Abstract
A competitive-inhibition enzyme-linked immunosorbent assay (cELISA) was developed for detection of equine antibodies specific for Babesia caballi. The assay used recombinant B. caballi rhoptry-associated protein 1 (RAP-1) and monoclonal antibody (MAb) 79/17.18.5, which is reactive with a peptide epitope of a native 60-kDa B. caballi antigen. The gene encoding the recombinant antigen was sequenced, and database analysis revealed that the gene product is a rhoptry-associated protein. Cloning and expression of a truncated copy of the gene demonstrated that MAb 79/17.18.5 reacts with the C-terminal repeat region of the protein. The cELISA was used to evaluate 302 equine serum samples previously tested for antibodies to B. caballi by a standardized complement fixation test (CFT). The results of cELISA and CFT were 73% concordant. Seventy-two of the 77 serum samples with discordant results were CFT negative and cELISA positive. Further evaluation of the serum samples with discordant results by indirect immunofluorescence assay (IFA) demonstrated that at a serum dilution of 1:200, 48 of the CFT-negative and cELISA-positive serum samples contained antibodies reactive with B. caballi RAP-1. Four of five CFT-positive and cELISA-negative serum samples contained antibodies reactive with B. caballi when they were tested by IFA. These data indicate that following infection with B. caballi, horses consistently produce antibody to the RAP-1 epitope defined by MAb 79/17.18.5, and when used in the cELISA format, recombinant RAP-1 is a useful antigen for the serologic detection of anti-B. caballi antibodies.
The monoclonal antibody (MAb)-based competitive-inhibition enzyme-linked immunosorbent assay (cELISA) has been shown to be a reliable and sensitive technique for the detection of antibody against pathogens that cause persistent infections (2, 9–11, 15). Assay specificity is determined by an MAb which is inhibited from binding to its epitope when antibody to that epitope is present in the test sera. The inherent specificity of the cELISA format allows use of rather crude antigen mixtures such as infected tissue lysates, tissue culture extracts, and bacterial lysates containing recombinant proteins. The key to successful application of the cELISA is the identification of a MAb that recognizes an epitope that is conserved within the pathogen and that consistently stimulates antibody production following infection. In such cases, the cELISA has proven to be superior in sensitivity to currently used assays such as the complement fixation test (CFT).
Babesia caballi is a tick-transmitted protozoan that causes a persistent infection of horses. Identification of infected erythrocytes in stained blood smears is possible only during the acute stage of disease, and therefore, routine diagnosis of persistently infected horses is by detection of equine anti-B. caballi antibodies. CFT and the indirect immunofluorescence assay (IFA) have been the standard tests for detection of antibodies to B. caballi. The CFT has several problems when applied to equine sera. Sera containing anticomplementary activity or antierythrocyte antibodies cannot be tested by the CFT, and a predominance of antibodies of the immunoglobulin G(T) [IgG(T)] isotype, which does not fix complement by the classical pathway (13), can result in false-negative results.
In this study, development of a cELISA for detection of B. caballi antibodies began with production of MAbs to parasite antigens. The MAbs were tested with their native antigens in a preliminary cELISA, and then a gene encoding an antigen with diagnostic utility was cloned and expressed in Escherichia coli. The selected antigen is homologous to the rhoptry-associated protein 1 (RAP-1) family of proteins described for many Babesia species. Comparison of CFT and the recombinant antigen-based cELISA with 302 equine serum samples from geographically distinct regions around the world demonstrated that the cELISA identified 25% more sera as having antibody to B. caballi than the CFT.
MATERIALS AND METHODS
Parasite and serum sources and antigen production.
B. caballi-infected whole blood was obtained from the National Veterinary Services Laboratory, U.S. Department of Agriculture, (Ames, Iowa), after serial passage through three splenectomized ponies. A fourth splenectomized pony was inoculated with 180 ml of whole blood and developed a parasitemia of 6.3% infected erythrocytes (iRBCs). From this blood, washed iRBCs, IFA slides, and stabilates were made by standard techniques and were stored at −80°C (8). B. caballi parasites were isolated by a Percoll gradient method as done previously for Babesia bigemina (12).
B. caballi was maintained in microaerophilous stationary-phase tissue culture in HL-1 medium (BioWhittaker) containing 20% horse serum as reported previously (6). Parasite antigens were labeled with 35S-methionine in methionine-free M199 medium and were processed as described previously (8). Immunoprecipitation of labeled antigens with MAb and horse sera was also performed as described previously (8).
Three hundred two equine serum samples previously tested for antibodies to Babesia equi and B. caballi by CFT were obtained from the National Veterinary Services Laboratories, U.S. Department of Agriculture. These sera originated from 21 countries in North and South America and Europe and from South Africa and Qatar. Thirteen of the serum samples could not be tested by CFT due to anticomplementary activity. Six equine serum samples used as negative controls in the cELISA were from a breeding herd maintained at Washington State University (Pullman, Wash.).
Anti-Babesia equi sera from horses designated H5, H016, and H020 were derived by experimental infection (9). Sera from these horses were collected 34 (horse H5) and 9 (horses H016 and H020) months postinfection. The IFA titers of these sera on these dates were 1:6,400, 1:6,400, and 1:12,800, respectively. Horse H5 was infected intravenously, and horses H016 and H020 were infected via tick transmission (9).
Production of MAbs.
MAbs were produced by immunizing BALB/c mice with Percoll gradient-purified parasites solubilized in Freund’s incomplete adjuvant for the first immunization, followed by two immunizations in Freund’s incomplete adjuvant and a final intravenous immunization with Percoll gradient-purified parasites in phosphate-buffered saline (PBS). Spleen cells from immunized mice were fused with SP2/0 hybridoma cells and were maintained by a standard technique (16). Hybridoma supernatants were screened by fixed IFA, and cells of reactive wells were cloned by limiting dilution. Selected cloned hybridomas were cultured in a capillary-flow culture system (CellMax; Cellco, Germantown, Md.). The heavy-chain isotype of the MAb was determined by enzyme-linked immunosorbent assay (ELISA), and the MAb concentration was determined by immunodiffusion.
Immunoaffinity purification of native protein.
Native B. caballi proteins were isolated by immunoaffinity chromatography. The MAb from the hybridoma supernatant was purified by protein A chromatography (AffiGel); Bio-Rad Laboratories and was bound to CNBr-activated Sepharose 4B according to the manufacturer’s directions (Pharmacia). Twenty-five milliliters of washed, packed iRBCs was solubilized in a lysis buffer (8) containing 1% Nonidet P-40 (NP-40), and the solution was passed over a column containing the bound MAb. The column was washed in TEN buffer (0.02 M Tris, 0.005 M EDTA, 0.1 M NaCl [pH 7.6]) containing 1% NP-40 followed by washing with the same buffer containing 2 M NaCl and 0.1 M glycine, and the columns was then eluted with TEN buffer–0.5% deoxycholate–2 M KSCN. Eluted fractions were dialyzed extensively with PBS before being analyzed by silver staining and Western blotting of sodium dodecyl sulfate (SDS)-polyacrylamide gels.
Periodate treatment and epitope characterization.
The affinity-purified native proteins were boiled in sample buffer (8), separated on SDS–7.5 to 17.5% polyacrylamide gradient gels, and transferred to nitrocellulose. After transfer to nitrocellulose, the membrane was rinsed in 50 mM sodium acetate (pH 4.5) and was cut into strips. Sodium m-periodate was diluted in the same buffer to achieve final concentrations of 0, 0.1, 1.0, and 10 mM. Strips containing each antigen were incubated in the different concentrations of periodate for 1 h. After the strips were again rinsed in sodium acetate, the strips were treated with 50 mM sodium borohydride in PBS for 30 min, followed by development of blots with the respective MAbs, with horseradish peroxidase-labeled anti-mouse IgG, IgM, and IgA antibodies, and by enhanced chemiluminescence (NEN). Periodate sensitivity was validated by including a negative control (periodate-resistant epitope), B. equi EMA-1 and EMA-2 proteins with MAb 36/133.97 (7), and a positive control (periodate-sensitive epitope), the Neospora caninum 65-kDa protein with MAb 4A4-2 (2).
RNA isolation and gene cloning.
Total RNA was isolated from iRBCs with the Trizol reagent as directed by the manufacturer (Gibco BRL). Poly(A) RNA was purified from the total RNA by an a oligo(dT) selection method (Pharmacia). cDNA synthesis was also performed according to the manufacturer’s directions (Pharmacia), and the resulting cDNAs were ligated into the bacteriophage lambda ZAP II vector (Stratagene). The amplified library was screened for expression of proteins reactive with MAb 79/17.18.5 as reported previously (9), except that an iodinated anti-mouse IgG antibody was used (NEN). Recombinant pBluescript plasmids containing clones were recovered from plaque-purified lambda phage by in vivo excision (Stratagene).
Sequence analysis.
Cloned inserts in the pBluescript plasmid were sequenced by automated sequencing technology. Sequence information was assembled with the Wisconsin Package, version 9.1 (Genetics Computer Group, Madison, Wis.), on a Unix computer. Database searches were performed through the BLAST program (1), and sequence analysis and alignment were performed with Genetics Computer Group programs.
Production of recombinant antigen.
Bacterial lysate containing recombinant protein was produced following transformation of competent E. coli DH5α with 1 ng of the recombinant pBluescript plasmid. The entire transformation reaction mixture was added to 250 ml of Luria-Bertani broth containing 12.5 mg of ampicillin and 1 mM isopropyl-β-d-thiogalactopyranoside, and the culture was incubated, with shaking, overnight, at 37°C and 250 rpm. The bacteria were pelleted by centrifugation at 1,000 × g for 10 min, and the pellet was resuspended in lysis buffer without NP-40 and repelleted. This pellet was resuspended in 10 ml of lysis buffer containing 1 mg of lysozyme per ml, and the mixture was incubated on ice for 20 min. Then, NP-40 was added to a concentration of 1%, and the solution was mixed and incubated on ice for an additional 10 min. The lysate was sonicated twice for 20 s each time at 100 W while on ice. The lysate was then centrifuged at 12,000 × g for 10 min, and the supernatant was recovered and stored at −20°C until it was used as an antigen in the cELISA.
cELISA.
Immulon 2 plates (Dynatech) were coated overnight with recombinant bacterial lysate diluted in PBS containing 20 mM MgCl2. Optimal concentrations of recombinant lysate antigen and MAb 79/17.18.5 were determined by checkerboard dilutions. For cELISA, coated wells were blocked for 1 h in PBS–0.2% Tween 20–20% nonfat dry milk, followed by the addition of undiluted test horse serum to duplicate wells. After 15 min, diluted MAb 79/17.18.5 was added to the test wells. The wells were washed three times in PBS–Tween 20, followed by the addition of biotinylated anti-mouse IgG antibody, avidin-alkaline phosphatase complex (VectaStain ABC-AP; Vector Laboratories), and p-nitrophenyl phosphate substrate. The plates were read spectrophotometrically at 405 nm. Six normal equine control serum samples were run in duplicate on each plate and were used to calculate the mean normal optical density on each test plate. An equine serum sample was declared positive for antibodies if it inhibited the binding of the MAb by greater than 30% of that observed with the pool of normal control sera. Percent inhibition was calculated by the following formula: 100 − [(OD of the test sample/OD of the mean normal control serum panel) × 100], where OD is optical density.
CFT was performed at the National Veterinary Services Laboratory, Veterinary Services, Animal and Plant Health Inspection Service, U.S. Department of Agriculture, by previously described methods (5). Sera yielding discrepant results between the cELISA and CFT were tested at a 1:200 dilution by IFA with smears of washed iRBCs that had been fixed for 30 s in acetone and developed with fluorescein isothiocyanate-labeled goat anti-equine immunoglobulin antibody.
Nucleotide sequence accession numbers.
The GenBank accession nos. for the sequences reported in this paper are AF092736 (full-length clone) and AF092735 (truncated clone).
RESULTS
Antigens recognized by polyclonal immune sera and MAb 79/17.18.5.
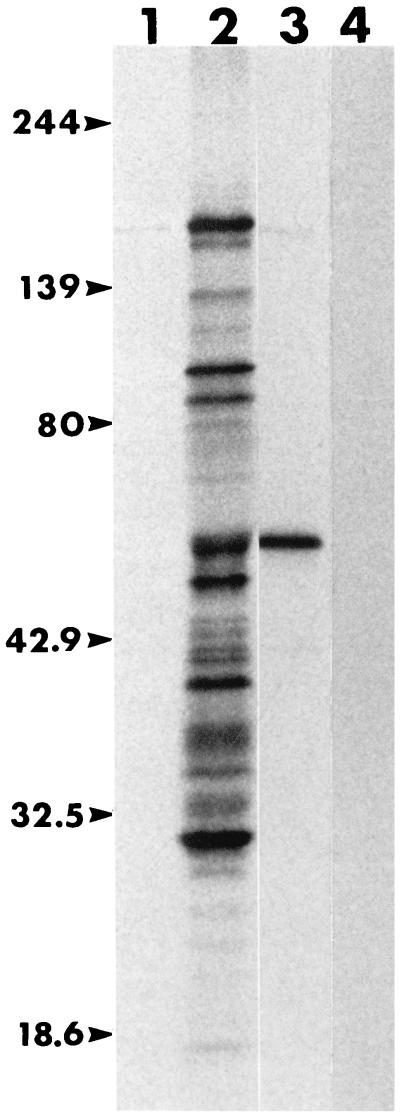
B. caballi immune serum was made by inoculating an Arabian foal (horse A2034), with an intact spleen, intravenously with 2 ml of stabilate (6.3% parasitized erythrocytes) at 1 and 7 months of age. Serum was collected 1 month following the second inoculation for use as immune serum. The predominant 35S-methionine-labeled antigens that this serum immunoprecipitated were 160, 103, 93, 60, 53, 39, and 23 kDa (Fig. 1, lane 2). MAb 79/17.18.5, identified as having the IgG1 isotype, immunoprecipitated a 60-kDa antigen from the 35S-methionine-labeled mixture of proteins (Fig. 1, lane 3). By the fixed IFA, MAb 79/17.18.5 showed a polar reactivity to parasites, suggesting that the antigen is a constituent of an apical complex organelle (data not shown). There was no reactivity of the MAb to B. equi antigens by the fixed IFA, nor did an isotype control MAb immunoprecipitate any proteins from the labeled B. caballi proteins (Fig. 1, lane 4).
FIG. 1.
Immunoprecipitated, metabolically labeled B. caballi proteins recognized by horse sera and MAbs. Lane 1, preinoculation serum from horse A2034; lane 2, immune serum from horse A2034; lane 3, anti-RAP-1 MAb 79/17.18.5; lane 4, IgG1 isotype control MAb 18.185. Molecular size markers (in kilodaltons) are given on the left (Kaleidoscope Standards; Bio-Rad Laboratories).
Periodate treatment of affinity-purified protein.
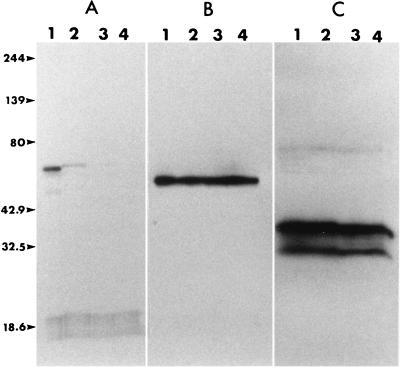
Western blotting of native protein fractions isolated from the MAb 79/17.18.5 affinity column also demonstrated that this MAb bound to a 60-kDa protein (Fig. 2B). This antigen was used to determine whether the epitope bound by MAb 79/17.18.5 was sensitive to periodate treatment. Results showed that with 0.1 M periodate, the positive control antigen, the N. caninum 65-kDa protein, lost reactivity to its MAb (Fig. 2A, lane 2), while negative control antigens, B. equi EMA-1 and EMA-2 proteins, retained their reactivities to their MAbs even with periodate at a 10 mM concentration (Fig. 2C, lanes 1 to 4). In a similar way, the B. caballi 60-kDa protein retained reactivity to MAb 79/17.18.5 (Fig. 2B, lanes 1 to 4), suggesting that the MAb reacts with a peptide rather than a carbohydrate epitope.
FIG. 2.
Western-immunoblotted proteins treated with periodate followed by development with MAbs. (A) N. caninum 65-kDa protein; (B) B. caballi native RAP-1; (C) B. equi EMA-1/EMA-2 proteins. Lane 1, no treatment; lanes 2, 3, and 4, treatment with 0.1, 1.0, and 10 mM periodate, respectively. Numbers on the left are in kilodaltons.
Sequence analysis of gene encoding recombinant antigen.
Two cDNAs were isolated on the basis of their ability to express the epitope recognized by MAb 79/17.18.5. The first was a 1,943-bp cDNA that contained a 1,467-bp open reading frame beginning with a start codon (ATG) at position 119 and ending at the stop codon at position 1586 (TAG) (see GenBank accession no. AF092736). The open reading frame encodes a 488-amino-acid protein with a molecular mass of 63,923 Da. A putative signal peptide cleavage is predicted after the valine at amino acid position 23. Removal of the 2,582-Da signal peptide would result in a 61,341-Da peptide, which is similar to the size (60 kDa) measured for the immunoprecipitated and affinity-purified native protein. There are four N-linked glycosylation consensus sequences (NXS/T), but the use of these sites is not suggested by the size of the native polypeptide. Figure 3 shows an alignment of the C-terminal 165 amino acids, which contain four repeats of 35 residues each that are only slightly degenerate from each other. A final repeat is truncated by the stop codon that terminates the open reading frame.
FIG. 3.
C-terminal repeats of B. caballi RAP-1. The residues in reverse type (black background) were conserved in three or more of the repeats. The truncated clone begins at the start of repeat 3 (residue 394). The asterisk indicates the stop codon for the protein. MAb 79/17.18.5 binds within this sequence of RAP-1. Residue numbering on the right is consistent with the numbering in the full-length clone (GenBank accession no. AF092736).
A second cDNA that expressed the epitope recognized by MAb 79/17.18.5 is an apparent truncated cDNA (see GenBank accession no. AF092735). It is a 670-bp fragment with a 287-bp open reading frame that begins at the corresponding position (position 1297) of the full-length cDNA. The start of the deduced amino acid sequence of the truncated clone corresponds exactly to the start of the third repeat (residue 394 in Fig. 3) and continues through the end of the coding sequence. There is a one-base substitution in the coding sequence of the truncated clone in comparison to the sequence of the full-length clone. The G-to-A substitution in the truncated clone results in an amino acid substitution of serine for glycine. It is not known whether this substitution is due to a cloning or a sequencing event or if the change actually existed in the organism. That MAb 79/17.18.5 binds to the truncated clone, which consists only of a portion of the C-terminal repeats, indicates that the epitope for MAb 79/17.18.5 is within the 35-bp repeats (Fig. 3).
A BLAST search with the deduced amino acid sequence revealed a high degree of homology between this sequence and the RAP-1 family of proteins of other Babesia spp. BESTFIT comparison of the B. caballi RAP-1 with other babesial RAP-1 revealed 45.11% identity (53.93% similarity) to Babesia ovis (B48572), 40.71% identity (48.94% similarity) to Babesia bigemina (M60878), 35.80% identity (43.62% similarity) to Babesia bovis (M38218), 33.48% identity (43.61% similarity) to Babesia canis (M91168), and 31.01% identity (37.96% similarity) to Babesia divergens (Z49818) (parenthetical designations are GenBank accession numbers). In addition, this B. caballi sequence is 100% identical to a previously reported PCR product from B. caballi DNA with homology to the RAP-1 family of proteins (U46551). Finally, a cDNA that contains a portion of the C-terminal repeats has been filed with GenBank (accession no. AF048748). The cDNA is 100% identical to the truncated clone reported in this paper through the length that they share in common, which includes the G-to-A substitution.
cELISA with recombinant bacterial lysate.
Bacterial lysate was titrated against MAb 79/17.18.5 by coating the wells of Immulon 2 plates with dilutions equivalent to 1.0 to 0.001 μl of lysate contained in a volume of 100 μl. In the titration, the amount of MAb 79/17.18.5 tested corresponded to dilutions containing 1.65 to 0.000165 μg in a volume of 10 μl. One hundred microliters of undiluted normal horse serum was added to each well, followed by the addition of 10 μl of diluted MAb. Further titration was done with the high-titer anti-B. caballi serum and normal horse serum on the same plate such that at each given MAb and antigen concentration, the range in inhibition between negative and positive sera could be evaluated. A volume of 0.025 μl of bacterial lysate and 0.0165 μg of MAb per well was chosen to yield the largest range in OD values between positive and negative sera.
Three hundred two serum samples submitted to the National Veterinary Services Laboratory for testing by CFT were also tested by cELISA. Thirteen samples had anticomplementary activity when tested by CFT and were not considered when comparing the results for CFT and cELISA. Of the remaining 289 serum samples, CFT identified 76 as positive (26.3%) and 213 as negative for antibody to B. caballi. The cELISA, however, indicated that 143 of the 289 serum samples were positive (49.5%). Therefore, 72 serum samples were CFT negative and cELISA positive, while 5 serum samples tested CFT positive and cELISA negative. As a result of these 77 samples (27%) with discordant results, the concordance between CFT and cELISA is 73%.
The 72 CFT-negative and cELISA-positive serum samples were retested to determine how many were positive by fixed IFA. The 72 samples with discordant results were tested at a 1:200 dilution to ensure specificity. Samples for IFA were tested blind with reference to the cELISA and CFT results. Even at the 1:200 dilution, 67% (48 of 72) of the CFT-negative and cELISA-positive serum samples were strongly positive by IFA. Three serum samples from horses with experimental B. equi infections (horses H5, H016, and H020) (9) showed no reactivity to B. caballi antigens by IFA, even with a 1:10 dilution, demonstrating that the IFA results for field sera were not due to cross-reactivity derived from coinfection with B. equi.
Five samples with discrepant results were CFT positive and cELISA negative. Application of these samples diluted 1:200 to fixed IFA demonstrated that four of five were positive for B. caballi antibody. These five serum samples represent approximately 6.5% of the discordance betweeen the two tests, and four of these serum samples were apparently false negative by cELISA. These five serum samples originated from five different countries on two continents representing the Old and the New World, suggesting that there is no correlation between parasite strain and lack of reactivity in the cELISA.
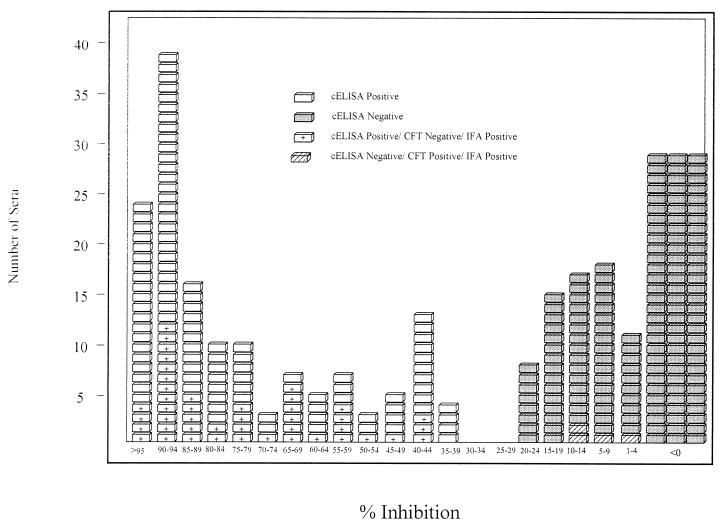
Figure 4 depicts the distribution of the cELISA results according to percent inhibition and shows the distribution of the IFA-positive samples among the serum samples with discrepant results. The majority of cELISA-positive samples (85%) had inhibition of greater than 50%. Also, the majority of cELISA-negative samples (95%) inhibited the MAb by less than 20%, with 55% of the cELISA-negative samples showing no inhibition. Therefore, most sera tested by cELISA were either clearly positive or clearly negative. The 30% inhibition cutoff was determined by identifying the median point between the negative sample with the highest level of inhibition and the positive sample with the lowest level of inhibition.
FIG. 4.
Distribution of cELISA results by percent inhibition. Samples with discrepant results that were found to be positive by IFA have an additional mark: plus sign, the sample was IFA positive, in agreement with the cELISA; cross-hatching, the sample was IFA positive, in agreement with CFT; white blocks, the samples were cELISA positive irrespective of the CFT results; shaded blocks, the samples were cELISA negative, irrespective of the CFT results.
DISCUSSION
The cELISA with MAb 79/17.18.5 and recombinant RAP-1 is an improved test for the detection of antibody to B. caballi in horses. The format overcomes the problems inherent in CFT, including the inability to run samples with anticomplement activity and antierythrocyte antibodies and the potential for false-negative results due to the inability of IgG(T) isotype antibodies to fix complement via the classical pathway. Also, in contrast to CFT and IFA, the cELISA format allows sera to be tested undiluted, thus maximizing test sensitivity. In addition, the use of recombinant antigen replaces the need to infect horses to derive antigen and provides an inexpensive and easily reproducible antigen source.
The data presented here indicate that MAb 79/17.18.5 binds to a peptide epitope within the C-terminal repeat region of B. caballi RAP-1. The evidence supporting this conclusion includes (i) the lack of predicted N-linked glycosylation sites in the truncated recombinant clone, (ii) the lack of a hydrophobic sequence in the clone of the repeat region capable of directing cleavage and replacement by a glycosylphosphatidyl inositol anchor, and (iii) the fact that the binding site for MAb 79/17.18.5 is resistant to periodate treatment. A final proof is that the MAb identifies the recombinant protein, for which E. coli is unlikely to produce posttranslational modifications involving the addition of carbohydrates and lipids to polypeptides. The fact that MAb 79/17.18.5 binds to the repeats suggests that there may be multiple binding sites for the MAb on each RAP-1 molecule.
There are differences in the reported sizes of the major B. caballi antigens recognized by the equine humoral response (3). It is not possible to reconcile the differences here, but we speculate that variations in gel systems and measurement techniques may be responsible. For instance, Bose (4) reported on a 48-kDa antigen that has a punctate immunofluorescence pattern and that is consistently recognized by all European and South American horse sera tested. Interestingly, MAb 79/17.18.5 reacts solely to an approximately 48-kDa molecule in Western blots of total iRBC lysate (data not shown). We suspect that the presence of the combination of erythrocyte and babesial antigens in the preparation forces anomalous migration of B. caballi RAP-1 in SDS-polyacrylamide gels in comparison to that of the purified molecule as reported in this paper. Therefore, we cannot exclude the possibility that Bose’s 48-kDa protein and RAP-1 are the same entity.
A problem associated with the use of IFA in this work for the purpose of defining the true status of sera with discordant results is test sensitivity and specificity. In order to ensure specificity in the IFA, sera with discordant results were diluted 1:200. The need to dilute these sera then leads to the potential of decreased sensitivity. Therefore, the data comparing CFT and cELISA can be presented only as concordance. The results reported in this paper indicate that the cELISA identified 25% more field sera as positive for B. caballi antibodies than the CFT. Use of the IFA (even at a 1:200 dilution) showed that 67% of the sera that tested cELISA positive and CFT negative were true positives.
In contrast, only five serum samples were cELISA negative and CFT positive, and four of these were shown to be true positives by IFA. Each of these had low titers by CFT, although four samples were clearly IFA positive. Since the sera were not clustered from one place of origin, it does not suggest a problem in the conservation of the repeats. On the contrary, B. bovis also has repeats at the C terminus of RAP-1, and the repeats are highly conserved in parasites from geographically distinct sources (14). Also, a recent submission to GenBank (accession no. AF048748) representing an independently derived cDNA from B. caballi shares 100% homology with the truncated cDNA described in this report. The lack of reactivity of these five samples may represent sera retrieved from horses early after the onset of infection, prior to the development of a significant quantity of antibody to the epitope defined by MAb 79/17.18.5, and in fact represents false-negative cELISA results.
Infections of horses with the causative protozoan parasites (B. equi and B. caballi) of equine piroplasmosis severely restrict the international movement of horses. Several countries, including the United States, restrict the entry of horses on the basis of their serologic status for these protozoa. Due to in part to the limited knowledge concerning the competence of certain ticks to transmit these hemoparasites, the movement of infected horses is restricted in order to prevent the establishment of new areas of endemicity. In addition to restricting equine commerce, infections with these protozoa also affect international equestrian events, such as the 1996 Olympics in Atlanta, Ga. Due to the problems inherent in the use of CFT for the detection of specific antibody in horses, a recombinant antigen-based cELISA was developed for the detection of anti-B. equi antibody (9). This paper describes the development of a cELISA for the detection of anti-B. caballi antibodies, and that cELISA may be used in parallel with the cELISA for the detection of anti-B. equi antibodies. These recombinant antigen-based cELISAs overcome the problems inherent to CFT and will allow accurate epidemiological studies. The use of recombinant antigens in the cELISA will allow the international standardization of the testing of horses for persistent infection with the causative protozoans of equine piroplasmosis.
ACKNOWLEDGMENTS
This work was supported by the Agricultural Research Service, U.S. Department of Agriculture (grant CWU 5348-32000-010-00D).
We acknowledge Debra Alperin, Bruce Mathison, and Judy Nicholson for excellent technical assistance.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Meyers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Baszler T V, Knowles D P, Dubey J P, Gay J M, Mathison B A, McElwain T F. Serological diagnosis of bovine neosporosis by Neospora caninum monoclonal antibody-based competitive inhibition enzyme-linked immunosorbent assay. J Clin Microbiol. 1996;34:1423–1428. doi: 10.1128/jcm.34.6.1423-1428.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bose R, Daeman K. Demonstration of the humoral immune response of horses to Babesia caballi by Western blotting. Int J Parasitol. 1992;22:627–630. doi: 10.1016/0020-7519(92)90011-9. [DOI] [PubMed] [Google Scholar]
- 4.Bose R. Polyclonal antibody characterization of Babesia caballi antigens. Int J Parasitol. 1994;24:511–517. doi: 10.1016/0020-7519(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 5.Frerichs W M, Holbrook A A, Johnson A J. Equine piroplasmosis: complement fixation titers of horses infected with Babesia caballi. Am J Vet Res. 1969;30:697–702. [PubMed] [Google Scholar]
- 6.Holman P J, Frerichs W M, Chieves L, Wagner G G. Culture confirmation of the carrier status of Babesia caballi-infected horses. J Clin Microbiol. 1993;31:698–701. doi: 10.1128/jcm.31.3.698-701.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kappmeyer L S, Perryman L E, Knowles D P. A Babesia equi gene encodes a surface protein with homology to Theileria species. Mol Biochem Parasitol. 1993;62:121–124. doi: 10.1016/0166-6851(93)90185-z. [DOI] [PubMed] [Google Scholar]
- 8.Knowles D P, Perryman L E, Goff W L, Miller C D, Harrington R D, Gorham J R. A monoclonal antibody defines a geographically conserved surface protein epitope of Babesia equi merozoites. Infect Immun. 1991;59:2412–2417. doi: 10.1128/iai.59.7.2412-2417.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knowles D P, Kappmeyer L S, Stiller D, Hennager S G, Perryman L E. Antibody to a recombinant merozoite protein epitope identifies horses infected with Babesia equi. J Clin Microbiol. 1992;30:3122–3126. doi: 10.1128/jcm.30.12.3122-3126.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knowles D P, de Echaide S T, Palmer G, McGuire T, Stiller D, McElwain T. Antibody against an Anaplasma marginale MSP5 epitope common to tick and erythrocyte stages identifies persistently infected cattle. J Clin Microbiol. 1996;34:2225–2230. doi: 10.1128/jcm.34.9.2225-2230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H, Shen D T, Knowles D P, Gorham J R, Crawford T B. Competitive-inhibition enzyme-linked immunosorbent assay for antibody in sheep and other ruminants to a conserved epitope of malignant catarrhal fever virus. J Clin Microbiol. 1994;32:1674–1679. doi: 10.1128/jcm.32.7.1674-1679.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McElwain T F, Perryman L E, Davis W C, McGuire T C. Antibodies define multiple proteins with epitopes exposed on the surface of live Babesia bigemina merozoites. J Immunol. 1987;138:2298–2304. [PubMed] [Google Scholar]
- 13.McGuire T C, Van Hoosier G L, Jr, Henson J B. The complement-fixation reaction in equine infectious anemia: demonstration of inhibition by IgG(T) J Immunol. 1971;107:1738–1744. [PubMed] [Google Scholar]
- 14.Suarez C E, Palmer G H, Hotzel I, McElwain T F. Structure, sequence, and transcriptional analysis of the Babesia bovis rap-1 multigene locus. Mol Biochem Parasitol. 1998;93:215–224. doi: 10.1016/s0166-6851(98)00032-2. [DOI] [PubMed] [Google Scholar]
- 15.Torioni de Echaide S, Knowles D P, McGuire T C, Palmer G H, Suarez C E, McElwain T F. Detection of cattle naturally infected with Anaplasma marginale in a region of endemicity by nested PCR and a competitive enzyme-linked immunosorbent assay using recombinant major surface protein 5. J Clin Microbiol. 1998;36:777–782. doi: 10.1128/jcm.36.3.777-782.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokoyama W M. Production of monoclonal antibodies. In: Coligan J E, editor. Current protocols in immunology. New York, N.Y: Wiley Intersciences; 1994. pp. 2.2.1–2.5.17. [Google Scholar]