Abstract
Articular cartilage is characterized by zonal organizations containing dual gradients of biochemical cues and mechanical cues. However, how biochemical gradient interacts with the mechanical gradient to drive the cartilage zonal development remains largely unknown. Here, we report the development of a dual-gradient hydrogel platform as a 3D niche to elucidate the relative contributions of biochemical and mechanical niche gradients in modulating zonal-specific chondrocyte responses and cartilage zonal organization. Chondroitin sulfate (CS), a major constituent of cartilage extracellular matrix, was chosen as the biochemical cue. Poly(ethylene glycol), a bioinert polymer, was used to create the stiffness gradient. Dual-gradient hydrogels upregulated cartilage marker expressions and increased chondrocyte proliferation and collagen deposition in a zonal-dependent manner. Hydrogels with CS gradient alone exhibited poor mechanical strength and degraded prematurely after 1 week of culture. While CS gradient alone did not support long-term culture, adding CS gradient to mechanical-gradient hydrogels substantially enhanced cell proliferation, glycosaminoglycan production, and collagen deposition compared to mechanical-gradient hydrogels alone. These results suggest that biochemical and mechanical gradient cues synergize to enhance cartilage zonal organization by chondrocytes in 3D. Together, our results validate the potential of dual-gradient hydrogels as a 3D cell niche for cartilage regeneration with zonal organization and may be used to recreate other tissue interfaces.
Keywords: dual gradient, hydrogels, biochemical, mechanical, zonal organization, cartilage
Graphical Abstract
INTRODUCTION
As articular cartilage transitions from the superficial zone to the deep zone, the extracellular matrix (ECM) of the cartilage is characterized by increasing stiffness and higher amounts of ECM constituents such as glycosaminoglycans (sGAGs).1 Zonal organization plays an important role in cartilage structure and function. To mimic the biochemical ligands that are present in the ECM, various cartilage-derived ECM molecules, such as chondroitin sulfate (CS) and hyaluronic acid (HA), have been incorporated into 3D hydrogels to promote chondrogenesis in vitro and in vivo.2,3 To better mimic cartilage structure and function, there is a critical need to develop methods to facilitate the recreation of the zonal complexities of native articular cartilage.4 To induce zone- specific cellular responses, cells have been encapsulated in multilayered hydrogels using polymers including poly(ethylene glycol) (PEG), agarose, and alginate.1,5,6 While the multi-layered hydrogels enabled studies on cell behaviors in response to various matrix cues, the distinct transition and poor mechanical properties at the interfaces between layers remained suboptimal for recapitulating the continuous transition of niche cues in native tissues.
To overcome these limitations, recent studies have shown that gradient scaffolds can be fabricated with immobilized or soluble gradients of biological agents. Single-gradient hydrogels containing biochemical cues have been shown to induce cell migration,7 axonal guidance,8 and cell differentiation9 in a gradient-dependent manner. In addition to biochemical cues, physical cues such as matrix stiffness have also been shown to play an important role in directing cell fates in 2D and 3D.10,11 Hydrogels with a gradient of stiffness12 or porosity13 have been reported to mimic tissue zonal organization14 or to facilitate high-throughput screening of cell-niche interactions.15,16 Recently, we reported a PEG-based tissue-scale hydrogel with stiffness gradient ranging from 2 to 60 kPa, which induced zonal-specific responses of chondrocytes and mesenchymal stem cells (MSCs) and results in extracellular deposition that mimics zonal organization of articular cartilage.14
Despite the progress of harnessing gradient hydrogels to mimic tissue organization, most studies so far were limited to investigating the effects of only biochemical gradient or mechanical gradient. In contrast, cell niche is a multifactorial microenvironment containing both biochemical and mechanical cues,17–20 which interact in a complex manner to modulate cell fates and tissue formation21. One recent study reported a method to create dual-gradient hydrogels as a 3D cell niche using a syringe pumping system.22 Although the method supported high cell viability after encapsulation in dual-gradient hydrogels, no long-term cell culture was performed for analyses of tissue regeneration.22 How mechanical and biochemical gradients interact to regulate cell fate and tissue formation in 3D remains largely unknown.
The goal of the present study was to engineer tissue scale (on the centimeter scale) hydrogels containing dual gradients of biochemical and mechanical cues as 3D cell niche to elucidate how biochemical gradient and mechanical gradient interact to drive cartilage zonal development by chondrocytes in 3D. To mimic the increasing concentration of sGAG from superficial zone to deep zone cartilage, chondroitin sulfate (CS), a major proteoglycan found in native cartilage, was chosen as the biochemical cue.23 To mimic the increasing stiffness gradient in native cartilage from superficial zone to deep zone, we chose PEG given its bioinert nature and efficacy in guiding zonal-specific cartilage development.14 To determine the respective contributions of biochemical and mechanical signal gradients to cellular responses in 3D, chondrocytes were encapsulated in a dual-gradient hydrogel as well as in biochemical or mechanical gradient only hydrogels as controls. All groups were cultured in chondrocyte growth medium for up to 3 weeks. Outcomes were analyzed using quantitative gene expression, biochemical assays, and mechanical testing. Comparisons of cell proliferation, cartilage matrix production, and mechanical properties across the three types of gradient hydrogels revealed the relative contributions of biochemical and mechanical gradients in driving cartilage zonal organization.
MATERIALS AND METHODS
Fabrication of 3D-Gradient Hydrogels and Cell Encapsulation.
8-arm PEG norbornene (10 kDa) and PEG dithiol (1.5 kDa) were synthesized as previously reported.43,44 CS was synthesized with a 25% methacrylation ratio in accordance with a previous protocol.24 Dual-gradient hydrogels were prepared utilizing a gradient maker (Hoefer SG-15 Amersham Biosciences) with two interconnected chambers filled with (20% w/v PEG, 3% w/v CS) and (2% w/v PEG, 8% w/v CS) solutions homogeneously mixed with 15 million/mL isolated chondrocytes. Hydrogels containing only a mechanical gradient were fabricated with precursor solutions of (20% w/v PEG, 3% w/v CS) and (2% w/v PEG, 3% w/v CS) in two chambers. Hydrogels with a biochemical gradient only were made by mixing precursor solutions of 3% and 8% (w/v) of CS without any PEG. The effluent from the mixing chamber was collected in a customized mold (3 cm × 1 cm × 3 mm) made of Teflon sheets. Gradient hydrogels were cross-linked using photopolymerization as previously reported.14
Chondrocyte Isolation and Culture.
Neonatal bovine chondrocytes were isolated from hyaline articular cartilage tissue as previously reported.24 After encapsulation, chondrocytes were cultured with standard chondrocyte growth medium25 for 3 weeks. Cell viability was assessed with the LIVE/DEAD Viability/ Cytotoxicity Kit (Invitrogen; the kit contains calcein-AM and ethidium homodimer-1) 24 h after encapsulation using a Zeiss fluorescence microscope (Observer Z1).
Mechanical Testing.
Compressive modulus was measured using unconfined compression tests with the Instron 5944 (Interface Inc.) as previously reported.26 Hydrogel samples (n = 3) were biopsied using a 6 mm biopsy punch (AcuPunch) from five evenly divided zones into a cylindrical shape. The test was conducted at a rate of 1% strain s–1 to a maximum strain of 30%. A preload force of 1 mN was applied. The compressive modulus was then calculated using the linear-curve fits of stress versus strain for a range of 10–20% strain.
sGAG Assay.
To characterize the sGAG content of gradient hydrogels, samples were first evenly divided into five zones (n = 3) and prepared using papain solution (Worthington Biochemical) as previously reported.14 sGAG content was measured spectrophotometrically using the 1,9-dimethylmethylene blue (DMMB) dye-binding assay (pH 3.0) with shark chondroitin sulfate (Sigma) as standard (0.0884 mg/mL).
Gene Expression RT-PCR.
Gradient hydrogels were manually cut into five zones based on the dimensionality after 1 week of culturing chondrocytes in chondrocyte growth medium. Total cellular RNA was extracted and purified with TRIzol reagent (Invitrogen) and the RNeasy Mini Kit (Qiagen). Reverse transcription was performed using SuperScript III reverse transcriptase (Invitrogen). A 7500 Fast Real-Time PCR System and SYBR Green master mix (Applied Biosystems) were used to perform quantitative reverse transcription polymerase chain reaction. The relative expression levels of genes were determined using the comparative double delta CT method (Qiagen). The expression levels of genes of interest (n = 3 hydrogels/ group), including genes encoding ras homologue gene family member A (RhoA) rho-associated, coiled-coil-containing protein kinase 1 and 2 (ROCK 1 and 2), SRY-related protein 9 (sox9), aggrecan, matrix metallopeptidase-13 (MMP-13), and collagen types I, II, and X, were first normalized to that of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase and followed by secondary normalization of the control group (day 1 neonatal chondrocytes).
Biochemical Assays of DNA (PicoGreen) and Collagen (Hydroxyproline).
For biochemical analysis after 3 weeks of culture, encapsulated chondrocytes in gradient hydrogels were harvested andevenly divided before using papain solution for preparation (Worthington Biochemical). DNA content (n = 3) was quantified using the PicoGreen assay (Molecular Probes), and acellular controls were used to subtract background (n = 3). Total collagen content (n = 3) was quantified from digested hydrogels using acid hydrolysis followed by p-dimethylaminobenzaldehyde and chloramine T assays (Sigma).
Histological Analysis and Immunofluorescence.
To visualize neocartilage deposition and distribution, immunofluorescence of collagen I, II, and X and histological staining of sGAG were performed on encapsulated chondrocytes after 3 weeks in culture. Cell-laden hydrogels were first fixed with 4% paraformaldehyde and dehydrated using 30% sucrose solution overnight before freezing in OCT compound (Tissue-Tek). Sections were prepared and stained for type I, II, and X collagen using rabbit polyclonal antibodies (Abcam) in accordance with a previously reported immunostaining protocol.24 For sGAG histological staining, sections were prepared and stained as previously reported25 using hematoxylin solution (Sigma-Aldrich) for nuclei staining and 0.1% safranin-O solution (Sigma) for staining sGAG.
Statistical Analysis.
Data are represented as mean ± standard deviation. Statistical differences across five zones were analyzed with ANOVA one-way, two-way, and post-tests with False Detection Rate correction. Statistical differences within each zone and across gradient types were analyzed using one-way ANOVA and post-tests. All analyses were done in GraphPad Prism 7 (Graphpad Software), except a custom R script (RStudio) was used to assess monotonic trend. For all statistical analyses, a threshold of α = 0.05 was chosen, and p-values at or below 0.05 indicated significance.
RESULTS
Dual-Gradient Hydrogels Exhibit Zonal-Dependent Stiffness and Biochemical Gradients.
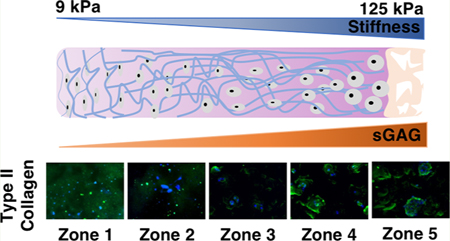
The goal of designing dual-gradient scaffolds for cartilage regeneration is to provide cells with an environment that recapitulates the composition, structure, and mechanics of native tissue in order to restore joint function (Figure 1A). As articular cartilage transitions from the superficial zone to the deep zone, the ECM of the cartilage is characterized by increased stiffness and more ECM constituents, such as sGAG.1 Here, compressive mechanical testing confirmed that the stiffness of dual-gradient hydrogels increased linearly from zone 1 (9.2 kPa) to zone 5 (124.7 kPa) (Figure 1B); this mechanical property was mainly controlled through initial precursor PEG concentrations. Similarly, quantitative assays of sGAG incorporated in the hydrogels confirmed that the biochemical gradient increased from zone 1 to zone 5 (Figure 1C) in the same direction as the mechanical gradient. In the present platform, we used photopolymerization for gelation, which prepares 3D-gradient scaffolds for many cell types and has been optimized to reduce cytotoxicity.27 After 24 h and 7 days of encapsulation and culture, chondrocytes displayed high cell viability (>90%) and homogeneous cellular distributions in all zones (Figure S1), confirming that our fabrication process is cell-friendly.
Figure 1.
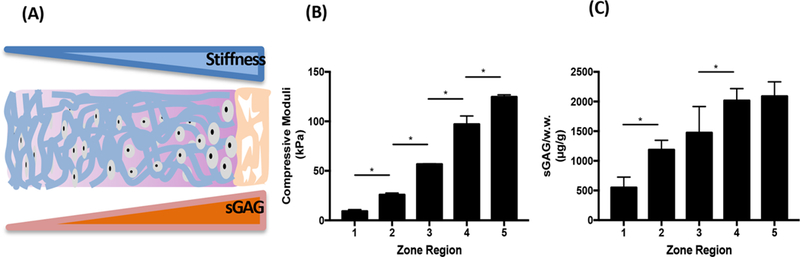
Schematic and characterization of dual-gradient hydrogels. (A) Schematic representation of the dual-gradient hydrogel design mimicking the trend of stiffness and sGAG content in native cartilage extracellular matrix. (B) Compressive modulus increased from zone 1 (9 kPa) to zone 5 (125 kPa) in dual-gradient hydrogels. (C) sGAG content increased from zone 1 to zone 5. Statistical analyses were performed using one-way analysis of variance (ANOVA), with Benjamin-Hotchberg correction for multiple posthoc comparisons of all adjacent zones. n = 3/group; *P < 0.05.
Chondrocytes Upregulated Cartilage Gene Expression in 3D Hydrogels in Response to Stiffness and Biochemical Gradients.
We evaluated the effects of dual-gradient hydrogels on chondrocytes by first investigating whether chondrocytes perceived their microenvironment differently when encapsulated within five zones of stiffness. As expected, cells that were encapsulated within stiffer zones showed higher expression of ROCK than cells encapsulated within softer matrix (Figures 2A and S2A), suggesting that cells are indeed sensing their microenvironment as soft or stiff through the Rho-ROCK pathway. We have shown in a previous study that chondrocytes cultured within homogeneous scaffolds showed mild changes in cartilage-marker expression, and changes in matrix production were purely driven by changes in cell proliferation.24 In contrast, chondrocytes cultured within dual-gradient hydrogels for 1 week only exhibited markedly enhanced upregulation of cartilage-specific gene expression compared to day 1 in a zonal-dependent manner. Specifically, increasing hydrogel stiffness and CS content significantly increased cartilage-related gene expression, including expression of the genes encoding aggrecan and type II collagen (Figure 2B,C). Given that neonatal chondrocytes initially exhibit negligible type I collagen expression on day 1, the upregulation of collagen I gene expression (Figure 2D) by day 7 did not yield significant production of type I collagen by day 21 (Figure 3), suggesting that the deposited cartilage is predominately hyaline-like. Collagen X is a hypertrophy marker of cartilage that is more prevalent in the deep zone.28 As stiffness and CS levels increased from zone 1 to zone 5, chondrocytes were more hypertrophic, as demonstrated by increased Collagen X staining (Figure 2E). We also examined the effects of dual gradients on the expression of the gene encoding MMP-13, which is a marker of cartilage remodeling.29,30 In zone 5, which was the stiffest and had the highest amount of CS, chondrocytes exhibited more extensive matrix degradation and remodeling than chondrocytes in other zones (Figure 2F).
Figure 2.
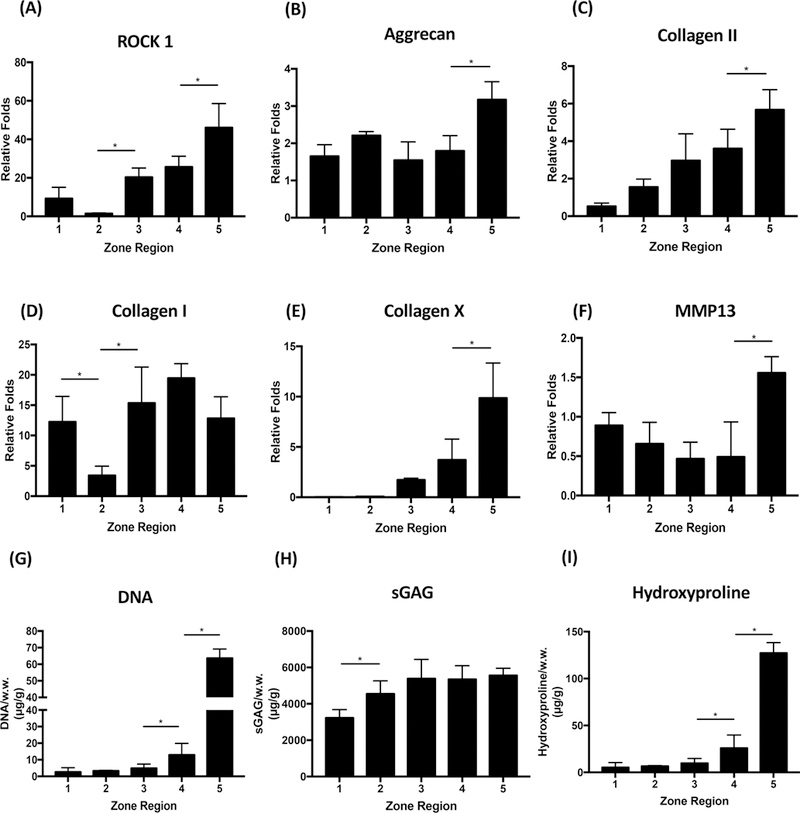
Zonal response and cartilage formation by chondrocytes in dual-gradient hydrogels. (A−F) Quantitative gene expressions of cartilage markers at day 7 normalized to day 1. (G−I) Cell proliferation and accumulated matrix deposition by chondrocytes in dual-gradient hydrogels at day 21. Statistical analyses were performed using one-way analysis of variance (ANOVA), with Benjamin-Hotchberg correction for multiple posthoc comparisons of all adjacent zones. n = 3/group; *P < 0.05.
Figure 3.
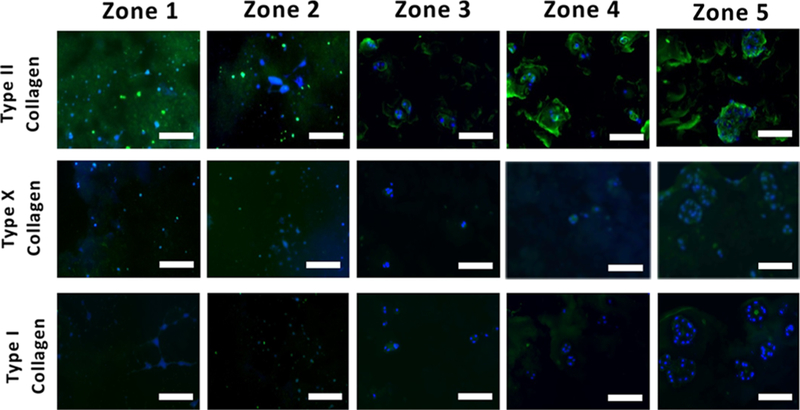
Immunostaining of cartilage matrix proteins within each zone of dual-gradient hydrogel. Increasing CS and stiffness in dual-gradient hydrogels led to larger nodules of neocartilage and more ECM matrix deposition with minimal hypertrophic phenotype and fibrocartilage observed. The encapsulated cells retained a rounded morphology in stiffer/high-CS zones (i.e., zone 4/5) while the cells present in softer/low-CS zones (i.e., zone 1/2) became elongated and spread. Scale bar = 50 μm.
Dual-Gradient Hydrogels Induced Chondrocyte Proliferation and Cartilage Matrix Production in a Zonal- Dependent Manner.
Consistent with the trend in gene expression at day 7 (Figure 2B,C), at day 21, the cumulative protein level, cellular proliferation, and hydroxyproline production increased as the stiffness and CS content increased in a zonal-dependent manner from zone 1 to zone 5, evidenced via quantitative biochemical assays including DNA and hydroxyproline (Figure 2G,I). Even though measurements of sGAG did not reveal a distinct zonal trend of deposition (Figure 2H), histology revealed increased nodule size of cellular deposited matrix as the stiffness and CS content increased from zone 1 to zone 5 (Figure S3), which mimics the sGAG distribution in native cartilage.1
To further characterize the effects of dual-gradient hydrogels on cell proliferation and cartilage formation within zones, we investigated the distribution and morphology of major cartilage matrix proteins such as proteoglycans and collagens I, II, and X after 3 weeks of culture. Encapsulated chondrocytes retained a rounded morphology in stiffer and high-CS zones 4 and 5, while cells in softer, low-CS zones 1 and 2 became elongated and stretched (Figure 3). In order to investigate sGAG nodule morphology and their distribution within the zones of the dual-gradient hydrogel, safranin-O staining was performed, and the histological staining showed dramatic morphology and distribution differences such that there was more extensive sGAG deposition in zones 4 and 5 compared to zones 1 and 2 (Figure S3). Cells residing in zone 5 proliferated and formed large cell clusters with extensive sGAG and type II collagen (Figures 2G,H, 3, and S3). In contrast, cells in softer zones did not cluster and had marginal and diffusive new type II collagen and sGAG deposition (Figures 3 and S3). Increasing CS concentration and stiffness in dual-gradient hydrogels led to larger nodules of neocartilage and more ECM deposition perhaps through the facilitation of cellular remodeling of ECM through degradation (Figure 2F). Chondrocytes in all zones produced minimal type I collagen (Figure 3). Minimal to no staining for type X collagen was observed in zones 1 to 4, while zone 5 showed a relatively higher amount of Type X collagen (Figure 3).
Dual-Gradient Hydrogels Are More Beneficial than Single-Gradient Hydrogels in Supporting Chondrocyte- Based Cartilage Zonal Development.
We evaluated the benefits of dual-gradient versus single-gradient hydrogels in supporting cartilage formation by comparing three types of gradient hydrogels: mechanical-only, biochemical-only, and dual-gradient (Figure 4). As revealed by quantitative biochemical assays, mechanical-only gradients had the lowest cell proliferation among the three hydrogel types (Figure 5A). In terms of matrix production, although CS-gradient hydrogels induced more sGAG accumulation (Figure 5B), hydroxypro- line levels in these gels were much lower than in the other two types of gradient hydrogels (Figure 5C). Most importantly, although CS-gradient hydrogels initially outperformed both mechanical-gradient hydrogels and dual-gradient hydrogels in sGAG matrix production and cellular proliferation (Figure 5A,B), the mechanical properties of CS-gradient hydrogels were the lowest. The CS-gradient hydrogel lost its integrity after 1 week of culture (Figure 4A), suggesting that it may be less suitable for translational applications than dual-gradient hydrogels. Compared to the mechanical only-gradient hydrogel, the dual-gradient hydrogel induced more cellular proliferation and neocartilage formation (both sGAG and hydroxyproline) with desirable mechanical properties (Figures 4A and 5). Taken together, these results suggest that dual-gradient hydrogels that mimic native cartilage properties are important in inducing organized cellular phenotypes and optimizing ECM formation.
Figure 4.
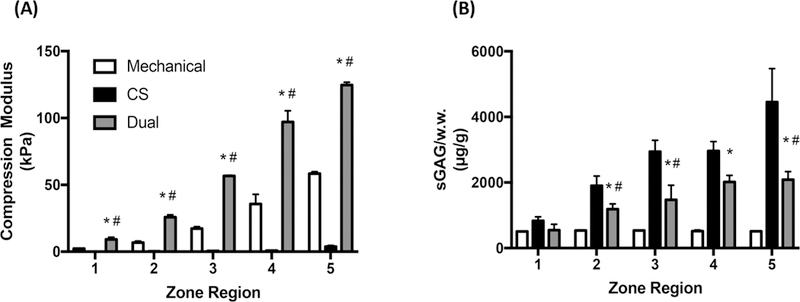
Characterizations of compressive modulus (A) and sGAG content (B) in dual-gradient vs single-gradient hydrogels at day 1. Statistical analyses were performed using two-way ANOVA. *P < 0.01 compared to mechanical; #P < 0.01 compared to CS; posthoc student’s t test corrected using Benjamin-Hotchberg procedure (n = 3/group).
Figure 5.
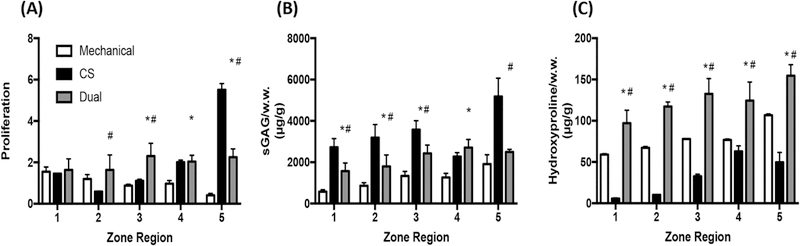
Cell proliferation and matrix production (sGAGs and hydroxyproline) by chondrocytes at day 7 in single-gradient (mechanical or CS) vs dual-gradient hydrogels. The amount of sGAG and hydroxyproline reported here is cell-deposited matrix, which is calculated by subtracting values of acellular hydrogels from cellular hydrogels. The comparison was performed at day 7 because CS only-gradient hydrogels fully degraded afterward. (A) Fold of cell proliferation normalized to day 1, (B) sGAG per wet weight, and (C) hydroxyproline per wet weight. Statistical analyses were performed between dual-gradient hydrogels vs mechanical only- or CS only-gradient hydrogels. Two-way ANOVA was performed followed by posthoc student’s t test corrected using the Benjamin-Hotchberg procedure (n = 3/group). *P < 0.01 compared to mechanical; #P < 0.01 compared to CS.
Biochemical or Mechanical Gradient, Respectively, Had an Opposite Effect in Modulating Chondrocyte Proliferation and Matrix Deposition in 3D and Exhibited an Additive Effect When Combined.
By analyzing matrix production per cell for the three types of gradient hydrogels, we identified the respective roles of biochemical and mechanical cues in cell fate at the early time point. With the mechanical-only gradient, chondrocytes proliferated less as stiffness increased but had more cartilage production per cell (Figure 6A,B). In contrast, cells encapsulated within biochemical only-gradient hydrogels showed the opposite trend, with markedly more cell proliferation but lower matrix production in high-CS zones 4 and 5 (Figure 6C,D). In dual-gradient hydrogels, cellular proliferation and matrix production balanced out from zones 1 to 5 due to additive cellular responses to cues present in the dual-gradient hydrogels (Figure 6E,F), suggesting that cells sensed the biochemical and mechanical cues equivalently at the early time point in culture.
Figure 6.
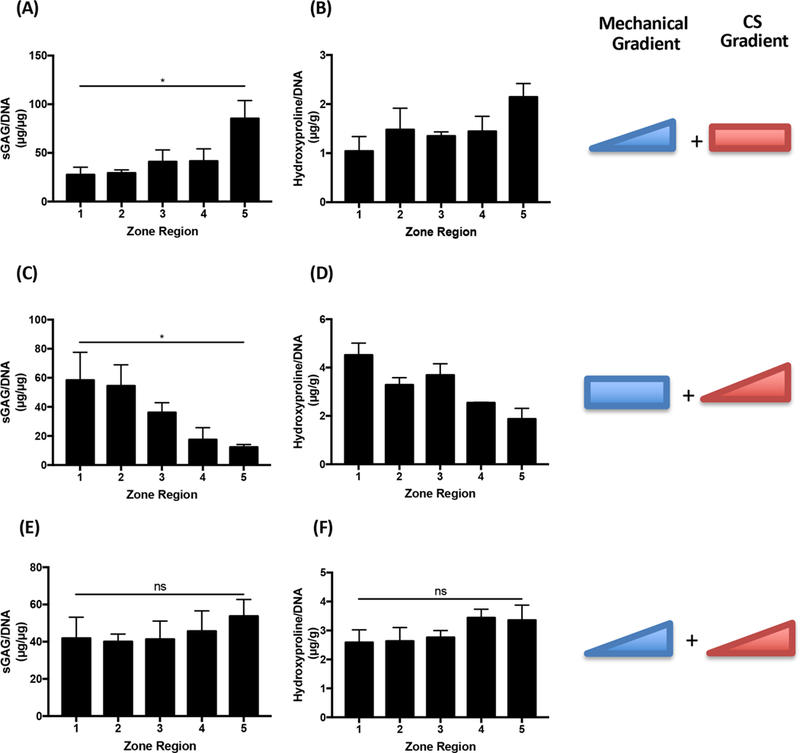
Cartilage matrix production per cell in single- vs dual-gradient hydrogels, with differential chondrocytes responses at an individual cell level. The comparison was performed at day 7 because CS only-gradient hydrogels fully degraded afterward. Mechanical-only gradient and CS-only gradient resulted in opposite trends in cartilage production per chondrocyte, which was balanced out in dual-gradient hydrogels. *P < 0.05; Mann-Kendall test which indicates a monotonic trend across all zones in the gradient hydrogels (n = 3 per group).
DISCUSSION
In this study, we demonstrated the advantage of dual-gradient hydrogels that enhance zonal organization of engineered cartilage: these dual-gradient hydrogels better mimic native tissue than single-gradient hydrogels (Figure 5). Dual-gradient hydrogels led to zonal-dependent upregulation of the expression of genes encoding cartilage components as well as the deposition of ECM molecules including collagens and sGAGs (Figures 2 and 3). Although CS only-gradient hydrogels initially induced more chondrocyte proliferation and matrix production (Figure 5), the stiffness of CS only- gradient hydrogels was <5 kPa, which is 90% lower than the other two gradient hydrogels (Figure 4A). Further, CS only- gradient hydrogels degraded prematurely after only 1 week of culture and failed to support long-term cell culture (data not shown). CS can be modified with more methacrylation in order to increase the stiffness of the resulting hydrogels;31 however, varying the CS methacrylation can also lead to undesirable changes in the hydrogel’s stiffness gradient. Compared to stiffness only-gradient hydrogels, dual-gradient hydrogels resulted in more robust cartilage-matrix deposition across all zones by day 7, while maintaining cellular zonal responses in 3D (Figure 5).
Cellular phenotypes and the matrix properties of articular cartilage vary with depth from the surface. For the superficial zone, chondrocytes secrete relatively little sGAG and aggrecan and display a correspondingly low compressive modulus, allowing the superficial zone to conform to the opposing tissue surface and to distribute stress.32,33 Collagen type X is a marker for chondrocyte hypertrophy and is expressed mainly in the deep zone of articular cartilage.34 Here, the zonal organization of engineered cartilage using dual-gradient hydrogels mimics these aspects of native cartilage, with softer, low-CS zones containing chondrocytes expressing lower levels of the gene encoding aggrecan (Figure 2B) and minimal expression of the gene encoding type X collagen (Figure 2E) compared to cells in stiffer, high-CS zones. A previous study reported that stiffer hydrogels promoted a hypertrophic phenotype of MSCs used for chondrogenesis.35 Similarly, zone 5 of our dual-gradient hydrogels induced more hypertrophic cellular phenotypes and more sGAG (Figure 2E,H), indicating a transition to a phenotype detected in chondrocytes at the osteochondral interface.36 Gene expression changes occur before changes at the protein level.37 Our data further confirm that chondrocyte phenotypes and gene expression at day 7 relate well with later biochemical assays of total collagen (Figure 2I) and immunostaining of type X collagen (Figure 3). Interestingly, we observed minimal type I collagen deposition throughout the five zones of our dual-gradient hydrogels (Figure 3), indicating that the newly formed cartilage did not express a fibrocartilage phenotype, making it suitable for articular cartilage repair.
While the CS-gradient hydrogels are not suitable for supporting cartilage regeneration due to poor mechanical properties (Figure 4A), adding a CS gradient into mechanical- gradient hydrogels is beneficial, as indicated by substantially enhanced cell proliferation, sGAG production, and hydroxyproline (Figure 5). Compared to hydrogels with a mechanical gradient alone, dual-gradient hydrogels including a CS gradient supported more chondrocyte proliferation and new cartilage ECM deposition (Figures 2 and 5). Previous work reported that adding CS into PEG hydrogels enhanced MSC chondrogenesis, as shown by upregulation of genes encoding cartilage components as well as the upregulation of the production of cartilage ECM.2,19 CS can contribute to morphogenesis and tissue homeostasis via directly interacting with cells,38 binding secreted paracrine factors,39 or facilitating tissue remodeling through cell-medicated enzymatic degradation.2 Such mechanisms may explain the enhanced cartilage formation observed here in dual-gradient hydrogels (Figure 5). Immunostaining also revealed higher numbers of large cell clusters in dual-gradient hydrogels compared to mechanical only-gradient hydrogels (Figure 3). During cartilage development, cells condense and organize into precartilage cell aggregates.40. Therefore, the observed clustering of cells likely serves as an important step in cartilage tissue formation. Importantly, immobilized CS segments of the scaffold undergo degradation in response to cellular processes (Figure 2F), paving the way for cell growth and matrix deposition in a less restricted microenvironment.
Given the large molecular weight of natural ECM molecules such as CS, increasing the ECM molecule concentration to vary biochemical cues often results in simultaneous changes in matrix stiffness.41–43 This challenge makes it difficult to determine the relative contributions of a biochemical ligand or matrix stiffness on stem-cell fate using ECM-containing hydrogels. To minimize the effects of varying ECM concentrations on scaffold stiffness, previous studies used cartilage ECM molecules at concentrations that are orders of magnitude lower than their physiological range.44,45 In the present study, we chose to use physiologically relevant concentration of CS to better mimic the native cartilage composition, and we reduced the degree of methacrylation of CS (~25%) to reduce the impact on hydrogel stiffness.31 One confounding factor of our dual-gradient hydrogel design is that incorporating methacrylated CS also causes a simultaneous increase in the hydrogel stiffness, though PEG is the dominant factor in determining the hydrogel stiffness. To minimize the impact of varying CS concentration on changing hydrogel stiffness, CS with lower methacrylation may be used to decouple the changes in biochemical and mechanical cues of hydrogels.31
In addition to recapitulating tissue zonal organization, the gradient hydrogels may also be harnessed as a tool for high-throughput screening of how niche cues impact cell fates in 3D. Previous studies showed that increasing cell proliferation is generally accompanied by decreasing matrix production per cell.46 Similarly, in the CS-gradient hydrogels, increasing CS concentration led to higher cell proliferation and lower matrix production per cell (Figure 6C,D). Interestingly, an opposite trend was observed in the mechanical only-gradient hydrogels. Specifically, increasing hydrogel stiffness increased the matrix production per chondrocyte (Figure 6A,B), which is consistent with the trend from our previous study.14 When chondrocytes were encapsulated in dual-gradient hydrogels, the effects of biochemical and mechanical cues appeared to balance out on cartilage production per cell, with no significant changes across zones (Figure 6E,F). Although the cartilage production per cell was comparable across different zones in dual-gradient hydrogels (Figure 6), total cartilage matrix still exhibits a zonal-dependent manner by day 21 due to the significant difference in cell proliferation across the five zones in dual-gradient hydrogels (Figure 2G). Cells encapsulated in zones with higher stiffness and CS dosage exhibited significantly more proliferation and total amount of sGAG and collagen (Figure 2H,I).
While this study focused on chondrocytes to show proof of concept, the gradient hydrogel platform may be easily adapted to analyze the responses of other cell types to dual-gradient niche cues. Depending on the cell type and its ability to degrade and remodel ECM, the range of niche gradient cues needs to be reoptimized. Unlike chondrocytes, the ability of MSCs to degrade PEG hydrogel is much more limited and slower. A previous study reported that MSCs produced more cartilage when encapsulated in soft hydrogels than stiffer hydrogels.47 In addition to CS, other types of biochemical cues such as hyaluronic acid may also be incorporated in gradient hydrogels for optimizing cartilage zonal development in 3D.35,48,49
CONCLUSION
Here, we report the development and characterization of a tissue-scale hydrogel containing both biochemical and mechanical gradients as a 3D niche for regenerating cartilage with biomimetic zonal organization. Encapsulation of chondrocytes in dual-gradient hydrogels led to zonal-dependent upregulated expression of genes encoding cartilage components and deposition of cartilage ECM. Although CS only-gradient hydrogels initially induced more cell proliferation and matrix production, CS only-gradient hydrogels suffered from poor mechanical properties and premature degradation. While a CS gradient alone was not suitable for supporting cartilage formation over the long-term, adding a biochemical gradient into the hydrogels containing a mechanical gradient substantially enhanced cartilage formation in a zonal-dependent manner. This dual-gradient platform can serve as a valuable tool to elucidate the interplay of mechanical and biochemical cues in regulating cell fate, including those of stem cells and other cell types. In addition, this platform may be broadly applicable for mimicking general tissue zonal organization and interfacial tissue engineering (osteochondral, muscle/tendon/ bone interfaces).
Supplementary Material
Figure S1, chondrocyte viability within 3D dual-gradienthydrogels; Figure S2, additional quantitation of gene expressions; Figure S3, safranin-O staining of sGAG deposition within dual-gradient hydrogels; Figure S4, total wet weights of mechanical-only, CS-only, and dualgradient hydrogels (PDF)
ACKNOWLEDGMENTS
This work was supported by the following grants: NIH R01DE024772 (F.Y.), NSF CAREER award (CBET-1351289) (F.Y.), and California Institute for Regenerative Medicine Tools and Technologies Award (RT3–07804) (F.Y.). The authors also acknowledge funding from the Stanford Chem-H Institute (F.Y.), Stanford Bio-X Interdisciplinary Initiative Program (F.Y.), the Stanford Child Health Research Institute Faculty Scholar Award (F.Y.), and Alliance for Cancer Gene Therapy Young Investigator award grant (F.Y.). D.Z. would like to thank Stanford Graduate Fellowship and Stanford Bio-X Interdisciplinary Program SIGF Fellowship for support.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsbiomaterials.8b00775.
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Sharma B; et al. Designing zonal organization into tissue- engineered cartilage. Tissue Eng. 2007, 13 (2), 405–14. [DOI] [PubMed] [Google Scholar]
- (2).Varghese S; et al. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol. 2008, 27 (1), 12–21. [DOI] [PubMed] [Google Scholar]
- (3).Kim IL; Mauck RL; Burdick JA Hydrogel design for cartilage tissue engineering: a case study with hyaluronic acid. Biomaterials 2011, 32 (34), 8771–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Bian L; et al. Enhanced MSC chondrogenesis following delivery of TGF-beta3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials 2011, 32 (27), 6425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Ng KW; et al. A layered agarose approach to fabricate depth-dependent inhomogeneity in chondrocyte-seeded constructs. J. Orthop. Res. 2005, 23 (1), 134–41. [DOI] [PubMed] [Google Scholar]
- (6).Gleghorn JP; et al. Adhesive properties of laminated alginate gels for tissue engineering of layered structures. J. Biomed. Mater. Res., Part A 2008, 85A (3), 611–618. [DOI] [PubMed] [Google Scholar]
- (7).DeLong SA; Moon JJ; West JL Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials 2005, 26 (16), 3227–34. [DOI] [PubMed] [Google Scholar]
- (8).Kapur TA; Shoichet MS Immobilized concentration gradients of nerve growth factor guide neurite outgrowth. J. Biomed. Mater. Res. 2004, 68A (2), 235–243. [DOI] [PubMed] [Google Scholar]
- (9).Ilkhanizadeh S; Teixeira AI; Hermanson O Inkjet printing of macromolecules on hydrogels to steer neural stem cell differentiation. Biomaterials 2007, 28 (27), 3936–43. [DOI] [PubMed] [Google Scholar]
- (10).Huebsch N; et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 2010, 9 (6), 518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Engler AJ; et al. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126 (4), 677–89. [DOI] [PubMed] [Google Scholar]
- (12).Wong JY; et al. Directed Movement of Vascular Smooth Muscle Cells on Gradient-Compliant Hydrogelsf. Langmuir 2003, 19 (5), 1908–1913. [Google Scholar]
- (13).Dubruel P; et al. Porous Gelatin Hydrogels: 2. In Vitro Cell Interaction Study. Biomacromolecules 2007, 8 (2), 338–344. [DOI] [PubMed] [Google Scholar]
- (14).Zhu D; et al. Mimicking Cartilage Tissue Zonal Organization by Engineering Tissue-Scale Gradient Hydrogels as 3D Cell Niche. Tissue Eng., Part A 2018, 24, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Johnson PM; et al. High throughput kinetic analysis of photopolymer conversion using composition and exposure time gradients. Polymer 2005, 46 (10), 3300–3306. [Google Scholar]
- (16).Tirella A; et al. A microfluidic gradient maker for toxicity testing of bupivacaine and lidocaine. Toxicol. In Vitro 2008, 22 (8), 1957–64. [DOI] [PubMed] [Google Scholar]
- (17).Martens PJ; Bryant SJ; Anseth KS Tailoring the degradation of hydrogels formed from multivinyl poly(ethylene glycol) and poly(vinyl alcohol) macromers for cartilage tissue engineering. Biomacromolecules 2003, 4 (2), 283–92. [DOI] [PubMed] [Google Scholar]
- (18).Park Y; et al. Bovine primary chondrocyte culture in synthetic matrix metalloproteinase-sensitive poly(ethylene glycol)-based hydrogels as a scaffold for cartilage repair. Tissue Eng. 2004, 10 (3–4), 515–22. [DOI] [PubMed] [Google Scholar]
- (19).Nguyen LH; et al. Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials 2011, 32 (29), 6946–52. [DOI] [PubMed] [Google Scholar]
- (20).Moutos FT; Freed LE; Guilak F A biomimetic threedimensional woven composite scaffold for functional tissue engineering of cartilage. Nat. Mater. 2007, 6 (2), 162–7. [DOI] [PubMed] [Google Scholar]
- (21).Nii M; et al. The effects of interactive mechanical and biochemical niche signaling on osteogenic differentiation of adipose- derived stem cells using combinatorial hydrogels. Acta Biomater. 2013, 9 (3), 5475–83. [DOI] [PubMed] [Google Scholar]
- (22).Jeon O; et al. Biochemical and physical signal gradients in hydrogels to control stem cell behavior. Adv. Mater. 2013, 25 (44), 6366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Watanabe H; Yamada Y; Kimata K Roles of aggrecan, a large chondroitin sulfate proteoglycan, in cartilage structure and function. J. Biochem. 1998, 124 (4), 687–93. [DOI] [PubMed] [Google Scholar]
- (24).Lai JH; et al. Stem cells catalyze cartilage formation by neonatal articular chondrocytes in 3D biomimetic hydrogels. Sci. Rep. 2013, 3, 3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Zhu D; et al. Elastin-like protein-hyaluronic acid (ELP-HA) hydrogels with decoupled mechanical and biochemical cues for cartilage regeneration. Biomaterials 2017, 127, 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Tong X; Yang F Sliding Hydrogels with Mobile Molecular Ligands and Crosslinks as 3D Stem Cell Niche. Adv. Mater. 2016, 28 (33), 7257–7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Chatterjee K; Young MF; Simon CG Jr. Fabricating gradient hydrogel scaffolds for 3D cell culture. Comb. Chem. High Throughput Screening 2011, 14 (4), 227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).van der Kraan PM; van den Berg WB Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis and Cartilage 2012, 20 (3), 223–232. [DOI] [PubMed] [Google Scholar]
- (29).Goldring MB Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther. Adv. Musculoskeletal Dis. 2012, 4 (4), 269–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Borzi RM; et al. MMP-13 loss associated with impaired ECM remodelling disrupts chondrocyte differentiation by concerted effects on multiple regulatory factors. Arthritis Rheum. 2010, 62 (8), 2370–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Wang T; et al. Chondrogenic differentiation of adipose- derived stromal cells in combinatorial hydrogels containing cartilage matrix proteins with decoupled mechanical stiffness. Tissue Eng., Part A 2014, 20 (15–16), 2131–9. [DOI] [PubMed] [Google Scholar]
- (32).Klein TJ; et al. Tissue engineering of stratified articular cartilage from chondrocyte subpopulations. Osteoarthritis Cartilage 2003, 11 (8), 595–602. [DOI] [PubMed] [Google Scholar]
- (33).Maroudas A Physicochemical Properties of Cartilage in the Light of Ion Exchange Theory. Biophys. J. 1968, 8 (5), 575–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Gannon JM; et al. Localization of type X collagen in canine growth plate and adult canine articular cartilage. J. Orthop. Res. 1991, 9 (4), 485–94. [DOI] [PubMed] [Google Scholar]
- (35).Bian L; et al. The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy. Biomaterials 2013, 34 (2), 413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Pacifici M; et al. Hypertrophic chondrocytes. The terminal stage of differentiation in the chondrogenic cell lineage? Ann. N. Y. Acad. Sci. 1990, 599, 45–57. [DOI] [PubMed] [Google Scholar]
- (37).Bian L; et al. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc. Natl. Acad. Sci. U. S. A. 2013, 110 (25), 10117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Hubbell JA Materials as morphogenetic guides in tissue engineering. Curr. Opin. Biotechnol. 2003, 14 (5), 551–8. [DOI] [PubMed] [Google Scholar]
- (39).Taipale J; Keski-Oja J Growth factors in the extracellular matrix. FASEB J. 1997, 11 (1), 51–9. [DOI] [PubMed] [Google Scholar]
- (40).Oberlender SA; Tuan RS Expression and functional involvement of N-cadherin in embryonic limb chondrogenesis. Development 1994, 120 (1), 177–187. [DOI] [PubMed] [Google Scholar]
- (41).Li Q; et al. Photocrosslinkable polysaccharides based on chondroitin sulfate. J. Biomed. Mater. Res. 2004, 68A (1), 28–33. [DOI] [PubMed] [Google Scholar]
- (42).Burdick JA; et al. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules 2005, 6 (1), 386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Erickson IE; et al. Macromer density influences mesenchymal stem cell chondrogenesis and maturation in photocrosslinked hyaluronic acid hydrogels. Osteoarthritis Cartilage 2009, 17 (12), 1639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Hwang NS; et al. Regulation of osteogenic and chondrogenic differentiation of mesenchymal stem cells in PEG-ECM hydrogels. Cell Tissue Res. 2011, 344 (3), 499–509. [DOI] [PubMed] [Google Scholar]
- (45).Guo Y; et al. Hydrogels of collagen/chondroitin sulfate/ hyaluronan interpenetrating polymer network for cartilage tissue engineering. J. Mater. Sci.: Mater. Med. 2012, 23 (9), 2267–79. [DOI] [PubMed] [Google Scholar]
- (46).Detamore MS; Athanasiou KA Effects of growth factors on temporomandibular joint disc cells. Arch. Oral Biol. 2004, 49 (7), 577–83. [DOI] [PubMed] [Google Scholar]
- (47).Wang T; et al. Modulating stem cell-chondrocyte interactions for cartilage repair using combinatorial extracellular matrix-containing hydrogels. J. Mater. Chem. B 2016, 4 (47), 7641–7650. [DOI] [PubMed] [Google Scholar]
- (48).Erickson IE; et al. Macromer density influences mesenchymal stem cell chondrogenesis and maturation in photocrosslinked hyaluronic acid hydrogels. Osteoarthritis and Cartilage 2009, 17, (12) 1639–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Chung C; Burdick JA Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng. Part A 2009, 15 (2), 243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1, chondrocyte viability within 3D dual-gradienthydrogels; Figure S2, additional quantitation of gene expressions; Figure S3, safranin-O staining of sGAG deposition within dual-gradient hydrogels; Figure S4, total wet weights of mechanical-only, CS-only, and dualgradient hydrogels (PDF)


