Fig. 3 |. Functional niche of unconventional T cells.
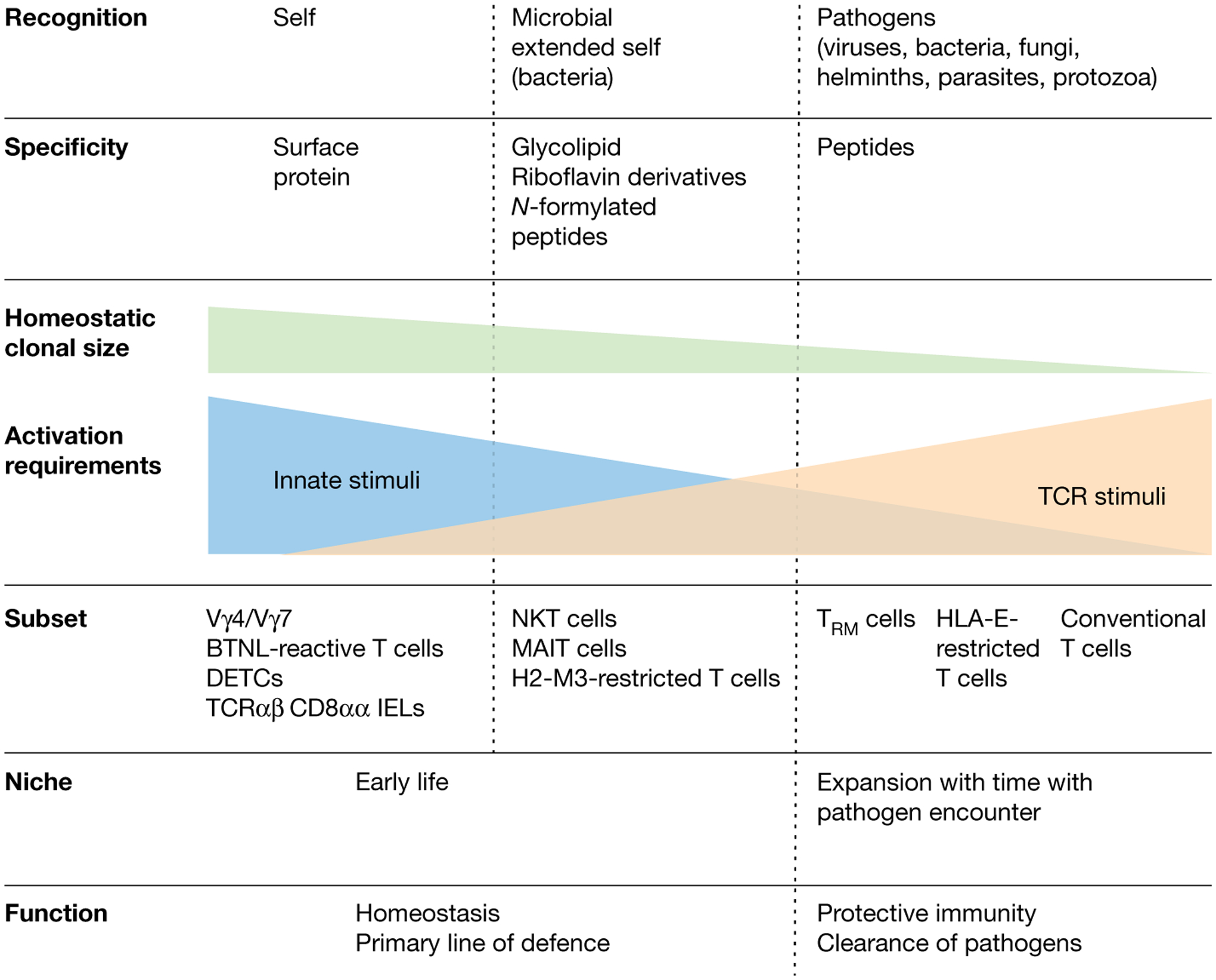
The T cell compartment is shown on a gradient from conventional T cells (right) to unconventional T cell subsets (left) according to the classifiers in bold. Classical adaptive T cells occupy a specific niche in terms of the antigenic universe they recognize (that is, MHC–peptide complexes), and they colonize tissues as tissue-resident memory T cells (TRM) only after being activated and having expanded in peripheral lymph nodes in response to an infection. By contrast, unconventional T cells recognize a broad spectrum of antigens ranging from self-molecules, to microbial extended self and non-self, to formylated peptides and to peptides. The clonal size at homeostasis for unconventional T cell subsets like NKT, MAIT and BTNL-reactive TCRγδ T cells is large, as these cells expand in tissues early in life and in the case of NKT and MAIT cells can occupy multiple tissues. The role of innate immune signals versus TCR-mediated signals varies in the activation of the different unconventional T cell subsets; innate signals have a more critical role in the innate-like unconventional T cells that expand and acquire an effector phenotype either in response to self in the periphery (BTNL-reactive TCRγδ T cells) or in the thymus during their development (MR1-, CD1d- and H2-M3-restricted T cells) than in HLA-E-restricted T cells that become activated in the periphery, similarly to conventional T cells. Of note, tissue-resident memory T cells also acquire the ability to respond to innate signals after establishing residence in tissues and are distinct in that regard from circulating memory and effector memory T cells. Finally, the unconventional T cell compartment constitutes a primary line of defence and also has an important role in tissue homeostasis and healing.
