Abstract
Cardiopulmonary exercise testing (CPET) is a dynamic clinical tool for determining the cause for a person's exercise limitation. CPET provides clinicians with fundamental knowledge of the coupling of external to internal respiration (oxygen and carbon dioxide) during exercise. Subtle perturbations in CPET parameters can differentiate exercise responses among individual patients and disease states. However, perhaps due to challenges in interpretation given the amount and complexity of data obtained, CPET is underutilized. In this article, we review fundamental concepts in CPET data interpretation and visualization. We also discuss future directions for how to best utilize CPET results to guide clinical care. Finally, we share a novel three-dimensional (3D) graphical platform for CPET data that simplifies conceptualization of organ system-specific (cardiac, pulmonary, and skeletal muscle) exercise limitations. Our goal is to make CPET testing more accessible to the general medical provider and make the test of greater use in the medical toolbox.
Keywords: Cardiopulmonary exercise testing, exercise intolerance, exertional dyspnea, interpretation, visualization
In Brief
This article discusses fundamental concepts and future directions for cardiopulmonary exercise testing interpretation and visualization.
Introduction
Cardiopulmonary exercise testing (CPET) is a dynamic tool with wide application in clinical medicine, including assessment of a person’s exercise limitation. CPET data are typically generated with temporal oxygen (O2) and carbon dioxide (CO2) gas exchange analysis during progressive maximal exercise on a cycle ergometer or treadmill over approximately 10 minutes. In addition to quantifying cardiorespiratory fitness and its cost-effective role in identifying disease-specific function-based prognosis, interpretation of integrated CPET and clinical data can distinguish the cause of unexplained dyspnea and exercise intolerance from among multiple potential etiologies (1). However, in part due to the amount and complexity of data generated from currently available CPET software packages, CPET is underutilized for the application of diagnosing exercise-limiting pathophysiology (2, 3). As such, CPET is not widely used outside of large medical centers having experienced consultant providers with advanced training—generally, cardiologists, pulmonologists, or clinical exercise physiologists. Further, interpretation of CPET results to distinguish the etiologies of exercise-induced dyspnea and exercise limitation are often poorly understood; therefore, the study is underutilized by general practitioners (4, 5). Here, we review current paradigms and challenges in CPET data interpretation. We then discuss the potential for future visualization tools to improve general clinical use of CPET for the assessment of exercise limitation.
CPET indications
CPET is indicated for the assessment of clinical diagnosis, prognosis, and response to therapy in a variety of clinical populations (2, 6). CPET measures physiological limitations in a broad range of disease states, such as ischemic heart disease and heart failure (2, 3, 7) obstructive and restrictive lung disease, pulmonary vascular disease (8, 9) and skeletal muscle abnormalities, peripheral vascular disease and muscle mitochondrial myopathies (2, 10). CPET is also a cost-effective method for cardiometabolic risk screening prior to surgery (11, 12). American College of Cardiology/American Heart Association class I (good level of evidence) indications for CPET include: 1) evaluation of exercise capacity and response to therapy in heart failure patients being considered for transplantation; and 2) differentiation of cardiac versus pulmonary limitation for patients with dyspnea on exertion (6, 13, 14). A Class IIa (weight of opinion) indication is to evaluate exercise capacity when indicated for medicine reasons when subjective estimates (exercise test time or work rate) are unreliable (6, 13, 14). Class IIb (efficacy less established) indications include: 1) evaluation of response to interventions in which improvement of exercise tolerance is an important end point; and 2) determination of exercise training intensity for cardiac rehabilitation (6, 15). CPET is not indicated (class III - not recommended) for routine use to evaluate exercise capacity (6, 13, 14). However, the use of CPET for this purpose is common in research settings.
Physiological basis of CPET
Interpretation of CPET data is based on knowledge of O2 and CO2 gas transport coupling among three organ systems at the core of exercise responsiveness and capacity: pulmonary, cardiac, and skeletal muscle (2, 3). Understanding this coupled physiology is essential for the facile and accurate interpretation of CPET-generated data. The pulmonary system transfers inspired O2 from the air to deoxygenated blood in pulmonary circulation. The cardiac system then pumps oxygenated blood from pulmonary circulation through the arterial vascular tree, to capillaries in the peripheral circulation of exercising muscles. Skeletal muscle mitochondria utilize O2 derived from the peripheral circulation for aerobic cellular energy metabolism to fuel movement and locomotion. CO2 generated from both aerobic and anaerobic cellular metabolism is then transferred in reverse order from skeletal muscle back to the heart, through the venous circulation, where it is subsequently expired through the lungs. Given the interconnectedness from internal to external respiration, CPET assessment of inspired O2 and expired CO2 can identify small perturbations in each of these vital organ systems, leading to shared clinical manifestations of dyspnea and fatigue contributing to exercise intolerance.
Key terms in CPET
Multiple terms are essential for proper interpretation of data obtained via ventilatory gas exchange analysis during CPET. Oxygen consumption (VO2) is a measure of cardiorespiratory fitness in absolute (mL/min) or relative (mL/kg/min) values. Peak VO2 is the greatest rate of oxygen consumption during progressive exercise. Respiratory exchange ratio (RER) is the molar ratio of CO2 produced per O2 consumed; it is progressive from less than 0.80 to greater than 1.0 during progressive exercise, is related to progressive use of glucose as more muscle glycolytic fibers are recruited as work load increases, and is a measure of cardiometabolic stress during CPET. Oxygen-pulse (O2-pulse) is the amount of oxygen uptake per heartbeat (VO2/HR; mL/beat); it is a surrogate measure for stroke volume (16). Breathing reserve or dyspnea index (VE/MVV) is the ratio of peak minute ventilation (VE; volume of air exhaled per min; L/min) during exercise to maximum voluntary ventilation (MVV; maximal volume of air a person can exhale in a minute; L). VE/VCO2 is the slope of VE versus carbon dioxide production (VCO2); it is a measure of ventilatory efficiency or dead space ventilation (17). Ventilatory threshold (VT) is the point at which ventilation disproportionally increases compared to oxygen consumption, reflecting increased energy demands from anaerobic metabolism (expressed as VO2 at VT (mL/min), or HR at VT (beats/min)); it is roughly equivalent to lactate threshold, when muscles begin to rely on anaerobic glycolysis for ATP production. Of note, additional testing—such as electrocardiography (ECG), blood pressure monitoring, and pulse oximetry—are commonly performed during CPET, however detailed discussion of changes in these parameters during exercise is beyond the scope of this communication.
CPET data reporting
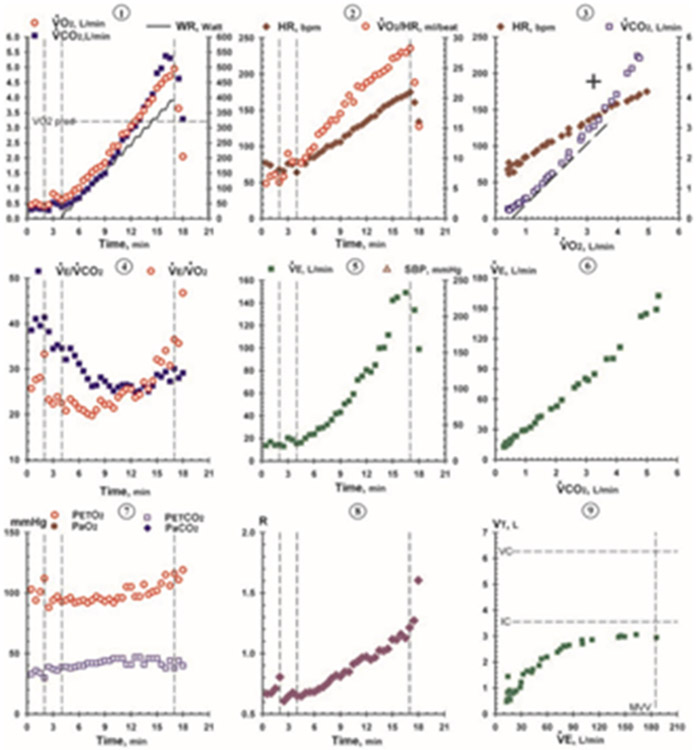
Each CPET data report displays simultaneously measured heart rate, ventilation, and gas exchange data as averaged values over fixed intervals (i.e., every 30 seconds). Tabular data are used to identify key physiologic responses to exercise at test end (i.e., VO2, VE, and RER at peak exercise). However, further interpretation of CPET data (i.e., for identification of organ specific limitations to exercise) requires graphical visualization of relationships between multiple key variables. For the past few decades, the gold standard for CPET data visualization has been the Wasserman “nine-panel plot”, which incorporates multiple channels of data into a one-page summary containing nine distinct scatter plots (Figure 1) (1, 18).
Figure 1.
CPET nine-panel plot. Example from Wasserman K, Hansen JE, Sue DY, Stringer W, Sietsema K, Sun XG, Whipp BJ. Principles of exercise testing and interpretation: Including pathophysiology and clinical applications: Fifth edition. Fifth edition. ed: Wolters Kluwer; 2011 (used by permission from Wolters Kluwer Health).
CPET data interpretation
Standard interpretation of CPET data requires evaluation of data both during and at the end of testing; visualization of relationships between key physiologic variables using tools such as the Wasserman nine-panel plot are often used by experienced readers. Identification of specific etiologies of exercise intolerance or exertional dyspnea using CPET is made by trained clinicians with the assistance of various algorithms and flowcharts (1, 6). Here, we summarize a common CPET data interpretation pathway we use in clinical practice:
Step 1) Evaluate pulmonary function tests.
Spirometry is commonly—but not universally—performed in exercise testing laboratories prior to CPET. Key respiratory parameters assessed by spirometry include forced vital capacity (FVC) and forced expiratory volume (FEV1). FVC (L) is the maximal volume of air a person can exhale in one breath. FEV1 (L) is the maximal volume of air a person can exhale in one second. A FEV1/FVC ratio of less than 0.80 suggests an obstructive pulmonary limitation. FEV1 can also be used to estimate MVV (i.e., MVV = FEV1 x 35 or 40).
Step 2) Evaluate test adequacy.
During progressive maximal aerobic exercise, increased lactic acid production from anaerobic metabolism results in greater bicarbonate buffering and subsequent CO2 exhalation, leading an increase in RER. An RER greater than 1.05 during CPET is generally considered suggestive of maximal effort at peak exercise and allows for interpretation of the test using standard procedures (19). An RER less than 1.05 at test end suggests either a submaximal test or the presence of a severe exercise limitation (i.e., advanced chronic obstructive pulmonary disease, interstitial lung disease, or peripheral arterial disease); interpretation should then take into consideration other factors such as percent predicted heart rate at peak exercise (i.e., greater than 85% estimated max heart rate), rating of perceived exertion (i.e., greater than 17 on Borg 6-20 scale), and clinical symptoms associated with stopping exercise (i.e., dyspnea, muscle pain) (20).
Step 3) Evaluate total oxidative capacity.
VO2 at peak exercise is compared to predicted values based on the patient’s age, gender, and weight (21, 22). VO2 peak values (mL/kg or mL/kg/min) less than predicted indicate a limitation in total oxidative capacity; further evaluation regarding organ-specific limitations (i.e., cardiac versus pulmonary versus skeletal muscle) can then be performed. Interpretation of total oxidative capacity should also consider that percent predicted values for VO2 peak will vary based on the use of absolute (mL/kg) versus relative (mL/kg/min) terms (i.e., obese persons may have a normal absolute VO2 peak but low relative VO2 peak) and the reference equation used for prediction (23).
Step 4) Evaluate for cardiac limitation.
For a healthy non-athlete during progressive exercise, heart rate and rate of inspired oxygen should both (i.e., the O2-pulse) linearly increase at a near-constant rate until peak exercise (24, 25). In a patient with a cardiac system limitation (i.e., myocardial ischemia or congestive heart failure), oxygen utilization (VO2) begins to decrease relative to heart rate prior to maximal exercise (26). This is due to a drop on the amount of blood ejected from the heart per beat, requiring an increase in heart rate to maintain needed cardiac output (mL blood per beat x beats per minute) for aerobic exercise. Thus, a cardiac limitation to exercise can be assessed via analysis of the O2-pulse curve shape and comparison of the O2-pulse at peak exercise to predicted values. A downsloping or abnormally shallow O2-pulse curve indicates a cardiac limitation to exercise.
Alternative cardiac limitation methods.
The cardiac limitation can also be defined by CPET parameters other than the change in O2-pulse (2, 27). For example, exercise-induced myocardial ischemia is hallmarked by an abnormal trajectory of the change in VO2 versus change in workload (ΔVO2/ΔW) on cycle ergometer. Further, the VE/VCO2 slope is a measure of dead space ventilation with strong associations and prognostic implications in heart failure and pulmonary arterial hypertension (2, 28). VE/VCO2 slope values < 30 are generally considered normal, while values > 40 are abnormal; however, abnormalities are also seen in patients with predominantly pulmonary limitations, such as chronic obstructive pulmonary disease or interstitial lung disease (29, 30). Further, electrocardiogram changes (i.e., ST segment abnormalities) and angina symptoms during exercise also suggest cardiac limitation (2, 3).
Step 5) Evaluate for pulmonary limitation.
During progressive exercise, a healthy person’s volume and rate of breathing increases but should never reach potential maximum, unless they have pulmonary disease or extremely high aerobic fitness (31). Thus, a pulmonary limitation to exercise can be identified when a patient’s breathing reserve (VE/MVV) is greater than 0.80 during peak exercise.
Alternative pulmonary limitation methods.
As discussed above, an abnormal VE/VCO2 slope can be seen in both pulmonary- and cardiac-limited patients. Desaturation during exercise (i.e., saturation of peripheral oxygen less than 95% or decrease by greater than 5%) is also suggestive of pulmonary limitation (2, 3).
Step 6) Evaluate for skeletal muscle limitation.
A healthy person uses balanced aerobic and anaerobic cellular metabolism for fuel during progressive exercise until VT, when skeletal muscle anaerobic metabolism begins to outpace aerobic metabolism. A patient with a skeletal muscle system limitation (i.e., deconditioning or mitochondrial myopathy) will reach VT earlier than expected and inefficiently use anaerobic systems to complete the work of exercise. Therefore, the lower the VT value compared to predicted (% of predicted maximum VO2 peak) and the longer relative time spent after VT during progressive maximal exercise, the more limiting to exercise is the skeletal muscle contribution.
Alternative skeletal muscle limitation methods.
Similar to the cardiac and pulmonary parameters, there are multiple CPET variables that can inform peripheral O2 utilization and skeletal muscle impairment other than VT (6). For example, an elevation in the slope of change in cardiac output—measured by adjunctive methods such as inert gas rebreathing—relative to change in VO2 (ΔQ/ΔVO2) is suggestive of a mitochondrial myopathy. In addition, an abnormal elevation in the VE/VO2 ratio at peak exercise also raises the consideration of a mitochondrial myopathy; however, VE/VO2 can be significantly elevated in primarily pulmonary disorders as well.
Challenges in CPET data visualization
As previously noted, CPET data visualizations to assist interpretation are widely based on seminal work by Wasserman and the nine-panel plot (1). The nine-panel plot was revolutionary because of its ability to distill complex CPET data into the most important parameters necessary to inform diagnostic evaluation. Still, CPET interpretation using this excellent visualization tool requires training and extensive experience to incorporate into clinical practice. Indeed, CPET data visualization using the Wasserman nine-panel plot method is not universally applied; there remains significant inter-individual variability in CPET interpretation (5, 9).
Subsequent CPET data visualizations attempt to refine the nine-panel plot method for CPET clinical interpretation. Relatively simple methods for improving CPET data visualization include optimization of graphical scaling, stylizing, and plot standardization (32). Other alternative visualizations include multidimensional graphs focusing on fewer CPET variables to inform more specific clinical questions (i.e., heart failure functional classification) (33). The European Association of Cardiovascular Prevention and Rehabilitation (EACPR) and American Heart Association (AHA) suggest using color-coded diagrams for diagnostic and prognostic stratification of patients with both unexplained dyspnea and disease-specific exercise limitations (2, 3). CPET data assessment consensus recommendations—including recommendations from the EACPR/AHA—also advise incorporating more recent reference thresholds with the nine-panel plot method and other revised algorithms (2, 3, 7). Future iterations of CPET data visualization will likely be refined by large-scale projects to define CPET data reference standards; but, these projects have thus far been limited by a relative paucity of non-white participants and disease-specific populations (34-37). Further, differences in CPET terminology, data reporting, and system operability add to the challenge of sharing and interpretation of CPET data across clinical and academic sites. Projects such as the Health Level 7 (HL7) Terminology Harmonization in Exercise Medicine and Exercise Science (THEMES) promise to standardize CPET data elements to remove these interoperability barriers for the benefit of future CPET data analytic platforms (https://www.hl7.org/implement/standards/product_brief.cfm?product_id=475).
Example of novel CPET data visualization
To show how some of these challenges might be addressed, we created an alternative visualization to the Wasserman nine-panel plot with the goal of producing a simpler representation of CPET generated data. The goal of this novel, three-dimensional graphical representation of CPET data is to provide non-experts with categorical structure as an initial step to diagnosing organ-specific etiologies of exercise intolerance. To this end, we use simplified formulas to inform CPET data visualization of the three major diagnostic categories of exercise limitation: cardiac, pulmonary, and skeletal muscle.
In each of the three limitation categories, we defined formulas identifying limitation on a scale of 0% to 100%; 0% means no limitation, and 100% means maximum limitation. Percent limitation for each category was calculated via algorithmic approach from raw CPET data based on relationships between variables deemed essential to determining individual organ system-specific limitations; organ system-specific limitations in this sense are relative and do not refer to normal versus abnormal.
We define the cardiac limitation formula as the difference between the O2-pulse slopes (m) during the second and last quarters (denoted as m25-50% and m75-100%, respectively) of the test. To calculate m for both quarters we used a linear regression of the set of O2 pulse values in the respective quarter. If the slope of m25-50% was not discernibly linear, then the slope of m50-75% was used instead. We then transformed both values of m into polar coordinates to obtain the angle, in radians, between them and the x-axis. The ratio of absolute value of the difference of the two angles to π/2 radians was the cardiac limitation.
The pulmonary limitation was defined as the ratio of subject’s rate of ventilation at peak exercise to their maximum voluntary ventilation (VE/MVV). Peak VE was determined as the VE (L/min) at test end, while MVV (L) corresponded with the value evaluated prior to testing.
The skeletal muscle limitation formula was defined as the percentage of total exercise time spent after VT until peak exercise (i.e., ratio of time spent after VT to total test time). To clarify, we define VT as the point in time, ignoring the first 20% of data points due to noise at the beginning of the test, when the slope of the VCO2/VO2 relationship starts to consistently increase by greater than 0.1 from the previous VCO2/VO2 slope.
In brief, individual CPET data used for graphical visualization was prepped using Python3 and the formulas for organ-specific exercise limitation were applied to create output extensible 3D (X3D) and JavaScript Object Notation (JSON) files. These files were then used as input for the Data-Driven-Documents (D3.js) and X3DOM visualizations. As shown in Figures 2-4, our visualization consists of one 3D plot and three two-dimensional (2D) plots, respectively. 3D plots present the three limitations together in a single three-dimensional graph. The 3D plot has multiple pre-programmed viewpoints for the plot, and the plot can be panned, zoomed, and rotated. 2D plots represent the main variables that are considered when calculating the respective limitation to provide supporting information for the 3D plot. In this visualization, the rectangular prisms in the 3D plots make it easy to quickly determine the extent of pulmonary, cardiac, and skeletal muscle limitations; and to associate distinguishable prism shapes to aid disease specific evaluation.
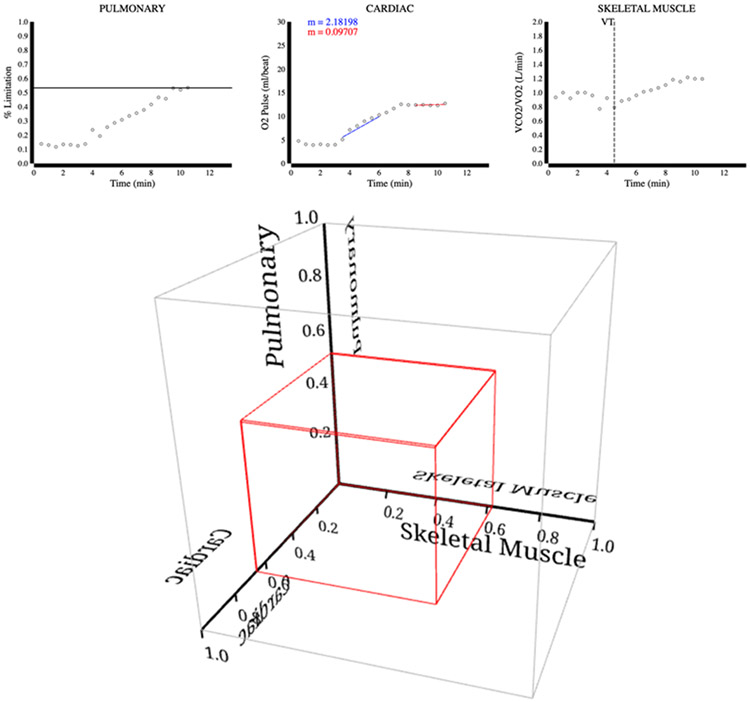
Figure 2.
Chronic heart failure. The upper panel depicts supporting 2D plots for the pulmonary, cardiac, and skeletal muscle limitations. The bottom panel depicts the 3D plot, which identifies the subject’s major limitation to exercise in the cardiac system.
Figure 4.
Mitochondrial myopathy. The upper panel depicts supporting 2D plots for the pulmonary, cardiac, and skeletal muscle limitations. The bottom panel depicts the 3D plot, which identifies the subject’s major limitation to exercise in the skeletal muscle system.
To evaluate our visualization as a proof-of-concept, we deployed it on eight CPET use case examples from Wasserman’s Principles of Exercise Testing and Interpretation (used and published with permission from the publisher, Wolters Kluwer Health) (1). In this communication, we discuss diagnostic evaluation using this CPET data visualization platform in three of those cases. Visualizations for the other five cases (case 1: normal man; case 2: normal athletic man; case 20: myocardial ischemia; case 53: mild pulmonary asbestosis; case 68: mixed disorder) are presented online (http://metagrid2.sv.vt.edu/~nickhrdy/cpet.html).
Cardiac limitation case
CPET example case #15 describes a 71-year-old man with chronic left ventricular systolic heart failure. The test was stopped as he complained of leg fatigue and shortness of breath (1). Evaluation of his CPET data revealed a VO2 peak of 1.03 L/min (52.2% of predicted 1.97 L/min), an abnormal O2 pulse tradjectory, VE/MVV of 0.60, and VT of 0.75 L/min (80.6% of predicted 0.93 L/min). As shown in Figure 2, the subject shows signs of partial limitation in all three domains. However, the most prominent limitation is cardiac, which fits the patient’s clinical diagnosis of heart failure. The cardiac limitation is supported by the difference in slope denoted in the second and last quarters of the cardiac 2D plot.
Pulmonary limitation case
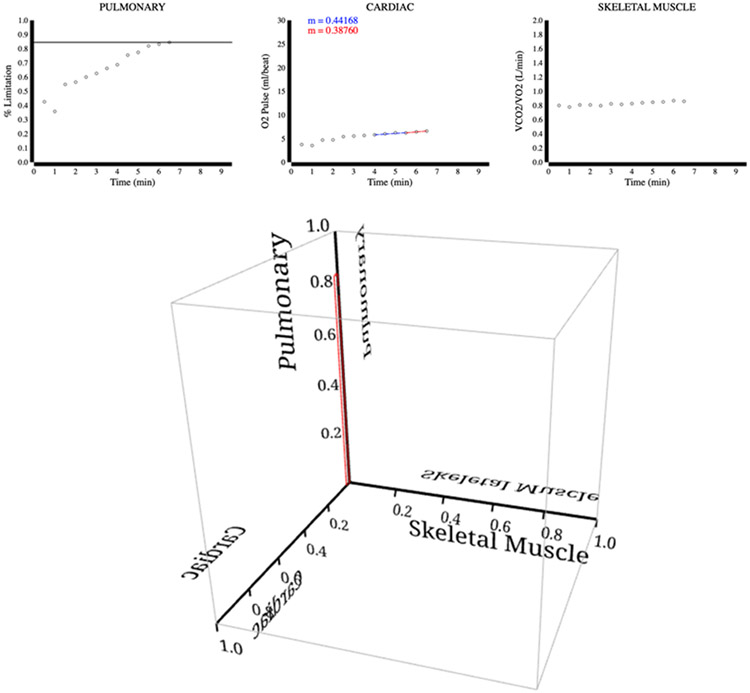
Figure 3 shows CPET example case #47 from a 65-year-old man with a history of asbestos and cigarette exposure (1). The test was stopped as the patient complained about shortness of breath. Evaluation of his CPET data revealed a peak VO2 peak of 0.90 L/min (42.7% of predicted 2.11 L/min), a normal O2 pulse trajectory, high VE/MVV of 0.90, and VT was not reached. Notably, his MVV was well below the predicted value and at peak exercise the patient's VE/MVV ratio was > 0.80; this observation is reflected in the 3D graph as a pulmonary limitation percentage. The 3D graph in Figure 3 shows a box extending further along the pulmonary axis well beyond those of the other two axes, consistent with a primary diagnosis of emphysema.
Figure 3.
Severe emphysema. The upper panel depicts supporting 2D plots for the pulmonary, cardiac, and skeletal muscle limitations. The bottom panel depicts the 3D plot, which identifies the subject’s major limitation to exercise in the pulmonary system.
Skeletal muscle limitation case
Figure 4 shows results from CPET example case #66 of a 52-year-old woman with clinical symptoms of increasing exercise intolerance. Though she reports being athletic during young adulthood, she recently started experiencing fatigue and weakness with heavy exertion. At peak exercise, she reported weakness, shortness of breath, and required 10 minutes of recovery before being able to stand independently (1). Evaluation of her CPET data revealed a VO2 peak of 0.62 L/min (40.2% of predicted 1.54 L/min), flattening of O2 pulse, VE/MVV of 0.36, and VT of 0.49 L/min (62.8% of predicted 0.78 L/min). From the 2D plot to the right, it is evident that she reached ventilatory threshold shortly after beginning the test. The 3D plot shows that all three axes have limitation signs; however, the skeletal muscle system is most limited. Supporting evidence of early onset VT and high relative time spent in VT prior to peak exercise suggests that her main limiting factor is skeletal muscle in nature. In this case, subsequent testing revealed a mitochondrial myopathy as her primary diagnosis.
Future directions
In comparison to the more detailed Wasserman nine-panel plot and other CPET assessment methods, the primary intention of our CPET data visualization is to provide a simpler tool for diagnostic assessment and monitoring of exercise intolerance. As a result, the primary drawback of our visualization tool is the lack of granularity in providing more detailed information regarding a patient’s primary exercise limitation. For example, if a patient’s greatest limitation is in the cardiac system, our visualization tool in its current form is unable to accurately differentiate from among potential specific diagnoses, such valvular heart disease, hypertrophic cardiomyopathy, pulmonary hypertension, or myocardial ischemia. Still, we envision that simplified CPET data visualization could be used to assist more advanced CPET interpretation and guide further diagnostic testing (i.e., laboratory studies, echocardiography, cross-sectional imaging).
While there are surprisingly few CPET variables that are actually needed to inform organ-specific limitations, there is a plethora of supporting data generated by CPET adding precision and accuracy to diagnostic evaluation (7, 38). Advanced organ- and disease-specific algorithms and machine learning tools (i.e., artificial neural networks) might utilize these additional CPET—as well as clinical—variables to refine current diagnostic paradigms (39, 40). Subsequent visualization platforms could also allow for real-time viewing and magnification of specific variables during the course of the exercise test to permit more detailed user-generated analysis of CPET as desired.
For future work, there we anticipate opportunities to build more robust CPET data visualization platforms using additional real-world cases to improve upon the organ-specific limitation formulas presented here. Moving forward, with the goal of introducing this visualization to a wider community, we plan to gather feedback from users (i.e., medical practitioners, clinical exercise physiologists, and patients) on the effectiveness of various graphical displays. Finally, there will be the opportunity for JSON and X3D data for the visualization presented here to be integrated into the HL7 THEMES framework.
Conclusions
CPET is a powerful multipurpose tool for clinicians caring for patients with cardiopulmonary disorders and exercise intolerance. Utilizing CPET for diagnostic evaluation requires multiple elements, including: 1) in-depth knowledge of exercise physiology and external to internal gas exchange coupling; 2) a well-equipped and maintained exercise physiology lab with experienced staff; 3) proper interpretation of CPET data by experienced practitioners. Unfortunately, each of these elements may be a barrier to utilizing CPET to greater extent in clinical practice. Novel CPET data visualization platforms hold promise to make CPET more accessible and improve clinician-patient communication regarding the etiology, work-up, and management of disease-specific exercise limitations. The addition of advanced computer-assisted diagnostic reasoning tools to these CPET platforms may also provide new insights into exercise testing and prescription for the experienced physiologist.
Here, we presented a novel visualization of CPET data to inform diagnostic evaluation for the general medical practitioner. In our visualization, the rectangular prisms in the 3D plots make it easy to quickly determine the extent of pulmonary, cardiac, and skeletal muscle limitations and to associate distinguishable shapes for each limitation. This intuitive visualization allows the user to read and present the CPET data more succinctly to others, including medical providers and patients unfamiliar with the details of test interpretation; however, we acknowledge that new platforms such as the novel visualization presented here need to be further refined and cross-validated against currently accepted tools such as the Wasserman nine-panel plot and the EACPR/AHA color-coded diagrams. Subsequent refinements of visualization platforms such as this are needed to fully realize the potential of CPET as a first line diagnostic, prognostic, and disease monitoring tool in the clinician’s armamentarium.
Acknowledgements
Funding from the NIH grant R03AG067949, the Duke Pepper Center REC Career Development Award, and the Rauch Family Research Scholarship (to BJA) supported this project. We would like to thank Dr. Dan M. Cooper, Dr. W. Ed Hammond, and Davera Gabriel for their assistance in the conceptualization of this project.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest and do not have any financial disclosures
REFERENCES:
- 1.Wasserman K, Hansen JE, Sue DY, et al. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications, 5th edition. Philadelphia (PA): Wolters Kluwer; 2011. [Google Scholar]
- 2.Guazzi M, Adams V, Conraads V, et al. European Association for Cardiovascular Prevention and Rehabilitation/American Heart Association Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126(18):2261–74. Epub 2012/09/07. doi: 10.1161/CIR.0b013e31826fb946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guazzi M, Arena R, Halle M, Piepoli MF, Myers J, Lavie CJ. 2016 focused update: Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2016;133(24):e694–711. Epub 2016/05/05. doi: 10.1161/CIR.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 4.Huddart S, Young EL, Smith RL, Holt PJ, Prabhu PK. Preoperative cardiopulmonary exercise testing in England - a national survey. Perioper Med (Lond). 2013;2(1):4. Epub 2014/01/30. doi: 10.1186/2047-0525-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reeves T, Bates S, Sharp T, et al. Cardiopulmonary exercise testing (CPET) in the United Kingdom-a national survey of the structure, conduct, interpretation and funding. Perioper Med (Lond). 2018;7:2. Epub 2018/02/10. doi: 10.1186/s13741-017-0082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balady GJ, Arena R, Sietsema K, et al. Clinician's guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122(2):191–225. Epub 2010/06/30. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 7.Guazzi M, Bandera F, Ozemek C, Systrom D, Arena R. Cardiopulmonary exercise testing: What is its value? J Am Coll Cardiol. 2017;70(13):1618–36. Epub 2017/09/25. doi: 10.1016/j.jacc.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Holverda S, Bogaard HJ, Groepenhoff H, Postmus PE, Boonstra A, Vonk-Noordegraaf A. Cardiopulmonary exercise test characteristics in patients with chronic obstructive pulmonary disease and associated pulmonary hypertension. Respiration. 2008;76(2):160–7. Epub 2007/10/26. doi: 10.1159/000110207. [DOI] [PubMed] [Google Scholar]
- 9.Myers J Applications of cardiopulmonary exercise testing in the management of cardiovascular and pulmonary disease. Int J Sports Med. 2005;26 Suppl 1:S49–55. Epub 2005/02/11. doi: 10.1055/s-2004-830515. [DOI] [PubMed] [Google Scholar]
- 10.Baloch ZQ, Abbas SA, Marone L, Ali A. Cardiopulmonary exercise testing limitation in peripheral arterial disease. Ann Vasc Surg. 2018;52:108–15. Epub 2018/05/20. doi: 10.1016/j.avsg.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Chakkera HA, Angadi SS, Heilman RL, et al. Cardiorespiratory fitness (peak oxygen uptake): Safe and effective measure for cardiovascular screening before kidney transplant. J Am Heart Assoc. 2018;7(11). Epub 2018/06/02. doi: 10.1161/JAHA.118.008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbertson NM, Gaitan JM, Osinski V, et al. Pre-operative aerobic exercise on metabolic health and surgical outcomes in patients receiving bariatric surgery: A pilot trial. PLoS One. 2020;15(10):e0239130. Epub 2020/10/03. doi: 10.1371/journal.pone.0239130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunt SA, Abraham WT, Chin MH, et al. American College of Cardiology/American Heart Association 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112(12):e154–235. Epub 2005/09/15. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 14.Albouaini K, Egred M, Alahmar A, Wright DJ. Cardiopulmonary exercise testing and its application. Heart. 2007;93(10):1285–92. Epub 2007/09/25. doi: 10.1136/hrt.2007.121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Squires RW, Kaminsky LA, Porcari JP, Ruff JE, Savage PD, Williams MA. Progression of exercise training in early outpatient cardiac rehabilitation: An official statement from the American Association of Cardiovasular and Pulmonary Rehabilitation. J Cardiopulm Rehabil Prev. 2018;38(3):139–46. Epub 2018/04/27. doi: 10.1097/HCR.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 16.Arena R, Myers J, Harber M, Wisloff U, Stensvold D, Kaminsky LA. Peak oxygen pulse responses during maximal cardiopulmonary exercise testing: Reference standards from FRIEND (Fitness Registry and the Importance of Exercise: an International Database). Int J Cardiol. 2020;301:180–2. Epub 2019/11/24. doi: 10.1016/j.ijcard.2019.11.106. [DOI] [PubMed] [Google Scholar]
- 17.Arena R, Myers J, Harber M, et al. The V E/V CO2 slope during maximal treadmill cardiopulmonary exercise testing: Reference standards from Fitness Registry and the Importance of Exercise: A National Database. J Cardiopulm Rehabil Prev. 2021. Epub 2021/01/21. doi: 10.1097/HCR.0000000000000566. [DOI] [PubMed] [Google Scholar]
- 18.Wasserman K Coupling of external to cellular respiration during exercise: the wisdom of the body revisited. Am J Physiol. 1994;266(4 Pt 1):E519–39. Epub 1994/04/01. doi: 10.1152/ajpendo.1994.266.4.E519. [DOI] [PubMed] [Google Scholar]
- 19.Mehra MR, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J Heart Lung Transplant. 2016;35(1):1–23. Epub 2016/01/19. doi: 10.1016/j.healun.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 20.American College of Sports Medicine. Guidelines for Exercise Testing and Prescription, 11th edition. Philadelphia (PA): Wolters Kluwer; 2021. [Google Scholar]
- 21.Peterman JE, Arena R, Myers J, et al. Development of global reference standards for directly measured cardiorespiratory fitness: A report from the Fitness Registry and Importance of Exercise National Database (FRIEND). Mayo Clin Proc. 2020;95(2):255–64. Epub 2019/12/31. doi: 10.1016/j.mayocp.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Koch B, Schaper C, Ittermann T, et al. Reference values for cardiopulmonary exercise testing in healthy volunteers: the SHIP study. Eur Respir J. 2009;33(2):389–97. Epub 2008/09/05. doi: 10.1183/09031936.00074208. [DOI] [PubMed] [Google Scholar]
- 23.Brawner CA, Ehrman JK, Shafiq A, et al. Challenges with percent predicted maximal VO2 in patients with heart failure. Med Sci Sports Exerc. 2018;50(2):204–10. Epub 2017/09/25. doi: 10.1249/MSS.0000000000001431. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira RB, Myers J, Araujo CG. Long-term stability of the oxygen pulse curve during maximal exercise. Clinics (Sao Paulo). 2011;66(2):203–9. Epub 2011/04/13. doi: 10.1590/s1807-59322011000200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conconi F, Ferrari M, Ziglio PG, Droghetti P, Codeca L. Determination of the anaerobic threshold by a noninvasive field test in runners. J Appl Physiol Respir Environ Exerc Physiol. 1982;52(4):869–73. Epub 1982/04/01. doi: 10.1152/jappl.1982.52.4.869. [DOI] [PubMed] [Google Scholar]
- 26.Lavie CJ, Milani RV, Mehra MR. Peak exercise oxygen pulse and prognosis in chronic heart failure. Am J Cardiol. 2004;93(5):588–93. Epub 2004/03/05. doi: 10.1016/j.amjcard.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 27.Belardinelli R, Lacalaprice F, Carle F, et al. Exercise-induced myocardial ischaemia detected by cardiopulmonary exercise testing. Eur Heart J. 2003;24(14):1304–13. Epub 2003/07/23. doi: 10.1016/s0195-668x(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 28.Arena R, Myers J, Abella J, et al. Defining the optimal prognostic window for cardiopulmonary exercise testing in patients with heart failure. Circ Heart Fail. 2010;3(3):405–11. Epub 2010/03/05. doi: 10.1161/CIRCHEARTFAILURE.109.906446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torchio R, Guglielmo M, Giardino R, et al. Exercise ventilatory inefficiency and mortality in patients with chronic obstructive pulmonary disease undergoing surgery for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2010;38(1):14–9. Epub 2010/04/02. doi: 10.1016/j.ejcts.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 30.Glaser S, Noga O, Koch B, et al. Impact of pulmonary hypertension on gas exchange and exercise capacity in patients with pulmonary fibrosis. Respir Med. 2009;103(2):317–24. Epub 2008/09/23. doi: 10.1016/j.rmed.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Stickland MK, Butcher SJ, Marciniuk DD, Bhutani M. Assessing exercise limitation using cardiopulmonary exercise testing. Pulm Med. 2012;2012:824091. Epub 2012/12/06. doi: 10.1155/2012/824091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dumitrescu D, Rosenkranz S. Graphical data display for clinical cardiopulmonary exercise testing. Ann Am Thorac Soc. 2017;14(Supplement_1):S12–S21. Epub 2017/05/26. doi: 10.1513/AnnalsATS.201612-955FR. [DOI] [PubMed] [Google Scholar]
- 33.Hansen JE, Sun XG, Stringer WW. A simple new visualization of exercise data discloses pathophysiology and severity of heart failure. J Am Heart Assoc. 2012;1(3):e001883. Epub 2012/11/07. doi: 10.1161/JAHA.112.001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaminsky LA, Arena R, Myers J. Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing: Data from the Fitness Registry and the Importance of Exercise National Database. Mayo Clin Proc. 2015;90(11):1515–23. Epub 2015/10/13. doi: 10.1016/j.mayocp.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaminsky LA, Imboden MT, Arena R, Myers J. Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing using cycle ergometry: Data from the Fitness Registry and the Importance of Exercise National Database (FRIEND) Registry. Mayo Clin Proc. 2017;92(2):228–33. Epub 2016/12/13. doi: 10.1016/j.mayocp.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Brawner CA, Ehrman JK, Keteyian SJ. Are international standards for exercise capacity ready for prime time? Mayo Clin Proc. 2020;95(2):218–20. Epub 2020/02/08. doi: 10.1016/j.mayocp.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 37.Ades PA, Savage PD, Brawner CA, et al. Aerobic capacity in patients entering cardiac rehabilitation. Circulation. 2006;113(23):2706–12. Epub 2006/06/07. doi: 10.1161/CIRCULATIONAHA.105.606624. [DOI] [PubMed] [Google Scholar]
- 38.Milani RV, Lavie CJ, Mehra MR, Ventura HO. Understanding the basics of cardiopulmonary exercise testing. Mayo Clin Proc. 2006;81(12):1603–11. Epub 2006/12/15. doi: 10.4065/81.12.1603. [DOI] [PubMed] [Google Scholar]
- 39.Kader MN, Moiz JA, Bhati P, Ali MS, Talwar D. Diagnostic validity of cardiopulmonary exercise testing for screening pulmonary hypertension in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev. 2020;40(3):189–94. Epub 2019/11/13. doi: 10.1097/HCR.0000000000000456. [DOI] [PubMed] [Google Scholar]
- 40.Zignoli A, Fornasiero A, Stella F, et al. Expert-level classification of ventilatory thresholds from cardiopulmonary exercising test data with recurrent neural networks. Eur J Sport Sci. 2019;19(9):1221–9. Epub 2019/03/19. doi: 10.1080/17461391.2019.1587523. [DOI] [PubMed] [Google Scholar]