Abstract
Protein Kinase D1 (PrKD1) functions as a tumor and metastasis suppressor in several human cancers by influencing cell-cycle progression. However, the exact mechanism of cell-cycle regulation by PrKD1 is unclear. Overexpression and ectopic expression of PrKD1 induces G1 arrest in cancer cell lines. Because checkpoint kinases (CHEKs) are known to play a role in progression through the G1 phase, we downregulated CHEK1, which did not overcome the G1 arrest induced by PrKD1. Using in vitro phosphorylation and Western blot assays, we showed that PrKD1 phosphorylates all CDC25 isoforms (known substrates of CHEK kinases), independent from CHEK kinases, suggesting that direct phosphorylation of CDC25 by PrKD1 may be an alternate mechanism of G1 arrest. The study has identified a molecular mechanism for the influence of PrKD1 in cell-cycle progression.
Keywords: CDC25, cell cycle, protein kinase D1
1 ∣. INTRODUCTION
Protein kinase D1 (PrKD1) is a novel serine threonine kinase that is downregulated in several human cancers including breast, colon, and prostate.1 In particular, PrKD1 is downregulated in advanced prostate cancer.2 Among several known mechanisms of action, PrKD1 is associated with advanced cancer by contributing to cell-cycle progression.3 While all three PrKD1 isoforms 1, 2, and 3 are known to play a role in cell-cycle progression in various cancers, the exact molecular mediators of PrKD1 on the cycle progression is yet unclear.4 In silico analysis identified cell-division cycle (CDC) 25 proteins as potential substrate for PrKD1. CDC25 proteins are phosphatases that belong to the tyrosine phosphatase family and play a critical role in regulating cell-cycle progression by dephosphorylating cyclin-dependent kinases at inhibitory residues.5 The CDC25 isoforms A, B, and C are involved in G1 and G2 transitions, and have been shown to be upregulated in prostate cancer.6 The CDC25 A and B have been shown to be involved in interaction with androgen receptor (AR), which is a known key driver in prostate cancer.7,8 In this study, we demonstrate that CDC25 A, B, and C are phosphorylated by PrKD1. Another key regulator of CDC25 phosphatases is checkpoint kinase (CHEK kinase). However, our studies showed that PrKD1 was able to regulate cell-cycle progression by phosphorylating CDC25 independent of CHEK kinase, which is a novel alternate pathway of CDC25 regulation in cancer cells.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Cell culture and transfection
LNCaP cells (lymph node metastasis of prostate carcinoma, # CRL-1740; ATCC, Manassas, VA), C4-2 cells (LNCaP-derived cells, prostate cancer, #CRL3314, UROCORe, Oklahoma City, OK), DU145 (brain metastasis of prostate carcinoma, # HTB-81; ATCC), HCT-116 (colorectal carcinoma, # CCL-24; ATCC), and MDA-MB-231 (breast adenocarcinoma, #HTB-26; ATCC) were cultured in appropriate growth medium. The first three cell lines were cultured in RPMI 1460 (HyClone, Logan, UT) and the last two were cultured in Dulbecco’s modified Eagle’s medium high glucose (HyClone), both supplemented with fetal bovine serum (10%; Invitrogen) and Antibiotic-Antifungal solution (1%; Invitrogen). Cells were maintained in 37°C and 5% CO2 incubator. When appropriate for the experimental design, cells were treated with Bryostatin-1 for 30 hours to activate exogenous PrKD1. Cells were transfected with PEGFP-empty vector or PEGFP vector containing PrKD1 gene using FuGENE HD transfection reagent (# E2311; Promega, Madison, WI) in a serum-free media for 16 hours, recovered in standard growth medium for 24 hours, selected in the presence of 400 μg/mL of G418 (Invitrogen, Carlsbad, CA), and used for the experiments within one to two passages after transfection. The expression of the CHEK family was inhibited using small interfering RNA (siRNA) for CHEK1 (SR300794; Origene, Rockville, MD) and nonsilencing control (SR30004; Origene). In selected experiments, γ-radiation (10 Gray, single dose) was used to induce DNA damage.
2.2 ∣. Cell-cycle analysis
Cell-cycle analysis was performed by quantification of DNA content of the cells using Propidium Iodide Flow Cytometry Kit (#ab139418; Abcam, Cambridge, MA) following producer protocol and BD Accuri C6 flow cytometry device (BD Biosciences, San Jose, CA).
2.3 ∣. Western blot analysis
Cells treated with bryostatin-1, a marine-derived macrolactone that is a known PrKD1 activator, were lysed using Pierce radioimmuno-precipitation buffer (#89900; Thermo Fisher Scientific, Watham, MA) containing proteinase inhibitor. Equal concentration of protein was loaded on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS PAGE) gels and transferred onto a polyvinylidene difluoride membrane (Millipore, Billerica, MA). Primary phosphorylated antibodies for CDC25 A (phospho-CDC25A (S124), #ab156574; Abcam, Cambridge, MA), B (phospho-CDC25B (S323), #ab53103; Abcam), and C (phospho-CDC25C (S216), #4901S; Cell Signaling Technology, Danvers, MA) followed by horse-radish peroxidase-conjugated secondary anti-mouse or anti-rabbit antibody (Cell Signaling Technology). The bands in the Western blot analysis were visualized using conjugation with chemiluminescence substrate (34094; Thermo Fisher Scientific) via LAS 3000 imaging system (Fuji Photo Film). Total protein loading was assessed using glyceraldehyde 3-phosphate dehydrogenase (GAPDH, #ab845; Abcam). Bands were quantified using ImageJ software to analyze the density of electrophoretic Western blot analysis bands. The values were normalized to GAPDH expression. The experiments were performed in triplicate.
2.4 ∣. In vitro phosphorylation assay
Human recombinant protein of CDC25A (#H00000993-P01; Abnova, Walnut, CA), CDC25B (#H00000994-P01; Abnova), or CDC25C (#H00000995-P01; Abnova) were mixed with human recombinant protein kinase D1 (rhPrKD1, #PV3791; Life technology, Waltham, MA) in the manufacturer’s recommended buffer plus 10 μmol/L adenosine triphosphate (ATP) and 10 μCi [γ-32P] ATP (3,000 Ci/mmol) and incubated for 15 minutes and finally arrested by 4× SDS Laemmli buffer. rhPrKD1 was activated by incubating with ATP in the presence of PS-DAG before the reaction. Reaction was carried out for 25 minutes at 37°C. The in vitro phosphorylated proteins were separated on gradient SDS PAGE gel, which was dried using gel-drying system and finally autoradiography carried out by exposing film to the dried gel. Syntide-2 was used as a positive control and rhIGF-1 as a negative control for substrate.
3 ∣. RESULTS
3.1 ∣. Protein kinase D1 affects cell cycle by arresting cells in G1 phase
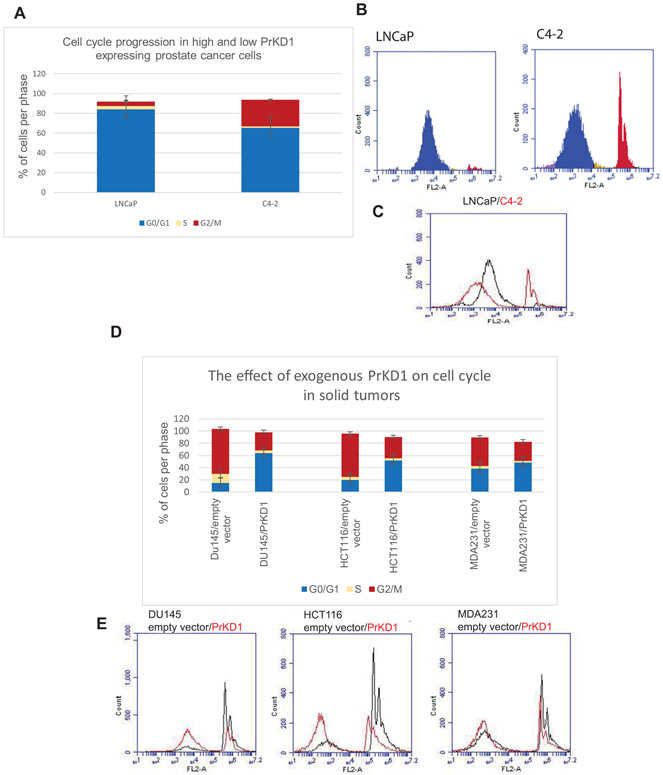
To investigate the role of PrKD1 in cell cycle, we compared the cell-cycle phases in LNCaP cells (with very high expression of PrKD1) and its more aggressive derivative C4-2 cells (with relatively low expression of PrKD1). The analysis showed that the amount of cells in G1 phase is significantly higher in LNCaP cells compared to C4-2 cells (Figure 1A-C), suggesting that PrKD1 may have a role in cell-cycle progression. To confirm the findings, we overexpressed PrKD1 in three aggressive cancer cell lines with very low expression of PrKD1 (DU145 prostate cancer cells, MDA-MB-231 breast cancer cells, and HCT-116 colorectal cancer cells) and showed that the overexpression of PrKD1 increased the number of cells in G1 phase in all three cell lines compared to vector only controls (Figure 1D-E), confirming that PrKD1 influences G1 phase transition.
FIGURE 1.
Comparative analysis of the cell cycle showed that protein kinase D1 (PrKD1) arrests the cells in G0/G1 phase. A shows percentage of cells in each phase of the cell cycle. B shows a representative of flow cytometry of LNCaP and C4-2 cells for evaluating DNA content. In each flow cytometry diagrams, the first curve shows the amount of cells in G1 and second curves shows the amount of cells in G2 phase. The distance between the two curves shows the amount of cells in the S phase. C is a histogram showing the percentage of cells in different cell-cycle phases in LNCaP cells compared to C4-2 cells. A-C, G1 phase is significantly longer in LNCaP cells (with high endogenous PrKD1) compared to its aggressive derivative C4-2 cells (with relatively low PrKD1). D-E, Inducing the expression of PrKD1 in aggressive tumor cells derived from different solid tumors prolonged G1 phase significantly (DU145: aggressive prostate cancer cell line, HCT-116: aggressive colon cancer cell line, MDA-MB-231: aggressive breast cancer cell line. In all three cell lines, the expression of PrKD1 is very low). D compares the percentage of cells in each phase of the cell cycle among different cell lines. E is a series of histograms comparing cell-cycle phases between PrKD1 overexpressed cells & control
The dysregulation of G1 phase transition has been shown to be a key in oncogenesis and the transition is fundamentally regulated by derepression of cyclins by phosphorylation events mediated by cyclin-dependent kinases (CDKs).9 In our experimental model, we induced G1 cell-cycle arrest by γ-radiation, which simulated the effects of radiotherapy in clinical practice. After γ-radiation, the number of cells in G1 phase was higher in C4-2/PrKD1 cells compared to vector control C4-2 cells (Figure S1A). The results suggest that PrKD1, at least partially, contributes to radiation-induced G1 cell-cycle arrest. To discriminate whether the arrest of cells in G1 phase is because of PrKD1 overexpression, γ-radiation, or both, we analyzed cell-cycle phases in C4-2/PrKD1 cells before and after γ-radiation. There were no significant changes in cell-cycle phases, suggesting that PrKD1 overexpression induces cell-cycle arrests in G1 phase (Figure S1B).
3.2 ∣. PrKD1 regulates cell cycle through phosphorylation of CDC25 phosphatases
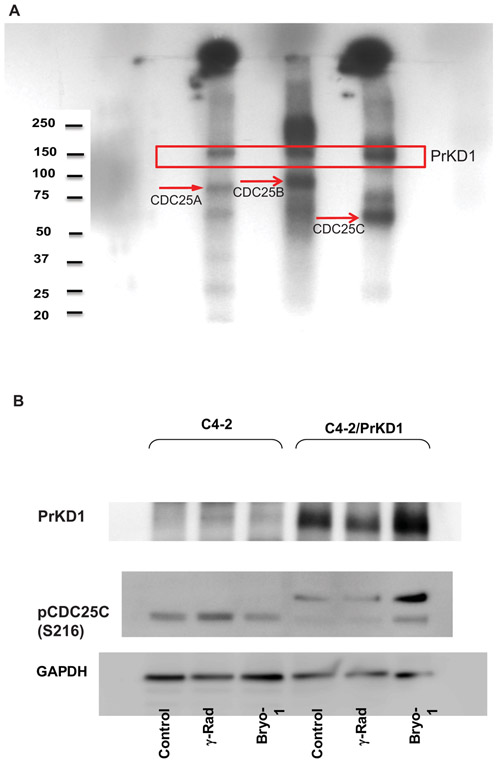
The in silico analysis identified CDC25 phosphatases as potential substrate for PrKD1. The analysis showed several serine and threonine residues with PrKD1 consensus motif LxRxxS within CDC25, suggesting that all three CDC25 isoforms could undergo substrate phosphorylation by PrKD1 at multiple sites. Of the various potential substrate phosphorylation sites, we chose to study the most extensively studied sites in each of the CDC25 isoforms: S124 for CDC25A, S323 for CDC25B, and S216 for CDC25C. We first performed in vitro kinase assay using the three CDC25 isoforms' recombinant proteins as substrates. The results showed that PrKD1 phosphorylates all three CDC25 isoforms (Figure 2A). To confirm the in vitro phosphorylation assay results, we performed phosphorylation assay by Western blot analysis using a variety of cancer cell lines. There was a significant increase in the amount of phosphorylated CDC25 isoforms in the cell lines with a higher expression of PrKD1 compared to low PrKD1 expressing cell lines (Figure 2B and S2). Further, activation of PrKD1 by Bryostatin, a marine-derived macrolactone that is a known PrKD1 activator, increased the phosphorylation of all three CDC25 isoforms with most profound and consistent effect on CDC25C at serine 216 (Figure 2B).
FIGURE 2.
PrKD1 phosphorylates CDC25 phosphatase. A, In vitro phosphorylation assay showed that PrKD1 phosphorylates CDC25 phosphatases at their common phosphorylation site: CDC25 A, CDC25B and CDC25C. B, Western blot analysis for CDC25C phosphorylation at serine 216 with reference to PrKD1 and activation of C4-2. γ-Radiation (γ-rad) was performed for DNA damage induction. Bryostatin (bryo-1) treatment was used for activation of exogenous PrKD1 after induction. PrKD1: protein kinase D1
3.3 ∣. The effect of PrKD1 on cell cycle is independent of checkpoint kinase 1
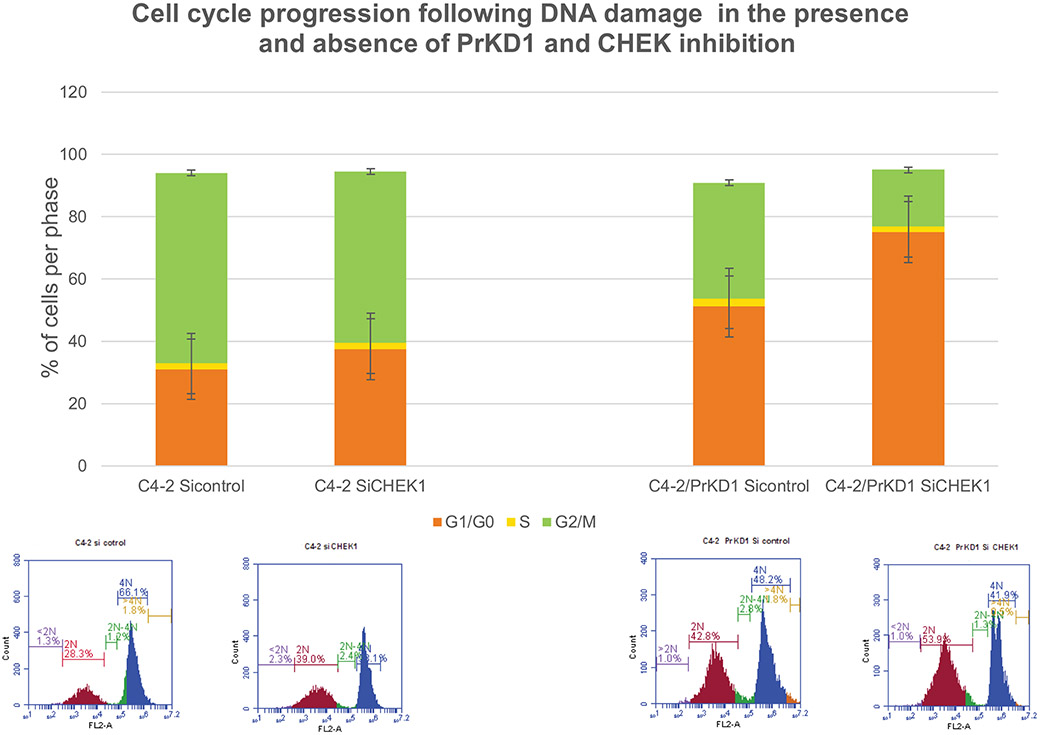
CHEK family of proteins are one of the main regulators of cell cycle at different phases and CHEK1 has been shown to be involved in G1 transition.10 CHEK1 mediates its effects on cell cycle by inhibiting CDC25 isoforms A, B, and C, which are phosphatases that activate CDK proteins. We investigated whether PrKD1 effect on cell-cycle progression is dependent on CHEK1. In stably transfected C4-2/PrKD1 prostate cancer cells and nontransfected control cells, we downregulated CHEK1 expression using siRNA and carried out cell-cycle analysis. In spite of downregulation of CHEK1, overexpression of PrKD1 continued to cause G1 arrests suggesting that PrKD1 effects on cell-cycle progression may be independent of CHEK1 (Figure 3).
FIGURE 3.
Cell-cycle progression after DNA damage in the presence and absence of PrKD1 and checkpoint kinase 1 (CHK1) small interfering RNA (siRNA). C4-2 cells and stably transfected C4-2/PrKD1 cells (overexpressed PrKD1) were transfected with siRNA for CHK1 or control siRNA (does not inhibit any RNA). Cell-cycle phases analyzed by flow cytometry, 48 hours after induction of DNA damage by γ-radiation (10 Gray, single dose). Upper panel is a bar diagram based on cell-cycle phases in flow cytometry (lower panel). The cell-cycle phases were identified based on DNA content where G1 has 2N, G2 phase has 4N DNA and the S phase is the area between the two curves. PrKD1: protein kinase D1
4 ∣. DISCUSSION
The cell-cycle control machinery is regulated tightly to ensure flawless duplication of genome and cells. Many proteins and enzymes are involved in the multistep control of the cell cycle. CDC25 phosphatases and CHEKs are the good examples of such proteins. Our results showed that PrKD1 is another protein involved in the regulation of cell cycle. PrKD1 maintains the cells in G1 phase and contributes to homeostasis of cell division. However, in pathologic conditions like progressive prostate cancer, where the expression of PrKD1 is downregulated, this balance is disturbed, the cells pass through G1 phase faster, which in turn, increases cell-cycle progression and causes cancer progression.
PrKD1, a serine/threonine kinase, was first discovered as a member of protein kinase C family (formerly called PKC mu) in 1994.3 There is constitutive low-level expression of the human PKC mu gene in many normal tissues.3 However, the highest expression of PrKD1 in normal tissues has been reported in testis and prostate by RNA sequencing.11 The higher levels of expression of PrKD1 in prostate tissue compared to several other human organs may dispose to downregulation of PrKD1 in progressive prostate cancer. In addition, PrKD1 is downregulated in other cancer including breast and colon cancers, all of which are associated with high cellular proliferation, suggesting a direct correlation between PrKD1 down-regulation and cell proliferation.
In this study, we show that PrKD1 causes cell-cycle arrest through phosphorylating CDC25 phosphatases independent from CHEK kinase. To study the phosphorylation of CDC25 A, B, and C, we identified the heavily studied phosphorylation site in each of the CDC25 isoforms: S124 for CDC25A, S323 for CDC25B, and S216 for CDC25C. Our results show that all CDC25 isoforms are phosphorylated by PrKD1. Further, the results of our in vitro phosphorylation assays have been confirmed by Western blot analysis. The phosphorylation band for CDC25B was fainter in our experiments compared to CDC25 A and C. We tried a variety of other commercially available antibodies for phospho-CDC25B, all of which produced similar results suggesting a limitation of the potency of available antibodies or unstable nature of phospho-CDC25B.12
A key driver of prostate cancer progression is the transcription factor, AR. AR upregulates CDC25C by inhibiting proteosomal and lysosomal degradation pathways.13 Moreover, CDC25A functions as a corepressor of AR8 and CDC25B is a coactivator of steroid receptors including AR.14 Depending on predominant expression of a CDC25 isoform in normal or tumor issue, there may be different results on androgen receptor function. It is conceivable that CDC25 phosphorylation by PrKD1 may be yet another novel mechanism to regulate AR activity.
There are about 300 known coactivators for nuclear receptors, of which, nuclear receptor coactivator 1 (NCOA/SRC1) in known to interact with the amino terminal of AR.15 Interestingly, unbiased transcriptome analysis of AR-dependent MDA PCa 2b prostate cancer cells with NCOA1 knockdown demonstrated prominent upregulation of PrKD1.15 In the same study, several AR binding sites identified in the PrKD1 gene and PrKD1 expression were negatively regulated by AR. The data suggest that NCOA1 may play a coregulatory function of AR and PrKD1 in prostate cancer.
In summary, our study suggests that PrKD1 inhibits cell-cycle progression through phosphorylation of CDC25 isoforms at the specific sites. The sites are also known to be phosphorylated by CHEK kinases, which could be activated by DNA damage. It is conceivable, that cells undergo G1 arrest after DNA damage to allow adequate time for repair and prevent deleterious mutations. PrKD1 has a synergic effect on cell-cycle checkpoint mechanisms and loss of PrKD1 dysregulates this important cell-cycle checkpoint mechanisms, which could lead to cell-cycle progression without proper DNA repair and contribute to cancer progression. Our study shows that PrKD1 is involved in cell-cycle inhibitory regulation by arresting cells in G1 phase. The downregulation of PrKD1 leads to the lack of phosphorylation of CDC25 phosphatases independent of CHEK kinases, which release the inhibitor signals on CDK leading to cell cycle and cancer progression.
Supplementary Material
ACKNOWLEDGMENT
The current work was supported by Veterans Administration Merit Review Grant, “IO1BX001536”, NIH grant “K12 GM102773” from NIGMS.
Funding information
National Institute of General Medical Sciences, Grant/Award Number: K12 GM102773; Health Services Research and Development, Grant/Award Number: IO1BX001536; Veterans Administration Merit Review; NIH, Grant/Award Number: K12 GM102773
Footnotes
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interests.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Sundram V, Chauhan SC, Jaggi M. Emerging roles of protein kinase D1 in cancer. Mol Cancer Res. 2011;9(8):985–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaggi M, Rao PS, Smith DJ, Hemstreet GP, Balaji KC. Protein kinase C mu is down-regulated in androgen-independent prostate cancer. Biochem Biophys Res Commun. 2003;307(2):254–260. [DOI] [PubMed] [Google Scholar]
- 3.Johannes FJ, Prestle J, Eis S, Oberhagemann P, Pfizenmaier K. PKCu is a novel, atypical member of the protein kinase C family. J Biol Chem. 1994;269(8):6140–6148. [PubMed] [Google Scholar]
- 4.Papazyan R, Doche M, Waldron RT, Rozengurt E, Moyer MP, Rey O. Protein kinase D isozymes activation and localization during mitosis. Exp Cell Res. 2008;314(16):3057–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norbury C, Nurse P. Cyclins and cell cycle control. Curr Biol. 1991;1(1):23–24. [DOI] [PubMed] [Google Scholar]
- 6.Ozen M, Ittmann M. Increased expression and activity of CDC25C phosphatase and an alternatively spliced variant in prostate cancer. Clin Cancer Res. 2005;11(13):4701–4706. [DOI] [PubMed] [Google Scholar]
- 7.Ngan ES, Hashimoto Y, Ma ZQ, Tsai MJ, Tsai SY. Overexpression of Cdc25B, an androgen receptor coactivator, in prostate cancer. Oncogene. 2003;22(5):734–739. [DOI] [PubMed] [Google Scholar]
- 8.Chiu YT, Han HY, Leung SC, et al. CDC25A functions as a novel Ar corepressor in prostate cancer cells. J Mol Biol. 2009;385(2):446–456. [DOI] [PubMed] [Google Scholar]
- 9.Bertoli C, Skotheim JM, de Bruin RA Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol. 2013;14(8):518–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patil M, Pabla N, Dong Z. Checkpoint kinase 1 in DNA damage response and cell cycle regulation. Cell Mol Life Sci. 2013;70(21):4009–4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fagerberg L, Hallstrom BM, Oksvold P, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014;13(2):397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sur S, Agrawal DK. Phosphatases and kinases regulating CDC25 activity in the cell cycle: clinical implications of CDC25 overexpression and potential treatment strategies. Mol Cell Biochem. 2016;416(1-2):33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou YW, Zhang L, Muniyan S, et al. Androgens upregulate Cdc25C protein by inhibiting its proteasomal and lysosomal degradation pathways. PLoS One. 2013;8(4):e61934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chua SS, Ma Z, Ngan E, Tsai SY. Cdc25B as a steroid receptor coactivator. Vitam Horm. 2004;68:231–256. [DOI] [PubMed] [Google Scholar]
- 15.Luef B, Handle F, Kharaishvili G, et al. The AR/NCOA1 axis regulates prostate cancer migration by involvement of PRKD1. Endocr Relat Cancer. 2016;23(6):495–508. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.