Summary
Background:
Magnetic resonance elastography (MRE) is a reliable non-invasive, alternative to liver biopsy for assessing liver fibrosis. There are limited data regarding an association between liver fibrosis by MRE and risk of cardiovascular disease.
Aim:
To investigate the association of high risk cardiovascular disease phenotype determined by coronary artery calcification (CAC) with liver fibrosis by MRE in patients with non-alcoholic fatty liver disease (NAFLD).
Method:
This was a cross-sectional analysis of well-characterized, prospective cohorts including 105 patients with NAFLD (MR imaging-derived proton density fat fraction ≥ 5%) with contemporaneous cardiac computed tomography (CT) and MRE. Patients were assessed using MRE for liver stiffness, and cardiac CT for presence of CAC (defined as coronary artery calcium score > 0). Odds of presence of CAC were analyzed using logistic regression analysis.
Results:
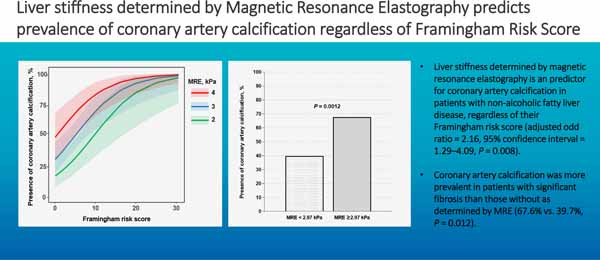
The average age and body mass index were 54.9 years and 32.9kg/m2, respectively. 49.5% of patients had CAC and 35.2% had significant liver fibrosis (defined as MRE ≥2.97kPa). Compared to patients without CAC, those with CAC were older (50.0[39.0–59.0] vs 63.0[55.5–67.5], P < 0.001), and had higher Framingham risk score (FRS, 1.0 [0.5–3.5] vs. 6.0 [2.0–12.0], P < 0.001). In multivariable-adjusted analysis, liver stiffness as a continuous trait on MRE was independently associated with the presence of CAC in a sex and age-adjusted model (adjusted odd ratios [aOR] = 2.23, 95% confidence interval [CI] = 1.31–4.34, P = 0.007) as well as in a FRS-adjusted model (aOR = 2.16, 95% CI = 1.29–4.09, P = 0.008). When analyzed as a dichotomous trait, significant fibrosis (MRE-stiffness ≥ 2.97 kPa) remained independently associated with the presence of CAC in both FRS-adjusted model and sex and age-adjusted model (aOR = 3.21–3.53, P = 0.013–0.017). In addition, CAC was more prevalent in patients with significant fibrosis than those without as determined by MRE (67.6% vs. 39.7%, P = 0.012).
Conclusion:
Liver stiffness determined by MRE is an independent predictor for presence of CAC in patients with NAFLD. Patients with NAFLD and significant fibrosis by MRE should be considered for further cardiovascular risk assessment, regardless of their FRS.
Keywords: NAFLD, liver stiffness, fibrosis, MRE, cardiovascular disease, coronary artery calcification
Graphical Abstract
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is one of the most prevalent chronic liver diseases worldwide, affecting approximately one-fourth of the Western population1. NAFLD is a liver manifestation of metabolic syndrome characterized by hepatic fat accumulation ≥ 5%.2,3 Although NAFLD can progress to fibrosis, cirrhosis and hepatocellular carcinoma, the most common cause of death in adults with NAFLD is cardiovascular disease (CVD).4,5
Many epidemiological studies have reported the association of NAFLD with increased risk of CVD.6 While the evidence from these studies is robust, most non-invasive assessments of CVD risk, such as Framingham Risk Score (FRS) and Atherosclerotic Cardiovascular Disease risk algorithm, do not include the presence of NAFLD in calculating CVD risk.7,8
Due to NAFLD’s high prevalence and heterogeneity of clinical course, it is important to identify predictors for incident CVD among patients with NAFLD.4 Prior studies have reported that advanced fibrosis (stage ≥ 3) is associated with all-cause mortality in patients with NAFLD.9,10 A recent study demonstrated the association of advanced fibrosis with incident CVD in a biopsy-proven NAFLD cohort.11 However, liver biopsy is limited in clinical practice due to its invasiveness and sampling variability.12 Non-invasive assessments for fibrosis based on indirect serum biomarkers such as NAFLD fibrosis score (NFS) and fibrosis-4 (FIB-4) index are also limited due to a significant proportion of patients being classified as having intermediate risk.13
Magnetic resonance elastography (MRE) is one of the most accurate non-invasive modalities for assessing liver stiffness14 and has low technical failure rates compared with ultrasound based elastography.14 Recently, we demonstrated that liver stiffness by MRE is associated with increased CVD risk in patients with type 2 diabetes.15 However, there is no data regarding association of liver stiffness by MRE with CVD risk in patients with NAFLD.
This study aimed to investigate the association of liver stiffness by MRE with high risk CVD phenotype based on the presence of coronary artery calcification (CAC) in patients with NAFLD. Then, we further analyzed the data with FRS of the patients to investigate association of significant fibrosis with risk of CVD.
METHODS
Study Design and Data Collection
This was a cross-sectional analysis of a single-center study from two previous studies of adults with Type 2 diabetes (Non-invasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE) or non-alcoholic steatohepatitis (NASH, Magnetic Resonance Imaging and Elastography in Ezetimibe Versus Placebo for the Assessment of Response to Treatment in NASH trial, Supplementary Figure 1). 16,17 All participants were prospectively recruited at the University of California at San Diego (UCSD) NAFLD Research Center from January 2013 to August 2014. A research study visit included demographics, anthropometric measurements, physical exam, biochemical testing, coronary calcium scan, MRE and magnetic resonance image derived proton density fat fraction (MRI-PDFF). The eligibility criteria for the two studies are described in previous publications.16,17 Main exclusion criteria were as followed: non-NAFLD chronic liver diseases, steatogenic medications, significant systemic illness, renal insufficiency, excessive alcohol use, human immunodeficiency virus, pregnancy, documented history of cardiovascular disease (CVD), such as acute coronary syndrome (ST elevation myocardial infarction, non-ST elevation myocardial infarction, unstable angina), stable angina, history of angioplasty or stent placement, cerebrovascular disease (ischemic or hemorrhagic stroke), and peripheral vascular disease for type 2 diabetes study17, and significant coronary artery disease for NASH study.16 The studies received UCSD institutional review board approval (approval numbers: UCSD IRB #121508 and #121314) and all patients provided written informed consent prior to enrollment.
Non-invasive fibrosis scores such as the NFS and FIB-4 index were calculated and categorized accordingly.18,19 The FRS was calculated using the algorithm from a previous publication.8 Homoeostatic model assessment of insulin resistance (HOMA-IR) was calculated using the following formula: HOMA-IR = [glucose (mg/dL) * insulin (mIU/mL)]/405. Adipose tissue IR (Adipo-IR) was calculated using the following formula: Adipo-IR = free fatty acids (mmol/L) * insulin (mIU/mL). Both insulin resistance tests underwent in a fasting state.
Primary and secondary outcome
Primary outcome of the study was defined as the presence of CAC in patients with NAFLD defined by MRI-PDFF ≥ 5%. The secondary outcome of the study was defined as the prevalence of CAC in patients with NAFLD and significant fibrosis defined by MRE-stiffness ≥ 2.97 kPa.
MRE and MRI-PDFF
All MR examinations were performed by the UCSD Liver Imaging Group at the MR3T Research laboratory using a 3T research scanner (GE Signa EXCITE HDxt; GE Healthcare, Waukesha, WI, USA). Trained and experienced MR technologists performed all the MR examinations. Patients were instructed to fast for a minimum of four hours before the MR scan to reduce potential physiological confounding factors. A torso phased-array coil was placed over the abdomen as the patient lay supine during imaging. Two MR techniques were utilized in the study: for NAFLD diagnosis, hepatic PDFF was estimated by chemical-shift-encoded MRI; for liver fibrosis assessment and diagnosis of significant fibrosis, hepatic stiffness was estimated by MRE. Significant fibrosis was defined as MRE-stiffness ≥ 2.97 kPa according to previous published study.14 Trained image analysts under the supervision of a faculty radiologist performed the PDFF and stiffness measurements blinded to clinical and biochemical data.
Cardiac computed tomography for CAC
A non-contrast cardiac prospective electrocardiogram-triggered volumetric computed tomography (CT) was performed using a 320-slice CT scanner (Aquilion One, Toshiba Medical Systems, Otawara, Japan). No administered medications for heart rate control or vasodilation were administered before the scan. At the end of inspiration, the patient held their breath as the scan ranged from the base of the heart to the carina; the field of view was 220 mm while the scan collimation was 320 × 0.5 mm. As determined by the SUREExposure 3D scanner software, a tube current ranging from 40 mA to 580 mA (± 10) at 120 kVp was administered. Rotation time was 0.35 s. Using five filter revolutions, 3 mm thick reconstruction slices were made. The Agatston scoring method, previously described by a fellowship-trained cardiac radiologist using independent post-processing software (Vital Images, Inc., Minnetonka, MN, USA)20, was used to quantify CAC. The presence of CAC was defined as coronary artery calcium score > 0.
Statistical analysis
Continuous data were shown with a mean and standard deviation (mean ± SD) or median with interquartile range. Continuous data were compared using Student’s t-test or the Mann-Whitney U test after Shapiro-Wilk normality testing. Categorical data were compared by a χ-squared test or Fisher’s exact test when more than 20% of cells expected frequencies below 5. The predictive factors for the presence of CAC were analyzed by a logistic regression model with stepwise backward elimination for odds ratio (OR). All statistical analyses were performed using R software (version 3.0, http://cran.r-project.org/, install. packages(“devtools”)). Logistic regression model-based plotting for probability of the presence of CAC were generated using ggplot2. A two tailed P value of ≤0.05 was used to determine statistical significance.
RESULTS
Baseline characteristics
105 patients with NAFLD who underwent MRE and cardiac CT for coronary calcium were included in this study. Median interval of cardiac CT and MRE with MRI-PDFF was 4.0 days and not more than 6 months. The average age and body mass index was 54.9 years and 32.9 kg/m2, respectively. 37 (35.2%) patients had significant liver fibrosis defined as MRE-stiffness ≥ 2.97kPa. 52 (49.5%) patients had CAC, with a median coronary artery calcium score of 121.0 [47.0–516.0]. The baseline characteristics of participants with and without CAC are shown in Table 1. Compared to those without CAC, patients with CAC were more likely to be older (50.0[39.0–59.0] vs 63.0[55.5–67.5], P < 0.001), more likely to have hypertension (48.1% vs 76.5%, P = 0.006), had higher FRS (1.0 [0.5–3.5] vs. 6.0 [2.0–12.0], P < 0.001), had lower platelet counts (271.0 [221.0–317.0] vs. 236.5 [192.5–274.0], P = 0.002), had higher HOMA-IR (5.3 [3.4–7.9] vs 7.0 [4.6–13.0], P = 0.016), had higher FIB-4 (0.8 [0.5–1.0] vs. 1.3 [0.9–1.7], P < 0.001) and NFS (−1.6 ± 1.3 vs −0.4 ± 1.2, P < 0.001), and were more likely to have higher median MRE-stiffness (2.7 [2.4–3.0] vs. 2.9 [2.4–3.6], P=0.037) and significant fibrosis (defined as MRE-stiffness ≥ 2.97kPa) (22.6% vs 48.1%, P = 0.012).
Table 1.
Baseline characteristics
| Absence of CAC group (N=53) |
Presence of CAC group (N=52) |
P value | |
|---|---|---|---|
| Male | 20 (37.7) | 30 (57.7) | 0.064 |
| Age, years | 50.0 [39.0–59.0] | 63.0 [55.5–67.5] | < 0.001* |
| BMI, kg/m2 | 33.3 ± 5.7 | 32.6 ± 5.0 | 0.529 |
| Waist circumference, cm | 101.0 [96.0–112.5] | 105.5[98.8–115.8] | 0.139 |
| Race/ethnicity, n (%) | |||
| White | 31 (58.5) | 34 (65.4) | 0.599 |
| Hispanic | 17 (32.1) | 10 (19.2) | 0.200 |
| Hypertension, n (%) | 25 (48.1) | 39 (76.5) | 0.006* |
| Diabetes mellitus, n (%) | 34 (64.2) | 45 (86.5) | 0.015* |
| History of smoking, n (%) | |||
| Current smoker | 4 (7.7) | 3 (5.8) | 1.000 |
| Ex-smoker | 14 (26.9) | 21 (40.4) | 0.213 |
| Biochemical data | |||
| Platelet counts, x109/L | 271.0 [221.0–317.0] | 236.5 [192.5–274.0] | 0.002* |
| AST, U/L | 30.0 [23.0–45.5] | 31.0 [20.5–41.0] | 0.430 |
| ALT, U/L | 31.0 [20.0–55.0] | 24.0 [18.5–34.0] | 0.030* |
| Total bilirubin, mg/dL | 0.4 [0.3–0.6] | 0.5 [0.3–0.7] | 0.245 |
| Albumin, g/dL | 4.5 ± 0.2 | 4.5 ± 0.3 | 0.356 |
| Alkaline phosphatase, U/L | 72.0 [57.0–87.0] | 73.0 [64.5–89.0] | 0.463 |
| GGT, U/L | 30.0 [21.5–49.5] | 32.0 [24.5–50.5] | 0.595 |
| Lipid profile | |||
| Total cholesterol, mg/dL | 186.6 ± 39.4 | 173.8 ± 33.2 | 0.075 |
| HDL, mg/dL | 49.0 [40.5–60.0] | 49.0 [38.5–59.0] | 0.753 |
| LDL, mg/dL | 99.0 [80.0–119.0] | 88.0 [70.5–112.5] | 0.141 |
| TG, mg/dL | 153.0 [113.0–189.0] | 147.5 [107.5–189.5] | 0.703 |
| Metabolic data | |||
| Fasting glucose, mg/dL | 111.0 [99.0–135.0] | 132.0 [98.5–160.5] | 0.158 |
| HOMA-IR | 5.3 [3.4–7.9] | 7.0 [4.6–13.0] | 0.016* |
| Adipo-IR | 8.7 [6.5–12.6] | 11.8 [6.9–22.6] | 0.053 |
| Use of statin, n (%) | 15 (28.3) | 30 (57.7) | 0.004* |
| Framingham risk score | 1.0 [0.5–3.5] | 6.0 [2.0–12.0] | < 0.001* |
| ≥ 20%, n (%) | 1 ( 1.9) | 8 (15.4) | 0.031* |
| ≤ 10%, n (%) | 45 (86.5) | 30 (57.7) | 0.002* |
| MRI PDFF, % | 12.6 [9.6–20.5] | 12.3 [6.9–17.8] | 0.213 |
| ≥ 15.7%, n (%) | 19 (35.8) | 19 (36.5) | 1.000 |
| MRE, kPa | 2.7 [2.4–3.0] | 2.9 [2.4–3.6] | 0.037* |
| ≥ 4.69, n (%) | 0 (0.0) | 7 (13.5) | 0.006* |
| ≥ 3.62, n (%) | 6 (11.3) | 12 (23.1) | 0.181 |
| ≥ 2.97, n (%) | 12 (22.6) | 25 (48.1) | 0.012* |
| FIB-4 | 0.8 [0.5–1.0] | 1.3 [0.9–1.7] | < 0.001* |
| > 2.67, n (%) | 0 (0.0) | 6 (11.5) | 0.013* |
| NFS | −1.6 ± 1.3 | −0.4 ± 1.2 | < 0.001* |
| ≥ 0.676, n (%) | 2 (3.8) | 9 (17.3) | 0.052 |
| Coronary artery calcium score | 121.0 [47.0–516.0] |
P value < 0.05
Data are expressed as median or mean, and interquartile range (IQR) or numbers (%).
Calculated by Student’s t test (or the Mann-Whitney U test, if appropriate) and chi-squared test (or Fisher’s exact test, if appropriate)
Presence of coronary artery calcification is defined as coronary artery calcium score > 0
CAC, coronary artery calcification; BMI, body mass index; AST, aspartate transaminase; ALT, alanine transaminase; GGT, gamma glutamyl transferase; HDL, high density lipoprotein; LDL, low density lipoprotein; TG, triglyceride; HOMA-IR, homoeostatic model assessment of insulin resistance; Adipo-IR, Adipose tissue insulin resistance; MRI PDFF, magnetic resonance imaging proton density fat fraction; MRE, magnetic resonance elastography; FIB-4, fibrosis-4; NFS, non-alcoholic fatty liver disease fibrosis score
Association between liver stiffness and CAC in patients with NAFLD
The association between the presence of CAC and liver stiffness is shown in Table 2. In the unadjusted analysis, sex, age, FRS, and liver stiffness were significant factors associated with the presence of CAC. In sex and age-adjusted analysis, liver stiffness (aOR = 2.23, 95% confidence interval [CI] = 1.31–4.34, P = 0.007) was independently associated with the presence of CAC (Figure 1a). In FRS-adjusted analysis, liver stiffness (aOR = 2.16, 95% CI = 1.29–4.09, P = 0.008) was also independently associated with the presence of CAC (Figure 1b).
Table 2.
Association between presence of coronary artery calcification and liver stiffness by MRE
| Male | Age, years | MRE, kPa | FRS | |||
|---|---|---|---|---|---|---|
| Unadjusted analysis | OR (95% CI) | 2.25 (1.04–4.98) | 1.11 (1.07–1.17) | 1.99 (1.24–3.56) | 1.17 (1.08–1.28) | |
| P value | 0.042* | < 0.001* | 0.010* | < 0.001* | ||
|
| ||||||
| Sex and age-adjusted model |
FRS-adjusted model§ |
|||||
| Male | Age, years | MRE, kPa | FRS | MRE, kPa | ||
|
| ||||||
| Multivariable-adjusted analysis | OR (95% CI) | 5.76 (2.04–18.31) | 1.13 (1.08–1.21) | 2.23 (1.31–4.34) | 1.18 (1.09–1.30) | 2.16 (1.29–4.09) |
| P value | 0.002* | < 0.001* | 0.007* | < 0.001* | 0.008* | |
P value < 0.05
FRS includes age, sex, history of smoking, total cholesterol, high density lipoprotein cholesterol, systolic blood pressure, medication for hypertension, diabetes, and history of vascular disease.
FRS, Framingham risk score; MRE, magnetic resonance elastography
Figure 1.
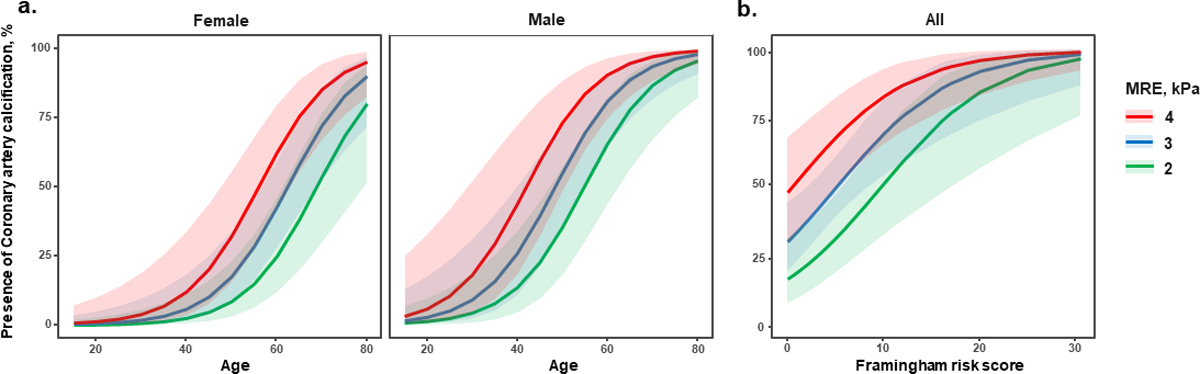
Predicted probability of presence of coronary artery calcification (defined as coronary artery calcium score > 0) according to liver stiffness by magnetic resonance elastography in patient with non-alcoholic fatty liver disease (a) Sex and age-adjusted model, (b) Framingham risk score-adjustedmodel
*The area covered by the prediction intervals is 95% confidence interval.
MRE, magnetic resonance elastography
Association between significant fibrosis and CAC in patients with NAFLD
Significant fibrosis (defined as MRE-stiffness ≥ 2.97 kPa) was independently associated with the presence of CAC in sex and age-adjusted analysis (aOR = 3.53, 95% CI = 1.29–10.48, P = 0.017, Figure 2) as well as in FRS-adjusted analysis (aOR = 3.21, 95% CI = 1.30–8.28, P = 0.013). In addition, the presence of CAC was more prevalent in patients with significant fibrosis than those without (67.6% vs. 39.7%, P = 0.012, Figure 3).
Figure 2.
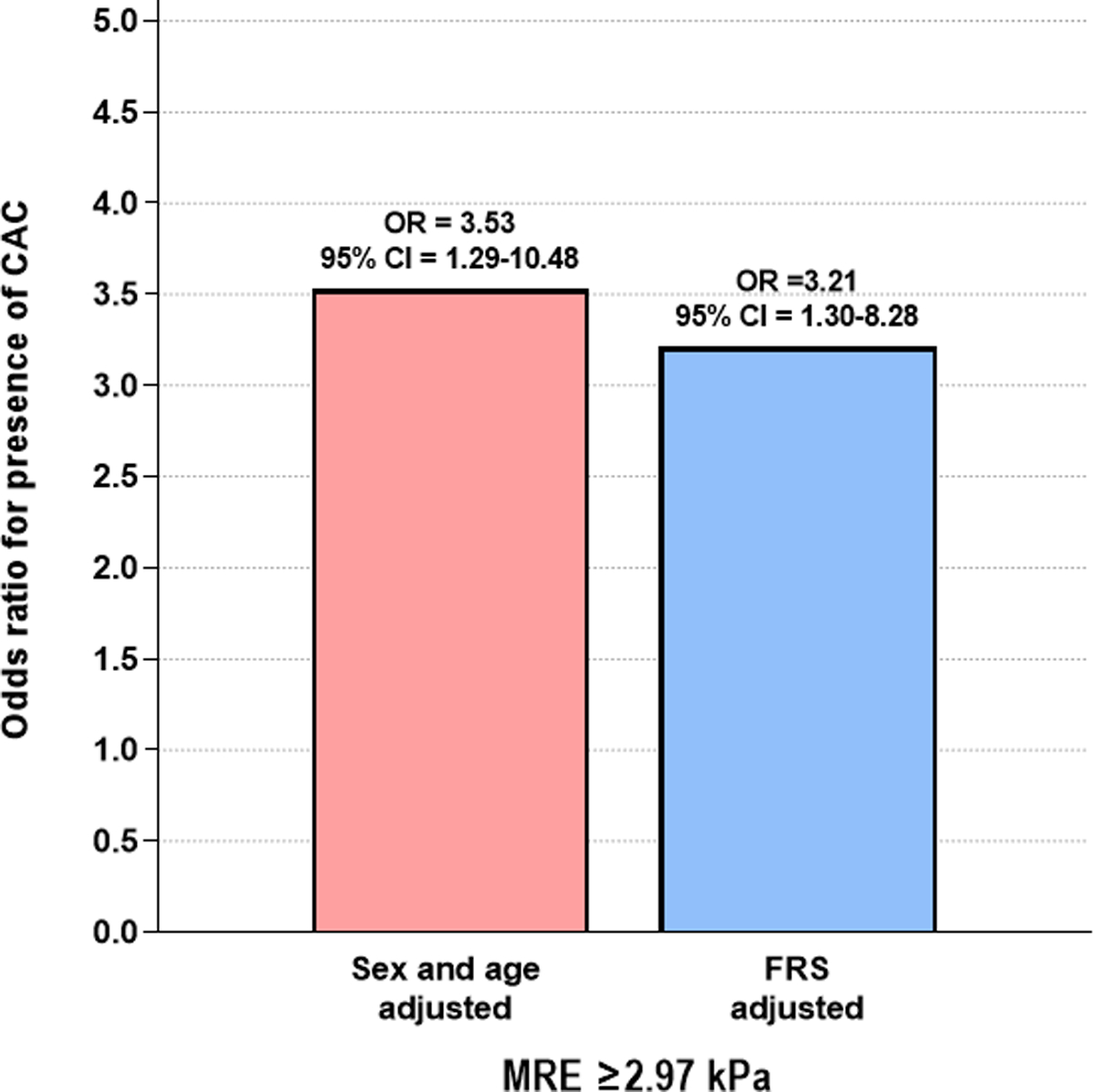
Odds ratio for the presence of coronary artery calcification (defined as coronary artery calcium score > 0) in sex and age-adjusted and FRS-adjusted logistic analysis in patients with non-alcoholic fatty liver disease and significant fibrosis (defined as MRE ≥2.97kPa)
MRE, magnetic resonance elastography; FRS, Framingham Risk Score; CAC, coronary artery calcification
Figure 3.
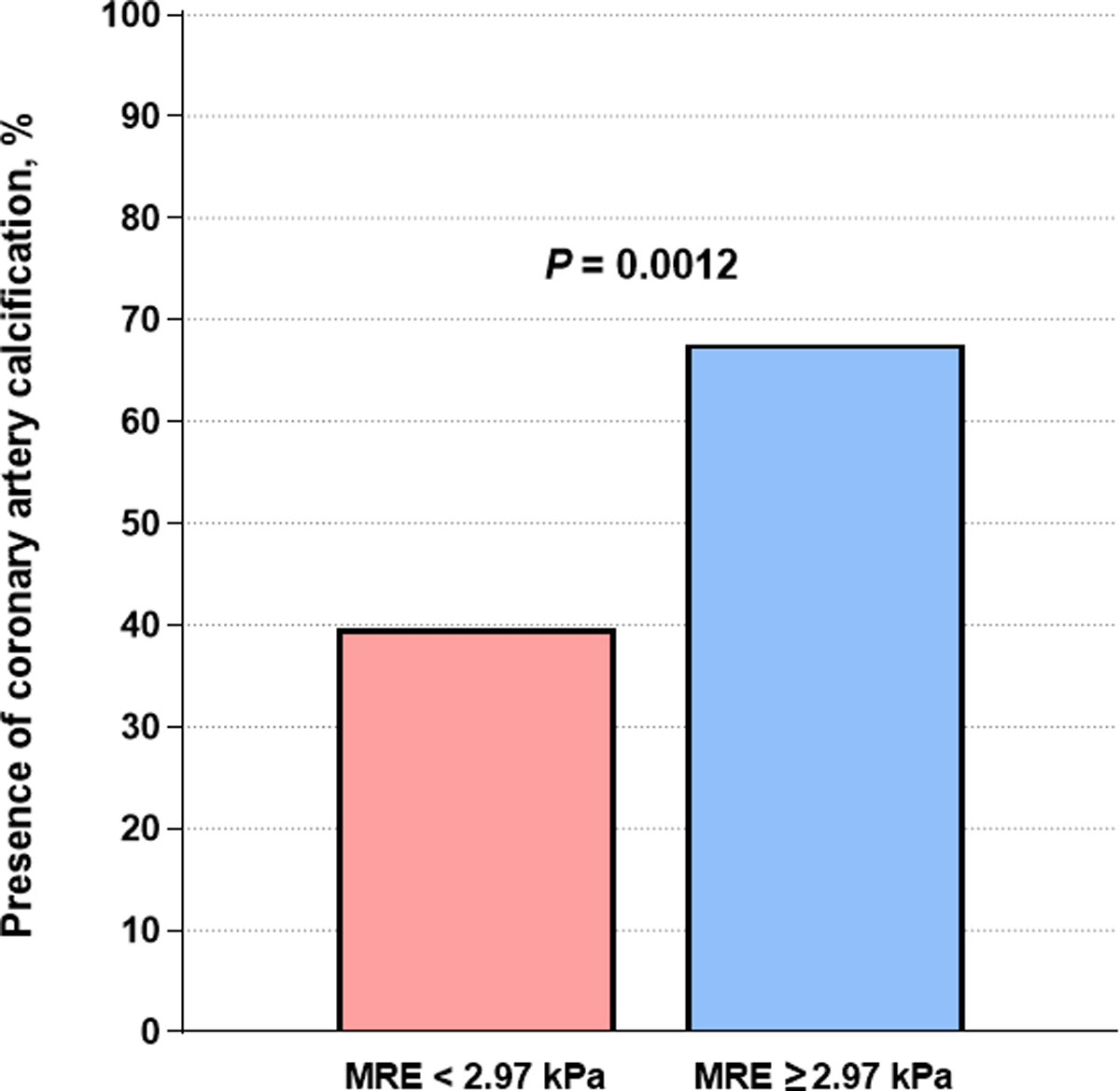
Prevalence of the presence of coronary artery calcification (defined as coronary artery calcium score > 0) in patients with non-alcoholic fatty liver disease according to significant fibrosis (defined as MRE≥2.97kPa)
MRE, magnetic resonance elastography
DISCUSSION
Main findings
We demonstrated that liver stiffness by MRE was an independent predictor for the presence of CAC in a well-characterized NAFLD cohort using both sex and age-adjusted and FRS-adjusted analysis. For patients with NAFLD, significant fibrosis (defined by MRE-stiffness ≥ 2.97 kPa) was an also independent predictor for the presence of CAC. In addition, CAC was more prevalent in patients with significant fibrosis.
These findings demonstrate that patients with NAFLD and significant fibrosis would be at higher risk for CVD regardless of their traditionally classified risk of CVD. Since NAFLD is prevalent in one fourth of the global population, traditional CVD risk scoring, such as FRS, may not be enough to assess the risk of CVD without accounting for NAFLD. Thus, MRE may benefit patients with NAFLD by helping to predict their risk of CVD.
In context with published literature
Association of NAFLD with CAC has been reported in several cohort studies.21–24 Most of these studies have demonstrated that NAFLD is an independent risk factor among the traditional risk factors.21–23 A recent study demonstrated the association of both NAFLD and alcoholic fatty liver disease with CAC in young and middle-aged population.23 While they also demonstrated association of NAFLD with their severity based on FIB-4 with CAC, their results had limited power as very few subjects had advanced fibrosis.23
Association of hepatic fibrosis with the risk of CVD in patients with NAFLD has been reported in several studies.9,11,25–28 NASH related fibrosis is associated with increased small dense low-density lipoprotein and oxidized phospholipids leading to oxidative stress and mitochondrial damage which may also increase the risk of atherosclerosis.29,30 Neutralization of these oxidized phospholipids leads to resolution of NASH as well as reduction of atherosclerosis.30 Most of clinical studies have demonstrated this association using non-invasive assessment for fibrosis based on indirect serum biomarkers, such as NFS and FIB-4 index.25,26 Only a few studies have demonstrated an association of hepatic fibrosis with the risk of CVD in patients with biopsy-proven NAFLD.9,11,27 To the best of our knowledge, this is the first study to evaluate the association of CAC with MRE-determined liver stiffness in patients with NAFLD. Unlike other studies9,11,25,26 which have demonstrated the association of advanced (stage ≥3) fibrosis with the risk of CVD, we found that significant (stage ≥ 2) fibrosis was associated with CAC. As the presence of CAC, a highly specific feature of subclinical coronary atherosclerosis, is associated with subclinical CVD31, we believe that the presence of CAC is associated with significant fibrosis rather than advanced fibrosis. Traditional CVD risk scoring models included established risk factors of CVD, such as age, sex, body mass index, race, smoking status, hypertension, diabetes, and dyslipidemia, which predicted 10-year risk of CVD.7,8 When traditional risk scoring model leads to unclear results, cardiac CT for CAC has been considered to assist treatment decisions.7 The presence of CAC is known to be associated with increased risk for CVD in many cohort studies.31 In this study, the prevalence of CAC was approximately 25% higher in patients with significant fibrosis compared to those without. Thus, we believe that MRE in patients with NAFLD may help reduce the burden for radiation exposure in cardiac CT for CAC.
Strengths and limitations
The strengths of this study are as followed: first, this is a cross-sectional study derived from two well-characterized prospective cohorts. Second, unlike previous studies, NAFLD and liver stiffness was defined by MRI-PDFF and MRE, which is one of most reliable modality for assessing liver fibrosis non-invasively. Previous studies using liver biopsy have shown limitations for diagnosing significant fibrosis due to inter- and intra-observer variability. Thus, this study provides new evidence of clinical significance regarding the risk of CVD in patients with NAFLD and significant fibrosis.
However, there are several limitations in this study. First, this study is a single-center study with a relatively small number of patients; therefore, a large scaled, longitudinal study is needed to validate the findings. Second, there was no study investigating association of liver stiffness by MRE or significant fibrosis with CAC, thus we were not able to perform a power analysis. Third, since the patients enrolled in this study were referred to participate in the clinical trial to be evaluated for NAFLD, they had relatively severe NASH compared to a random, general population. In addition, different eligibilities from two studies could cause selection bias. Therefore, further validations from the general population are needed.
Future implications
Even though some patients with NAFLD are classified as low risk of CVD by the traditional risk scoring model, significant fibrosis was an independent predictor for subclinical CVD. These results suggest that FRS is not enough to assess the risk of CVD in patients with NAFLD. Thus, in order to evaluate the risk of CVD in patients with NAFLD, liver stiffness should be considered. In addition, models for assessing the risk of CVD in patients with NAFLD, including traditional risk factors for CVD, need to be investigated in the near future.
In conclusion, liver stiffness by MRE is an independent factor for the risk of the presence of CAC in patients with NAFLD. Patients with MRE ≥ 2.97 kPa should be considered for cardiovascular risk assessment, regardless of their FRS.
Supplementary Material
Supplement figure 1. Flow chart of the study
Acknowledgments
Funding: RL receives funding support from NIEHS (5P42ES010337), NCATS (5UL1TR001442), DOD PRCRP (W81XWH-18–2-0026), NIDDK (U01DK061734, R01DK106419, R01DK121378, R01DK124318, P30DK120515), NHLBI (P01HL147835), and NIAAA (U01AA029019). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations:
- Adipo-IR
Adipose tissue insulin resistance
- CAC
coronary artery calcification
- CT
computed tomography
- CVD
cardiovascular disease
- FIB-4
fibrosis-4
- FRS
Framingham risk score
- HOMA-IR
homoeostatic model assessment of IR
- MRE
magnetic resonance elastography
- MRI-PDFF
magnetic resonance imaging proton density fat fraction
- NAFLD
nonalcoholic fatty liver disease
- NFS
NAFLD fibrosis score
- UCSD
University of California San Diego
Footnotes
Conflict of interests: Dr. Rohit Loomba serves as a consultant for Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Inipharm, Intercept, Ionis, Janssen Inc., Madrigal, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Pfizer, Sagimet, 89 bio, and Viking Therapeutics. In addition, his institution has received grant support from Allergan, Astrazeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Genfit, Gilead, Intercept, Inventiva, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Pfizer and Siemens. He is also co-founder of Liponexus, Inc.
Dr. Sirlin reports grants from GE, Siemens, Philips, Bayer, Foundation of NIH, Gilead; personal consultation fees from Blade, Boehringer, and Epigenomics; consultation under the auspices of the University to AMRA, BMS, Exact Sciences, GE Digital, and IBM-Watson; lab service agreements from Enanta, Gilead, ICON, Intercept, Nusirt, Shire, Synageva, Takeda; royalties from Wolters Kluwer for educational material outside the submitted work; and honoraria from Medscape for educational material outside the submitted work.
References
- 1.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013;10(11):686–690. [DOI] [PubMed] [Google Scholar]
- 2.Yoo JJ, Kim W, Kim MY, et al. Recent research trends and updates on nonalcoholic fatty liver disease. Clin Mol Hepatol 2019;25(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67(1):328–357. [DOI] [PubMed] [Google Scholar]
- 4.Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017;66(6):1138–1153. [DOI] [PubMed] [Google Scholar]
- 5.Loomba R, Adams LA. The 20% Rule of NASH Progression: The Natural History of Advanced Fibrosis and Cirrhosis Caused by NASH. Hepatology 2019;70(6):1885–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. Journal of hepatology 2016;65(3):589–600. [DOI] [PubMed] [Google Scholar]
- 7.Goff DC Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(25 Pt B):2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Agostino RB Sr., Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117(6):743–753. [DOI] [PubMed] [Google Scholar]
- 9.Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015;61(5):1547–1554. [DOI] [PubMed] [Google Scholar]
- 10.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2015;149(2):389–397 e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henson JB, Simon TG, Kaplan A, Osganian S, Masia R, Corey KE. Advanced fibrosis is associated with incident cardiovascular disease in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2020;51(7):728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang Y, Kim JI, Lee B, et al. Clinical application of ultrasonography-guided percutaneous liver biopsy and its safety over 18 years. Clin Mol Hepatol 2020;26(3):318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castera L, Friedrich-Rust M, Loomba R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019;156(5):1264–1281 e1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu C, Caussy C, Imajo K, et al. Magnetic Resonance vs Transient Elastography Analysis of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Pooled Analysis of Individual Participants. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2019;17(4):630–637 e638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mangla N, Ajmera VH, Caussy C, et al. Liver Stiffness Severity is Associated With Increased Cardiovascular Risk in Patients With Type 2 Diabetes. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2020;18(3):744–746 e741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loomba R, Sirlin CB, Ang B, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology 2015;61(4):1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doycheva I, Cui J, Nguyen P, et al. Non-invasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE. Aliment Pharmacol Ther 2016;43(1):83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45(4):846–854. [DOI] [PubMed] [Google Scholar]
- 19.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43(6):1317–1325. [DOI] [PubMed] [Google Scholar]
- 20.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15(4):827–832. [DOI] [PubMed] [Google Scholar]
- 21.Kim D, Choi SY, Park EH, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology 2012;56(2):605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinn DH, Kang D, Chang Y, et al. Non-alcoholic fatty liver disease and progression of coronary artery calcium score: a retrospective cohort study. Gut 2017;66(2):323–329. [DOI] [PubMed] [Google Scholar]
- 23.Chang Y, Ryu S, Sung KC, et al. Alcoholic and non-alcoholic fatty liver disease and associations with coronary artery calcification: evidence from the Kangbuk Samsung Health Study. Gut 2019;68(9):1667–1675. [DOI] [PubMed] [Google Scholar]
- 24.Kim SH, Park HY, Lee HS, et al. Association between non-alcoholic fatty liver disease and coronary calcification depending on sex and obesity. Sci Rep 2020;10(1):1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreno-Del Castillo MC, Sanchez-Rodriguez A, Hernandez-Buen Abad JJ, et al. Importance of Evaluating Cardiovascular Risk and Hepatic Fibrosis in Patients With Newly Diagnosed Nonalcoholic Fatty Liver Disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2019;17(5):997–999. [DOI] [PubMed] [Google Scholar]
- 26.Baratta F, Pastori D, Angelico F, et al. Nonalcoholic Fatty Liver Disease and Fibrosis Associated With Increased Risk of Cardiovascular Events in a Prospective Study. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2020;18(10):2324–2331 e2324. [DOI] [PubMed] [Google Scholar]
- 27.Niikura T, Imajo K, Ozaki A, et al. Coronary Artery Disease is More Severe in Patients with Non-Alcoholic Steatohepatitis than Fatty Liver. Diagnostics (Basel) 2020;10(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology 2018;155(2):443–457 e417. [DOI] [PubMed] [Google Scholar]
- 29.Imajo K, Hyogo H, Yoneda M, et al. LDL-migration index (LDL-MI), an indicator of small dense low-density lipoprotein (sdLDL), is higher in non-alcoholic steatohepatitis than in non-alcoholic fatty liver: a multicenter cross-sectional study. PLoS One 2014;9(12):e115403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun X, Seidman JS, Zhao P, et al. Neutralization of Oxidized Phospholipids Ameliorates Non-alcoholic Steatohepatitis. Cell Metab 2020;31(1):189–206 e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary Calcium Score and Cardiovascular Risk. J Am Coll Cardiol 2018;72(4):434–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement figure 1. Flow chart of the study


