Abstract
Ketogenic dietary therapies (KDTs) have been in use for refractory paediatric epilepsy for a century now. Over time, KDTs themselves have undergone various modifications to improve tolerability and clinical feasibility, including the Modified Atkins diet (MAD), medium chain triglyceride (MCT) diet and the low glycaemic index treatment (LGIT). Animal and observational studies indicate numerous benefits of KDTs in paediatric neurological conditions apart from their evident benefits in childhood intractable epilepsy, including neurodevelopmental disorders such as autism spectrum disorder, rarer neurogenetic conditions such as Rett syndrome, Fragile X syndrome and Kabuki syndrome, neurodegenerative conditions such as Pelizaeus-Merzbacher disease, and other conditions such as stroke and migraine. A large proportion of the evidence is derived from individual case reports, case series and some small clinical trials, emphasising the vast scope for research in this avenue. The term 'neuroketotherapeutics' has been coined recently to encompass the rapid strides in this field. In the 100th year of its use for paediatric epilepsy, this review covers the role of the KDTs in non-epilepsy neurological conditions among children.
Keywords: Bioenergetics, ketogenic dietary therapy, modified atkins diet, neuroketotherapeutics
INTRODUCTION
Ketogenic Dietary Therapies (KDTs) have traditionally been used in the treatment of paediatric refractory epilepsy.[1,2] Over nearly 100 years (since 1921) of use for paediatric epilepsy, and better understanding about the mechanisms of action, the spectrum of therapeutic indications of KDTs seems to be evolving, with parallel substantial increment in publications on KD in the last decade.
There are four forms of KDT available, which include the classic KD, the modified Atkins diet (MAD), the medium-chain triglyceride diet (MCT) and the low glycaemic index treatment (LGIT).[3] The classic KD used a 3:1 to 4:1 ratio of fat to carbohydrates (in grams). In the MAD, carbohydrates are restricted to 20 g per day and fats are increased. The major advantage of the MAD is that it can be initiated on an outpatient basis, unlike the classic KD. The MCT diet involves the use of medium-chain triglycerides as a rich ketogenic source thereby permitting relative liberalisation of carbohydrates in the diet. In the LGIT diet, complex carbohydrates with high glycaemic index are employed.
Even among epilepsy patients, additional effects of the KDTs were noted apart from seizure control, namely improvement in alertness, behaviour, sleep and cognition. These effects were not totally explained by either seizure control or reduction in antiseizure medication dosages. However, apart from these effects in epilepsy, the ketogenic diet was shown to confer a host of benefits including improved energy, decreased negative affect, improved social functioning, working memory and quality of life.[4] The broad range of non-epilepsy conditions in which the KDTs have been studied with varying degrees of therapeutic efficacy includes autism spectrum disorders, Angelman syndrome, mitochondrial diseases, headache, traumatic brain injury, Alzheimer's disease, Parkinson's disease, sleep disorders, amyotrophic lateral sclerosis, brain tumours, etc.
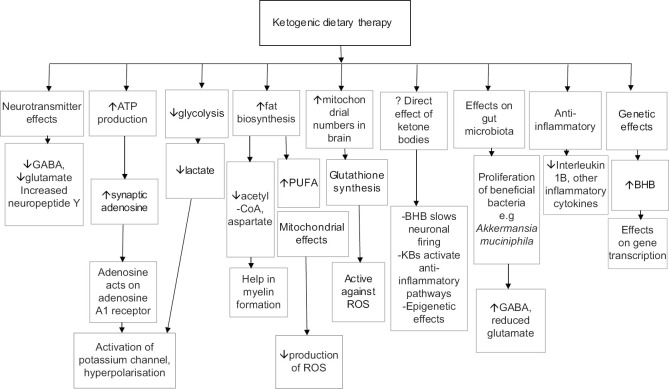
Bioenergetic dysregulation, including energy failure as seen in mitochondrial conditions, is increasingly considered to be a key mechanism underlying neuronal death in neurodegenerative conditions, leading to considerable interest in the repurposed role of the ketogenic diet in these diseases. The various mechanisms underlying the efficacy of the KDT in both epilepsy and non-epilepsy conditions are summarised in Figure 1. The term 'neuroketotherapeutics' was recently coined to refer to the use of ketosis for the improvement of diseased conditions of the nervous system. The target interval of ketone blood concentrations is from >0.2 up to 5-8 mM, which is in the range of moderate ketosis and has been reported to be safe and helpful.[5,6,7]
Figure 1.
Mechanisms of action of ketogenic dietary therapy. ATP= adenosine triphosphate; BHB= betahydroxybutyrate; GABA= gamma aminobutyric acid; PUFA= polyunsaturated fatty acids; ROS=reactive oxygen species
In the 100th year anniversary of its use for epilepsy, this paper aims to explore the utility of the ketogenic use in non-epileptic conditions in children.
SEARCH METHODOLOGY
We have conducted a narrative review using PubMed database which was searched and all published data available on uses of the ketogenic diet in child neurology other than epilepsy up to December 2020 was reviewed, using the search terms 'ketogenic diet' or 'ketogenic dietary therapy' or 'Modified Atkins diet' and 'paediatric neurology' or 'child neurology.' Additional cross-search was conducted for specific conditions such as 'stroke', 'migraine', 'autism spectrum disorder', 'Rett syndrome', 'global developmental delay', 'Fragile X syndrome', 'Kabuki syndrome', 'Pelizaeus Merzbacher disease' and 'Subacute sclerosing panencephalitis.' All types of studies including reviews, clinical studies, case series, case reports were included in the review. The abstracts were screened for relevance to the review topic.
KD IN NEURODEVELOPMENTAL DISORDERS
Autism spectrum disorder (ASD)
ASD is characterised by impairments in social communication and social interaction, and the presence of repetitive, restricted, patterns of behaviour and interests. No specific therapy is currently available for social deficits and repetitive behaviours.
KDT has been shown to reduce cortical excitability, restore bioenergetics dysfunction, improve myelin formation and white matter development and improve gut microbiota [Figure 1].[8] At the molecular level, the role of chronic neuroinflammation and altered ATP synthesis have been suggested to underlie some aspects of ASD.[9,10] Recently, insight into possible genetic-environmental interactions as a cause of ASD have provided a clue to the possible role of physiological abnormalities in the functioning of mitochondria.[11]
Animal data
Several animal studies have demonstrated that ketogenic diet might be effective in treating an ASD-like behavioural phenotype by improving sociability and decreasing repetitive behaviour.[12,13,14]
Clinical data
In human trials also [Table 1], ketogenic diet was observed to be effective in mitigating deficits in social behaviour, communication and repetitive behavioural patterns. The KD has been studied for ASD in two uncontrolled and one controlled open-label clinical trials and the MAD has been studied in one controlled open-label trial. Most commonly, majority of the children tolerated the diet and demonstrated moderate improvement in ASD symptoms, as monitored by Childhood Autism Rating Scale (CARS) or Autism Diagnostic Observation Schedule (ADOS) scores.[15,16,17,18]
Table 1.
Some neurological conditions for which KDTs has been used in human studies among children
| Author/ year | Study population | Intervention | Outcomes | Type of study |
|---|---|---|---|---|
|
| ||||
| Autism Spectrum Disorder | ||||
| Mu et al./ 2020[17] | 10 typically developed controls and 17 children with ASD at baseline | 3 months of treatment with a modified KD regimen | KD restored lower selenium levels in ASD to that of controls; Negative correlation between the changes in selenium and behaviour scores. | Clinical |
| El-Rashidy et al./ 2017[18] | 45 children with ASD randomised to three groups- MAD, GFCF and normal balanced diet | Randomised for 6 months to one of three groups | 19% children in MAD showed reduction in CARS scores compared to 11% in GFCF group and 0.7% in control group. MAD also scored better in cognition and sociability compared to GFCF group. | Clinical |
| Lee et al./2014[16] | 15 children with ASD aged 2-17 years | Modified ketogenic gluten-free diet regimen with supplemental MCT for 3 months | Significant improvement in core autism features noted on ADOS-2 and CARS; 6/15 had >30% reduction in ADOS-2 | Clinical |
| Evangeliou et al./ 2003[15] | 30 children with ASD aged 4-10 years | KD given for 6 months; 4 weeks continuously followed by two-week diet free interval | 18/30 had reduction in CARS scores (2 had >12 point reduction, 8 had 8-12 point reduction, 8 had 2-8 point reduction in CARS scores) | Clinical |
|
| ||||
| Global developmental delay/ Intellectual delay | ||||
|
| ||||
| Zhu et al./2017[19] | Prospective case control study among children with GDD MAD (n = 40) and conventional treatment group (n = 37) along with comprehensive rehabilitation training> |
MAD versus conventional treatment Assessed at 3, 6 and 9 months |
At 3, 6 and 9 months, MAD arm showed significantly greater improvement in scores of adaptive, fine motor and language quotients of the Gessell Development Scale Significantly greater improvements in the CBCL scores and S-M scale |
Clinical |
|
| ||||
| Attention Deficit Hyperactivity Disorder | ||||
|
| ||||
| Packer et al./ 2016[20] | RCT with crossover design Dogs with idiopathic epilepsy |
MCT with a standardized placebo diet on canine behaviour over 3 months and then switched to the alternative diet over 3 months | MCT-fed dogs showed significant improvement in the ADHD-associated behavioural factor chasing and decrease in stranger-directed fear versus placebo | Animal |
| Murphy et al./ 2006[77] | Adult male Long-Evans rats | Subjects were placed on either KD or a control diet Two experiments done |
Experiment 1: examined time frame and effect of diet on activity. Experiment 2: Assessed relationship between activity and anxiety level. KD was seen to cause decreased activity levels within 24 h Experiment 2 showed that decrease in activity not related to anxiety level |
Animal |
| Murphy et al./ 2005[78] | Wistar rats | Subjects placed on either KD or control diet Two experiments done |
Experiment 1: Rats placed on one of three diets: a control diet, a 6.3:1 KD, 4:1 KD Experiment 2: Rats placed either on a control diet or a 4:1 KD. Activity level, time spent immobile, grooming, and in exploratory behaviour measured for 600 s After initiation of the diets, KD-fed rats showed lower activity level than control rats |
Animal |
|
| ||||
| Rett syndrome | ||||
|
| ||||
| Haas et al./ 1986[79] | 7 girls between 5-10 years of age | Treatment with ketogenic diets using MCT oil | Slight improvement in behavior and motor concerns reported in 5 patients. 5 had additionally improved seizure control. | Clinical |
| Mantis et al./ 2009[26] | Male Mecp2 (308/y) mice | KD and a standard rodent chow diet (SD) over 30 days | Performance in motor behaviour, anxiety worse in male RTT mice compared to wild-type mice. Restriction of either KD or SD improved motor behaviour and anxiety. | Animal |
|
| ||||
| Fragile X syndrome | ||||
|
| ||||
| Westmark et al./ 2020[27] | Fmr1KO mice | KD versus regular diet | KD selectively decreased seizures in male mice but not female mice | Animal |
|
| ||||
| Alternating hemiplegia of childhood | ||||
|
| ||||
| Ulate-Campos et al./ 2014[80] | 10-year old girl with AHC with mutations in the ATP1A3 gene and insertion/ deletion in the SLC2A1 gene | KD initiated | Ketogenic diet initiation after failure of flunarizine led to dramatic reduction in the frequency of attacks, along with improvement in motor function | Clinical |
| Roubergue et al./ 2015[56] | 4 years 8-month-old girl | MAD initiated | MAD initiation at 3 y 5 months of age led to complete disappearance of attacks of hemiplegia over 15 months of follow up | Clinical |
| Schirinzi et al./ 2018[57] | 20 months boy symptomatic since three months of life | KD initiated | On initiation of KD at 20 months, both seizures and paroxysmal attacks reduced significantly at 2 years and 7 months | Clinical |
|
| ||||
| Inborn errors of metabolism | ||||
|
| ||||
| GLUT-1 deficiency | ||||
| Ramm-Pettersen et al./ 2014[41] | 6 patients with GLUT-1 deficiency | KD or MAD initiated. Neuropsychological assessment done at baseline and minimum 6 months after treatment | Improvement in various neuropsychological facets, 6-17 months after diet therapy | Clinical |
| Pyruvate dehydrogenase complex (PDC) deficiency Sofou et al./ 2017[43] | 19 patients with PDC deficiency | KD initiated for a median of 2.9 years | Improvement in epilepsy, ataxia, sleep, speech and language | Clinical |
|
| ||||
| Pelizaeus-Merzbacher disease | ||||
|
| ||||
| Stumpf et al./ 2019[54] | PMD mouse model with preserved blood–brain barrier | KD initiated | KD improved CNS myelination, slowed axonal degeneration and normalized motor functions in PMD mouse model | Animal |
|
| ||||
| Subacute Sclerosing Panencephalitis | ||||
|
| ||||
| Bautista et al./ 2003[49] | 9-years-old male with myoclonus unresponsive to valproate and clonazepam | KD initiated | Myoclonic jerks stopped within two weeks of initiation of the diet. However, these reappeared three months later and were refractory. |
Clinical |
| Nathan et al./ 2019[48] | Eight-year old male with SSPE, Risk and Haddad stage 3a | KD initiated | Myoclonic jerks ceased at 11 months. Cognition and physical abilities improved at 36 months. | Clinical |
|
| ||||
| Ischemic stroke | ||||
|
| ||||
| Shaafi et al./ 2019[69] | 24 rats studied in 3: Main, control, and sham . | Main group received KD plus MCT oil from 3 days prior to stroke induction Ischemic stroke of the middle cerebral artery induced in Main and Control group |
Following stroke, KD rats exhibited better movement and hand activity compared to control group | Animal |
| Yang et al./ 2017[70] | Mice fed KD for three weeks compared to mice on high carbohydrate (HC) diet or standard chow (control group) | Reversible MCA occlusion performed subsequent to dietary intervention | KD-fed mice had significantly reduced infarct volume, increase in regional cerebral blood flow and adenosine levels in both ischemic and reperfusion stages compared to HC and control groups | Animal |
|
| ||||
| Kabuki syndrome | ||||
|
| ||||
| Benjamin et al./ 2017[30] | Kmt2d+/βGeo mice | KD initiated | KD modulated H3ac and H3K4me3 in the granule cell layer, and improvement in neurogenesis defect and hippocampal memory abnormalities | Animal |
|
| ||||
| Glycogen storage disease type V | ||||
|
| ||||
| Løkken et al./ 2020[45] | Three diet regimens for 3 weeks among patients with glycogen storage disease type V | 2 participants completed diet 1 and 3 each completed diets 2 and 3 (Diet #1: 65%/15%/20%; Diet #2: 75%/15%/10%, or Diet #3: 80%/15%/5%, with % indicating proportion of fat/protein/carbohydrate) | Five patients had symptom relief, on diets #2 and #3. All 3 diet regimes improved exercise capacity | Clinical |
| Busch et al./ 2005[44] | 55-year-old male | Ketogenic diet initiated | Follow up at one year showed increased exercise tolerance (3 to 60-fold), maximum strength and activity | Clinical |
AHC= Alternating hemiplegia of childhood; ADHD= Attention deficit hyperactivity disorder; ASD= Autism spectrum disorder; ADOS= Autism Diagnostic Observation Schedule; CARS= Childhood autism rating scale; CBCL= Child Behaviour Checklist; GDD= Global developmental delay; GFCF= gluten free casein free; GLUT-1=Glucose transporter type 1; KD= Ketogenic diet; MAD= Modified Atkins diet; MCT= medium chain triglyceride; PDC= Pyruvate dehydrogenase complex deficiency; PMD= Pelizaeus-Merzbacher disease; RTT= Rett syndrome; S-M scale= Infants-Junior High School Students’ Social Life Abilities Scale; SSPE= Subacute sclerosing panencephaliti
Developmental delay
Global developmental delay is defined as significant delay in two or more developmental domains. There is very limited data on the role of KDTs in this entity.
Clinical data
To explore the role of KDTs in children with global developmental delay, Zhu et al. conducted a prospective case-control study for hospitalised children with global developmental delay who were divided into a KD treatment group or a conventional treatment group [Table 1].[19] The authors concluded that the KD can improve neurobehavioral development and emotional behaviours in children with global developmental delay, with few adverse effects. However, this study did not mention effects on comorbid epilepsy or seizures.[19]
Attention deficit hyperactivity disorder (ADHD)
ADHD in children is characterised by impulsivity, motor hyperactivity, inappropriate behaviour and inattention. These symptoms are attributed to altered neurotransmission and reduced prefrontal cortex metabolism.
Animal data
Several animal studies have shown that administration of KD caused reduction in hyperactivity and decreased exploratory behaviour compared to controls [Table 1].[20]
Clinical data
Although there is no human study so far aiming to study the effect of KD in ADHD, a case study investigated behaviour and development in children with severe seizures before commencing a ketogenic diet.[21] KD was found to significantly reduce seizure frequency within one year along with significant improvement in motor functioning, self-help skills, attention and social skills with ketogenic diet in children with abnormal emotional and behavioural responses.[21] Although the primary outcome in this study was seizure control, it suggested possible beneficial implications of ketogenic diet on ADHD-like symptoms in children.
Balance of evidence
Further studies are required, specifically focusing on the effects of ketogenic diet in ADHD to directly assess its efficacy.
KD IN NEUROGENETIC CONDITIONS
Rett syndrome (RTT)
RTT, an X-linked neurodevelopmental disorder caused by mutations in the MECP2 gene, is one of the leading causes of intellectual disability in female children. The GABAergic signalling system has been implicated as a key pathway commonly disturbed in neurodevelopmental diseases including RTT.[22] The GABA-A receptor is a therapeutic target for neurodevelopmental disorders. One of the proposed mechanisms of action of the KD is increase in the synthesis of GABA and decrease in synthesis of excitatory neurotransmitters, such as glutamate [Figure 1].[23]
Animal data
It has been shown that mice with Mecp2-deficiency exclusively in GABAergic neurons rapidly develop forepaw stereotyped movements, compulsive grooming, learning and memory deficits, abnormal social behaviour, electroencephalography hyperexcitability, lack of motor coordination, severe respiratory dysrhythmias and premature lethality [Table 1].[24]
KDTs have been shown promising results in controlling refractory epilepsy associated with RTT.[25] Though reports on application of this diet to RTT cases and in RTT-mice models are scarce, Mantis et al. in their study on Mecp2 null mice models, observed that the KD ameliorated anxiety and motor measurements.[26] However, further studies are required to explore the role of KDTs in non-epileptic symptomatology spectrum in RTT.
Fragile X syndrome (FXS)
FXS is a neurodevelopmental disorder clinically characterised by intellectual disability (overall IQ < 70), autistic-like behaviours and seizures. FXS results from a triplet repeat expansion mutation in FMR1 gene on the X chromosome.
Animal data
Westmark et al. tested the effects of chronic ketogenic diet treatment on seizures, body weight, ketone and glucose levels, diurnal activity levels, learning and memory, and anxiety behaviours in affected mice models [Table 1].[27] They observed that KD selectively attenuates seizures in male mice but not female mice and differentially affects weight gain and diurnal activity levels dependent on genotype, sex and age.[27] Since there is growing evidence that KDTs might be beneficial in ASD, future research is warranted to delineate their potential utility in this syndrome.
Kabuki syndrome
Kabuki syndrome is a monogenic disorder, the manifestations of which include intellectual disability, postnatal growth retardation, immunological dysfunction, and characteristic facial features. The underlying pathophysiology involves defects in opening of chromatin, a process essential for transcription of genes. Hence, agents that promote open chromatin states, such as histone deacetylase inhibitors (HDACis), could have a role in ameliorating disease progression.[28] Recently, beta-hydroxybutyrate (BHB), a ketone body, has been shown to have HDACi activity.[29]
Animal data
Benjamin et al. demonstrated rescue of hippocampal memory defects and deficiency of adult neurogenesis in a mouse model of Kabuki syndrome by imposing a ketogenic diet [Table 1]. This strategy might have worked by raising the level of the ketone beta-hydroxybutyrate, an endogenous HDACi. This work suggests that KDTs might be considered a feasible treatment option for Kabuki syndrome.[30]
Prader-Willi Syndrome (PWS)
PWS is the most common genetic cause of obesity caused by mutations in the paternal genes on chromosome 15q11-q13. During childhood, patients develop insatiable appetite, hyperphagia and obesity. Various dietary strategies have been used for weight management in PWS.
Clinical data
Felix et al. conducted a study to test the use of the MAD (low carbohydrate and high fat) for children with PWS ages 6–12 years who were overweight/obese [Table 1]. They observed positive effects on hyperphagia and behaviour in patients, with minimal adverse effects.[31]
Though the sample size was small, this study could act as pilot study for larger clinical trials investigating the role of KDTs in children with PWS.
KD AND BRAIN TUMOURS
There is preferential dependence of malignant cells on glucose and aerobic glycolysis as a source of energy (Warburg effect) which renders them susceptible to strategies that reduce glucose availability.[32] As rapidly proliferating cancer cells are dependent on abundant levels of glucose, a ketogenic diet may put the cancer cells under metabolic stress and inhibit or delay the cancer's growth. Other possible mechanisms of action could be potential anti-angiogenic and anti-proliferative effects, decreased substrates for macromolecular synthesis, as well as possible effects on oxidative stress, the NLRP3 inflammasome, and regulation of gene expression.[33,34]
Clinical data
There are limited studies demonstrating the benefit to this diet for patients with central nervous system (CNS) malignancies, more so in paediatric population. There have been few studies in adults exploring the role of KDTs in CNS tumours. They have observed beneficial effect, but all had limitations of very small sample size, concurrent treatment, and lack of uniformity regarding diagnosis and stage of disease.[35,36]
Nebeling et al. reported positive responses in two children with astrocytoma.[37] A systematic review evaluating the role of KDTs in therapeutic management of adult and paediatric gliomas revealed that though the diet is well tolerated, there is insufficient evidence to suggest any therapeutic effect of KDTs in the management of gliomas. They recommended further high-quality research to determine effectiveness of KDTs in management of gliomas.[38] KD has also been tried in recurrent diffuse intrinsic pontine glioma and subependymal giant cell tumour (SEGCT) among children with tuberous sclerosis (TSC) with no success.[39,40] There are various ongoing prospective clinical trials evaluating the effectiveness of KDTs in management of CNS malignancies. (NCT03451799, NCT03328858, NCT03160599, NCT02694094)
KDT IN INBORN ERRORS OF METABOLISM (IEM)
The KD diet has been effective in improving cognition and behaviour in glucose transporter protein 1 deficiency syndrome, pyruvate dehydrogenase complex deficiency and glycogen storage disease type V.
Glucose transporter protein 1 (GLUT-1) deficiency
GLUT-1 deficiency syndrome, associated usually with SLC2A mutations, presents phenotypically with developmental delay, epilepsy, microcephaly and movement disorders.
Ramm-Pettersen et al. demonstrated improvements in general alertness, expressive language, articulation and cognitive index after starting the KD in patients diagnosed with glucose transporter protein 1 deficiency complex.[41] In another study, MAD was tried in 10 patients with improvement in epilepsy as well as movement disorders observed.[42]
Pyruvate dehydrogenase complex (PDC) deficiency
KD use has been reported for PDH deficiency since 1976. In PDC deficiency, the end product of glycolysis, pyruvate, cannot be optimally routed through the tricarboxylic acid pathway, leading to lactate accumulation and impaired ATP production. The ketone bodies produced during ketogenesis provide alternate fuel sources to the brain.
Sofou et al. implemented the KD on 19 children diagnosed with pyruvate dehydrogenase complex deficiency and observed improvements in speech and language, ataxia, sleep disturbances, behaviour and social functioning.[43]
Glycogen storage disease type V
Glycogen storage disease type V (GSDV), also known as McArdle disease, is a rare inborn error of carbohydrate metabolism in skeletal muscle caused by mutations in the muscle glycogen phosphorylase gene. Absence of myophosphorylase results in inability to mobilise muscle glycogen stores. Accordingly, exercise tolerance is limited and varies as a function of the availability of alternative fuels. Currently, there is no satisfactory treatment for GSDV. Since ketone bodies can act as an alternative fuel to muscle glycogenolysis during exercise, the role of KD in this disorder needs to be explored.
There is one case report in literature, where administration of KD to a 55-year-old patient for one year led to improvement in exercise tolerance.[44] Lokken et al. conducted their study in 10 patients and observed that there was improvement in exercise tolerance assessed by heart rate changes during constant load cycling along with reduction in perceived exertion by the patients [Table 1].[45]
Larger, adequately powered trials are needed to investigate the role of KD in this disorder.
KDT IN NEURODEGENERATIVE DISORDERS
Glucose hypometabolism, mitochondrial dysfunction and increased reactive oxygen species (ROS) production are common features in several neurodegenerative diseases, including ALS, AD, Parkinson's (PD) and Huntington's (HD) diseases, linking deficient energy metabolism to neurodegeneration. Hence, the use of energy fuels such as ketones has been suggested as a strategy for the treatment of neurodegenerative diseases.[46]
Apart from this altered energy metabolism, the other proposed mechanisms of action of KD in neurodegenerative conditions are reduction of oxidative stress and neuroinflammation as observed in the nervous system of animal models.
Subacute sclerosing panencephalitis (SSPE)
SSPE is a chronic, progressive, inflammatory, and degenerative affliction of the brain caused by a persistent infection by, or, mutation of the measles virus (MV). The pathogenesis of SSPE is related to direct infection of the central nervous system (CNS) by measles virus, causing progressive inflammation and sclerosis (neurodegeneration) of the brain due to an impaired immune response.[47]
Clinical data
Since the KD was found effective in refractory epilepsy, with anti-inflammatory and neuroprotective properties in neurodegenerative conditions, Nathan et al. explored its role in SSPE.[48] They observed that treatment of an 8-year-old boy diagnosed with SSPE, led to a significant improvement in the physical parameters and cognition and resulted in the cessation of the myoclonic jerks within a month of starting the therapy. Bautista et al. also reported a 9-year old patient with SSPE whose myoclonus improved significantly, albeit temporarily, after being placed on the ketogenic diet.[49] However, a multicentre, randomised controlled trial would be helpful in determining the potential of the KD therapy in SSPE.
Other neurodegenerative disorders
KDTs have been studied in a limited manner in very small studies in other neurodegenerative conditions, such as Lafora body disease (LBD). In a preliminary observation among five patients with LBD, KD was found to not impact disease progression.[50]
In patients with neuronal ceroid lipofuscinosis (NCL), the KD was shown to be complementary for seizure control to drug therapy among patients with CLN2 disease.[51]
In a prospective open-label study, four patients (7-20 years) with North Sea Progressive Myoclonic Epilepsy underwent the modified Atkins diet. The primary outcome, which was health-related quality of life, improved in one patient but remained unchanged in the remaining three patients.[52]
Pelizaeus–Merzbacher disease (PMD)
PMD is a potentially fatal leukodystrophy characterised by pathological hypomyelination resulting in nystagmus, hypotonia, spasticity, ataxia and retarded cognitive development. Currently, PMD lacks any definitive therapeutic option. It has been observed in vitro and in vivo that lipid supplementation can enhance myelination in hypomyelinating pathologies and thereby promote repair. However, under physiological conditions, plasma cholesterol cannot cross the intact blood–brain barrier (BBB).[53]
Animal data
Stumpf et al. in their experiment on PMD mouse model with preserved BBB demonstrated that a high-fat/low-carbohydrate ketogenic diet restored oligodendrocyte integrity and increased CNS myelination [Table 1]. This dietary intervention also ameliorated axonal degeneration and normalized motor functions. They suggested that KDTs could have a potential therapeutic role in this disorder.[54]
Alternating hemiplegia of childhood (AHC)
AHC is a severe neurological disorder with infantile-onset recurrent episodes of hemiplegia in either side of the body. Pathogenic variants of ATP1A3 primarily cause AHC with altered function of the Na+-K+- ATPase pump, which maintains the resting state of the cell and functions as a signal transducer.[55]
Human data
Isolated case reports and case series described the potential beneficial effect of the ketogenic diet.[56,57,58] They observed reduced frequency and severity of attacks, prolonged attack-free periods (12-15 months), reduced seizure frequency and anti-epileptic drugs burden and improved neurocognition and motor skills [Table 1]. The mechanism of action of KD could be improvement in brain glucose hypometabolism and activation of widely distributed Na+-K+- ATPase channels, which are present in an inhibited state in AHC.
KDT IN ACUTE NEUROLOGICAL EMERGENCIES
Traumatic brain injury
Animal data
Animal studies have suggested that the brain's ability to use glucose as a fuel is impaired after brain injury. In addition, there is evidence that in state of acquired brain injury, ketone uptake and metabolism is favoured.
Many studies have shown that a ketogenic diet is an effective treatment therapy for traumatic brain injuries in rat models.[59,60,61] Therefore, ketogenic diet therapy may be a potential therapeutic option in patients with acquired brain injury in paediatric ICUs. The various mechanisms implicated are reduced ability of brain to utilise glucose in times of oxidative stress and ketones being more energy efficient, tend to improve the cellular metabolism, reduce apoptosis and free radical generation and lead to improvement in cerebral blood flow.[62]
A more recent animal study was conducted to assess the neuropathological changes in the ipsilateral cortex homogenates and mitochondria for markers of oxidative and antioxidant expression and mitochondrial function in an animal-based injury model fed with KD. They observed that the markers of oxidative stress were significantly attenuated and mitochondrial dysfunction was prevented with the KD. It could be concluded that ketogenic diet may improve cerebral metabolism in TBI.[63]
Clinical data
Apart from animal models, two studies have reported adults with traumatic brain injuries treated with a ketogenic diet.[64,65] They observed that ketogenic diets avoided states of hyperglycemia in post-traumatic brain injury, despite providing sufficient calories. Such biochemical changes were shown to be neuro-protective, without any serious adverse safety events.
Balance of evidence
Based on all the above-mentioned studies, it is evident that ketone bodies might play a vital role in the neurological recovery. Though most evidence stems mainly from animal studies and small clinical studies, there is evident need for randomised clinical studies to understand a stronger correlation between introduction of KD and its effect on recovery post-TBI. A pilot study about the safety and feasibility of Ketogenic Diet in Traumatic Brain Injury (KETI) is currently underway. (https://clinicaltrials.gov/ct2/show/NCT03982602)
Ischemic stroke
Ischemic strokes cause brain damage by excitotoxicity and apoptosis within hours of insult. Few animal studies have demonstrated that ketones are associated with a decrease in biomarkers of apoptosis thereby implying relative neuron sparing effect after stroke. Ketone bodies protect neurons and prolong their survival from mechanisms that precipitate excitotoxicity and eventually death thereby exerting neuroprotective effects.[66]
The KD has been demonstrated to ameliorate free radical injury to neurons post-stroke by improving the neuron's ability to eliminate free radicals and protecting mitochondria from oxidative stress-induced damage by an improvement of mitochondrial antioxidant capacity.[67]
Animal data
Julio-Ampilas et al. in their study on animal models also concluded that the KD leads to decreased cerebral oedema formation, infarct volume and reactive oxygen production after an ischemic insult.[68] Shaafi et al. in their study to evaluate the effects of KD preconditioning on early behaviour-motor outcome of rats with induced cerebral ischemic stroke observed that rats in the KD group were able to adjust their steps quickly and efficiently and were also able to have more symmetrical hand movements compared to other groups. This suggested the putative role of KD in preconditioning with improvement of early motor and behavioural outcomes after ischemic stroke.[69] In another study by Yang et al., KD-fed mice were compared to mice fed low fat, high carbohydrate (HC group) diet and standard chow (control group) after diet administration for 3 weeks followed by reversible middle cerebral artery occlusion.[70] KD-fed mice showed low volume of infarct development and better regional cerebral blood flow, increased extracellular adenosine, increased expression of hypoxia inducible factor (HIF)- related genes, and erythropoietin and VEGF protein levels compared to the other two groups [Table 1].
Balance of evidence
These observations support the therapeutic potential of KD in ischemic insults, which are associated with energy impairment and oxidative stress and which require treatments that can be effective when administered after the insult.
KDT IN OTHER NEUROLOGICAL CONDITIONS
Migraine
Migraine is a common, often underdiagnosed problem in children. The pathophysiology of the disorder presumably involves recurrent attacks of neurovascular pain triggered by a hyperexcitable and hypometabolic brain. Even in absence of robust scientific evidence, there has been a long debate regarding certain foods triggering the migraine attacks and role of dietary restrictions in prevention.[71]
This led to the thought of exploring the possible role of KDTs in preventive treatment of migraine. Experimental evidence indicates that KD may favourably act at different stages of migraine pathophysiology. It might help in restoring brain metabolism and excitability by inhibiting propagation of cortical spreading depression, improving the unbalance between excitatory and inhibitory neurotransmission, counteracting neuroinflammation and oxidative stress.[72]
Animal data
Animal studies have revealed KD significantly enhances brain metabolism by upregulation of genes encoding energy metabolism enzymes/mitochondrial proteins and increases the number of mitochondrial profiles and the phosphocreatine/creatine ratio.[73]
Clinical data
Several human trials have also observed beneficial role of KDTs in migraine prophylaxis, causing significant reduction in attack frequency and intensity.[74,75,76]
Balance of evidence
Although KDTs seem a promising therapeutic tool for migraine prevention in adults; however, well-designed therapeutic trials evaluating benefits of KDTs in paediatric migraine are warranted.
The indications for KDT in neurological conditions in children are summarised in Table 2, based on efficacy.
Table 2.
Summary of conditions other than epilepsy with probable/ possible benefit from KDT [Adapted from Kossoff et al.][81]
| Conditions where KD is beneficial with >70% response |
| Angelman syndrome |
| Complex I mitochondrial disease |
| Glucose-1 transporter deficiency syndrome |
| PDH deficiency |
| Conditions where KD is beneficial with 40-70% response (based on one case report or case series) |
| Adenylosuccinate lyase deficiency |
| CDKL5 encephalopathy |
| Glycogenosis type V |
| Lafora body disease |
| Phosphofructokinase deficiency |
| Rett syndrome |
| Subacute sclerosing panencephalitis (SSPE) |
FUTURE AVENUES FOR RESEARCH
The above review presents the evidence for KDTs in a host of conditions, apart from epilepsy.
More clarity is required in the mechanism of action of KDT, and how these mechanisms vary in different indications. It is clear that the evidence is in continual evolution, and data from high-quality clinical trials is the need of the hour. Future research must focus on finetuning selection of patients who would benefit from the diet. Additionally, in resource constrained settings, the efficacy and tolerability of KD variants such as the MAD must also be assessed in randomised trials, as these are better accessible and affordable.
Further longitudinal data is also necessary on long-term implications and possible adverse effects of KDTs, especially among patients with progressive neurological conditions who will require the KDT for prolonged durations.
CONCLUSIONS
The use of KDTs demonstrates numerous metabolic ramifications in the brain. As a consequence, these dietary therapies may be of benefit in several neurological conditions, apart from established benefit in epilepsy. However, this field is continuing to evolve, and much of the present evidence emanates from animal studies, case reports, case series and small studies. However, this may be worth trying in desperate situations, neurodegenerative conditions, several intellectual disability and malignant brain tumours. Apart from research settings, KDTs may be offered in selected patients after weighing risks and benefits with caretakers and family members. The understanding that the ketogenic diet needs regular medical supervision must be imparted. These dietary therapies must always be offered in concert with standard treatment and supportive care.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kossoff EH, McGrogan JR. Worldwide use of the ketogenic diet. Epilepsia. 2005;46:280–9. doi: 10.1111/j.0013-9580.2005.42704.x. [DOI] [PubMed] [Google Scholar]
- 2.Kossoff EH, Nabbout R. Use of dietary therapy for status epilepticus. J Child Neurol. 2013;28:1049–51. doi: 10.1177/0883073813487601. [DOI] [PubMed] [Google Scholar]
- 3.Verrotti A, Iapadre G, Pisano sS, Coppola G. Ketogenic diet and childhood neurological disorders other than epilepsy: An overview. Expert Rev Neurother. 2017;17:461–73. doi: 10.1080/14737175.2017.1260004. [DOI] [PubMed] [Google Scholar]
- 4.Grigolon RB, Gerchman F, Schöffel AC, Hawken ER, Gill H, Vazquez GH, et al. Mental, emotional, and behavioral effects of ketogenic diet for non-epileptic neuropsychiatric conditions. Prog Neuropsychopharmacol Biol Psychiatry. 2020;102:109947. doi: 10.1016/j.pnpbp.2020.109947. [DOI] [PubMed] [Google Scholar]
- 5.Koppel SJ, Swerdlow RH. Neuroketotherapeutics: A modern review of a century-old therapy. Neurochem Int. 2018;117:114–25. doi: 10.1016/j.neuint.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veech RL. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids. 2004;70:309–19. doi: 10.1016/j.plefa.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Hashim SA, VanItallie TB. Ketone body therapy: From the ketogenic diet to the oral administration of ketone ester. J Lipid Res. 2014;55:1818–26. doi: 10.1194/jlr.R046599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mychasiuk R, Rho JM. Genetic modifications associated with ketogenic diet treatment in the BTBRT+Tf/J mouse model of autism spectrum disorder. Autism Res. 2017;10:456–71. doi: 10.1002/aur.1682. [DOI] [PubMed] [Google Scholar]
- 9.Herbert MR, Buckley JA. Autism and dietary therapy: Case report and review of the literature. J Child Neurol. 2013;28:975–82. doi: 10.1177/0883073813488668. [DOI] [PubMed] [Google Scholar]
- 10.Cheng N, Rho JM, Masino SA. Metabolic dysfunction underlying autism spectrum disorder and potential treatment approaches. Front Mol Neurosci. 2017;10:34. doi: 10.3389/fnmol.2017.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossignol DA, Frye RE. Mitochondrial dysfunction in autism spectrum disorders: A systematic review and meta-analysis. Mol Psychiatry. 2012;17:290–314. doi: 10.1038/mp.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verpeut JL, DiCicco-Bloom E, Bello NT. Ketogenic diet exposure during the juvenile period increases social behaviors and forebrain neural activation in adult Engrailed 2 null mice. Physiol Behav. 2016;161:90–8. doi: 10.1016/j.physbeh.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Smith J, Rho JM, Teskey GC. Ketogenic diet restores aberrant cortical motor maps and excitation-to-inhibition imbalance in the BTBR mouse model of autism spectrum disorder. Behav Brain Res. 2016;304:67–70. doi: 10.1016/j.bbr.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Newell C, Bomhof MR, Reimer RA, Hittel DS, Rho JM, Shearer J. Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder. Mol Autism. 2016;7:37. doi: 10.1186/s13229-016-0099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evangeliou A, Vlachonikolis I, Mihailidou H, Spilioti M, Skarpalezou A, Makaronas N, et al. Application of a ketogenic diet in children with autistic behavior: Pilot study. J Child Neurol. 2003;18:113–8. doi: 10.1177/08830738030180020501. [DOI] [PubMed] [Google Scholar]
- 16.Lee RWY, Corley MJ, Pang A, Arakaki G, Abbott L, Nishimoto M, et al. A modified ketogenic gluten-free diet with MCT improves behavior in children with autism spectrum disorder. Physiol Behav. 2018;188:205–11. doi: 10.1016/j.physbeh.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mu C, Corley MJ, Lee RWY, Wong M, Pang A, Arakaki G, et al. Metabolic framework for the improvement of autism spectrum disorders by a modified ketogenic diet: A pilot study. J Proteome Res. 2020;19:382–90. doi: 10.1021/acs.jproteome.9b00581. [DOI] [PubMed] [Google Scholar]
- 18.El-Rashidy O, El-Baz F, El-Gendy Y, Khalaf R, Reda D, Saad K. Ketogenic diet versus gluten free casein free diet in autistic children: A case-control study. Metab Brain Dis. 2017;32:1935–41. doi: 10.1007/s11011-017-0088-z. [DOI] [PubMed] [Google Scholar]
- 19.Zhu D-N, Li P, Wang J, Yuan JY, Zhang GY, Liang JF, et al. [Prospective study of ketogenic diet in treatment of children with global developmental delay] Zhongguo Dang Dai Er Ke Za Zhi. 2017;19:1038–43. doi: 10.7499/j.issn.1008-8830.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Packer RMA, Law TH, Davies E, Zanghi B, Pan Y, Volk HA. Effects of a ketogenic diet on ADHD-like behavior in dogs with idiopathic epilepsy. Epilepsy Behav. 2016;55:62–8. doi: 10.1016/j.yebeh.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Pulsifer MB, Gordon JM, Brandt J, Vining EP, Freeman JM. Effects of ketogenic diet on development and behavior: Preliminary report of a prospective study. Dev Med Child Neurol. 2001;43:301–6. doi: 10.1017/s0012162201000573. [DOI] [PubMed] [Google Scholar]
- 22.Braat S, Kooy RF. The GABAA receptor as a therapeutic target for neurodevelopmental disorders. Neuron. 2015;86:1119–30. doi: 10.1016/j.neuron.2015.03.042. [DOI] [PubMed] [Google Scholar]
- 23.Rogawski MA, Löscher W, Rho JM. Mechanisms of action of antiseizure drugs and the ketogenic diet. Cold Spring Harb Perspect Med. 2016;6:a022780. doi: 10.1101/cshperspect.a022780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chao H-T, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–9. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caraballo R, Vaccarezza M, Cersósimo R, Rios V, Soraru A, Arroyo H, et al. Long-term follow-up of the ketogenic diet for refractory epilepsy: Multicenter Argentinean experience in 216 pediatric patients. Seizure. 2011;20:640–5. doi: 10.1016/j.seizure.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Mantis JG, Fritz CL, Marsh J, Heinrichs SC, Seyfried TN. Improvement in motor and exploratory behavior in Rett syndrome mice with restricted ketogenic and standard diets. Epilepsy Behav. 2009;15:133–41. doi: 10.1016/j.yebeh.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 27.Westmark PR, Gutierrez A, Gholston AK, Wilmer TM, Westmark CJ. Preclinical testing of the ketogenic diet in fragile X mice. Neurochem Int. 2020;134:104687. doi: 10.1016/j.neuint.2020.104687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fahrner JA, Bjornsson HT. Mendelian disorders of the epigenetic machinery: Tipping the balance of chromatin states. Annu Rev Genomics Hum Genet. 2014;15:269–93. doi: 10.1146/annurev-genom-090613-094245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339:211–4. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benjamin JS, Pilarowski GO, Carosso GA, Zhang L, Huso DL, Goff LA, et al. A ketogenic diet rescues hippocampal memory defects in a mouse model of Kabuki syndrome. Proc Natl Acad Sci U S A. 2017;114:125–30. doi: 10.1073/pnas.1611431114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Felix G, Kossoff E, Barron B, Krekel C, Testa EG, Scheimann A. The modified Atkins diet in children with Prader-Willi syndrome. Orphanet J Rare Dis. 2020;15:135. doi: 10.1186/s13023-020-01412-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strickland M, Stoll EA. Metabolic reprogramming in glioma. Front Cell Dev Biol. 2017;5:43. doi: 10.3389/fcell.2017.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woolf EC, Scheck AC. The ketogenic diet for the treatment of malignant glioma. J Lipid Res. 2015;56:5–10. doi: 10.1194/jlr.R046797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Youm Y-H, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263–9. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt M, Pfetzer N, Schwab M, Strauss I, Kämmerer U. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: A pilot trial. Nutr Metab (Lond) 2011;8:54. doi: 10.1186/1743-7075-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panhans CM, Gresham G, Amaral LJ, Hu J. Exploring the feasibility and effects of a ketogenic diet in patients with CNS malignancies: A retrospective case series. Front Neurosci. 2020;14:390. doi: 10.3389/fnins.2020.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nebeling LC, Miraldi F, Shurin SB, Lerner E. Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: Two case reports. J Am Coll Nutr. 1995;14:202–8. doi: 10.1080/07315724.1995.10718495. [DOI] [PubMed] [Google Scholar]
- 38.Martin-McGill KJ, Srikandarajah N, Marson AG, Tudur Smith C, Jenkinson MD. The role of ketogenic diets in the therapeutic management of adult and paediatric gliomas: A systematic review. CNS Oncol. 2018;7:CNS17. doi: 10.2217/cns-2017-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Louw EJTM, Reddingius RE, Olieman JF, Neuteboom RF, Catsman-Berrevoets CE. Ketogenic diet treatment in recurrent diffuse intrinsic pontine glioma in children: A safety and feasibility study. Pediatr Blood Cancer. 2019;66:e27561. doi: 10.1002/pbc.27561. [DOI] [PubMed] [Google Scholar]
- 40.Chu-Shore CJ, Thiele EA. Tumor growth in patients with tuberous sclerosis complex on the ketogenic diet. Brain Dev. 2010;32:318–22. doi: 10.1016/j.braindev.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Ramm-Pettersen A, Stabell KE, Nakken KO, Selmer KK. Does ketogenic diet improve cognitive function in patients with GLUT1-DS? A 6- to 17-month follow-up study. Epilepsy Behav. 2014;39:111–5. doi: 10.1016/j.yebeh.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 42.Amalou S, Gras D, Ilea A, Greneche MO, Francois L, Bellavoine V, et al. Use of modified Atkins diet in glucose transporter type 1 deficiency syndrome. Dev Med Child Neurol. 2016;58:1193–9. doi: 10.1111/dmcn.13167. [DOI] [PubMed] [Google Scholar]
- 43.Sofou K, Dahlin M, Hallböök T, Lindefeldt M, Viggedal G, Darin N. Ketogenic diet in pyruvate dehydrogenase complex deficiency: Short- and long-term outcomes. J Inherit Metab Dis. 2017;40:237–45. doi: 10.1007/s10545-016-0011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Busch V, Gempel K, Hack A, Müller K, Vorgerd M, Lochmüller H, et al. Treatment of glycogenosis type V with ketogenic diet. Ann Neurol. 2005;58:341. doi: 10.1002/ana.20565. [DOI] [PubMed] [Google Scholar]
- 45.Løkken N, Hansen KK, Storgaard JH, Ørngreen MC, Quinlivan R, Vissing J. Titrating a modified ketogenic diet for patients with McArdle disease: A pilot study. J Inherit Metab Dis. 2020;43:778–86. doi: 10.1002/jimd.12223. [DOI] [PubMed] [Google Scholar]
- 46.Bough KJ, Rho JM. Anticonvulsant mechanisms of the ketogenic diet. Epilepsia. 2007;48:43–58. doi: 10.1111/j.1528-1167.2007.00915.x. [DOI] [PubMed] [Google Scholar]
- 47.Garg RK. Subacute sclerosing panencephalitis. J Neurol. 2008;255:1861–71. doi: 10.1007/s00415-008-0032-6. [DOI] [PubMed] [Google Scholar]
- 48.Nathan J, Khedekar Kale D, Naik VD, Thakker F, Bailur S. Substantial remission in subacute sclerosing panencephalitis by following the ketogenic diet: A case report. Cureus. 2019;11:e5485. doi: 10.7759/cureus.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bautista RED. The use of the ketogenic diet in a patient with subacute sclerosing panencephalitis. Seizure. 2003;12:175–7. doi: 10.1016/s1059-1311(02)00268-6. [DOI] [PubMed] [Google Scholar]
- 50.Cardinali S, Canafoglia L, Bertoli S, Franceschetti S, Lanzi G, Tagliabue A, et al. A pilot study of a ketogenic diet in patients with Lafora body disease. Epilepsy Res. 2006;69:129–34. doi: 10.1016/j.eplepsyres.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Kohan R, Cismondi IA, Oller-Ramirez AM, Guelbert N, Anzolini TV, Alonso G, et al. Therapeutic approaches to the challenge of neuronal ceroid lipofuscinoses. Curr Pharm Biotechnol. 2011;12:867–83. doi: 10.2174/138920111795542633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Egmond ME, Weijenberg A, van Rijn ME, Elting JW, Gelauff JM, Zutt R, et al. The efficacy of the modified Atkins diet in North Sea Progressive Myoclonus Epilepsy: An observational prospective open-label study. Orphanet J Rare Dis. 2017;12 doi: 10.1186/s13023-017-0595-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saher G, Rudolphi F, Corthals K, Ruhwedel T, Schmidt KF, Löwel S, et al. Therapy of Pelizaeus-Merzbacher disease in mice by feeding a cholesterol-enriched diet. Nat Med. 2012;18:1130–5. doi: 10.1038/nm.2833. [DOI] [PubMed] [Google Scholar]
- 54.Stumpf SK, Berghoff SA, Trevisiol A, Spieth L, Düking T, Schneider LV, et al. Ketogenic diet ameliorates axonal defects and promotes myelination in Pelizaeus-Merzbacher disease. Acta Neuropathol. 2019;138:147–61. doi: 10.1007/s00401-019-01985-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samanta D. Management of alternating hemiplegia of childhood: A review. Pediatr Neurol. 2020;103:12–20. doi: 10.1016/j.pediatrneurol.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 56.Roubergue A, Philibert B, Gautier A, Kuster A, Markowicz K, Billette de Villemeur T, et al. Excellent response to a ketogenic diet in a patient with alternating hemiplegia of childhood. JIMD Rep. 2014;15:7–12. doi: 10.1007/8904_2013_292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schirinzi T, Graziola F, Cusmai R, Fusco L, Nicita F, Elia M, et al. ATP1A3-related epileptic encephalopathy responding to ketogenic diet. Brain Dev. 2018;40:433–8. doi: 10.1016/j.braindev.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 58.Vila-Pueyo M, Pons R, Raspall-Chaure M, Marcé-Grau A, Carreño O, Sintas C, et al. Clinical and genetic analysis in alternating hemiplegia of childhood: Ten new patients from Southern Europe. J Neurol Sci. 2014;344:37–42. doi: 10.1016/j.jns.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 59.Appelberg KS, Hovda DA, Prins ML. The effects of a ketogenic diet on behavioral outcome after controlled cortical impact injury in the juvenile and adult rat. J Neurotrauma. 2009;26:497–506. doi: 10.1089/neu.2008.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davis LM, Pauly JR, Readnower RD, Rho JM, Sullivan PG. Fasting is neuroprotective following traumatic brain injury. J Neurosci Res. 2008;86:1812–22. doi: 10.1002/jnr.21628. [DOI] [PubMed] [Google Scholar]
- 61.Deng-Bryant Y, Prins ML, Hovda DA, Harris NG. Ketogenic diet prevents alterations in brain metabolism in young but not adult rats after traumatic brain injury. J Neurotrauma. 2011;28:1813–25. doi: 10.1089/neu.2011.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hasselbalch SG, Madsen PL, Hageman LP, Olsen KS, Justesen N, Holm S, et al. Changes in cerebral blood flow and carbohydrate metabolism during acute hyperketonemia. Am J Physiol. 1996;270:E746–51. doi: 10.1152/ajpendo.1996.270.5.E746. [DOI] [PubMed] [Google Scholar]
- 63.Greco T, Glenn TC, Hovda DA, Prins ML. Ketogenic diet decreases oxidative stress and improves mitochondrial respiratory complex activity. J Cereb Blood Flow Metab. 2016;36:1603–13. doi: 10.1177/0271678X15610584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robertson CS, Goodman JC, Narayan RK, Contant CF, Grossman RG. The effect of glucose administration on carbohydrate metabolism after head injury. J Neurosurg. 1991;74:43–50. doi: 10.3171/jns.1991.74.1.0043. [DOI] [PubMed] [Google Scholar]
- 65.Ritter AM, Robertson CS, Goodman JC, Contant CF, Grossman RG. Evaluation of a carbohydrate-free diet for patients with severe head injury. J Neurotrauma. 1996;13:473–85. doi: 10.1089/neu.1996.13.473. [DOI] [PubMed] [Google Scholar]
- 66.Maalouf M, Sullivan PG, Davis L, Kim DY, Rho JM. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience. 2007;145:256–64. doi: 10.1016/j.neuroscience.2006.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheng B, Yang X, An L, Gao B, Liu X, Liu S. Ketogenic diet protects dopaminergic neurons against 6-OHDA neurotoxicity via up-regulating glutathione in a rat model of Parkinson's disease. Brain Res. 2009;1286:25–31. doi: 10.1016/j.brainres.2009.06.060. [DOI] [PubMed] [Google Scholar]
- 68.Julio-Amilpas A, Montiel T, Soto-Tinoco E, Gerónimo-Olvera C, Massieu L. Protection of hypoglycemia-induced neuronal death by β-hydroxybutyrate involves the preservation of energy levels and decreased production of reactive oxygen species. J Cereb Blood Flow Metab. 2015;35:851–60. doi: 10.1038/jcbfm.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shaafi S, Sharifi-Bonab M, Ghaemian N, Mokhtarkhani M, Akbari H. Early motor-behavioral outcome of ischemic stroke with ketogenic diet preconditioning: Interventional animal study. J Stroke Cerebrovasc Dis. 2019;28:1032–9. doi: 10.1016/j.jstrokecerebrovasdis.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 70.Yang Q, Guo M, Wang X, Zhao Y, Zhao Q, Ding H, et al. Ischemic preconditioning with a ketogenic diet improves brain ischemic tolerance through increased extracellular adenosine levels and hypoxia-inducible factors. Brain Res. 2017;1667:11–8. doi: 10.1016/j.brainres.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 71.Martin VT, Vij B. Diet and headache: Part 2. Headache. 2016;56:1553–62. doi: 10.1111/head.12952. [DOI] [PubMed] [Google Scholar]
- 72.de Almeida Rabello Oliveira M, da Rocha Ataíde T, de Oliveira SL, de Melo Lucena AL, de Lira CE, Soares AA, et al. Effects of short-term and long-term treatment with medium- and long-chain triglycerides ketogenic diet on cortical spreading depression in young rats. Neurosci Lett. 2008;434:66–70. doi: 10.1016/j.neulet.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 73.Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60:223–35. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- 74.Urbizu A, Cuenca-León E, Raspall-Chaure M, Gratacòs M, Conill J, Redecillas S, et al. Paroxysmal exercise-induced dyskinesia, writer's cramp, migraine with aura and absence epilepsy in twin brothers with a novel SLC2A1 missense mutation. J Neurol Sci. 2010;295:110–3. doi: 10.1016/j.jns.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 75.Di Lorenzo C, Coppola G, Sirianni G, Di Lorenzo G, Bracaglia M, Di Lenola D, et al. Migraine improvement during short lasting ketogenesis: A proof-of-concept study. Eur J Neurol. 2015;22:170–7. doi: 10.1111/ene.12550. [DOI] [PubMed] [Google Scholar]
- 76.Di Lorenzo C, Coppola G, Bracaglia M, Di Lenola D, Evangelista M, Sirianni G, et al. Cortical functional correlates of responsiveness to short-lasting preventive intervention with ketogenic diet in migraine: A multimodal evoked potentials study. J Headache Pain. 2016;17:58. doi: 10.1186/s10194-016-0650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murphy P, Burnham WM. The ketogenic diet causes a reversible decrease in activity level in Long-Evans rats. Exp Neurol. 2006;201:84–9. doi: 10.1016/j.expneurol.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 78.Murphy P, Likhodii SS, Hatamian M, McIntyre Burnham W. Effect of the ketogenic diet on the activity level of Wistar rats. Pediatr Res. 2005;57:353–7. doi: 10.1203/01.PDR.0000150804.18038.79. [DOI] [PubMed] [Google Scholar]
- 79.Haas RH, Rice MA, Trauner DA, Merritt TA. Therapeutic effects of a ketogenic diet in Rett syndrome. Am J Med Genet Suppl. 1986;1:225–46. doi: 10.1002/ajmg.1320250525. [DOI] [PubMed] [Google Scholar]
- 80.Ulate-Campos A, Fons C, Artuch R, Castejón E, Martorell L, Ozelius L, et al. Alternating hemiplegia of childhood with a de novo mutation in ATP1A3 and changes in SLC2A1 responsive to a ketogenic diet. Pediatr Neurol. 2014;50:377–9. doi: 10.1016/j.pediatrneurol.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 81.Kossoff EH, Zupec-Kania BA, Auvin S, Ballaban-Gil KR, Christina Bergqvist AG, Blackford R, et al. Optimal clinical management of children receiving dietary therapies for epilepsy: Updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open. 2018;3:175–92. doi: 10.1002/epi4.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]