Abstract
Aims:
Intravenous (IV) misuse of the μ opioid analgesic oxymorphone has caused significant public health harms; however, no controlled data on its IV abuse potential are available. The primary aim of this pilot study was to directly compare IV oxymorphone to IV oxycodone, morphine and hydromorphone on a subjective measure of drug liking and to assess relative potency.
Methods:
Participants (n=6) with opioid use disorder, physical dependence and current IV use completed this two-site, within-subject, double-blind, placebo-controlled, inpatient pilot study. During each session, one IV dose (mg/70 kg) was administered: oxymorphone (1.8, 3.2, 5.6, 10, 18, 32), hydromorphone (1.8, 3.2, 5.6, 10, 18), oxycodone (18, 32, 56), morphine (18, 32) and placebo. Data were collected before and for 6 h after dosing. Primary outcomes included safety/physiological effects, subjective reports of drug liking and relative potency estimates.
Results:
All active test drugs produced prototypical, dose-related μ opioid agonist effects (e.g., miosis). Oxymorphone was more potent than the comparator opioids on several measures, including drug liking and respiratory depression (p <0.05). Across abuse-related subjective outcomes, oxymorphone was 2.3 – 2.8 fold more potent than hydromorphone and 12.5 –14 fold more potent than oxycodone (p<0.05).
Conclusions:
Despite the relatively small sample size, this pilot study detected robust oxymorphone effects. Oxymorphone was far more potent than the comparator opioids, particularly on abuse potential outcomes. Overall, these findings may help explain surveillance reports that demonstrate, after adjusting for prescription availability, oxymorphone is injected at the highest frequency, relative to other prescription opioids.
Keywords: oxymorphone, intravenous, opioid, abuse potential, potency, oxycodone, hydromorphone, morphine
INTRODUCTION
Oxymorphone (14-hydroxydihydromorphinone) is a semisynthetic opioid agonist that displays a high degree of μ opioid receptor selectivity and intrinsic activity (Volpe et al., 2011; Olson et al., 2019). Oxymorphone (oral, parental formulations) has a long history as an effective analgesic for cancer-related (Coblentz and Bierman, 1956; Eddy and Lee, 1959; Ciliberti and Eddy, 1961; Beaver and Feise, 1977; Beaver et al., 1977) and non-cancer pain conditions (Gabrail et al., 2004; Gimbel and Ahdieh, 2004; Gimbel et al., 2005; Hale et al., 2005, 2007; Sloan et al., 2005; Aqua et al., 2007; Rauck et al., 2008). These studies, along with proprietary data that are not publicly available, contributed to the published analgesic potency conversion tables that report oral oxymorphone is twice as potent as oxycodone, hydrocodone and methadone, and three-fold more potent than morphine for the treatment of pain (Endo Pharmaceuticals, 2016). However, comparatively few data are available regarding the analgesic potency of parenteral oxymorphone. The limited number of controlled studies that have tested parenteral oxymorphone have indicated that it is 8 – 13-fold more potent than morphine (Coblentz and Bierman, 1956; Eddy and Lee, 1959; Beaver et al., 1977), 50-fold more potent than meperidine, 2-fold more potent than hydromorphone (Coblentz and Bierman, 1956) and 14-fold more potent than oral oxymorphone on peak analgesic effect (Beaver and Feise, 1977). Nonetheless, the prescribing information for injectable oxymorphone only contains one opioid conversion – parenteral oxymorphone is 10-fold more potent than parenteral morphine for pain relief (Endo Pharmaceuticals, 2006).
Although the analgesic efficacy of oxymorphone has been well characterized, there are limited data regarding its abuse potential. Two controlled studies have examined the effects of oral oxymorphone in the human laboratory, enrolling participants with histories of occasional opioid misuse. The first compared the physiological and behavioral effects of controlled-release (CR) formulations of oral oxymorphone (15, 30 mg) to oral oxycodone doses that were two-fold greater (30, 60 mg) (Schoedel et al., 2010, 2011). The results indicated that when the 1: 2 (oxymorphone: oxycodone) dose ratios were compared, oxymorphone displayed less abuse potential, and less physiological and cognitive impairment relative to oxycodone. However, the comparator doses tested were not physiologically equipotent (i.e., the miotic effects of oxycodone [30 mg] were greater than those produced by oxymorphone [30 mg]). A follow-up study was conducted to examine the effects of equal doses of oral immediate-release oxycodone and oxymorphone (10, 20, 40 mg) on experimental pain outcomes, abuse potential and overall potency (Babalonis et al., 2016). That report revealed that oral oxymorphone was approximately two-fold less potent than oxycodone on experimental pain outcomes (e.g., cold pressor, pressure algometer) and physiological outcomes (e.g., pupil diameter, end-tidal carbon dioxide [EtCO2] concentrations). However, at the highest dose tested (40 mg), oxymorphone produced abuse-related effects that were comparable to 40 mg oxycodone. These data suggest that oral oxymorphone may have greater abuse potential than oxycodone, given 1) that oxymorphone doses (e.g., 40 mg) which did not reliably produce analgesia and were less potent on physiological endpoints, produced comparatively greater ratings of abuse potential and 2) the poor oral bioavailability of oxymorphone (~ 10%) relative to oxycodone (approx. 60%), routes of administration with greater bioavailability (intranasal, IV) may pose even greater potential for misuse.
Outside of controlled laboratory studies, there is historical and epidemiological evidence suggesting that oxymorphone has high abuse potential, particularly when used intravenously. Oxymorphone products were introduced into the U.S. market in 1959 (Numorphan®); however, the manufacturer (Endo Pharmaceuticals) voluntarily removed the oral products from the market in 1979, citing commercial reasons. However, reports at the time indicated that oxymorphone was being misused, particularly via injection, with some users indicating that they preferred it over heroin (Watkins and Chambers, 1972). The same manufacturer reintroduced oral formulations to the market in 2006 under a new trade name (Opana®, Opana ER®). After these products (including re-formulated product introduced in 2012) were on the U.S. market for several years, reports began to emerge detailing oxymorphone misuse. Users were manipulating the extended-release mechanism in the oral products to gain access to the full dose for intranasal or IV misuse (FDA, 2017; Broz et al., 2018). Surveillance data also suggested that oxymorphone was being injected at inordinately high frequency, relative to the low number of prescriptions written (Butler et al., 2013; Cassidy et al., 2014; Cicero et al., 2016; FDA, 2017). This intravenous misuse produced significant public health problems. For example, in 2015, there was an HIV outbreak (n=181) in rural Indiana associated with sharing needles to inject ER oxymorphone – 88% of the infected in individuals (n=159) reported injecting ER oxymorphone and 92% also tested positive for HCV (Peters et al., 2016). Injection misuse of ER oxymorphone has also caused acute kidney injury (Ambruzs et al., 2014; Bonnecaze et al., 2018) and blood vessel and blood clotting disorders (i.e., thrombotic microangiopathy; thrombotic-thrombocytopenic purpura-like syndrome; hemolytic uremic syndrome) (Rane et al., 2014; Ban et al., 2017; Thakur et al., 2017) due to excipients (i.e., chemical stabilizers, binders embedded in pills) and not oxymorphone itself (Hunt et al., 2017). These mounting health concerns led the FDA to convene two independent advisory committees in March 2017 to hear the evidence related to the public health harms of oxymorphone misuse (FDA, 2017). In June 2017, the FDA asked Endo to remove the product from the market primarily due to injection-related harms and the sponsor ultimately complied. Although Opana ER® is no longer marketed in the U.S., generic formulations of ER and immediate-release oxymorphone products are still available.
Despite this long history of oxymorphone misuse, it is unclear whether its IV misuse is primarily due to non-pharmacological reasons (e.g., greater availability in certain geographic regions, price) or one or more pharmacological factors (e.g., potency, high degree of intrinsic activity). Thus, the primary aim of this dose-finding, double-blind, placebo-controlled two-site pilot study was to 1) determine doses of IV oxymorphone that would produce ratings of drug liking that were comparable to dose ranges of other full μ opioid agonists (oxycodone, morphine, hydromorphone), 2) compare IV oxymorphone, oxycodone, morphine, and hydromorphone on other subjective effects related to abuse potential (e.g., good drug effects, high), as well as physiological and observer-rated effects, and 3) calculate the relative potency of IV oxymorphone on outcomes related to abuse potential and physiological/safety outcomes. This study also served as a pilot study to identify doses of oxymorphone and the comparator opioids that produced equieffective effects (e.g., peak effects on drug liking) for a subsequent randomized study of abuse liability and self-administration.
METHODS
Participants
Participants were adults ages 18–55 (Kentucky site) or 21–55 (New York site) who were physically dependent on opioids and met DSM-5 criteria for moderate-to-severe opioid use disorder. All participants completed in-person screening evaluations that included substance use and psychiatric assessments, medical history and physical exam, blood chemistry, urinalysis, and ECG. Additional inclusion criteria included: BMI of 18–35 kg/m2 and a body weight between 110 and 220 lbs (due to weight-based dosing), recent (e.g., past month) IV opioid use, minimum of 21 days of opioid use in the 30 days prior to enrollment, physical dependence for a minimum of 1 month prior to enrollment, observed urine samples testing positive for opioids (or if testing negative, presenting with opioid withdrawal symptoms) and/or exhibiting opioid withdrawal signs after naloxone administration (0.2 – 3 mg, intramuscular), and medical clearance by study physician (based on medical history, physical exam, labs, ECG). Exclusion criteria included other current physiological drug dependence requiring medical intervention (e.g., benzodiazepines, alcohol), clinically significant lab or ECG results, significant medical (e.g., seizure disorder) or psychiatric conditions requiring medication or that would interfere with data collection (e.g., bipolar disorder, suicidality), current prescription medications (aside from oral contraceptives), seeking treatment for opioid use disorder, currently in treatment and taking medications for opioid use disorder (e.g., buprenorphine, methadone), physical dependence on long-acting opioids, oxygen saturation of < 92% at screening, QTc interval > 450 ms at screening (or personal or family history of prolonged QT interval), and current pregnancy or lactation/breastfeeding.
All participants provided sober, written informed consent during screening and were compensated for their participation. The study was approved by the Institutional Review Boards at the University of Kentucky and New York State Psychiatric Institute and was conducted in accordance with the Helsinki guidelines for ethical research. A Certificate of Confidentiality was also obtained from the FDA. This study was conducted under an investigator-initiated Investigational New Drug Application (FDA IND 137,611).
Drug Doses
Participants were stabilized on morphine (30 mg, p.o.), administered four times per day: 7 am, 1 pm, 6 pm, 10 pm. On experimental session days, two doses were omitted (1 and 6 pm) for safety purposes; however, on occasion, the 6 pm dose was administered at investigator discretion (e.g., complaints of opioid withdrawal). During each experimental session, one IV dose was administered at 11 am. The IV drug doses (mg/70 kg) included in the data analysis: oxymorphone 1.8, 3.2, 5.6, 10, 18, and 32 mg; oxycodone: 18, 32, and 56 mg; morphine: 18 and 32 mg; hydromorphone: 1.8, 3.2, 5.6, 10, and 18 mg; and placebo (saline vehicle). Qualification session doses were IV morphine 56 mg/70 kg and placebo. All six participants included in the data analysis completed the qualification sessions and received all 17 experimental doses, with one exception – one participant did not receive the 18 mg/70 kg dose (n=5 in this condition, n=6 in all others).
Morphine (56 mg/70 kg) and oxymorphone (56 mg/70 kg) were initially included as test conditions but were not administered to all participants due to adverse events (AEs) – these doses are not included in the current data analysis. Oxymorphone (56 mg/70 kg) produced profound sedation (n=2) and was withheld for all subsequent participants. Morphine produced an overall unpleasant experience for some participants; AEs included: headache (n=5), tachycardia (n=3), pruritus (n=3), diaphoresis (n=3), hypotension (n=2), depressed respiration rate (n=2), flushing/feeling hot (n=2), numbness/tingling (n=2), chest pain/tightness (n=2), rapid respiration rate (n=1), sedation (n=1), hypertension (n=1). These AEs occurred most frequently during the qualification sessions, but also when 56 mg/70 kg (n=4) and 32 mg/70 kg (n=2) were administered during test sessions. The morphine 32 mg/70 kg was administered to all participants (and is included in the data analysis), but morphine 56 mg/70 kg was not administered to all participants and is not included in the current analysis.
Drugs
Commercially available liquid morphine (Mallinckrodt Pharmaceuticals, Bedminster Township, NJ [New York site]; SPECGX LLC, St. Louis, MO [Kentucky site]) was used for the oral maintenance doses. Commercially available parenteral morphine (West-Ward Pharmaceuticals, Columbus, OH) and hydromorphone (Akorn Pharmaceuticals, Lake Forest, IL) were used for the IV doses. IV preparations of oxycodone and oxymorphone were not commercially available and were created by a compounding pharmacy using powder-based oxymorphone and oxycodone (both obtained from Mallinckrodt Pharmaceuticals) and bacteriostatic sodium chloride saline (0.9%; Hospira, Lake Forest, IL); salt weight (HCl) was used for drug weight calculations. All IV drug preparation was completed under sterile conditions. Saline was used for placebo, for dilution for weight-based dosing and to create uniform volumes. Each IV dose was 20 mL in volume, administered across 30 sec and was injected into either an indwelling peripheral catheter or peripherally inserted central catheter (PICC).
Dose Selection Rationale
The IV doses of oxycodone, morphine and hydromorphone as well as the oral doses of morphine were selected based on two previous studies that maintained participants on morphine (30 mg QID, p.o.) (Comer et al. 2008; Walsh et al. 2017) and administered 18 mg of intramuscular hydromorphone (Walsh et al., 2017) and 50 mg/70 kg of IV oxycodone and morphine (Comer et al. 2008) – all parenteral doses produced drug liking and were well tolerated. There are limited controlled data on the effects of parenteral administration of oxymorphone. In a previous oral dosing study, equal doses of oxycodone and oxymorphone produced comparable outcomes on drug liking (Babalonis et al., 2014). Thus, we included equal doses of oxymorphone and oxycodone and a range of oxycodone doses that produced strong drug liking. Lower doses of oxymorphone were also included to capture a full dose response and to encompass the reported 1:10 oxymorphone: morphine IV potency ratio (Endo Pharmaceuticals, 2006).
Study Design
This two-site study utilized a within-subject, double-blind, placebo-controlled crossover design. Doses were administered in a quasi-randomized order for safety – all active doses of a given opioid were administered in ascending order, but active and placebo drug schedules were otherwise randomized. All participants completed two qualification sessions (3.5 hr), followed by 17 experimental sessions (7 hr) up to 5 days per week over the course of an approximately 5-week inpatient stay.
General Methods
After medical clearance, participants were admitted to the inpatient unit and began morphine maintenance. Before each session, observed urine samples were collected and tested for drugs of abuse (Discover™ Drug Test Card; American Screening LLC, Shreveport, LA; Magenta Dip, All Test North American, Gilbert, AZ) and for pregnancy in female participants (hCG Test Card, Teco Diagnostics, Anaheim, CA; hCG Test Cassette, Henry Schein, Melville, NY); breath samples were also obtained and tested for the presence of alcohol (AlcoMate Premium AL7000, Advance Safety Devices LLC, Chatswort, CA; Alco-Sensor III, Intoximeters Inc., St. Louis, MO). Participants were provided with a light, standardized caffeine-free breakfast to be completed 4 h before drug administration; participants were not permitted to smoke for 1 h prior to IV drug administration and were permitted to resume after session completion. Data were collected before and for 6 h after the test dose, and participants were not permitted to leave the session area until their vital signs met safety criteria (e.g., oxygen saturation >95% with minimal sedation).
Qualification Sessions
After participants were stabilized on morphine (e.g., Clinical Opioid Withdrawal Scale (Wesson and Ling, 2003) score ≲ 5), which generally occurred approximately 5 days after admission, sessions were initiated. Two qualification sessions (3.5 h long) were conducted during which participants received IV morphine (56 mg/70kg) or placebo, with order of presentation randomized across participants. Qualification criteria included: 1) safely tolerating the morphine dose (respiration rate, blood pressure within safety criteria), 2) reporting liking for morphine (i.e., peak visual analog scale [VAS] score of ≥ 60 on a bipolar scale (i.e., 0=strong disliking, 50= neutral, 100= strong liking), and 3) little to no drug liking for placebo (i.e., peak VAS response of ≥ 40, but <60). Participants who did not meet qualification criteria were discharged.
Experimental Sessions
There were 17 test sessions (16 active dose conditions, placebo), and each session was 7 h in duration (30 mins baseline and 6 h post-dose monitoring).
Physiological Measures
Heart rate, blood pressure, oxygen saturation (Dinamap Non-Invasive Patient Monitor, GE Medical Systems, Tampa, FL; Criticare Poet Plus 8100, Criticare Technologies, Warwick RI), expired end-tidal carbon dioxide (EtCO2), respiration rate (N-85 Capnograph, Nellcor, Boulder, CO; Criticare Poet Plus 8100) and pupil diameter (PLR-200 and VIP-300 models, NeurOptics, Irvine, CA) were collected before and immediately after IV drug administration every 5 min for the first 15 mins and in 15 min intervals thereafter. Anesthesiologists were present or readily available for all sessions for safety purposes.
Subject- and Observer-Rated Drug Effects
Subjective effects measures included a 7-item VAS (adapted from Walsh et al., 2008). The item, “Do you like the drug effect you are feeling now?” (described above) was presented on a bipolar scale; the remaining 6 questions were presented on a unipolar VAS scale (0= not at all; 100 = extremely): “Do you feel any drug effects?”, “Do you feel high?”, “Do you feel good drug effects?”, “Do you feel bad drug effects?” and “Do you want to take the drug again?” and “Do you have any desire to use opioids?” The VAS items were administered at baseline, every min for the first 10 min post-dose, 15, 30 min and in 30 min intervals thereafter. Street value ($US) and the Opioid Symptom Scale [OSS – an assessment of opioid side effects]) were collected at baseline, 15 and 30 min post-dose and every 30 min thereafter. At the end of each session, participants were asked to identify the drug they received from a list of opioid agonists (e.g., heroin, hydromorphone) and placebo (adapted from Jasinski et al., 1977). Observers rated 12-item opioid agonist scale (Observer Rated Opioid Agonist Effects) from 0 (not present) to 4 (frequent) (Fraser et. al., 1961).
Statistical Analyses
All measures were initially analyzed as raw time course data using a two factor repeated measures model (drug condition, time) with an autoregressive covariance structure. Peak/trough scores were calculated for individual participants within each dose condition and analyzed in a one-factor model (drug condition) with a compound symmetry covariance structure. These multi-level analyses were run with Proc Mixed in SAS 9.3 (SAS Institute, Inc; Cary, NC, USA), which is suited for data with repeated measures, correlations among observations within an individual subject and missing data. The response of individual subjects is first modeled, and then the estimates for each individual are combined in a group analysis (Singer, 1998; Ballinger 2004; Diggle et al. 1996; Gibbons et al. 1993; Kreft and De Leeuw 1998). Tukey’s post-hoc tests compared active doses to placebo. Results were considered significant when p ≤ 0.05 and means (standard errors) are reported unless otherwise indicated.
For primary outcome measures for which significant peak effects were observed (relative to placebo), the Finney parallel lines bioassay (Finney, 1964) was employed to assess oxymorphone relative potency. This assay utilizes a six-point method (3 active doses of each drug) and valid analyses are obtained if the dose response curves are linear, parallel, overlapping and have slopes that are significantly different from zero. Two potency comparisons were conducted: 1) oxymorphone and hydromorphone (3.2, 5.6, 10 mg); and 2) oxymorphone (1.8, 3.2, 5.6 mg) and oxycodone (18, 32, 56 mg). Morphine was excluded due to an insufficient number of test doses.
RESULTS
Participants
A total of 17 participants were enrolled in the study. A total of 10 participants did not complete the study and these individuals are not included in the data analysis (2 did not meet qualification criteria, 5 chose to discharge, 3 were discharged by the investigators [e.g., behavioral concerns]). A total of seven participants completed the study; however, one participant provided unintelligible data (not time- or dose-related) and their data could not be used.
Six participants were included in the data analysis: one woman and five men; one African American and five Caucasian participants. Their mean age was 33 (± 3.4) years, BMI = 22 (± 1.5) kg/m2; five participants were daily tobacco smokers (9.7 [±2.7] cigarettes per day) and one was a non-smoker. Participants reported using IV heroin and/or fentanyl: 29 (± 0.5) days in the 30 days prior to enrollment. Other past 30-day drug use included: alcohol (n=2), cocaine (n=4), benzodiazepine (n=1), methamphetamine (n=1), prescription opioid use (n=3).
Physiological Outcomes
Figure 1A displays trough pupil diameter as a function of dose, with all drugs producing dose-dependent decreases in pupil diameter (F [16,79]= 10.9, p<0.05). Oxymorphone (5.6, 10, 18, 32 mg/70 kg), hydromorphone (5.6, 10, 18) and oxycodone (32, 56 mg/70 kg) produced significant miosis (p<0.05) compared to placebo, while morphine did not (p>0.05). Similarly, peak end-tidal carbon dioxide (EtCO2) concentrations (Figure 1B) were significantly increased (F [16,79]= 9.7, p<0.05), with post-hoc analysis revealing differences from placebo for oxymorphone (18, 32 mg/70 kg) and hydromorphone (18 mg/70 kg) (p<0.05), but not morphine or oxycodone (p>0.05).
Figure 1.
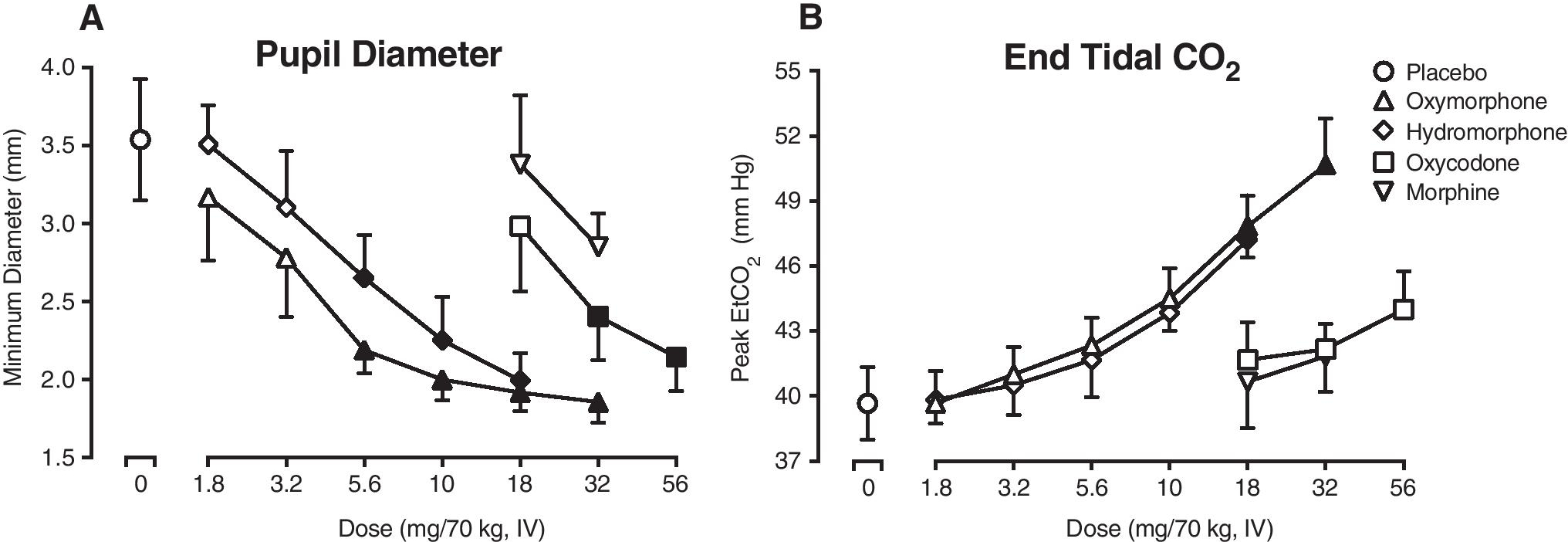
Panel A displays trough pupil diameter and Panel B displays peak end-tidal carbon dioxide concentrations (EtCO2) as a function of opioid agonist drug (line functions on graphs) and dose (x-axis, doses expressed as mg/70 kg, IV) (n=6, error bars ± 1 SEM). Filled symbols indicate a significant difference from the placebo condition (Tukey post-hoc, p<0.05). A time course analysis detected a significant effect of dose x time interaction for pupil diameter (F (432, 2105) = 1.3, p<0.05) and a main effect of dose for EtCO2 (F (16, 79) = 9.0, p<0.05).
Time course analysis detected a main effect of dose on heart rate, diastolic blood pressure, oxygen saturation, and manual respiratory rate (F [16, 79] = 2.0 −13.3, p<0.05). A main effect of dose was also detected on trough oxygen saturation (F [16,79]= 3.9, p<0.05), with post-hoc testing indicating a significant effect of 18 mg oxymorphone (p<0.05). A main effect of dose on peak heart rate (F [16,79]= 2.1, p<0.05) however, post-hoc testing did not detect any significant drug versus placebo differences (p>0.05).
Subjective Effects
Figure 2 displays mean time course data for the VAS item, “Do feel any drug effects?” for the four test drugs (separate panels). All active IV doses produced increased ratings on this measure with maximal drug effects emerging almost immediately after infusion. However, the magnitude and duration of effects varied as a function of drug and dose. Oxymorphone doses ≥ 5.6 mg/70 kg (Panel A) and the high dose of hydromorphone (18 mg/70 kg; Panel B) produced effects of greatest magnitude; however, oxymorphone doses ≥ 5.6 mg/70 kg were not uniformly dose-related (but were on peak ratings, as displayed in Fig 3). All doses of oxycodone (Panel C) and low doses of oxymorphone and hydromorphone produced effects lasting approx. 3–4 h, while oxymorphone ≥ 10 mg/70 kg and the highest dose of hydromorphone (18 mg) were detected up to 6 h post-dose. Morphine (Panel D) displayed the shortest time course, with little to no effects detected 2 h post-dose.
Figure 2.
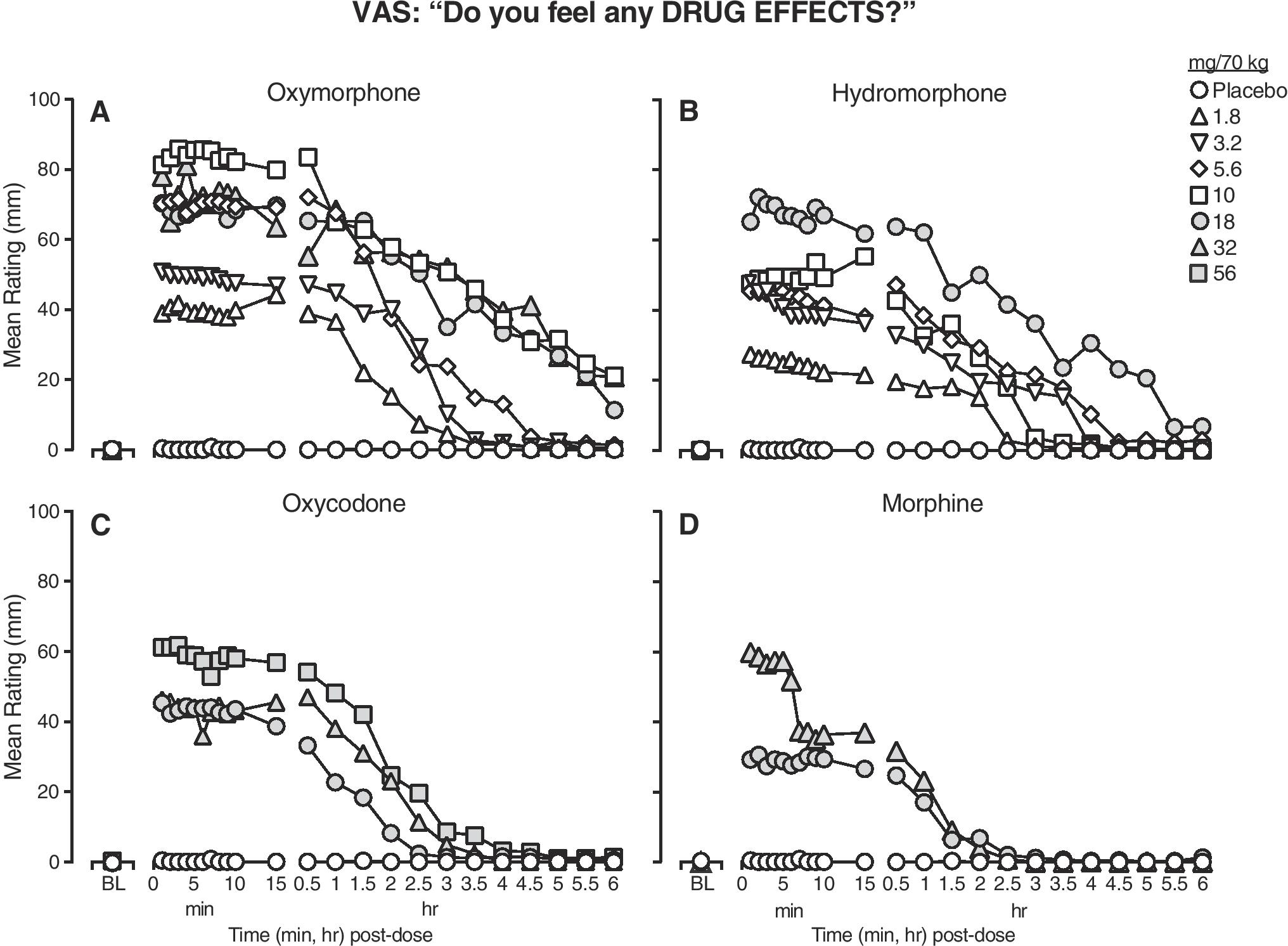
Mean ratings (n=6) for the VAS item, “Do you feel a drug effect?”, as a function of time (x-axis) from baseline (BL) through 6 h post-dose, across a dose range of oxymorphone (panel A), hydromorphone (panel B), oxycodone (panel C) and morphine (panel D) (doses expressed as mg/70 kg, IV). Error bars were omitted for clarity. Time course analysis indicated significant dose x time interaction on this measure (F [16,79] = 3.4, p <0.05). Note that filled symbols do not denote statistical significance – grey shading was used to create unique symbols for the highest dose conditions.
Figure 3.
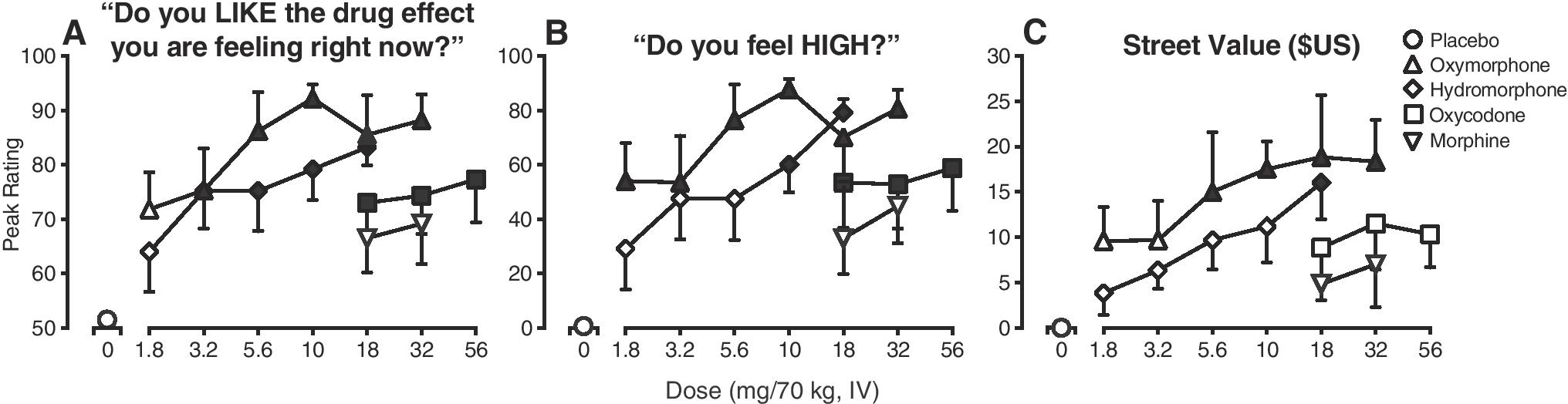
Peak ratings the VAS item “Do you like the drug effect you are feeling right now?” (Panel A) and VAS item “Do you feel high?” (Panel B), and Street Value estimates (Panel C). Values are displayed as a function of opioid agonist drug (line functions on graphs) and dose (x-axis, doses expressed as mg/70 kg, IV) (n=6; error bars ± 1 SEM). The filled symbols indicate a significant difference from the placebo condition (Tukey post-hoc, p<0.05). Time course analysis indicated significant main effect of dose for drug liking (F [16,79] = 3.1, p <0.05), a dose x time interaction for ratings of high (F [368, 1769] = 1.5, p <0.05) and a main effect of dose for street value (F [16,79] = 3.2, p <0.05).
Figure 3A displays the peak effects on the VAS scale item, “Do you like the drug effect you are feeling right now?” (main effect of dose: F [16,79]= 5.6, p<0.05). All doses of oxymorphone, hydromorphone and oxycodone produced significant, dose-related effects relative to placebo (p<0.05), while morphine did not (p>0.05). In general, the 5.6 mg/70 kg dose of oxymorphone produced ratings of drug liking that were greater than all comparator doses. Figure 3B displays peak VAS ratings of “Do you feel high?” (main effect of dose: (F [16,79]= 5.5, p<0.05). All doses of oxymorphone produced significant effects relative to placebo (p<0.05), as did the two highest doses of hydromorphone (10, 18 mg/70 kg), and all doses of oxycodone (18, 32, 56 mg/70). Morphine did not produce any significant effects (p>0.05). A similar profile of effects was observed on other VAS outcomes including ratings of good drug effects and willingness to take the drug again (F [16,79]= 4.8 – 6.4, p<0.05). No significant effects of dose were detected on VAS ratings of bad drug effects or desire to use opioids (F [16,79]= 1.1 – 1.5, p>0.05).
Oxymorphone produced greater effects than the comparator drugs on ratings of street value, as displayed in Figure 3C (main effect of dose: F [16,79]= 4.2, p<0.05). The four highest doses of oxymorphone (5.6, 10, 18, 32 mg/70 kg) produced significant peak street value ratings (p<0.05) in the range of $15.00 (± 6.60) to $18.30 (± 4.60), while the high dose of hydromorphone (18 mg/70 kg) produced ratings of $16.00 (± 4.00) (p<0.05). None of the other drug conditions produced effects significantly different from placebo (p>0.05).
On the Opioid Symptoms Scale, a main effect of dose was detected on the total score (F [16, 79] = 5.0, p<0.05), with post-hoc testing indicating that only oxymorphone (5.6 – 32 mg/70 kg) produced effects significantly different from placebo (p<0.05). Oxymorphone also increased ratings of individual items (itchiness, dry mouth, drowsy, difficult to pass urine, confused, nausea); ratings of itchiness were also increased by oxycodone (56 mg/70 kg) (p<0.05).
Observer Ratings
A main effect of dose was detected for the total score of observer-rated opioid agonist effects (F [16,79] = 9.6, p<0.05) and on ten of the individual items (skin itchy, friendly, nodding, relaxed, coasting/spaced out, talkative, heavy/sluggish, sleepy, drunken, good mood) (p<0.05). Oxymorphone (5.6 – 32 mg) increased ratings across 9 items (as listed above, with the exception of sleepy), while the other opioids were less consistent (i.e., hydromorphone increased ratings on 5 items, oxycodone on 3 items, morphine increasing none).
Drug Identification
Participants were highly accurate identifying active drug vs. placebo. Placebo was reliably identified and there were only a few instances (5 occasions across all participants and dosing conditions) in which active drug was identified as placebo. However, participants were generally unable to identify the specific opioid administered with responses distributed in a random pattern.
Potency Estimates
Potency analyses were conducted on a subset of two physiologic outcomes (pupil diameter and EtCO2 concentrations); however, only one comparison met validity criteria – oxymorphone produced effects on EtCO2 concentrations that were slightly (1.2-fold) more potent than hydromorphone (0.82 mg oxymorphone: 1 mg hydromorphone) (p<0.05; Table 1).
Table 1.
Relative Potency Comparisons
| Oxymorphone vs. Hydromorphone | VAS Drug Liking | VAS Drug Effect | VAS High | Street Value | Pupil Diameter | EtCO2 |
|---|---|---|---|---|---|---|
|
|
|
|||||
| oxymorphone relative potency (mg of oxymorphone ≈ 1 mg hydromorphone) |
0.41 | 0.43 | 0.36 | 0.41 | - - | 0.82 |
|
Oxymorphone vs. Oxycodone
|
||||||
| oxymorphone relative potency (mg of oxymorphone ≈ 1 mg oxycodone) |
0.07 | 0.08 | - - | 0.07 | - - | - - |
Relative potency comparisons were made between 1) equal doses of oxymorphone and hydromorphone (3.2, 5.6, 10 mg, IV) and 2) oxymorphone (1.8, 3.2, 5.6 mg, IV) and oxycodone (18, 32, 56 mg, IV). Six outcome measures were included in each analysis. Invalid outcomes are denoted with a double-dash (- -). Valid outcomes reported above are based upon the following criteria outlined by Finney (1964): no significant differences in preparation, linearity or parallelism and a significant regression. Relative potency is expressed as mg of oxymorphone necessary to produce an effect equivalent to 1 mg of the test agent.
Potency comparisons were conducted on a total of four subjective effect measures (drug liking, drug effect, high, street value). When oxymorphone and hydromorphone were compared, oxymorphone was 2.3 – 2.8-fold more potent across the four measures (i.e., 0.36 – 0.43 mg oxymorphone produced equivalent effects to 1 mg hydromorphone) (p<0.05). When oxymorphone and oxycodone were compared, one outcome (ratings of high) did not meet the validity criteria, but for the other three outcomes, oxymorphone was 12.5 –14-fold more potent than oxycodone (0.07 – 0.08 mg oxymorphone: 1 mg oxycodone) (p<0.05; Table 1).
DISCUSSION
This within-subject, double-blind, placebo-controlled, dose-finding pilot study examined the subjective, physiological and observer-related effects and potency of a wide range of doses of IV oxymorphone compared to hydromorphone, morphine and oxycodone in participants with moderate-to-severe opioid use disorder. Oxymorphone, hydromorphone and oxycodone produced prototypical dose-related opioid effects on pupil diameter and increased ratings of abuse-related drug effects, while morphine produced weaker effects across all measures. Overall, oxymorphone was more potent than the comparator drugs (i.e., oxymorphone > hydromorphone > oxycodone > morphine) and produced overall greater magnitude of effects on all outcomes tested. These findings are novel as this is the first study to examine the subjective, physiological and overall relative potency of intravenous oxymorphone.
Oxymorphone produced the greatest degree of miosis, a measure that has historically been used as a physiological index of intrinsic μ-opioid activity (Fraser et al., 1954; Martin, 1983). For example, a moderate dose of oxymorphone (10 mg) produced trough miotic effects that were largely greater than or equal to the effects produced by the highest doses of all the other drugs (10 mg oxymorphone: 1.99 [±0.13] cm; highest doses of the comparator drugs ranged from 1.99 [±0.17] to 2.85 [±0.22] cm). Oxymorphone (18, 32 mg) also significantly increased peak EtCO2 concentrations, similar to the high dose of hydromorphone (18 mg) and oxymorphone was 1.2-fold more potent than hydromorphone on this outcome. For example, at a dose (18 mg) tested across all of the opioids, respiratory depression was the greatest with oxymorphone (47.8 ± 1.4), followed by hydromorphone (47.2 ± 0.8), oxycodone (41.7 ± 1.7) and morphine (40.7 ± 2.1) (placebo: 39.7). However, neither oxycodone nor morphine produced significant effects on peak EtCO2 (p>0.05); although this appears antithetical given the supratherapeutic doses tested, this participant sample was quite opioid-tolerant (i.e., physically dependent on opioids, maintained on opioids during the study). The physiological effects of IV oxymorphone have not previously been thoroughly examined – most studies have reported on its effects when combined with other drugs in anesthesia preparations (e.g., Appleton, 1960). However, in one previous study, the respiratory depressant effect of IV oxymorphone (1 mg/70 kg) was examined in a healthy, non-drug using sample – oxymorphone produced prototypical opioid-induced respiratory depression: baseline = 24.9 [±11.9] l/min; peak effect post-dose = 14.1 [±4.9] l/min (Johnstone et al., 1975). Overall, at equal doses (e.g., 18 mg) oxymorphone and hydromorphone produced robust respiratory depression, while oxycodone and morphine produced placebo-like effects. Thus, oxymorphone may pose more a greater risk of respiratory depression, relative to equal doses of other prescription opioids, due to its high potency.
On measures of abuse potential (e.g., high, drug liking, street value), oxymorphone was highly potent, such that a moderate dose of oxymorphone (5.6 mg/70 kg) produced peak effects that were greater than or equal to all other comparator doses. In some instances, the lowest dose of oxymorphone tested (1.8 mg/70 kg) produced peak effects that were greater than or equal to even the highest doses of oxycodone and morphine (e.g., VAS ratings of high; Fig. 3B). Oxymorphone also produced a somewhat longer duration of effects (Fig. 2) compared to the other opioids. For example, 10 – 32 mg/70 kg oxymorphone produced subjective effects that were detected up to 6 h post-dose. This duration was comparable to 18 mg hydromorphone, but longer than the effects produced by the high doses of morphine and oxycodone which returned to baseline at 3 and 4 h post-dose, respectively. Similar time course effects of oxymorphone have been reported when intramuscular and subcutaneous doses have been administered for pain relief (Eddy and Lee, 1959; Beaver and Feise, 1977; Beaver et al., 1977). Oxymorphone also produced increases in estimates of street value, with 5.6 – 32 mg/70 kg oxymorphone producing ratings (range: $15.00 - $18.30) that were comparable to 18 mg hydromorphone ($16.00). The street value of oxymorphone has been reported in at least three studies: $0.73 - $2.90 per mg (in contrast to oxycodone: $0.12 to $1.07 per mg) in a crowdsourced study (Lebin et al., 2019); $1.57 - $1.64 per mg in a black market surveillance study (Dasgupta et al., 2013); and $3 to $4 per mg in a study interviewing participants in Austin, Indiana (the site of the HIV outbreak associated with injection oxymorphone use) who reported that one 40 mg oxymorphone pill was valued between $120 - $160 (Broz et al., 2018). These participants also reported sharing a full 40 mg pill amongst 7 people or sharing one-quarter of pill (i.e., 10 mg) amongst 2–3 people (equivalent to 3.3 – 5.7 mg of IV oxymorphone per person), which aligns with the dose range that produced significant ratings of high and drug liking in the current study. Similarly, one previous controlled study that examined the abuse potential of parenteral oxymorphone reported that in non-tolerant former opioid users (n=5), 1.5 – 2 mg subcutaneous oxymorphone produced “intense morphine-like effects” and the authors concluded that oxymorphone had high addiction liability (Fraser and Isbell, 1955). Taken together, these data suggest that oxymorphone displays a high degree of abuse potential at relatively low doses and is much more potent than the comparator opioids: 2.3 to 2.8-fold more potent than hydromorphone and 12.5 to 14-fold greater than oxycodone (p<0.05).
The current data, which indicate that oxymorphone displays greater potency and greater abuse potential, align with the epidemiological reports on IV oxymorphone misuse. Surveillance data indicate that even though oxymorphone is not widely prescribed (the extended-release product accounted for 5% of extended-release/long-acting opioid sales in 2015 [IMS Health, 2015, as cited by FDA, 2017]), the frequency of oxymorphone injection is disproportionately high compared to other prescription opioids (Butler et al., 2013; Cicero et al., 2016; FDA, 2017), with one estimate indicating injection prevalence up to 7 times higher than other prescription opioids (NAVIPPRO® Report, as cited by FDA, 2017). This disproportionately high prevalence of injection has caused serious health complications, including HIV transmission (Peters et al., 2016), kidney injury (Ambruzs et al., 2014; Bonnecaze et al., 2018) and serious blood vessel and blood clotting disorders (Rane et al., 2014; Ban et al., 2017; Thakur et al., 2017) and have also been implicated in fatal overdose (FDA, 2017; also see Garside et al., 2009; Crum et al., 2013). Several factors are hypothesized to have contributed to this increase in injection, including: 1) the low oral bioavailability of oxymorphone, which may increase misuse via other routes with greater bioavailability, similar to other licit (e.g. methylphenidate [Volkow and Swanson, 2003]) and illicit (e.g., heroin [Girardin et al., 2003]) drugs of abuse, 2) easy manipulation of the extended-release product so that high quantities of the drug could be accessed for injection (FDA, 2017; Broz et al., 2018), 3) the pharmacological action of oxymorphone, including a high degree of specificity, binding affinity and intrinsic activity at the μ opioid receptor (Carliss et al., 2009; Volpe et al., 2011; Olson et al., 2019), 4) its rapid transport across the blood-brain barrier (Sadiq et al., 2013), and 5) its high relative potency, particularly as it relates to its abuse profile – as demonstrated in the current study.
Although the current data provide rather clear evidence of the relative high abuse potential of IV oxymorphone, there were several study limitations. First, the sample size was small (n=6), as this study was powered as a dose-finding pilot study to identify doses for a subsequent and more thorough evaluation of abuse potential. In addition, the dose order was not fully randomized for safety reasons and constrained so that lower doses preceded higher doses. Further, the morphine qualification dose (56 mg/70 kg) produced adverse effects in several participants. A similar dose (50 mg/70 kg) was well-tolerated and produced drug liking responses in a previous study (Comer et al., 2008) and it is unclear why the current dose produced adverse effects; however, this dose was not ideal for qualification or test session doses in this participant sample. Nonetheless, the findings are orderly, dose-dependent, and robust and produced statistically significant effects across the broad array of outcomes.
Taken together, these data indicate that IV oxymorphone is highly potent, particularly on outcomes related to abuse potential. When administered intravenously, oxymorphone has abuse potential that far exceeded the comparator opioids and produced significant abuse-related effects at comparatively low doses (1.8 – 5.6 mg/70 kg). Along with epidemiological and medical reports that have detailed health-related harms associated with injected oxymorphone, these data suggest that IV oxymorphone use may pose a disproportionately high degree of risk and public health harm relative to other full-agonist IV prescription opioids.
Acknowledgments
Funding from the U.S. Food and Drug Administration (HHSF223201710119C [SDC]; HHSF223201710120C [SLW]) and the National Center for Advancing of Translational Sciences (UL1TR001998 [UK CTSA]) provided support for this research. We wish to thank the staff at the University of Kentucky (UK) Center on Drug and Alcohol Research and the New York State Psychiatric Institute (NYSPI) Clinical Research Unit for research support: Ida Holt RN, Janet Murray RN, Claudia Tindall APRN, Andrea Woodson RN, Rebecca Abbott, Nur Ali, Nicholas Allwood, Ben Foote, Jocelyn Nichols, Lauren Noble, Victoria Vessels and Vincent Woolfolk; the investigational pharmacists at UK Investigational Pharmacy, Dr. Seth Larkin and Dr. Thomas Lyman and NYSPI, Dr. Robert Jung and Dr. Lindsey Tesseyman for preparing study medication; and Dr. Samy-Claude Elayi and Dr. Ronnie Zeidan for patient support.
Conflicts of Interest
In the past three years Sandra Comer has provided consulting and advisory board services to pharmaceutical and health services companies (Alkermes, Charleston Labs, Clinilabs, Collegium, Epiodyne, Mallinckrodt, Nektar, Opiant, Osmotica, Otsuka, Sun Pharma), her university has received research contracts from companies for drug-related research for which she serves as Principal or Co-Principal Investigator (Alkermes, BioXcel, Corbus, GoMedical, Intra-cellular Therapies, Lyndra, and Janssen), and she received honoraria for writing critical reviews for the World Health Organization. Sharon Walsh has served as a consultant or scientific advisor for Opiant Pharmaceuticals, Cerevel Therapeutics, AstraZeneca, Camurus, Otsuka, BrainsWay, Trevi Therapeutics, Summit Biosciences and World Meds over the past three years on topics related to opioid use disorder and its treatment but not directly related to the topic of this report. Jermaine Jones is currently the recipient of an investigator-initiated grant from Merck Pharmaceuticals and has served as a consultant for Alkermes for the past three years. Shanna Babalonis, Michelle Lofwall, Kevin Hatton, Jeanne Manubay, Robert Whittington and Paul Nuzzo have no conflicts to report related to this project.
REFERENCES
- Ambruzs JM, Serrell PB, Rahim N, Larsen CP (2014) Thrombotic microangiopathy and acute kidney injury associated with intravenous abuse of an oral extended-release formulation of oxymorphone hydrochloride: kidney biopsy findings and report of 3 cases. Am J Kidney Dis 63: 1022–6. [DOI] [PubMed] [Google Scholar]
- Appleton JC (1960) Clinical evaluation and observation of 14-hydroxydihydromorphinone (Numorphan). Anesth Analg 39: 505–10. [PubMed] [Google Scholar]
- Aqua K, Gimbel JS, Singla N, Ma T, Ahdieh H, Kerwin R (2007) Efficacy and tolerability of oxymorphone immediate release for acute postoperative pain after abdominal surgery: a randomized, double-blind, active- and placebo-controlled, parallel-group trial. Clin Ther 29: 1000–12. [DOI] [PubMed] [Google Scholar]
- Babalonis S, Lofwall MR, Nuzzo PA, Walsh SL (2016) Pharmacodynamic effects of oral oxymorphone: abuse liability, analgesic profile and direct physiologic effects in humans. Addict Biol 21: 146–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger GA (2004) Using generalized estimating equations for longitudinal data analysis. Organ Res Methods 7: 127–150. [Google Scholar]
- Ban BH, Verma A, Tudor M, Sethi J (2017) Opana-induced thrombotic microangiopathy masquerading as thrombotic thrombocytopenic purpura. Oxf Med Case Reports 2017: omx026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver WT, Feise GA (1977) A comparison of the analgesic effect of oxymorphone by rectal suppository and intramuscular injection in patients with postoperative pain. J Clin Pharmacol 17: 276–91. [DOI] [PubMed] [Google Scholar]
- Beaver WT, Wallenstein SL, Houde RW, Rogers A (1977) Comparisons of the analgesic effects of oral and intramuscular oxymorphone and of intramuscular oxymorphone and morphine in patients with cancer. J Clin Pharmacol 17: 186–98. [DOI] [PubMed] [Google Scholar]
- Bierman HR, Coblentz A (1956) The analgesic properties of numorphan (14-hydroxy dihydromorphinone), a new synthetic narcotic. N Engl J Med 255: 694–8. [DOI] [PubMed] [Google Scholar]
- Bonnecaze AK, Wilson MW, Dharod A, Fletcher A, Miller PJ (2018) Acute kidney injury is common with intravenous abuse of extended-release oral oxymorphone and delayed renal recovery rates are associated with increased KDIGO staging. Nephrology (Carlton) 23: 921–926. [DOI] [PubMed] [Google Scholar]
- Broz D, Zibbell J, Foote C, Roseberry JC, Patel MR, Conrad C, Chapman E, Peters PJ, Needle R, McAlister C, Duwve JM (2018) Multiple injections per injection episode: High-risk injection practice among people who injected pills during the 2015 HIV outbreak in Indiana. Int J Drug Policy 52: 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SF, Cassidy TA, Chilcoat H, Black RA, Landau C, Budman SH, Coplan PM (2013) Abuse rates and routes of administration of reformulated extended-release oxycodone: initial findings from a sentinel surveillance sample of individuals assessed for substance abuse treatment. J Pain 14: 351–8. [DOI] [PubMed] [Google Scholar]
- Carliss RD, Keefer JF, Perschke S, Welch S, Rich TC, Weissman AD (2009) Receptor reserve reflects differential intrinsic efficacy associated with opioid diastereomers. Pharmacol Biochem Behav 92: 495–502. [DOI] [PubMed] [Google Scholar]
- Cassidy TA, DasMahapatra P, Black RA, Wieman MS, Butler SF (2014) Changes in prevalence of prescription opioid abuse after introduction of an abuse-deterrent opioid formulation. Pain Med 15: 440–51. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Kasper ZA (2016) A tale of 2 ADFs: differences in the effectiveness of abuse-deterrent formulations of oxymorphone and oxycodone extended-release drugs. Pain 157: 1232–8. [DOI] [PubMed] [Google Scholar]
- Ciliberti BJ, Eddy NB (1961) Pre-anaesthetic medication: morphine, anileridine, oxymorphone and placebo. Bull Narc 3:1–8. [Google Scholar]
- Comer SD, Sullivan MA, Whittington RA, Vosburg SK, Kowalczyk WJ (2008) Abuse liability of prescription opioids compared to heroin in morphine-maintained heroin abusers. Neuropsychopharmacology 33: 1179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum ED, Bailey KM, Richards-Waugh LL, Clay DJ, Gebhardt MA, Kraner JC (2013) Validation of blood and liver oxymorphone analysis using LC-MS-MS: concentrations in 30 fatal overdoses. J Anal Toxicol 37: 512–6. [DOI] [PubMed] [Google Scholar]
- Dasgupta N, Freifeld C, Brownstein JS, Menone CM, Surratt HL, Poppish L, Green JL, Lavonas EJ, Dart RC (2013) Crowdsourcing black market prices for prescription opioids. J Med Internet Res 15: e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle PJ, Liang K, Zeger SL (1996) Analysis of Longitudinal Data. Oxford University Press, Inc. [Google Scholar]
- Eddy NB, Lee LE Jr. (1959) The analgesic equivalence to morphine and relative side action liability of oxymorphone (14-hydroxydihydro morphinone). J Pharmacol Exp Ther 125: 116–21. [PubMed] [Google Scholar]
- Endo Pharmaceuticals (2016). Prescribing Information and Package Insert, Opana ER® (oxymorphone hydrochloride) extended-release tablets (5 mg, 7.5 mg, 10 mg, 15 mg, 20 mg, 30 mg, 40 mg). Malvern, PA. [Google Scholar]
- Endo Pharmaceuticals (2006). Prescribing Information and Package Insert, Opana® (oxymorphone hydrochloride) injection (1 mg/mL ampules). Chadds Ford, PA. [Google Scholar]
- Finney DJ (1964) Statistical method in biological assay. 2nd ed. Hafner: New York. [Google Scholar]
- Fraser HF, Nash TL, Vanhorn GD, Isbell H (1954) Use of miotic effect in evaluating analgesic drugs in men. Arch Int Pharmacodyn Ther 98: 443–451. [PubMed] [Google Scholar]
- Fraser HF, Isbell H (1955) Addictive properties of morphine derivatives. Published abstracts from the American Society for Pharmacology and Experimental Therapeutics. Charlottesville, VA, September. 6-8, 1954. [Google Scholar]
- Fraser HF, Van Horn GD, Martin WR, Wolbach AB, Isbell H (1961) Methods for evaluating addiction liability. (A) “Attitude” of opiate addicts toward opiate-like drugs. (B) a short-term “direct” addiction test. J Pharmacol Exp Ther 133, 371–387. [PubMed] [Google Scholar]
- Gabrail NY, Dvergsten C, Ahdieh H (2004) Establishing the dosage equivalency of oxymorphone extended release and oxycodone controlled release in patients with cancer pain: a randomized controlled study. Curr Med Res Opin 20: 911–8. [DOI] [PubMed] [Google Scholar]
- Garside D, Hargrove RL, Winecker RE (2009) Concentration of oxymorphone in postmortem fluids and tissue. J Anal Toxicol 33: 121–8. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Hedeker D, Elkin I, Waternaux C, Kraemer HC, Greenhouse JB, Shea MT, Imber SD, Sotsky SM, Watkins JT (1993) Some conceptual and statistical issues in analysis of longitudinal psychiatric data. Application to the NIMH treatment of Depression Collaborative Research Program dataset. Arch Gen Psychiatry 50: 739–50. [DOI] [PubMed] [Google Scholar]
- Gimbel J, Ahdieh H (2004) The efficacy and safety of oral immediate-release oxymorphone for postsurgical pain. Anesth Analg 99: 1472–7; table of contents. [DOI] [PubMed] [Google Scholar]
- Girardin F, Rentsch KM, Schwab MA, Maggiorini M, Pauli-Magnus C, Kullak-Ublick GA, Meier PJ, Fattinger K (2003) Pharmacokinetics of high doses of intramuscular and oral heroin in narcotic addicts. Clin Pharmacol Ther 74: 341–52. [DOI] [PubMed] [Google Scholar]
- Hale ME, Ahdieh H, Ma T, Rauck R, Oxymorphone ERSG (2007) Efficacy and safety of OPANA ER (oxymorphone extended release) for relief of moderate to severe chronic low back pain in opioid-experienced patients: a 12-week, randomized, double-blind, placebo-controlled study. J Pain 8: 175–84. [DOI] [PubMed] [Google Scholar]
- Hale ME, Dvergsten C, Gimbel J (2005) Efficacy and safety of oxymorphone extended release in chronic low back pain: results of a randomized, double-blind, placebo- and active-controlled phase III study. J Pain 6: 21–8. [DOI] [PubMed] [Google Scholar]
- Hunt R, Yalamanoglu A, Tumlin J, Schiller T, Baek JH, Wu A, Fogo AB, Yang H, Wong E, Miller P, Buehler PW, Kimchi-Sarfaty C (2017) A mechanistic investigation of thrombotic microangiopathy associated with IV abuse of Opana ER. Blood 129: 896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IMS Health (2016) National Prescription Audit™: 2010–2015. [Google Scholar]
- Jasinski DR, Griffith JD, Pevnick JS, Gorodetzky C, Cone E, Kay D (1977) Progress Report from the Clinical Pharmacology Section of the NIDA Addiction Research Center, 39th Annual Meeting, The Committee on the Problems of Drug Dependence, National Research Council, National Academy of Sciences, Washington, D.C., 133–168. [Google Scholar]
- Johnstone RE, Lief PL, Kulp RA, Smith TC (1975) Combination of delta9-tetrahydrocannabinol with oxymorphone or pentobarbital: Effects on ventilatory control and cardiovascular dynamics. Anesthesiology 42: 674–84. [DOI] [PubMed] [Google Scholar]
- Kreft I, De Leeuw J (1998) Introducing Multilevel Modeling. Sage Publications, Ltd. [Google Scholar]
- Lebin JA, Murphy DL, Severtson SG, Bau GE, Dasgupta N, Dart RC (2019) Scoring the best deal: Quantity discounts and street price variation of diverted oxycodone and oxymorphone. Pharmacoepidemiol Drug Saf 28: 25–30. [DOI] [PubMed] [Google Scholar]
- Martin WR (1983) Pharmacology of opioids. Pharmacol Revs 35:283–323. [PubMed] [Google Scholar]
- NAVIPPRO® (2016) Study Report. [Google Scholar]
- Olson KM, Duron DI, Womer D, Fell R, Streicher JM (2019) Comprehensive molecular pharmacology screening reveals potential new receptor interactions for clinically relevant opioids. PLoS One 14: e0217371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters PJ, Pontones P, Hoover KW, Patel MR, Galang RR, Shields J, Blosser SJ, Spiller MW, Combs B,Rane M, Aggarwal A, Banas E, Sharma A (2014) Resurgence of intravenous Opana as a cause of secondary thrombotic thrombocytopenic purpura. Am J Emerg Med 32: 951 e3–4. [DOI] [PubMed] [Google Scholar]
- Rauck R, Ma T, Kerwin R, Ahdieh H (2008) Titration with oxymorphone extended release to achieve effective long-term pain relief and improve tolerability in opioid-naive patients with moderate to severe pain. Pain Med 9: 777–85. [DOI] [PubMed] [Google Scholar]
- Sadiq MW, Bostrom E, Keizer R, Bjorkman S, Hammarlund-Udenaes M (2013) Oxymorphone active uptake at the blood-brain barrier and population modeling of its pharmacokinetic-pharmacodynamic relationship. J Pharm Sci 102: 3320–31. [DOI] [PubMed] [Google Scholar]
- Schoedel KA, McMorn S, Chakraborty B, Potts SL, Zerbe K, Sellers EM (2011) Positive and negative subjective effects of extended-release oxymorphone versus controlled-release oxycodone in recreational opioid users. J Opioid Manag 7: 179–92. [DOI] [PubMed] [Google Scholar]
- Schoedel KA, McMorn S, Chakraborty B, Zerbe K, Sellers EM (2010) Reduced cognitive and psychomotor impairment with extended-release oxymorphone versus controlled-release oxycodone. Pain Physician 13: 561–73. [PubMed] [Google Scholar]
- Singer JD (1998) Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat 24: 323–355. [Google Scholar]
- Sloan P, Slatkin N, Ahdieh H (2005) Effectiveness and safety of oral extended-release oxymorphone for the treatment of cancer pain: a pilot study. Support Care Cancer 13: 57–65. [DOI] [PubMed] [Google Scholar]
- Switzer WM, Conrad C, Gentry J, Khudyakov Y, Waterhouse D, Owen SM, Chapman E, Roseberry JC, McCants V, Weidle PJ, Broz D, Samandari T, Mermin J, Walthall J, Brooks JT, Duwve JM, Indiana HIVOIT (2016) HIV Infection Linked to Injection Use of Oxymorphone in Indiana, 2014–2015. N Engl J Med 375: 229–39. [DOI] [PubMed] [Google Scholar]
- Thakur K, Agrawal V, Kass A, Dimarino LM, Dorion RP, Vadakara J (2017) Thrombotic microangiopathy secondary to intravenous abuse of Opana® ER. Case Rep Hematol 2017: 1623907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM (2003) Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry 160:1909–18. [DOI] [PubMed] [Google Scholar]
- Volpe DA, McMahon Tobin GA, Mellon RD, Katki AG, Parker RJ, Colatsky T, Kropp TJ, Verbois SL (2011) Uniform assessment and ranking of opioid mu receptor binding constants for selected opioid drugs. Regul Toxicol Pharmacol 59: 385–90. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Comer SD, Lofwall MR, Vince B, Levy-Cooperman N, Kelsh D, Coe MA, Jones JD, Nuzzo PA, Tiberg F, Sheldon B, Kim S (2017) Effect of buprenorphine weekly depot (CAM2038) and hydromorphone blockade in individuals with opioid use disorder: a randomized clinical trial. JAMA Psychiatry 74: 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Nuzzo PA, Lofwall MR, Holtman JR Jr. (2008) The relative abuse liability of oral oxycodone, hydrocodone and hydromorphone assessed in prescription opioid abusers. Drug Alcohol Depend 98: 191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins TD, Chambers CD (1972) Oxymorphone Abuse Among Current Narcotic Addicts. In: Drug Abuse: Current Concepts and Research. Keup W(ed). Charles C. Thomas: Springfield, Ill. pp. 307–312. [Google Scholar]
- Wesson DR, Ling W (2003) The clinical opiate withdrawal scale (COWS). J Psychoactive Drugs. 35:253–259. [DOI] [PubMed] [Google Scholar]
- United States Food and Drug Administration (2017) Joint Meeting of the Drug Safety and Risk Management (DSaRM) Advisory Committee and the Anesthetic and Analgesic Drug Products Advisory Committee (AADPAC) Meeting, March 13–14, 2017. https://www.fda.gov/advisory-committees/anesthetic-and-analgesic-drug-products-advisory-committee/2017-meeting-materials-anesthetic-and-analgesic-drug-products-advisory-committee. [Google Scholar]


