The muscle-specific miR-1 and miR-133a RNAs lock cardiomyocytes in a differentiated state by suppressing OSMR/FGFR signaling.
Abstract
Dedifferentiation of cardiomyocytes is part of the survival program in the remodeling myocardium and may be essential for enabling cardiomyocyte proliferation. In addition to transcriptional processes, non-coding RNAs play important functions for the control of cell cycle regulation in cardiomyocytes and cardiac regeneration. Here, we demonstrate that suppression of FGFR1 and OSMR by miR-1/133a is instrumental to prevent cardiomyocyte dedifferentiation and cell cycle entry in the adult heart. Concomitant inactivation of both miR-1/133a clusters in adult cardiomyocytes activates expression of cell cycle regulators, induces a switch from fatty acid to glycolytic metabolism, and changes expression of extracellular matrix genes. Inhibition of FGFR and OSMR pathways prevents most effects of miR-1/133a inactivation. Short-term miR-1/133a depletion protects cardiomyocytes against ischemia, while extended loss of miR-1/133a causes heart failure. Our results demonstrate a crucial role of miR-1/133a–mediated suppression of Osmr and Ffgfr1 in maintaining the postmitotic differentiated state of cardiomyocytes.
INTRODUCTION
Adult mammalian cardiomyocytes are characterized by a limited proliferative potential contributing to the low regenerative capacity of the heart (1). In contrast, embryonic and fetal cardiomyocytes are highly proliferative, enabling fetal hearts to compensate for the loss of up to 50% of cardiac tissue (2). Mouse cardiomyocytes cease proliferation shortly after birth around postnatal day 5 (P5) but continue to undergo DNA replication and karyokinesis without cytokinesis, resulting in the formation of binucleated and polyploid adult cardiomyocytes, which varies considerably in different species (3, 4). In stark contrast to adult hearts, the proliferative capacity of neonatal mammalian cardiomyocytes enables heart regeneration shortly after birth (5, 6). Thus, induction of cardiomyocyte proliferation has become a major focus to improve cardiac function and prevent heart failure in adult hearts (7–9). Several conditions and molecules are known to induce proliferation of adult cardiomyocyte including hypoxia (10), overexpression of combinations of cell cycle regulators (7), concomitant inactivation of Meis1 and Hoxb13 (11), changes of components in the extracellular matrix (ECM), e.g., agrin (12), and others. Furthermore, growth factors such as interleukin-6 or members of the fibroblast growth factor (FGF) family can stimulate proliferation of cardiomyocytes, particularly at the neonatal stage when cardiomyocytes are immature and still not fully differentiated (13–15).
The biophysical constrains exerted by sarcomeres, which occupy huge parts of mature adult cardiomyocytes, have led to the hypothesis that cytokinesis of cardiomyocytes requires a partial disassembly of the sarcomeric contractile apparatus and down-regulation of structural proteins (16–18). Dedifferentiation of cardiomyocytes becomes apparent after myocardial infarction and during heart failure reflected by expression of genes typically present in immature embryonic, fetal, and early postnatal cardiomyocytes including smooth muscle cell genes (19, 20). A major mediator of cardiomyocyte dedifferentiation and ECM regulation is oncostatin M (OSM) (20, 21). OSM regulates dedifferentiation through activation of the mitogen-activated protein kinase (MAPK) kinase (MEK)/extracellular signal–regulated kinase (ERK) pathway and provides strong cardioprotection after acute myocardial infarction in mice (20, 22). A further potent regulator of cardiomyocyte dedifferentiation is the FGF receptor 1 (FGFR1) activating growth factor FGF2, which, in contrast to OSM, needs cofactors such as triiodothyronine to induce fetal reprogramming (23). In contrast to OSM, which primarily induces dedifferentiation of cardiomyocytes, FGF signaling contributes to proliferation of neonatal and adult cardiomyocytes (15, 24, 25).
So far, the activity of the OSM and FGF pathways was assumed to rely mostly on the release of growth factors or intracellular signaling events, while information about a potential posttranscriptional regulation of components of the OSM and FGF signaling machinery is critically missing. To fill this gap, we focused on microRNAs (miRNAs), which potently regulate multiple targets at the posttranscriptional level and modulate various steps during cardiovascular development (26). Numerous miRNAs have been described to regulate cardiomyocyte proliferation and heart repair [reviewed in (27, 28)]. Examples include miR-195 (29) and miR-99/100 (30), which have been identified to promote expression of cell cycle genes in cardiomyocytes after knockdown or promote cardiomyocyte dedifferentiation and proliferation. However, neither miR-195 nor miR-99/100 is specifically expressed in cardiomyocytes. Similarly, hsa-miR-590 and hsa-miR-199a, identified by screening for miRNAs promoting proliferation of neonatal cardiomyocytes (31), are not exclusively expressed in the cardiac lineage, which may suggest that cardiomyocyte-specific pathways have so far escaped identification. Likewise, several long noncoding RNAs were found to direct cardiomyocyte proliferation and cardiac regeneration, including ECRAR (endogenous cardiac regeneration-associated regulator), CRRL (cardiomyocyte regeneration-related lncRNA), and CAREL (cardiac regeneration-related lncRNA) [reviewed in (28)].
Here, we searched for miRNAs specifically expressed in muscle cells that efficiently target effectors of cardiomyocyte dedifferentiation and proliferation. We found that the OSM receptor β (Osmr) and Fgfr1 are direct miR-1 and miR-133a targets. Cardiomyocyte-specific inactivation of both miR-1/133a clusters increased expression of Osmr and Fgfr1, resulting in persistent activation of OSM and FGF signaling in adult mice, and cell cycle entry. Concomitant inactivation of both miR-1/133a clusters and Osmr or Fgfr1 normalized numerous changes in miR-1/133a mutants, demonstrating that Osmr or Fgfr1 are major targets of miR-1/133a. Our findings suggest that miR-1/133a maintain the mature, differentiated, and postmitotic state of cardiomyocytes by repressing Osmr and Fgfr1 to prevent dedifferentiation, which, in the long run, results in impaired cardiac contractility and heart failure.
RESULTS
Individual miRNAs from the miR-1/133a clusters selectively regulate Osmr1 and Fgfr1 expression in cardiomyocytes
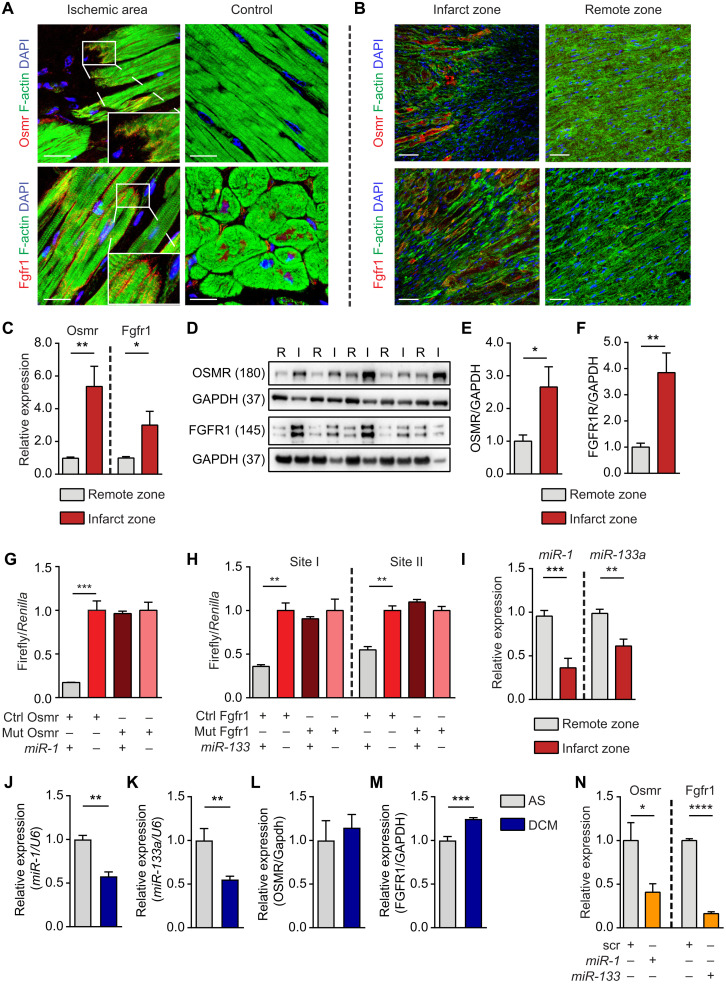
To determine the role of posttranscriptional regulatory processes for cardiomyocyte dedifferentiation and proliferation, we screened for putative miRNA binding sites in mRNAs coding for OSM (OSMR) and FGF (FGFR1) receptors that are up-regulated in the infarct border zone of patients with ischemic cardiomyopathy (ICM) compared to control samples (Fig. 1A and figs. S1 and S2) and in mice after acute myocardial infarction (Fig. 1B). Up-regulation of Osmr and Fgfr1 in the border zone was also verified by reverse transcription quantitative polymerase chain reaction (RT-qPCR; Fig. 1C) and Western blot analysis (Fig. 1, D to F). The miRNA target prediction tools TargetScan and miRanda identified one potential miR-1 binding site within the 3′ untranslated region (UTR) of Osmr and two miR-133a binding sites in Fgfr1 (fig. S2, A and B), which were functionally validated by luciferase reporter assays (Fig. 1, G and H). miR-1 and miR-133a are clustered in the genome and represent a functional unit, suggesting a common regulatory mechanism for the up-regulation of both receptors during myocardial infarction. In line with this assumption, we detected a significant down-regulation of miR-1 and miR-133a in the infarct border zone (Fig. 1I). Although reduction of miR-1 and miR-133a might be influenced by a changed cell composition in the border zone, this observation revealed an inverse relation between reduced miR-1/133a and increased Osmr and Fgfr1 expression. The same relation was also found in human patients with dilated cardiomyopathy (DCM) (Fig. 1, J to M). To investigate whether the miR-1/133a cluster is able to suppress expression of OSMR and FGFR1, we transfected isolated neonatal mouse cardiomyocytes (P0 to P3) with miRNAs directed against Osmr and Fgfr1. RT-qPCR analysis revealed a strong reduction of Osmr and Fgfr1 expression (Fig. 1N), indicating that miR-1/133a are indeed able to suppress expression of these important receptors.
Fig. 1. Fgfr1 and Osmr are direct targets of miR-1 and miR-133a.
(A and B) Immunofluorescence staining for OSMR and FGFR1 in patients with ICM [scale bars, 30 μm (A)] and mouse hearts 7 days after transient LAD (left anterior descending coronary artery) ligation [scale bars, 20 μm (B)]. DAPI, 4′,6-diamidino-2-phenylindole. (C) RT-qPCR analysis of Osmr and Fgfr1 expression in infarct compared to remote zone 7 days after transient LAD ligation (n = 7/7; two-tailed unpaired t test). (D to F) Western blot analysis of OSMR and FGFR1 expression in mouse hearts 7 days after LAD ligation in infarct compared to remote zone (n = 5/5; two-tailed unpaired t test). (G and H) Luciferase reporter assays (n = 3 each group; one-tailed unpaired t test). One miR-1 binding site is within the Osmr 3′UTR, and two miR-133a binding sites are within the Fgfr1 3′UTR. Each of the two sites was tested in separate assays. (I) RT-qPCR analysis of miR-1 and miR-133a expression in mouse hearts 7 days after transient LAD ligation (n = 7/7; two-tailed unpaired t test). Normalized to U6. (J and K) RT-qPCR analysis of miR-1 and miR-133a expression in patients with DCM (n = 5) compared to aortic stenosis (AS) (n = 3; two-tailed unpaired t test). Normalized to U6. (L and M) RT-qPCR analysis of Osmr and Fgfr1 expression in DCM (n = 5) compared to AS (n = 3; two-tailed unpaired t test). (N) RT-qPCR analysis of Osmr (n = 3 each; one-tailed unpaired t test) and Fgfr1 (scr, n = 2; miR-treated, n = 3; one-tailed unpaired t test) expression in neonatal mouse cardiomyocytes after transfection with miR-1 or miR-133a miRNA mimics. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
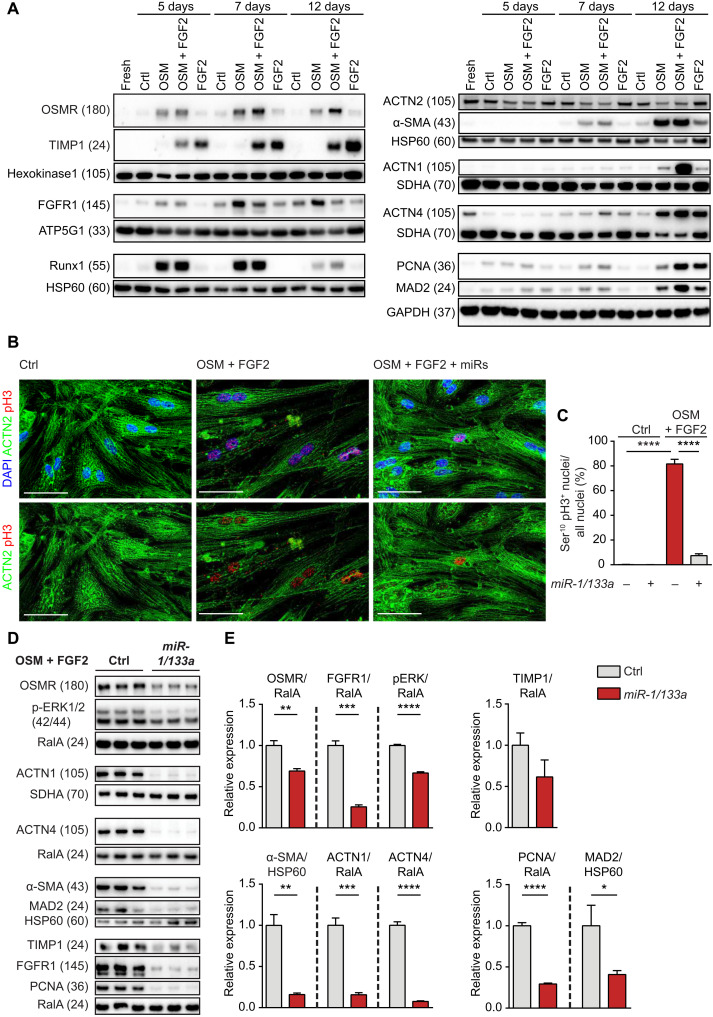
To further explore the effects of miR-1/133a on receptor regulation and cardiomyocyte dedifferentiation, we treated adult rat cardiomyocytes in culture with OSM and FGF2 (Fig. 2A and fig. S3). OSM strongly induced expression of OSMR and FGFR1 as well as of Runx1, α–smooth muscle actin (α-SMA), actinin alpha 1 (ACTN1), and actinin alpha 4 (ACTN4). FGF2 also induced α-SMA, tissue inhibitor of metalloproteinases 1 (TIMP1), and ACTN4, while expression of OSMR and ACTN1 increased only slightly. Costimulation with OSM plus FGF2 induced expression of OSMR, α-SMA, ACTN1, and ACTN4 synergistically (Fig. 2A). Along the same line, combined treatment with OSM or FGF2 enhanced expression of the cell cycle markers proliferating cell nuclear antigen (PCNA) and Mitotic Arrest Deficient-2 (MAD2) and resulted in accumulation of phospho-H3 positive nuclei in cardiomyocytes, indicating cell cycle reentry of adult cardiomyocytes, although no signs of increased cytokinesis were detected (Fig. 2, B and C). In contrast, separate treatment with either OSM or FGF2 only caused a moderate increase of PCNA and MAD2 expression, confirming potential synergistic interactions (Fig. 2A). Overexpression of miR-1/133a nearly abolished effects of OSM or FGF2 on adult rat cardiomyocytes. Increased expression of dedifferentiation marker and cell cycle genes and accumulation of phospho-H3 positive nuclei after OSM and FGF2 treatment were essentially absent after experimental elevation of miR-1/133a levels (Fig. 2, B to E). We concluded that miR-1/133a may play an important role in the regulation of cardiomyocyte dedifferentiation by preventing synergistic activities of OSMR and FGFR1 signaling.
Fig. 2. Synergistic induction of cardiomyocyte dedifferentiation and cell cycle entry by OSMR and FGFR1 is inhibited by miR-1/133a.
(A) Western blot analysis of increased OSMR and FGFR1 expression and of markers related to cell cycle, fibrosis, and contractility after treatment of adult rat cardiomyocytes with OSM and FGF2. Different loading controls [HK1, HSP60, ATP5G1, SDHA, and GAPDH (glyceraldehyde-3-phosphate dehydrogenase)] were used for normalization to account for differences in molecular weights of proteins of interest on the respective membranes. (B and C) Immunofluorescence staining of adult rat cardiomyocytes showing increase of pH3 after stimulation with OSM and FGF2, which is inhibited by miR-1/133a (scale bars, 50 μm; n = 5 each group; unpaired two-tailed t test). ACTN2 staining was used to confirm the identity of cardiomyocytes. (D) Western blot analysis of OSMR and FGFR1 after stimulation of adult rat cardiomyocytes with OSM and FGF2 and miR-1/133a treatment. Expression of pERK and markers related to fibrosis, contractility, and cell cycle are shown as well. (E) Quantification of results shown in (D) (n = 3 each group; one-tailed unpaired t test). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Inactivation of miR-1/133a genes increases expression of the Osmr and Fgfr1 resulting in activation of the MEK/ERK pathway
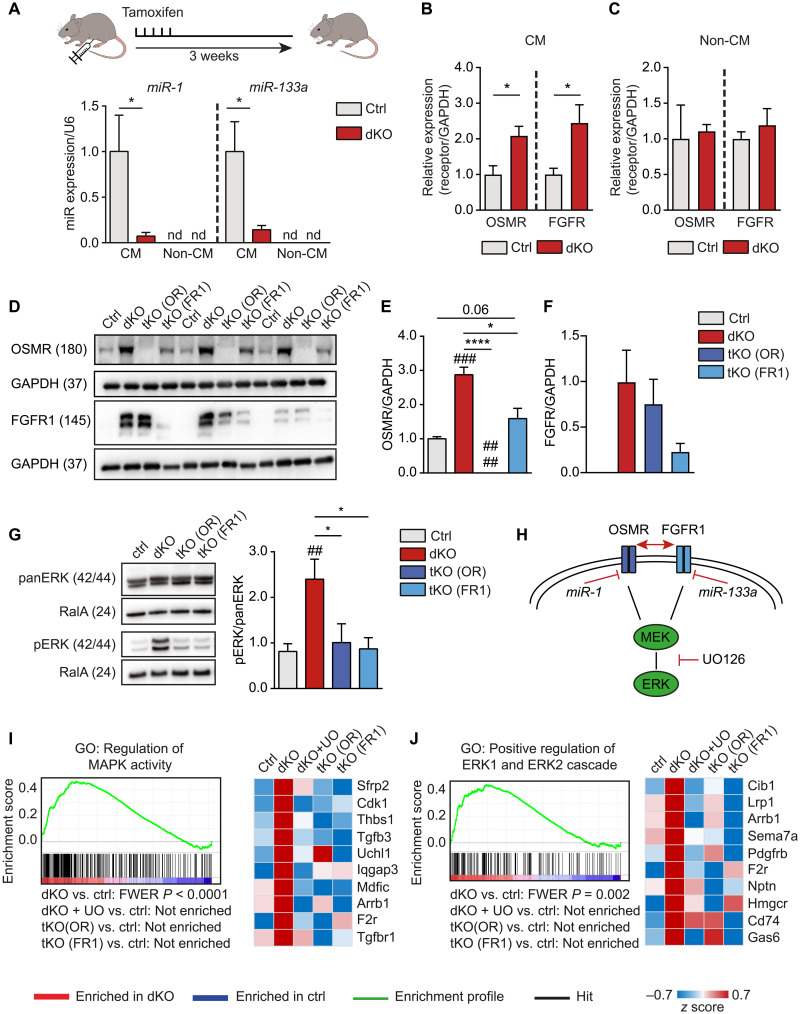
To investigate whether miR-1/133a prevent cardiomyocyte dedifferentiation and cell cycle reentry in vivo, we conditionally inactivated both miR-1/133a gene clusters expressed in adult cardiomyocytes (32). We used a tamoxifen-inducible alpha-myosin heavy chain (Myh6)–MerCreMer (33) in combination with previously described alleles of miR-1/133a (34), which yielded Myh6-MerCreMerpos/miR-1-1/133a-2−/−/miR-1-2/133a-1fl/fl mice (miR-1/133a dKO or dKO in short) (Fig. 3A and fig. S4, A to F). In addition, we used Osmr (35) and Fgfr1 (36) conditional alleles to generate Myh6-MerCreMerpos/miR-1-1/133a-2−/−/miR-1-2/133a-1fl/fl/OSMR−/− [tKO (OR)] and Myh6-MerCreMerpos/miR-1-1/133a-2−/−/miR-1-2/133a-1fl/fl/Fgfr1fl/fl [tKO (FR1)] knockout mice, which allowed us to address the role of Fgfr1 and Osmr for miR-1/133a–dependent effects in the adult myocardium. qRT-PCR analysis revealed an increase of Fgfr1 and Osmr expression in freshly isolated dKO cardiomyocytes but not in noncardiomyocytes after tamoxifen treatment of adult mice (Fig. 3, B and C). The increased expression of Fgfr1 and Osmr was linked to a highly significant up-regulation of genes related to MAPK activity and ERK signaling. Western blot analysis of adult hearts confirmed the strong up-regulation of both receptors in dKO mice and corroborated efficient inactivation of respective genes in tKO hearts (Fig. 3, D to F). Notably, deletion of Fgfr1 in dKO mice reduced induction of OSMR, while deletion of Osmr did not result in a major reduction of FGFR1, although we cannot completely rule out cross-reactivity of the FGFR1 antibody with other FGFRs or FGFR1 expression in non-cardiomyocytes of the heart. We also detected a strong increase in ERK phosphorylation in adult dKO hearts, which was completely abrogated by additional deletion of either Osmr or Fgfr1 (Fig. 3G). Since FGFR1 and OSMR signal via the MEK-ERK signaling cascade, normalization of ERK phosphorylation in tKO (OR) and tKO (FR1) cardiomyocytes indicates that increased ERK signaling after loss of miR-1/133a is caused by derepression of Fgfr1 and Osmr. To confirm this hypothesis, we specifically suppressed MEK/ERK signaling downstream of OSMR and FGFR1 in miR-1/133a dKO mice by the small-molecule inhibitor UO126 (Fig. 3H). Next, we performed microarray analysis to detect transcriptional changes in hearts of dKO and of UO126-treated dKO, tKO (OR), and tKO (FR1) animals in comparison to hearts from control animals. Inhibition of MEK1/2 signaling or inactivation of either Osmr or Fgfr1 in dKO hearts normalized expression of genes related to MAPK and ERK signaling that were up-regulated in the absence of miR-1/133a (Fig. 3, I and J).
Fig. 3. Synergistic effects of OSMR and FGFR1 in mouse hearts after inactivation of miR-1/133a are mediated by MAPK-ERK.
(A) RT-qPCR analysis of miR-1/133a expression in adult dKO and control cardiomyocytes (n = 6, each) 3 weeks after tamoxifen administration. miR-1/133a is not detected (nd) in noncardiomyocytes (non-CM; ctrl, n = 5; dKO, n = 6; one-tailed unpaired t test). (B and C) RT-qPCR analysis of OSMR and FGFR1 expression in adult cardiomyocytes (B) (n = 4 each) and non-CM (C) (OSMR ctrl, n = 4; dKO, n = 3; FGFR1 ctrl, n = 5; dKO, n = 4; two-tailed unpaired t tests) of ctrl and dKO mice normalized to GAPDH 3 weeks after tamoxifen treatment. (D to F) Western blot analysis of OSMR and FGFR1 expression in ctrl, dKO, and tKO hearts (n = 3 each; one-tailed unpaired t test), normalized to GAPDH. (G) Western blot analysis of phosphorylated ERK in adult dKO compared to control and tKO hearts [ctrl, n = 5; dKO, n = 5; tKO (OR), n = 3; tKO (FR1), n = 3; one-tailed unpaired t test]. (H) Model of OSMR and FGFR1 repression by miR-1 and miR-133a. (I and J) Gene set enrichment analysis (GSEA) of microarray analysis data from adult mouse hearts. Genes related to MEK-ERK signaling are enriched in dKO versus ctrl hearts. Heatmaps of the 10 most up-regulated genes in dKO hearts derived from respective gene sets. Treatment with UO126 or additional deletion of OSMR (tKO OR) or FGFR1 (tKO FR1) [ctrl: n = 4; dKO, dKO + UO, tKO (OR), and tKO (FR1): n = 3] normalizes expression of up-regulated genes in dKO hearts. *P < 0.05 and ****P < 0.0001 compared to dKO; ##P < 0.01 and ###P < 0.001 compared to WT.
Previous studies showed that the primary sources of OSM in the heart are infiltrating leukocytes, especially neutrophils and macrophages (20, 37, 38). We therefore analyzed whether miR-1/133a mutant hearts harbor increased numbers of leucocytes, which provide the OSMR ligand. Immunofluorescence and qRT-PCR analysis revealed accumulation of CD11b-positive leucocytes in the myocardium of miR-1/133a mutants and increased expression of OSM (fig. S5, A to D), which, together with derepression of Osmr, explains activation of the MEK/ERK pathway.
miRNAs usually regulate large numbers of target genes. Hence, we wondered whether miR-1/133a also regulate other mRNAs in the adult heart. Transcriptional profiling of neonatal and adult miR-1/133a dKO hearts revealed a strong increase of the miR-1 and miR-133a targets Myocardin and Kcnmb1 (39) and an increase of Calponin 1 (Cnn1), Calponin 2 (Cnn2), and Myotrophin, which also carry putative miR-1 or miR-133a binding sites. Luciferase miRNA reporter vector assays confirmed that Myotrophin, Cnn1, and Cnn2 are potential direct targets of miR-1 and/or miR-133a in the postnatal heart (fig. S6). However, we did not further interrogate the role of these primary miR-1/133 targets during the further course of the study.
Repression of Osmr and Fgfr1 by miR-1/133a is associated with cell cycle arrest of cardiomyocytes and inhibition of dedifferentiation
Overexpression of miR-1/133a nearly abolished cardiomyocyte dedifferentiation and cell cycle entry induced by combined treatment with OSM or FGF2. Thus, we wondered whether repression of FGFR1 and OSMR signaling by miR-1/133a prevents dedifferentiation and cell cycle entry in vivo. RT-qPCR analysis of miR-1/133a dKO hearts showed increased expression of the cell cycle regulatory genes Pdk3, Cdk1, Ccna2, Ccnb1, E2f3 and the stem cell marker Runx1 (fig. S7, A to F), which was normalized by the MEK/ERK inhibitor UO126. Similarly, we found that treatment with UO126 (0.15 nM) prevented enhanced expression of the cell cycle markers Cdk1, Ccna2, and Ccnb1 in response to stimulation of isolated neonatal cardiomyocytes with FGF1 and OSM (fig. S8, A to C). In line with this analysis, perinatal deletion of miR-1/133a in MCK-miR-1/133a dKO mice (MCK-Crepos/miR-1-1/133a-2−/−/miR-1-2/133a-1fl/fl) (fig. S8, D and E) revealed a twofold increase in the number of phospho-Histone3–positive cardiomyocytes in miR-1/133a dKO compared to control hearts (fig. S8, F and G). Furthermore, we found that treatment with agrin (12) stimulates 5-ethynyl-2′-deoxyuridine (EdU) incorporation more strongly in miR-1/133a MCK dKO compared to control cardiomyocytes, suggesting that the pro-proliferative state instigated by loss of miR-1/133a makes cardiomyocytes more receptive to pro-proliferative signals (fig. S8, H and I). Continued failure to suppress dedifferentiation by inactivation of miR-1/133a resulted in DCM at the perinatal stage (fig. S8J).
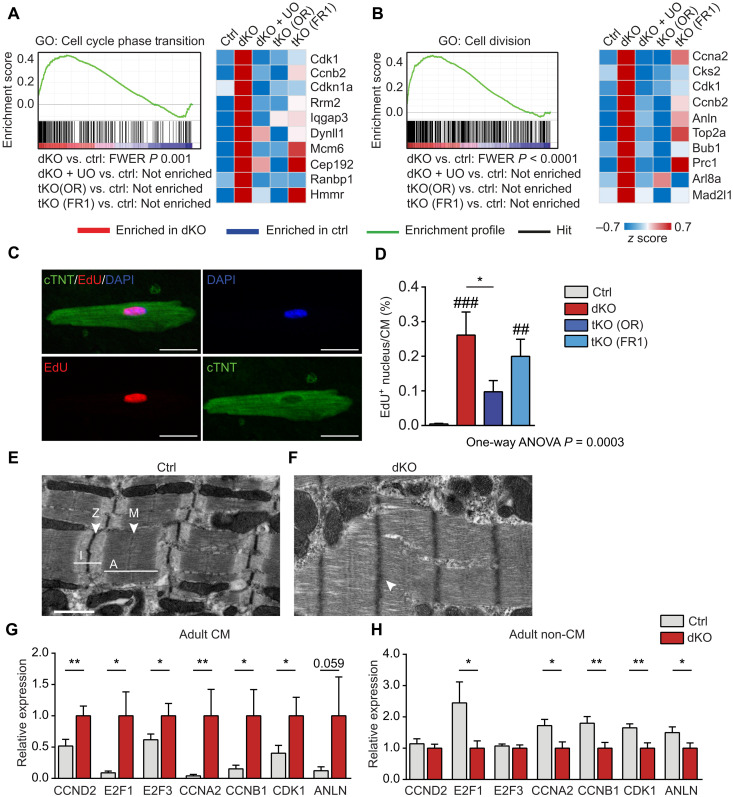
Next, we asked whether activity of Osmr and Fgfr1 in miR-1/133a dKO is sufficient to initiate dedifferentiation and cell cycle entry of adult cardiomyocytes. Unbiased analysis of gene ontology (GO) terms revealed activation of processes related to proliferation, which were reversed in tKO (OR) mice, in animals treated with UO126, and, to a lesser extent, in the tKO (FR1) strain (Fig. 4, A and B). We did not observe a consistent enrichment of components of the Notch, Hippo, and WNT pathways with increased expression. Injection of the thymidine analog EdU (39) for 7 days into 10-week-old miR-1/133a dKO and control mice after administration of tamoxifen resulted in the appearance of EdU-positive cardiomyocytes in dKO but not in controls hearts (Fig. 4, C and D). Notably, EdU-incorporating cardiomyocytes often showed signs of dedifferentiation as indicated by reduced presence of cross-striations and diminished cardiac Troponin T2 (cTNT) staining (fig. S9A). Moreover, electron microscopy revealed signs of sarcomere disorganization and fuzzy Z-discs (Fig. 4, E and F). Stimulation of isolated adult rat cardiomyocytes with OSM/FGF2 for 7 days also resulted in EdU+ and pH3+ nuclei, substantiating the induction of cell cycle reentry of adult cardiomyocytes upon activation of OSMR/FGFR signaling (fig. S9, B and C). The percentage of EdU+ cardiomyocytes was strongly reduced in tKO (OR) and, to a lesser extent, in tKO (FR1) compared to dKO mice, which matched the corresponding transcriptional changes (Fig. 4, A to D). The majority of EdU-positive nuclei were found in mononucleated cardiomyocytes, although we occasionally detected EdU incorporation in binucleated cardiomyocytes (fig. S9D). To validate the transcriptome analysis and to ascertain that regulated cell cycle–associated genes are exclusively up-regulated in cardiomyocytes, we separated cardiomyocytes and noncardiomyocytes from 10-week-old mice. qRT-PCR analysis confirmed a strong increase of Cyclin D2 (Ccnd2), E2 transcription factor 1 (E2F1), E2F3, Cyclin A2 (Ccna2), Ccnb1, Cyclin dependent kinase 1 (Cdk1), and Anln expression in cardiomyocytes (Fig. 4G) but not in noncardiomyocytes (Fig. 4H). Increased expression of Anillin, a cytokinesis marker (40), in miR-1/133a dKO mutant cardiomyocytes indicated that mutant cardiomyocytes might undergo cytokinesis (Fig. 4G). In line with this assumption, we detected a significant increase of mononucleated cardiomyocytes in miR-1/133a dKO mutant compared to control hearts (ca. 30 versus 20%), suggesting increased formation of new cardiomyocytes after postnatal loss of miR-1/133a, since newly formed cardiomyocytes initially show only a single nucleus (fig. S9E). However, we were unable to observe proliferation of isolated adult miR-1/133a cKO cardiomyocytes in vitro, which might be due to the relatively modest number of cardiomyocytes initiating cytokinesis. Additional deletion of Osmr1 or Fgfr1 in miR-1/133a dKO mutant did not reduce numbers of mononucleated cardiomyocytes to control levels, which corresponds to the EdU incorporation assay.
Fig. 4. Loss of miR-1/133a facilitates cell cycle reentry of cardiomyocytes.
(A and B) GSEA of microarray data from adult mouse hearts showing enrichment of genes related to cell cycle phase transition (A) and cell division (B) after inactivation of miR-1/133a. UO126 treatment or additional knockout of Osmr (tKO OR) or Fgfr1 (tKO FR1) abolished enrichment. Heatmaps of the 10 most up-regulated genes in dKO hearts derived from the respective gene sets confirm normalization of gene expression [ctrl: n = 4; dKO, dKO + UO, tKO (OR), tKO (FR1): n = 3]. (C and D) EdU incorporation in an isolated cTNT+ adult miR-1/133a mutant cardiomyocyte and quantification of EdU incorporation in cardiomyocytes of dKO, tKO, and control mice after EdU administration for 7 days before isolation [ctrl, n = 13; dKO, n = 10; tKO (OR), n = 14; tKO (FR1), n = 11 animals; one-way analysis of variance (ANOVA)]. Scale bars, 20 μm. (E and F) Electron microscope images of adult cardiomyocytes (ctrl, n = 3; dKO, n = 2). Scale bars, 1 μm. Sarcomeres of dKO cardiomyocytes show fuzzy Z-discs (arrowhead in inlet) and signs of disorganization. (G and H) qRT-PCR analysis of cell cycle markers in isolated adult dKO (n = 7 to 9) and control cardiomyocytes (n = 8 to 12) and noncardiomyocytes (ctrl non-CM, n = 6 to 7; dKO, n = 6 to 7; one-tailed unpaired t test). *P < 0.05 and **P < 0.01 compared to dKO; ##P < 0.01 and ###P < 0.001 compared to WT.
Increased cell cycle activity of dKO cardiomyocytes was associated with a pronounced dedifferentiation of cardiomyocytes as indicated by the presence of SMA (ACTA2) (fig. S10, A to C), which is strongly induced by the OSM-ERK cascade in adult cardiomyocytes and normally only found in embryonic cardiomyocytes and smooth muscle cells (20). Similarly, expression of Myh7, which is predominantly present during prenatal development of the mouse, was strongly increased (fig. S10, B and D). Up-regulation of SMA (Acta2) in dKO hearts was also confirmed by immunofluorescence staining (fig. S10E) and Western blot analysis (fig. S10, F and G). Changes in the expression of genes coding for contractile proteins were not reversed by additional deletion of Osmr and Fgfr1 or UO126 treatment, suggesting that miR-1/133 regulate these genes by different pathways (39, 41). Continued expression of fetal cardiac myosin heavy chain or smooth muscle genes may have adverse effects on the contractility of the heart. Therefore, we examined the physiological consequences of an extended inactivation of miR-1/133a genes in the heart. Tamoxifen-induced deletion of miR-1/133a did not cause an apparent clinical phenotype at early stages. However, 50 days after tamoxifen treatment, miR-1/133a dKO mice developed a massive enlargement of the ventricles and the atria and thinning of the ventricular walls (fig. S10, H and I), which was not prevented by additional inactivation of Osmr and Fgfr1 or by UO126 administration. We concluded that long-lasting dedifferentiation of cardiomyocytes is not compatible with normal cardiac function and that miR-1/133a is essential to maintain a differentiated mature phenotype, critical to preventing heart failure.
Enhanced levels of Osmr and Fgfr1 in miR-1/133a dKO hearts alter expression of ECM genes and causes a metabolic shift
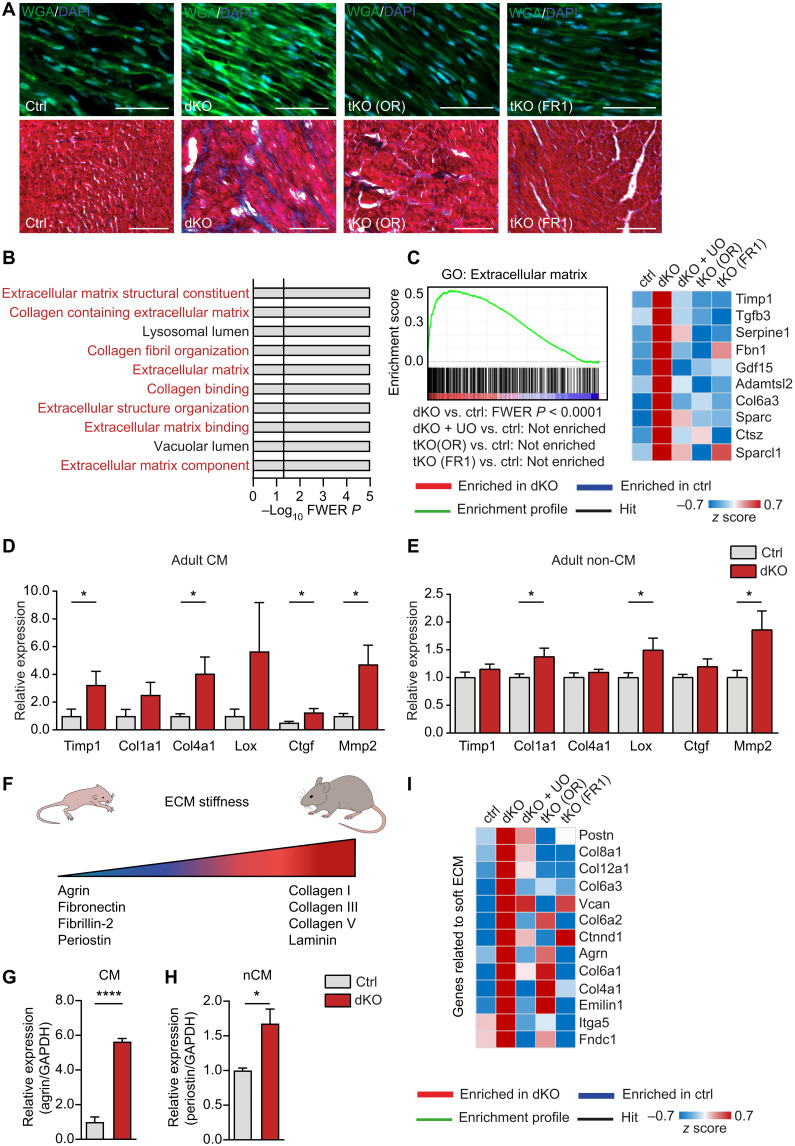
Changes in the transcriptional pattern of cell cycle–related genes in dKO hearts were associated with increased deposition of ECM components (Fig. 5A) and marked enrichment of gene sets related to the ECM, which were, again, prevented by additional inactivation of Osmr and Fgfr1 (Fig. 5, B and C). qRT-PCR analysis confirmed a strong increase of Timp1, Col1a1, Col4a1, Ctgf, and Mmp2 in adult cardiomyocytes (Fig. 5D). Moderate up-regulation of Col1a1, Lox, and Mmp2 was also observed in noncardiomyocytes, probably due to paracrine activity of cardiomyocytes (Fig. 5E). Some genes identified by expression analysis are closely associated with ECM stiffness, which changes during heart development. Lower stiffness of the ECM is found in neonatal compared to adult hearts and was reported to support heart regeneration and cardiomyocyte proliferation in injured neonatal hearts (42). Further expression analysis by qRT-PCR analysis revealed increased expression of agrin and periostin in dKO hearts, which are representative for a neonatal-like ECM composition (Fig. 5, F to H) (12, 43). Inactivation of Osmr or Fgfr1 or treatment with UO126 abrogated the transcriptional signature characteristic for neonatal-like ECM in dKO hearts, similar to the inhibition of cell cycle reentry (Fig. 5I), suggesting that induction of neonatal-like ECM composition and cell-cycle reentry are closely linked to support cardiac regeneration.
Fig. 5. miR-1/133a control expression of ECM components in cardiomyocytes via OSMR and FGFR1.
(A) Wheat germ agglutinin (WGA) immunofluorescence and trichrome staining of ECM deposition in heart sections from adult ctrl and knockout mice [ctrl, n = 2; dKO, n = 2; tKO (OR), n = 5; tKO (FR1), n = 5]. Scale bars, 20 μm. (B) Top 10 GO terms enriched in dKO compared to ctrl hearts. Terms associated with ECM are in red. (C) Microarray-based GSEA of adult ctrl and knockout hearts and heatmap of the 10 most up-regulated genes in dKO hearts derived from the respective gene set. Additional knockout of Osmr (tKO OR) or Fgfr1 (tKO FR1) or treatment with UO126 [ctrl: n = 4; dKO, dKO + UO, tKO (OR), tKO (FR1): n = 3] abolished enrichment in dKO hearts. (D and E) RT-qPCR analysis of different ECM genes in adult dKO (n = 4) and control cardiomyocytes (n = 5) and noncardiomyocytes (control, n = 4; dKO, n = 2 to 3) from 15-week-old mice, 3 weeks after tamoxifen administration. (F) Depiction of expression changes in neonatal and adult mice hearts of genes affecting ECM stiffness. (G and H) RT-qPCR analysis of agrin (F) (ctrl, n = 4; dKO, n = 3) and periostin (G) (ctrl, n = 4; dKO, n = 4) expression in isolated cardiomyocytes and noncardiomyocytes (n = 5) from adult dKO hearts compared to controls (two-tailed unpaired t tests). (I) Heatmap of genes related to soft ECM increased in miR-1/133a dKO versus wild-type (WT) hearts. Additional deletion of Osmr (tKO OR) or Fgfr1 (tKO FR1) or treatment with UO126 abolished increased expression. *P < 0.05 and ****P < 0.0001.
Cardiomyocytes undergo a pronounced switch during maturation from glycolytic metabolism to oxidative phosphorylation, which is accompanied by massively enhanced mitochondrial biogenesis at early postnatal stages (44). Since dedifferentiation of adult cardiomyocytes may result in a MEK/ERK mediated resistance to ischemia (18, 45), we performed a GO term analysis of metabolic changes in miR-1/133a dKO hearts. We detected a general decline of metabolic processes in adult dKO hearts and a pronounced shift from fatty acid metabolism/oxidative decarboxylation to glycolysis (Fig. 6), indicating that depletion of miR-1/133a forces cardiomyocytes to acquire metabolic features, reminiscent of immature cardiomyocytes (Fig. 6, A and B).
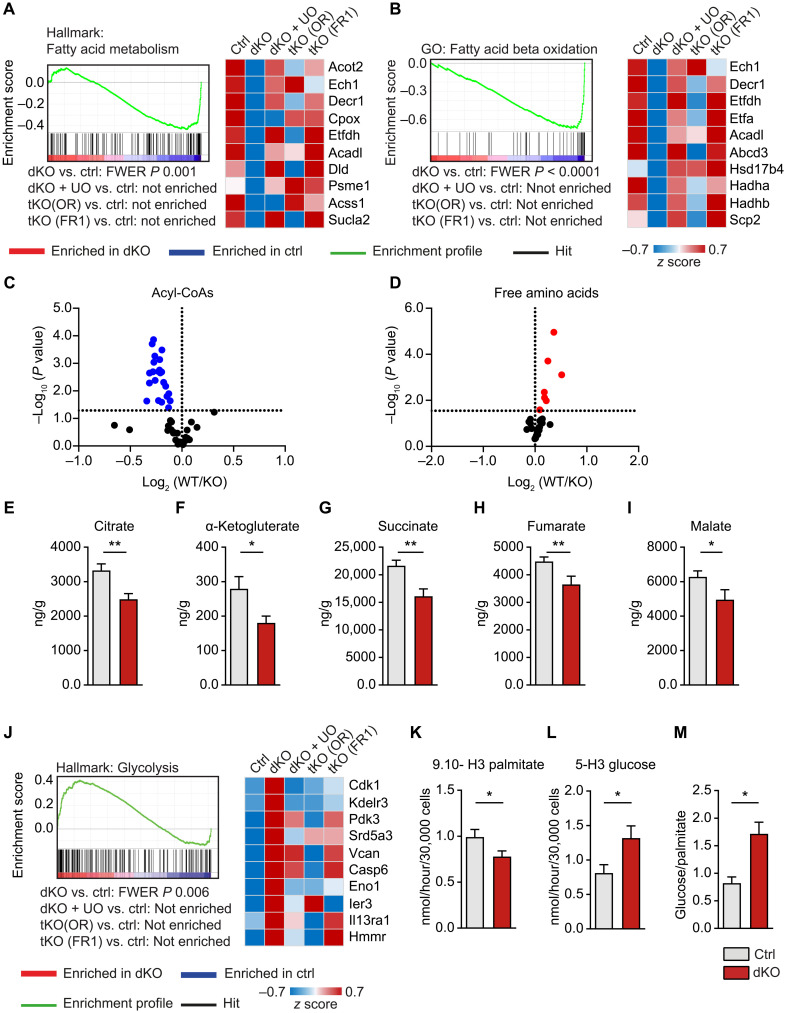
Fig. 6. Loss of miR-1/133a induces a metabolic shift in adult cardiomyocytes.
(A and B) GSEA of miR-1/133a knockout (dKO) versus WT hearts demonstrating down-regulation of genes related to fatty acid metabolism. Additional deletion of Osmr (tKO OR) or Fgfr1 (tKO FR1) in miR-1/133a dKO mice or UO126 treatment abolishes down-regulation. Heatmaps of the 10 most down-regulated genes in dKO hearts derived from the respective gene sets [ctrl: n = 4; dKO, dKO + UO, tKO (OR), tKO (FR1): n = 3]. (C to I) Metabolome analysis of dKO (n = 9) compared to control hearts (n = 12) showing significant decline of all detected acyl-CoA species (C) and increase of all detected free amino acids (D). Each dot in the volcano plots represents one specific metabolite. (E to I) Concentrations of metabolites demonstrate decreased TCA activity in miR-1/133a dKO hearts. (J) GSEA of microarray data from adult mouse hearts and heatmap of the 10 most up-regulated genes in dKO hearts derived from the respective gene set, showing enrichment of genes related to glycolysis in miR-1/133a (dKO) versus WT hearts. Additional knockout of Osmr (tKO OR) or Fgfr1 (tKO FR1) or UO126 treatment of dKO hearts abolished enrichment. (K to M) Assessment of palmitate and glucose catabolism, demonstrating decreased fatty acid oxidation (K) but increased glycolysis (L) and increased glycolysis/fatty acid oxidation ratios (M) in miR-1/133a dKO cardiomyocytes compared to ctrl (n = 8 to 9; one-tailed unpaired t test). *P < 0.05 and **P < 0.01.
Further metabolomic analysis supported results of the transcriptional analysis and revealed down-regulation of the fatty acid metabolism with a massively reduced amount of acyl–coenzyme A (CoA) concentrations in dKO hearts (Fig. 6C). We also observed a significant increase in some free amino acids (Fig. 6D), probably due to reduced oxidative phosphorylation in dKO cardiomyocytes. Moreover, we found a decrease in metabolites of the tricarboxylic acid (TCA) cycle (Fig. 6, E to I), while gene set enrichment analysis (GSEA) suggested increased activity of genes related to glycolysis in dKO hearts (Fig. 6J), suggesting a shift from fatty acid oxidation to glycolysis in dKO mice. To corroborate this assumption, we analyzed cardiomyocyte metabolism by direct measurement of the consumption of fatty acids and glucose in isolated cardiomyocytes. Freshly isolated cardiomyocytes were incubated in medium containing either tritium-labeled palmitate or glucose to quantify fatty acid oxidative and glycolytic activity. We observed a decrease in fatty acid oxidation and increased glycolysis in dKO cardiomyocytes (Fig. 6, K to M), supporting the results of the transcriptome and metabolomic analysis and confirming the hypothesis that adult dKO cardiomyocytes undergo a metabolic shift.
Loss of miR-1/133a leads to decreased scar size after myocardial infarction
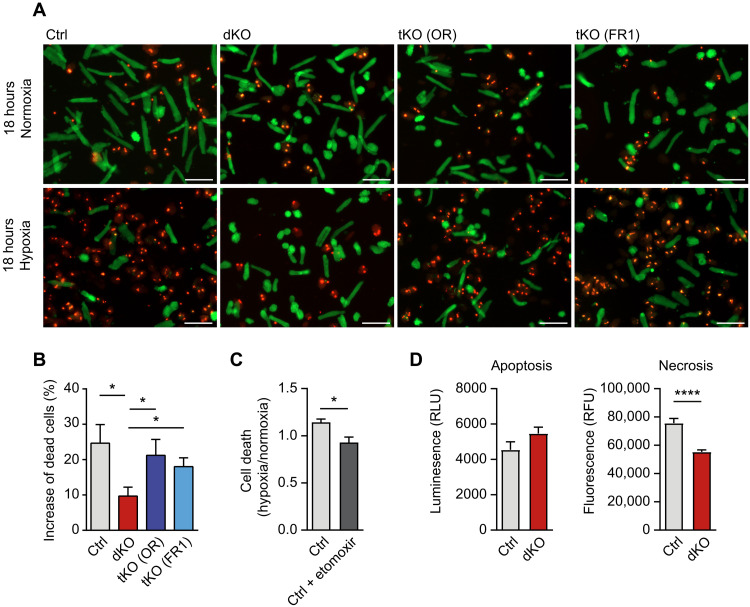
The reduced expression of contractile proteins and the metabolic switch from oxygen-consuming oxidative phosphorylation suggested that miR-1/133a dKO cardiomyocytes may be more resistant to hypoxia. To examine this possibility, we cultured isolated ventricular cardiomyocytes for 18 hours at 1% O2. Cell viability assays revealed a substantial increase of surviving miR-1/133a dKO cardiomyocytes compared to controls. As expected, additional inactivation of Osmr and Fgfr1 neutralized the beneficial effects of miR-1/133a deletion (Fig. 7, A and B). Treatment of adult cardiomyocytes with etomoxir improved survival of cardiomyocytes under hypoxia, partially mimicking the phenotype of miR-1/133a dKO cardiomyocytes, characterized by reduction of fatty acid oxidation (Fig. 7C). Notably, we did not observe a significant decline of apoptosis in miR-1/133a dKO cardiomyocytes under hypoxia but a significant drop of necrosis, suggesting that enhanced survival does not depend on a decrease of programmed cell death (Fig. 7D). Since extended loss of maturity in cardiomyocytes due to loss of miR-1/133a will compromise cardiac function, we decided to investigate potential beneficial effects of miR-1/133a depletion for cardiac function after myocardial infarction by transient suppression of miR-1/133a activity. Locked nucleic acids (LNAs) directed against miR-1/133a were injected 7 days before transient ligation of left anterior descending coronary artery (LAD) into miR-1-1/133a-2 mutant mice, which still carry the intact miR-1-2/133a-1 gene cluster and do not show any apparent changes in cardiomyocyte physiology (32). Quantification of scar sizes 14 days after myocardial infarction revealed a decrease in scar size by approximately 50% after miR-1/133a LNA injection compared to control-injected miR-1-1/133a-2 mutant hearts (Fig. 8, A to C, and fig. S11). Unexpectedly, when injections were done at the same time as the LAD ligation, LNA–miR-1/133a increased scar sizes (fig. S12). We concluded that it requires a certain time before the beneficial impact of a loss of miR-1/133a becomes effective. Moreover, the cellular changes induced by loss of miR-1/133a are apparently operative during the time of injury, which argues for protective roles of the reprogramed metabolism and ECM after loss of miR-1/133a rather than for beneficial effects of cell cycle entry of dedifferentiated cardiomyocytes or stimulation of cardiac regeneration.
Fig. 7. Loss of miR-1/133a increases survival of adult cardiomyocytes under hypoxia.
(A and B) Cell viability assays of adult miR-1/133a dKO compared to ctrl cardiomyocytes under normoxia and hypoxia (18 hours; 1% O2). Increased survival of miR-1/133a dKO cardiomyocytes is prevented in tKO (OR) and tKO (FR1) mice [ctrl, n = 9; dKO, n = 7; tKO (OR), n = 5; tKO (FR1), n = 6; one-tailed unpaired t test]. Scale bars, 100 μm. (C) Cell viability assay of adult mouse cardiomyocytes without and with etomoxir (100 μM, 12 hours) under normoxia and hypoxia (18 hours, 1% O2). Etomoxir treatment increases the number of viable cardiomyocytes under hypoxia (n = 3 each; two-tailed unpaired t test). (D) Assays for apoptosis and necrosis using isolated adult mouse cardiomyocytes reveals no significant difference in apoptosis, but reduced necrosis of miR-1/33a dKO compared to control cardiomyocytes (n = 2 animals each, 4 technical replicates each). RLU, relative luminescence units; RFU, relative fluorescence units.
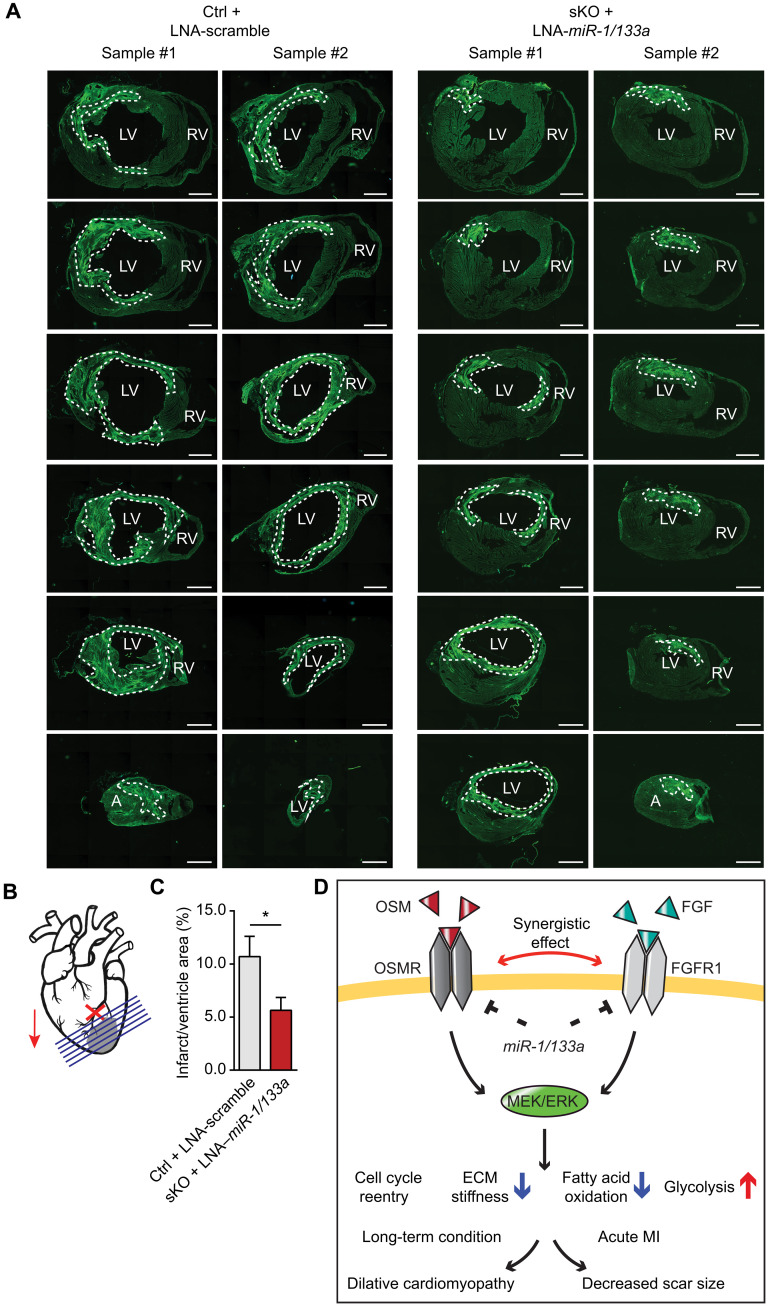
Fig. 8. Loss of miR-1/133a reduces scar size after myocardial infarction.
(A and B) Analysis of myocardial scar formation by WGA staining 14 days after ischemia and reperfusion (I/R) injury. Injection of control and miR-1/133a sKO mice with scrambled control and LNA–miR-1/133a, respectively, was done 7 days before I/R injury. The dashed line indicates quantified infarct areas. Sections were taken every 50 μM along the basal-apical axis. Scale bars, 100 μm. LV, left ventricle; RV, right ventricle. (C) Quantification of scars relative to whole ventricular areas in LNA–miR-1/133 and control-injected animals (ctrl, n = 14; sKO, n = 12; two-tailed unpaired t test). *P < 0.05. (D) Model of the putative regulatory mechanisms of cardiomyocyte dedifferentiation controlled by OSMR, FGFR1, and miR-1/133a. Activation of OSMR and FGFR1 leads to increased MEK/ERK signaling, which is required for induction of cardiomyocyte dedifferentiation, including enhanced cell cycle entry, reduced ECM stiffness, decreased fatty acid oxidation, and increased glycolysis. miR-1/133a suppresses expression of FGFR1 and OSMR receptors and thereby locks cardiomyocytes in a differentiated state. Down-regulation of miR-1/133a in the border zone of infarcted hearts or inactivation of miR-1/133a facilitates dedifferentiation of cardiomyocytes by allowing increased FGFR1 and OSMR expression. Inactivation of Osmr or Fgfr1 normalizes gene expression and prevents cardiomyocyte dedifferentiation in miR-1/133a dKO hearts.
DISCUSSION
Maintenance of a fully functional contractile state in cardiomyocytes is essential to master the enormous work load of the adult heart. On the other hand, acquisition of a less mature, dedifferentiated state may be beneficial for cardiomyocytes to withstand an adverse environment and initiate regeneration. Osmr and Fgfr1 are important components of the molecular machinery regulating cardiomyocyte dedifferentiation and proliferation. Several studies demonstrated that OSMR is a major driver of cardiomyocyte dedifferentiation, increases the propensity to enter the cell cycle, enhances resistance to hypoxia, and transiently protects the myocardium (20, 21, 23), while FGFR1 supports myocardial regeneration in zebrafish (46) and reentry of cardiomyocytes into the cell cycle (24, 47–50). After inhibition of p38 signaling, FGF1 also stimulates proliferation of postnatal mammalian cardiomyocytes (15). Here, we found that OSM and FGF2 act synergistically to induce dedifferentiation of isolated adult rat cardiomyocytes. Furthermore, we obtained evidence for a regulatory cross-talk between the OSMR and FGFR1. Osmr1 and Fgfr1 are both strongly up-regulated in the border zone after myocardial infarction, which suggests that cardiomyocytes attempt to shed the differentiated state under certain pathological conditions to acquire resilience toward adverse environmental conditions and enter the cell cycle.
The up-regulation of OSMR1 and FGFR1 has been mostly attributed to positive feedback loops, in which receptor concentration increase when concentrations of corresponding ligands rise (20). On the basis of a search for potential miRNAs binding sites, we found an additional layer of regulation. We found that miR-1/133a posttranscriptionally represses Osmr and Fgfr1 expression and thus act as critical regulators of cardiomyocyte dedifferentiation and cell cycle entry. Up-regulation of OSMR and FGFR1 in the infarct border zone matches the down-regulation of miR-1/133a after myocardial infarction and during DCM (51–53), suggesting that loss of differentiated features is a common modality to cope with pathological cues. miR-1 and miR-133a are the most abundant miRNAs in the heart and known to repress multiple targets during prenatal heart development (41, 54–56). However, their function in adult hearts has remained enigmatic because of embryonic lethality of miR-1/133 compound mutants (39). So far, the role of miR-1 and miR-133a genes has only been studied separately (41, 54–56), although the miR-1/133a gene cluster represents a functional unit (39).
miRNAs are assumed to target many different genes, often in the same pathway. We observed the same phenomenon for miR-1/133a in the adult heart and validated several potential miR-1/133a targets using luciferase assays. However, the majority of effects caused by inactivation of both miR-1/133a gene clusters were clearly attributed to the up-regulation of Osmr and Fgfr1. Analysis of miR-1/133a/Osmr or miR-1/133a/Fgfr1 triple mutants unequivocally demonstrated that effects of miR-1/133a on cell cycle activity, ECM composition, and metabolism were mediated by Osmr and Fgfr1. In contrast, regulation of genes coding for contractile proteins was not normalized by inactivation of by Osmr and Fgfr1, suggesting additional modes of action. Activation of Osmr and Fgfr1 expression by loss of miR-1/133a led to enhanced ERK activity, which is instrumental for OSMR-mediated cardiomyocyte dedifferentiation (20). Inhibition of ERK reversed several aspects of the miR-1/133a dKO phenotype and normalized expression of most of the up-regulated genes, essentially phenocopying the effects of Osmr and Fgfr1 inactivation. This finding reveals that ERK signaling has a central role in miR-1/133a–mediated repression of OSMR and FGFR1, albeit either receptor uses several different signal transduction routes (57, 58). Nevertheless, we do not claim that miR-1/133a exert their functions exclusively via OSMR and FGFR1. We observed up-regulation of the miR-1/133a targets Myocardin and Kcnmb1 (39) and an increase of Calponin 1 (Cnn1), Calponin 2 (Cnn2), and Myotrophin, which also carry putative miR-1 or miR-133a binding sites. Cnn1 and Cnn2 are smooth muscle–specific, actin-, tropomyosin-, and calmodulin-binding proteins (59, 60) that are expressed in embryonic cardiomyocytes (61). Myotrophin exhibits multiple functions including actin-binding (62) and stimulation of myocyte growth by nuclear translocation of p65 (63). Obviously, such molecules might also play a role in promoting dedifferentiation and acquisition of a less mature phenotype of cardiomyocyte.
Previous studies reported that constitutive activation of Erbb2 in adult cardiomyocytes promotes cardiomyocyte dedifferentiation and proliferation, which is completely abrogated by inhibition of ERK signaling (64). The authors attributed the loss of regenerative capacity and lack of cardiomyocyte proliferation in the adult heart to the down-regulation of Erbb2 during the first postnatal week. In contrast to the up-regulation of OSMR and FGFR after myocardial infarction, expression of ERBB2 further declines after myocardial infarction, essentially ruling out that activation of ERK and cardiomyocyte dedifferentiation in the border zone relies on ERBB2. In addition to ERBB2, other pathways were found to contribute to the transition from proliferation to cell cycle arrest including the Hippo pathway (65), the homeobox protein Meis1 (66), redox signaling (67), and inflammatory macrophages (68). It remains to be seen whether these pathways can be as readily manipulated in the adult heart as the miR-1/133a-OSMR pathway to stimulate responses, which protect the myocardium and potentially facilitate regeneration.
Although enhanced cell cycle entry is an interesting feature of dedifferentiated cardiomyocytes, potentially enabling a proliferative response to replace lost cardiomyocytes and regenerate the heart, the profound metabolic changes that occur during dedifferentiation might provide a direct benefit for the ischemic heart. Recent publications highlight that inhibition of fatty acid oxidation (69) or stimulation of glycolysis (70) promotes cardiomyocyte proliferation, which is in line with our data. The switch from oxidative phosphorylation to glycolytic metabolism reduces oxygen consumption, which also might be supported by reduced energy requirements due to partial disassembly of sarcomeres. In essence, dedifferentiated cardiomyocytes enter a protective hibernation state, which reduces contractile power but might enhance cellular survival. miR-1/133a are important for preventing untimely transition into such a state. Our studies reveal that metabolic reprogramming of cardiomyocytes following inactivation of miR-1/133a occurs via up-regulation of Fgfr1 and Osmr. Although the induction of metabolic changes by OSM in cultured cardiomyocytes (71) argues for an indirect function of miR-1/133a via OSMR, other direct miR-1 targets such as the estrogen-related receptor β (Errβ), regulating glycolysis, glycogenesis, and the expression of sarcomeric proteins (55) might also contribute.
Changes in the composition of the ECM are another major hallmark of dedifferentiation. The ECM stiffness changes during development with higher ECM stiffness in adult compared to neonatal hearts. Reduced ECM stiffness might support cardiomyocyte proliferation and augment the regenerative potential of the neonatal heart after injury (42). Several studies demonstrated that a specific composition of the ECM facilitates heart regeneration (72). In particular, two proteins, agrin and periostin, were reported to play important roles during these processes (12, 43), which were both strongly up-regulated after loss of miR-1/133a together with several other ECM components. We hypothesize that the metabolic switch and changes in the composition of the ECM are most relevant for enhanced resilience of miR-1/133a dKO cardiomyocytes under hypoxia. Since we did not observe a major surge in cardiomyocyte cytokinesis after loss of miR-1/133a despite increased cell cycle entry, we reason that miR-1/133a dKO cardiomyocytes acquire a more pro-proliferative state, making them more receptive to signals that induce cardiomyocyte division. This conclusion is supported by increased EdU incorporation of miR-1/133a dKO compared to control cardiomyocytes after treatment with agrin. However, despite acquisition of a pro-proliferative state, we did not obtain convincing evidence that the pathways activated by loss of miR-1/133a are sufficient to induce cardiac regeneration.
The massive differences between short-term suppression of miR-1/133a and long-term inactivation, i.e., smaller myocardial scars versus cardiac failure, highlight the necessity to carefully control cardiomyocyte dedifferentiation and proliferation in a temporal and spatial manner. Transient induction of dedifferentiation clearly has beneficial effects and protects the heart when done 7 days before myocardial infarction. In contrast, long-term dedifferentiation leads to down-regulation of contractile proteins and adverse cardiac remodeling and reduces contractility of the heart, resulting in DCM and cardiac failure. In summary, we propose that down-regulation of miR-1/133a in the infarction and border zone will increase expression of Osmr and Fgfr1, promote dedifferentiation and cell cycle reentry, and increase resistance against hypoxia due to the metabolic shift from fatty acid oxidation to glycolysis (Fig. 8D). Continued or increased expression of miR-1/133a will reinforce the differentiated state of cardiomyocytes in unaffected regions of the heart, which has to compensate for the loss of contractile power to maintain sufficient perfusion. Although down-regulation or loss of miR-1/133a alone is not sufficient to trigger regeneration, up-regulation of the miR-1/133a targets Osmr and Fgfr1 may set the stage for cardiac regeneration by creating a pro-proliferative state. We expect that the mounting knowledge about pathways regulating cardiomyocytes dedifferentiation and proliferation will empower combinatorial approaches to overcome the multiple barriers that prevent productive division of cardiomyocytes.
MATERIALS AND METHODS
Ethics statement
All animal experiments were in accordance with German animal protection laws and were approved by the local governmental animal protection committee (B2_1008/B2_1022/B2_1084/B2_1196). All experiments performed in this study comply with the Declaration of Helsinki and were approved by the responsible ethical committee. Informed consent of the patients was given in agreement with the requirements of the responsible ethical committees.
Human myocardial samples
Cardiac tissue from patients with ICM and a left ventricular ejection fraction below 25% was obtained during heart transplantation. Tissue samples originating from subvalvular myocardial resections of patients with aortic stenosis and preserved ejection fraction (>60%) were used as controls. The ventricular architecture and differentiation status of the control myocardium were preserved. Samples showed very little inflammatory infiltrates and no signs of dedifferentiation as determined by immunofluorescence. All tissue samples were flash-frozen and kept at −80°C until usage. Before heart transplantation, all patients had a longstanding history of myocardial infarction. The diagnosis of ICM was based on clinical history, echocardiography, and cardiac catheterization data. Patients were functionally classified according to the New York Heart Association criteria and medically treated according to the guidelines of the European Society of Cardiology.
Knockout, transgenic mice, and injections of inhibitors
Wild-type genomic DNA of the miR-1-2/miR-133a-1 (mouse chr. 18) locus was recovered by Pvu II digestion of a previously described targeting vector (39), generated by recombination with a bacterial artificial chromosome containing the relevant genomic DNA (129S7AB2.2; bMQ332I08). A loxP site flanked by an Eco RV and a Nhe I restriction site (AACTTCGTATAGCATACATTATACGAAGTTATgatatcgctagcAT, gctagcgatatcATAACTTCGTATAATGTATGCTATACGAAGTTAT) was inserted into the Pac I site 3′ of the mir-1-2/133a-1 coding region. A neomycin resistance cassette was inserted into a Bsa BI site 5′ of the mir-1/miR-133a coding region. The cassette was flanked by Asc I sites, flp recombination sequences, and contained a loxP site at the 5′ end. After homologous recombination in embryonic stem cells and germline transmission of the allele, the neomycin resistance cassette was removed by flp recombination (73). Mice were mated to miR-1-1//miR-133a-2 mutant mice (39), Mck-Cre (74), and Myh6-MerCreMer (33) mice to delete the miR-1/133a locus (dKO) at different developmental time points. For the additional deletion of Osmr [tKO(OR)] or Fgfr1 (tKOFR1), we used previously generated Osmr (35) and Fgfr1 (36) alleles to generate Myh6-MerCreMerpos/miR-1-1/133a-2−/−/miR-1-2/133a-1fl/fl/Osmr−/− [tKO (OR)] and Myh6-MerCreMerpos/miR-1-1/133a-2−/−/miR-1-2/133a-1fl/fl/ Fgfr1fl/fl [tKO (FR)] animals. To activate Cre recombinase in adult mice tamoxifen (200 mg/kg, intraperitoneally) was injected at five consecutive days in 8- to 10-week-old mice. UO126 (0.2 mg/kg) was administered by intraperitoneal injection three weeks after the first tamoxifen injection for three consecutive days. Heart tissue was isolated 30 min after the last injection. Deletion of the conditional allele was induced in 8- to 10-week-old adult mice (mean, 7.4 ± 1.8 weeks) if not stated otherwise. Animals of both sexes were euthanized for molecular analyses 4.1 ± 1.4 weeks and for histology 7.2 ± 1.3 weeks after induction of recombination. Unless stated otherwise, wild-type littermates were used as controls. Neonatal mice were analyzed from P0 to P5. LNA antimirs [Exiqon, #199900; 3.5 mg/kg body weight (BW) i-mmu-miR-1a-3p and i-mmu-miR-133a-3p or 7 mg/kg BW scrambled negative control] were injected intravenously 7 days before LAD ligation or concomitant with LAD ligation into sKO (miR-1-2/133–1−/−) or control animals.
Microarrays and quantitative RT-PCR
Total RNA from control and knockout whole hearts (neonatal P5, n = 3 and adult hearts, n = 3) was extracted using Peqlab Gold TriFast according to the manufacturer’s instructions. RNA quality was analyzed using the Agilent Bioanalyzer and the RNA 6000 Nano Kit. Affymetrix DNA microarray reactions were performed according to standard protocols, and samples were hybridized to Affymetrix GeneChip Mouse Gene 1.0 ST arrays, processed, scanned, analyzed, and normalized (RMA with Affymetrix Expression console). Fold changes were calculated, and data were analyzed by GSEA (GSEA 4.0). GSEA data were corrected for multiple testing using false discovery rate (FDR) or family-wise error rate (FWER) method.
Reverse transcription of purified RNA was done either according to the Invitrogen SuperScript II Reverse Transcriptase (18064-014) protocol or following the Applied Biosystems TaqMan MicroRNA Reverse Transcription Kit (4366596). For analysis of relative gene expression, the following TaqMan gene expression assays were used: pri-miRNA mmu-mir-1a-1 Mm03306163_pri, pri-miRNA mmu-mir-1b Mm03308741_pri, pri-miRNA mmu-mir-133a-1 Mm03306281_pri, pri-miRNA mmu-mir-133a-2 Mm03307401_pri, hsa-miR-1 002222, hsa-miR-133a 002246, U6 snRNA 001973, Gapdh 4352339E, Myh7 Mm01319006_g1, Acta2 Mm00725412_s1, Cdk1 Mm00772472_m1, Ccnd2 Mm00438070_m1, Nppa Mm01255748_g1, Anln Mm00503748_m1, Ccnb1 Mm03053893_Gh, Ccna2 Mm00438063_m1, Prkcd Mm00440891_m1, Pdk3 Mm00455220_m1, Myc Mm00487804_m1, Kit Mm00445212_m1, Klf4 Mm00516104_m1, Cdk1 Mm00772472_m1, Runx1 Mm01213404_m1, Fgf1 Mm00438906_m1, Fgfr1 Mm00438930_m1 and Hs00384276_m1, E2f1 Mm00432936_m1, E2f3 Mm01138833_m1, Myh6 Mm00440359_m1, Osm Mm_01193966_m1, Osmr Mm01307326_m1 and Hs00384276_m1, Sprr1a Mm01962902_s1, Myh11 Mm00443013_m1, Agrin Mm01264855_m1, and Periostin Mm01284919_m1.
SYBR Green RT-qPCRs were performed using the Applied Biosystems Step-OnePlus system. RT-qPCR reactions were done with the help of the FastStart Universal SYBR Green Mastermix (Roche) or the KAPA SYBR FAST qPCR Master Mix (KAPA Biosystems) using the following oligonucleotides: Cnn1 (forward, CATCAAAGCCATTACCAAG and reverse, CTTGACTCCCACATTGACTTTG), Cnn2 (forward, GAACAAGCTGCAGCCAGGCTC and reverse, GTCCACAGGGTTCATGCCGTAG), Mtpn (forward, GAAGCCTCTTCATTATGCTGC and reverse, GACAGAAGCAATTTCACGCAG), and Osmr (forward, AGCCGCCAATCGTGCCAACA and reverse, TGCACTGGCACGGGTGGTTC). Data were normalized to Gapdh (ACCACAGTCCATGCCATCAC and CATGCCAGTGAGCTTCCCGT). Relative expression levels were calculated by the ΔΔCT method.
Morphological analysis, EdU staining, and immunohistochemistry
Hearts of neonatal and adult control and knockout mice were isolated, fixed in 4% paraformaldehyde (PFA)/phosphate-buffered saline (PBS) and equilibrated in 15% sucrose/PBS for 1 hour and 30% sucrose/PBS overnight at 4°C, followed by embedding in Tissue-Tek and cryosectioning (10 μm). Hematoxylin and eosin staining was performed using standard protocols (39). Trichrome staining was performed using the standard protocol supplied by the manufacturer [trichrome stains (Masson): HT15, Sigma-Aldrich; Weigert’s iron hematoxylin solution: HT1079, Sigma-Aldrich]. For immunohistochemical staining, sections were washed with 1× PBS and incubated in blocking solution containing 5% normal goat serum, 1% bovine serum albumin, and 0.3% Triton X-100 for 1 hour at room temperature. Antibodies were incubated in blocking solution 1:200 overnight at 4°C. After washing with PBS, secondary antibodies and 4′,6-diamidino-2-phenylindole was applied for 1 hour at room temperature, followed by 3× 5 min washing in PBS and embedding in Fluoromount. EdU staining was performed according to the manufacturer’s protocol.
The following antibodies were used: monoclonal anti–α-actinin (1:200, sarcomeric; Sigma-Aldrich, A7811), rabbit anti–atrial–natriuretic peptide (1:200; Chemicon International, AB5490), monoclonal anti-SMA (1:1000; Sigma-Aldrich, C6198), rabbit anti–phospho–Histone 3 (1:200; Ser10, Upstate Cell Signaling Solutions, no. 06-570), monoclonal anti–troponin I (1:200; Sigma-Aldrich, T 6277), anti–cardiac troponin T antibody (1:200; Abcam, ab105439), monoclonal anti-mouse CD11b fluorescein isothiocyanate (FITC; 1:50; eBioscience, 11-0112-85), rabbit anti-mouse OSM (1:100; ProteinTech, 10982-1-AP), and lectin from Triticum vulgaris FITC conjugate (1:250; Sigma-Aldrich, no. L4895). The following secondary antibodies were used: anti-rabbit Alexa Fluor 594 (1:1000; A11012, Invitrogen) anti-rabbit Alexa Fluor 546 (1:1000; A11035, Invitrogen), anti-mouse Alexa Fluor 594 (1:1000; A11005, Invitrogen), and anti-rabbit Alexa Fluor 488 (1:1000; A11070, Invitrogen).
Isolation of adult mouse cardiomyocytes
Isolation of adult control and knockout cardiomyocytes was performed as described (32). Cells were plated on 0.1% laminin-coated plates and allowed to attach for 3 hours. Cells were harvested for RNA/protein isolation or fixed with 4% PFA/PBS for immunofluorescence staining.
Isolation of adult rat cardiomyocytes
Ventricular cardiomyocytes were isolated from 200- to 250-g Sprague Dawley rats and cultured exactly as previously described (20). Myocytes were plated in basic medium at a density of 1.5 × 104 cells/cm2 on laminin (10 μg/ml; Millipore)–coated dishes (Nunc) and allowed to recover for 1 day. Then, cardiomyocytes were kept continuously in basic medium containing 5% fetal calf serum (Sigma-Aldrich).
Isolation of neonatal cardiomyocytes and transfection
Neonatal cardiomyocytes (P0 to P3) were isolated using Neonatal Dissociation and Neonatal Cardiomyocyte Isolation kits (Miltenyi Biotec, 130-098-373/130-100-825) following protocols provided by the manufacturer. Cardiomyocytes were plated on 10% fibronectin/PBS-coated 24-well plates in standard cell culture medium at 37°C, 5% CO2. Cells were incubated for 3 days before transfection or stimulation. Transfection of miRIDIAN microRNA mimic miR-1, miR-133a, or scramble controls (GE Healthcare, C310376-07, C-310408-07, and CN-001000-01) was performed in triplicates using the DharmaFECT 1 Transfection Reagent (GE Healthcare, T-2001) following instructions of the manufacturer. Cells were harvested for RNA extraction after 24 hours.
Stimulation/inhibition of neonatal cardiomyocytes
Neonatal (P0 to P3) cardiomyocytes were isolated and cultured as described above. Neonatal cardiomyocytes were stimulated for 24 hours with recombinant FGF1 (45 nM; Invitrogen, 13241-013) or recombinant OSM (10 ng/500 μl; R&D Systems), or combination of both cytokines was done for 24 hours. Cells were then harvested for further analysis. Agrin treatment (100 ng/ml) of neonatal cardiomyocytes was performed as previously described (12).
Stimulation of adult rat cardiomyocytes
Cultures were stimulated with human FGF2 (20 ng/ml; PeproTech), mouse OSM (20 ng/ml; OSM; Sigma-Aldrich), and albumin for the controls (20 ng/ml; Sigma-Aldrich) as indicated in the figures. Medium was exchanged every other day. Cultures were processed for Western blot and immunofluorescence analysis as described previously (20). Transfection of cardiomyocyte cultures was performed with the DharmaFECT transfection reagent (Dharmacon) 1 day before stimulation using 0.05 μM miRs and miR controls as described for the neonatal mouse cardiomyocytes.
EdU injections
Ten- to 12-week-old mice were injected daily for 1 week with EdU (50 mg/kg, intraperitoneally; Life Technologies, A10044) 2 weeks after the first tamoxifen injection, followed by isolation of cardiomyocytes. EdU labeling was performed using the Click-iT plus EdU Imaging Kit (Invitrogen C10639).
Western blot analysis
For Western blot analysis, 10 μg of protein were loaded on NuPAGE Novex 4–12% bis-tris Gels (Invitrogen) and processed as described (32). The following antibodies were used: rabbit anti-FGFR1 (1:500; no. 9740, Cell Signaling Technology), goat anti-OSMRβ (1:500; AF662, R&D Systems), mouse anti-myotrophin (1:500; 611830, BD), rabbit anti-GAPDH (1:5000; no. 2118, Cell Signaling Technology), rabbit anti-pERK (1:500; no. 9101, Cell Signaling Technology), mouse anti-panERK (1:500; 612641, BD), rabbit anti-ACTN1 (1:1000; ab68194, Abcam), rabbit anti-ACTN4 (1:1000; ab108198, Abcam), mouse anti-αSMA (1:1000; A5228, Sigma-Aldrich), rabbit anti–hexokinase I (1:1000; no. 2024, Cell Signaling Technology), mouse anti-MAD2 (1:1000; 610679, BD), mouse anti-Myh (1:1000; LS-B6307, LSBio), mouse anti-myomesin (1:100; clone M5, gift of H. M. Eppenberger), mouse anti-PCNA (1:1000; 555566, Bionity), rabbit anti–phospho–Histone H3 (Ser10) (1:1000; no. 9701, Cell Signaling Technology), rabbit anti–phospho-MEK1/2 (1:1000; no. 3958, Cell Signaling Technology), mouse anti–Ral A (1:5000; 610222, BD), rabbit anti-RUNX1 (1:1000; ab92336, Abcam), rabbit anti-SDHA (1:1000; no. 5839, Cell Signaling Technology), mouse anti-TIMP1 (1:1000; MAB9801, R&D Systems), and anti-goat Alexa Fluor 594 (1:1000; A-21468, Invitrogen).
Northern blot analysis
Total RNA from heart or liver tissue was isolated using the TRIzol method (Invitrogen). Five micrograms of RNA were loaded per lane, subjected to electrophoresis, blotted, and probed with (γ-32P)ATP-labeled miR-1 or miR-133a and U6 snRNA as described (32).
Hypoxia chamber and cell viability assay
Isolated adult mouse cardiomyocytes were seeded in 12-well plates and placed into the Hypoxystation (Whitley H45 HEPA) for 18 hours. For etomoxir treatment, isolated cardiomyocytes were seeded in 24-well plates and placed into the Hypoxystation for 18 hours after 12-hour incubation with medium with or without 100 μM etomoxir. To create a hypoxic environment, oxygen concentration was decreased to 1% O2. Cell viability was determined using a LIVE/DEAD cell viability kit for mammalian cells according to the protocol of the manufacturer (L3224, Thermo Fisher Scientific). The degree of apoptosis and necrosis was determined using RealTime-Glo Annexin V Apoptosis and Necrosis Assay according to the protocol of the manufacturer (JA1011, Promega).
Fatty acid oxidation and glycolysis assay
Fatty acid oxidation and glycolysis was analyzed as described previously (75), with modifications. Briefly, 30,000 isolated adult mouse cardiomyocytes were incubated in culture medium supplemented with 2.2 μCi glucose d-[5-3H(N)] or 5 μCi palmitic acid [9,10-3H(N)] for 6 hours at 37°C and 5% CO2. After incubation, the supernatant was transferred into glass vials containing hanging wells with prewetted Whatman paper. Glass vials were sealed with rubber stoppers and 3H2O was allowed to equilibrate between medium and prewetted Whatman paper by incubation of glass vials for 48 hours at 37°C. Labeled water captured in the hanging wells and measured in a scintillation counter using LSC-cocktail (Ultima Gold, PerkinElmer). Data were normalized to the amounts of seeded cells.
Metabolomics
Heart tissue sample were snap-frozen immediately after dissection. Frozen heart tissues were grinded with mortar and pestle on dry ice. Thirty to 50 mg per experiment/kit from each sample was used for metabolomic measurements. Different metabolites were purified, measured, and analyzed as described in the following chapters.
Quantification of TCA cycle metabolites
Tissue samples were lysed in ice-cold 85% methanol (10 μl/mg) with two freeze-thaw cycles and centrifuged (15,000g, 5 min, 4°C). An equal volume of supernatant was collected; isotope labeled internal standard were added, and the samples were evaporated to dryness in a Concentrator Plus (Eppendorf, Wesseling-Berzdorf, Germany). Samples were reconstituted in 50 μl of water + 0.5% formic acid, transferred to Autosampler vials and subsequently analyzed by liquid chromatography–tandem mass spectrometry (LC-MS/MS).
Liquid chromatography was performed on an Agilent 1290 Infinity LC system (Agilent, Waldbronn, Germany) consisting a 1290 Bin Pump, a 1290 TCC column oven, a 1290 Sampler, and a 1290 Thermostat. The reversed-phase LC separation was performed using a Waters Acquity UPLC HSS T3 column (150 mm by 2.1 mm, 1.8 μm; Waters, Eschborn, Germany) at 40°C. Gradient elution was performed with 0.5% formic acid in water (mobile phase A) and 0.5% formic acid in methanol (mobile phase B) at a flow rate of 400 μl/min. Gradient conditions were 2% B for 1.5 min, followed by a 3-min gradient to 100% B, followed by a cleaning and equilibration step, resulting in 10 min of total LC run time. Injection volume was 2.5 μl for all samples. Autosampler temperature was 6°C. MS was performed on a QTrap 5500 mass spectrometer (Sciex, Darmstadt, Germany) equipped with an ESI TurboIonSpray source. Electrospray ionization at 400°C and −4500 V in negative ionization mode was used. Ion source gas parameters were as follows: CUR (Curtain Gas), 30 psi; GS1 (Ion Source Gas 1), 45 psi; and GS2 (Ion Source Gas 2), 25 psi. The specific MRM (Multiple reaction monitoring) transition for every compound was normalized to appropriated isotope labeled internal standards. Calibration curves were established with authentic standards. Analyst 1.6.2 and MultiQuant 3.0 (both from Sciex, Darmstadt, Germany) were used for data acquisition and analysis, respectively.
Quantification of free amino acids in tissue
Tissue samples were lysed in ice-cold 85% methanol (10 μl/mg) with two freeze-thaw cycles and centrifuged (15,000g, 5 min, 4°C). An equal volume of supernatant was collected. Sample preparation was performed using the EZ:faast LC-MS free amino acid analysis kit (Phenomenex, Aschaffenburg, Germany) according to the manufacturer’s instructions, with minor modifications. Only 10 μl of the internal standard mix was applied to all samples and to the standard curve. After processing, the sample preparation samples and standards were evaporated and resolved in 75 μl in 66.6% methanol containing 10 mM ammonium formate. Analysis of metabolites was performed by LC-MS/MS using the EZ:faast AAA-MS HPLC column on an Agilent 1290 Infinity LC system (Agilent, Waldbronn, Germany) coupled to a QTrap 5500 mass spectrometer (Sciex, Darmstadt, Germany). The column temperature was set to 35°C. Gradient elution was performed with 10 mM ammonium formate in water (mobile phase A) and 10 mM ammonium formate in methanol (mobile phase B). Conditions for the separation were a 13-min gradient from 68% B to 83% B, followed by an equilibration step. The flow rate was set to 250 μl/min. The injection volume was 1 μl. Electrospray ionization in positive mode was used. The ion source parameter was as follows: CUR, 25 psi; IS, 4000°C; transmission electron microscopy (TEM), 425°C; GS1, 40 psi; and GS2 40 psi. Calibration curves were established with the authentic standards from the EZ:faast kit. The intensity of the measured metabolite was normalized to internal standards. Analyst 1.6.2 and MultiQuant 3.0 (Sciex, Darmstadt, Germany) were used for data acquisition and analysis, respectively.
Quantification of acyl-CoA in tissues
Snap-frozen tissue samples were mixed with acetonitrile/isopropanol (3:1, 20 μl/mg). One hundred microliters of 0.1 M KH2PO4 (pH 6.7) was added to each vial. Ten microliters of internal standards mix was spiked into each sample. Samples were vortexed and centrifuged for 5 min at 15,000g. The supernatant was transferred to a new vial and acidified with 150 μl of acetic acid. Samples were transferred to preconditioned (1 ml of 9:3:4:4 acetonitrile/isopropanol/water/acetic acid) SPE columns [2-(2-pyridyl)ethyl silica gel (54127-U), Supelco, Bellefonte, USA]. Columns were washed with acetonitrile/isopropanol/water/acetic acid (9:3:4:4) and immediately dried under vacuum. Samples were eluted with 1 ml of methanol/200 mM ammonium format (4:1, pH 7). Samples were dried, reconstituted in 50 μl of 80% methanol, transferred to Autosampler vials, and measured by LC-MS.
Analysis of acyl-CoAs was performed by LC-MS/MS using Acquity BEH C18 column (1.7 μm, 2.1 mm by 75 mm, Waters, Milford, USA) on an Agilent 1290 Infinity LC system (Agilent, Waldbronn, Germany) coupled to a QTrap 5500 mass spectrometer (Sciex, Darmstadt, Germany). The column temperature was set to 35°C. Gradient elution was performed with 10 mM ammonium acetate in water [pH 8.5, with InfinityLab deactivator additive (1 ml/liter); Agilent, Waldbronn, Germany] as mobile phase A and 10 mM ammonium acetate in 90% acetonitrile [with InfinityLab deactivator additive (1 ml/liter)] as mobile phase B. Acyl-CoAs were eluted with a 5 min gradient starting with 5% B for 0.2 min, increasing to 45% B in 0.8 min, than to 65% B in 2 min and up to 100% B in 0.1 min, holding 100% B for 2 min, followed by wash an equilibration steps. The flow rate was set to 700 μl/min. The injection volume was 5 μl. Electrospray ionization in positive mode was used. The ion source parameter was as follows: CUR, 20 psi; IS, 3500 V; TEM, 460°C; GS1, 40 psi; and GS2, 40 psi. Calibration curves were performed with authentic standards. The intensity of the measured metabolite was normalized to internal standards. Analyst 1.6.2 and MultiQuant 3.0 (Sciex, Darmstadt, Germany) were used for data acquisition and analysis, respectively.
Induction of myocardial infarction and validation of infarct size measurement
Myocardial infarction was induced by ischemia and reperfusion (I/R). The LAD was ligated for 30 min at distal position causing myocardial ischemia. Afterward, the ligature was released. Hearts were harvested 14 days after I/R. Animals were anesthetized with isoflurane (5 mg/liter). After intubation, anesthesia was maintained during the operation by supplying isoflurane (1.5 mg/liter). Each animal was given 5 IU of heparin sodium (Liquaemin) by subcutaneous injection 20 min before surgery. Pain control was accomplished by subcutaneous preoperative injection of buprenorphine, followed by metamizole treatment in the drinking water. For infarct size measurement, hearts were sectioned and stained. The measurement of the scar size was done using the Fiji package of the ImageJ software.
Luciferase reporter assay
Wild-type and mutant binding sites were cloned into the pmirGLO Dual-Luciferase Vector (E13330, Promega) using Nhe I and Xho I restriction sites. The binding sites were cloned in quadruplicates via phosphorylated oligonucleotides. Sequences of binding sites are as follows: Fgfr1 (miR-133a binding site no. 1), CTAGCTCCTCCTTCCCAGGTGTTGGACCAAGATCCTCCTTCCCAGGTGTTGGACCAAGATCCTCCTTCCCAGGTGTGGACCAAGATCCTCCTTCCCAGGTGTTG GACCAAGAC; Fgfr1 (miR-133a binding site no. 2), CTAGCTACTAATTTGCTTTGCTGACCAAATATACTAATTTGCTTTGCTGACCAAATATACTAATTTGCTTTGCTGACCAAATATACTAATTTGCTTTGCTGACCAA ATAC; Osmrβ (miR-1 binding site), CTAGCAAGAAAATTCTGTCTCACATTCCTATAAGAAAATTCTGTCTCACATTCCTATAAGAAAATTCTGTCTCACATTCCTATAAGAAAATTCTGTCTCACATTC CTATC; Runx1 (miR-1 binding site), CTAGCCTAGAAATATTGTTTACATTAAACTCTAGAAATATTGTTTACATTAAACTCTAGAAATATTGTTTACATTAAACTCTAGAAATATTGTTTACATTAAACTC; Cnn1 (miR-133a binding site), CTAGCGTTCCTACATGATGAAGGACCAACTCCAGTTCCTACATGATGAAGGACCAACTCCAGTTCCTACATGATGAAGGACCAACTCCAGTTCCTACATGATG AAGGACCAACTCCAC; Cnn2 (miR-1 binding site), CTAGCTTTCTAAGAGTAAAGTACATTCCTGATTTCTAAGAGTAAAGTACATTCCTGATTTCTAAGAGTAAAGTACATTCCTGATTTCTAAGAGTAAAGTACATT CCTGAC; Cnn2 (miR-133a binding site), CTAGCGTGAAATTCCCTGTGTGGACCAAAAAAGTGAAATTCCCTGTGTGGACCAAAAAAGTGAAATTCCCTGTGTGGACCAAAAAAGTGAAATTCCCTGTGTGGACCAAAAAAC; and Mtpn (miR-1 binding site), CTAGCGAGTGTATATTGTCTTATCATTCCAGAGAGTGTATATTGTCTTATCATTCCAGAGAGTGTATATTGTCTTATCATTCCAGAGAGTGTATATTGTCTTAT CATTCCAGAC.
Human embryonic kidney cells were transfected at 70% confluency with or without 50 pmol of miRIDIAN microRNA mimic miR-1 or miR-133a (GE Healthcare, C-310376-07 and C-310408-07) and 50 ng of plasmid using Lipofectamine 2000 (Invitrogen). Transfections were done in triplicate in 24-well formats, and cells were harvested 24 hours after transfection. Measurements were done according to the manufacturer’s instructions using the Dual-Luciferase Reporter assay (Promega) and the Mithras LB940plate reader (Berthold).
In silico screen for miR-1 and miR-133a targets
Predicted miRNA target sites for miR-1 and miR-133a (conserved, miRanda August 2010 release and TargetScan release 6.2 June 2012) were matched to expression levels using Affymetrix transcriptome data. Up-regulated transcripts (fold change >1.2 cKO versus control) predicted by TargetScan or miRanda were considered as potential targets. To screen for targets potentially affecting OSM and FGF signaling pathways, predicted targets were filtered with respect to their GO term annotation (GO:0038165, oncostatin-M–mediated signaling pathway; GO:0008543, fibroblast growth factor receptor signaling pathway), resulting in three (Osmr, Fgfr1, and Ctgf) potential target molecules out of 42 listed genes.
Gene set enrichment analysis
GSEA (76, 77) was performed using linear Affymetrix microarray data and GSEA standard settings.
Statistics
The data were tested for normal distribution allowing us to select the appropriate parametric or nonparametric statistical tests. Data are expressed as means ± SEM unless otherwise indicated. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 were regarded to be significant compared to dKO, and #P < 0.05, ##P < 0.01, ###P < 0.001, and ####P < 0.0001 were regarded to be significant compared to control. For statistical analyses, the GraphPad Prism 8 software package was used.
Acknowledgments
We thank B. Matzke, S. Thomas, M. Wiesnet, and K. Richter for excellent technical support.
Funding: German Research Foundation Transregional Collaborative Research Centre 267 TP A05 (T.Br. and T.Bo.), German Research Foundation Research Training Group RTG2355 (T.Bo.), German Research Foundation Collaborative Research Centre 1213 TP B02 (T.Br.), German Research Foundation Transregional Collaborative Research Centre 81 TP A02 (T.Br.), German Research Foundation Excellence Cluster Cardiopulmonary Institute (CPI), EXC2026 project ID 390649896 (T.Br.), Bundesministerium für Bildung und Forschung German Centre for Cardiovascular Research, and Partner Site Rhein-Main project ID 81Z0200302 (T.Br.)
Author contributions: Conceptualization: T.Bo. and T.Br. Methodology: M.V., J.B., K.W.-L., and S.Z. Investigation: M.V., J.B., K.W.-L., and T.K. Human samples: M.R. Supervision: T.Bo. and T.Br. Writing (original draft): M.V., J.B., and T.Bo. Writing (review and editing): T.Bo. and T.Br.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Microarray data are available at ArrayExpress (E-MTAB-10226; www.ebi.ac.uk/arrayexpress/experiments/E-MTAB-10226/.
Supplementary Materials
This PDF file includes:
Figs. S1 to S12
REFERENCES AND NOTES
- 1.Bergmann O., Bhardwaj R. D., Bernard S., Zdunek S., Barnabe-Heider F., Walsh S., Zupicich J., Alkass K., Buchholz B. A., Druid H., Jovinge S., Frisen J., Evidence for cardiomyocyte renewal in humans. Science 324, 98–102 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drenckhahn J. D., Schwarz Q. P., Gray S., Laskowski A., Kiriazis H., Ming Z., Harvey R. P., Du X. J., Thorburn D. R., Cox T. C., Compensatory growth of healthy cardiac cells in the presence of diseased cells restores tissue homeostasis during heart development. Dev. Cell 15, 521–533 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Li F., Wang X., Capasso J. M., Gerdes A. M., Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J. Mol. Cell. Cardiol. 28, 1737–1746 (1996). [DOI] [PubMed] [Google Scholar]
- 4.Zebrowski D. C., Jensen C. H., Becker R., Ferrazzi F., Baun C., Hvidsten S., Sheikh S. P., Polizzotti B. D., Andersen D. C., Engel F. B., Cardiac injury of the newborn mammalian heart accelerates cardiomyocyte terminal differentiation. Sci. Rep. 7, 8362 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porrello E. R., Mahmoud A. I., Simpson E., Hill J. A., Richardson J. A., Olson E. N., Sadek H. A., Transient regenerative potential of the neonatal mouse heart. Science 331, 1078–1080 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haubner B. J., Schneider J., Schweigmann U., Schuetz T., Dichtl W., Velik-Salchner C., Stein J. I., Penninger J. M., Functional recovery of a human neonatal heart after severe myocardial infarction. Circ. Res. 118, 216–221 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Mohamed T. M. A., Ang Y.-S., Radzinsky E., Zhou P., Huang Y., Elfenbein A., Foley A., Magnitsky S., Srivastava D., Regulation of cell cycle to stimulate adult cardiomyocyte proliferation and cardiac regeneration. Cell 173, 104–116.e12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y., Feng J., Song S., Li H., Yang H., Zhou B., Li Y., Yue Z., Lian H., Liu L., Hu S., Nie Y., gp130 controls cardiomyocyte proliferation and heart regeneration. Circulation 142, 967–982 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Judd J., Lovas J., Huang G. N., Defined factors to reactivate cell cycle activity in adult mouse cardiomyocytes. Sci. Rep. 9, 18830 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakada Y., Canseco D. C., Thet S., Abdisalaam S., Asaithamby A., Santos C. X., Shah A. M., Zhang H., Faber J. E., Kinter M. T., Szweda L. I., Xing C., Hu Z., Deberardinis R. J., Schiattarella G., Hill J. A., Oz O., Lu Z., Zhang C. C., Kimura W., Sadek H. A., Hypoxia induces heart regeneration in adult mice. Nature 541, 222–227 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Nguyen N. U. N., Canseco D. C., Xiao F., Nakada Y., Li S., Lam N. T., Muralidhar S. A., Savla J. J., Hill J. A., Le V., Zidan K. A., El-Feky H. W., Wang Z., Ahmed M. S., Hubbi M. E., Menendez-Montes I., Moon J., Ali S. R., Le V., Villalobos E., Mohamed M. S., Elhelaly W. M., Thet S., Anene-Nzelu C. G., Tan W. L. W., Foo R. S., Meng X., Kanchwala M., Xing C., Roy J., Cyert M. S., Rothermel B. A., Sadek H. A., A calcineurin–Hoxb13 axis regulates growth mode of mammalian cardiomyocytes. Nature 582, 271–276 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bassat E., Mutlak Y. E., Genzelinakh A., Shadrin I. Y., Baruch Umansky K., Yifa O., Kain D., Rajchman D., Leach J., Riabov Bassat D., Udi Y., Sarig R., Sagi I., Martin J. F., Bursac N., Cohen S., Tzahor E., The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 547, 179–184 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou B., Honor L. B., He H., Ma Q., Oh J.-H., Butterfield C., Lin R. Z., Melero-Martin J. M., Dolmatova E., Duffy H. S., Gise A., Zhou P., Hu Y. W., Wang G., Zhang B., Wang L., Hall J. L., Moses M. A., McGowan F. X., Pu W. T., Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J. Clin. Invest. 121, 1894–1904 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Przybyt E., Krenning G., Brinker M. G., Harmsen M. C., Adipose stromal cells primed with hypoxia and inflammation enhance cardiomyocyte proliferation rate in vitro through STAT3 and Erk1/2. J. Transl. Med. 11, 39 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engel F. B., Hsieh P. C., Lee R. T., Keating M. T., FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc. Natl. Acad. Sci. U.S.A. 103, 15546–15551 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahuja P., Perriard E., Perriard J. C., Ehler E., Sequential myofibrillar breakdown accompanies mitotic division of mammalian cardiomyocytes. J. Cell Sci. 117, 3295–3306 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Jopling C., Boue S., Izpisua Belmonte J. C., Dedifferentiation, transdifferentiation and reprogramming: Three routes to regeneration. Nat. Rev. Mol. Cell Biol. 12, 79–89 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Szibor M., Poling J., Warnecke H., Kubin T., Braun T., Remodeling and dedifferentiation of adult cardiomyocytes during disease and regeneration. Cell. Mol. Life Sci. 71, 1907–1916 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taegtmeyer H., Sen S., Vela D., Return to the fetal gene program: A suggested metabolic link to gene expression in the heart. Ann. N. Y. Acad. Sci. 1188, 191–198 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kubin T., Poling J., Kostin S., Gajawada P., Hein S., Rees W., Wietelmann A., Tanaka M., Lorchner H., Schimanski S., Szibor M., Warnecke H., Braun T., Oncostatin M is a major mediator of cardiomyocyte dedifferentiation and remodeling. Cell Stem Cell 9, 420–432 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Stawski L., Trojanowska M., Oncostatin M and its role in fibrosis. Connect. Tissue Res. 60, 40–49 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu J., Zhang L., Zhao Z., Zhang M., Lin J., Wang J., Yu W., Man W., Li C., Zhang R., Gao E., Wang H., Sun D., OSM mitigates post-infarction cardiac remodeling and dysfunction by up-regulating autophagy through Mst1 suppression. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 1951–1961 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Gosteli-Peter M. A., Harder B. A., Eppenberger H. M., Zapf J., Schaub M. C., Triiodothyronine induces over-expression of alpha-smooth muscle actin, restricts myofibrillar expansion and is permissive for the action of basic fibroblast growth factor and insulin-like growth factor I in adult rat cardiomyocytes. J. Clin. Invest. 98, 1737–1744 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novoyatleva T., Sajjad A., Pogoryelov D., Patra C., Schermuly R. T., Engel F. B., FGF1-mediated cardiomyocyte cell cycle reentry depends on the interaction of FGFR-1 and Fn14. FASEB J. 28, 2492–2503 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Itoh N., Ohta H., Nakayama Y., Konishi M., Roles of FGF signals in heart development, health, and disease. Front. Cell Dev. Biol. 4, 110 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boettger T., Braun T., A new level of complexity: The role of microRNAs in cardiovascular development. Circ. Res. 110, 1000–1013 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Dong X., Dong X., Gao F., Liu N., Liang T., Zhang F., Fu X., Pu L., Chen J., Non-coding RNAs in cardiomyocyte proliferation and cardiac regeneration: Dissecting their therapeutic values. J. Cell. Mol. Med. 25, 2315–2332 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan T., Krishnan J., Non-coding RNAs in cardiac regeneration. Front. Physiol. 12, 650566 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porrello E. R., Johnson B. A., Aurora A. B., Simpson E., Nam Y. J., Matkovich S. J., Dorn G. W. II, van Rooij E., Olson E. N., MiR-15 family regulates postnatal mitotic arrest of cardiomyocytes. Circ. Res. 109, 670–679 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aguirre A., Montserrat N., Zacchigna S., Nivet E., Hishida T., Krause M. N., Kurian L., Ocampo A., Vazquez-Ferrer E., Rodriguez-Esteban C., Kumar S., Moresco J. J., Yates J. R. 3rd, Campistol J. M., Sancho-Martinez I., Giacca M., Izpisua Belmonte J. C., In vivo activation of a conserved microRNA program induces mammalian heart regeneration. Cell Stem Cell 15, 589–604 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eulalio A., Mano M., Dal Ferro M., Zentilin L., Sinagra G., Zacchigna S., Giacca M., Functional screening identifies miRNAs inducing cardiac regeneration. Nature 492, 376–381 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Besser J., Malan D., Wystub K., Bachmann A., Wietelmann A., Sasse P., Fleischmann B. K., Braun T., Boettger T., MiRNA-1/133a clusters regulate adrenergic control of cardiac repolarization. PLOS ONE 9, e113449 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sohal D. S., Nghiem M., Crackower M. A., Witt S. A., Kimball T. R., Tymitz K. M., Penninger J. M., Molkentin J. D., Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ. Res. 89, 20–25 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Wust S., Drose S., Heidler J., Wittig I., Klockner I., Franko A., Bonke E., Gunther S., Gartner U., Boettger T., Braun T., Metabolic maturation during muscle stem cell differentiation is achieved by miR-1/133a-mediated inhibition of the Dlk1-Dio3 mega gene cluster. Cell Metab. 27, 1026–1039.e6 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Tanaka M., Hirabayashi Y., Sekiguchi T., Inoue T., Katsuki M., Miyajima A., Targeted disruption of oncostatin M receptor results in altered hematopoiesis. Blood 102, 3154–3162 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Trokovic R., Trokovic N., Hernesniemi S., Pirvola U., Vogt Weisenhorn D. M., Rossant J., McMahon A. P., Wurst W., Partanen J., FGFR1 is independently required in both developing mid- and hindbrain for sustained response to isthmic signals. EMBO J. 22, 1811–1823 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lorchner H., Poling J., Gajawada P., Hou Y., Polyakova V., Kostin S., Adrian-Segarra J. M., Boettger T., Wietelmann A., Warnecke H., Richter M., Kubin T., Braun T., Myocardial healing requires Reg3β-dependent accumulation of macrophages in the ischemic heart. Nat. Med. 21, 353–362 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Abe H., Takeda N., Isagawa T., Semba H., Nishimura S., Morioka M. S., Nakagama Y., Sato T., Soma K., Koyama K., Wake M., Katoh M., Asagiri M., Neugent M. L., Kim J. W., Stockmann C., Yonezawa T., Inuzuka R., Hirota Y., Maemura K., Yamashita T., Otsu K., Manabe I., Nagai R., Komuro I., Macrophage hypoxia signaling regulates cardiac fibrosis via oncostatin M. Nat. Commun. 10, 2824 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wystub K., Besser J., Bachmann A., Boettger T., Braun T., miR-1/133a clusters cooperatively specify the cardiomyogenic lineage by adjustment of myocardin levels during embryonic heart development. PLOS Genet. 9, e1003793 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Straight A. F., Field C. M., Mitchison T. J., Anillin binds nonmuscle myosin II and regulates the contractile ring. Mol. Biol. Cell 16, 193–201 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heidersbach A., Saxby C., Carver-Moore K., Huang Y., Ang Y.-S., de Jong P. J., Ivey K. N., Srivastava D., microRNA-1 regulates sarcomere formation and suppresses smooth muscle gene expression in the mammalian heart. eLife 2, e01323 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams C., Quinn K. P., Georgakoudi I., Black L. D. III, Young developmental age cardiac extracellular matrix promotes the expansion of neonatal cardiomyocytes in vitro. Acta Biomater. 10, 194–204 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Z., Xie J., Hao H., Lin H., Wang L., Zhang Y., Chen L., Cao S., Huang X., Liao W., Bin J., Liao Y., Ablation of periostin inhibits post-infarction myocardial regeneration in neonatal mice mediated by the phosphatidylinositol 3 kinase/glycogen synthase kinase 3β/cyclin D1 signalling pathway. Cardiovasc. Res. 113, 620–632 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doenst T., Nguyen T. D., Abel E. D., Cardiac metabolism in heart failure: Implications beyond ATP production. Circ. Res. 113, 709–724 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kubin T., Cetinkaya A., Kubin N., Bramlage P., Sen-Hild B., Gajawada P., Akinturk H., Schonburg M., Schaper W., Choi Y. H., Barancik M., Richter M., The MEK/ERK module is reprogrammed in remodeling adult cardiomyocytes. Int. J. Mol. Sci. 21, 6348 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poss K. D., Getting to the heart of regeneration in zebrafish. Semin. Cell Dev. Biol. 18, 36–45 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Dell’Era P., Ronca R., Coco L., Nicoli S., Metra M., Presta M., Fibroblast growth factor receptor-1 is essential for in vitro cardiomyocyte development. Circ. Res. 93, 414–420 (2003). [DOI] [PubMed] [Google Scholar]
- 48.Mima T., Ueno H., Fischman D. A., Williams L. T., Mikawa T., Fibroblast growth factor receptor is required for in vivo cardiac myocyte proliferation at early embryonic stages of heart development. Proc. Natl. Acad. Sci. U.S.A. 92, 467–471 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seyed M., Dimario J. X., Fibroblast growth factor receptor 1 gene expression is required for cardiomyocyte proliferation and is repressed by Sp3. J. Mol. Cell. Cardiol. 44, 510–519 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Sheikh F., Fandrich R. R., Kardami E., Cattini P. A., Overexpression of long or short FGFR-1 results in FGF-2-mediated proliferation in neonatal cardiac myocyte cultures. Cardiovasc. Res. 42, 696–705 (1999). [DOI] [PubMed] [Google Scholar]
- 51.Bostjancic E., Zidar N., Glavac D., MicroRNA microarray expression profiling in human myocardial infarction. Dis. Markers 27, 255–268 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Danowski N., Manthey I., Jakob H. G., Siffert W., Peters J., Frey U. H., Decreased expression of miR-133a but not of miR-1 is associated with signs of heart failure in patients undergoing coronary bypass surgery. Cardiology 125, 125–130 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Ferreira L. R., Frade A. F., Santos R. H., Teixeira P. C., Baron M. A., Navarro I. C., Benvenuti L. A., Fiorelli A. I., Bocchi E. A., Stolf N. A., Chevillard C., Kalil J., Cunha-Neto E., MicroRNAs miR-1, miR-133a, miR-133b, miR-208a and miR-208b are dysregulated in chronic chagas disease cardiomyopathy. Int. J. Cardiol. 175, 409–417 (2014). [DOI] [PubMed] [Google Scholar]
- 54.Liu N., Bezprozvannaya S., Williams A. H., Qi X., Richardson J. A., Bassel-Duby R., Olson E. N., microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev. 22, 3242–3254 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wei Y., Peng S., Wu M., Sachidanandam R., Tu Z., Zhang S., Falce C., Sobie E. A., Lebeche D., Zhao Y., Multifaceted roles of miR-1s in repressing the fetal gene program in the heart. Cell Res. 24, 278–292 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao Y., Ransom J. F., Li A., Vedantham V., von Drehle M., Muth A. N., Tsuchihashi T., McManus M. T., Schwartz R. J., Srivastava D., Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell 129, 303–317 (2007). [DOI] [PubMed] [Google Scholar]
- 57.Hermanns H. M., Oncostatin M and interleukin-31: Cytokines, receptors, signal transduction and physiology. Cytokine Growth Factor Rev. 26, 545–558 (2015). [DOI] [PubMed] [Google Scholar]
- 58.Ornitz D. M., Itoh N., The fibroblast growth factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 4, 215–266 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strasser P., Gimona M., Moessler H., Herzog M., Small J. V., Mammalian calponin. Identification and expression of genetic variants. FEBS Lett. 330, 13–18 (1993). [DOI] [PubMed] [Google Scholar]
- 60.Jin J. P., Wu D., Gao J., Nigam R., Kwong S., Expression and purification of the h1 and h2 isoforms of calponin. Protein Expr. Purif. 31, 231–239 (2003). [DOI] [PubMed] [Google Scholar]
- 61.Samaha F. F., Ip H. S., Morrisey E. E., Seltzer J., Tang Z., Solway J., Parmacek M. S., Developmental pattern of expression and genomic organization of the calponin-h1 gene. A contractile smooth muscle cell marker. J. Biol. Chem. 271, 395–403 (1996). [DOI] [PubMed] [Google Scholar]
- 62.Bhattacharya N., Ghosh S., Sept D., Cooper J. A., Binding of myotrophin/V-1 to actin-capping protein: Implications for how capping protein binds to the filament barbed end. J. Biol. Chem. 281, 31021–31030 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Das B., Gupta S., Vasanji A., Xu Z., Misra S., Sen S., Nuclear co-translocation of myotrophin and p65 stimulates myocyte growth. Regulation by myotrophin hairpin loops. J. Biol. Chem. 283, 27947–27956 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.D’Uva G., Aharonov A., Lauriola M., Kain D., Yahalom-Ronen Y., Carvalho S., Weisinger K., Bassat E., Rajchman D., Yifa O., Lysenko M., Konfino T., Hegesh J., Brenner O., Neeman M., Yarden Y., Leor J., Sarig R., Harvey R. P., Tzahor E., ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat. Cell Biol. 17, 627–638 (2015). [DOI] [PubMed] [Google Scholar]
- 65.Heallen T., Morikawa Y., Leach J., Tao G., Willerson J. T., Johnson R. L., Martin J. F., Hippo signaling impedes adult heart regeneration. Development 140, 4683–4690 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mahmoud A. I., Kocabas F., Muralidhar S. A., Kimura W., Koura A. S., Thet S., Porrello E. R., Sadek H. A., Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature 497, 249–253 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Puente B. N., Kimura W., Muralidhar S. A., Moon J., Amatruda J. F., Phelps K. L., Grinsfelder D., Rothermel B. A., Chen R., Garcia J. A., Santos C. X., Thet S., Mori E., Kinter M. T., Rindler P. M., Zacchigna S., Mukherjee S., Chen D. J., Mahmoud A. I., Giacca M., Rabinovitch P. S., Aroumougame A., Shah A. M., Szweda L. I., Sadek H. A., The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 157, 565–579 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aurora A. B., Porrello E. R., Tan W., Mahmoud A. I., Hill J. A., Bassel-Duby R., Sadek H. A., Olson E. N., Macrophages are required for neonatal heart regeneration. J. Clin. Invest. 124, 1382–1392 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lim G. B., Inhibiting fatty acid oxidation promotes cardiomyocyte proliferation. Nat. Rev. Cardiol. 17, 266–267 (2020). [DOI] [PubMed] [Google Scholar]
- 70.Fukuda R., Marín-Juez R., El-Sammak H., Beisaw A., Ramadass R., Kuenne C., Guenther S., Konzer A., Bhagwat A. M., Graumann J., Stainier D. Y., Stimulation of glycolysis promotes cardiomyocyte proliferation after injury in adult zebrafish. EMBO Rep. 21, e49752 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Poling J., Gajawada P., Richter M., Lorchner H., Polyakova V., Kostin S., Shin J., Boettger T., Walther T., Rees W., Wietelmann A., Warnecke H., Kubin T., Braun T., Therapeutic targeting of the oncostatin M receptor-β prevents inflammatory heart failure. Basic Res. Cardiol. 109, 396 (2014). [DOI] [PubMed] [Google Scholar]
- 72.Hortells L., Johansen A. K. Z., Yutzey K. E., Cardiac fibroblasts and the extracellular matrix in regenerative and nonregenerative hearts. J. Cardiovasc. Dev. Dis. 6, 29 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rodriguez C. I., Buchholz F., Galloway J., Sequerra R., Kasper J., Ayala R., Stewart A. F., Dymecki S. M., High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat. Genet. 25, 139–140 (2000). [DOI] [PubMed] [Google Scholar]
- 74.Bruning J. C., Michael M. D., Winnay J. N., Hayashi T., Horsch D., Accili D., Goodyear L. J., Kahn C. R., A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol. Cell 2, 559–569 (1998). [DOI] [PubMed] [Google Scholar]
- 75.Schweisgut J., Schutt C., Wust S., Wietelmann A., Ghesquiere B., Carmeliet P., Drose S., Korach K. S., Braun T., Boettger T., Sex-specific, reciprocal regulation of ERα and miR-22 controls muscle lipid metabolism in male mice. EMBO J. 36, 1199–1214 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mootha V. K., Lindgren C. M., Eriksson K. F., Subramanian A., Sihag S., Lehar J., Puigserver P., Carlsson E., Ridderstrale M., Laurila E., Houstis N., Daly M. J., Patterson N., Mesirov J. P., Golub T. R., Tamayo P., Spiegelman B., Lander E. S., Hirschhorn J. N., Altshuler D., Groop L. C., PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273 (2003). [DOI] [PubMed] [Google Scholar]
- 77.Subramanian A., Tamayo P., Mootha V. K., Mukherjee S., Ebert B. L., Gillette M. A., Paulovich A., Pomeroy S. L., Golub T. R., Lander E. S., Mesirov J. P., Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 102, 15545–15550 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S12