Abstract
While adoption studies have provided key insights into the influence of the familial environment on IQ scores of adolescents and children, few have followed adopted offspring long past the time spent living in the family home. To improve confidence about the extent to which shared environment exerts enduring effects on IQ, we estimated genetic and environmental effects on adulthood IQ in a unique sample of 486 biological and adoptive families. These families, tested previously on measures of IQ when offspring averaged age 15, were assessed a second time nearly two decades later ( M offspring age = 32 years). We estimated the proportions of the variance in IQ attributable to environmentally mediated effects of parental IQs, sibling-specific shared environment, and gene-environment covariance to be .01 [95% CI .00, .02], .04 [95% CI .00, .15], and .03 [95% CI .00, .07] respectively; these components jointly accounted for 8 percent of the IQ variance in adulthood. The heritability was estimated to be .42 [95% CI .21, .64]. Together, these findings provide further evidence for the predominance of genetic influences on adult intelligence over any other systematic source of variation.
Keywords: Intelligence, adoption, heritability, vocabulary, polygenic scores
Introduction
Cognitive ability tends to aggregate strongly in families, with both genetic and environmental factors contributing to family resemblance. A long history of twin and family studies has found that between 50 and 80 percent of variance in IQ is associated with genetic factors in industrialized countries, with perhaps 50 percent attributable to additive genetic factors alone (Hunt, 2011; Polderman et al., 2015). However, this imprecision owes in part to the inclusion of individuals over a wide range of ages, and studies of fully-adult individuals have found a heritability of intelligence closer to .80 (Warne, 2020). While these estimates are largely derived from twins and parent-offspring pairs, adoption studies have the ability to more precisely disentangle genetic and environmental sources of variance, as adopted individuals share all of their rearing environments but none of their genetic variance with their adoptive families (Plomin et al., 2013). For this reason, researchers consider adoption to be one of the most powerful ways to test for the presence of environmental influence on the development of intelligence (Plomin & DeFries, 1980; Horn et al., 1979).
Observational studies of adopted children and adolescents have consistently found a significant positive effect of their rearing environment on their developing IQs. The role of the rearing environment can be examined both through the correlations between the IQs of adopted individuals and their adoptive family members and through the difference between their IQs and those of their biological relatives remaining in their original rearing environments. Since samples of adopted individuals with known biological parents are rare, much of our current understanding comes from correlations between adoptive relatives; however, several studies looking at the “treatment effect” of adoption have found consistent results. For example, a meta-analysis conducted by van Ijzendoorm et al. (2005) reported that adoption generally improves the IQs of children relative to their biological parents. One such classic study found a mean increase in IQ of adopted-out children relative to their birth mothers roughly equivalent to 13 points after correcting for the Flynn Effect (Skodak & Skeels, 1949; Flynn, 1993).
The question of persistence is perhaps the most important in considering the effects of the rearing environment on IQ, especially since other types of studies have documented a “fadeout” of environmental improvements over time (e.g., Protzko, 2015). Kendler and colleagues have used a cosibling control design to examine the effect of the rearing environment on IQ in a sample of 436 adoptive-biological sibships (Kendler et al., 2015). These male, 18–20 year old adopted Swedish conscripts showed a mean gain in 4.41 IQ points relative to their biological siblings, who were raised by the original biological family. This finding, which they replicated in a larger sample of half-sibs (with a mean gain of 3.18 IQ points associated with adoption), is a strong indicator that IQ can be, to some extent, affected up to late adolescence by the family environment. These results are consistent with those from the classic cross-fostering study of 14-year-old French children by Capron & Duyme (1989).
Studies such as these do suggest that although this effect is small relative to the genetic effects on IQ, it is not zero; however, the size of this effect diminishes substantially after adolescence. Sandra Scarr, a pioneer of modern IQ adoption studies, was perhaps the first to note this fadeout phenomenon (e.g., Scarr & Weinberg, 1978). With respect to the tapering correlations in IQ between children and their adoptive parents as the child matured, observed in the Minnesota Adolescent Adoption Study, Scarr & Weinberg remarked:
We interpret the results to mean that younger children, regardless of their genetic relatedness, resemble each other intellectually because they share a similar rearing environment. Older adolescents, on the other hand, resemble one another only if they share genes. Our interpretation is that older children escape the influences of the family and are freer to select their own environments. Parental influences are diluted by the more varied mix of adolescent experiences. (Scarr & Weinberg, 1983, p. 264)
Scarr’s observation would form the basis of a set of hypotheses regarding gene-environment correlation (rGE) and its three types of expression: passive, evocative (often referred to as “reactive”), and active (Scarr & McCartney, 1983; Plomin et al., 1977). While traditional twin and adoption studies of heritable human traits typically strive to estimate how much variation in a phenotype is due to differences among genotypes, Scarr’s popularization and exploration of this new terminology sought to move the focus onto processes as they occur over time, rather than static estimates at a given time point. Consequently, this exploration has led to many new questions about developmental processes and trajectories.
Gene-environment correlation
Today, gene-environment correlation is widely recognized among IQ researchers, with some authors estimating that its effects might account for up to 30 percent of the variance in adult IQ (Johnson et al., 2011). And while the heritability of IQ gradually increases over the lifespan, research has also shown that this may be explained in part by a concordant increase in active gene-environment correlation (Haworth et al., 2010). A continuous increase of active gene-environment correlation over the life course may also explain the high genetic correlation between childhood and adult IQ inferred from biometrical studies and GWAS in the simultaneous presence of the increasing heritability of IQ (Briley & Tucker-Drob, 2013; Sniekers et al., 2017). Passive gene-environment correlation, by contrast, occurs necessarily in the presence of parental effects, but can be inflated by mechanisms unrelated to parental involvement. For example, it can sometimes be induced by population stratification (Balbona et al., 2021). In biological families, passive gene-environment correlation driven by the parents can manifest in a number of ways. For example, a child may inherit from her parents both genes for high verbal ability and access to the means to use this ability—such as encouragement in learning to read by her highly verbal parents—which may increase the child’s verbal ability over time.
A formalization of this notion is a path model with directed edges going from the parental phenotypes to the phenotype of their offspring (Figure 1). Models of this type were proposed by Eaves (1976) and Cloninger et al. (1979) among others. If the phenotype is verbal ability, say, then a nonzero path coefficient means that a highly verbal parent exerts a positive environmentally mediated effect on the offspring’s own verbal ability. This mechanism results in passive gene-environment correlation because offspring receiving genes for a particular level of ability also tend to be raised by parents providing an environment with correlated effects on ability (Figure 1). In a study of both biological and adoptive parent-offspring sets, one can use biometrical methods to estimate the extent of passive gene-environment correlation induced by this mechanism.
Figure 1:
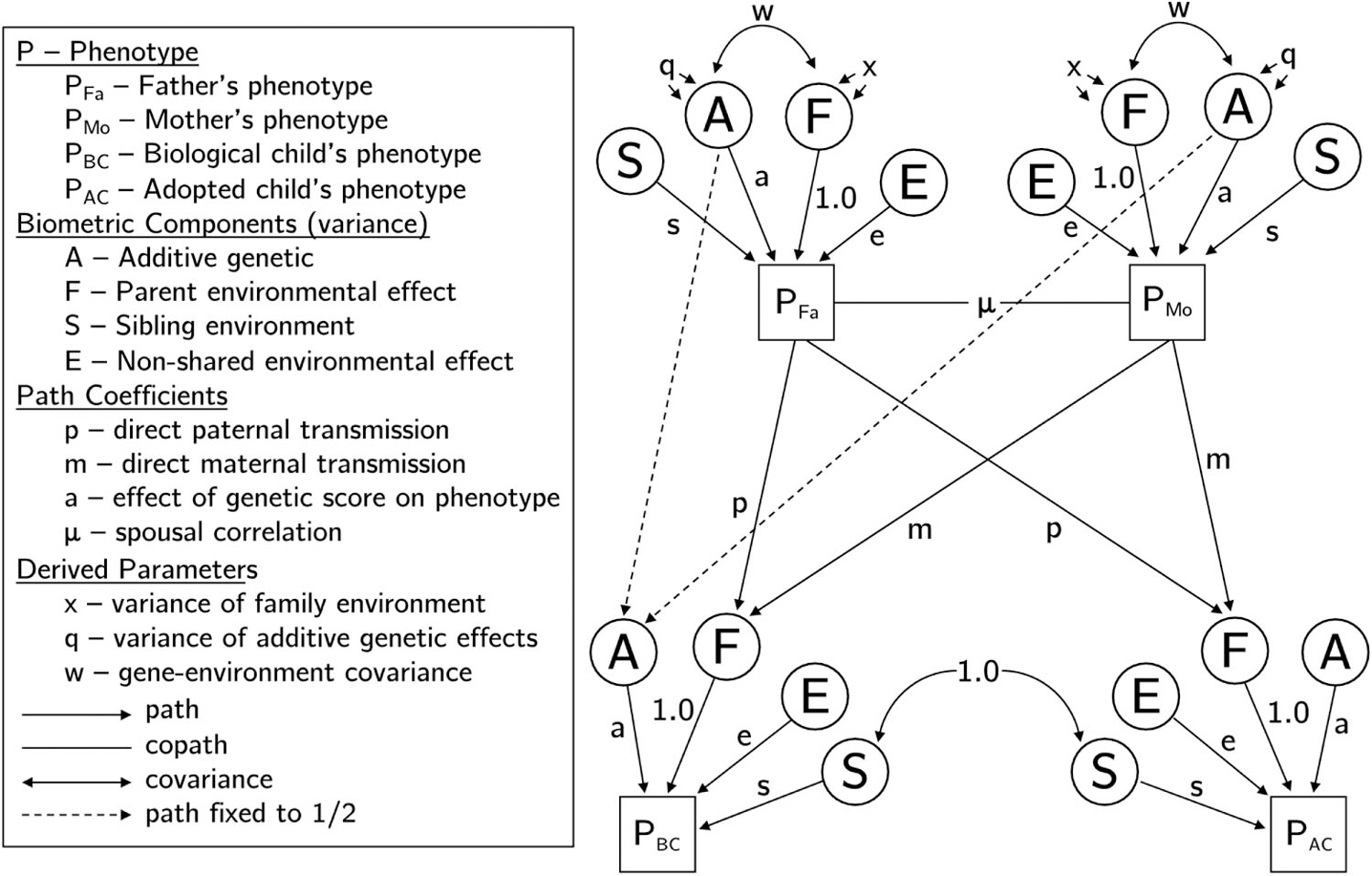
Path diagram following Figure 1 from Keller et al. (2009) illustrating variance components and effects in an example family consisting of a father, a mother, one biological offspring, and one adopted offspring. Values for paths are as labeled except for the dashed paths from the genetic value of the parent to the genetic value of the biological offspring, which are fixed to 1/2 according to standard genetic theory. Components S and E have variance = 1; variance of F (x) and A (q) are noted in supplementary section 1.
Current study
At the Minnesota Center for Twin and Family Research (MCTFR), the Sibling Interaction and Behavior Study (SIBS) has followed a sample of adoptive and biological Minnesota families for nearly two decades. With initial IQ assessments having been conducted when offspring were approximately 15 years of age, the current SIBS assessment wave will comprise the first study of cognitive ability in an adoption sample that includes fully genotyped individuals, one or both parents, and sibling pairs of offspring who now average above age 30, well past the typical rearing period of American families (Dey & Pierret, 2014). With several measures of IQ from both childhood and adulthood, our central aim was to conduct biometric decomposition to estimate the proportions of heritability, non-genetic familial influence, and gene-environment covariance that contribute to the IQ scores of adult offspring using an adaptation of the extended twin-family design “Cascade” model (Keller et al., 2009; Eaves, 2009).
Although adoption studies are a powerful tool for investigating genetic and non-genetic effects on IQ, they are not without their potential issues. One common criticism of biometrical estimates derived from adoptive samples (e.g., of twins reared apart) is that such estimates are unreliable because adopted offspring may have been selectively placed in their adoptive families based on some perceived similarity to the adopting parents (Loehlin et al., 1997). Although previous research has shown that moderate placement effects do not strongly influence parent-offspring correlations (Bouchard Jr. & McGue, 1990; Loehlin et al., 1997) and such effects are unlikely to occur in the present sample, who are predominantly international placements with limited information on birth background (McGue et al., 2007), a placement effect for IQ in adoptive families could nevertheless pose a threat to the validity of its biometrical estimates.
We tested for placement effects using polygenic scores for educational attainment ( PGSEA ) derived from the largest genome-wide association study to date (Lee et al., 2018). These scores, which were available for approximately 90% of the present sample, are able to predict 11–13 percent of the variance in years of education and 8–10 percent in IQ, with which it is highly correlated. In addition to validating the predictive accuracy of PGSEA on IQ phenotypes in the current sample, parent and offspring scores enable an empirical test of adoption placement for IQ and related phenotypes. We test for the presence of placement effects by comparing the correlations between parent and offspring PGSEA to their theoretically predicted values, which—in the absence of placement effects—should not differ significantly from zero in adoptive families.
It is important to understand that placement bias and passive gene-environment correlation are not equivalent concepts. Placement bias is a threat to the validity of inferences drawn from adoption studies, whereas gene-environment correlation is usually thought of as a natural phenomenon occurring in populations of intact biological families. If adoptees with genotypes causing higher IQ are placed in home environments that also foster higher IQ, then we have a case of both placement bias and a kind of passive gene-environment correlation in this sample of adoptive families. In the majority of families, however, there may be no passive gene-environment correlation at all. In this paper we use “gene-environment correlation” and related terms to mean heritable traits of the parents exerting environmentally mediated effects on the same traits in their biological offspring. If there is no placement bias in the adoptive families, then this kind of gene-environment correlation does not occur among them. Note that in Figure 1 this situation is represented by the various paths connecting the A and F of the biological offspring but not those of the adopted offspring.
Method
Participants
Participating families were originally assessed on IQ measures between 1998 and 2004 as part of the Sibling Interaction and Behavior Study (SIBS) (McGue et al., 2007), a sample of adoptive and biological families recruited by the Minnesota Center for Twin and Family Research (MCTFR). Adoptive and biological families were identified from adoption agencies and Minnesota birth records, and are composed of at least one parent and two offspring who were adolescents at the time of intake (M age = 14.9 years, SD = 1.6). Study eligibility was limited to those families living within driving distance of the research lab and having adolescent offspring within five years of age of each other. In addition, adolescents in adoptive families were required to have been placed for adoption before reaching two years of age (M = 4.7 months, SD = 3.4 months). At least one parent from each participating family was interviewed to establish family eligibility, most of whom agreed to participate in the study (63 percent of the adoptive families and 57 percent of the biological families). Valid measures of IQ are available from this intake sample for 461 mothers, 46 fathers, 690 adoptive and 538 biological offspring. No information on the biological parents was available for all adopted offspring.
Eligible parents and sibling pairs from this sample began their third follow-up assessment in 2017 via phone interview and mailed or online survey. At the end of this follow-up in summer 2020, at least two members from a total of 486 families had participated in current IQ assessment, which includes 226 adoptive families, 164 biological families, and 96 mixed families which have both biological and adopted offspring. A total of 764 offspring, composed of 415 adopted and 347 biological individuals, now average 31.8 years of age (SD = 2.7) and are all fully adult (minimum age = 24.7 years; maximum age = 40.5 years). Comparison of non-participants to current participants on intake measures reveals a small amount of attrition associated with IQ, though all standardized mean differences (d) were less than .20 in absolute value, suggesting minimal attrition bias (SI Table S1). An overview of the sample at intake and follow-up 3 is shown in Table 1.
Table 1:
Description of SIBS sample
| Sample | Number of individuals | M age at intake (SD) | M age at follow-up 3 (SD) |
|---|---|---|---|
| Parents | |||
| Mothers | 419 | 46.6 (4.2) | 63.9 (4.8) |
| Fathers | 201 | 48.2 (4.4) | 65.4 (4.7) |
| Adopted offspring | |||
| Female | 235 | 15.2 (2.2) | 32.5 (2.8) |
| Male | 127 | 14.8 (1.8) | 31.6 (2.5) |
| Biological offspring | |||
| Female | 186 | 14.9 (1.9) | 31.5 (2.5) |
| Male | 120 | 14.8 (1.8) | 31.1 (2.6) |
Note: Number of individuals listed represents those with valid scores for either Vocabulary or the ICAR-16 at follow-up 3. Valid N for each intake measure are shown in SI Table S2.
Sample ethnicity.
The ethnic composition of offspring in this sample is additionally unique; while 95 percent of parents and biological offspring are of non-Hispanic white European ancestry, 21 percent of the adoptive offspring are white, 66 percent are Asian, and 13 percent are of other ethnicities (McGue et al., 2007; Miller et al., 2012). McGue et al. (2007) reported minimal ethnicity effects in the SIBS sample at intake, which we largely replicate in the current follow-up assessment. While rearing family socioeconomic status and polygenic scores were both moderately higher among Asian offspring (Cohen’s = d .36–.46; p < .01 ), no measure of cognitive ability differed significantly between offspring of different ethnicities. See SI Table S6 for these and other comparisons, along with a discussion of their relevance.
Measures
In addition to the measures described below, age, years of education, and highest degree achieved were also included in some analyses, along with family socioeconomic status (SES), which is computed as a standardized composite of Hollingshead job status, years of education, and income as determined at initial assessment. Total valid entries, means and standard deviations for each scale and demographic measure for mothers, fathers and offspring for adoptive and biological families are given in SI Table S2.
Cognitive ability scales
All eligible offspring were assessed on IQ at intake, and one parent from each family was assessed at the first follow-up. IQ scores were assessed using an abbreviated form of either the Wechsler Adult Intelligence Scale–Revised (WAIS-R) (Wechsler, 1981) for participants age 16 years and older (26.7 percent of the offspring sample), or the Wechsler Intelligence Scale for Children–Revised (WISC-R) (Wechsler, 1974) for those younger than 16 (73.3 percent of sample). Available measures at intake include age-scaled Verbal, Performance and Total IQ, as well as age-scaled subtest scores for Vocabulary, Information, Block Design, and Picture Completion. Following intake assessment, one individual with an IQ below 70 was dropped from subsequent assessments (McGue et al., 2007).
At follow-up 3, offspring were again administered the Vocabulary subtest; both parents and offspring were administered the ICAR-16, a measure of general intelligence. The ICAR-16 is a 16-item short form of the International Cognitive Ability Resource assessment, a public-domain cognitive assessment tool created by Condon & Revelle (2014). This reliable measure (see SI Tables S3, S4 and S5) of cognitive ability is useful as a short-form stand in for general intelligence.
Intercorrelations across all measured variables are shown for the full sample, both parents and offspring in each family type in SI Tables S8–S10. Adopted and biological offspring differed significantly in ICAR-16 and intake Information scores; these differences are shown in SI Table S7.
Polygenic scores
Approximately 90 percent of the SIBs follow-up 3 sample have valid genotype data, which was assessed as part of an MCTFR genome-wide association study (Miller et al., 2012) and from which polygenic scores (PGS) for educational attainment were derived in the current sample. Participating individuals were genotyped on 527,829 single-nucleotide polymorphisms (SNPs) using the Illumina Human660W-Quad array. A polygenic score, often called the polygenic risk score in disease prediction, is calculated from a set of SNPs that are tested in the initial sample for association with a trait of interest. We used a PGS for educational attainment ( PGSEA ) derived from Lee et al. (2018) to test for placement effects and predictive utility for measures of IQ in the current sample. See Supplementary Information: Polygenic scores for methodological detail on PGS construction, and Miller et al. (2012) for additional details on sampling, ancestry control, assessment, quality control, and imputation performed on the MCTFR samples.
Parent-offspring correlations for polygenic score use the mean PGSEA of mother and father, henceforth denoted as the “midparent” value. Since PGSEA were available for the majority of parents in this sample (79 percent of fathers; 89 percent of mothers), midparent PGSEA analyses were conducted using only families with valid scores for both parents. Under genetic theory, one would expect midparent-offspring PGS correlations close to their theoretically predicted values of for biological parent-offspring pairs (Wright, 1931) and ~ 0 for adoptive pairs; in adoptive pairs, significant deviation from zero would suggest that adoption placement effects may be operating on IQ, with which educational attainment is highly genetically correlated (Lee et al., 2018). While such an observed deviation would constitute positive evidence for placement, the lack of such an effect would not definitely show that placement for IQ is implausible, as educational attainment may not be the only relevant variable related to potential parenting effects on IQ.
Predictive utility of PGSEA on cognitive phenotypes was assessed separately for white and Asian subsamples. A total of 810 white and 365 Asian individuals (including parents and offspring) had valid PGSEA and full-scale IQ data, affording the ability to detect a correlation between PGSEA and full-scale IQ as small as .12 ( r2 = .014 ) in the white sample, and a correlation as small as .18 ( r2 = .032 ) in the Asian sample at p < .01 and with 80% power. For the testing of placement effects, a total of 258 midparent-offspring pairs had valid PGSEA in the adoptive Asian sample, affording the ability to detect a placement correlation as small as .21 at p < .01 and with 80% power.
Biometric modeling
To estimate variance components, including the heritability, we adapted Keller et al. (2009)’s extended twin-family design (ETFD) “Cascade” model, which is itself a development of earlier work (Heath et al., 1985; Fulker, 1988; Truett et al., 1994). After applying Fisher’s z-transformation (e.g., Eaves et al., 2008), we adjusted parameter estimates to minimize the squared differences between the empirical correlations and theoretical (model-predicted) correlations. Each sum of squared differences term was weighted by the reciprocal of its variance ( N ‒3, where N is the number of pairs in the correlation). We constrained any variance to be non-negative. To perform statistical inference, we took bootstrap resamples of our families and re-estimated the parameters iteratively. Additionally, we constrained the dominance genetic variance to equal zero. Since there is compelling theory and evidence for most genetic variance being additive (Hill et al., 2008; Maki-Tanila & Hill, 2014) and dominance variance being negligible (Hivert et al., 2021; Pazokitoroudi et al., 2021), and the point estimate of this parameter was usually zero for most IQ phenotypes, we constrained this parameter to zero in order to improve statistical inference about other parameters.
For each phenotype, heritability ( h2 ) = a2q ; sum of maternal + paternal environment (Cmp ) = m2 + p2 + 2mpμ ; sum of maternal + paternal G-E covariance is equivalent to 2wa. Although maternal and paternal environment parameters were estimated independently for each phenotype (SI Table S12), no evidence was observed for significantly different contributions between parents, perhaps due to the relatively small sample of fathers. μ refers to the phenotypic correlation between parents, which is modeled with a copath (edge with no arrows) via special rules clarified by van Eerdewegh (1982). The modeling assumes that the correlation between parents is entirely the result of phenotypic matching, the effects of which on the additive genetic variance have reached an equilibrium. A special type of edge with distinctive path-tracing properties is required because a correlation due to phenotypic matching is not explained by one variable affecting the other or both being affected by a common cause, as assumed in elementary accounts of structural equation modeling (Lee, 2012). Details of parameter estimates and variance components, along with model assumptions and justifications, can be found in the Supplementary Material.
Decomposition of variance terms presented in Table 3 were computed from these parameters. Refer to Supplementary Information (“Methods”) for more detail.
Table 3:
Decomposition of variance [95% CI] for each measure and subtest of cognitive ability
| A | C | G-E covariance | E | ||
|---|---|---|---|---|---|
|
|
|
||||
| Heritability ( h2 ) | Parental environment | Sibling environment | Non-shared environment | ||
| Intake | |||||
| Performance IQ | .26 [.14, .40] | .02 [.01, .07] | .08 [.01, .14] | .06 [.01, .11] | .58 [.50, .64] |
| Verbal IQ | .36 [.26, .46] | .03 [.00, .10] | .07 [.00, .15] | .10 [.04, .14] | .44 [.34, .53] |
| Total IQ | .32 [.20, .45] | .04 [.01, .10] | .06 [.00, .13] | .11 [.04, .15] | .47 [.38, .56] |
| Block Design | .32 [.19, .45] | .03 [.01, .07] | .03 [.00, .10] | .09 [.03, .13] | .53 [.46, .60] |
| Picture Completion | .08 [.00, .21] | .02 [.00, .06] | .03 [.00, .10] | .01 [.00, .04] | .86 [.80, .89] |
| Vocabulary | .37 [.27, .49] | .04 [.01, .09] | .02 [.00, .09] | .12 [.05, .16] | .45 [.37, .55] |
| Information | .28 [.19, .39] | .02 [.00, .06] | .12 [.05, .18] | .05 [.01, .08] | .53 [.48, .60] |
| Follow-up 3 | |||||
| ICAR-16 | .42 [.21, .64] | .01 [.00, .03] | .04 [.00, .15] | .03 [.00, .07] | .50 [.44, .57] |
| Vocabulary | .12 [.00, .25] | .10 [.03, .23] | .06 [.00, .15] | .08 [.00, .13] | .64 [.53, .75] |
Note: 95% CIs are computed from each parameter’s 200 bootstrap iterations (Efron & Tibshirani, 1993) for each scale. Non-shared environment is computed by subtracting the heritability, parental environment, sibling environment, and gene-environment (G-E) covariance from 1. For full parameter estimates, see SI Table S12. Column values add up to 1, total phenotypic variance.
Results
Measures of full-scale IQ taken at follow-up 3 were moderately correlated over time for both offspring and parents (all p < .001). For offspring, ICAR-16 scores (follow-up 3) correlated with intake Total IQ at r = .46 [95% CI .40, .52]; for parents, this correlation was also r = .46 [95% CI .38, .53]. The moderate size of these correlations partially reflects the approximately 17-year gap between testing sessions, especially for offspring who were of adolescent age at intake assessment (see, e.g., Hoekstra et al., 2007), but are nevertheless within the range reported by Condon & Revelle (2014) for the ICAR-16 and various measures of general ability.
Offspring Vocabulary scores taken at intake correlated with their Vocabulary scores at follow-up 3 at r = .53 [95% CI .47, .58]. SI Tables S8–S10 show full correlation tables between these and other intake measures of IQ for each sub-sample of individuals.
Familial correlations
At intake, parent-offspring correlations were significant and moderately sized only in biological families, with two exceptions (Table 2). Picture Completion showed very low correlations between all relatives except father and biological child ( r = .30 ). Vocabulary was an exception in that the correlations between adoptive relatives were also moderate in size.
Table 2:
Observed and model-predicted correlations for each measure and sub-measure of IQ and ICAR-16 score
| Parent correlations | Sibling correlations | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Mom/bio | Dad/bio | Mom/adopt | Dad/adopt | Dad/Mom | Bio/bio | Adopt/bio | Adopt/adopt | SRMR | |
| Intake measures | |||||||||
| Performance IQ | .10 | ||||||||
| Observed | .28 | .41 | .12 | −.08 | .08 | .26 | .27 | .08 | |
| Expected | .28 | .25 | .12 | .10 | .08 | .30 | .13 | .11 | |
| Verbal IQ | .04 | ||||||||
| Observed | .35 | .41 | .07 | .19 | .40 | .43 | .24 | .07 | |
| Expected | .35 | .46 | .07 | .19 | .40 | .42 | .16 | .12 | |
| Total IQ | .05 | ||||||||
| Observed | .36 | .46 | .09 | .16 | .30 | .34 | .30 | .09 | |
| Expected | .34 | .43 | .11 | .20 | .30 | .39 | .16 | .11 | |
| Block Design | .11 | ||||||||
| Observed | .32 | .41 | .14 | −.10 | .03 | .28 | .32 | ~ 0 | |
| Expected | .32 | .28 | .14 | .10 | .03 | .31 | .11 | .07 | |
| Picture Completion | .08 | ||||||||
| Observed | .09 | .30 | .04 | .01 | .13 | .07 | .19 | .02 | |
| Expected | .09 | .18 | .04 | .13 | .13 | .11 | .06 | .05 | |
| Vocabulary | .05 | ||||||||
| Observed | .39 | .44 | .07 | .26 | .37 | .39 | .24 | .04 | |
| Expected | .38 | .50 | .09 | .21 | .37 | .40 | .13 | .07 | |
| Information | .02 | ||||||||
| Observed | .23 | .28 | .04 | .15 | .34 | .37 | .15 | .13 | |
| Expected | .24 | .33 | .04 | .13 | .34 | .34 | .17 | .14 | |
| Follow-up 3 measures | |||||||||
| ICAR-16 | .02 | ||||||||
| Observed | .27 | .31 | −.03 | .10 | .19 | .27 | .05 | .07 | |
| Expected | .24 | .33 | −.01 | .08 | .19 | .30 | .06 | .05 | |
| Vocabulary | .04 | ||||||||
| Observed | .33 | .45 | .18 | .32 | .37 | .24 | .16 | .25 | |
| Expected | .30 | .42 | .20 | .32 | .37 | .30 | .21 | .18 | |
Note: Expected correlations are derived from the best-fitting estimates of the biometrical parameters. SRMR refers to the standardized root mean residual. See SI Table S11 for 95% CIs for observed family correlations.
At follow-up 3, familial correlations for the ICAR-16 are largely consistent with the expected patterns of correlations between biological and adoptive relatives for a heritable trait little affected by shared environment (Table 2). The correlations between biological relatives are significant and moderate in size, whereas those between adoptive relatives are consistently smaller. Vocabulary, by contrast, shows surprisingly persistent correlations between both adoptive and biological relatives in adulthood. The correlations specifically between parent and offspring are displayed in Figure 2.
Figure 2:
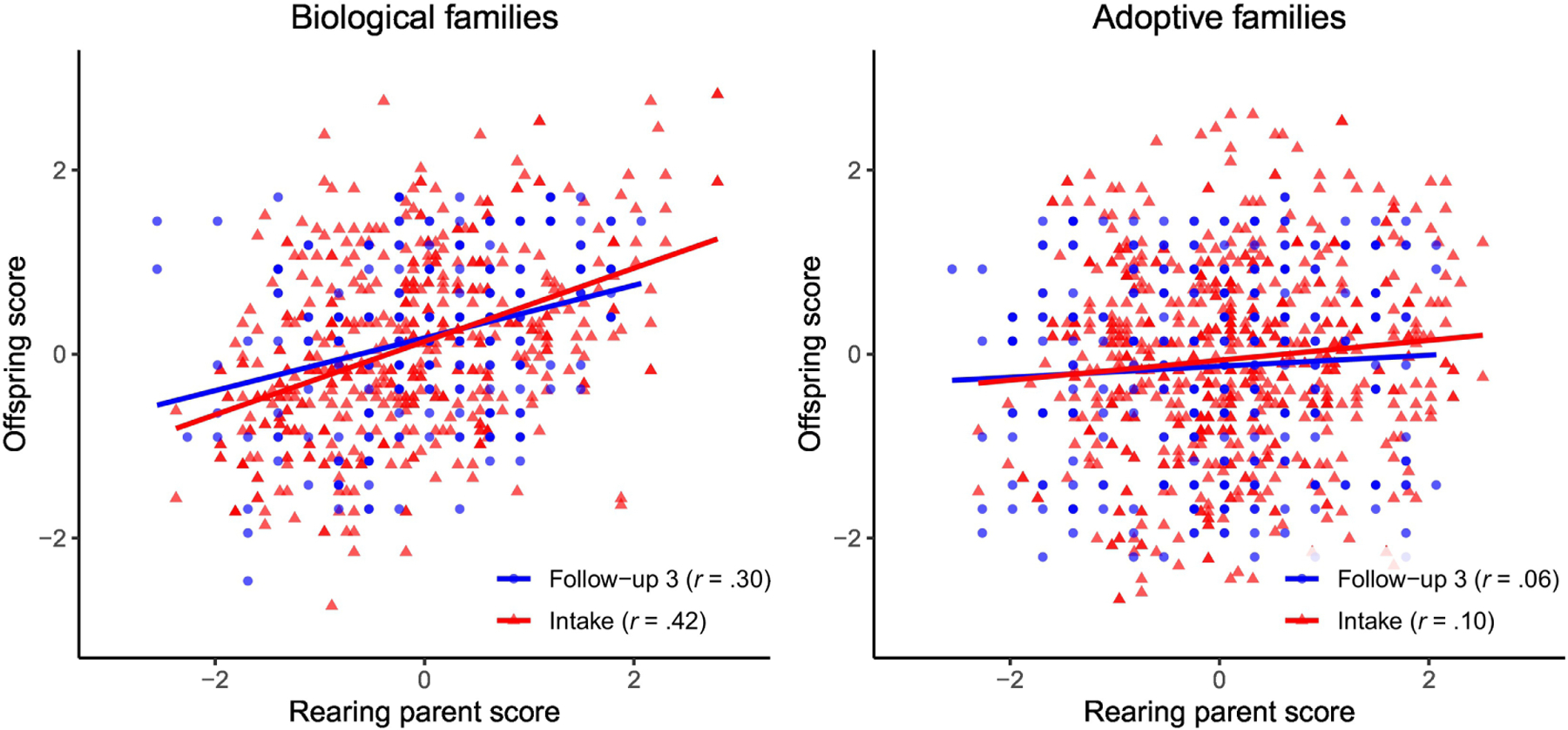
Scatter plots and associated regression lines for measures of cognitive ability g taken at intake and follow-up 3 for both biological (left panel) and adopted (right panel) offspring and their rearing parents. Intake measure of g is full-scale Wechsler IQ score, and follow-up 3 measure is ICAR-16 score. All parent-offspring pairs are included, which means that the data points are not independent. All values are standardized.
To model the expected correlation for each type of relationship, we adapted the expressions for the correlations between relatives from Keller et al. (2009) to include adoptive relationships. The model allows for parent-to-offspring transmission through both genetic (biological families only) and environmental (both family types) pathways. The model also allows for assortative mating and environmental effects shared by siblings reared together that are not induced by the phenotypes of their parents. Observed and model-predicted correlations for each relationship pair are shown in Table 2, with 95% CIs for observed correlations in SI Table S11. We evaluated model fit for each scale with the standardized root mean residual (SRMR), a summary of the magnitude of difference between observed and predicted correlations where “good fit” is generally considered to be < .08 (Hu & Bentler, 1999). Note that we follow the recommendation of McDonald (2010) to report each individual discrepancy, and our finding no consistent difference between observed and model-predicted correlation suggests that there was no general bias associated with our modeling approach. Model fit was good for most IQ measures with the exception of the Picture Completion and Block Design subtests, which contributed to the high SRMR for Performance IQ. Across all cognitive ability measures, the mean SRMR was .06.
Variance decomposition
For intake measures, variance decomposition for each ability measure revealed substantial contributions of genetics. The column parental environment in Table 3 refers to the proportion of variance in the offspring test score attributable to any environmentally mediated effects of the parental test scores. The largest such proportion can be seen for Vocabulary, suggesting that the vocabularies of parents may affect those of their children through environmental mechanisms, perhaps by exposing them to more or fewer vocabulary words during childhood. The percentage of the variance attributable to the parental scores, however, is quite modest (.04). The column sibling environment in Table 3 refers to the proportion of variance in the offspring test score attributable to aspects of the environment shared by siblings reared in a common home, other than the parental phenotypes, and it can be seen that estimates of this parameter are fairly small.
When a trait is both heritable and affected by the parental phenotypes through some environmental mechanism, then we have a case of passive gene-environment correlation. The column “G-E covariance” in Table 3 gives the proportion of the variance attributable to this correlation. A relatively large proportion of the variance in Vocabulary (.12) is the result of G-E covariance.
At follow-up 3, variance decomposition revealed a strong effect of genetics on ICAR-16 scores with little evidence for contributions of the familial environment. Vocabulary scores showed a moderate influence of parental environment (.10) and, unexpectedly, a smaller genetic contribution than is seen for the same measure at intake.
Polygenic scores and adoption placement effects
In an aggregated sample consisting of all white participants (offspring and both parents), an R2 of .154 [95% CI .113, .195] in our prediction of Verbal IQ with a PGS surpasses all previous benchmarks known to us (Rietveld et al., 2014; Selzam et al., 2017; Sniekers et al., 2017; Savage et al., 2018; Lee et al., 2018; Allegrini et al., 2019), and an R2 of .114 [95% CI .077, .151] for Total IQ is near the upper end of previous predictions (SI Table S13). However, we acknowledge that our sample is not large by the standards of PGS validation.
PGSEA were generally less predictive for Asian than for white offspring, which is expected because when the validation sample is different from GWAS sample, predictive accuracy falls due to different SNP correlations in different populations. Standardized mean PGSEA were significantly different between these ethnicities (Cohen’s d = .87; see SI Table S6), as well as between adopted (21 percent white) and biological (95 percent white) offspring (Cohen’s d = −.27; see SI Table S7). Despite these mean differences, PGSEA remained strongly predictive ( p < .001) of all IQ phenotypes except Performance IQ and Picture Completion in both adoptive and biological offspring (SI Table S14).
Nevertheless, to account for the possibility of biased predictions introduced by population stratification, the test of placement effects was conducted separately for white ( N = 83) and Asian ( N = 258) adoptees. In the sample of biological offspring ( N = 271, all white), midparent correlated with offspring PGSEA at r = .65 (95% CI [.58, .71]; p < .001), which is very close to the theoretical prediction of . There may be collider bias as a result of excluding individuals with very low IQs from the study (Pearl, 2009; Lee, 2012), spuriously reducing the correlation below , but any such reduction appears to be small. At the same time, the correlation between midparent and offspring PGSEA in adoptive families was not significantly different from zero for either Asian ( r = −.04; 95% CI: −.16, .08) or white adoptees ( r = .07; 95% CI: −.14, .28). The similarity of these correlations to their theoretically predicted values provides evidence that the placement of adoptees in their homes was not strongly purposive or selective, implying that the adoption process may somewhat approximate a true experiment. Although educational attainment variance is slightly higher in adoptive offspring (SI Table S2), a nominally significant difference ( F = 1.4, p = .002), range restriction for education in adoptive and biological parents is unlikely to bias these parent-offspring correlations (McGue et al., 2007).
Discussion
While the high heritability of IQ is now well known, the question of whether and to what extent general intelligence is malleable by the influences of parental environment has remained a topic of great interest in behavior genetics. IQ has been subject to a large number of twin and adoption studies, many of which have found a small but significant effect of parental transmission in adoptive samples up until late adolescence. Kendler et al. (2015), for example, found in a sample of 18–20-year-old half-sibships that adoption was associated with a gain of approximately 3.18 IQ points (SE = 0.34). Our biometric decomposition of variance is consistent with this figure: the parental environment contributing 4 percent of the variance in full-scale IQs at age ~ 15 (Table 3), with a standard deviation of 14.2 for full-scale IQ in adopted offspring, indicates that a 1-SD increase in the quality of the parental environment would increase IQ by approximately 2.83 points (i.e., ; Burks, 1938).
The evidence for parenting effects on Wechsler IQ subtests is more equivocal, and biometric decomposition reveals a moderate but significant effect of gene-environment covariance on Vocabulary in childhood. While a similarly-sized G-E covariance is observed for childhood Total IQ, this effect has completely disappeared in adulthood; the same cannot be said unambiguously for Vocabulary, which retains weak evidence in adulthood for a persistent parenting effect.
Nevertheless, we acknowledge that this indeterminate evidence does not conclusively answer the question of parenting effects on Vocabulary. Indeed, either interpretation of the validity of parenting effects on Vocabulary would be consistent with existing proposals. Vocabulary is a particularly interesting facet of human intelligence for this reason; while such a test may intuitively seem to reflect only culturally-obtained word knowledge—and, indeed, it has been shown to be the most “culturally-loaded” (Georgas et al., 2003) of all Wechsler subtests—Vocabulary has also emerged as the IQ subtest that shares the most genetic variance with general intelligence (Johnson et al., 2007). If substantial and persistent parenting effects on offspring Vocabulary reflect the reality of how verbal ability develops over time, this may be consistent with proposals that have posited gene-environment correlation as an important factor in the high apparent heritability of certain verbal abilities (e.g., Kan et al., 2014).
Our study is limited by a number of factors that may contribute to this lack of definitive evidence for Vocabulary in the present sample. The current wave of SIBS assessments, though reasonably large by the standards of historical adoption studies, may be underpowered for reliable estimates of variance components for Vocabulary. Additionally, the lack of diversity in IQ measures at follow-up 3 makes it impossible to directly compare the effects of Vocabulary to other WAIS-R subtests that were only administered to this cohort in childhood. In particular, it is difficult to know why the heritability of Vocabulary at follow-up 3 is surprisingly low (e.g. Rijsdijk et al., 2002; Johnson et al., 2007; DeFries et al., 1979; Capron & Duyme, 1996) without additional WAIS-R subtests administered at follow-up 3 to compare.
Despite these limitations, this study brings a number of novel insights to the ongoing discussion of genetic and environmental contributions to IQ. Our PGS for educational attainment provide what is, at moment of writing, the largest R2 estimates for any cognitive phenotype (.113 for Total IQ; .154 for Verbal IQ). The particularly high predictive validity for PGSEA for verbal IQ is perhaps to be expected given that verbal IQ correlates more strongly than other IQ subscales with educational attainment, particularly for parents (SI Table S9). These scores also enable a unique test for the so-called “placement effect,” wherein adoptees (typically twins reared apart) are thought by some skeptics to resemble their adoptive parents prior to placement, thus biasing biometrical estimates. By demonstrating a total lack of evidence ( p = .514) for a correlation between parents and adoptive offspring in polygenic scores, we provide support for the validity of at least some adoption studies in establishing causal inference.
Another strength of our study is the use of parents and offspring to estimate the heritability of intelligence. Such designs are less susceptible than twin studies to non-additive genetic variance inflating estimates of the narrow-sense heritability (Lynch & Walsh, 1998). The broad-sense heritability, including both additive and non-additive sources of genetic variance, is less useful a measure than the narrow-sense heritability for a number of reasons. For instance, although the broad-sense heritability is well estimated by the correlation between monozygotic twins reared apart, it is the narrow-sense heritability to which PGS predictions should converge as GWAS grow in size and reduce the so-called missing heritability. Our estimate of .42, for an IQ test with a reliability of .64, is fully consistent with previous parent-offspring studies supporting a narrow-sense heritability between .4 and .6 (DeFries et al., 1979; Plomin et al., 1997; Loehlin et al., 1997; Scarr, 1997; Black et al., 2009; Björklund et al., 2010).
Our heritability estimate is consistent with estimates of the narrow-sense heritability obtained by applying the genomic-relatedness method to GWAS data (Davies et al., 2011), although this method is limited to capturing only the heritability attributable to genetic variants above a certain allele frequency (Yang et al., 2010; Lee & Chow, 2014). A recent study assaying all variants with a minor allele frequency greater than .00002 estimated the heritability of a fluid-reasoning test to be .39 (Evans et al., 2018, SI Table 10). All of these results are consistent with any missing heritability being merely undiscovered rather than truly missing; traditional biometrical studies and GWAS are in good agreement.
Perhaps most importantly, our unique sample of families with fully adult offspring enables the first investigation of parenting effects on IQ in adopted offspring over the age of 30. Generalizations about how genetic and environmental effects on intelligence change with age tend to rely heavily on studies of the young and the elderly (Hunt, 2011; Mackintosh, 2011). Our study, which consists of offspring over the age of 30, therefore contributes to a better sampling of the entire life course. Vocabulary results are somewhat difficult to interpret, the lack of evidence for parenting effects on general intelligence in adulthood is far clearer. By examining parent-offspring resemblance in a sample of offspring that are among the oldest of any adoption study of IQ to date, we have effectively tested for the presence of parenting effects that would have persisted for more than a decade after the conclusion of the typical rearing period. No such persistence is found to occur in our unique sample. Although molecular methods have profoundly changed the face of behavior genetics, it is clear that adoption studies, which have been a cornerstone of the field throughout the 20th century, can continue to bear new fruit in the search for causal bases to psychological traits.
Supplementary Material
Highlights.
Genetic and environmental sources of variance in IQ were estimated from 486 adoptive and biological families with adult offspring (M age = 31.8 years; S D = 2.7)
Families include 419 mothers, 201 fathers, 415 adopted offspring, and 347 bio logical offspring
Proportions of the variance in IQ attributable to environmentally mediated effects of parental IQs, sibling-specific shared environment, and gene-environment co variance were estimated to be .01 [95% CI .00, .02], .04 [95% CI .00, .15], and .03 [95% CI .00, .07] respectively
Heritability was estimated to be .42 [95% CI .21, .64]
Parent-offspring correlations for educational attainment polygenic scores show no evidence of adoption placement effect
Acknowledgements
This research was made possible by a grant from the John Templeton Foundation as part of their Genetics and Human Agency initiative (Grant Number 60780), and original assessment data collection was funded by the National Institutes of Health (Grant Numbers MH066140 and AA011886). We also thank our collaborators and staff at the Minnesota Center for Twin and Family Research for their dedication in data collection and entry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Science Practices
Biometric code used in key analyses is available on the Open Science Framework: osf.io/q3cx5.
References
- Allegrini AG, Selzam S, Rimfeld K, von Stumm S, Pingault J-B, & Plomin R (2019). Genomic prediction of cognitive traits in childhood and adolescence. Molecular Psychiatry, 24, 819–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbona JV, Kim Y, & Keller MC (2021). Estimation of parental effects using polygenic scores. Behavior Genetics, 51, 264–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund A, Eriksson KH, & Jäntti M (2010). IQ and family background: Are associations strong or weak? Berkeley Electronic Journal of Economic Analysis and Policy, 10, Article 2. [Google Scholar]
- Black SE, Devereux PJ, & Salvanes KG (2009). Like father, like son? a note on the intergenerational transmission of IQ scores. Economics Letters, 105, 138–140. [Google Scholar]
- Bouchard TJ Jr., & McGue M (1990). Genetic and rearing environmental influences on adult personality: An analysis of adopted twins reared apart. Journal of Personality, 58, 263–292. [DOI] [PubMed] [Google Scholar]
- Briley DA, & Tucker-Drob EM (2013). Explaining the increasing heritability of cognitive ability across development: A meta-analysis of longitudinal twin and adoption studies. Psychological Science, 24, 1704–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burks BS (1938). On the relative contributions of nature and nurture to average group differences in intelligence. Proceedings of the National Academy of Sciences, 24, 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capron C, & Duyme M (1989). Assessment of effects of socio-economic status on IQ in a full cross-fostering study. Nature, 340, 552–554. [Google Scholar]
- Capron C, & Duyme M (1996). Effect of socioeconomic status of biological and adoptive parents on WISC-R subtest scores of their French adopted children. Intelligence, 22, 259–275. [Google Scholar]
- Cloninger CR, Rice J, & Reich T (1979). Multifactorial inheritance with cultural transmission and assortative mating II. A general model of combined polygenic and cultural inheritance. American Journal of Human Genetics, 31, 176–198. [PMC free article] [PubMed] [Google Scholar]
- Condon DM, & Revelle W (2014). The international cognitive ability resource: Development and initial validation of a public-domain measure. Intelligence, 43, 52–64. [Google Scholar]
- Davies G, Tenesa A, Payton A, Yang J, Harris SE, Liewald D, Ke X, Le Hellard S, Christoforou A, Luciano M, McGhee K, Lopez LM, Gow AJ, Corley J, Redmond P, Fox HC, Haggarty P, Whalley LJ, McNeill G, Goddard ME, Espeseth T, Lundervold AJ, Reinvang I, Pickles A, Steen VM, Ollier W, Porteous DJ, Horan M, Starr JM, Pendleton N, Visscher PM, & Deary IJ (2011). Genome-wide association studies establish that human intelligence is highly heritable and polygenic. Molecular Psychiatry, 16, 996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFries JC, Johnson RC, Kuse AR, McClearn GE, Polovina J, Vandenberg SG, & Wilson JR (1979). Familial resemblance for specific cognitive abilities. Behavior Genetics, 9, 23–43. [DOI] [PubMed] [Google Scholar]
- Dey JG, & Pierret CR (2014). Independence for young millennials: Moving out and boomeranging back. Monthly Labor Review: U.S. Bureau of Labor Statistics, December 2014. [Google Scholar]
- Eaves L (2009). Putting the ‘human’ back in genetics: Modeling the extended kinships of twins. Twin Research and Human Genetics, 12, 1–7. [DOI] [PubMed] [Google Scholar]
- Eaves LJ (1976). The effect of cultural transmission on continuous variation. Heredity, 37, 41–57. [DOI] [PubMed] [Google Scholar]
- Eaves LJ, Hatemi PK, Prom-Womley EC, & Murrelle L (2008). Social and genetic influences on adolescent religious attitudes and practices. Social Forces, 86, 1621–1646. [Google Scholar]
- van Eerdewegh P (1982). Statistical selection in multivariate systems with applications in quantitative genetics (unpublished Ph.D. dissertation). St. Louis: Washing University. [Google Scholar]
- Efron B, & Tibshirani R (1993). An Introduction to the Bootstrap. Monographs on Statistics and Applied Probability. New York, N.Y.: Chapman and Hall. [Google Scholar]
- Evans LM, Tahmasbi R, Vrieze SI, Abecasis GR, Das S, Gazal S, Bjelland DW, de Candia TR, Haplotype Reference Consortium, Goddard ME, Neale BM, Yang J, Visscher PM, & Keller MC (2018). Comparison of methods that use whole genome data to estimate the heritability and genetic architecture of complex traits. Nature Genetics, 50, 737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JR (1993). Skodak and Skeels: The inflated mother-child IQ gap. Intelligence, 17, 557–561. [Google Scholar]
- Fulker DW (1988). Genetic and cultural transmission in human behavior. In Weir BS, Eisen EJ, Goodman MM, & Namkoong G (Eds.), Proceedings of the Second International Conference on Quantitative Genetics (pp. 318–340). Sunderland, MA: Sinauer. [Google Scholar]
- Georgas J, van de Vijver FJR, Weiss LG, & Saklofske DH (2003). A cross-cultural analysis of the WISC-III. In Culture and children’s intelligence: Cross-cultural analysis of the WISC-III. (pp. 277–313). Georgas, James: Department of Psychology, University of Athens, Athens, Greece, 15784: Academic Press. [Google Scholar]
- Haworth CMA, Wright MJ, Luciano M, Martin NG, de Geus EJC, van Beijsterveldt CEM, Bartels M, Posthuma D, Boomsma DI, Davis OSP, Kovas Y, Corley R, DeFries JC, Hewitt JK, Olson RK, Rhea S-A, Wadsworth SJ, Iacono WG, McGue M, Thompson LA, Hart SA, Petrill SA, Lubinski D, & Plomin R (2010). The heritability of general cognitive ability increases linearly from childhood to young adulthood. Molecular Psychiatry, 15, 1112–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Kendler KS, Eaves LJ, & Markell D (1985). The resolution of cultural and biological inheritance: Informativeness of different relationships. Behavior Genetics, 15, 439–465. [DOI] [PubMed] [Google Scholar]
- Hill WG, Goddard ME, & Visscher PM (2008). Data and theory point to mainly additive genetic variance for complex traits. PLOS Genetics, 4, e1000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hivert V, Sidorenko J, Rohart F, Goddard ME, Yang J, Wray NR, Yengo L, & Visscher PM (2021). Estimation of non-additive genetic variance in human complex traits from a large sample of unrelated individuals. American Journal of Human Genetics, 108, 786–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra RA, Bartels M, & Boomsma DI (2007). Longitudinal genetic study of verbal and nonverbal IQ from early childhood to young adulthood. Learning and Individual Differences, 17, 97–114. [Google Scholar]
- Horn JM, Loehlin JC, & Willerman L (1979). Intellectual resemblance among adoptive and biological relatives: The Texas adoption project. Behavior Genetics, 9, 177–201. [DOI] [PubMed] [Google Scholar]
- Hu L, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6, 1–55. [Google Scholar]
- Hunt E (2011). Human Intelligence. New York, NY: Cambridge University Press. [Google Scholar]
- van Ijzendoorm MH, Juffer F, & Poelhius CW (2005). Adoption and cognitive development: A meta-analytic comparison of adopted and nonadopted children’s IQ and school performance. Psychological Bulletin, 131, 301–316. [DOI] [PubMed] [Google Scholar]
- Johnson W, Bouchard TJ Jr., McGue M, Segal NL, Tellegen A, Keyes M, & Gottesman I (2007). Genetic and environmental influences on the Verbal-Perceptual-Image Rotation (VPR) model of the structure of mental abilities in the Minnesota study of twins reared apart. Intelligence, 35, 542–562. [Google Scholar]
- Johnson W, Penke L, & Spinath FM (2011). Heritability in the era of molecular genetics: Some thoughts for understanding genetic influences on behavioural traits. European Journal of Personality, 25, 254–266. [Google Scholar]
- Kan K-J, Wicherts J, Dolan C, & van der Maas HLJ (2014). On the nature and nurture of intelligence and specific cognitive abilities: The more heritable, the more culture dependent. Psychological Science, 24, 2420–2428. [DOI] [PubMed] [Google Scholar]
- Keller MC, Medland SE, Duncan LE, Hatemi PK, Neale MC, Maes HH, & Eaves LJ (2009). Modeling extended twin family data I: Description of the cascade model. Twin Research and Human Genetics, 12, 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Turkheimer E, Ohlsson H, Sundquist J, & Sundquist K (2015). Family environment and the malleability of cognitive ability: A Swedish national home-reared and adopted-away cosibling control study. Proceedings of the National Academy of Sciences, 112, 4612–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ (2012). Correlation and causation in the study of personality. European Journal of Personality, 26, 372–390 (2012). [Google Scholar]
- Lee JJ, & Chow CC (2014). Conditions for the validity of SNP-based heritability estimation. Human Genetics, 133, 1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M, Nguyen-Viet TA, Bowers P, Sidorenko J, Karlsson Linnér R, Fontana MA, Kundu T, Lee C, Li H, Li R, Royer R, Timshel PN, Walters RK, Willoughby EA, Yengo L, Alver M, Bao Y, Clark DW, Day FR, Furlotte NA, Joshi PK, Kemper KE, Kleinman A, Langenberg C, Mägi R, Trampush JW, Verma SS, Wu Y, Lam M, Zhao JH, Zheng Z, Boardman JD, Campbell H, Freese J, Harris KM, Hayward C, Herd P, Kumari M, Lencz T, Luan J, Malhotra AK, Metspalu A, Milani L, Ong KK, Perry JRB, Porteous DJ, Ritchie MD, Smart MC, Smith BH, Tung JY, Wareham NJ, Wilson JF, Beauchamp JP, Conley DC, Esko T, Lehrer SF, Magnusson PKE, Oskarsson S, Pers TH, Robinson MR, Thom K, Watson C, Chabris CF, Meyer MN, Laibson DI, Yang J, Johannesson M, Koellinger PD, Turley P, Visscher PM, Benjamin DJ, & Cesarini D (2018). Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nature Genetics, 50, 1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loehlin JC, Horn JM, & Willerman L (1997). Heredity, environment, and IQ in the Texas Adoption Project. In Sternberg RJ, & Grigorenko EL (Eds.), Intelligence, Heredity, and Environment (pp. 105–125). Cambridge, UK: Cambridge University Press. [Google Scholar]
- Lynch M, & Walsh B (1998). Genetics and the Analysis of Quantitative Traits. Sunderland, MA: Sinauer. [Google Scholar]
- Mackintosh NJ (2011). IQ and Human Intelligence. (2nd ed.). New York, NY: Oxford University Press. [Google Scholar]
- Maki-Tanila A, & Hill WG (2014). Influence of gene interaction on complex trait variation with multilocus models. Genetics, 198, 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald RP (2010). Structural models and the art of approximation. Perspectives on Psychological Science, 5, 675–686. [DOI] [PubMed] [Google Scholar]
- McGue M, Keyes M, Sharma A, Elkins I, Legrand L, Johnson W, & Iacono WG (2007). The environments of adopted and non-adopted youth: Evidence on range restriction from the Sibling Interaction and Behavior Study (SIBS). Behavior Genetics, 37, 449–462. [DOI] [PubMed] [Google Scholar]
- Miller MB, Basu S, Cunningham J, Eskin E, Malone SM, Oetting WS, Schork N, Sul JH, Iacono WG, & Mc Gue M (2012). The Minnesota Center for Twin and Family Research genome-wide association study. Twin Research and Human Genetics, 15, 767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazokitoroudi A, Chiu AM, Burch KS, Pasaniuc B, & Sankararaman S (2021). Quantifying the contribution of dominance deviation effects to complex trait variation in biobank-scale data. American Journal of Human Genetics, 108, 799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl J (2009). Causality: Models, Reasoning, and Inference. (2nd ed.). New York, NY: Cambridge University Press. [Google Scholar]
- Plomin R, & DeFries JC (1980). Genetics and intelligence: Recent data. Intelligence, 4, 15–24. [Google Scholar]
- Plomin R, DeFries JC, Knopik VS, & Neiderhiser JM (2013). Behavioral genetics (6th edition). New York, NY: Worth Publishers. [Google Scholar]
- Plomin R, DeFries JC, & Loehlin JC (1977). Genotype–environment interaction and correlation in the analysis of human behavior. Psychological Bulletin, 84, 309–322. [PubMed] [Google Scholar]
- Plomin R, Fulker DW, Corley R, & DeFries JC (1997). Nature, nurture, and cognitive development from 1 to 16 years. Psychological Science, 8, 442–447. [Google Scholar]
- Polderman TJ, Benyamin B, De Leeuw CA, Sullivan PF, Van Bochoven A, Visscher PM, & Posthuma D (2015). Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nature Genetics, 47, 702–709. [DOI] [PubMed] [Google Scholar]
- Protzko J (2015). The environment in raising early intelligence: A meta-analysis of the fadeout effect. Intelligence, 53, 202–210. [Google Scholar]
- Rietveld CA, Esko T, Davies G, Pers TH, Turley P, Benyamin B, Chabris CF, Emilsson V, Johnson AD, Lee JJ, de Leeuw C, Marioni RE, Medland SE, Miller MB, Rostapshova O, van der Lee SJ, Vinkhuyzen AAE, Amin N, Conley D, Derringer J, van Duijn CM, Fehrmann R, Franke L, Glaeser EL, Hansell NK, Hayward C, Iacono WG, Ibrahim-Verbaas CA, Jaddoe VWV, Karjalainen J, Laibson DII, Lichtenstein P, Liewald DCM, Magnusson PKE, Martin NG, McGue M, McMahon G, Pedersen NL, Pinker S, Porteous DJ, Posthuma D, Rivadeneira F, Smith BH, Starr JM, Tiemeier H, Timpson NJ, Trzaskowski M, Uitterlinden AG, Verhulst FC, Ward ME, Wright MJ, Davey Smith G, Deary IJ, Johannesson M, Plomin R, Visscher PM, Benjamin DJ, Cesarini D, & Koellinger PD (2014). Common genetic variants associated with cognitive performance identified using the proxy-phenotype method. Proceedings of the National Academy of Sciences USA, 111, 13790–13794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijsdijk FV, Vernon PA, & Boomsma DI (2002). Application of hierarchical genetic models to raven and WAIS subtests: A Dutch twin study. Behavior Genetics, 32, 199–210. [DOI] [PubMed] [Google Scholar]
- Savage JE, Jansen PR, Stringer S, Watanabe K, Bryois J, de Leeuw CA, Nagel M, Awasthi S, Barr PB, Coleman JRI, Grasby KL, Hammerschlag AR, Kaminski JA, Karlsson R, Krapohl E, Lam M, Nygaard M, Reynolds CA, Trampush JW, Young H, Zabaneh D, Hägg S, Hansell NK, Karlsson IK, Linnarsson S, Montgomery GW, Muñoz-Manchado AB, Quinlan EB, Schumann G, Skene NG, Webb BT, White T, Arking DE, Avramopoulos D, Bilder RM, Bitsios P, Burdick KE, Cannon TD, Chiba-Falek O, Christoforou A, Cirulli ET, Congdon E, Corvin A, Davies G, Deary IJ, DeRosse P, Dickinson D, Djurovic S, Donohoe G, Conley ED, Eriksson JG, Espeseth T, Freimer NA, Giakoumaki S, Giegling I, Gill M, Glahn DC, Hariri AR, Hatzimanolis A, Keller MC, Knowles E, Koltai D, Konte B, Lahti J, Le Hellard S, Lencz T, Liewald DC, London E, Lundervold AJ, Malhotra AK, Melle I, Morris D, Need AC, Ollier W, Palotie A, Payton A, Pendleton N, Poldrack RA, Räikkönen K, Reinvang I, Roussos P, Rujescu D, Sabb FW, Scult MA, Smeland OB, Smyrnis N, Starr JM, Steen VM, Stefanis NC, Straub RE, Sundet K, Tiemeier H, Voineskos AN, Weinberger DR, Widen E, Yu J, Abecasis G, Andreassen OA, Breen G et al. (2018). Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nature Genetics, 50, 912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarr S (1997). Behavior-genetic and socialization theories of intelligence: Truce and reconciliation. In Sternberg RJ, & Grigorenko E (Eds.), Intelligence, Heredity, and Environment (pp. 3–41). Cambridge, UK: Cambridge University Press. [Google Scholar]
- Scarr S, & McCartney K (1983). How people make their own environments: A theory of genotype–environment effects. Child Development, 54, 424. [DOI] [PubMed] [Google Scholar]
- Scarr S, & Weinberg RA (1978). The influence of “family background” on intellectual attainment. American Sociological Review, 43, 674–692. [Google Scholar]
- Scarr S, & Weinberg RA (1983). The Minnesota adoption studies: Genetic differences and malleability. Child Development, 54, 260–267. [PubMed] [Google Scholar]
- Selzam S, Krapohl E, Von Stumm S, O’Reilly PF, Rimfeld K, Kovas Y, Dale PS, Lee JJ, & Plomin R (2017). Predicting educational achievement from DNA. Molecular Psychiatry, 22, 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skodak M, & Skeels HM (1949). A final follow-up study of 100 adopted children. Journal of Genetic Psychology, 75, 85–125. [DOI] [PubMed] [Google Scholar]
- Sniekers S, Stringer S, Watanabe K, Jansen PR, Coleman JR, Krapohl E, Taskesen E, Hammerschlag AR, Okbay A, Zabaneh D, Amin N, Breen G, Cesarini D, Chabris CF, Iacono WG, Ikram MA, Johannesson M, Koellinger P, Lee JJ, Magnusson PK, McGue M, Miller MB, Ollier WE, Payton A, Pendleton N, Plomin R, Rietveld CA, Tiemeier H, Van Duijn CM, & Posthuma D (2017). Genome-wide association meta-analysis of 78,308 individuals identifies new loci and genes influencing human intelligence. Nature Genetics, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truett KR, Eaves LJ, Waiters EE, Heath AC, Hewitt JK, Meyer JM, Silberg J, Neale MC, Martin NG, & Kendler KS (1994). A model system for analysis of family resemblance in extended kinships of twins. Behavior Genetics, 24, 35–49. [DOI] [PubMed] [Google Scholar]
- Warne R (2020). In the Know: Debunking 35 Myths about Human Intelligence. Cambridge, U.K.: Cambridge University Press. [Google Scholar]
- Wechsler D (1974). Manual for the Wechsler Intelligence Scale for Children–Revised. New York, NY: Psychological Corporation. [Google Scholar]
- Wechsler D (1981). Wechsler Adult Intelligence Scale-Revised. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Wright S (1931). Statistical Methods in Biology. Journal of the American Statistical Association, 26, 155–163. [Google Scholar]
- Yang J, Benyamin B, McEvoy BP, Gordon S, Henders AK, Nyholt DR, Madden PA, Heath AC, Martin NG, Montgomery GW, Goddard ME, & Visscher PM (2010). Common SNPs explain a large proportion of the heritability for human height. Nature Genetics, 42, 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


