Abstract
Rates of diabetic retinopathy (DR) and diabetic macular edema (DME), a common ocular complication of diabetes mellitus, are increasing worldwide. There is a substantial burden concerning the detection and management of this condition, particularly in low-resource settings, due to limitations such as the time, cost, and labor associated with current screening and treatment methods. Artificial intelligence (AI) is a modality of pattern recognition that has the potential to combat these limitations in a reliable and cost-effective way. This review explores the various applications of AI on the screening, management, and treatment of DR and DME. AI applications for detecting referable DR and DME have been the most thoroughly researched applications for this condition. While some studies exist using AI to stratify DR patients based on the risk of progression, predict treatment outcomes to anti-VEGF therapy, and explore the utilization of AI for clinical trials to develop new treatments for DR, further validation studies on larger datasets are warranted.
Keywords: Artificial intelligence, deep learning, diabetic retinopathy, diabetic macular edema
Introduction
The worldwide prevalence of diabetes mellitus (DM) continues to increase rapidly and is expected to reach over 592 million people by 2035.1, 2 The most common microvascular complication of DM is diabetic retinopathy (DR), the leading cause of new cases of blindness in adults aged 17–34 in the world.3 According to a meta-analysis performed on 35 studies between 1998 and 2008, the prevalence of DR and vision-threatening DR was 34.6% and 10.2% respectively.4 In this same study, the prevalence of proliferative diabetic retinopathy (PDR) was 7.0% and the presence of diabetic macular edema (DME) was 6.81%.4 Interventions exist to effectively halt or reverse retinopathy in many cases and thus the key to preventing vision loss is expedient recognition and treatment of retinopathy. However, given the high prevalence of DM worldwide, ensuring that all patients are screened and have effective follow-up adds a significant burden on healthcare systems. This paper will explore how artificial intelligence (AI) can minimize the burden of DR by addressing issues with detection, monitoring progression, and predicting treatment outcomes for DR and DME.
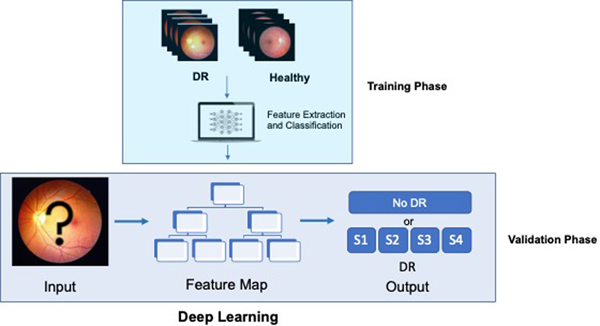
In computer science, machine learning is the study of using machines to make intelligent decisions by extracting patterns and learning from data. A branch in machine learning called deep learning is largely inspired by our knowledge of the human brain. Specifically, deep learning consists of artificial neural networks consisting of a relatively large number of layers or “neurons,” hence the term “deep.”5 Deep learning has performed remarkably well in image processing tasks and has outperformed other computer vision methods including in medical imaging, with reported accuracy similar to that of expert humans in some cases.6, 7 Figure 1 describes the traditional method of staging diabetic retinopathy whereas Figure 2 describes a deep learning model.
Figure 1 –
Image Classification by Trained Personnel: This figure shows the process of having trained personnel use their experience and traditional algorithms to determine which stage of DR a fundus image is.
Figure 2 –
Deep Learning: In deep learning, photos of DR and healthy images are initially introduced to the machine. Through complex mathematical equations resembling neural networks, the machine identifies features from the input data. The model creates a map using these features so that it can determine the proper DR staging when presented with an unknown fundus image using what it learned during the training phase.
1. Diabetic Retinopathy Detection and Screening
Screening for DR is important for early detection and prevention of vision loss. Nationwide screening programs identify referable DR (characterized by moderate or severe diabetic retinopathy or the presence of diabetic macular edema) and refer them to ophthalmology clinics where patients can receive a full ophthalmic examination. Estimates predict that over $500 million could be saved in healthcare related costs if DR is detected earlier in the United States (U.S.) alone.8 Traditionally, the gold standard for DR screening is considered a funduscopic examination by a trained ophthalmologist.9 However, due to the rapidly increasing rates of diabetes and the costs associated with screening (monetary, time, labor), ensuring all diabetic patients receive the required screening and treatment proves to be a challenge.
Telemedicine has become a popular method for diagnosing DR during screening by taking color fundus photographs and sending them to ophthalmologists to grade the images remotely and has been shown to improve screening rates, treatment rates, and reduce vision loss due to diabetes.10, 11 Even with imaging using only telemedicine, it is estimated that 32 million retinal images would have to be evaluated to ensure that the need for DR screening is met.12 Recent advances in AI provide promise that an automated diagnostic tool may be developed so that screening will be accessible to people in a wide range of settings.
Using AI to screen for “referable diabetic retinopathy” is one of the most advanced applications of AI within ophthalmology. Many researchers have developed algorithms that are able to “learn” to diagnose DR and tested the accuracy of their programs on repositories of retinal images with high sensitivity and specificity results.6, 13–21 (Table 1) The FDA approved a program in April 2018 known as IDx-DR which uses pictures from a nonmydriatic retinal camera (NW400, Topcon Medical Systems, Oakland, NJ) and then uses an AI program to diagnose DR in a clinical setting.22 The imaging system on the IDX-DR is costly, and thus there are numerous studies exploring the use of similar AI programs on smartphone based fundus cameras such as the Remidio Fundus on Phone (FOP).23–27 The use of automated AI programs on smartphones provides a relatively low cost and effective method of screening DR. Several studies have validated screening programs for diabetic retinopathy in the clinical setting, both with the use of smartphone based fundus cameras and traditional fundus imaging, in international settings including Singapore,28, 29 United Kingdom,30 India,25 Australia,31 and Thailand.32–34
Table 1:
Identification of Referable DR (Moderate NPDR or Worse)
| Author | Title | Year | Sensitivity | Specificity | AUC | Accuracy | Validation Data Set | Type of Image |
|---|---|---|---|---|---|---|---|---|
| Abramoff et al13 | Improved automated detection of diabetic retinopathy on a publicly available data set through integration of deep learning | 2016 | 96.8 | 87 | 0.98 | Not Reported | 1748 (Messidor 2) | Fundus |
| Abramoff et al14 | Pivotal trial of an automated AI-based diagnostic system for detection of diabetic retinopathy in primary care offices | 2018 | 87.2 | 90.7 | Not Reported | Not Reported | 819 patients (Acquired Images) | OCT |
| Bellemo et al31 | Artificial intelligence using deep learning to screen for referable and vision-threatening diabetic retinopathy in Africa: a clinical validation study | 2019 | 92.3 | 89 | 0.973 | Not Reported | 1574 patients (Acquired Images) | Fundus |
| Bhaskaranad et al15 | The Value of Automated Diabetic Retinopathy Screening with the EyeArt System: A Study of More Than 100,000 Consecutive Encounters from People with Diabetes. | 2019 | 91.3 | 91.1 | Not Reported | 91.20% | 850908 images (Acquired Images) | Fundus |
| Gargeya et al16 | Automated identification of diabetic retinopathy using deep learning | 2017 | 94 | 98 | 0.97 | Not Reported | 75137 (Kaggle Images) | Fundus |
| Gulshan et al11 | Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs | 2016 | 87–90.3 | 98.1–98.5 | Not Reported | Not Reported | 128175 (EYEPACS-1 and Messidor-2) | Fundus |
| He et al17 | Artificial intelligence-based screening for diabetic retinopathy at community hospital. | 2019 | 91.2 | 98.8 | 0.95 | Not Reported | 889 patients (Acquired Images) | Fundus |
| Heydon et al32 | Prospective evaluation of an artificial intelligence enabled algorithm for automated diabetic retinopathy screening of 30 000 patients | 2020 | 95.7 | 54 | Not Reported | 89.4 | 30405 (Acquired Images) | Fundus |
| Keel et al29 | Visualizing deep learning models for the detection of referable diabetic retinopathy and glaucoma. | 2018 | 92.3 | 93.7 | Not Reported | Not Reported | 96 Patients (Acquired Images) | Fundus |
| Kermany et al18 | Identifying medical diagnoses and treatable diseases by image-based deep learning | 2018 | 97.8 | 97.4 | 0.99 | 96.60% | 4686 patients | OCT |
| Natarajan et al24 | Diagnostic accuracy of community-based diabetic retinopathy screening with an offline artificial intelligence system on a smartphone | 2019 | 100% | 81.90% | Not Reported | Not Reported | 231 patients (Acquired Images) | Smartphone fundus |
| Rajalakshmi et al23 | Automated diabetic retinopathy detection in smartphone-based fundus photography using artificial intelligence. | 2018 | 99.3 | 68.8 | Not Reported | Not Reported | 296 patients (Acquired Images) | Smartphone Fundus |
| Ramachandran et al19 | Diabetic retinopathy screening using deep neural network | 2018 | 84.6/96 | 79.7/90 | 0.90/0.98 | Not Reported | 485 Images (Otago)/1200 Images (Messidor) | Fundus |
| Ruamviboonsuk et al30 | Deep learning versus human graders for classifying diabetic retinopathy severity in a nationwide screening program. | 2019 | 96.8 | 95.6 | 0.987 | Not Reported | 7517 patients (Acquired Images) | Fundus |
| Sahlsten et al43 | Deep Learning Fundus Image Analysis for Diabetic Retinopathy and Macular Edema Grading. | 2019 | 89.6 | 97.4 | 0.987 | Not Reported | 7304 images (Messidor) | Fundus |
| Takahashi et al35 | Applying artificial intelligence to disease staging: Deep learning for improved staging of diabetic retinopathy | 2017 | Not Reported | Not Reported | 0.81 | 96% | 496 (Acquired Images) | Fundus |
| Ting et al36 | Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. | 2017 | 90.5 | 91.6 | 0.936 | Not Reported | 71896 images (Singapore National Diabetic Retinopathy Screening Program) | Fundus |
| Xu K et al85 | Deep Convolutional Neural Network-Based Early Automated Detection of Diabetic Retinopathy Using Fundus Image. | 2017 | 95.8 | 80.2 | 0.945 | 94.50% | 400 (Kaggle Images) | Fundus |
2. Assessing Progression of DR and DME Using AI
Once referable diabetic retinopathy is detected, the main goal is to prevent progression to vision threatening diabetic retinopathy, which is defined as severe non-proliferative diabetic retinopathy (NPDR), PDR or the presence of DME.4 In order to do this, it is essential to identify the stage of diabetic retinopathy and monitor for progression. As management regimens vary by the severity of diabetic retinopathy, it is crucial to 1) assess the severity of diabetic retinopathy by staging DR and determining the presence of DME, 2) identify which patients are at high-risk for advancing to vision threatening diabetic retinopathy, and 3) establish ideal screening intervals based on a patient’s risk stratification scores.
2a. Staging DR and Evaluating for Presence of DME
Vision threatening diabetic retinopathy (VTDR), particularly PDR or the presence of DME, is commonly associated with severe vision loss and thus identifying VTDR to initiate prompt treatment (i.e. anti-VEGF and/or laser treatment) is crucial while managing a diabetic patient.35 However, studies have shown that reliability among graders staging DR is only 70%.36 Deep learning algorithms developed by researchers have found reliability of grading up to 95% and sensitivities for staging ranging from 90.5% to 97%.6, 29, 32, 37, 38 (Table 2)
Table 2:
Staging Diabetic Retinopathy
| Author | Title | Year | Sensitivity | Specificity | AUC | Accuracy | Validation Data Set | Type of Image | Type of Staging |
|---|---|---|---|---|---|---|---|---|---|
| Abbas et al35 | Automatic recognition of severity level for diagnosis of diabetic retinopathy using deep visual features | 2017 | 92.2 | 94.5 | 0.924 | Not Reported | 750 images (Combination of DIARETB1, FAZ, Messidor, Prv-DR datasets) | Fundus | Identifies 5 Severity Levels of DR |
| Gulshan et al11 | Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. | 2016 | 84.0/87.8 | 98.8/98.2 | Not Reported | Not Reported | 8788 Images (EyePACS-1)/ 1745 Images (Messidor-2) | Fundus | Identification of severe or worse DR |
| He et al17 | Artificial intelligence-based screening for diabetic retinopathy at community hospital. | 2019 | 80.4 | 99.4 | 0.9 | Not Reported | 889 patients (Acquired Images) | Fundus | Identification of PDR |
| Rajalakshmi et al23 | Automated diabetic retinopathy detection in smartphone-based fundus photography using artificial intelligence. | 2018 | 99.1/78.1 | 80.4/89.8 | Not Reported | Not Reported | 296 patients (Acquired Images) | Fundus | Identification of severe or worse DR |
| Ruamviboonsuk et al30 | Deep learning versus human graders for classifying diabetic retinopathy severity in a nationwide screening program. | 2019 | 93.6 | 98.2 | 0.991 | Not Reported | 7517 patients (Acquired Images) | Fundus | Identification of severe or worse DR |
| Ting et al36 | Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes | 2017 | 100 | 91.1 | 0.958 | Not Reported | 71896 images (Singapore National Diabetic Retinopathy Screening Program) | Fundus | Identification of severe or worse DR |
Diabetic macular edema (DME) is a complication of DR characterized by retinal thickening in the macula due to fluid accumulation. Center-involved (ci)- DME is the primary cause of moderate vision loss for diabetic patients and is an indication for referral and treatment. The standard for identifying ci-DME was established by several major clinical trials measuring macular thickening based on optical coherence tomography (OCT).39 OCT machines are more costly than fundus cameras and thus the presence of hard exudates within one optic disc diameter on fundus photographs is commonly used as a proxy for photographic screeing.9 However, this alternative method of determining ci-DME has been shown to have variable results.40 Several researchers have developed a deep learning algorithm using fundus photographs to determine the presence of ci-DME, validated by OCT or fundus images, with a similar sensitivity and higher specificity than human graders.41–44 (Table 3) Sahlsten et al developed a program that accurately graded diabetic macular edema according to a commonly used diabetic macular edema severity scale (PIMEC).45 Lastly, similar to fundus images, conclusions drawn from OCT images are limited by interobserver variability amongst readers and are prone to error given the large number of images.46 Several studies have presented methods to utilize deep learning to detect, differentiate, and quantify macular fluid objectively for a range of exudative macular diseases, including DME.47–49 (Table 3)
Table 3:
Detecting Diabetic Macular Edema
| Author | Title | Year | Sensitivity | Specificity | AUC | Accuracy | Validation Data Set | Type of Image | Type of Staging | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Alsaih et al42 | Machine learning techniques for diabetic macular edema (DME) classification on SD-OCT images. | 2017 | 87.5 | 87.5 | Not Reported | Not Reported | 32 volumes (Singapore Eye Research Institute Database) | OCT | Detects DME | *Each volume has 128 B-Scan slices |
| Chan et al41 | Fusing Results of Several Deep Learning Architectures for Automatic Classification of Normal and Diabetic Macular Edema in Optical Coherence Tomography. | 2018 | 93.75 | 93.75 | Not Reported | 93.75 | 115 volumes (from Singapore Eye Research Institute and Chinese University of Hong Kong dataset) | OCT | Detects DME | *Each volume has 128 B-Scan slices |
| Gulshan et al11 | Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. | 2016 | 91/90.4 | 99/98.8 | Not Reported | Not Reported | 8788 Images (EyePACS-1)/ 1745 Images (Messidor-2) | Fundus | Detects DME | |
| Sahlsten et al43 | Deep Learning Fundus Image Analysis for Diabetic Retinopathy and Macular Edema Grading. | 2019 | Not Reported | Not Reported | 0.981 | 93.4 | 7304 images (Messidor) | Fundus | Identifies DME Stage based on PIMEC | |
| Schlegl et al45 | Fully Automated Detection and Quantification of Macular Fluid in OCT Using Deep Learning. | 2018 | Not Reported | Not Reported | 0.94/0.92 | Not Reported | 1200 scans (Acquired Scans using Cirrus and Spectralis) | OCT | Detects and quantifies intraretinal cystoid fluid /subretinal fluid | |
| Sun et al46 | Fully automated macular pathology detection in retina optical coherence tomography images using sparse coding and dictionary learning. | 2017 | 100/99.7 | Not Reported | Not Reported | Not Reported | 45 patients (Duke Spectral Domain)/678 Images (Acquired) | OCT | Detects DME | Also detects AMD |
| Syed et al47 | Automated diagnosis of macular edema and central serous retinopathy through robust reconstruction of 3D retinal surfaces. | 2016 | 100 | 96.7 | Not Reported | 98.9 | 90 Volumes (Armed Forces Institute of Opthalmology) | OCT | Detects DME | Also detects central serous retinopathy |
| Varadarajan et al39 | Predicting optical coherence tomography-derived diabetic macular edema grades from fundus photographs using deep learning. | 2020 | 85 | 80 | 0.89 | 81 | 1033 images (Thailand Dataset) | Fundus | Detects ci-DME |
2b. Risk Stratification for Progression to VTDR
Another useful modality of AI in DR is risk stratification. Since progression can be mitigated by intensive glycemic management and, when necessary, intervention with laser photocoagulation, intraocular corticosteroids, or anti-vascular endothelial growth factor (VEGF) agents, identifying which individuals are most likely to progress to VTDR is critical.50,51 While studies have theorized several risk factors (i.e. age, gender, HbA1c levels, blood pressure, proteinuria) and predictors (i.e. microaneurysm turnover), there are still many unknown factors contributing to an individual’s risk profile, making it difficult for ophthalmologists to know which individuals are more likely to progress quickly.52–58 Several studies have used AI to help in this task. (Table 4) Arcadu et al used deep learning to predict DR progression, defined as 2-step worsening according to the Early Treatment Diabetic Retinopathy Study severity scale, using a 7-field color fundus photograph taken during the initial visit. They compared their predictions to DR severity scores assessed by masked, well-trained human reading center graders at various time points. The deep learning model accurately predicted progression of DR at 6,12, and 24 months with an area under the curve of 0.68, 0.79, and 0.77 respectively.59 Similarly, several studies have utilized retinal vasculature to predict progression from early NPDR to PDR with AUCs as high as 0.968 using deep learning.60–62 Another study by Bora et al determined the risk of diabetic patients with no DR developing DR within two years using deep learning with an AUC up to 0.71.63 Identifying high-risk patients can help determine which patients need more intensive treatment, support clinical decision-making, and potentially aid in recruitment for clinical trials. While further studies are necessary on larger data sets to further these results, these studies provide promise that an algorithm to identify higher risk patients to determine an ideal management schedule is possible.
Table 4:
Progression of Diabetic Retinopathy or Diabetic Macular Edema
| Author | Title | Year | Sensitivity | Specificity | AUC | Accuracy | Validation Data Set | Type of Image | Type of Staging | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Arcadu et al40 | Deep Learning Predicts OCT Measures of Diabetic Macular Thickening From Color Fundus Photographs. | 2019 | 87.50% | 96.50% | 0.97 (Central Subfield Thickness) and 0.91 (Central Foveal Thickness) | Not Reported | 17997 Images (RIDE/RISE DME Database) | Fundus | Predicts OCT Measures of Diabetic Macular Thickening based on Fundus Photographs | Predicts central subfield thickness >250 um and Central foveal thickness >250 um |
| Arcadu et al57 | Deep learning algorithm predicts diabetic retinopathy progression in individual patients. | 2019 | 66%, 91%, 79% | 77%, 65%, 72% | 0.68,0.79,0.77 | 529, 528, 499 patients (Acquired Images) | Fundus | Predicts Progression of DR at 6, 12, 24 months | DR progression defined as 2-step worsening on the EDTRS scale | |
| Bora et al61 | Predicting Risk of Developing Diabetic Retinopathy using Deep Learning | 2020 | Not Reported | Not Reported | 0.71 | Not Reported | 4762 images (EyePACS and Thailand Dataset) | Fundus | Predicts risk of Progression to DR | |
| Leontidis et al58 | A new unified framework for the early detection of the progression to diabetic retinopathy from fundus images. | 2017 | Not Reported | Not Reported | 0.821–0.968 | Not Reported | 127 images | Fundus | Progression from no DR to DR | Identified retinal vascular geometry as a feature that predicts progression from no DR to DR |
| Orlando et al60 | Proliferative diabetic retinopathy characterization based on fractal features: Evaluation on a publicly available dataset. | 2017 | Not Reported | Not Reported | 0.093 | Not Reported | 1200 images (Messidor dataset) | Fundus | Progression to PDR on patients with no NPDR or mild NPDR | Identified higher fractal dimension has a predictor for PDR |
| Welikala et al59 | Automated detection of proliferative diabetic retinopathy using a modified line operator and dual classification | 2017 | 86.2 | 94.4 | 0.9632 | Not Reported | 60 images (Messidor and Acquired) | Fundus | Identifies progression to PDR | Detects PDR via detecting new blood vessels |
2c. Establishing Optimal Screening Intervals Based on Risk Profile
Determination of high-risk patients can also help establish optimal screening intervals for these patients, which would improve cost-effectiveness of screening. Several studies have established the cost-effectiveness of population-based screening programs that have been optimized for individuals based on their risk assessment.64–66 AI can be used to help determine the risk for progression of patients with DR as well as define the ideal screening frequency based on this risk profile. For example, in order to account for a variety of factors such as risk profile, resource availability, an individual’s lifestyle, and genetics, the current guidelines for DR screening intervals are reported in ranges (i.e. 3–6 months for moderate NPDR). By using AI to develop a more individualized screening interval, low-risk patients can alleviate the burden on the health system by spacing out screening intervals whereas high-risk patients can have more frequent follow-up to ensure adequate detection and treatment. Furthermore, just as recommendations for screening intervals vary based on the resource setting,67 deep learning algorithms can be tested on a variety of different situations so that referral guidelines can be optimized based on a given set of parameters. Eleuteri et al used machine learning to develop a generalizable risk calculation engine to assign individualized screening intervals for patients with diabetic retinopathy based on risk.68 Once more algorithms have been validated to accurately predict progression of DR, further research investigating optimal screening intervals using deep learning models integrating various risk factors and resource settings for diabetic retinopathy are warranted.
3. Predicting Treatment Outcomes with anti-VEGF for DR and DME with AI
After stratifying patients based on their risk of progression to VTDR, the next step will be to determine which patients will benefit the most from certain treatments. Management for patients vary greatly based on the stage of DR, the presence of DME, and their risk profile. Better understanding of which patients will benefit the most from different treatment options can help ophthalmologists develop targeted plans to improve treatment outcomes while minimizing complications.69
For example, anti-VEGF agents such as aflibercept, bevacizumab, and ranibizumab are the main modalities of treatment for ci-DME. Although they have been shown to generally improve visual acuity, the therapeutic response to anti-VEGF treatment in these patients varies greatly due to the complicated pathogenesis of diabetic retinopathy.70 Some patients, despite frequent anti-VEGF injections, continue to exhibit signs of disease activity (incomplete responders) and may benefit from earlier intravitreal corticosteroid treatment, however, reliable features for identifying these patients remain elusive. Identifying such factors that distinguish one type of patient from the other, including differences in phenotype and genotype, to predict response would be extremely beneficial considering the high-cost burden associated with anti-VEGF injections.71 AI has the potential to help determine these factors, providing the potential to select an individualized, more effective first-line therapy.
Several studies have attempted to develop deep learning algorithms to predict early anatomical and functional factors associated with favorable outcomes with anti-VEGF injections.72–78 (Table 5) Takahashi et al used neural networks to predict which treatments would be most beneficial for patients with 96% accuracy but had a false negative rate of 12%.73 Two studies used pre-treatment OCT scans to develop a deep learning algorithm that was able to predict responsiveness to anti-VEGF therapy for up to 95% of patients.74, 76 Other studies have identified specific anatomical features, such as presence of baseline subretinal fluid or intraretinal cystic fluid in the outer nuclear layer, that are associated with better outcomes after anti-VEGF treatment for patients with diabetic macular edema using deep learning techniques.77, 78 These studies highlight the potential for artificial intelligence to aid the clinical decision-making process in selecting treatment for patients with VTDR.
Table 5:
Determining Treatment Outcomes based on anti-VEGF Injections
| Author | Title | Year | Sensitivity | Specificity | AUC | Accuracy | Validation Data Set | Type of Image | Type of Staging | Tx of DME/DR/Nonspecific? |
|---|---|---|---|---|---|---|---|---|---|---|
| Arcadu et al70 | Deep learning algorithm for patient-level prediction of diabetic retinopathy (DR) response to vascular endothelial growth factor (VEGF) inhibition. | 2019 | 87/81/84 | 72/82/82 | .82/,85/.87 | Not Reported | 759 patients (RISE/RIDE data) | Fundus | Predicts >2 step improvement on Diabetic Retinopathy Severity Scale in response to ranibizumab treatment for 6 mo/12 mo/24 mo | DR |
| Chen et al73 | A Novel Machine Learning Algorithm to Automatically Predict Visual Outcomes in Intravitreal Ranibizumab-Treated Patients with Diabetic Macular Edema. | 2018 | Not Reported | Not Reported | Not Reported | Not Reported | 464 Eyes (DRCR.net database) | Patient data (sex, age, diabetes type or condition, systemic diseases, eye status, treatment time tables) | Reported correlation coefficients (0.7 at 52 weeks, 0.55 at 78 weeks, and 0.81 at 104 weeks) | DME |
| Gerendas et al75 | Computational image analysis for prognosis determination in DME. | 2017 | Not Reported | Not Reported | Not Reported | R^2=0.21/0.23 | 629 patients (Acquired Images) | OCT | Predicts prognosis of DME patients undergoing anti-VEGF therapy | DME |
| Prahs et al72 | OCT-based deep learning algorithm for the evaluation of treatment indication with anti-vascular endothelial growth factor medications | 2017 | 90.1 | 96.2 | 0.968 | 95.5 | 5358 images (Acquired Images) | OCT | Classifies images that have no injection vs injection | Nonspecific |
| Rasti et al74 | Deep learning-based single-shot prediction of differential effects of anti-VEGF treatment in patients with diabetic macular edema. | 2020 | 80.1 | 85 | 0.866 | Not Reported | 127 patients (Acquired Images) | OCT | Response to VEGF was defined as at least 10% reduction in retinal thickness following treatment | DME |
| Roberts et al76 | Quantification of Fluid Resolution and Visual Acuity Gain in Patients With Diabetic Macular Edema Using Deep Learning: A Post Hoc Analysis of a Randomized Clinical Trial. | 2020 | Not Reported | Not Reported | Not Reported | Not Reported | 570 patients (Acquired Images) | OCT | Used an automated algorithm to quantify intraretinal and subretinal fluid to assess treatment outcomes with anti-VEGF treatment | DME |
| Takahashi et al71 | Applying artificial intelligence to disease staging: Deep learning for improved staging of diabetic retinopathy. | 2017 | Not Reported | Not Reported | Not Reported | 96 | 4,709 images (Acquired Images) | Fundus | Suggests treatments for DR based on staging | DR |
4. Potential Impact on Patients and Clinical Workflow
With effective screening methods, artificial intelligence will decrease the potential need for less urgent ophthalmology office visits by identifying patients with referable DR. In addition to decreasing the number of appointments, this benefits the patient economically with reduced transportation and co-pay costs. Diabetic patients are particularly susceptible to loss to follow-up; the poorer a patient’s glycemic control, the more likely he or she is to miss appointments.79 Minimizing the number of appointments will help increase rates of adherence as part of the multi-dimensional management of diabetes.80 Tufail et al conducted a detailed analysis on the cost-effectiveness of automated DR screening and concluded that automated screening of DR was significantly cost-effective, particularly in rural settings.30 Another factor to consider is the time it takes to schedule a follow-up appointment with a specialty service. One study found that the average wait time to schedule a follow-up appointment for patients in six high-volume clinics referred to an ophthalmologist from a primary care physician for DR was seven months.81 This delay in evaluation and treatment may lead to worse outcomes and higher costs due to managing DR at a later stage.
In addition to helping manage screening, diagnosis and treatment of DR clinically, AI can also help the clinical workflow. Through more effective screening, AI can also reduce the load on ophthalmologists’ offices, allowing for better utilization of resources for higher acuity patients. With the current projection of 592 million people having diabetes by 2035, around 3 million eyes will have to be evaluated every year, which would overwhelm the capacity of most healthcare institutions.82 This burden would be significantly alleviated if only moderate/severe DR patients had to be referred to an ophthalmologist.
Furthermore, the amount of time it takes to identify diabetic retinopathy is significantly reduced with AI; the median time required to manually stage DR in retinal images with seven fields was 12.8 min per patient whereas staging level for DR in Abbas et al’s study only took 2.12 seconds. 37 AI/machine learning can also help clinicians quantify retinal vasculature using fluorescein angiography (FA), a task which is very time consuming and prone to errors due to contrast.83 A study using this algorithm found it only took a mean of 22.1 seconds to calculate macular retinal vessel density from FA images and found it correlated with baseline visual acuity in patients with PDR.84
Potential Impacts on Clinical Trials
Lastly, AI can help randomized control trials (RCTs) study new, more personalized therapies for diabetic retinopathy. Considering the complex pathogenesis of diabetic retinopathy and the varied clinical presentations that complicate the diagnosis, targeting specific therapies for subsets of patients may result in more individualized, effective therapies. For example, Roberts et al used deep learning to determine aflibercept was superior in specifically reducing baseline subretinal fluid, which serves as a potential explanation as to why aflibercept has been shown to be particularly beneficial in DME patients.78 However, performing randomized controlled trials as therapies become more individualized becomes increasingly difficult as there is a smaller subset of individuals that are eligible for the study. AI can help identify certain subsets of individuals that may be eligible for the therapy, reducing the burden of having to screen a large number of individuals and making it easier to perform more focused trials and develop novel therapies.
Another benefit of utilizing AI for clinical trials is the standardization of outcome. When performing RCTs on novel drugs for diabetic retinopathy, the endpoint (i.e. severity of diabetic retinopathy or resolution of hemorrhage) is often determined by interpreting either a fundus image (i.e. decrease in exudates or microaneurysms) or an OCT image (i.e. decrease in intraretinal fluid). However, as mentioned earlier in this review, these endpoints are often subject to interobserver variability. AI has been shown to be able to determine these endpoints with higher sensitivities and specificities than human graders with minimal variability between readings. Lastly, given AI’s ability to accurately predict the outcome of a disease with and without established interventions,74 future developments in AI may be able to predict natural progression of diabetic retinopathy to the extent that no control arm is necessary. This would further reduce the number of subjects required to perform RCTs.85 Additionally, one of the biggest deterrents for patients joining an RCT is the prospect of randomization to a placebo,86 and thus having artificial intelligence model the placebo arm may increase participation in these trials. For example, if a randomized control trial was being conducted on anti-VEGF therapy for patients at high risk for progressing to PDR, deep learning could be used to model a control arm to model the natural progression of diabetic retinopathy in this patient population without anti-VEGF treatment. This may also address an ethical issue encountered in RCTs where an intervention is clearly shown to be better for a certain patient subgroup. Though further research needs to be performed to validate that the modeled control arm acts similarly to the patient population subset, there is promise that AI can serve as an adjunct to RCTs to more effectively manage DR.
Conclusion
Using AI, there is potential for exponential gain using automated programs to screen, monitor and manage DR leading to reliable and cost-effective screening protocols. This will lead to an effective utilization of resources, help alleviate the growing burden of managing these patients, and improve treatment outcomes especially in light of the rising incidence of diabetes. While there are many technologies that hold promise to fundamentally change the landscape of DR management, further validation studies on larger datasets need to be performed, particularly to help with risk stratification and treatment prediction outcomes for DR. Similarly, more studies are necessary to evaluate how AI can help augment randomized clinical trials to further develop novel therapeutics for this condition. Another issue to be addressed in the clinical use of AI is interpretability since the basis for recommendations, in particular when using deep learning methods, may not be apparent the clinician using an AI system. Developments in explainable AI will lead to greater trust and utilization of AI and allow for clinicians to better judge when a recommendation by an AI system may not be appropriate for a particular patient. Ultimately, the hope is to integrate AI into DR screening programs resulting in an optimization of patients referred to specialists, timely diagnoses, and early treatment to preserve vision.
Footnotes
Conflict of Interest: No conflicting relationship exists for any author.
References
- 1.Guariguata L, Whiting DR, Hambleton I, et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–149. [DOI] [PubMed] [Google Scholar]
- 2.Organization WH. Global Report on Diabetes Available: www.who.int/news-room/fact-sheets/detail/diabetes.
- 3.Fong DS, Aiello L, Gardner TW, et al. Retinopathy in diabetes. Diabetes Care. 2004;27 Suppl 1:S84–87. [DOI] [PubMed] [Google Scholar]
- 4.Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436–444. [DOI] [PubMed] [Google Scholar]
- 6.Gulshan V, Peng L, Coram M, et al. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. JAMA. 2016;316(22):2402–2410. [DOI] [PubMed] [Google Scholar]
- 7.Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542(7639):115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoenfeld ER, Greene JM, Wu SY, et al. Patterns of adherence to diabetes vision care guidelines: baseline findings from the Diabetic Retinopathy Awareness Program. Ophthalmology. 2001;108(3):563–571. [DOI] [PubMed] [Google Scholar]
- 9.Harding SP, Broadbent DM, Neoh C, et al. Sensitivity and specificity of photography and direct ophthalmoscopy in screening for sight threatening eye disease: the Liverpool Diabetic Eye Study. BMJ. 1995;311(7013):1131–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tozer K, Woodward MA, Newman-Casey PA. Telemedicine and Diabetic Retinopathy: Review of Published Screening Programs. J Endocrinol Diabetes. 2015;2(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pradeepa R, Rajalakshmi R, Mohan V. Use of Telemedicine Technologies in Diabetes Prevention and Control in Resource-Constrained Settings: Lessons Learned from Emerging Economies. Diabetes Technol Ther. 2019;21(S2):S29–S216. [DOI] [PubMed] [Google Scholar]
- 12.Abramoff MD, Niemeijer M, Suttorp-Schulten MS, et al. Evaluation of a system for automatic detection of diabetic retinopathy from color fundus photographs in a large population of patients with diabetes. Diabetes Care. 2008;31(2):193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faust O, Acharya UR, Ng EY, et al. Algorithms for the automated detection of diabetic retinopathy using digital fundus images: a review. J Med Syst. 2012;36(1):145–157. [DOI] [PubMed] [Google Scholar]
- 14.Wang S, Zhang Y, Lei S, et al. Performance of deep neural network-based artificial intelligence method in diabetic retinopathy screening: a systematic review and meta-analysis of diagnostic test accuracy. Eur J Endocrinol. 2020;183(1):41–49. [DOI] [PubMed] [Google Scholar]
- 15.Abramoff MD, Lou Y, Erginay A, et al. Improved Automated Detection of Diabetic Retinopathy on a Publicly Available Dataset Through Integration of Deep Learning. Invest Ophthalmol Vis Sci. 2016;57(13):5200–5206. [DOI] [PubMed] [Google Scholar]
- 16.Abramoff MD, Lavin PT, Birch M, et al. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018;1:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhaskaranand M, Ramachandra C, Bhat S, et al. The Value of Automated Diabetic Retinopathy Screening with the EyeArt System: A Study of More Than 100,000 Consecutive Encounters from People with Diabetes. Diabetes Technol Ther. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gargeya R, Leng T. Automated Identification of Diabetic Retinopathy Using Deep Learning. Ophthalmology. 2017;124(7):962–969. [DOI] [PubMed] [Google Scholar]
- 19.He J, Cao T, Xu F, et al. Artificial intelligence-based screening for diabetic retinopathy at community hospital. Eye (Lond). 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kermany DS, Goldbaum M, Cai W, et al. Identifying Medical Diagnoses and Treatable Diseases by Image-Based Deep Learning. Cell. 2018;172(5):1122–1131 e1129. [DOI] [PubMed] [Google Scholar]
- 21.Ramachandran N, Hong SC, Sime MJ, et al. Diabetic retinopathy screening using deep neural network. Clin Exp Ophthalmol. 2018;46(4):412–416. [DOI] [PubMed] [Google Scholar]
- 22.Ratner M. FDA backs clinician-free AI imaging diagnostic tools. Nat Biotechnol. 2018;36(8):673–674. [DOI] [PubMed] [Google Scholar]
- 23.Raman R, Srinivasan S, Virmani S, et al. Fundus photograph-based deep learning algorithms in detecting diabetic retinopathy. Eye. 2019;33(1):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fenner BJ, Wong RLM, Lam WC, et al. Advances in Retinal Imaging and Applications in Diabetic Retinopathy Screening: A Review. Ophthalmol Ther. 2018;7(2):333–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajalakshmi R, Subashini R, Anjana RM, et al. Automated diabetic retinopathy detection in smartphone-based fundus photography using artificial intelligence. Eye (Lond). 2018;32(6):1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Natarajan S, Jain A, Krishnan R, et al. Diagnostic Accuracy of Community-Based Diabetic Retinopathy Screening With an Offline Artificial Intelligence System on a Smartphone. JAMA Ophthalmol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalavar M, Watane A, Sridhar J. Screening for Diabetic Retinopathy Using Artificial Intelligence and Smartphone-Based Fundus Images. Invest Ophthalmol Vis Sci. 2020;61(9). [Google Scholar]
- 28.Nguyen HV, Tan GS, Tapp RJ, et al. Cost-effectiveness of a National Telemedicine Diabetic Retinopathy Screening Program in Singapore. Ophthalmology. 2016;123(12):2571–2580. [DOI] [PubMed] [Google Scholar]
- 29.Ting DSW, Pasquale LR, Peng L, et al. Artificial intelligence and deep learning in ophthalmology. Br J Ophthalmol. 2019;103(2):167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tufail A, Rudisill C, Egan C, et al. Automated Diabetic Retinopathy Image Assessment Software: Diagnostic Accuracy and Cost-Effectiveness Compared with Human Graders. Ophthalmology. 2017;124(3):343–351. [DOI] [PubMed] [Google Scholar]
- 31.Keel S, Wu J, Lee PY, et al. Visualizing Deep Learning Models for the Detection of Referable Diabetic Retinopathy and Glaucoma. JAMA Ophthalmol. 2019;137(3):288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruamviboonsuk P, Krause J, Chotcomwongse P, et al. Deep learning versus human graders for classifying diabetic retinopathy severity in a nationwide screening program. NPJ Digit Med. 2019;2:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellemo V, Lim ZW, Lim G, et al. Artificial intelligence using deep learning to screen for referable and vision-threatening diabetic retinopathy in Africa: a clinical validation study. The Lancet Digital Health. 2019;1(1):e35–e44. [DOI] [PubMed] [Google Scholar]
- 34.Heydon P, Egan C, Bolter L, et al. Prospective evaluation of an artificial intelligence-enabled algorithm for automated diabetic retinopathy screening of 30 000 patients. Br J Ophthalmol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akiyode O, Tran C. Overview of Ocular Anti-Vascular Endothelial Growth Factor Therapy in the Management of Diabetic Eye Complications. Diabetes Spectr. 2016;29(1):44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Funatsu H, Yamashita H, Shimada H, et al. [Reliability of evaluating grade of diabetic retinopathy]. Nippon Ganka Gakkai Zasshi. 1993;97(3):396–402. [PubMed] [Google Scholar]
- 37.Abbas Q, Fondon I, Sarmiento A, et al. Automatic recognition of severity level for diagnosis of diabetic retinopathy using deep visual features. Med Biol Eng Comput. 2017;55(11):1959–1974. [DOI] [PubMed] [Google Scholar]
- 38.Ting DSW, Cheung CY, Lim G, et al. Development and Validation of a Deep Learning System for Diabetic Retinopathy and Related Eye Diseases Using Retinal Images From Multiethnic Populations With Diabetes. JAMA. 2017;318(22):2211–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120(10):2013–2022. [DOI] [PubMed] [Google Scholar]
- 40.Wang YT, Tadarati M, Wolfson Y, et al. Comparison of Prevalence of Diabetic Macular Edema Based on Monocular Fundus Photography vs Optical Coherence Tomography. JAMA Ophthalmol. 2016;134(2):222–228. [DOI] [PubMed] [Google Scholar]
- 41.Varadarajan AV, Bavishi P, Ruamviboonsuk P, et al. Predicting optical coherence tomography-derived diabetic macular edema grades from fundus photographs using deep learning. Nat Commun. 2020;11(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arcadu F, Benmansour F, Maunz A, et al. Deep Learning Predicts OCT Measures of Diabetic Macular Thickening From Color Fundus Photographs. Invest Ophthalmol Vis Sci. 2019;60(4):852–857. [DOI] [PubMed] [Google Scholar]
- 43.Chan GCY, Kamble R, Muller H, et al. Fusing Results of Several Deep Learning Architectures for Automatic Classification of Normal and Diabetic Macular Edema in Optical Coherence Tomography. Conf Proc IEEE Eng Med Biol Soc. 2018;2018:670–673. [DOI] [PubMed] [Google Scholar]
- 44.Alsaih K, Lemaitre G, Rastgoo M, et al. Machine learning techniques for diabetic macular edema (DME) classification on SD-OCT images. Biomed Eng Online. 2017;16(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sahlsten J, Jaskari J, Kivinen J, et al. Deep Learning Fundus Image Analysis for Diabetic Retinopathy and Macular Edema Grading. Sci Rep. 2019;9(1):10750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toth CA, Decroos FC, Ying GS, et al. Identification of Fluid on Optical Coherence Tomography by Treating Ophthalmologists Versus a Reading Center in the Comparison of Age-Related Macular Degeneration Treatments Trials. Retina. 2015;35(7):1303–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlegl T, Waldstein SM, Bogunovic H, et al. Fully Automated Detection and Quantification of Macular Fluid in OCT Using Deep Learning. Ophthalmology. 2018;125(4):549–558. [DOI] [PubMed] [Google Scholar]
- 48.Sun Y, Li S, Sun Z. Fully automated macular pathology detection in retina optical coherence tomography images using sparse coding and dictionary learning. J Biomed Opt. 2017;22(1):16012. [DOI] [PubMed] [Google Scholar]
- 49.Syed AM, Hassan T, Akram MU, et al. Automated diagnosis of macular edema and central serous retinopathy through robust reconstruction of 3D retinal surfaces. Comput Methods Programs Biomed. 2016;137:1–10. [DOI] [PubMed] [Google Scholar]
- 50.Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103(12):1796–1806. [PubMed] [Google Scholar]
- 51.Diabetes C, Complications Trial Research G, Nathan DM, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. [DOI] [PubMed] [Google Scholar]
- 52.Klein R, Klein BE, Moss SE, et al. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102(4):520–526. [DOI] [PubMed] [Google Scholar]
- 53.Marshall G, Garg SK, Jackson WE, et al. Factors influencing the onset and progression of diabetic retinopathy in subjects with insulin-dependent diabetes mellitus. Ophthalmology. 1993;100(8):1133–1139. [DOI] [PubMed] [Google Scholar]
- 54.Gallego PH, Craig ME, Hing S, et al. Role of blood pressure in development of early retinopathy in adolescents with type 1 diabetes: prospective cohort study. BMJ. 2008;337:a918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology. 1998;105(10):1801–1815. [DOI] [PubMed] [Google Scholar]
- 56.Pappuru RKR, Ribeiro L, Lobo C, et al. Microaneurysm turnover is a predictor of diabetic retinopathy progression. Br J Ophthalmol. 2019;103(2):222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lachin JM, Genuth S, Nathan DM, et al. Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial--revisited. Diabetes. 2008;57(4):995–1001. [DOI] [PubMed] [Google Scholar]
- 58.Hove MN, Kristensen JK, Lauritzen T, et al. The relationships between risk factors and the distribution of retinopathy lesions in type 2 diabetes. Acta Ophthalmol Scand. 2006;84(5):619–623. [DOI] [PubMed] [Google Scholar]
- 59.Arcadu F, Benmansour F, Maunz A, et al. Deep learning algorithm predicts diabetic retinopathy progression in individual patients. NPJ Digit Med. 2019;2:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leontidis G, Al-Diri B, Hunter A. A new unified framework for the early detection of the progression to diabetic retinopathy from fundus images. Comput Biol Med. 2017;90:98–115. [DOI] [PubMed] [Google Scholar]
- 61.Welikala RA, Dehmeshki J, Hoppe A, et al. Automated detection of proliferative diabetic retinopathy using a modified line operator and dual classification. Comput Methods Programs Biomed. 2014;114(3):247–261. [DOI] [PubMed] [Google Scholar]
- 62.Orlando JI, van Keer K, Barbosa Breda J, et al. Proliferative diabetic retinopathy characterization based on fractal features: Evaluation on a publicly available dataset. Med Phys. 2017;44(12):6425–6434. [DOI] [PubMed] [Google Scholar]
- 63.Bora A, Balasubramanian S, Babenko B, et al. Predicting Risk of Developing Diabetic Retinopathy using Deep Learning. arXiv preprint arXiv:200804370. 2020. [DOI] [PubMed] [Google Scholar]
- 64.Agardh E, Tababat-Khani P. Adopting 3-year screening intervals for sight-threatening retinal vascular lesions in type 2 diabetic subjects without retinopathy. Diabetes Care. 2011;34(6):1318–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lund SH, Aspelund T, Kirby P, et al. Individualised risk assessment for diabetic retinopathy and optimisation of screening intervals: a scientific approach to reducing healthcare costs. Br J Ophthalmol. 2016;100(5):683–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aspelund T, Thornorisdottir O, Olafsdottir E, et al. Individual risk assessment and information technology to optimise screening frequency for diabetic retinopathy. Diabetologia. 2011;54(10):2525–2532. [DOI] [PubMed] [Google Scholar]
- 67.Updated 2017: ICO Guidelines for Diabetic Eye Care. International Council of Ophthalmology 2017. [Google Scholar]
- 68.Eleuteri A, Fisher AC, Broadbent DM, et al. Individualised variable-interval risk-based screening for sight-threatening diabetic retinopathy: the Liverpool Risk Calculation Engine. Diabetologia. 2017;60(11):2174–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tan GS, Cheung N, Simo R, et al. Diabetic macular oedema. Lancet Diabetes Endocrinol. 2017;5(2):143–155. [DOI] [PubMed] [Google Scholar]
- 70.Agarwal A, Soliman MK, Sepah YJ, et al. Diabetic retinopathy: variations in patient therapeutic outcomes and pharmacogenomics. Pharmgenomics Pers Med. 2014;7:399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmid MK, Reich O, Faes L, et al. Comparison of Outcomes and Costs of Ranibizumab and Aflibercept Treatment in Real-Life. PLoS One. 2015;10(8):e0135050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arcadu F, Benmansour F, Maunz A, et al. Deep learning algorithm for patient-level prediction of diabetic retinopathy (DR) response to vascular endothelial growth factor (VEGF) inhibition. Investigative Ophthalmology & Visual Science. 2019;60(9):2806–2806. [Google Scholar]
- 73.Takahashi H, Tampo H, Arai Y, et al. Applying artificial intelligence to disease staging: Deep learning for improved staging of diabetic retinopathy. PLoS One. 2017;12(6):e0179790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prahs P, Radeck V, Mayer C, et al. OCT-based deep learning algorithm for the evaluation of treatment indication with anti-vascular endothelial growth factor medications. Graefes Arch Clin Exp Ophthalmol. 2018;256(1):91–98. [DOI] [PubMed] [Google Scholar]
- 75.Chen SC, Chiu HW, Chen CC, et al. A Novel Machine Learning Algorithm to Automatically Predict Visual Outcomes in Intravitreal Ranibizumab-Treated Patients with Diabetic Macular Edema. J Clin Med. 2018;7(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rasti R, Allingham MJ, Mettu PS, et al. Deep learning-based single-shot prediction of differential effects of anti-VEGF treatment in patients with diabetic macular edema. Biomed Opt Express. 2020;11(2):1139–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gerendas BS, Bogunovic H, Sadeghipour A, et al. Computational image analysis for prognosis determination in DME. Vision Res. 2017;139:204–210. [DOI] [PubMed] [Google Scholar]
- 78.Roberts PK, Vogl WD, Gerendas BS, et al. Quantification of Fluid Resolution and Visual Acuity Gain in Patients With Diabetic Macular Edema Using Deep Learning: A Post Hoc Analysis of a Randomized Clinical Trial. JAMA Ophthalmol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Karter AJ, Parker MM, Moffet HH, et al. Missed appointments and poor glycemic control: an opportunity to identify high-risk diabetic patients. Med Care. 2004;42(2):110–115. [DOI] [PubMed] [Google Scholar]
- 80.Watane A, Kalavar M, Cavuoto K, et al. Factors Associated with Follow-up Non-Compliance in Patients Presenting to an Emergency Department with Non-Proliferative Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2020;61(7). [Google Scholar]
- 81.Quade R. Evaluation of the Expanding Access to Diabetic Retinopathy Screening Initiative. Quade & Associates 2011. [Google Scholar]
- 82.Gegundez-Arias MCO J Garrido, B Ponte, F. Alvarez, D Marin. Inter-observer reliability and agreement study on early diagnosis of diabetic retinopathy and diabetic macular edema risk. Bioinformatics and Biomedical Engineering IWBBIO. 2016;9656 of Lecture Notes in Bioinformatics:369–379. [Google Scholar]
- 83.Ding L, Bawany MH, Kuriyan AE, et al. A Novel Deep Learning Pipeline for Retinal Vessel Detection In Fluorescein Angiography. IEEE Trans Image Process. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bawany MH, Ding L, Ramchandran RS, et al. Automated vessel density detection in fluorescein angiography images correlates with vision in proliferative diabetic retinopathy. PLoS One. 2020;15(9):e0238958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee CS, Lee AY. How Artificial Intelligence Can Transform Randomized Controlled Trials . Transl Vis Sci Technol. 2020;9(2):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jenkins V, Farewell V, Farewell D, et al. Drivers and barriers to patient participation in RCTs. Br J Cancer. 2013;108(7):1402–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]