Structured Abstract
Background:
Heart failure-related cardiogenic shock (HF-CS) accounts for an increasing proportion of CS cases in contemporary cardiac intensive care units (CICUs). Whether HF disease chronicity identifies distinct clinical profiles of HF-CS is unknown.
Methods and Results:
We evaluated CICU admissions for HF-CS from 28 centers using data from the Critical Care Cardiology Trials Network (CCCTN) registry (2017-2020). HF-CS was defined as CS due to ventricular failure in the absence of AMI and was classified as "de novo" vs. "acute-on-chronic" based on the absence or presence of a prior diagnosis of HF, respectively. Clinical features, resource utilization, and outcomes were compared between groups. Among 1,405 HF-CS admissions, 370 had de novo HF-CS (26.3%) and 1,035 had acute-on-chronic HF-CS (73.7%). Patients with de novo HF-CS had a lower prevalence of hypertension, diabetes, coronary artery disease, atrial fibrillation, and chronic kidney disease (all p<0.01). Median SOFA scores were higher in those with de novo HF-CS (8; 25th-75th: 5–11) vs. acute-on-chronic HF-CS (6; 25th-75th: 4–9, p<0.01), as was the proportion of SCAI shock stage E (46.1% vs. 26.1%, p<0.01). After adjustment for clinical covariates and preceding cardiac arrest, risk of in-hospital mortality was higher in de novo HF-CS as compared to acute-on-chronic HF-CS (adjusted hazard ratio [HR] 1.36, 95% confidence interval [CI]: 1.05 to 1.75, p = 0.02).
Conclusions:
Despite having fewer comorbidities, patients with de novo HF-CS had more severe shock presentations and worse in-hospital outcomes. Whether HF disease chronicity is associated with time-dependent compensatory adaptations, unique pathobiological features, and responses to treatment in patients presenting with HF-CS warrants further investigation.
Keywords: heart failure, cardiogenic shock, critical care cardiology
Lay Summary:
Inadequate blood flow to vital organs related to a failing heart is now caused by something other than a new heart attack in the majority of cases (67%). One out of every 4 of these patients have no prior history of heart failure. Such patients with new heart failure and shock have more severe shock presentations, greater organ injury, and higher rates of death as compared to those with a history of heart failure. These findings underscore the need to understand possible compensatory adaptations in patients with longstanding heart failure.
Proposed Tweet:
New data from #CCCTN now out in @JCardFail - @ankeetbhatt @ddbergMD and colleagues find 1 out of 4 patients with HF-related CS had no prior HF hx. De novo HF-CS more severe, with higher mortality vs. acute-on-chronic HF-CS, despite fewer comorbidities. @ShashankSinhaMD @JasonKatzMD @TIMIStudyGroup
Introduction:
Cardiogenic shock (CS) is characterized by life-threatening end-organ hypoperfusion resulting from a low cardiac output state.1 Although clinical trials and observational evaluations in CS have historically focused on acute myocardial infarction-related CS (AMI-CS), more recent epidemiological evidence suggests that AMI-CS now accounts for the minority of CS cases in contemporary cardiac intensive care units (CICUs).2,3 The spectrum of CS related to causes other than AMI is diverse but is most commonly due to myocardial dysfunction.2,4,5 This group has been variably defined as heart failure-related CS (HF-CS).6,7 The shifting epidemiology of CS, specifically the increasing proportion of HF-CS, is likely a function of declining prevalence and improved acute management of acute coronary syndromes (ACS). Furthermore, the rise of chronic comorbidities and treatments to reduce sudden cardiac death may predispose to HF.8,9
Despite a greater burden of non-cardiovascular comorbidities (e.g., chronic kidney disease, pulmonary disease, and liver disease) compared to patients with AMI-CS, patients with HF-CS tend to have lower in-hospital mortality and less mechanical circulatory support (MCS) use.4,10,11 One potential explanation for these observations is that acute hemodynamic disturbances are more poorly tolerated in patients with acute myocardial dysfunction (e.g., AMI-CS) than they are in patients with chronic HF states. Whether HF disease chronicity identifies distinct clinical profiles among those without AMI-CS that might warrant different management strategies is unknown. Therefore, we aimed to compare the clinical features and outcomes of patients admitted to the CICU with HF-CS according to the absence (de novo) or presence (acute-on-chronic) of a prior history of HF.
Methods
Study Population
The Critical Care Cardiology Trials Network (CCCTN) is a collaborative research network of American Heart Association Level 1 CICUs12 located in North America coordinated by the Thrombolysis in Myocardial Infarction (TIMI) Study Group (Brigham and Women's Hospital, Boston, MA).13 In this data analysis from 2017 to 2020, each participating center (n=28) contributed an annual 2-month snapshot of all consecutive medical admissions to the CICU; all consecutive admissions were recorded, but the months of capture could vary by year and by site. The CCCTN Registry protocol and waiver of informed consent were approved by the Institutional Review Board at Mass General Brigham and at each participating center. De-identified clinical data are recorded through comprehensive clinical review into electronic centralized case report forms.13
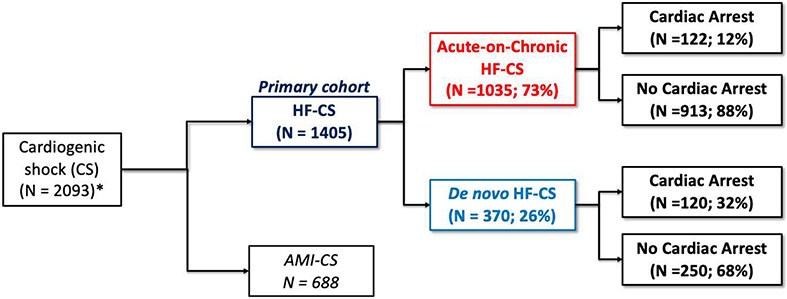
We included patients with CS, defined by sustained hemodynamic impairment (systolic blood pressure <90 mm Hg for ≥30 minutes or the need for inotropic or vasopressor support to maintain blood pressure) and evidence of end-organ hypoperfusion (altered mental status, oliguria, acute kidney injury, hepatic injury, or elevated serum lactate [>2 mmol/L]) due to a low cardiac output state. When pulmonary artery catheter data were available, cardiac index thresholds of 1.8 and 2.2 L/min/m2 were used for those without and with inotropic support, respectively, along with elevated filling pressures to define CS. When pulmonary artery catheter data were not available, assessment of CS was based on clinical features.2 Patients with CS from causes not due to primary ventricular failure (e.g., severe valvular disease, arrhythmia, post-cardiotomy, tamponade) were excluded.14 Included admissions were stratified into AMI-CS or HF-CS. HF-CS was further classified as having "de novo" vs. "acute-on-chronic" presentations based on the absence or presence, respectively, of a prior diagnosis of HF, inclusive of both reduced and preserved ejection fraction (Figure 1).
Figure 1(Take Home Figure): Study Population by CS Classification.
Of patients with cardiogenic shock (n=2,093) identified at contemporary North American CICUs, 1,405 (67%) had heart failure related cardiogenic shock (HF-CS), defined as cardiogenic shock in the absence of acute myocardial infarction. Approximately 1 in 4 patients with HF-CS did not have a prior history of HF (de novo HF-CS). A greater proportion of patients with de novo HF-CS had preceding cardiac arrest as compared to those with acute-on-chronic HF-CS.
* Excludes post-cardiotomy shock and shock primarily due to severe valvular disease, arrhythmia, or tamponade
Statistical Analysis
Baseline patient characteristics, presenting clinical features, and CICU resource utilization were summarized according to CS type (de novo HF-CS vs. acute-on-chronic HF-CS). All group comparisons were made between those with de novo HF-CS and acute-on-chronic HF-CS; demographic and resource utilization data for AMI-CS were included for reference. Categorical variables are reported as counts and percentages based on the number of patients with available data for each variable; continuous variables are reported as medians (25th-75th percentiles). The Pearson χ2 test was used to compare categorical variables and Wilcoxon rank-sum test was used to compare continuous variables.
Shock severity was assessed by Society of Cardiovascular Angiography and Intervention (SCAI) classification15 and the degree of end-organ injury by Sequential Organ Failure Assessment (SOFA) score.16 The proportion of patients managed with varying CICU resources including mechanical circulatory support (MCS) were compared; MCS included intra-aortic balloon pump counter-pulsation, Impella percutaneous ventricular assist systems (2.5, CP, 5.0, 5.5, RP), TandemHeart percutaneous ventricular assist systems, venoarterial extracorporeal membrane oxygenation (VA-ECMO), and surgically implanted, non-durable MCS devices (e.g., CentriMag™). Hemodynamic values were compared among patients who underwent placement of a pulmonary artery catheter as part of their routine clinical course. Given a disproportionate number of cardiac arrest cases among patients with de novo HF-CS, we performed sensitivity analyses excluding those with cardiac arrest preceding CICU admission.
In-hospital mortality was compared between groups using a Fine-Gray subdistribution model to account for the competing risk of hospital discharge (due to differences in hospital length of stay). The model was stratified by site and adjusted for potential confounders selected a priori, including age, sex, history of coronary artery disease, diabetes, hypertension, cerebrovascular disease, peripheral artery disease, chronic kidney disease, atrial fibrillation, pulmonary hypertension, history of ventricular arrhythmias, SOFA score, SCAI shock stage, serum lactate and cardiac arrest prior to CICU admission. A nominal α level of 0.05 was used to assess for statistical significance. All reported P values were 2-sided. All statistical computations were performed with SAS System V9.4 (SAS Institute Inc, Cary, NC).
Results
Epidemiology of heart failure-related cardiogenic shock
Overall, 2,093 admissions with CS were included. Of these admissions, 688 (32.9%) had AMI-CS. The analysis cohort of HF-CS included 1,405 (67.1%) admissions, 370 of which had de novo HF-CS (26.3%), while the remainder had acute-on-chronic HF-CS (Figure 1). Cardiac arrest preceding CICU admission occurred more frequently in patients with de novo HF-CS than in those with acute-on-chronic HF-CS (32.4% vs. 11.8%; p<0.01).
Baseline Characteristics
Baseline characteristics according to CS type are summarized in Table 1. Patients with HF-CS had a median age of 62 (25th-75th: 52-71) years, 33% were female, and 358 (25%) were Black. Among patients presenting with acute-on-chronic HF-CS, most (n=925, 91.0%) had pre-existing left ventricular systolic dysfunction (i.e., LVEF <50%). Patients with de novo HF-CS had fewer cardiovascular risk factors and comorbidities than those with acute-on-chronic HF-CS, including a lower prevalence of hypertension, diabetes mellitus, coronary artery disease, atrial fibrillation, pulmonary hypertension, and chronic kidney disease, among others (all p<0.01). Presenting LVEF was higher in patients with de novo HF-CS as compared to those with acute-on-chronic HF-CS (Table 1). Median time from hospital admission to CICU admission was 0.1 (25th-75th: 0.0-1.1) days in patients with de novo HF-CS and 0.3 (25th-75th: 0.0-3.2) days in those with acute-on-chronic HF-CS.
Table 1:
Baseline characteristics by CS Type
| n (%) unless otherwise noted |
De Novo HF-CS N=370 |
Acute-on-Chronic HF- CS N=1035 |
P-value* | AMI-CS N=688 |
|---|---|---|---|---|
| Demographics | ||||
| Age, median (25th-75th), y | 61.0 (50.0-70.0) | 62.0 (53.0-71.0) | 0.11 | 67.0 (58.0-76.0) |
| Female sex | 138 (37.3%) | 326 (31.5%) | 0.04 | 223 (32.4%) |
| Race/Ethnicity | 0.41 | |||
| White | 227 (61.4%) | 605 (58.5%) | 433 (62.9%) | |
| Black | 84 (22.7%) | 274 (26.5%) | 78 (11.3%) | |
| Other | 59 (15.9%) | 156 (15.1%) | 177 (25.7%) | |
| BMI, median (25th-75th), kg/m 2 | 27.8 (24.0-32.1) | 27.5 (23.4-32.1) | 0.76 | 27.8 (24.4-31.6) |
| Comorbidities | ||||
| Current Smoker | 66 (17.8%) | 115 (11.3%) | <0.01 | 158 (23.0%) |
| Diabetes mellitus | 102 (27.6%) | 388 (37.5%) | <0.01 | 311 (45.2%) |
| Hypertension | 181 (48.9%) | 573 (55.4%) | 0.03 | 474 (68.9%) |
| Coronary artery disease | 97 (26.2%) | 449 (43.4%) | <0.01 | 258 (37.5%) |
| Cerebrovascular disease | 22 (5.9%) | 111 (10.7%) | 0.01 | 63 (9.2%) |
| Peripheral artery disease | 22 (5.9%) | 88 (8.5%) | 0.12 | 71 (10.3%) |
| Active cancer | 35 (9.5%) | 60 (5.8%) | 0.02 | 44 (6.4%) |
| Atrial fibrillation | 64 (17.3%) | 444 (42.9%) | <0.01 | 80 (11.6%) |
| Ventricular arrhythmia | 14 (3.8%) | 184 (17.8%) | <0.01 | 19 (2.8%) |
| Severe valvular disease | 28 (7.6%) | 222 (21.4%) | <0.01 | 40 (5.8%) |
| Pulmonary hypertension | 29 (7.8%) | 131 (12.7%) | 0.01 | 11 (1.6%) |
| Congenital heart disease | 11 (3.0%) | 27 (2.6%) | 0.71 | 5 (0.7%) |
| Chronic kidney disease | 50 (13.5%) | 433 (41.8%) | <0.01 | 130 (18.9%) |
| On dialysis | 10 (20.0%) | 43 (9.9%) | 0.03 | 29 (22.3%) |
| Pulmonary disease | 47 (12.7%) | 181 (17.5%) | 0.03 | 75 (10.9%) |
| Liver disease | 5 (1.4%) | 49 (4.7%) | <0.01 | 9 (1.3%) |
| Shock Presentation | ||||
| Presentation LVEF | <0.01 | |||
| <20% | 145 (39.2%) | 518 (50.1%) | 152 (22.1%) | |
| 20-<30% | 70 (18.9%) | 285 (27.6%) | 189 (27.5%) | |
| 30-<40% | 44 (11.9%) | 88 (8.5%) | 118 (17.2%) | |
| 40-<50% | 24 (6.5%) | 28 (2.7%) | 84 (12.2%) | |
| ≥50% | 68 (18.4%) | 73 (7.1%) | 79 (11.5%) | |
| Unknown | 19 (5.1%) | 43 (4.2%) | 66 (9.6%) | |
P-value indicates the comparison between de novo HF-CS and acute-on-chronic HF-CS
LVEF = left ventricular ejection fraction; ED= emergency department; HF-CS = Heart failure associated cardiogenic shock; AMI-CS = acute myocardial infarction related cardiogenic shock
Shock Severity and End Organ Injury
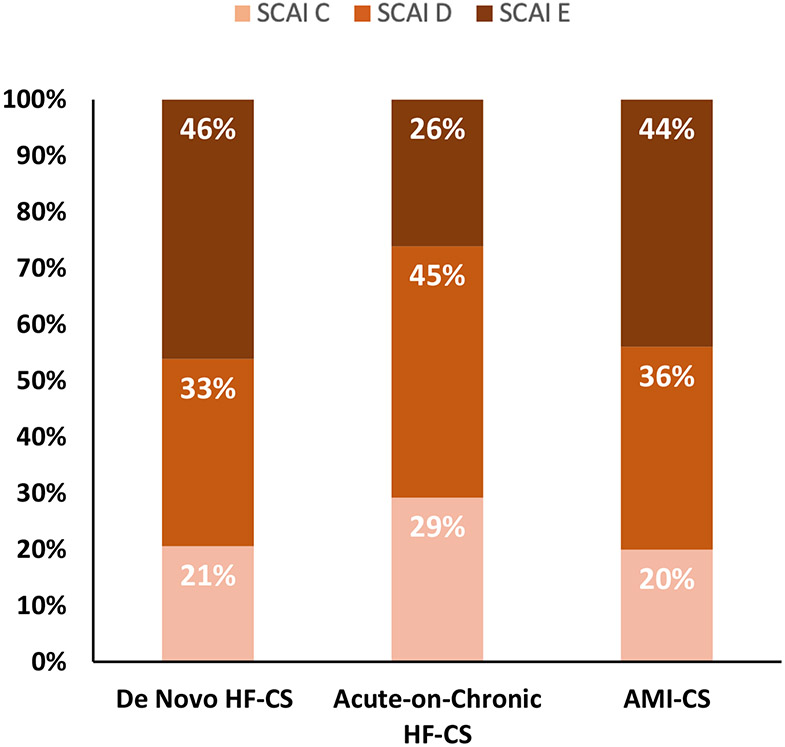
Despite lower comorbidity burden and higher presenting LVEF, patients with de novo HF-CS had more severe shock presentations, including a higher proportion of SCAI shock stage E (46.1% vs. 26.1%, p<0.01). SCAI shock severity classifications in those with de novo HF-CS, acute-on-chronic HF-CS, and AMI-CS are shown in Figure 2. End-organ dysfunction as assessed by SOFA score was also greater in those with de novo HF-CS (median, 8 [25th-75th: 5-11]) as compared to those with acute-on-chronic HF-CS (6 [4-9], p<0.01). Additional markers of end-organ injury, including elevations in alanine aminotransferase or aspartate aminotransferase ≥150 units/L and serum lactate ≥4 mmol/L, occurred more frequently in patients with de novo HF-CS as compared to those with acute-on-chronic HF-CS (Table 2). Greater shock severity and end-organ injury persisted in patients with de novo HF-CS after exclusion of admissions with preceding cardiac arrest (Supplementary Figure 1 and Supplementary Table 1)
Figure 2: Distribution of Society of Cardiovascular Angiography and Intervention (SCAI) shock severity by CS classification.
*Among patients with available data on SCAI shock stage
Table 2:
End-Organ Perfusion during CICU stay by CS Type
|
Median (25th-75th), unless
otherwise noted |
De Novo HF-CS N=370 |
Acute-on-Chronic HF-CS N=1035 |
P-value* | AMI-CS N=688 |
|---|---|---|---|---|
| Perfusion Markers | ||||
| Lactate, mmol/L | 3.9 (1.9-8.0) | 2.7 (1.7-4.8) | <0.01 | 4.2 (2.2-8.0) |
| Lactate≥4 mmol/L, n(%) | 164 (49.4%) | 284 (31.8%) | <0.01 | 312 (51.8%) |
| Arterial pH | 7.3 (7.2-7.4) | 7.4 (7.3-7.4) | <0.01 | 7.3 (7.2-7.4) |
| Total bilirubin, mg/dL | 1.2 (0.7-2.2) | 1.6 (0.9-2.8) | <0.01 | 1.0 (0.6-1.6) |
| Creatinine, mg/dL | 1.8 (1.2-2.9) | 2.1 (1.5-3.2) | <0.01 | 1.8 (1.3-3.2) |
| ALT, mg/dL# | 88.0 (40.0-425.0) | 53.0 (25.0-239.0) | <0.01 | 88.0 (45.0-327.0) |
| AST, mg/dL# | 135.0 (54.0-702.0) | 65.5 (33.0-269.0) | <0.01 | 229.0 (87.0-749.0) |
| ALT≥150 and/or AST≥150# n(%) | 104/213 (48.8%) | 222/627 (35.4%) | <0.01 | 255/403 (63.3%) |
| SOFA score≥8 | 191 (51.6%) | 423 (40.9%) | <0.01 | 382 (55.5%) |
P-value indicates the comparison between de novo HF-CS and acute-on-chronic HF-CS.
ALT = alanine aminotransferase; AST = aspartate aminotransferase; HF-CS = Heart failure associated cardiogenic shock; AMI-CS = acute myocardial infarction related cardiogenic shock; SOFA = Sequential Organ Failure Assessment
Data available for a subset of all patients.
Hemodynamics
A total of 598 (43%) HF-CS patients underwent invasive hemodynamic assessment with pulmonary artery (PA) catherization during CS presentation. Hemodynamic monitoring by PA catheterization occurred less frequently in patients with de novo HF-CS (43.2%) vs. acute-on-chronic HF-CS (55.4%, p <0.01), a pattern that was consistent among admissions without cardiac arrest (44.0% vs. 55.9%, p <0.01). In the selected sub-cohort of patients with available invasive hemodynamic data, cardiac indices (1.8 [1.5-2.5] L/min/m2 vs. 1.9 [1.5-2.3] L/min/m2; p=0.90) and cardiac power outputs were similar (0.6 [0.5-0.9] W/m2 vs. 0.6 [0.5-0.8] W/m2; p=0.87) between patients with de novo HF-CS vs acute-on-chronic HF-CS. Pulmonary capillary wedge pressures were also similar between groups (23.5 [17.5-29.0] mmHg vs. 24.0 [19.0-30.0] mmHg, p = 0.11). Despite equivalent elevations in right atrial pressure (15.0 [10.0-20.0] mmHg vs. 15.0 [10.0-20.0]; p=0.67), patients with de novo HF-CS vs. acute-on-chronic HF-CS had lower overall mean pulmonary arterial pressures (31.0 [24.7-38.3] mmHg vs. 35.0 [27.7-41.3] mmHg; p<0.01), and median pulmonary arterial pulsatility indices [PAPi] of 1.5 [0.8-2.5] vs. 1.7 [1.1-2.6], respectively (p = 0.13; Table 3). Hemodynamic findings were similar in those without preceding cardiac arrest (Supplementary Table 2).
Table 3:
Invasive hemodynamics by CS Type
| Median (25th-75th), unless otherwise noted | n | De Novo HF-CS | n | Acute-on-Chronic HF- CS |
P-value* | n | AMI-CS |
|---|---|---|---|---|---|---|---|
| Right Atrial Pressure (mmHg) | 117 | 15.0 (10.0-20.0) | 443 | 15.0 (10.0-20.0) | 0.67 | 234 | 13.0 (9.0-17.0) |
| PA Systolic Pressure (mmHg) | 119 | 44.0 (36.0-54.0) | 448 | 51.0 (41.0-61.0) | <0.01 | 243 | 42.0 (34.0-51.0) |
| PA Diastolic Pressure (mmHg) | 119 | 23.0 (18.0-30.0) | 448 | 26.0 (20.0-32.0) | 0.01 | 244 | 21.0 (17.0-27.0) |
| Mean PA Pressure (mmHg) | 119 | 31.0 (24.7-38.3) | 448 | 35.0 (27.7-41.3) | <0.01 | 243 | 28.3 (22.7-35.0) |
| PA Pulsatility Index (PAPi) | 115 | 1.5 (0.8-2.5) | 432 | 1.7 (1.1-2.6) | 0.13 | 229 | 1.6 (1.0-2.4) |
| Pulmonary Capillary Wedge Pressure (mmHg) | 104 | 23.5 (17.5-29.0) | 392 | 24.0 (19.0-30.0) | 0.11 | 198 | 20.0 (15.0-27.0) |
| Mean Arterial Pressure (MAP, mmHg) | 126 | 75.5 (68.0-86.0) | 464 | 76.0 (68.0-85.0) | 0.77 | 240 | 72.0 (62.0-84.0) |
| Cardiac index, L/min/m2 | 103 | 1.8 (1.5-2.5) | 353 | 1.9 (1.5-2.3) | 0.90 | 215 | 2.1 (1.7-2.8) |
| Cardiac power output, W/m2 | 102 | 0.6 (0.5-0.9) | 348 | 0.6 (0.5-0.8) | 0.87 | 200 | 0.7 (0.5-0.9) |
| Systemic Vascular Resistance (dynes/sec/cm −5) | 95 | 1401.5 (850.0-1876.7) | 336 | 1276.1 (977.7-1688.2) | 0.20 | 192 | 1110.0 (788.8-1472.5) |
| Pulmonary Vascular Resistance (dynes/sec/cm−5) | 85 | 152.1 (74.4-237.0) | 301 | 190.5 (101.8-296.0) | <0.01 | 176 | 139.7 (61.8-220.5) |
P-value indicates the comparison between de novo HF-CS and acute-on-chronic HF-CS.
PA= pulmonary artery; W= watts; HF-CS = Heart failure associated cardiogenic shock; AMI-CS = acute myocardial infarction related cardiogenic shock
Intensive Care Unit Resource Utilization
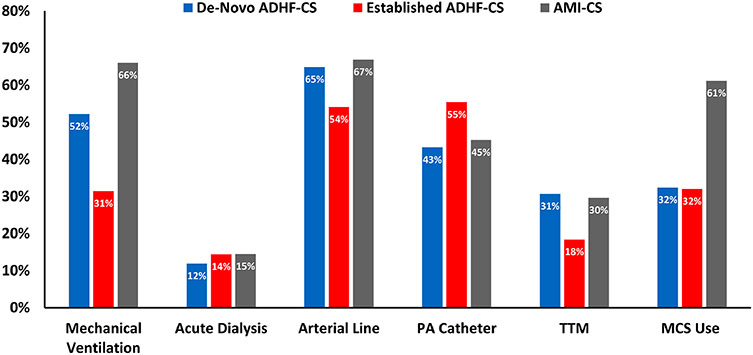
Resource utilization across HF-CS groups is shown in Figure 3. The use of mechanical ventilation (52.5% vs. 31.4%, p<0.01) was significantly higher in patients with de novo HF-CS vs. acute-on-chronic HF-CS. These differences were attenuated but remained present (33.6% vs. 25.1%, p<0.01) after exclusion of patients with preceding cardiac arrest (Supplementary Table 3). A median of 2 (25th-75th: 1-2) vasoactive therapies were used in patients with de novo HF-CS vs. 1 (25th-75th: 1-2) in those with acute-on-chronic HF-CS. Despite greater shock severity and higher SOFA scores, overall MCS use was similar between groups (32.4% vs. 32.0%, p=0.87) and much lower than MCS use in AMI-CS (61%). IABP was the predominant form of MCS (47% of all MCS for both de novo HF-CS and acute-on-chronic HF-CS), though the proportion of patients managed with VA-ECMO was higher among those with de novo HF-CS (21.7% vs. 8.2%) (Supplementary Table 4). Overall, most patients were managed with only a single MCS device during their hospitalization. Of 120 patients managed with MCS in the de novo HF-CS group, 24 (20%) had >1 temporary MCS device, whether concurrently or in series. Of 331 patients with MCS use in the acute-on-chronic HF-CS group,119 (35.9%) had >1 MCS device. Heart transplantation during hospitalization was less common in patients with de novo HF-CS (n=3, 0.8%) vs. acute-on-chronic HF-CS (n=62, 6.0%; p<0.01).
Figure 3: Intensive Care Unit Resource utilization by CS category.
CVC = central venous catheter; PA = pulmonary artery, TTM = targeted temperature management; MCS = mechanical circulatory support.
Mechanical circulatory support included intra-aortic balloon pump counter-pulsation, Impella percutaneous ventricular assist systems (2.5, CP, 5.0), TandemHeart percutaneous ventricular assist systems, venoarterial extracorporeal membrane oxygenation (VA-ECMO), and surgically implanted, non-durable MCS devices (e.g., CentriMag).
In-Hospital Outcomes
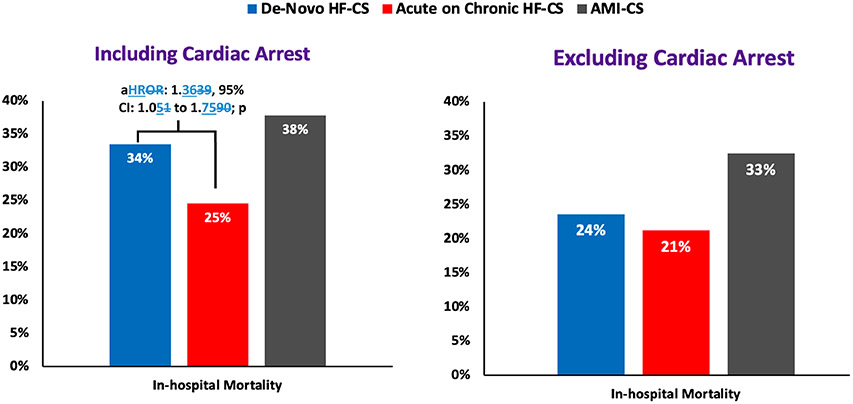
Full details of patient discharge disposition by type of CS are reported in Supplementary Table 5. In-hospital death was higher in patients with de novo HF-CS as compared to those with acute-on-chronic HF-CS (33.5% vs. 24.5%, p <0.01). Median time from CICU admission to death was longer in those with acute-on-chronic HF-CS (4.2 [25th-75th: 1.9-9.9] days) as compared to those with de novo HF-CS (2.4 [25th-75th: 1.3-8.0] days). After excluding patients with cardiac arrest prior to CICU admission, of whom approximately half died during their hospital course (de novo HF-CS: 54% vs. acute-on-chronic HF-CS: 49%), differences in in-hospital death persisted but were attenuated (Figure 4). After adjustment for clinical covariates and preceding cardiac arrest, risk of in-hospital mortality was higher in de novo HF-CS as compared to acute-on-chronic HF-CS (adjusted hazard ratio [HR] 1.36, 95% confidence interval [CI]: 1.05 to 1.75, p = 0.02).
Figure 4: In-Hospital Mortality by CS category, inclusive and exclusive of cardiac arrest.
Adjusted for age, sex, history of coronary artery disease, diabetes, hypertension, cerebrovascular disease, peripheral vascular disease, chronic kidney disease, atrial fibrillation, pulmonary hypertension, history of ventricular arrhythmias, and cardiac arrest prior to CICU admission
Discussion
In this analysis of over 2,000 CICU admissions with CS in North America, only 1 in 3 patients had AMI-CS. Approximately 1 in 4 patients presenting with HF-CS did not have a prior history of HF. Patients with de novo HF-CS had more severe shock presentations, greater end-organ injury, and higher in-hospital mortality as compared to those with acute-on-chronic presentations of HF-CS. After accounting for clinical characteristics and the greater prevalence of preceding cardiac arrest in patients with de novo HF-CS, adjusted mortality was greater in those with de novo HF-CS vs. acute-on-chronic HF-CS.
The majority of CS research to date has focused on patients who develop CS after AMI.17-21 However, the incidence of AMI, particularly STEMI, has been steadily declining.9 Concurrently, increases in the prevalence of chronic medical conditions have contributed to a greater population burden of HF.9,22,23 In our study, we found that over two-thirds of patients with CS had a primary cause not attributed to AMI. This group has been variably defined as HF-CS or acute decompensated HF-CS.14 As there is likely considerable heterogeneity in patients with HF-CS, defining clinical subgroups is a diagnostic and therapeutic priority.2 In our analysis of contemporary North American CICU patients, categorizing HF-CS by disease chronicity identified potentially distinct populations. These data may have implications for in-hospital prognostication and for multidisciplinary shock-team approaches to care for patients with HF-CS. They also highlight the salient need for future clinical trials in cardiogenic shock designed to address management strategies in patients without AMI-CS.
Patients with de novo HF-CS had a greater proportion of SCAI shock stage E, higher SOFA scores, more frequent cardiac arrest, and higher serum lactate levels as compared to those with acute-on-chronic HF-CS. In addition, among patients with available invasive hemodynamic data, patients with de novo HF-CS and acute-on-chronic HF-CS had similar presenting cardiac power outputs. Taken together, these findings raise the possibility that a relative lack of chronic adaptation to low cardiac output and/or elevated ventricular filling pressures in those with de novo HF-CS may, in part, explain greater shock severity and end-organ injury. Compensatory responses in chronic HF have been incompletely studied to date, but mechanistic data suggest that patients with longstanding HF develop clinically important physiological adaptations that enhance their tolerance of low cardiac output states and/or elevated ventricular filling pressures. For example, dilation of the left ventricular and elevated end-diastolic volumes may preserve cardiac output even at lower LVEFs. In addition, patients with chronic HF have been found to use different myosin isoforms in diaphragmatic muscles, facilitating enhanced oxidative capacity with a mechanism similar to that seen in limb muscles subject to endurance training.24 Similar adaptations may occur in cardiomyocytes when subject to prolonged elevated filling pressures.25 Analogous adaptations may occur in other end-organs as a consequence of chronic low-flow states.26-29 For example, hypoxic inducible factor (HIF) shifts metabolism from oxidative phosphorylation to anaerobic glycolysis and suppresses mitochondrial respiration in renal tubular cells subject to prolonged periods of low partial pressures of oxygen, which may be adaptive.26,28 Other compensatory responses have also been postulated in patients with chronic HF.30,31 An alternative potential explanation for our observations includes longer exposure of patients with acute-on-chronic HF-CS to disease-modifying HF pharmacotherapies with favorable reverse remodeling effects.32-34 Finally, the higher proportion of preceding cardiac arrest and potential post-arrest myocardial dysfunction among patients with de novo HF-CS may also have contributed to between-group differences; however, the sensitivity analyses of shock severity and hemodynamic profiles excluding cardiac arrest patients do not support cardiac arrest as the sole explanatory factor.
Despite similar shock severity classification and end-organ injury in patients with de novo HF-CS compared to AMI-CS, MCS was used about half as frequently, with proportions smaller than other contemporary HF-CS cohorts.6 Whether earlier mechanical or pharmacological support in addition to team-based shock care35 may be beneficial in these patients warrants further investigation.
Heart transplant and durable LVAD implantation were low among patients with de novo HF-CS despite a lower comorbidity burden; reasons are likely multifactorial but may include greater multiorgan dysfunction affecting candidacy for advanced therapies, lack of prior advanced therapy evaluations, and inability to communicate with patients while they are critically ill, perceived potential for myocardial recovery, and a greater proportion of cardiac arrest and resultant neurological impairments, among others.
Few data exist examining the epidemiology of HF-CS as categorized by HF chronicity.36 We found that patients with de novo HF-CS had a greater risk of in-hospital death as compared to those with acute-on-chronic HF-CS. A prior analysis using electronic health record data from the United States reported that among admissions for HF without CS, proportions of patients with progression to CS were similar between groups and in-hospital and 30-day post-discharge mortality were lower in de novo HF vs. acute-on-chronic HF.37 These results should be interpreted in the context of dramatic differences in overall risk; specifically, in-hospital death among our de novo HF-CS population was >10-fold higher in our study than those reported for the population with de novo HF without CS in the prior study.
Limitations
This analysis has several limitations that should be acknowledged. First, the CCCTN registry is focused on medical CICU admissions. Therefore, these data do not reflect admissions with HF-CS managed exclusively in cardiac surgical ICUs (i.e., post-cardiotomy CS) or other ICUs, which may influence the estimates of MCS use and type. However, those presenting to the CICU who then required transfer to another unit were captured within these data. Second, more specific data on the cause of HF-CS (i.e., myocarditis, stress-induced cardiomyopathy, etc.) were not captured within the CCCTN registry. Further investigation detailing HF cause among patients with de novo HF-CS may lend additional insight into drivers of outcome in this important CS phenotype. Third, the distinction between de novo and acute-on-chronic HF-CS was based on prior history of HF; however, HF duration among those with acute-on-chronic HF-CS was not recorded in this registry and would be of interest in future work. In addition, ascertainment of ‘prior history of HF’ relied in investigator report and therefore could be subject to misclassification; however, to support consistent definitions, all study staff underwent training by the central CCCTN team, and all case entries were individually reviewed by the Coordinating Center via automated consistency checks and manual review. Fourth, invasive hemodynamic profiles were only available in a subset of patients, and the timing of hemodynamic assessment was not standardized. Furthermore, patients may have been on varying levels of pharmacological or mechanical support at the time of data capture. Given the risk for selection bias, these particular data should be interpreted with caution.38 Fifth, only in-hospital outcomes were available, precluding assessment of short and long-term post-discharge outcomes in those surviving hospitalization. Finally, in the context of an observational registry, our findings can be interpreted only as associations and may be subject to residual unmeasured confounding.
Conclusion
Approximately 2 out of 3 patients with CS admitted to contemporary North American CICUs have HF-CS. Among such patients, those with de novo HF-CS appear to have distinct clinical presentations and outcomes from patients with acute-on-chronic HF-CS. Our findings reveal the need to investigate whether distinct pathobiological compensatory mechanisms and responses to treatment in patients presenting with HF-CS who do vs. do not have a preexisting HF history.
Supplementary Material
Key Points:
A majority (67%) of patients with cardiogenic shock (CS) admitted to North American CICUs have heart failure-related CS (HF-CS) unrelated to acute myocardial infarction.
Approximately 1 in 4 patients with HF-CS present with de novo HF-CS, defined as no known prior history of heart failure.
Compared to patients with acute-on-chronic HF-CS, those with de novo HF-CS have more severe shock, greater end-organ injury, and higher in-hospital mortality.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation 2008; 117: 686–97. [DOI] [PubMed] [Google Scholar]
- 2.Berg DD, Bohula EA, van Diepen S, et al. Epidemiology of shock in contemporary cardiac intensive care units. Circ Cardiovasc Qual Outcomes 2019; 12: e005618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jentzer JC, Ahmed AM, Vallabhajosyula S, et al. Shock in the cardiac intensive care unit: Changes in epidemiology and prognosis over time. Am Heart J; 232: 94–104. [DOI] [PubMed] [Google Scholar]
- 4.Harjola V-P, Lassus J, Sionis A, et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail 2015; 17: 501–9. [DOI] [PubMed] [Google Scholar]
- 5.van Diepen S, Katz JN, Albert NM, et al. Contemporary management of cardiogenic shock: A scientific statement from the american heart association. Circulation 2017; 136: e232–68. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez-Montfort J, Sinha SS, Thayer KL, et al. Clinical Outcomes Associated with Acute Mechanical Circulatory Support Utilization in Heart Failure Related Cardiogenic Shock. Circ Heart Fail 2021; published online April 27. DOI: 10.1161/CIRCHEARTFAILURE.120.007924. [DOI] [PubMed] [Google Scholar]
- 7.Thayer KL, Zweck E, Ayouty M, et al. Invasive Hemodynamic Assessment and Classification of In-Hospital Mortality Risk Among Patients With Cardiogenic Shock. Circ Heart Fail 2020; 13: e007099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berg DD, Bohula EA, Morrow DA. Epidemiology and causes of cardiogenic shock. Curr Opin Crit Care 2021; published online May 19. DOI: 10.1097/MCC.0000000000000845. [DOI] [PubMed] [Google Scholar]
- 9.Virani SS, Alonso A, Aparicio HJ, et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021; 143: e254–743. [DOI] [PubMed] [Google Scholar]
- 10.Jentzer JC, Wiley B, Bennett C, et al. Temporal Trends and Clinical Outcomes Associated with Vasopressor and Inotrope Use in The Cardiac Intensive Care Unit. Shock 2020; 53: 452–9. [DOI] [PubMed] [Google Scholar]
- 11.Berg DD, Barnett CF, Kenigsberg BB, et al. Clinical practice patterns in temporary mechanical circulatory support for shock in the critical care cardiology trials network (CCCTN) registry. Circ Heart Fail 2019; 12: e006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrow DA, Fang JC, Fintel DJ, et al. Evolution of critical care cardiology: transformation of the cardiovascular intensive care unit and the emerging need for new medical staffing and training models: a scientific statement from the American Heart Association. Circulation 2012; 126: 1408–28. [DOI] [PubMed] [Google Scholar]
- 13.Bohula EA, Katz JN, van Diepen S, et al. Demographics, care patterns, and outcomes of patients admitted to cardiac intensive care units: the critical care cardiology trials network prospective north american multicenter registry of cardiac critical illness. JAMA Cardiol 2019; 4: 928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varshney AS, Berg DD, Katz JN, et al. Use of temporary mechanical circulatory support for management of cardiogenic shock before and after the united network for organ sharing donor heart allocation system changes. JAMA Cardiol 2020; 5: 703–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawler PR, Berg DD, Park J-G, et al. The range of cardiogenic shock survival by clinical stage: data from the critical care cardiology trials network registry. Crit Care Med 2021; published online April 2. DOI: 10.1097/CCM.0000000000004948. [DOI] [PubMed] [Google Scholar]
- 16.Sampaio FB, Alves WA, Magalhães CK, Oliveira VN, Santos LP. The use of the SOFA score to analyze the profile and severity of organ dysfunction in patients with cardiovascular disorders. Crit Care 2005. [Google Scholar]
- 17.Henry TD, Tomey MI, Tamis-Holland JE, et al. Invasive management of acute myocardial infarction complicated by cardiogenic shock: A scientific statement from the american heart association. Circulation 2021; 143: e815–29. [DOI] [PubMed] [Google Scholar]
- 18.Ouweneel DM, Eriksen E, Sjauw KD, et al. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. J Am Coll Cardiol 2017; 69: 278–87. [DOI] [PubMed] [Google Scholar]
- 19.Thiele H, Akin I, Sandri M, et al. PCI Strategies in Patients with Acute Myocardial Infarction and Cardiogenic Shock. N Engl J Med 2017; 377: 2419–32. [DOI] [PubMed] [Google Scholar]
- 20.Thiele H, Zeymer U, Neumann F-J, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012; 367: 1287–96. [DOI] [PubMed] [Google Scholar]
- 21.Thiele H, Zeymer U, Thelemann N, et al. Intraaortic Balloon Pump in Cardiogenic Shock Complicating Acute Myocardial Infarction: Long-Term 6-Year Outcome of the Randomized IABP-SHOCK II Trial. Circulation 2018; published online Nov 11. DOI: 10.1161/CIRCULATIONAHA.118.038201. [DOI] [PubMed] [Google Scholar]
- 22.Conrad N, Judge A, Tran J, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet 2018; 391: 572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson SL, Tong X, King RJ, Loustalot F, Hong Y, Ritchey MD. National burden of heart failure events in the united states, 2006 to 2014. Circ Heart Fail 2018; 11: e004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tikunov B, Levine S, Mancini D. Chronic congestive heart failure elicits adaptations of endurance exercise in diaphragmatic muscle. Circulation 1997; 95: 910–6. [DOI] [PubMed] [Google Scholar]
- 25.Taegtmeyer H, Sen S, Vela D. Return to the fetal gene program: a suggested metabolic link to gene expression in the heart. Ann N Y Acad Sci 2010; 1188: 191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapitsinou PP, Haase VH. Molecular mechanisms of ischemic preconditioning in the kidney. Am J Physiol Renal Physiol 2015; 309: F821–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heusch G Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res 2015; 116: 674–99. [DOI] [PubMed] [Google Scholar]
- 28.Haase VH. Mechanisms of hypoxia responses in renal tissue. J Am Soc Nephrol 2013; 24: 537–41. [DOI] [PubMed] [Google Scholar]
- 29.Stenzel-Poore MP, Stevens SL, Xiong Z, et al. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet 2003; 362: 1028–37. [DOI] [PubMed] [Google Scholar]
- 30.De Keulenaer GW, Feyen E, Dugaucquier L, et al. Mechanisms of the Multitasking Endothelial Protein NRG-1 as a Compensatory Factor During Chronic Heart Failure. Circ Heart Fail 2019; 12: e006288. [DOI] [PubMed] [Google Scholar]
- 31.Lim HS, Howell N. Cardiogenic Shock Due to End-Stage Heart Failure and Acute Myocardial Infarction: Characteristics and Outcome of Temporary Mechanical Circulatory Support. Shock 2018; 50: 167–72. [DOI] [PubMed] [Google Scholar]
- 32.Januzzi JL, Prescott MF, Butler J, et al. Association of Change in N-Terminal Pro-B-Type Natriuretic Peptide Following Initiation of Sacubitril-Valsartan Treatment With Cardiac Structure and Function in Patients With Heart Failure With Reduced Ejection Fraction. JAMA 2019; : 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, et al. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol 2021; 77: 243–55. [DOI] [PubMed] [Google Scholar]
- 34.Lopaschuk GD, Verma S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl Sci 2020; 5: 632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moghaddam N, van Diepen S, So D, Lawler PR, Fordyce CB. Cardiogenic shock teams and centres: a contemporary review of multidisciplinary care for cardiogenic shock. ESC Heart Fail 2021; 8: 988–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones TL, Tan MC, Nguyen V, et al. Outcome differences in acute vs. acute on chronic heart failure and cardiogenic shock. ESC Heart Fail 2020; 7: 1118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greene SJ, Triana TS, lonescu-lttu R, et al. Patients hospitalized for de novo versus worsening chronic heart failure in the united states. J Am Coll Cardiol 2021; 77: 1023–5. [DOI] [PubMed] [Google Scholar]
- 38.Fang JC, Jones TL. Can a pulmonary artery catheter improve outcomes in cardiogenic shock? JACC Heart Fail 2020; 8: 914–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.