 (0.19) | 0.82 (0.24) | |
| Comorbidity | |||
| None | 0.82 (0.16) | 0.83 (0.22) | |
| Mild | 0.80 (0.18) | 0.83 (0.23) | |
| Moderate | 0.78 (0.19) | 0.82 (0.15) | |
| Severe | 0.79 (0.16) | 0.82 (0.15) | |
| Unknown/Missing | 0.80 (0.18) | 0.82 (0.24) | |
Appendix Table 3.
Variation in Utilities Based on EORTC QLC-30/QLC-LC13 at Baseline Survey
| Demographic | Patients with EORTC N (%) | EORTC Score* Mean (SD) | EORTC Score* Median (IQR) |
|---|---|---|---|
|
| |||
| All | 2212 (100%) | 21.94 (6.11) | 21.0 (9.0) |
|
| |||
| Sex | |||
| Male | 1171 (52.9%) | 21.79 (5.93) | 21.0 (9.0) |
| Female | 1041 (47.1%) | 22.10 (6.31) | 21.0 (9.0) |
| p-value† | 0.50 | ||
|
| |||
| Race/Ethnicity | |||
| Caucasian | 1655 (74.8%) | 21.75 (5.91) | 21.0 (8.0) |
| Latino | 92 (4.2%) | 21.16 (5.70) | 20.5 (8.0) |
| African American | 255 (11.5%) | 22.95 (6.79) | 22.0 (11.0) |
| Asian | 80 (3.6%) | 19.93 (4.88) | 20.0 (7.0) |
| Other | 130 (5.9%) | 24.07 (7.34) | 23.0 (9.0) |
| p-value‡ | 0.0001 | ||
|
| |||
| Age | |||
| <=54 | 339 (15.35) | 24.13 (7.26) | 23.0 (11.0) |
| 55-59 | 286 (12.9%) | 22.94 (5.97) | 23.0 (9.0) |
| 60-64 | 339 (15.3%) | 22.55 (6.28) | 21.0 (8.0) |
| 65-69 | 385 (17.4%) | 21.34 (5.95) | 21.0 (8.0) |
| 70-74 | 365 (16.5%) | 21.09 (5.56) | 20.0 (7.0) |
| 75-79 | 289 (13.1%) | 20.89 (5.06) | 21.0 (7.0) |
| 80+ | 209 (9.5%) | 20.07 (5.14) | 19.0 (7.0) |
| p-value‡ | <0.0001 | ||
|
| |||
| Stage at Diagnosis | |||
| I | 684 (30.9%) | 20.33 (5.64) | 19.0 (7.0) |
| II | 213 (9.6%) | 22.42 (5.79) | 22.0 (8.0) |
| III | 599 (27.1%) | 22.96 (6.28) | 22.0 (9.0) |
| IV | 587 (26.5%) | 22.58 (6.16) | 22.0 (8.0) |
| Unknown/Missing | 129 (5.8%) | 21.94 (6.39) | 21.0 (9.0) |
| p-value‡ | <0.0001 | ||
|
| |||
| Histology | |||
| NSCLC | 1869 (84.6%) | 21.74 (5.94) | 23.0 (10.0) |
| SCLC | 242 (10.9%) | 22.67 (6.87) | 21.0 (8.0) |
| Missing | 101 (4.6%) | 22.77 (6.92) | 21.5 (10.0) |
| p-value‡ | n.s. | ||
|
| |||
| Treatment | |||
| Surgery | 517 (23.4%) | 19.79 (5.50) | 19.0 (7.0) |
| Chemotherapy | 325 (14.7%) | 22.04 (6.19) | 21.0 (9.0) |
| Radiotherapy | 109 (4.9%) | 21.93 (6.10) | 21.0 (9.0) |
| Surgery w/Chemotherapy | 252 (11.4%) | 22.58 (5.76) | 22.0 (8.0) |
| Surgery w/Radiotherapy | 58 (2.6%) | 21.47 (5.69) | 21.0 (7.0) |
| Chemoradiotherapy | 639 (28.9%) | 23.21 (6.31) | 23.0 (8.0) |
| Surgery w/Chemo/Rad | 195 (8.8%) | 23.18 (6.11) | 23.0 (9.0) |
| No Treatment | 117 (5.3%) | 20.92 (5.68) | 20.0 (8.0) |
| p-value‡ | <0.0001 | ||
|
| |||
| Comorbidity | |||
| None | 388 (17.5%) | 21.67 (6.05) | 21.0 (9.0) |
| Mild | 686 (31.0%) | 21.89 (6.30) | 21.0 (8.0) |
| Moderate | 374 (16.9%) | 22.34 (6.07) | 21.0 (8.0) |
| Severe | 344 (15.6%) | 22.78 (6.18) | 22.0 (8.0) |
| Unknown/Missing | 420 (19%) | 21.21 (5.74) | 20.0 (8.0) |
| p-value‡ | 0.03 | ||
14 lung cancer-specific questions from EORTC QLC LC13, maximum total score is 56 (worse health).
Mann-Whitney U test.
Kruskal-Wallis test
Appendix Figure 1.
Appendix Figure 2.
Appendix Figure 3.
Appendix Figure 4.
References
- 1.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. Oxford University Press; New York: 1996. [Google Scholar]
- 2.Ware JEKM, Bjorner JB, et al. User's Manual for the SF-36v2 Health Survey. Quality Metric, Inc.; Lincoln, RI: 2007. [Google Scholar]
- 3.Earle C, Chapman R, Baker C, Bell C, Stone P, Sandberg E, et al. Systematic overview of cost- utility assessments in oncology. Journal of Clinical Oncology. 2000;18:3302–17. doi: 10.1200/JCO.2000.18.18.3302. [DOI] [PubMed] [Google Scholar]
- 4.Sturza J. A review and meta-analysis of utility values for lung cancer. Medical Decision Making. 2010 doi: 10.1177/0272989X10369004. Online before print. [DOI] [PubMed] [Google Scholar]
- 5.Kind P, Macran S. Eliciting social preference weights for Functional Assessment of Cancer Therapy-Lung health states. Pharmacoeconomics. 2005;23:1143–53. doi: 10.2165/00019053-200523110-00006. [DOI] [PubMed] [Google Scholar]
- 6.Ayanian JZ, Chrischilles EA, Fletcher RH, Fouad MN, Harrington DP, Kahn KL, et al. Understanding cancer treatment and outcomes: the Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2004;22:2992–6. doi: 10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 7.EuroQol--a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 8.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43:203–20. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Malin JL, Ko C, Ayanian JZ, Harrington D, Nerenz DR, Kahn KL, et al. Understanding cancer patients' experience and outcomes: development and pilot study of the Cancer Care Outcomes Research and Surveillance patient survey. Support Care Cancer. 2006;14:837–48. doi: 10.1007/s00520-005-0902-8. [DOI] [PubMed] [Google Scholar]
- 10.Agency for Healthcare Research and Quality . Calculating the U.S. Population-based EQ-5D Index Score. Rockville: Aug, p. MD2005. [Google Scholar]
- 11.Shaw JW, Johnson JA, Chen S, Levin JR, Coons SJ. Racial/ethnic differences in preferences for the EQ-5D health states: results from the U.S. valuation study. J Clin Epidemiol. 2007;60:479–90. doi: 10.1016/j.jclinepi.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42:851–9. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]
- 13.Bergman B, Aaronson NK, Ahmedzai S, Kaasa S, Sullivan M. The EORTC QLQ-LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur J Cancer. 1994;30A:635–42. doi: 10.1016/0959-8049(94)90535-5. [DOI] [PubMed] [Google Scholar]
- 14.Hinz A, Einenkel J, Briest S, Stolzenburg JU, Papsdorf K, Singer S. Is it useful to calculate sum scores of the quality of life questionnaire EORTC QLQ-C30? European journal of cancer care. 2012;21:677–83. doi: 10.1111/j.1365-2354.2012.01367.x. [DOI] [PubMed] [Google Scholar]
- 15.Hart A. Mann-Whitney test is not just a test of medians: differences in spread can be important. BMJ. 2001;323:391–3. doi: 10.1136/bmj.323.7309.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neumann PJ, Weinstein MC. Legislating against use of cost-effectiveness information. N Engl J Med. 2010;363:1495–7. doi: 10.1056/NEJMp1007168. [DOI] [PubMed] [Google Scholar]
- 17.Luo N, Johnson JA, Shaw JW, Coons SJ. Relative efficiency of the EQ-5D, HUI2, and HUI3 index scores in measuring health burden of chronic medical conditions in a population health survey in the United States. Med Care. 2009;47:53–60. doi: 10.1097/MLR.0b013e31817d92f8. [DOI] [PubMed] [Google Scholar]
- 18.Palta M, Chen HY, Kaplan RM, Feeny D, Cherepanov D, Fryback DG. Standard error of measurement of 5 health utility indexes across the range of health for use in estimating reliability and responsiveness. Med Decis Making. 2011;31:260–9. doi: 10.1177/0272989X10380925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fryback DG, Palta M, Cherepanov D, Bolt D, Kim JS. Comparison of 5 health-related quality-of- life indexes using item response theory analysis. Med Decis Making. 2010;30:5–15. doi: 10.1177/0272989X09347016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fryback DG, Dunham NC, Palta M, Hanmer J, Buechner J, Cherepanov D, et al. US norms for six generic health-related quality-of-life indexes from the National Health Measurement study. Med Care. 2007;45:1162–70. doi: 10.1097/MLR.0b013e31814848f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727–36. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15:708–15. doi: 10.1016/j.jval.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Grutters JP, Joore MA, Wiegman EM, Langendijk JA, de Ruysscher D, Hochstenbag M, et al. Health-related quality of life in patients surviving non-small cell lung cancer. Thorax. 2010;65:903–7. doi: 10.1136/thx.2010.136390. [DOI] [PubMed] [Google Scholar]
- 24.McHorney CA, Tarlov AR. Individual-patient monitoring in clinical practice: are available health status surveys adequate? Qual Life Res. 1995;4:293–307. doi: 10.1007/BF01593882. [DOI] [PubMed] [Google Scholar]
- 25.Bottomley A, Gaafar R, Manegold C, Burgers S, Coens C, Legrand C, et al. Short-term treatment- related symptoms and quality of life: results from an international randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an EORTC Lung-Cancer Group and National Cancer Institute, Canada, Intergroup Study. J Clin Oncol. 2006;24:1435–42. doi: 10.1200/JCO.2005.03.3027. [DOI] [PubMed] [Google Scholar]
- 26.Hollen PJ, Gralla RJ, Kris MG, Cox C, Belani CP, Grunberg SM, et al. Measurement of quality of life in patients with lung cancer in multicenter trials of new therapies. Psychometric assessment of the Lung Cancer Symptom Scale. Cancer. 1994;73:2087–98. doi: 10.1002/1097-0142(19940415)73:8<2087::aid-cncr2820730813>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 27.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. doi: 10.1186/1477-7525-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walters SJ, Brazier JE. What is the relationship between the minimally important difference and health state utility values? The case of the SF-6D. Health Qual Life Outcomes. 2003;1:4. doi: 10.1186/1477-7525-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cherepanov D, Palta M, Fryback DG. Underlying dimensions of the five health-related quality-of- life measures used in utility assessment: evidence from the National Health Measurement Study. Med Care. 2010;48:718–25. doi: 10.1097/MLR.0b013e3181e35871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen E, Nguyen J, Cramarossa G, Khan L, Leung A, Lutz S, et al. Symptom clusters in patients with lung cancer: a literature review. Expert review of pharmacoeconomics & outcomes research. 2011;11:433–9. doi: 10.1586/erp.11.56. [DOI] [PubMed] [Google Scholar]
- 31.Iyer S, Roughley A, Rider A, Taylor-Stokes G. The symptom burden of non-small cell lung cancer in the USA: a real-world cross-sectional study. Support Care Cancer. 2014;22:181–7. doi: 10.1007/s00520-013-1959-4. [DOI] [PubMed] [Google Scholar]
- 32.Chen L, Antras L, Duh MS, Levy N, Neary M, O'Brien ME, et al. Psychometric validation of the Patient Symptom Assessment in Lung Cancer instrument for small cell lung cancer. Current medical research and opinion. 2007;23:2741–52. doi: 10.1185/030079907x233331. [DOI] [PubMed] [Google Scholar]
- 33.Brazier JE, Rowen D, Mavranezouli I, Tsuchiya A, Young T, Yang Y, et al. Developing and testing methods for deriving preference-based measures of health from condition-specific measures (and other patient-based measures of outcome). Health Technol Assess. 2012;16:1–114. doi: 10.3310/hta16320. [DOI] [PubMed] [Google Scholar]
- 34.Johnson JA, Luo N, Shaw JW, Kind P, Coons SJ. Valuations of EQ-5D health states: are the United States and United Kingdom different? Med Care. 2005;43:221–8. doi: 10.1097/00005650-200503000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Tomlinson G, Bremner KE, Ritvo P, Naglie G, Krahn MD. Development and validation of a utility weighting function for the patient-oriented prostate utility scale (PORPUS). Med Decis Making. 2012;32:11–30. doi: 10.1177/0272989X11407203. [DOI] [PubMed] [Google Scholar]
- 36.Sundaram M, Smith MJ, Revicki DA, Miller LA, Madhavan S, Hobbs G. Estimation of a valuation function for a diabetes mellitus-specific preference-based measure of health: the Diabetes Utility Index. Pharmacoeconomics. 2010;28:201–16. doi: 10.2165/11313990-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Pickard AS, Ray S, Ganguli A, Cella D. Comparison of FACT- and EQ-5D-based utility scores in cancer. Value Health. 2012;15:305–11. doi: 10.1016/j.jval.2011.11.029. [DOI] [PubMed] [Google Scholar]