Abstract
Background:
Parkinson's disease (PD) is associated with brainstem dysfunction causing non-motor symptoms. Vestibular evoked myogenic potential (VEMP) and brainstem auditory evoked potential (BAEP) are electrophysiological tests to assess the vestibular and auditory pathways in the brainstem.
Objectives:
To study the abnormalities of cervical VEMP (cVEMP) and BAEP in PD and to correlate the findings with the symptoms related to brainstem involvement.
Patients and Methods:
cVEMP and BAEP were recorded in 25 PD patients and compared 25 age matched controls. The PD patients were assessed with the following clinical scales: REM Sleep Disorder Screening Questionnaire (RBD-SQ), Epworth Sleepiness Scale (ESS), mini-BESTest, Geriatric Depression Scale (GDS-15) and MMSE (Mini-mental state examination). The P13 and N23 peak latencies and the P13/N23 amplitude of cVEMP, the latencies of waves I, III and V, and the inter-peak latencies (IPL) of waves I-III, III-V and I-V of BAEP were measured.
Results:
The PD patients showed prolonged latencies and reduced amplitude in cVEMP responses. They had abnormal BAEP in the form of prolonged absolute latencies of wave V, followed by wave III and I–V IPL with no significant difference in waves I and I–III IPL. The cVEMP abnormality was correlated directly with RBD-SQ and inversely with mini-BESTest scores. There were no correlations between cVEMP/BAEP abnormality and disease severity, GDS-15, ESS and MMSE.
Conclusion:
PD is associated with cVEMP and BAEP abnormalities that suggest auditory and vestibular pathway dysfunction in the brainstem and cVEMP correlates with the symptoms of brainstem degeneration like RBD and postural instability.
Keywords: BAEP, cervical VEMP, evoked potentials, Parkinson's disease, RBD
INTRODUCTION
Parkinson's disease (PD) is an alphasynucleinopathy caused by the degeneration of midbrain dopaminergic neurons in substantia nigra.[1] It is characterized by the presence of motor symptoms of rest tremors, rigidity and bradykinesia. Along with these motor symptoms, PD patients manifest non-motor symptoms (NMS). These NMS include autonomic dysfunction, neuropsychiatric disturbances, sleep disturbances, fatigue, sensory symptoms and gastrointestinal disorders.[2] According to the Braak staging of progression of PD, alpha-synuclein deposition starts caudally from the dorsal motor vagal nucleus in the medulla, then ascends in the brainstem and finally involves the neocortex. Brainstem nuclei are involved in stages I to III of Braak staging.[3] Alpha-synuclein deposition also occurs in vestibular nuclei.[4] In PD, a functional misconnection between the brainstem and higher structures, involving mainly non-dopaminergic pathways, occur even in the earliest phase of the disease. The neurophysiological studies of the brainstem in PD indicate a mis-modulation of physiological afferent processing and brainstem control of spinal motor neurons.[5] The electrophysiological tool for the assessment of brainstem pathways are the brainstem auditory evoked potential (BAEP) and vestibular evoked myogenic potential (VEMP). Abnormalities in BAEP responses occur in upper brainstem (midbrain) lesions, while abnormalities in the VEMP responses are involved in the lower brainstem (pontine and upper medullary) lesions. Therefore, they have a localising value of brainstem dysfunction at different levels. Prior studies have shown impaired VEMP and BAEP responses in PD patients compared to controls which were attributed to the underlying brainstem dysfunction.[6,7] The present study was aimed at determining the presence of vestibular and auditory pathway abnormalities in PD as compared to the healthy age-matched controls and whether the presence of these abnormalities has a significant correlation with the presence of postural instability and few NMS like RBD, depression, ESS and cognitive impairment.
MATERIALS AND METHODS
Study design and subjects recruitment
The study was a prospective, cross-sectional, analytical study. A total of 50 subjects (25 IPD patients and 25 age-matched healthy controls) were recruited for the study. Patients with PD were recruited from the outpatient/in-patient care in the Department of Neurology of our tertiary care teaching institute. The study period was from 2016 to 2018. Idiopathic PD was diagnosed as per the United Kingdom Parkinson's Disease Society (UKPDS) Brain Bank clinical diagnostic criteria.[8] The exclusion criteria were those of the UKPDS and those with presbycusis, chronic vertigo and history of ear surgery were excluded. The participants were evaluated in the 'ON' phase without dyskinesia to reduce any artefacts due to the OFF-period tremors. The study protocol was approved by the Institutional Ethics committee (ID prot Ref number- SS-1/EC/01/2017). All subjects gave written informed consent.
Study methods and data collection
The demographic details, clinical characteristics and medication history of the patients were recorded in the pre-designed clinical proforma. Motor severity was assessed using the United Parkinson's Disease Rating Scale part III (UPDRS-III) both in the 'OFF' and 'ON' states.[9] The stage of the disease was assessed by the modified Hoehn and Yahr (H&Y) scale in the 'OFF' state.[10] The presence of the rapid eye movement (REM) Sleep Behaviour Disorder (RBD) was assessed using the RBD Screening Questionnaire (RBDSQ) in the 'ON' state.[11] The excessive daytime sleepiness was assessed using the Epworth Sleepiness Scale (ESS) in the 'ON' state.[12] The Geriatric Depression Scale (GDS-15) in the 'ON' state and the Mini Balance Evaluation Systems Test (Mini-BESTest) in the 'OFF' state were used to assess depression, balance and postural instability.[13,14] The mini-mental state examination (MMSE) was used for cognitive screening. Scores below 24 were used to diagnose cognitive impairment in the 'ON' state.[15]
Electrophysiological assessment
All subjects underwent pure tone audiometry to assess the peripheral auditory system function. Those with normal hearing threshold were included. VEMP and BAEP were performed in all the subjects in the 'ON' state using the Nihon Kohden Neuropack Software with preset parameters for each test performed.
VEMP assessment
Cervical VEMP (cVEMPs)
An auditory stimulus (loud click: 100 db nHL or louder with a duration of 0.1 msec and frequency of 3–5 hz) was delivered to an ear and a masking noise was subjected to the opposite ear. While turning the neck to the opposite side, an auditory response waveform with a latency of 10-40 msec was recorded from an electrode on the sternocleidomastoid muscle (SCM). The active electrode was placed at the midpoint of each SCM muscle, the reference electrode on the suprasternal notch and the ground electrode on the forehead. Measurement setting: Sensitivity-100 μV/div, filter-2 to 5 KHz/high cut filter: 1.5 to 2 KHz, Analysis time: 10 msec/div and number of averaging: 200 to 500. The peak latency of the first wave (p13) and the peak latency of the second wave (n23), peak to peak amplitude (p13/n23) and interside peak latency difference between the first waves are shown in Figure 1.
Figure 1.
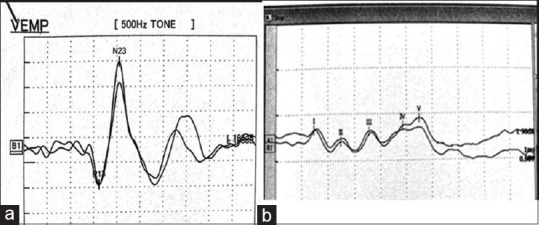
(a) Normal cVEMP waveforms; (b) Normal BAEP waveforms
BAEP
A two-channel recording was done using the vertex electrode as the reference electrode and the left and the right earlobe electrode was used as the active electrode. The skin electrode contact impedence less than 2 KΩ. The number of averaging was 1000–2000. The auditory stimulation (click sound) was presented to either side of the ear at an intensity of 80–90 db nHL at a rate of 10 Hz. The procedure was repeated twice to check the reproducibility. The latencies of wave I, III and V and inter-peak latencies I-V, I-III and III-V (IPLs) were recorded [Figure 1].
Statistical analysis
Statistical analysis was performed using the SPSS version 22. The qualitative variables were expressed as frequency and percentage and compared using Chi-square test. The quantitative variables were expressed as mean with standard deviation and compared using independent sample 't' test. The correlations between clinical and VEMP findings were performed by calculating the Spearman's correlation coefficient. Statistical significance was set at P value < 0.05.
RESULTS
A total of 50 subjects (IPD group – 25, Control group – 25) were included in the study. All subjects underwent cervical VEMP and BAEP analyses and the characteristic waveforms were analysed. In the IPD group, 19 were males (76%) and in the control group 15 were males (60%). The mean age of patients with IPD was 68.3 ± 9.0 years and in control subjects were 65.0 ± 7.9 years. The history of postural instability was present in 13 cases and dementia was present (MMSE < 23) in 15 cases of the IPD group. The demographic and clinical assessment data of the patient and control groups are presented in Table 1.
Table 1.
Clinical scales score
| PD (n=25) | Controls (n=25) | |
|---|---|---|
| Gender (M/F) | 19/6 | 15/10 |
| Age (years) | 68.3±8.9 | 65.0±7.9 |
| Duration (years) | 5.1±2.3 | - |
| H&Y stage score | 2.7±0.8 | - |
| UPDRS III | 47.2±8.0 | - |
| MMSE | 22.9±3.6 | 28.2±0.8 |
| RBDSQ | 6.1±1.2 | - |
| ESS | 9.8±3.6 | - |
| Mini-BESTest score | 14.5±6.3 | - |
| GDS-15 | 6.1±2.8 | - |
RBDSQ - Rapid eye movement sleep behaviour disorder screening questionnaire; UPDRS - Unified Parkinson’s disease rating scale; H&Y - Hoehn and Yahr; MMSE- mini-mental state examination; ESS - Epworth sleepiness scale; GDS-15 - Geriatric depression scale
VEMP assessment and results
All subjects underwent bilateral recording of cVEMP from the active sternocleidomastoid (SCM). Absent waveforms and prolonged peak latencies (P13 and N23) were considered as abnormal. Absent waveforms were recorded in three (out of 25) cases, unilaterally recorded in four (out of 25) cases (two cases - left and two cases - right) in the IPD group. All waveforms were recordable in the control group; however, one case was found to have prolonged peak latency. P13 peak latencies were found to be significantly prolonged in the IPD group (13 cases) measuring 16.98 ± 2.50 msecs in comparison to 14.19 ± 0.79 msecs (p = 0.03) of the controls. N23 peak latency was also significantly prolonged in the IPD group (12 cases) (26.82 ± 2.10 msecs vs 23.85 ± 0.77 msecs; P = 0.04). The mean P13–N23 amplitude was significantly reduced in the IPD group (15.69 ± 6.34 μV vs 19.57 ± 2.83; P = 0.04). RBDSQ score had a positive correlation with P13 peak latency (rho = 0.651, P = 0.012) and N23 latency (rho = 0.736, P = 0.003). Both P13 peak latency (rho = -0.64, P = 0.014) and N23 peak latency had a negative correlation with Mini-BESTesT score (rho = -0.641, P = 0.04). However, there were no significant correlations with disease severity (UPDRS III, H&Y stage), cognitive impairment, depression and ESS [Table 2].
Table 2.
Correlations of cVEMP response with clinical scales
| Parameters | RBD | Mini-BESTesT | UPDRS III | H&Y staging | GDS | ESS | MMSE |
|---|---|---|---|---|---|---|---|
| P 13 latency | |||||||
| Correlation coefficient (r) | 0.651 | -0.640 | -0.050 | 0.231 | 0.512 | 0.355 | 0.066 |
| P | 0.01* | 0.01* | 0.86 | 0.65 | 0.06 | 0.21 | 0.98 |
| N23 latency | |||||||
| Correlation coefficient (r) | 0.736 | -0.641 | 0.133 | 0.25 | 0.521 | 0.360 | -0.415 |
| P | 0.003* | 0.04* | 0.65 | 0.67 | 0.65 | 0.12 | 0.14 |
*P≤0.05 significant RBDSQ - Rapid eye movement sleep behaviour disorder screening questionnaire; UPDRS - Unified Parkinson’s disease rating scale; H&Y - Hoehn & Yahr; MMSE - mini-mental state examination; ESS - Epworth sleepiness scale; GDS-15 - Geriatric depression scale; cVEMP - cervical vestibular evoked myogenic potentials
BAEP assessment and results
All 50 cases underwent BAEP assessment. The absent peak (I, II, III, IV and V) and prolonged latencies of individual waves along with inter-peak latencies (IPL) (I-III, III-V and I-V) were considered as abnormal. All waveforms were recordable in the control group. In IPD group, BAEP was not recordable bilaterally in four cases and in two cases not recordable unilaterally. The latencies of wave V (5.64 ± 0.16 ms, P = 0.002) and wave III (3.98 ± 0.22 ms, P = 0.024) and I–V inter-peak (4.31 ± 0.12 ms, P = 0.002) latencies were significantly prolonged compared to control. The latencies of wave I, II and IV as well as I-III and III-V IPL were not significantly prolonged [Table 3]. In the IPD group, 12 cases had prolonged wave V latency, nine cases had prolonged wave III latency and 11 cases had prolonged I-V IPL. Among the controls, one subject had prolonged wave III and V latency and another subject had prolonged latency of wave V and prolonged I-V IPL. There were no significant correlations of BAEP findings to disease severity, dementia, RBD-SQ, ESS, GDS and Mini BESTesT scores.
Table 3.
Comparison of BAEP responses
| BAEP waveforms and IPL latency (ms) | PD (n=25) | Controls (n=25) | P |
|---|---|---|---|
| Wave I | 1.8±0.1 | 1.6±0.1 | 0.09 |
| Wave II | 2.9±0.1 | 2.8±0.3 | 1.00 |
| Wave III | 3.9±0.2 | 3.5±0.1 | 0.02* |
| Wave IV | 5.3±0.3 | 4.9±0.3 | 0.06 |
| Wave V | 5.6±0.2 | 5.2±0.2 | 0.002* |
| Wave I-III IPL | 2.4±0.1 | 2.1±0.1 | 0.06 |
| Wave III-V IPL | 2.3±0.1 | 2.2±0.8 | 0.06 |
IPL - inter-peak latency; *P<0.05; BAEP- Brainstem auditory evoked potentials
DISCUSSION
The present study was aimed at determining the presence of vestibular and auditory pathway abnormalities in PD as compared to the healthy age-matched controls and whether the presence of these abnormalities had significant correlation with the presence of postural instability and few NMS like RBD, depression, ESS and cognitive impairment. Electrophysiological studies have shown functional abnormalities at the level of spinal cord, brainstem, cerebellum, basal ganglia and motor cortex in PD. Both VEMP and BAEP assessments are non-invasive techniques to evaluate the vestibular and auditory pathways in neurological disorders including PD. There are few studies wherein VEMP and BAER have been used on PD patients.[16] The need for the study of vestibular pathway dysfunction in PD stems from the fact that alpha-synuclein deposition has been found in vestibular nuclei, dopamine D2 receptors have been identified in medial and lateral vestibular nuclei suggesting the modulatory role of dopamine on vestibular nuclei and vestibular nuclei neurons receive input from the locus coeruleus and dorsal raphe nucleus that are degenerated in PD and cause secondary involvement of vestibular nucleus.[17]
cVEMP in PD
cVEMP is a manifestation of the vestibulo-collic reflex (VCR), wherein the stimulation of the vestibular organs (with loud sound) evokes a muscle reflex. It is an ipsilateral reflex with biphasic positive–negative surface potential with peak latencies at about 13 and 23 msecs. The oligosynaptic pathway involves the saccule and utricle of the vestibular apparatus, vestibular nucleus in the pontomedullary junction, medial vestibulospinal tract, nucleus ambiguus in the medulla and the accessory nerve.[18] Prolongation of the latencies at 13 and 23 msecs or absence of waveform indicate saccular/utricular otolith afferent, vestibular nerve/vestibular nucleus or its connection dysfunction. Pollak et al. (2009) reported higher frequency of absent cVEMP responses (24 out of 54 patients) in their PD patients with no difference in the latencies as compared to the healthy controls.[19] De Natale ER, et al. (2015) showed higher frequency of absent VEMP response in PD patients with no latency differences.[7] Shalash AS, et al. (2017) showed prolonged P13 and N23 latencies (15 cases) with absent responses (three of 15 cases of PD). They also found that bilateral P13–N23 amplitudes were significantly decreased in the IPD group.[16] We evaluated 50 subjects (IPD – 25, Control – 25) with cVEMP and BAEP. The mean peak P13 and N23 latencies were significantly prolonged in IPD group with absent waveforms were recorded in 3 (out of 25) cases, unilaterally recorded in 4 (out of 25) cases (2 cases -left 2 cases- right) and reduced P13–N23 amplitudes. Age can be confounding factor in VEMP abnormalities due to a progressive decline in the number of peripheral receptors and excitability of nuclear vestibular neurons.[20] The mean age of IPD patients and healthy controls were similar so as to negate the confounding factor of age. Whether the reduction of VEMP responses in PD is a part of the widespread neural degeneration is not known as it is relatively preserved in olivopontocerebellar ataxia.[21] The previous studies have tried to correlate VEMP response abnormalities with the stage of the disease according to the modified H&Y stage, UPDRS-III score, postural instability/balance, sleep and depression. De Natale et al. (2015) showed that VEMP abnormalities correlated with RBD and postural instability but not with ESS, depression.[7] They found that cVEMP latency delay was significantly prevalent in early PD while low amplitude and absence were more observed in late PD.[22] Pollack et al. (2009) reported a correlation of cVEMP with depression.[19] We found significant correlation of RBDSQ score and Mini-BESTesT score with P13 and N23 peak latency but no correlation with depression, ESS and disease severity.
BAEP in PD
BAEP recording is an electrophysiological technique used to evaluate the auditory pathway. Wave I is produced by the auditory nerve, wave II by cochlear nucleus, wave III by superior olive in lower pons and wave IV/V by inferior colliculus in upper pons/lower midbrain. I-III IPL represents conduction from the eighth nerve to the lower pons, III-V from lower pons to upper pons and I-V from eighth nerve to upper pons. The BAEP abnormalities in PD have been reported with varied results. Tsuji et al. (1981) and Prasher et al. (1986) have reported normal BAEPs in PD.[23,24] Tachibana et al. (1989) showed increase in V wave peak latency and I–V and III–V IPLs in PD.[25] Liu C (2017) reported increased latencies of wave III and wave V and the IPL of III–V and I–V in PD.[6] Shalash et al. (2017) reported abnormal BAEP wave morphology, prolonged absolute latencies of wave V and I–V interpeak latencies in PD.[16] Ahmed et al. (2017) showed delay in the latencies of waves III, IV and V and IPL III-V in PD.[26] In our study, BAEP was not recordable bilaterally in four cases and unilaterally in two cases in PD. The latencies of wave V, wave III and I–V inter-peak latencies were significantly prolonged compared to control. The limitations were small sample size and no correlation with other NMS in PD. There was no correlation between BAEP and cVEMP in PD patients.
CONCLUSION
IPD is associated with cVEMP and BAEP abnormalities suggesting involvement of the auditory and vestibular pathways in PD. In particular, cVEMP is useful in clinical practice as its abnormalities correlate with RBD and postural instability. It may serve as a predictive marker for occurrence of RBD and postural instability in PD.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the forms, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
Authors thank the electrophysiology technician for conducting the electrophysiological tests.
REFERENCES
- 1.Tolosa E, Wenning G, Poewe W. The diagnosis of Parkinson's disease. Lancet Neurol. 2006;5:75–86. doi: 10.1016/S1474-4422(05)70285-4. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhuri KR, Odin P, Antonini A, Martinez-Martin P. Parkinson's disease: The non-motor issues. Parkinsonism Relat Disord. 2011;17:717–23. doi: 10.1016/j.parkreldis.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 3.Braak H, Del Tredici K, Rub U, De Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 4.Seidel K, Mahlke J, Siswanto S, Kruger R, Heinsen H, Auburger G, et al. The brainstem pathologies of Parkinson's disease and dementia with Lewy bodies. Brain Pathol. 2015;25:121–35. doi: 10.1111/bpa.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nonnekes J, Geurts AC, Oude Nijhuis AB. Reduced StartReact effect and freezing of gait in Parkinson's disease: Two of a kind? J Neurol. 2014;261:943–50. doi: 10.1007/s00415-014-7304-0. [DOI] [PubMed] [Google Scholar]
- 6.Chunyan L, Yaping Z, Weiguo T, Binda W, Bona W, Songbin H. Evoked potential changes in patients with Parkinson's disease. Brain Behav. 2017;7:e00703. doi: 10.1002/brb3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Natale ER, Ginatempo F, Paulus KS, Pes GM, Manca A, Tolu E, et al. Abnormalities of vestibular-evoked myogenic potentials in idiopathic Parkinson's disease are associated with clinical evidence of brainstem involvement. Neurol Sci. 2015;36:995–1001. doi: 10.1007/s10072-014-2054-4. [DOI] [PubMed] [Google Scholar]
- 8.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: A clinic-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahn S, Elton RL . UPDRS development committee. Unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson's Disease. Florham Park, NJ: Macmillan; 1987. pp. 153–63. [Google Scholar]
- 10.Hoehn MM, Yahr MD. Parkinsonism: Onset, progression and mortality. Neurology. 1967;17:427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 11.Stiasny-Kolster K, Mayer G, Schäfer S, Möller JC, Heinzel-Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire—a new diagnostic instrument. Mov Disord. 2007;22:2386–93. doi: 10.1002/mds.21740. [DOI] [PubMed] [Google Scholar]
- 12.Johns MW. A new method for measuring daytime sleepiness: The epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 13.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1982;83(17):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 14.Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the balance evaluation system's test: The mini-BESTest. J Rehabil Med. 2010;4:323–33. doi: 10.2340/16501977-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Shalash AS, Hassan DM, Elrassas HH, Salama MM, Méndez-Hernández E, Salas-Pacheco JM, et al. Auditory- and vestibular-evoked potentials correlate with motor and non-motor features of Parkinson's disease. Front Neurol. 2017;8:55. doi: 10.3389/fneur.2017.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halberstadt AL, Balaban CD. Selective anterograde tracing of the individual serotonergic and nonserotonergic components of the dorsal raphe nucleus projection to the vestibular nuclei. Neuroscience. 2007;147:207–23. doi: 10.1016/j.neuroscience.2007.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colebatch JG, Halmagyi GM, Skuse NF. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry. 1994;57:190–7. doi: 10.1136/jnnp.57.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollak L, Prohorov T, Kushnir M, Rabey M. Vestibulocervical reflexes in idiopathic Parkinson disease. Clin Neurophysiol. 2009;39:235–40. doi: 10.1016/j.neucli.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Birdane L, Incensulu A, Gurbuz MK, Ozbabalik D. Sacculocollic reflex in patients with dementia: Is it possible to use it for early diagnosis? Neurol Sci. 2012;33:17–21. doi: 10.1007/s10072-011-0595-3. [DOI] [PubMed] [Google Scholar]
- 21.Takegosh H, Murofushi T. Vestibular evoked myogenic potentials in patients with spinocerebellar degeneration. Acta Otolaryngol. 2000;120:821–4. doi: 10.1080/000164800750061660. [DOI] [PubMed] [Google Scholar]
- 22.de Natale ER, Ginatempo F, Paulus KS, Manca A, Mercante B, Pes GM, et al. Paired neurophysiological and clinical study of the brainstem at different stages of Parkinson's disease. Clin Neurophysiol. 2015;126:1871–8. doi: 10.1016/j.clinph.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Tsuji S, Muraoka S, Kuroiwa Y, Chen KM, Gajdusek CD. [Auditory brainstem evoked response (ABSR) of Parkinson-dementia complex and amyotrophic lateral sclerosis in Guam and Japan (author's transl)] Rinsho Shinkeigaku. 1981;21:37–41. [PubMed] [Google Scholar]
- 24.Prasher D, Bannister R. Brain stem auditory evoked potentials in patients with multiple system atrophy with progressive autonomic failure (Shy-Drager syndrome) J Neurol Neurosurg Psychiatry. 1986;49:278–89. doi: 10.1136/jnnp.49.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tachibana H, Takeda M, Sugita M. Short-latency somatosensory and brainstem auditory evoked potentials in patients with Parkinson's disease. Int J Neurosci. 1989;44:321–6. doi: 10.3109/00207458908986210. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed NG, Agrawal DK, Chughtai AM. Evaluation of Brainstem auditory evoked response (BAER) in Parkinson's disease. Int Arch Biomed Clin Res. 2017;3:90–4. [Google Scholar]


