Abstract
Background
Cerebrovascular events, dementia, and cancer can contribute to physical disability with activities of daily living (ADL). It is unclear whether low-dose aspirin reduces this burden in aging populations. In a secondary analysis, we now examine aspirin’s effects on incident and persistent ADL disability within a primary prevention aspirin trial in community-dwelling older adults.
Methods
The ASPREE (ASPirin in Reducing Events in the Elderly) trial of daily 100 mg aspirin versus placebo recruited 19 114 healthy adults aged 70+ years (65+ years if U.S. minority) in Australia and the United States. Six basic ADLs were assessed every 6 months. Incident ADL disability was defined as inability or severe difficulty with ≥1 ADL; persistence was confirmed if the same ADL disability remained after 6 months. Proportional hazards modeling compared time to incident or persistent ADL disability for aspirin versus placebo; death without prior disability was a competing risk.
Results
Over a median of 4.7 years, incident ADL disability was similar in those receiving aspirin (776/9525) and placebo (787/9589) with walking, bathing, dressing, and transferring the most commonly reported. Only 24% of incident ADL disability progressed to persistent. Persistent ADL disability was lower in the aspirin group (4.3 vs 5.3 events/1000 py; hazard ratio [HR] = 0.81, 95% confidence interval [CI]: 0.66–1.00), with bathing and dressing the most common ADL disabilities in both groups. Following persistent ADL disability, there were more deaths in the aspirin group (24 vs 12).
Discussion
Low-dose aspirin in initially healthy older people did not reduce the risk of incident ADL disability, although there was evidence of reduced persistent ADL disability.
Keywords: Aspirin, Clinical trials, Functional performance, Physical function, Preventive health care
Prolongation of healthy active life as we age is everyone’s hope, and of equal importance is that this extended life should preserve one’s capacity to function independently. Mobility and basic activities of daily living are tasks necessary to maintain independent functioning (1,2). A lot of difficulty with performing these activities, or an inability to perform independently, marks a serious decline in functional health, and foreshadows institutionalization and death (eg, (1)). Therefore, identification of interventions that can reduce or prevent disability, particularly in older persons, is a major public health goal.
Underlying causes of both death and disability in an aging population include major cardiovascular and cerebrovascular disease events (such as myocardial infarction [MI], heart failure, and stroke), dementia, and cancer (3,4). An altered incidence of any (or all) of these might affect the overall risk–benefit balance of a preventive therapy intended to delay or prevent physical disability in this age group. Low-dose aspirin has been considered as a potential candidate for exploration in this context due to prevention of atherothrombotic events and for targeting pro-inflammatory pathway activation (“inflammaging”) which has been proposed to contribute to these diseases of aging (5,6).
ASPirin in Reducing Events in the Elderly (ASPREE) was a primary prevention trial of daily low-dose aspirin in “healthy” older persons who, at baseline, did not have a life-limiting illness likely to result in death within 5 years (the original follow-up period of ASPREE) (7,8). ASPREE determined that aspirin did not extend disability-free survival, the primary endpoint which was a composite of death, dementia, or persistent physical disability (9). Persistent physical disability was assessed in the trial as a composite of self-reported persistent activities of daily living (ADL) disability and adjudicated “admission to care” for those without ADL data (9). The present post hoc analysis evaluated whether aspirin prevented incident or persistent ADL disability in ASPREE participants.
Method
Study Design and Population
Recruitment into the ASPREE trial took place in Australia (16 703) and the United States (2411) between March 2010 and December 2014 (7,8). Eligible participants were community-dwelling individuals aged 70 years or older, except for U.S. African American or Hispanic participants who were eligible if aged 65 years or older. ASPREE was approved by multiple Institutional Review Boards in the United States and Australia, registered with International Standard Randomized Controlled Trial Number Register (ISRCTN83772183) and clinicaltrials.gov (NCT01038583) and undertaken in accordance with the Declaration of Helsinki. The aspirin versus placebo intervention was stopped for futility for the composite primary endpoint by the sponsor (National Institute on Aging, in consultation with the Data Safety and Monitoring Board) in June 2017, after a median of 4.7 years of follow-up. The ASPREE Protocol (10) is available at aspree.org and includes details of randomization, inclusion/exclusion criteria, study measurements, and assessments and sample size calculations for the primary and secondary endpoints of the trial.
ASPREE participants were free of documented evidence of cardiovascular or cerebrovascular disease (CVD), dementia, and major physical disability at enrollment. Prior cancer or other illnesses were not an exclusion provided life expectancy was at least 5 years. Participants were excluded if they had any of the following: dementia diagnosis or a Modified Mini-Mental State (3MS) examination (11) score ≤ 77; a lot of difficulty or an inability to perform independently any one of the 6 basic ADLs (12); a current or recurrent condition with a high risk of major bleeding; anemia (hemoglobin level <12 g/dL for males, <11 g/dL for females); a history of a diagnosed CVD event (MI, congestive heart failure, angina pectoris ± nitrate use, stroke, transient ischemic attack, >50% carotid stenosis, or previous carotid endarterectomy or stenting, coronary artery angioplasty or stenting, coronary artery bypass grafting, or abdominal aortic aneurysm); a clinical diagnosis of atrial fibrillation; current continuous use of other anti-platelet drug or anticoagulant; pill-taking compliance below 80% during the placebo run-in phase; or poorly controlled hypertension (systolic blood pressure ≥ 180 mmHg or diastolic blood pressure ≥ 105 mmHg).
Participants were randomly assigned to receive 100 mg of enteric-coated aspirin daily or matching placebo. Medication compliance was assessed by annual pill counts from returned bottles (73% for those assigned aspirin and 74% for those assigned placebo group throughout the trial) (9). Analysis was on an intention-to-treat basis, so participants who ceased study medication for any reason were encouraged to remain in the trial and attend study visits for clinical event and other data collection.
Participants’ demographic variables and health measures were assessed at baseline and at annual in-person visits which were supplemented by 6-monthly telephone calls for additional information (8). Components of the primary and secondary endpoints were adjudicated by committees of clinical experts who were blinded to trial-group assignment. Details of the data collection schedules were previously described (7–9).
ADL Disability Assessment
At study enrollment, each participant was required to be able to perform every ADL independently but was permitted to have “a little” or “some” difficulty with each ADL. Persistent ADL disability was defined by confirmation after 6 months of the inability or a lot of difficulty with performance of one or more of 6 basic ADLs or that the participant required assistance to perform the ADL. Confirmation after 6 months of the same ADL disability was chosen to identify persistent rather than transient disability as this was more likely to represent permanent disability and therefore longer term adverse outcomes.
The set of 6 ADLs used in ASPREE was derived from the original Katz items (2) as used in the Established Populations for Epidemiologic Study of the Elderly (EPESE) (13) and Lifestyle Interventions and Independence for Elders (LIFE) (14) studies. The 6 ADLs include the following: walking across a room, bathing, dressing, transferring from a bed or chair, toileting, and eating. The following response options were offered: (i) no difficulty, (ii) a little difficulty, (iii) some difficulty, (iv)] a lot of difficulty, or (v) unable to perform. An additional question was asked for each ADL (regardless of the response to the parent question) about whether the participant usually requires assistance from another person, or does not. A self-report of (iv) or (v) or requiring assistance to complete was deemed an ADL disability, whereas (ii) or (iii) was considered an ADL limitation. The instrument was administered by interview at baseline, at every annual study visit, and every intervening 6 months by phone. Although the ASPREE Protocol (10) allowed for a proxy to be permitted to answer the ADL questions on behalf of a participant who was unable to answer due to illness or other reasons, operationally this did not happen because only participants’ self-reported ADLs were accepted, either in person or by phone.
As previously described (9), the original ASPREE physical disability endpoint was persistent loss of the same ADL and in 2016, the ASPREE Data Safety and Monitoring Board approved the expansion of the definition to include approval for “admission to permanent care” (nursing home placement or long-term care) in those cases where it was not possible to obtain confirmatory ADL information. Of the 412 persistent physical disability endpoints reported in the ASPREE primary outcome paper (9), there were 39 participants (8.5%) who were approved for admission to care and whom the adjudicators considered had reached a persistent physical disability endpoint. The present analysis of aspirin and ADL disability does not include these participants and only considered those participants from whom ADL information could be collected.
Clinical Events Preceding ADL Disability
In an exploratory analysis, we describe the preceding health events identified through the ASPREE clinical trial, as endpoints or hospitalizations, in an attempt to explain the transition from incident to persistent ADL loss and whether that was different with aspirin treatment. ASPREE’s adjudicated endpoints other than for disability (10) included major CVD events (fatal and nonfatal MI, fatal and nonfatal ischemic stroke, and hospitalization for heart failure), cancer (incident and metastatic, fatal, and nonfatal), dementia, all-cause mortality, and major hemorrhage. Major hemorrhage was defined as hemorrhagic stroke, non-stroke intracranial bleeding, or extracranial clinically significant bleeding (defined as bleeding requiring transfusion, hospitalization or prolongation of hospitalization, or surgery; or bleeding causing death (15)). Gastrointestinal bleeding, intracranial (non-stroke) bleeding, and major bleeding at other sites were the major contributors (>80%) to clinically significant bleeding events (16). For deaths, adjudicators examined the progression of the final illness or incident, and assigned an underlying cause of death, which was the single disease most likely to have initiated the trajectory toward death. Details of the adjudication criteria and processes for confirming trial endpoints were previously described (9,15–17). ASPREE also collected evidence of hospitalizations that were for reasons other than adjudicated endpoints and lasting for 24 hours or more (10). ASPREE did not collect supporting documentation on other major adverse health events such as chronic obstructive pulmonary disease, infections, or osteoarthritis.
Statistical Analysis
Data reported here arise from version 3 of the ASPREE longitudinal dataset. Intention-to-treat analyses were used throughout. Time-to-event for incident ADL disability was any ADL report of (iv) or (v) and for persistent ADL disability was taken as the first reported date of an ADL disability which was then confirmed approximately 6 months later (±1 month).
A competing risk Cox proportional hazards model (PH) (18) was used to compare the aspirin and placebo groups regarding time-to-event endpoints (19). In these analyses, individuals were censored at the time of death if they died without any prior ADL disability. This study focuses on a post hoc analysis of the persistent ADL disability, which was the main component of the ASPREE physical disability endpoint (9), with further exploratory analyses of incident ADL disability.
Prespecified subgroups, the same as those analyzed for the main ASPREE trial (19), included age, gender, race/ethnicity, country of recruitment, education (±12 years), smoking, cancer history, previous use of aspirin, diabetes mellitus, hypertension, body mass index, and Fried frailty phenotype (nonfrail, prefrail, or frail) (20). Non-prespecified subgroup analyses were also used to investigate the effect of aspirin versus placebo on disability and included chronic kidney disease, gait speed, grip strength, waist circumference, and any ADL difficulty at baseline.
Heterogeneity of treatment effect across subgroups was tested based on the subgroup–treatment interaction term in a Cox PH model, using a significance level of p ≤ .01 to account for the number of statistical analyses. PH assumptions were tested using Schoenfeld residuals; all p-values were found to be greater than .1, indicating satisfaction of the assumption for all endpoints. Cumulative incidences were used to show event risk, based on competing-risks regression models, to account for competing risks of death, and stratified according to trial group.
Exploratory analysis of health events preceding ADL disability, including adjudicated ASPREE events or numbers of hospitalizations in the preceding 6 months, aimed to determine whether such events could explain any effect of aspirin. Additionally, we used an illness-death model (18) and restricted mean survival time (21) to assess the effect of aspirin on time to death following persistent ADL disability. The former is a special case of a multistate model where the disease progress can be described as a transition between three states: health, disease, and death (18).
Since most subgroup comparisons were prespecified (19), p-values are included. For all other post hoc and exploratory analyses, p-values are not reported.
Results
Study Participants
At study enrollment, there were no differences by treatment group for country, race/ethnicity, smoking, cancer history, diabetes mellitus, hypertension, chronic kidney disease, depression, and body mass index, as reported previously (9). In addition, there were no differences by treatment group for other participant characteristics relevant to the development of physical disability, including body weight and waist circumference, gait speed and grip strength, frailty, and any difficulty with each ADL (Supplementary Table S1). Of those participants with any ADL limitation at baseline (70% in the aspirin group and 68% in the placebo group), most people reported “a little difficulty” with a single ADL (Supplementary Table S2).
Incident ADL Disability and Treatment With Aspirin
Of the total number of participants who reported any incident ADL disability (1563; 20.6 events/1000 person-years), similar numbers were observed in the aspirin and placebo groups (776 vs 787). The types of incident ADL disabilities were also similar across treatment groups (Table 1). From most to least common incident ADL disabilities were walking, bathing, transferring, or dressing (all 21%–24%), 6% with toileting and 3% with eating (Table 1). Most participants (70%) described only one ADL disability, 18% reported disability in 2 ADLs, and 12% reported disability in 3 or more ADLs at a time (Table 1). Aspirin did not affect the type or number of incident ADL disabilities (Table 1).
Table 1.
Incident ADL Disability (number and rate) by Type and Treatment Group
| Aspirin (N = 9525) | Placebo (N = 9589) | Total (N = 19 114) | |
|---|---|---|---|
| ADL disability, participants; N (%) | |||
| No ADL disability | 8749 (91.8) | 8802 (91.7) | 17 551 (91.8) |
| Any incident ADL disability | 776 (8.1) | 787 (8.2) | 1563 (8.1) |
| Rate/1000 py | Rate/1000 py | HR (95% CI) | |
| Incident ADL disability rate | 20.6 | 20.6 | 1.00 (0.91–1.10) |
| Concurrent ADL disability; N (%) | |||
| Single ADL | 535 (68.9) | 551 (70.0) | 1085 (69.5) |
| Two ADLs | 151 (19.5) | 130 (16.5) | 281 (18.0) |
| Three or more ADLs | 90 (11.6) | 106 (13.5) | 196 (12.5) |
| Total | 776 (100) | 787 (100) | 1562 (100) |
| Incident ADL disability by type; N (%)a | |||
| ADL 1 (walking across a room) | 291 (24.5) | 291 (23.9) | 582 (24.2) |
| ADL 2 (bathing) | 274 (23.1) | 289 (23.8) | 563 (23.4) |
| ADL 3 (dressing) | 264 (22.3) | 262 (21.5) | 526 (21.9) |
| ADL 4 (transferring from chair or bed) | 255 (21.5) | 258 (21.2) | 513 (21.4) |
| ADL 5 (toileting) | 65 (5.5) | 82 (6.7) | 147 (6.1) |
| ADL 6 (eating) | 37 (3.1) | 34 (2.8) | 71 (3.0) |
| Total | 1186 (100) | 1216 (100) | 2402 (100) |
Notes: Incident ADL disability (at one time point) was observed to convert in 24% of cases to persistent physical disability through confirmation 6 months later of the same ADL disability. The order of ADL disability is from most common to the least. The same order is used in subsequent tables. ADL = Activities of daily living; CI = Confidence interval.
a N = number of participants with event; each participant could lose more than one ADL at the same time.
Persistent ADL Disability, Treatment With Aspirin and Subgroup Analysis
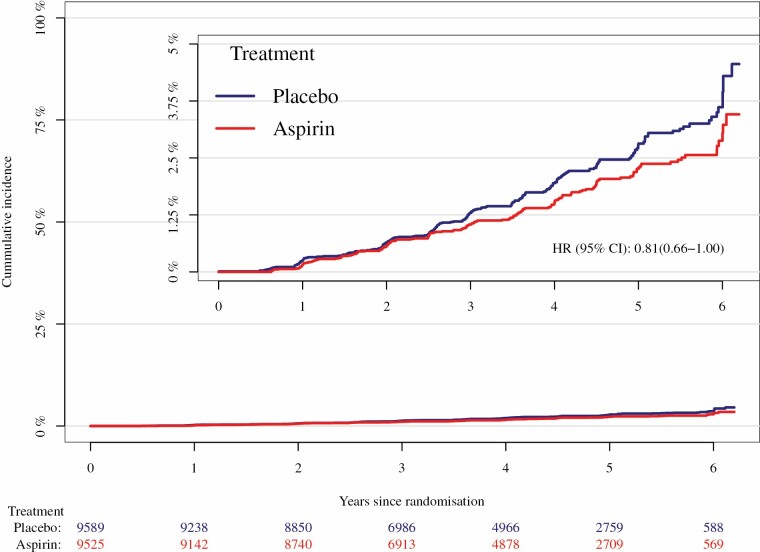
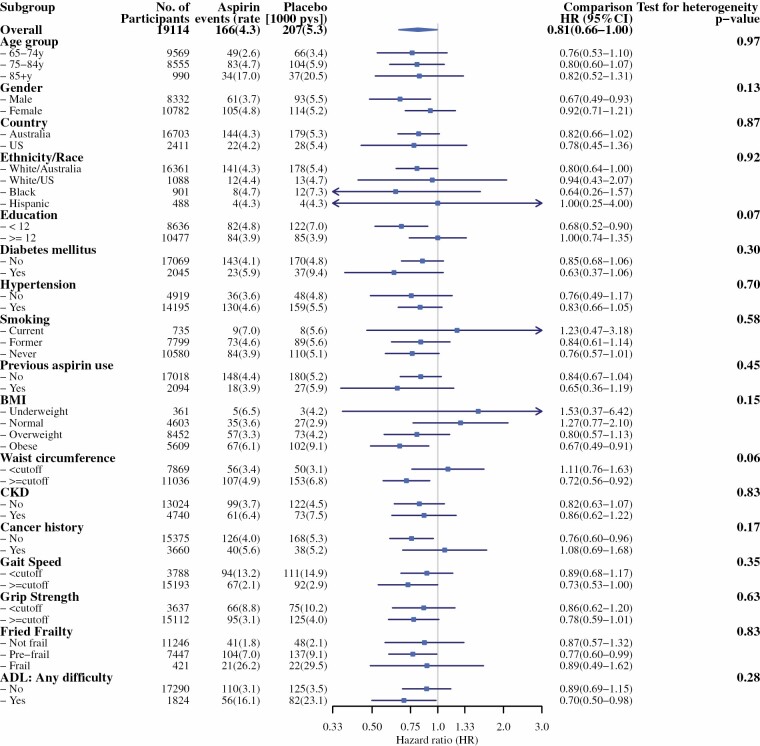
Of the 1563 participants with self-reported incident ADL disability, only 373 (24%) reported sustained or persistent ADL disability 6 months later (Table 2). Rates of persistent ADL disability are given in Table 2 and cumulative incidences are shown in Figure 1. Aspirin treatment appeared to reduce the risk of persistent ADL disability compared with placebo (4.3 vs 5.3 events/1000 person-years, respectively; hazard ratio [HR] = 0.81; 95% confidence interval [CI] 0.66–1.00). There were no significant interactions between treatment groups and any prespecified or non-prespecified subgroup for persistent ADL disability (Figure 2). Notably, one of the non-prespecified subgroups was having ADL limitations at baseline or not (Supplementary Table S2; Figure 2).
Table 2.
Persistent ADL Disability by Type and Treatment Group
| Aspirin (N = 9525) | Placebo (N = 9589) | Total (N = 19 114) | |
|---|---|---|---|
| Persistent physical disability, participants N (%) | |||
| No persistent ADL disability | 9337 (98.0) | 9365 (97.7) | 18 702 (97.8) |
| Persistent ADL disabilitya | 166 (1.7) | 207 (2.2) | 373 (2.0) |
| Rate/1000 py | Rate/1000 py | HR (95% CI) | |
| Persistent ADL disability | 4.3 | 5.3 | 0.81 (0.66–1.00) |
| Concurrent persistent ADL disabilitya | |||
| Single ADL | 111 (66.9) | 165 (79.7) | 276 (74.0) |
| Two ADLs | 34 (20.5) | 25 (12.1) | 59 (15.8) |
| Three or more ADLs | 21 (12.7) | 17 (8.2) | 38 (10.2) |
| Total | 166 (100) | 207 (100) | 373 (100) |
| Persistent ADL disability by type; N (%)a | |||
| ADL 1 (walking across a room) | 42 (16.3) | 42 (14.7) | 84 (15.5) |
| ADL 2 (bathing) | 87 (33.9) | 100 (35.0) | 187 (34.4) |
| ADL 3 (dressing) | 66 (25.7) | 79 (27.6) | 145 (26.7) |
| ADL 4 (transferring from chair or bed) | 48 (18.7) | 47 (16.4) | 95 (17.5) |
| ADL 5 (toileting) | 12 (4.7) | 14 (4.9) | 26 (4.8) |
| ADL 6 (eating) | 2 (0.8) | 4 (1.4) | 6 (1.1) |
| Total | 257 (100) | 286 (100) | 543 (100) |
Notes: ADL disability is defined as “a lot of difficulty,” “unable to perform” or needing assistance with the ADL. Data apply to the first occasion of any ADL disability that was subsequently confirmed as persistent. ADL = Activities of daily living; CI = Confidence interval.
a N = number of participants with event; each participant could lose more than one ADL at the same time.
Figure 1.
Cumulative incidence of persistent activities of daily living disability, by treatment group
Figure 2.
Forest plot of aspirin effect on persistent activities of daily living disability in prespecified and non-prespecified subgroups.
For participants with persistent ADL disability, this was predominantly a single ADL (74%) with 16% of participants reporting a lot of difficulty (or unable to perform) 2 ADLs and 10% 3 or more ADLs (Table 2). The most common ADLs contributing to persistent disability were bathing (33%) and dressing (27%) with transferring and walking disability occurring in less than 20% of cases (Table 2). Disability in toileting (5%) or eating (1%) was much less common (Table 2). Aspirin treatment did not affect the types of ADL disability that persisted for 6 months (Table 2). Compared to the placebo group, more participants in the aspirin group reported multiple concomitant persistent ADL disability (20% vs 33%, respectively; Table 2).
Where possible, attempts were made to determine whether any incident ADL disability that occurred in the 6 months prior to June 12, 2017, when the study intervention was stopped (9), were persistent after 6 months. A sensitivity analysis showed inclusion of these late confirmed ADL disabilities had little effect on the results reported above.
In a further exploratory analysis, persistent ADL disability was defined as any ADL disability that persisted for 6 months rather than only the same ADL. This resulted in an additional 110 participants with persistent ADL disability. Similar to aspirin’s effect with the stricter definition of persistence, there were fewer events in the aspirin group compared with the placebo group (229 [47%] vs 254 [53%], respectively).
ASPREE Clinical Events Preceding Incident or Persistent ADL Disability
Prior to incident ADL disability, there were 261 participants (17%) who experienced an adjudicated ASPREE endpoint including MI, heart failure, stroke, dementia, cancer, or clinically significant bleeding (Table 3). Of these participants, 89% experienced 1 event, 11% experienced 2 events, and 1 participant had 3 events. There was no difference between aspirin and placebo groups (Table 3). The most common ASPREE endpoint preceding incident ADL disability was cancer (45%), followed by CVD (31% comprised MI, stroke, and heart failure), dementia (20%), and clinically significant bleeding (17%).
Table 3.
ASPREE Adjudicated Endpoint Events (cancer, myocardial infarction, stroke, heart failure, clinically significant bleeding, and dementia) or Hospitalizations That Preceded Incident ADL Disability or Persistent ADL Disability, by Treatment Group
| Incident ADL Disability | |||
|---|---|---|---|
| Number With Preceding ASPREE Eventsa; N | Aspirin 776 | Placebo 787 | Total 1563 |
| No preceding events | 643 (82.9%) | 659 (83.7%) | 1302 (83.3%) |
| One or more | 133 (17.1%) | 128 (16.3%) | 261 (16.7%) |
| -1 | 115 (86.5%) | 115 (89.8%) | 230 (88.1%) |
| -2 or more | 18 (13.5%) | 13 (10.2%) | 31 (11.9%) |
| ASPREE event by typeb; N | 133 | 128 | 261 |
| Cancer | 61 (45.9%) | 57 (44.5%) | 118 (45.2%) |
| Myocardial infarction | 14 (10.5%) | 10 (7.8%) | 24 (9.2%) |
| Stroke | 20 (15.0%) | 19 (14.8%) | 39 (14.9%) |
| Heart failure | 8 (6.0%) | 10 (7.8%) | 18 (6.9%) |
| Clinically significant bleeding | 19 (14.3%) | 24 (18.8%) | 43 (16.5%) |
| Dementia | 30 (22.6%) | 22 (17.2%) | 52 (19.9%) |
| Any hospitalizations in previous 6 months | 179 (23.1%) | 183 (23.3%) | 362 (23.2%) |
| Persistent ADL disability | |||
| Number with preceding ASPREE eventsa; N | Aspirin 166 | Placebo 207 | Total 373 |
| No preceding events | 117 (70.5%) | 160 (77.3%) | 277 (74.3%) |
| One or more | 49 (29.5%) | 47 (22.7%) | 96 (25.7%) |
| 1 | 42 (85.7%) | 40 (85.1%) | 82 (85.4%) |
| 2 or more | 7 (14.3%) | 7 (14.9%) | 14 (14.6%) |
| ASPREE event by typeb; N | 49 | 47 | 96 |
| Cancer | 16 (32.7%) | 14 (29.8%) | 30 (31.3%) |
| Myocardial infarction | 5 (10.2%) | 8 (17.0%) | 13 (13.5%) |
| Stroke | 7 (14.3%) | 10 (21.3%) | 17 (17.7%) |
| Heart failure | 6 (12.2%) | 2 (4.3%) | 8 (8.3%) |
| Clinically significant bleeding | 7 (14.3%) | 10 (21.3%) | 17 (17.7%) |
| Dementia | 15 (30.6%) | 12 (25.5%) | 27 (28.1%) |
| Any hospitalizations in previous 6 months | 31 (18.7%) | 40 (19.3%) | 71 (19.0%) |
Note: N = number of participants; (%) = proportion of participants or proportion of events in participants. ADL = Activities of daily living.
aParticipants could have more than 1 preceding ASPREE adjudicated endpoint event, therefore (%) will not add to 100%. Stroke includes ischemic and hemorrhagic. bCancer, myocardial infarction, stroke, heart failure, dementia or major hemorrhage were adjudicated events in ASPREE. Hospitalizations for reasons other than ASPREE adjudicated events were reported from the 6 months prior to incident or persistent ADL disability.
Most participants with persistent ADL disability did not have a preceding ASPREE endpoint, though the proportion with a preceding event was higher (96 of 373; 26%) than prior to incident disability (Table 3). The majority (85%) of participants with an ASPREE endpoint prior to persistent ADL confirmation had one event and 15% experienced more than one event (Table 3). Prior to persistent ADL disability, 30% in the aspirin group and 23% in the placebo group experienced an ASPREE event (Table 3). Cardiovascular disease (40%), followed by cancer (31%) and dementia (28%) were the most common preceding events, with a similar event profile in the aspirin and placebo groups (Table 3).
In the 6 months prior to incident ADL disability, 23% of participants were hospitalized (for reasons other than adjudicated ASPREE endpoints): The corresponding proportion for persistent ADL disability was 19% (Table 3). The majority of participants in each case (86%–92%) reported only 1 hospital stay (Supplementary Table S3). The same proportion of participants in the aspirin group and placebo group were admitted to hospital for reasons other than ASPREE endpoints (Table 3). There was a similar time since hospitalization before reaching an ADL disability in the aspirin and placebo groups (Supplementary Table S3).
Deaths After Persistent ADL Disability
Within the period of the ASPREE trial, 36 participants died following ADL disability. Of these, 24 were in the aspirin group and 12 in the placebo group (HR = 2.58; 95% CI 1.29–5.16). This higher number of deaths in the aspirin group was across all causes of death except those attributed to CVD causes (Supplementary Table S4). In a post hoc analysis, and based on an illness death model (19), the restricted mean survival times were calculated for participants in the aspirin and placebo groups (Supplementary Table S5). The average disability-free time from randomization to persistent ADL disability was lower by 4.5–8 days across the ASPREE population of 19 114 participants (Supplementary Table S5). The restricted mean survival times for those with persistent ADL disability to death were shorter in the aspirin group compared with placebo by 76, 132, and 164 days at 5, 6, and 6.5 years, respectively (Supplementary Table S5).
Discussion
The primary results of the ASPREE trial found that low-dose aspirin had no significant effect on dementia and disability-free survival (9) in older community-dwelling people free of known CVD or other 5-year life-limiting illness (7,8). Nor did aspirin significantly affect the ASPREE composite physical disability endpoint (9). This study extends those initial findings, investigating the effect of aspirin on incident and persistent ADL disability and on subgroups of potential relevance to the risk of persistent disability. Here, we report that incident ADL disability occurred at 20.6 events/1000 person-years. Notably, the majority of participants did not report ADL disability over the median 4.7 years of the trial and there was a low rate of conversion from incident to persistent ADL disability (24%). Low-dose aspirin did not affect incident ADL disability but appeared to reduce persistent ADL disability (by 19%). This could not be accounted for by fewer preceding CVD events or other major ASPREE clinical endpoints, or fewer hospitalizations. On the other hand, persistent ADL disability was followed by twice as many deaths in the aspirin group compared with the placebo group.
Overall, the proportion of participants who progressed from incident to persistent ADL disability was low. This suggests that much of the incident ADL disability was transient, possibly due to joint replacement or other recoverable illness or injury, since many neurological, circulatory, sensory, or arthritic conditions are likely to persist. The low conversion to persistence also suggests that incident ADL disability may not be the best surrogate for permanent physical disability.
There were nearly double the number of cancer endpoints than CVD or dementia events preceding incident ADL disability. For persistent ADL disability, however, the preceding numbers were similar for cancer, CVD, and dementia endpoints. Perhaps, this difference reflects recovery from cancer treatment in some participants in the intervening 6 months between incident and persistent ADL loss.
Hospitalization-acquired disability has previously been reported as a leading cause of loss of independence in older persons (22,23), yet less than a quarter of ASPREE participants with ADL disability were hospitalized in the 6 months before the reported disability. In fact, less than half of all reported ADL disability, whether incident or persistent, was preceded by hospitalization or an ASPREE endpoint. Presumably most ADL cases were precipitated by nonhospitalizing events such as falls or chronic conditions (eg, respiratory or musculoskeletal) that were not captured by our endpoint process.
What could be the explanation for aspirin reducing persistent ADL disability? The answer does not appear in the preceding endpoints or hospitalizations because they were no less common in the aspirin-treated group. Indeed, preceding CVD events occurred in only a small proportion of those who developed persistent ADL disability (38 events in 373 participants) with numbers similar in the aspirin and placebo groups. Could there be a common etiology behind this aspirin effect to delay the progress of other chronic illnesses? Inflammation is a factor underlying chronic diseases, influencing weakness and fatigue (5,6). It is plausible that any beneficial effect of aspirin extending time to persistent disability could be through suppressing chronic inflammation, although 100 mg daily aspirin dose is yet to be shown sufficient by such a mechanism in healthy older persons.
At baseline, the aspirin and placebo groups were well balanced with respect to all subgroups shown in Figure 2, including mild physical disability categories (“a little” or “some” ADL difficulty). Aspirin’s treatment effect was not different across subgroups. As originally described by Katz (2) and many other studies since (eg, (22–25)), some ADL disabilities were more common than others. Walking, bathing, dressing, and transferring were similarly responsible (~21%–24%) for incident ADL disability with very few participants losing the ability to toilet (6%) or eat (3%) independently. By contrast, bathing (34%) and dressing (27%) were more common as persistent ADLs. These are physically complex tasks for which assistance may be needed but the participant could still live mostly independently and attend study visits to complete ADL assessments. Aspirin did not affect the ADL disability by type, whether incident or persistent.
The selection criteria for inclusion in ASPREE ensured that participants had generally lower age-matched levels of major disease and disability than the general populations of the United States and Australia (8). In this cohort, the incidence of ADL disability (20.6/1000 person-years) was lower than in the Cardiovascular Health Study (27/1000 person-years) (24). Other cohorts such as the Rush Memory and Aging Study reported a higher incidence of ADL disability (~30% over ~3.5 years follow-up in those who survived this period), though many in that study would not have met the CVD exclusion criteria of ASPREE, so may have been at higher risk of incident disability (25).
The risk of death following persistent ADL disability was low (0.5 and 0.9/1000 person-years for placebo and aspirin, respectively). Nevertheless, the risk was nearly twice as high in the aspirin group as in the placebo group and was not associated with any single cause of death. This higher risk of death is consistent with the overall ASPREE findings which showed excess mortality risk with aspirin compared to placebo (17). A possible factor contributing to this aspirin-related effect is the observation that higher numbers of participants in the aspirin group reported multiple ADL loss, which may reflect more extensive persistent physical disability.
Strengths of the ASPREE study include the size of the trial, particularly the large number of participants older than 70 years who began the study being independent with all ADLs, the low loss to follow up and confirmation of all ASPREE events with medical documentation and expert adjudication panels (8,9,15–17). Furthermore, the ADL questions were asked every 6 months alternately in person and by phone, maximizing the numbers of participants for whom there was regular ascertainment of ADLs throughout the trial.
Study limitations included the low number of persistent ADL disability cases, partly reflecting the overall health of the trial cohort at study enrollment (8), but also the limited length of the trial (median 4.7 years) (9). Even though many adverse health events were captured as ASPREE endpoints, morbidities such as musculoskeletal disorders and chronic respiratory conditions were not collected. What events may underlie the ADL disability cases, not preceded by ASPREE endpoints or hospitalizations, may be revealed by further analysis of self-reported health events, intervening illnesses or injuries (that have been shown to be associated with severe disability), reasons for hospitalizations, sociodemographic factors, mental health, and medication use (22,23,26–28). A further limitation is the exploratory, post hoc nature of these analyses resulting in the need for cautious interpretation.
Conclusion
Of the participants in the ASPREE clinical trial reporting incident ADL disability, only 24% progressed to persistent loss of the same ADL. Aspirin, at a dose of 100 mg/day for a median of 4.7 years, did not reduce the rate of incident ADL disability compared with the placebo group but there was some evidence of a reduction in persistent physical disability. Of the small number of participants who died after persistent ADL disability, but within the timeframe of the trial, death was hastened in the aspirin group.
Funding
The work was supported by the National Institute on Aging and the National Cancer Institute at the National Institutes of Health (U01AG029824); the National Health and Medical Research Council (NHMRC) of Australia (334047 and 1127060); Monash University and the Victorian Cancer Agency. J.R. is funded by a NHMRC Dementia Research Leader Fellowship (APP1135727). C.M.R. is supported through a NHMRC Principal Research Fellowship (APP1136372).
Supplementary Material
Acknowledgments
The authors acknowledge the dedicated and skilled staff in Australia and the United States for the conduct of the trial. The authors are also most grateful to the ASPREE participants, who so willingly volunteered for this study, and the general practitioners and medical clinics who supported the participants in the ASPREE study.
ASPREE Investigator Group listed on www.aspree.org
Conflict of Interest
A.M.M. reports receiving consulting and travel fees from Bayer, AG to present ASPREE primary results after their publication, and consulting fees from Alkahest, Inc. and reports grants from National Institute on Aging. M.R.N. reports receiving consulting and travel fees from Bayer, AG. R.L.W., S.E., L.T.P.T., M.E.E., J.R., R.W., R.C.S., S.A.W., E.S., C.M.R., J.E.L., S.G.O., R.E.T., S.M.F., N.P.S., J.D.W., J.J.M., and A.B.N. report no disclosures relevant to the manuscript. Bayer AG supplied study drug (aspirin) and matching placebo also produce BP lowering medication (no link with ASPREE’s participants) and had no other role in the trial. L.T.P.T. (PhD Biostat) completed the statistical analysis.
References
- 1. Guralnik JM, LaCroix AZ, Branch LG, Kasl SV, Wallace RB. Morbidity and disability in older persons in the years prior to death. Am J Public Health. 1991;81:443–447. doi: 10.2105/ajph.81.4.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016 [DOI] [PubMed] [Google Scholar]
- 3. Andrawes WF, Bussy C, Belmin J. Prevention of cardiovascular events in elderly people. Drugs Aging. 2005;22:859–876. doi: 10.2165/00002512-200522100-00005 [DOI] [PubMed] [Google Scholar]
- 4. Stamm BJ, Burke JF, Lin CC, Price RJ, Skolarus LE. Disability in community-dwelling older adults: exploring the role of stroke and dementia. J Prim Care Community Health. 2019;10:2150132719852507. doi: 10.1177/2150132719852507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. ASPREE Investigator Group. Study design of ASPirin in Reducing Events in the Elderly (ASPREE): a randomized, controlled trial. Contemp Clin Trials. 2013;36:555–564. doi: 10.1016/j.cct.2013.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McNeil JJ, Woods RL, Nelson MR, et al. ; ASPREE Investigator Group . Baseline characteristics of participants in the ASPREE (ASPirin in Reducing Events in the Elderly) study. J Gerontol A Biol Sci Med Sci. 2017;72:1586–1593. doi: 10.1093/gerona/glw342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McNeil JJ, Woods RL, Nelson MR, et al. Effect of aspirin on disability-free survival in the healthy elderly. N Engl J Med. 2018;379:1499–1508. doi: 10.1056/NEJMoa1800722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. ASPREE Protocol. https://aspree.org/aus/wp-content/uploads/sites/2/2014/04/ASPREE-Protocol-AUS-Version-9-Nov-2014-Monash-approved.pdf
- 11. Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 12. Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–727. doi: 10.1111/j.1532-5415.1983.tb03391.x [DOI] [PubMed] [Google Scholar]
- 13. Cornoni-Huntley J, Lafferty ME, eds. Established populations for epidemiologic studies of the elderly: Resource data book. Chapter 3. National Institute on Aging, 1986: 56–57. [Google Scholar]
- 14. Pahor M, Blair SN, Espeland M, et al. Effects of a physical activity intervention on measures of physical performance: results of the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) Study. J Gerontol A Biol Sci Med Sci. 2006;61:1157–1165. 10.1093/gerona/61.11.1157 [DOI] [PubMed] [Google Scholar]
- 15. Margolis KL, Mahady SE, Nelson MR, et al. Development of a standardized definition for clinically significant bleeding in the ASPirin in Reducing Events in the Elderly (ASPREE) trial. Contemp Clin Trials Commun. 2018;11:30–36. doi: 10.1016/j.conctc.2018.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McNeil JJ, Wolfe R, Woods RL, et al. ; ASPREE Investigator Group . Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509–1518. doi: 10.1056/NEJMoa1805819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519–1528. 10.1056/NEJMoa1803955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Statist. Med. 2007;26:2389–2430. 10.1002/sim.2712 [DOI] [PubMed] [Google Scholar]
- 19. Wolfe R, Murray AM, Woods RL, et al. The aspirin in reducing events in the elderly trial: Statistical analysis plan. Int J Stroke. 2018;13:335–338. doi: 10.1177/1747493017741383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 21. Royston P, Parmar MK. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol. 2013;13:152. doi: 10.1186/1471-2288-13-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gill TM, Allore HG, Gahbauer EA, Murphy TE. Change in disability after hospitalization or restricted activity in older persons. JAMA. 2010;304:1919–1928. doi: 10.1001/jama.2010.1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reichardt LA, Aarden JJ, van Seben R, et al. ; Hospital-ADL study group . Unravelling the potential mechanisms behind hospitalization-associated disability in older patients; the Hospital-Associated Disability and impact on daily Life (Hospital-ADL) cohort study protocol. BMC Geriatr. 2016;16:59. doi: 10.1186/s12877-016-0232-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jacob ME, Marron MM, Boudreau RM, Odden MC, Arnold AM, Newman AB. Age, race, and gender factors in incident disability. J Gerontol A Biol Sci Med Sci. 2018;73:194–197. doi: 10.1093/gerona/glx194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shah RC, Buchman AS, Leurgans S, Boyle PA, Bennett DA. Association of total daily physical activity with disability in community-dwelling older persons: a prospective cohort study. BMC Geriatr. 2012;12:63. doi: 10.1186/1471-2318-12-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Farias ST, Park LQ, Harvey DJ, et al. Everyday cognition in older adults: associations with neuropsychological performance and structural brain imaging. J Int Neuropsychol Soc. 2013;19:430–441. doi: 10.1017/S1355617712001609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stamm TA, Pieber K, Crevenna R, Dorner TE. Impairment in the activities of daily living in older adults with and without osteoporosis, osteoarthritis and chronic back pain: a secondary analysis of population-based health survey data. BMC Musculoskelet Disord. 2016;17:139. doi: 10.1186/s12891-016-0994-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gill TM, Han L, Gahbauer EA, Leo-Summers L, Murphy TE. Risk factors and precipitants of severe disability among community-living older persons. JAMA Netw Open. 2020;3:e206021. doi: 10.1001/jamanetworkopen.2020.6021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.