Abstract
(−)-Cannabidiol [(−)-CBD] has recently gained prominence as a treatment for neuro-inflammation and other neurodegenerative disorders; interest is also developing in its synthetic enantiomer, (+)-CBD, which has a higher affinity to CB1 / CB2 receptors than the natural stereoisomer. We have developed an inexpensive, stereoselective route to access ent-CBD derivatives using (+)-carvone as a starting material. In addition to (+)-CBD, we report the first syntheses of (+)-cannabidivarin, (+)-cannabidiphorol as well as C-6 / C-8 homologues.
Keywords: Cannabidiol (CBD), Cannabidivarin (CBDV), Cannabidiphorol (CBDP), Enantiomer Natural Products, Alkene Transposition
Graphical Abstract
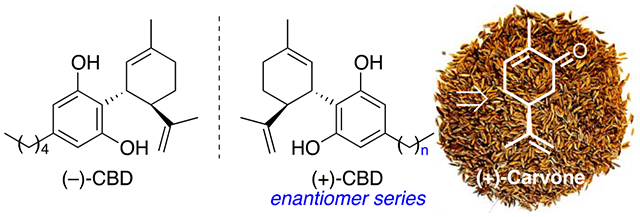
To create your abstract, type over the instructions in the template box below.
Fonts or abstract dimensions should not be changed or altered.
Introduction
Nature remains inspiring in its ability to manufacture a diverse array of chiral secondary metabolites from relatively simple starting compounds. Perhaps even more remarkable is the fact that many of these building blocks exist as achiral, sparsely functionalized materials that are transformed in vivo into highly decorated molecules that exist as single stereoisomers. Importantly, while epimeric / diastereomeric metabolites are oftentimes isolated,1 with very few exceptions [eg., (+)- and (−)-carvone], natural product enantiomers are rarely found in Nature,2 but rather are almost exclusively manufactured in the laboratory. More often than not, this occurs serendipitously, en route to the total synthesis of a compound with unknown, undefined, or otherwise ambiguous absolute stereochemical assignments.3 If the molecule is sufficiently small in size, a stereoselective synthesis may also be performed to probe the potentially unique activity of the non-natural ent-derivative, as there exists a prodigious amount of data that demonstrates the difference of one enantiomer versus the other in a biological context.4 Additionally, there has been at several studies that have documented the increased activity of a natural product diastereomer relative to the natural stereoisomer itself.5 Therefore, the targeted study of ent-natural products, and related stereoisomers, is a viable and valuable approach to the discovery of potential new leads for drug discovery.
Recently, terpene derived (−)-Cannabidiol [(−)-2, (−)-CBD, Fig. 1], the major non-psychoactive constituent found in hemp, has gained popularity amongst the synthetic community,6 as cannabinoids, in general, have been increasingly shown to possess potent anti-inflammatory activity,7 especially against a number of neurological ailments including, but not limited to Alzheimer’s8a and Parkinson’s disease.8b Additionally, many naturally occurring cannabinoids have been studied in animal / clinical trials for a number of other uses, exploiting their antiepileptic,8b anxiolytic,8c antiarthritic,8d and antiemetic8e properties. There is also emerging evidence that (−)-CBD can interact with endocannabinoid receptors in the brain and protect against oxidative stress in neural cells.8f This in turn helps to reduce inflammation, the effects of which can cause the buildup of neurotoxic substances over time and lead to neuro-degeneration.8b In recent years, neuroinflammation has been identified as contributing more to the pathogenesis of Alzheimer’s than even senile plaques and neurofibrillary tangles.9
Figure 1.
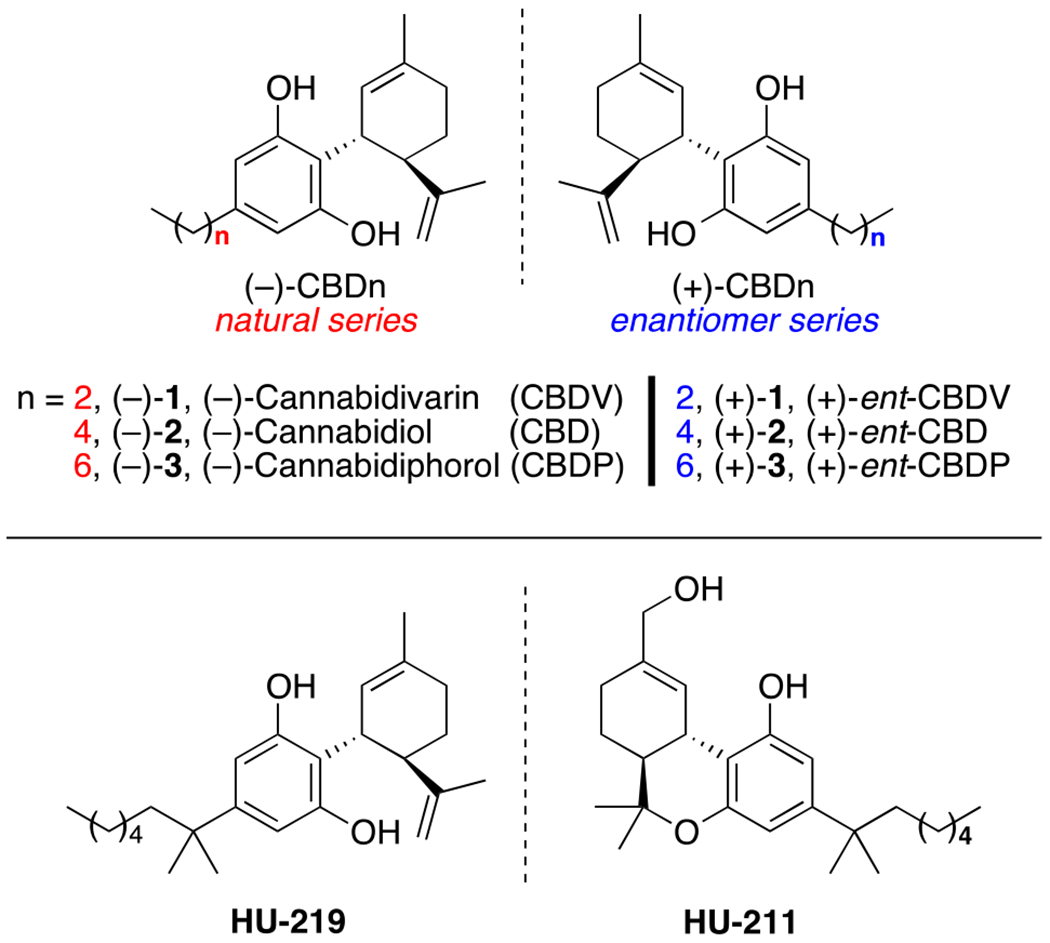
Cannabidiol and related analogs.
Both natural and synthetic cannabinoids have been involved in numerous clinical trials with several approved in multiple countries for their beneficial and quantifiable medicinal applications. While most of these treatments are CBD / THC mixtures, for example, Epidiolex,8b Cannador, 8b and Sativex10 (Nabiximol), some are pure THC-derived drugs, such as Nabilone8b [(±)-Cesamet] and Dronabinol.8b Also of significance, the cannabinoid drug Dexanabinol (HU-211, Fig. 1), based on the (+)-ent-cannabinoid skeletal structure, surprisingly has no affinity for CB1 or CB2 receptors, yet has significant non-competitive antagonist effects on N-methyl-d-aspartic acid.11 This is notable since it is based on HU-219, which is a synthetic and more potent derivative of (−)-CBD.11
While data suggests that (−)-CBD exhibits a low affinity for CB1 (found mainly in the brain) and CB2 (in peripheral cells), its non-natural synthetic enantiomer ent-CBD [(+)-2] and related derivatives are known to have a higher affinity for these same membrane receptors.12 We believe ent-CBD derivatives will continue to prove valuable as novel derivatives of (−)-CBD continue to be explored as potential new therapeutics. To help support this statement, Table 1 shows the nM binding affinities of select cannabinoids towards the CB1 and CB2 receptors, demonstrating that (+)-ent-2 has increased binding when compared to its natural stereoisomer.8c Interestingly, another trend that warrants attention is the increased binding affinity of Δ9-(−)-THC derivatives as their alkyl tails increase in length;13 (−)-THCP, which has a seven carbon tail, binds an order of magnitude tighter to CB1 and CB2 than Δ9-(−)-THC (Table 1).
Table 1.
| Cannabinoid | CB1 Ki (nM) | CB2 Ki (nM) |
|---|---|---|
| (−)-CBDV | >10,000 | >10,000 |
| (−)-CBD | >10,000 | >10,000 |
| (+)-CBD | 842 | 203 |
| (−)-THCV | 22–75 | 62–105 |
| (−)-THC | 18–40 | 36–42 |
| (−)-THCP | 1.2 | 6.2 |
In 2018, the Maio laboratory reported a new synthetic method that allowed for the expedient construction of non-natural CBD derivatives via the Lewis Acid mediated union of (−)-carvone, a readily available and inexpensive starting material, with resorcinol derivatives.14 Importantly, by using (+)-carvone, this protocol also allowed access to enantiomers of the CBD scaffold in only three synthetic operations, two of which are general and can be carried out on gram scale, yielding a relatively stable epoxy-carvone silyl ether. However, difficulty in Δ8 to Δ9–alkene transposition forced us to explore an alternative route for converting our scaffold into (+)-ent-CBD itself, as well as its C-3 and C-7 alkyl chain isomers, (+)-ent-cannabidivarin [(+)-1, ent-CBDV] and (+)-ent-cannabidiphorol [(+)-3, ent-CBDP], respectively, neither of which have been previously prepared in their non-natural, enantiomeric form. Our interest in these latter two derivatives stems from structure activity relationship data that demonstrate the importance of the alkyl chain length and how these derivatives may bind to CB1 and CB2 receptors (Table 1).15 Also of note, natural (−)-CBDV is in early clinical development for the treatment of autism spectrum disorders16 and recently, (−)-CBDP has emerged as a more potent cannabinoid than (−)-CBD itself, making it an alternative to THC therapy without the signature psychoactivity of the latter.17
At the onset of our synthetic campaign, we evaluated the currently known syntheses of (−)- and (+)-CBD, many of which involve the acid-catalyzed union of a terpene derivative with olivetol, several of which are noteworthy here. The report by Petrzilka utilized limonene-derived 5 as one of the coupling partners (Scheme 1), uniting this compound with olivetol (10) under mildly acidic conditions.18 While this processes does permit access to (−)-CBD, its key step suffers from a long reaction time (days), modest yield, and the overall number of steps in which 5 was derived from (+)-4.19 A separate approach, first pioneered by Cardillo8g and later employed by Mechoulam,8c utilized isopiperitenone [(−)-6] as a starting material. From this terpene, (+)-CBD could be accessed in two steps involving (1) LiA1H4 reduction, and (2) treatment of the resultant alcohol mixture (8 and 9) with 10 in the presence of BF3•OEt2. Unfortunately, the relatively high cost of isopiperitenone (in either enantio form, ~$1000/g) challenged us to think of potential ways to synthesize enantiopure 8 from more readily available starting compounds (Scheme 1).20 Recognizing the structural similarity between the southern hemisphere of 8 and (+)-carvone, we began to envision strategies to convert this inexpensive ($0.15/g), caraway-derived terpene into the requisite chiral, non-racemic isopiperitenol.
Scheme 1.
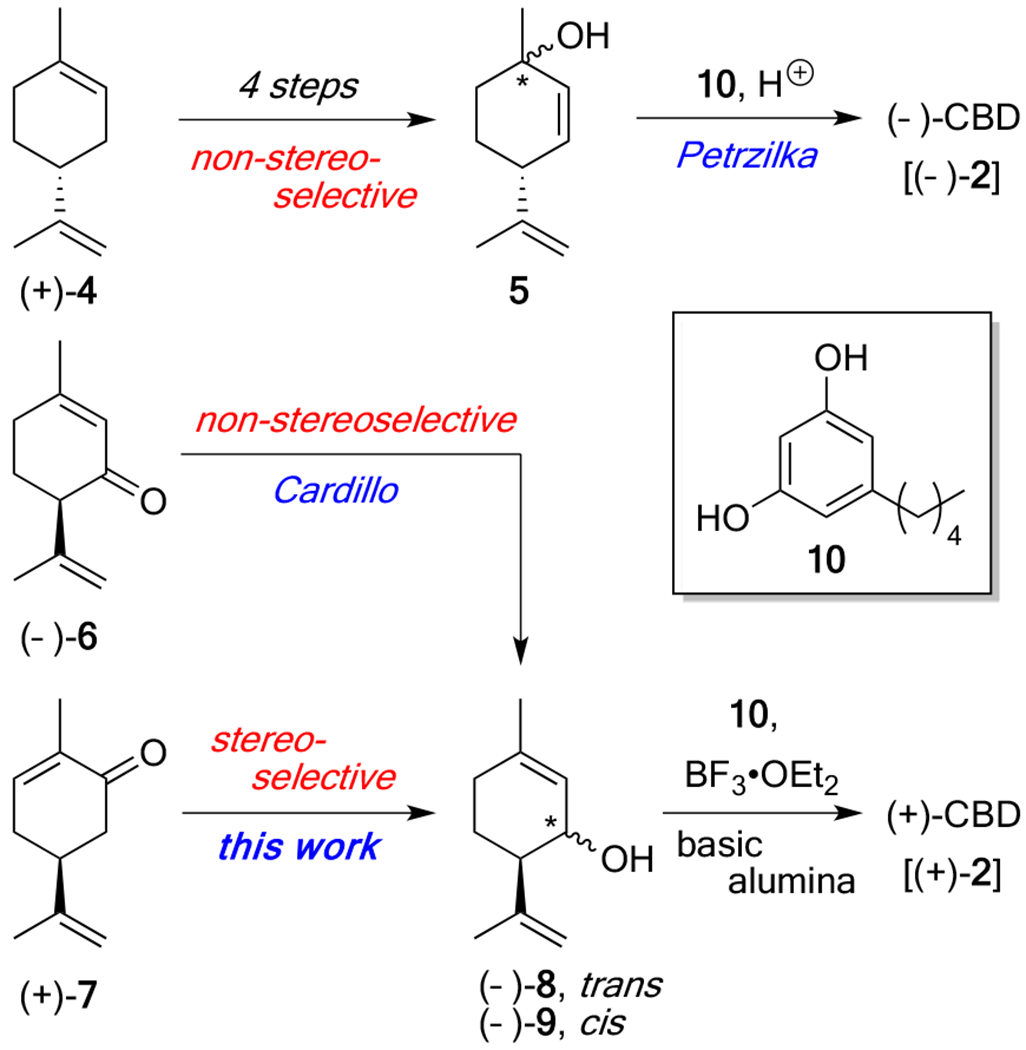
Previous syntheses in the context of this work
Results and Discussion
In terms of retrosynthesis, based on literature precedent, we believed it would be possible to access 8 from tosylhydrazone 12 by exploiting the McIntosh reduction / rearrangement chemistry, which would effectively transpose the alkene from the Δ8 to the Δ9 location (note: cannabinoid notation).21 Hydrazone 12, in turn, could be easily derived from hydroxycarvone 11, which is already known to be the major product formed upon the Rubottom oxidation of (+)-carvone.22
In the forward direction, treatment of (+)-carvone (7) with LDA, followed by the addition of TMSC1 to the in situ-generated enolate allowed access to the corresponding silyl enol ether, which was directly treated with m-CPBA to afford a mixture of α-hydroxycarvone isomers trans-(+)-11 (major) and cis-(+)-13 (minor), respectively. Although the diastereomer ratio and yield oftentimes varied, it consistently provided trans-hydroxycarvone (+)-11 as the major product. Pleasingly, this result was in good agreement with literature precedent for this reaction22b and these C(6)-epimers could be easily separated by flash column chromatography. Next, each of these compounds was separately treated with tosylhydrazide and the corresponding hydrazones [(−)-12 and (+)-14] were successfully subjected to a one-pot reduction / rearrangement21 sequence to afford the desired products, (1S, 6R)-isopiperitenol (−)-8 and (1R, 6R)-isopiperitenol (−)-9 in excellent overall yield (87% and 65%). Notably, the catechol-borane used for this step can be formed in situ for a fraction of the cost.23 Also of note, while previously demonstrated on related systems,21b this alkene transposition reaction has yet to be reported for α-hydroxycarvone. Importantly, this operationally simple and robust 4-step sequence can be carried out on gram scale, representing the first asymmetric total syntheses of (−)-8 and (−)-9 from (+)-carvone, circumventing the need to source these same alcohol products from costly (−)-isoperitenone (6).
Once synthetic (+)-isopiperitenol was in hand, we chose to repeat the Mechoulam buffered Lewis Acid protocol8c for the synthesis of (+)-ent-CBD [(+)-2] before exploiting this same method for the synthesis of novel cannabinoids (+)-ent-CBDV [(+)-1] and (+)-ent-CBDP [(+)-3] (Scheme 3). Pleasingly, when a solution of (−)-8 and olivetol (10) or, separately, (−)-9 and 10 was added to a solution of BF3•OEt2 and basic alumina at reflux, (+)-ent-CBD [(+)-2] was produced as the major product, along with its abnormal regioisomer (+)-abn-CBD [(+)-15] in only 10 seconds and in yields consistent with literature values.8c Also observed, as documented by Crombie,24 was the formation of bis-(+)-16 as a minor by-product. Importantly, these three reaction products have substantially different Rf values, making their separation by flash column chromatography an efficient way in which to separate them (see ESI for a photo of a representative TLC plate). Also, as an interesting side note, when Baek25 repeated this reaction protocol in the absence of basic alumina, union of (−)-8 and 10 was followed by rapid cyclization to form (+)-ent-THC. We found similar results were obtained when the basic alumina was flame-dried prior to use.
Scheme 3.
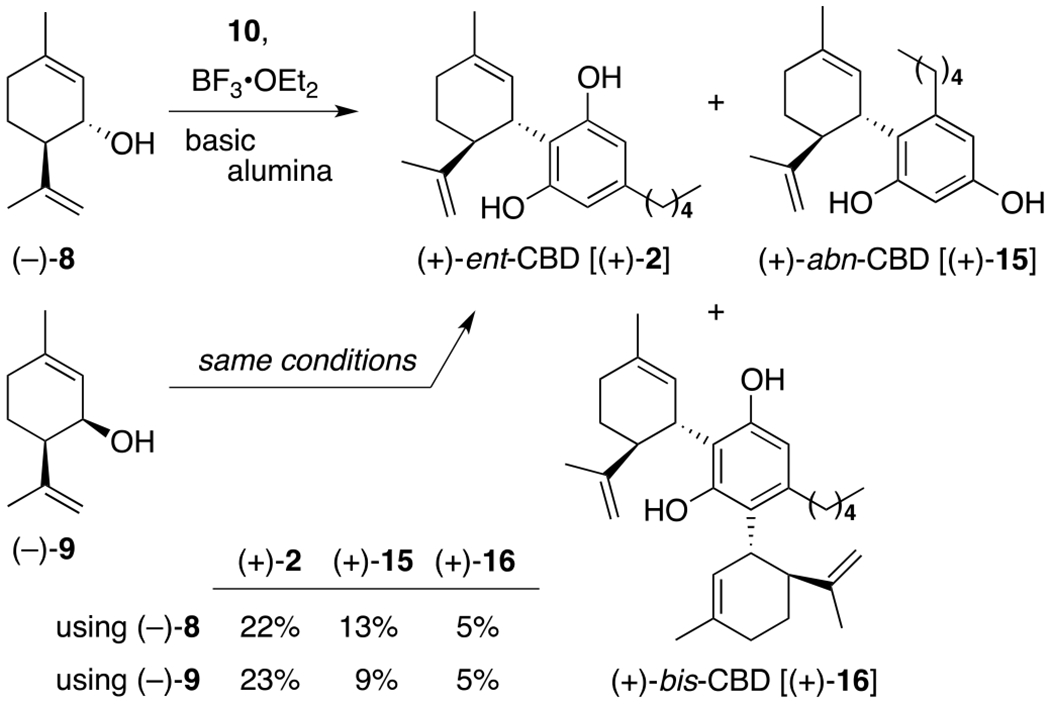
Continuation of the Mechoulam (+)-ent-CBD synthesis.
Encouraged by the successful repetition of the Mechoulam (+)-ent-CBD synthesis, our attention turned to the construction of the C-3 and C-7 alkyl chain isomers, (+)-ent-cannabidivarin [(+)-1] and (+)-ent-cannabidiphorol [(+)-3], the natural stereoisomers of which are both known compounds.24 It was during this time that we also began exploring the literature and discovered that the analogous C-6 isomer [(+)-17, CBD-Hex] was only reported in the patent literature,26 with no synthesis shown, and the C-8 isomer [(+)-18, CBD-Oct] had yet to be proposed. We believed this latter CBD derivative would be of value since a Δ8-(−)-THC-Oct derivative has been previously reported and showed optimal binding to the CB1 and CB2 receptor when compared to its heptyl, pentyl, butyl, and propyl derivatives.27 Clearly, the targeted synthesis of this congener in enantiomeric form, should prove valuable for future study.
In order to target these four derivatives, it was first necessary to synthesize their corresponding resorcinol fragments. In each case, this was easily accomplished in three steps involving, (1) olefination using 3,5-dimethoxybenzaldehyde and the appropriately sized ylide partner (see ESI for details), (2) hydrogenation of the resultant E/Z-alkene mixture, and (3) acid-catalyzed ether cleavage. It should be noted that all three of these operations are relatively high yielding and can be performed without intermediate purification, in a single 8 h period.
Once in hand, each of these C(6)-substituted resorcinol derivatives (19a-d) was separately united with (+)-isopiperitenol [(−)-8] using alumina buffered BF3•OEt2 to afford the corresponding ent-CBD derivative, along with the concomitant formation of their ent-abn-CSD and ent-bis-CBD congeners (Scheme 4, see ESI for full details). Importantly, this represents the first asymmetric total syntheses of (+)-CBDV [(+)-1] and (+)-CBDP [(+)-3], and the first targeted syntheses of the related congeners (+)-CBD-Hex [(+)-17] and CBD-Oct [(+)-18].
Scheme 4.
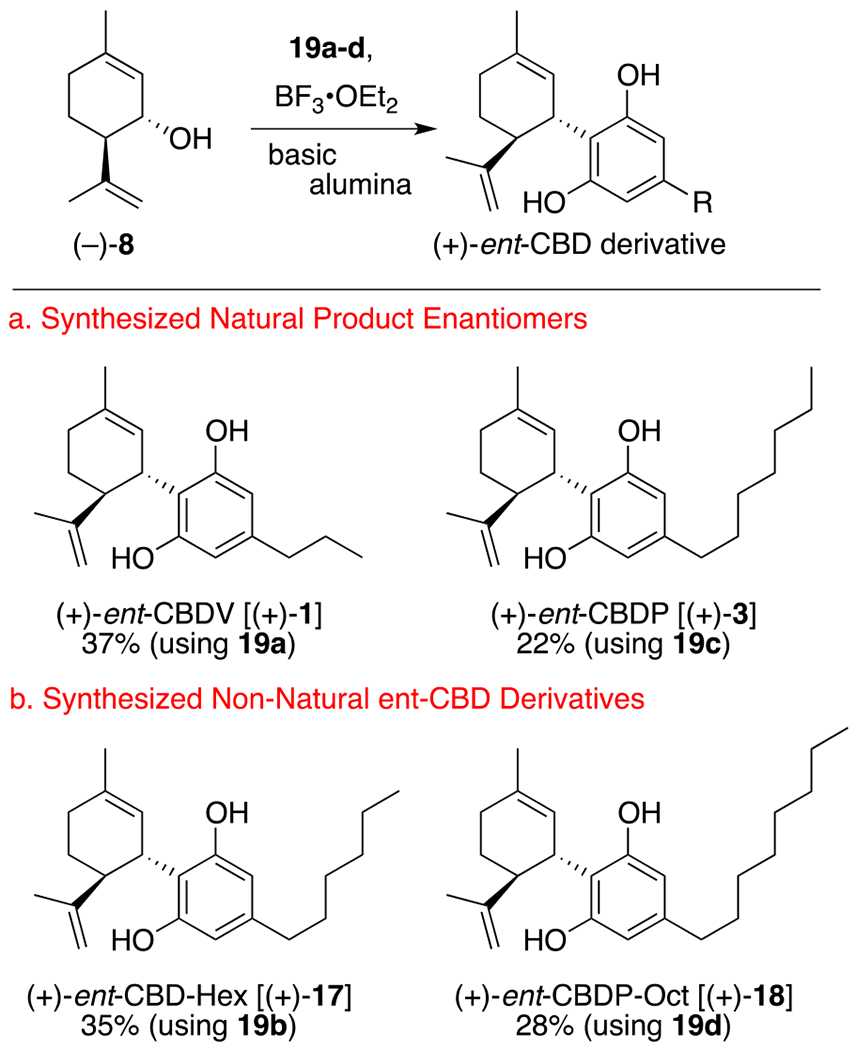
First asymmetric synthesis of (+)-ent-CBDV, CBDP, and related C-6 and C-8 alkyl chain derivatives.
Conclusion
In summary, we report here the first asymmetric synthesis of both (1S, 6R)-isopiperitenol (37% overall) and (1R, 6R)-isopiperitenol (5% overall) in four synthetic steps from (+)-carvone as a starting material. Of note, this was made possible by exploiting the McIntosh alkene trans-position reaction as a key step. We then demonstrated the utility of this protocol by synthesizing (in one additional step for each) the enantiomer of cannabidiol, (+)-CBD (22%), and the related congeners (+)-CBDV (37%), (+)-CBDP (22%), (+)-CBD-Hex (35%), and (+)-CBD-Oct (28%). Also of note, this manuscript reports the first documentation and characterization of nearly all of their associated abnormal and bis-addition byproducts. We believe these enantiomer CBD derivatives will be of great interest and may lead to the discovery of even more active CBD-analogs. We are currently investigating the biological potency of these new ent-CBD derivatives and our findings will be reported in due course.
Supplementary Material
Scheme 2.
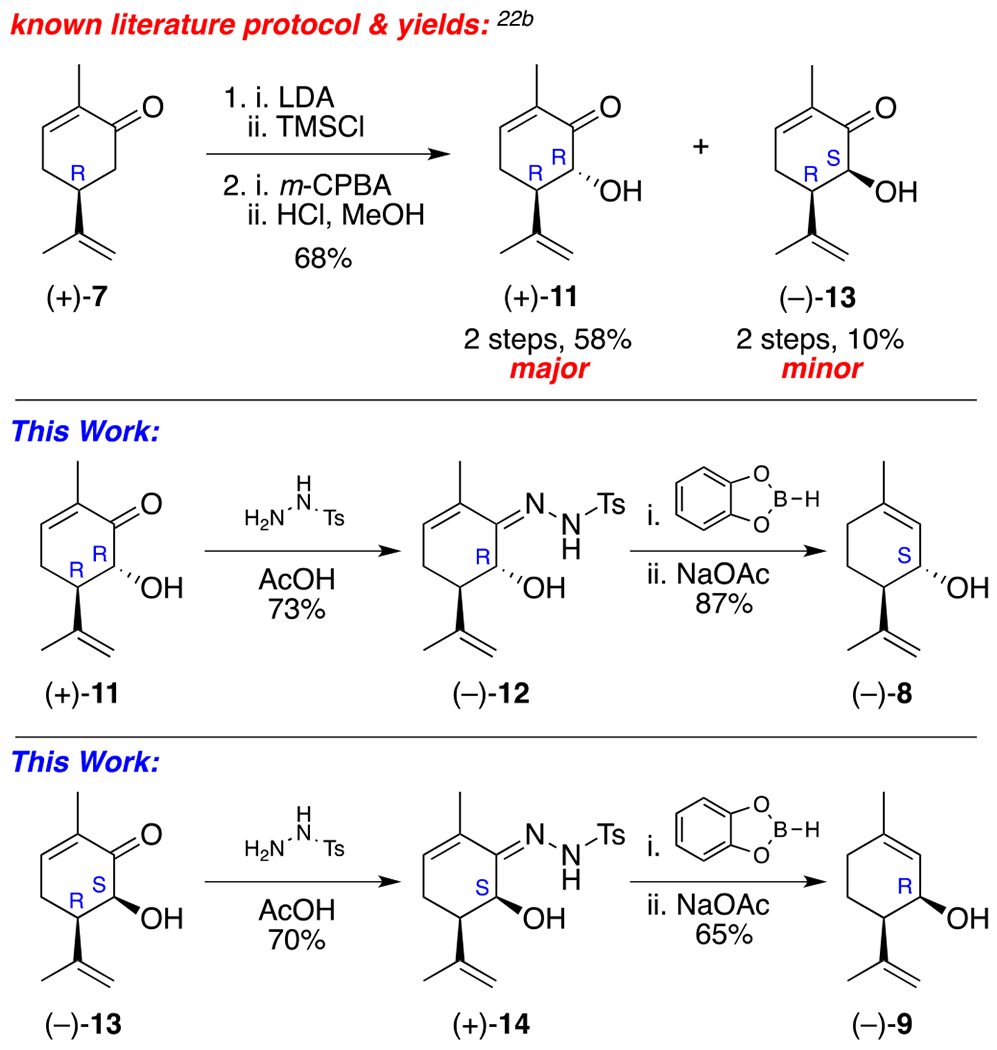
Synthesis of (1S, 6R)-isopiperitenol and (1R, 6R)-isopiperitenol.
Highlights.
Inexpensive production of trans-isopiperitenol from enantiopure carvone
Boron trifluoride mediated coupling of olivetol and isopiperitenol
Robust syntheses of (+)-cannabidiol (CBD), (+)-abn-CBD, and (+)-bis-CBD
First syntheses of (+)-ent-cannabidivarin (CBDV) and (+)-ent-cannabidiphorol (CBDP)
Novel Syntheses of hexyl (CBD-Hex) and octyl (CBD-Oct) CBD-derivatives
Acknowledgments
Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103451. The authors would also like to thank the National Science Foundation (1452489) for seed support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References and notes
- 1.Ito T; Ito H; Oyama M; Tanaka T; Murata J; Darnaedi D; Iinuma M Phytochem. Lett 2012, 5, 325–328. [Google Scholar]
- 2.Interesting examples include: limonene, pinene, and notoamide B, for a full review, see: Finefield JF; Sherman DH; Kreitman M; Williams RM Angew. Chem. Int. Ed 2012, 51, 4802–4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.For a recent example, see: Tello-Aburto R; Newar TD; Maio WA J. Org. Chem 2012, 77, 6271–6289. [DOI] [PubMed] [Google Scholar]
- 4.Mori K Chirality 2011, 23, 449–462. [DOI] [PubMed] [Google Scholar]
- 5.de Fatima A; Kohn LK; de Carvalho JE; Pilli RA Bioorg. Med. Chem 2006, 14, 622–631. [DOI] [PubMed] [Google Scholar]
- 6.(a) Pirrung MC J. Med. Chem 2020, 63, 12131–12136. [DOI] [PubMed] [Google Scholar]; (b) Jung B; Lee JK; Kim J; Kang EK; Han SY; Lee H-Y; Choi IS Chem. Asian. J 2019, 14, 3749–3762. For recent syntheses of (−)-CBD and related analogs, see: [DOI] [PubMed] [Google Scholar]; (c) Shultz ZP; Lawrence GA; Jacobson JM; Cruz EJ; Leahy JW Org. Lett 2018, 20, 381–384. [DOI] [PubMed] [Google Scholar]; (d) Kinney WA; McDonnell ME; Zhong HM; Liu C; Yang L; Ling W; Qian T; Chen L; Cai Z; Petkanas D; Brenneman DE ACS Med. Chem. Lett 2016, 7, 424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Gotz MR; Collado JA; Fernandez-Ruiz J; Fiebich BL; Garcia-Toscano L; Gomez-Canas M; Koch O; Leha A; Munoz E; Navarrete C; Pazos MR; Holzgrabe U Front. Pharmacol 2019, 10, 1284. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Gong X; Sun C; Abame MA; Shi W; Xie Y; Xu W; Zhu F; Zhang Y; Shen J; Aisa HA J. Org. Chem 2020, 85, 2704–2715. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Shabat S; Hanus LO; Katzavian G; Gallily RJ Med. Chem 2006, 49, 1113–1117 [DOI] [PubMed] [Google Scholar]
- 8.(a) Mazzoccanti G; Ismail OH; D’Acquarica I; Villani C; Manzo C; Wilcox M; Cavazzini A; Gasparrini F Chem. Commun 2017, 53, 12262–12265.; [DOI] [PubMed] [Google Scholar]; (b) Rosenberg EC; Tsien RW; Whalley BJ; Devinsky O Neurotherapeutics, 2015, 12, 747–768.; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hanus LO; Tchilibon S; Ponde DE; Breuer A; Fride E; Mechoulam R Org. Biomol. Chem 2005, 3, 1116–1123; [DOI] [PubMed] [Google Scholar]; (d) Papahatjis DP; Nahmias VR; Nikas SP; Andreou T; Alapafuja SO; Tsotinis A; Guo J; Fan P;Makriyannis AJ Med. Chem 2007, 50, 4048–4060.; [DOI] [PubMed] [Google Scholar]; (e) Fride E; Ponde D; Breuer A; Hanus L Neuropharmacology, 2005, 48, 1117–1129.; [DOI] [PubMed] [Google Scholar]; (f) Mechoulam R J. Clin. Pharmacol 2002, 42, 11S–19S. [DOI] [PubMed] [Google Scholar]; (g) Cardillo B; Merlini L, Servi S Tetrahedron Lett. 1972, 13, 945–948. [Google Scholar]; (h) Fride E; Feigin C; Ponde DE; Breuer A; Hanus L; Arshavsky N; Mechoulam R Eur. J. Pharmacol 2004, 506, 179–188. [DOI] [PubMed] [Google Scholar]
- 9.Heneka MT; Carson MJ; Landreth GE; Brosseron F; Feinstein DL; Jacobs AH; Wyss-Coray T; Victorica J; Ransohoff RM; Herrup K; Frautschy SA; Finsen B; Brown GC; Verkhratsky A; Yamanaka K; Koistinaho J; Latz E; Halle A; Petzold GC; Town T; Morgan D; Shinohara ML; Perry VH; Holmes C; Bazan NG; Brooks DJ; Hunot S; Joseph B; Deigendesch N; Garaschuk O; Boddeke E; Dinarello CA; Breitner JC; Cole GM; Golenbock DT; Kummer MP Lancet. Neurol 2015, 14, 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maccarrone M; Bab I; Biro T; Cabral GA; Dey SK; Di Marzo V; Konje JC; Kunos G; Mechoulam R; Pacher P; Sharkey KA; Zimmer A Trends in Pharm. Sci 2015, 36, 277–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pop E Curr. Pharm. Design, 2000, 6, 1347–1359. [DOI] [PubMed] [Google Scholar]
- 12.Bisogno T; Hanus L; De Petrocellis L; Tchilibon S; Ponde DE; Brandi I; Moriello AS; Davis JB; Mechoulam R; Di Marzo V Brit. J. Pharm 2001, 134, 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.An D; Peigneur S; Hendrickx LA; Tytgat J Int. J. Mol. Sci 2020, 21, 5046, 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey SJ; Sapkota RS; Golliher AE; Dungan B; Talipov M; Holguin FO; Maio WA Org. Lett 2018, 20, 4618–4621. [DOI] [PubMed] [Google Scholar]
- 15.Chung H; Fierro A; Pessoa-Mahana CD. PLoS ONE, 2019, 14, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong X; Sun C; Abama MA; Shi W; Xie Y; Xu W; Zhu F; Zhang Y; Shen J; Aisa HA J. Org. Chem. 2020, 85, 4, 2704–2715. [DOI] [PubMed] [Google Scholar]
- 17.Citti C; Linciano P; Russo F; Luongo L; Iannotta M; Maione S; Lagana A; Capriotti AL; Forni F; Vandelli MA; Gigli G; Cannazza G Sci. Rep 2019, 9, 20335, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrzilka T; Haefliger W; Sikemeier C; Ohloff G; Eschenmoser A Helv. Chim. Acta 1967, 50, 719–723. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinsons SM; Price J; Kassiou M Tetrahedron Lett. 2013, 54, 52–54. [Google Scholar]
- 20.For a recent review on chiral pool natural products, see: Brill ZG; Condakes ML; Ting CP; Maimone TJ Chem. Rev 2017, 117, 11753–11795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(a) Chai Y; Vicic DA; McIntosh MC Org. Lett. 2003, 5, 1039–1042. [DOI] [PubMed] [Google Scholar]; (b) Bateman DT; Joshi AL; Moon K; Galitovskaya EN; Upreti M; Chambers TC; McIntosh MC Bioorganic Med. Chem. Lett 2009, 19, 6898–6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.(a) Ardashov OV; Pavlova AV; Il’ina IV; Morozova EA; Korchagina DV; Karpova EV; Volcho KP; Tolstikova TG; Salakhutdinov NF J. Med. Chem 2011, 54, 3866–3874. [DOI] [PubMed] [Google Scholar]; (b) dos Santos RB; Brocksom TJ; Zanotto PR; Brocksom U Molecules, 2002, 7, 129–134. [Google Scholar]
- 23.Waltz KM; Hartwig JF J. Am. Chem. Soc 2000, 122, 11358–11369. [Google Scholar]
- 24.Crombie L; Crombie WML Phytochemistry 1975, 14, 213–220. [Google Scholar]
- 25.Baek S; Srebnik M; Mechoulam R; Tetrahedron Lett. 1985, 26, 1083–1086. [Google Scholar]
- 26.Horwitz A; D’Espaux L; Wong J; Bector R; Hjelmeland AK; Platt D; Ubersax J U.S. Patent 2020069214, 2019, (patent application).
- 27.Martin BR; Jefferson R; Winckler R; Wiley JL; Huffman JW; Crocker PJ; Saha B; Razdan RK J. Pharmacol. Exp. Ther 1999, 290, 1065–1079. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


