Abstract
COVID-19 is associated with myocardial injury caused by ischemia, inflammation, or myocarditis. Cardiovascular magnetic resonance (CMR) is the noninvasive reference standard for cardiac function, structure, and tissue composition. CMR is a potentially valuable diagnostic tool in patients with COVID-19 presenting with myocardial injury and evidence of cardiac dysfunction. Although COVID-19–related myocarditis is likely infrequent, COVID-19–related cardiovascular histopathology findings have been reported in up to 48% of patients, raising the concern for long-term myocardial injury. Studies to date report CMR abnormalities in 26% to 60% of hospitalized patients who have recovered from COVID-19, including functional impairment, myocardial tissue abnormalities, late gadolinium enhancement, or pericardial abnormalities. In athletes post–COVID-19, CMR has detected myocarditis-like abnormalities. In children, multisystem inflammatory syndrome may occur 2 to 6 weeks after infection; associated myocarditis and coronary artery aneurysms are evaluable by CMR. At this time, our understanding of COVID-19–related cardiovascular involvement is incomplete, and multiple studies are planned to evaluate patients with COVID-19 using CMR. In this review, we summarize existing studies of CMR for patients with COVID-19 and present ongoing research. We also provide recommendations for clinical use of CMR for patients with acute symptoms or who are recovering from COVID-19.
Key Words: cardiovascular magnetic resonance, COVID-19, ischemia, multisystem inflammatory syndrome, myocardial injury, myocarditis, SARS-CoV-2
Central Illustration
As of July 2021, the worldwide number of confirmed COVID-19 cases has reached more than 180 million, with almost 4 million related deaths (1). SARS-CoV-2, which causes COVID-19, preferentially infects epithelial cells of the respiratory tract via the angiotensin-converting enzyme 2 (ACE2) receptor (2). However, both the heart and myocardial vessels are also potential targets of SARS-CoV-2 via the ACE2 receptor. Myocardial injury in association with COVID-19 has been linked to greater risk of in-hospital mortality (3).
Cardiovascular magnetic resonance (CMR) is the reference standard for the evaluation of myocardial structure and function. In addition, CMR is unique in its capability to probe myocardial tissue composition. The American College of Cardiology, the European Society of Cardiology, and the Society for Cardiovascular Magnetic Resonance concur that CMR is a potentially valuable diagnostic tool in patients with COVID-19 presenting with myocardial injury and evidence of cardiac dysfunction (4, 5, 6, 7).
The purpose of this report is to review the use of CMR to evaluate cardiac disease in association with COVID-19. (The Society of Cardiovascular Magnetic Resonance has endorsed the contents of this report.) We assess the clinical evidence for myocardial injury and pathologic findings of COVID-19 relevant to the diagnostic use of CMR for patients. Next, we summarize reports to date that have used CMR for patients and athletes recovering from COVID-19. Expert opinion is presented regarding the appropriate use of CMR in the setting of COVID-19.
Background: Myocardial Injury in COVID-19
Manifestations of myocardial injury
Reports of myocardial injury in association with COVID-19 have included acute ischemic injury (type 1 myocardial infarction [8]) as well as nonischemic injury (ie, myocarditis) (9, 10, 11), stress cardiomyopathy (12), acute heart failure (13), and secondary cardiac injury caused by sepsis and critical illness (14). Mechanisms of myocardial injury may be direct (viral infection, thought to be less common) or indirect via systemic inflammatory response. Activation of a proinflammatory response secondary to an immune response to SARS-CoV-2 results in cytokine release and a prothrombotic state (15,16). Giustino et al (17) reported 305 patients hospitalized with COVID-19 from 7 hospitals in Milan and New York. Myocardial injury (defined as cardiac troponin [cTn] elevation above the 99th percentile upper reference limit [18]) at any time during admission was common—present in 62% of patients. Elevated cTn was associated with older age, pre-existing cardiovascular disease, COVID-19 severity, and clinical deterioration (15,19). In other studies, patients with elevated cTn were at higher risk for adverse events during hospitalization, including a higher death rate, acute respiratory distress syndrome, and malignant arrhythmias (19, 20, 21).
Myocardial injury in patients with COVID-19 can be detected by cardiac imaging. Giustino et al (17) indicated that nearly two thirds of patients with myocardial injury by cTn had major echocardiographic abnormalities. Abnormalities included left ventricle (LV) wall motion abnormalities (24%), right ventricular (RV) dysfunction (26%), global LV dysfunction (18%), diastolic dysfunction grades II or III (13%), and pericardial effusion (7%). In-hospital mortality was 5.2% without cardiac involvement but rose to 32% in those with myocardial injury and echocardiographic abnormalities (17). These findings were supported by Rath et al (22), who showed a significantly higher mortality in patients with impaired LV ejection fraction, impaired RV function, and tricuspid regurgitation. In 100 consecutive individuals hospitalized with COVID-19, Szekely et al (23) reported RV dilatation and dysfunction in 39% of patients. Dweck et al (24) performed a prospective multicenter survey of 1,216 hospitalized acute COVID-19 patients with clinical indications for echocardiography. They reported abnormal echocardiograms in 55% of patients. In most cases, the underlying cause of LV abnormalities was not identified. Thus, although echocardiography is a first-line imaging tool, its ability to discern specific diagnoses is suboptimal.
Given the high rate of acute COVID-19–associated cardiac abnormalities, concern exists for long-term myocardial injury in convalescent patients. In a report of 1,733 previously hospitalized patients evaluated 6 months after symptom onset, 11% of patients reported palpitations and 5% reported ongoing chest pain, raising the question of long-term cardiac injury (25). In a multicenter study, cardiopulmonary damage in 109 hospitalized patients and 37 outpatients recovering from COVID-19 was assessed (26). At follow-up, echocardiography revealed a high rate of diastolic dysfunction (55%), but only 2.8% had reduced LV ejection fraction; N-terminal pro–brain natriuretic peptide was elevated in 23% of patients with COVID-19 (26).
Histopathology evidence for myocardial injury in COVID-19
The histopathologic basis of myocardial injury caused by COVID-19 has been studied. In the heart, the ACE2 receptor is more highly expressed in pericytes that line the vasculature compared to myocytes (27). Basso et al (28) reported myocarditis (defined as lymphocytic infiltration plus myocyte necrosis) in 3 of 21 (14%) selected autopsy cases of COVID-19. Halushka and Vander Heide (29) reviewed 22 publications describing autopsy results in 277 patients who died of COVID-19. These investigators suggested that myocarditis was infrequent (1.4%). However, at least 1 acute, potentially COVID-19–related cardiovascular histopathology finding (eg, micro- or macrovascular thrombi, interstitial inflammation, and/or intraluminal megakaryocytes) was common (48% of cases) (29). Lindner et al (30) demonstrated the presence of SARS-CoV-2 viral particles in the heart in 24 of 39 (59%) consecutive autopsies. Of note, viral particles were not present in myocytes but, rather, within the interstitial space. In addition to the aforementioned inflammatory processes, Bois et al (31) reported microthrombi in association with COVID-19. In another series of 40 hearts from patients who died of COVID-19, myocardial necrosis (primarily of the LV) was present in 14 (35%); the majority of these had small (11/14) or large (2/14) vessel thrombosis (32).
In summary, myocardial injury in hospitalized patients with COVID-19 is frequent and portends a worse prognosis. Based on limited autopsy information, the pathogenesis of SARS-CoV-2 infection was infrequently lymphocytic myocarditis; instead, macrophage infiltration, inflammation, and microthrombi were more common at autopsy. Early evidence indicates that myocardial abnormalities are present in only a proportion of convalescent patients, and current data are limited. In the following section, we review information to date showing the use of CMR as a highly sensitive method to detect myocardial abnormalities in association with COVID-19.
CMR of Acute and Convalescent Patients with COVID-19
Assessment of myocardial injury using CMR
CMR identifies myocardial injury associated with both nonischemic and ischemic disease. CMR assesses both myocardial function and tissue characterization, including myocardial edema that is present in inflammatory disease. For acute myocarditis-like presentations, CMR may support or exclude active myocardial inflammation by use of the so-called Lake Louise criteria (33). The Lake Louise criteria comprise at least 1 T2-based criterion with at least 1 T1-based criterion (see Table 1 for definitions of CMR terminology). Supportive criteria include pericardial effusion and systolic LV dysfunction.
Table 1.
CMR Terminology and Methods for Tissue Characterization
| CMR Method or Terminology | Definition | CMR Application | Interpretation in Patients With COVID-19 |
|---|---|---|---|
| T1 relaxation parametersa | |||
| T1-weighted images | Images dominated by T1 relaxation magnetic relaxation. Signal intensity is relative (not quantitative). | Typically used for depiction of myocardial anatomy. Post–gadolinium administration images depict the distribution of the intravenous contrast agent | Acute: evidence for myocardial injury Chronic: evidence for myocardial fibrosis/scar |
| Native T1 mapping | Pixel-by-pixel presentation of T1 values (in milliseconds) of the myocardium without a gadolinium-based contrast agent. | Increased T1 times indicate increased interstitial space (eg, collagen or amyloid deposits) or increased (intracellular or extracellular) tissue water (ie, myocardial edema). Decreased T1 times indicate intracellular lipid or iron deposition. |
|
| Late gadolinium enhancement | T1-weighted images acquired 10-15 min after intravenous administration of a gadolinium-based contrast agent. | Infarction/scar: typically subendocardial involvement in a coronary artery distribution. Nonischemic necrosis/scar: typically mid or epicardial myocardial involvement, not in a coronary artery distribution. |
|
| ECV fraction | Proportion of the ECV in the myocardium compared to total myocardial volume. Estimated using native T1 and postgadolinium T1 mapping methods | Increased ECV is present in diffuse myocardial fibrosis and myocardial inflammation. ECV may also be elevated in infiltrative disease such as amyloidosis. | |
| T2 relaxation parametersb | |||
| T2-weighted images | Images dominated by effects of T2 magnetic relaxation. Signal intensity is relative (not quantitative). | Signal intensity is markedly increased in areas of tissue edema. | Evidence for myocardial edema may be associated with inflammation |
| T2 mapping | Pixel-by-pixel presentation of T2 values (in milliseconds) of the myocardium. | Increased T2 time indicates myocardial edema. |
CMR = cardiac magnetic resonance; ECV = extracellular volume.
T1 relaxation, or longitudinal magnetic relaxation time, in milliseconds. After a radiofrequency pulse, T1 is the time constant for regrowth of (1 − 1/e) or approximately 63% of its initial maximum magnetic strength.
T2 relaxation, or transverse magnetic relaxation time, in milliseconds. After a radiofrequency pulse, T2 is the time constant for transverse magnetization to fall to approximately 37% (1/e) of its initial value.
The Lake Louise criteria have been validated in the context of clinically suspected acute myocarditis; they have not been validated in patients recovering from acute COVID-19 or presenting with prolonged symptoms. Nevertheless, CMR allows the assessment of a wide range of functional and tissue characterization parameters (Table 1). Especially in patients with chronic inflammatory conditions, T2 mapping (reflecting myocardial edema) is reported to inform the CMR diagnosis (34). However, the optimal combination of CMR criteria to characterize myocardial disease in patients recovering from COVID-19 remains to be determined. Suggested Society for Cardiovascular Magnetic Resonance imaging protocols for patients with active or convalescent-phase COVID-19 infection have been reviewed by Kelle et al (35).
CMR for patients with acute COVID-19
The use of CMR in the acute setting has been infrequently reported, in part because of concerns of infection control in the hospital environment. Case reports have shown abnormal myocardial T2 and native T1 times, pericardial abnormalities (myopericarditis), and a nonischemic pattern of late gadolinium enhancement (LGE) (9,36). In patients with a high pretest probability for acute myocardial injury and myocarditis-like injury, CMR may improve diagnostic specificity, guide management decisions, and affect the prognosis (33). CMR can provide a noninvasive, biopsy-like method for identifying the imaging features of myocardial inflammation.
CMR for convalescent patients with COVID-19
Several early reports raised concern for myocardial injury in association with COVID-19. In an early study, Ng et al (37) reported results from 16 patients who had been hospitalized with COVID-19 and who had elevated cTn or abnormal electrocardiograms (ECGs) during the acute illness (Table 2 ). At 2 months after the initial COVID-19 diagnosis, CMR was abnormal in 9 of 16 (56%) patients. Three patients (19%) had CMR criteria for myocarditis-like injury. That study was buttressed by a report from Germany: Puntmann et al (38) performed a prospective study of 100 recovered patients, the majority (49%) of whom had mild to moderate COVID-19 and two-thirds of whom were not hospitalized. At 2 to 3 months after a positive test result, 78 of 100 patients with prior COVID-19 had an abnormal CMR finding. The mean LV and RV ejection fractions were lower, and median native T1 and T2 were higher (indicative of edema and/or collagen deposition), than in control individuals. Pericardial enhancement was frequent (22%). There were greater proportions of patients with ischemic (32% vs 17%) and nonischemic (20% vs 7%) LGE patterns than the risk factor-matched control group. The prevalence of CMR abnormalities was more frequent than identified by cardiac blood biomarkers (38). However, individuals not hospitalized for COVID-19 had fewer CMR abnormalities compared to the hospitalized patients. This result was confirmed by Joy et al (39), who evaluated 74 health care workers with mild or asymptomatic COVID-19; CMR abnormalities at 6 months post–SARS-CoV-2 infection were similar to those of control subjects.
Table 2.
Summary of Studies of CMR in Patients After Recovery From COVID-19
| First Author (Ref. #) Study Design | Number of Cases | Men, % | Age, ya | Timing of CMR | Patient Characteristics During Acute COVID-19 | Patient Characteristics During the Postacute Stage | Comparator(s) | LGE | Myocardial Parametric Mapping | LV/RV Structure and Function, Pericardial Disease |
|---|---|---|---|---|---|---|---|---|---|---|
| Ng et al (37) Retrospective observational study |
16 | 56 | 68 (53-69) | 56 days (median) after recovery |
|
At ≥2 wk postdischarge, 11 (69%) patients were asymptomatic; 5 (31%) had symptoms such as cough, shortness of breath, and mild chest pain. | None |
|
In 6 patients (all without LGE), 4 had elevated T1 only, 1 had elevated T2 only, and 1 had both elevated T1 and T2. | Not reported |
| Puntmann et al (38) Prospective observational cohort study |
100 | 53 | 49 ± 14 | 71 (64-92) days from positive test |
|
On day of CMR, 17 patients reported atypical chest pain, and 20 reported palpitations. Compared with pre–COVID-19 status, 36 patients (36%) reported ongoing shortness of breath and exhaustion; 5% had significant TnT elevation at time of CMR. |
|
|
|
|
| Huang et al (44) Retrospective observational study |
26 | 38 | 38 (32-45) | 47 (36-58) days from onset of cardiac symptoms |
|
All had ≥1 cardiac symptoms (chest pain: 12%; palpitation: 88%; chest: distress 23%) after discharge. Patients with a history of CAD or myocarditis were excluded. None had elevated hsTnT at the time of CMR. | Healthy control individuals (n = 20): age- and sex-matched control subjects |
|
|
|
| Raman et al (45) Prospective observational cohort study |
58 | 59 | 55.4 ± 13.2) | 2.3 months (IQR: 2.1-2.5) after COVID-19 onset |
|
Individuals with pre-existing severe/end-stage multisystem comorbidities were excluded. | Risk factor–matched control individuals (n = 30): matched on age, sex, BMI, smoking, hypertension, diabetes, CAD, and stroke |
|
|
Not reported |
| Li et al (40) Prospective observational cohort study |
40 | 60 | 54 ± 12 | 158 ± 18 days after admission and 124 ± 17 days after discharge |
|
Discharged for ≥90 d. Individuals with pre-existing CAD, myocarditis, abnormal ECG findings, abnormal blood cardiac biomarker levels, or cardiac symptoms were excluded. | Healthy control individuals (n = 25): age- and sex- matched control subjects without history of cardiovascular disease and with normal ECG, echo, and CMR findings |
|
|
|
| Wang et al (41) Prospective observational cohort study |
44 | 43.2 | 47.6 ± 13.3 | 102.5 ± 20.6 days from discharge |
|
Recovered and discharged for 12 wk. Individuals with the following pre-existing conditions were excluded: uncontrolled hypertension, CAD, valvular disease, atrial fibrillation, heart failure, myocarditis, cardiomyopathy, and pacemaker placement. | Healthy control individuals (n = 31): age and sex matched; known to have normal ECG, echo, and CMR findings |
|
|
|
| Knight et al (42) Prospective observational study |
29 | 83 | 64 ± 9 | 37 ± 10 days after diagnosis |
|
Recovered and discharged from the hospital. Individuals with ACS, PE, or known cardiac pathology likely to cause scar and those aged ≥80 y were excluded. | None |
|
|
|
| Kotecha et al (43) Prospective observational study Note: This study includes the 29 patients in a study by Knight et al (42) |
148 | 56 | 64 ± 12 | 68 days after diagnosis |
|
Recovered and discharged from the hospital. Patients with medical unsuitability for CMR assessed by the referring clinician (eg, severe comorbidities, frailty) or with ACS as the primary reason for hospitalization were excluded. |
|
|
|
|
| Joy et al (39) Prospective observational study |
74 | 42 | 37 (31-48) | 6 months postinfection |
|
At the time of CMR, 16 (11%) reported symptoms: 5 (3%) sore throat, 4 (3%) fatigue, 4 (3%) rhinorrhea, and 3 (2%) shortness of breath, with no difference between seropositive and seronegative subjects (8% vs 13%). | Matched control individuals (n = 75): seronegative health care workers matched on age, sex, and ethnicity |
|
|
|
ACS = acute coronary syndrome; BMI = body mass index; CAD = coronary artery disease; CMR = cardiovascular magnetic resonance; ECG = electrocardiogram; echo = echocardiogram; ECV = extracellular volume; hsTnl = high-sensitivity troponin I; hsTNT = high-sensitivity troponin T; LGE = late gadolinium enhancement; LV = left ventricle; LVEF = left ventricular ejection fraction; LVEDVi = left ventricular end-diastolic volume index; MI = myocardial infarction; NIV = noninvasive ventilation; PE = pulmonary embolus; RV = right ventricle; RVCI = right ventricular cardiac index; RVEDVi = right ventricular end-diastolic volume index; RVEF = right ventricular ejection fraction; RVSVi = right ventricular stroke volume index; TnT = troponin T.
Values for age are mean ± SD or median (interquartile range).
Elevated hsTnT indicates a level of >99th percentile upper reference limit; hsTnT, N-terminal pro–b-type natriuretic peptide.
CMR of patients hospitalized for COVID-19
Rates of CMR-identified abnormalities in patients hospitalized because of COVID-19 have shown wide variation. Li et al (40) used CMR to evaluate 40 patients who had been hospitalized with moderate to severe COVID-19. The investigators excluded patients with known cardiovascular disease or diabetes. At approximately 5 months after hospital discharge, 24 of 40 (60%) patients had elevated extracellular volume (ECV) compared to control individuals, and 28 of 40 had subclinical LV dysfunction (by global longitudinal strain). However, only 1 of the 40 patients had LGE. In 44 hospitalized patients with COVID-19 free from pre-existing baseline cardiovascular disease, Wang et al (41) found nonischemic LGE in 13 of 44 (30%) patients after 3 months. Patients with LGE had worse LV and RV function by strain analysis compared to control individuals.
Knight et al (42) described CMR findings in 29 patients who had been hospitalized with COVID-19 and who had unexplained cTn elevation during the acute illness. At a mean of approximately 1 month after hospital admission, 32% of patients had occult ischemic heart disease (by LGE or stress perfusion), and 45% had a “myocarditis-like” pattern of LGE. In an expanded report from the same group, Kotecha et al (43) reported convalescent CMR findings from 148 patients hospitalized with severe COVID-19. At 2 months after hospital discharge, the investigators reported a myocarditis-like pattern of LGE in 26% (39/148) and myocardial infarction or inducible ischemia in 22% (32/148).
Huang et al (44) published a retrospective study of 26 patients hospitalized with moderate to severe COVID-19, who underwent CMR postrecovery (at approximately 1.5 months) for investigation of cardiac symptoms (chest pain: 12%; palpitation: 88%; chest distress: 23%). Fifteen patients (58%) had abnormal CMR findings (defined as increased myocardial T2 time and/or the presence of LGE); these patients had lower RV function than control individuals (eg, lower ejection fraction and stroke volume). Their results suggested a link between LV myocardial inflammation and lower RV function, a proxy indicator of COVID-19 severity. Knight et al (42) also noted a link between sustained pulmonary and cardiac involvement, with high rates of persistent lung parenchymal changes (69%) and pleural effusion (14%) on postrecovery CMR. These observations give rise to the concept that cardiac involvement associated with COVID-19 may not be a specific effect on the heart but, rather, a consequence of pulmonary and systemic inflammatory processes. The concept of systemic inflammatory activity in multiple organs (rather than cardiac-specific injury) is also supported by the findings of Raman et al (45), indicating multiorgan involvement after recovery.
In summary, reports of CMR-identified abnormalities in patients hospitalized caused by COVID-range from 26% to 60% of individuals at 1 to 5 months after hospital discharge. Reassuringly, patients with mild COVID-19 and asymptomatic individuals are reported to have low rates of CMR abnormalities (38,39). Comparison of early CMR studies is hampered by variable times of patient follow-up, associated comorbidities, prevalence of cardiovascular risk factors, and potential in-hospital treatment. CMR methods have also varied among published reports. Future studies with standardized CMR protocols are needed to evaluate the longer-term (1 year or more) effect of COVID-19 disease on the heart. Because of the high sensitivity of CMR for myocardial scar (particularly in patients with cardiovascular risk factors and pre-existing cardiovascular disease), these longer-term follow-up studies should include carefully matched risk factor control groups (46).
Evaluation of Athletes After COVID-19
As a result of the close congregation and contact of players during practice and competition, there is an increased risk of COVID-19 infections among athletes. Exercise initiated too early after viral infection or in occult myocarditis may have serious consequences (47,48). Indeed, non–COVID-19 myocarditis accounts for 4% to 8% of sudden cardiac deaths in athletes (49,50) or may lead to long-term complications such as myocardial scarring, arrhythmias, and myocardial dysfunction.
CMR of athletes recovering from COVID-19
Table 3 summarizes publications to date that have used CMR to evaluate athletes recovering from COVID-19. In the first publication on CMR in athletes, Rajpal et al (51) studied 26 college athletes who underwent CMR 11 to 53 days after having tested positive for COVID-19 (Table 3). The investigators found that 46% had mild to moderate symptoms of COVID-19 infection and that 54% were asymptomatic. Twelve athletes (46%) had myocardial LGE, with 4 (15%) having myocarditis-like findings on CMR (52).
Table 3.
CMR of Athletes Recovered From COVID-19
| First Author (Ref. #) | Patient Cohort (Cases) |
LGE Positive | Abnormal T1 + T2, Myocarditis-like Findings on CMRa |
LGE Pattern/Location in Patients With Myocarditisa,b | Troponin in Patients With Myocarditisa | ECG and TTE in Patients With Myocarditisa | Pericardium Pathology | |||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Men, % | Age, y | ||||||||
| Rajpal et al (52) | 26 | 58 | 19 ± 1.5 | 12/26 (46) | 4/26 (15%)
|
|
No | No | Effusion: 2/26 (8%) in athletes with myocarditisa | |
| ||||||||||
| Brito et al (54) | 54 (48c) | 85 | 19 (19-21) | 1/48 (2) | 0% | not applicable | not applicable | not applicable | Pericardial LGE: 19/48 (40%) Effusion: 28/48 (58%) | |
| ||||||||||
| Małek et al (55) | 26 | 19 | 24 (21-27) | 1/24 (4) | 0% | not applicable | not applicable | not applicable | Effusion: 2/26 (8%) | |
| ||||||||||
| Vago et al (56) | 12 | 17 | 23 (20-23) | 0/12 (0) | 0% | not applicable | not applicable | not applicable | No | |
| ||||||||||
| Clark et al (57) | 59 | 37 | 20 (19-21) | 16/59 (27) | 2/59 (3%)
|
|
No | 1 of 2 patients developed LV dysfunction (LVEF 45%) on a follow-up TTE | Pericardial LGE: 1/59 (2%) | |
| ||||||||||
| Starekova et al (58) | 145 | 75 | 19.6 ± 1.3 | 42/145 (29) | 2 (1.4%)
|
|
1 of 2 | 1 of 2 patients new nonspecific ST-segment and T-wave ECG abnormalities and mild reduction in GLS in TTE | Pericardial LGE: 1 (in patient with myopericarditis) | |
| ||||||||||
| Martinez et al (59) | 789 (27c) | 99 | 25 ± 3 | 2/27 (7) | 3/27 (11%)d 0.4% of the total cohort; 3 symptomatic |
not applicable | 1 of 3 | 1 of 3 patients ECG abnormalities 1 of 3 patients regional wall motion, mildly reduced LVEF (50%), dilated RV by TTE | Pericardial LGE: 2/27 (7.4%) | |
| ||||||||||
| Hendrickson et al (60) | 137 (5c) | 68 | 20 (18-27) | 0/5 (0) | 0% | not applicable | not applicable | not applicable | Small effusion in TTE: 4/137 (2.9%) | |
| ||||||||||
| Moulson et al (61) | 3,018 (198e) | 68 | 20 ± 1 | not applicable | Definite, probable, or possible cardiac involvement overall: n = 21/3,018 (0.7%)
|
not applicable | not applicable | not applicable | not applicable | |
| Multicenter (n = 42) study | ||||||||||
| Daniels et al (53) | 2,461 (1,597c) | 67 | not reported | not applicable | Myocarditis: n = 37/1,597; range 0%-7% (overall: 2.3%; 95% CI: 1.6-3.2)
|
not applicable | not applicable | not applicable | not applicable | |
| Multicenter (n = 13) study | ||||||||||
Values are n, %, mean ± SD, or n/N (%). All were retrospective studies except for Rajpal et al (52) and Vago et al (56), which were prospective studies.
GLS = global longitudinal strain; not applicable = not applicable or not given; TTE = transthoracic echocardiography; other abbreviations as in Table 2.
Myocarditis diagnosis based on CMR findings as per updated Lake Louise criteria (58).
Segment location given according to 17-segment American Heart Association model of the LV.
Number who underwent CMR.
CMR criteria for myocarditis not specified.
Number who underwent primary screening CMR.
Subsequent publications have reported lower rates of CMR abnormalities (Table 3). Brito et al (53) described CMR findings in 48 college athletes at a median of 27 days (range 22-33 days) after a positive COVID-19 test. None had CMR-defined myocarditis, although approximately 1 in 3 athletes had pericardial abnormalities. Similarly, Małek et al (54) and Vago et al (55) reported on 26 and 12 athletes, respectively; none had CMR-defined myocarditis. Clark et al (56) described 59 college athletes recovering from COVID-19 with CMR at a median of 22 days (range 10-162 days) following diagnosis. Two athletes (3%) had myocarditis-like findings on CMR.
Starekova et al (57) studied a consecutive cohort of college athletes (N = 145) who underwent standardized screening including CMR. Two patients (1.4%) had myocarditis-like CMR findings. Finally, Martinez et al (58) reported the evaluation of 789 professional U.S. athletes after COVID-19 recovery. Twenty-seven patients underwent CMR, and 3 (11%) of them had myocarditis-like findings. However only a small fraction of the professional athletes underwent CMR. Hendrickson et al (59) evaluated 137 collegiate athletes, with 5 patients referred for CMR because of abnormal testing results (eg, elevated cTn, coronary artery ectasia [59]). No abnormal findings were detected by CMR, and no athlete had an abnormal ECG.
In the Outcomes Registry for Cardiac Conditions in Athletes (ORCCA) prospective registry, collegiate athletes with at least 1 positive component of a triad of initial testing (ECG, cTn, or transthoracic echocardiography) were more than 4 times more likely to have a positive CMR finding compared to primary screening CMR (15/119 [12.6%] vs 6/198 [3%], respectively) (60). Another large multicenter study conducted by Daniels et al (52) included 13 universities and 2,461 athletes, of whom 1,597 had CMR. In 37 of 1,597 (2.3%) athletes, myocarditis was diagnosed clinically. Of these 37 athletes, 31 had CMR findings meeting the Lake Louise criteria for myocarditis. The prevalence of abnormal CMR findings varied from 0% to 7.6% among the included institutions (52).
In summary, the prevalence of myocarditis-like findings on CMR for athletes after COVID-19 is highly variable across the studies (range 0%-15%) (Table 3). Larger multicenter studies have tended toward lower prevalence rates. Approximately 50% of athletes who had myocarditis-like findings on CMR in single-center studies were asymptomatic, and all but 2 had normal troponin and ECG. A potential false-positive CMR finding was LGE at the RV insertion point (0%-26% prevalence) that has been previously reported in association with athletic activity (61) and is unlikely to be related to COVID-19. An important limitation of these reports is either lack or insufficient matching of a control group (eg, by age, sex, or type of sport [endurance or strength]) (Supplemental Table 1).
Multisystem Inflammatory Syndrome in Children
Multisystem inflammatory syndrome in children (MIS-C), also called pediatric inflammatory multisystem syndrome, is characterized by a severe inflammatory response after SARS-CoV-2 infection. Multiple definitions of the syndrome have been published (62, 63, 64). The U.S. Centers for Disease Control and Prevention defines MIS-C as individuals younger than 21 years of age who have a fever for at least 24 hours, laboratory evidence of inflammation, multisystem involvement, severe illness requiring hospitalization, no alternative plausible diagnosis, and recent or current SARS-CoV-2 infection or exposure (63). MIS-C is believed to be a delayed immune response occurring after SARS-CoV-2 infection, typically occurring 2 to 6 weeks after infection (65, 66, 67). The immunologic profile for acute COVID-19 appears to be distinct from MIS-C (68).
Patients presenting with MIS-C are frequently otherwise healthy and may present with symptoms similar to Kawasaki disease (eg, rash, conjunctival injection) or myocarditis, sometimes in shock, in addition to frequent gastrointestinal symptoms (65). Older pediatric patients (13-20 years of age) more often present with myocarditis-like symptoms (73%) as opposed to younger patients (0-5 years of age [39%]), but younger patients more often present with symptoms similar to Kawasaki disease (48% vs 11% in older pediatric patients) (69). Patients have markedly abnormal laboratory testing results, including elevated inflammatory markers, thrombocytopenia, elevated B-type natriuretic peptide and/or cTn, and abnormal coagulation markers (67). Potential mechanisms contributing to the pathophysiology of MIS-C include a hyperactive postviral immunologic response to COVID-19 leading to systemic inflammation. However, this is the subject of ongoing investigation (70).
Cardiac involvement in children with MIS-C is common, including most frequently diminished LV systolic function in addition to arrhythmias, pericardial effusion, and coronary artery dilation and/or aneurysms (65). In a case series of 570 children, the median length of stay was 6 days, with 64% requiring intensive care, and although most children ultimately recover, the mortality rate was reported as 1% to 2% (65). Feldstein et al (71) described reduced LV ejection fraction in 172 of 503 (34%) of patients with MIS-C; all but 1 patient recovered function at 90 days (71). In the same study, coronary artery aneurysms were present in 57 of 424 (13%) patients, with normalization in all evaluated patients at 90 days.
There have been several small studies to date evaluating CMR findings in patients with MIS-C, encompassing more than 130 patients. CMRs that were performed during the initial hospitalization or soon after discharge frequently identified myocardial edema on T2-weighted images, hyperemia and capillary leak (using T1-weighted images before and immediately after gadolinium administration), and LGE (72, 73, 74, 75, 76, 77). However, in a few studies evaluating CMRs closer to 2 to 3 months after discharge, there were frequently no abnormalities (78,79).
CMR for Patients With COVID-19: Planned and Ongoing Studies
The use of CMR in the context of COVID-19 is driven by the accuracy and reproducibility of the method and its unique role in myocardial tissue characterization (Central Illustration ). Supplemental Table 2 is a list of planned and ongoing studies using CMR to investigate the cardiovascular manifestations of COVID-19, identified primarily from clinical trial registration sites. Overall, almost 10,000 participants will be included across all studies, which are planned in a range of settings and in diverse patient populations. Global sharing of CMR databases may further augment the power of these proposed investigations. Indeed, recent national and international initiatives have been established to create research databases of CMR studies of patients with COVID-19 (80,81).
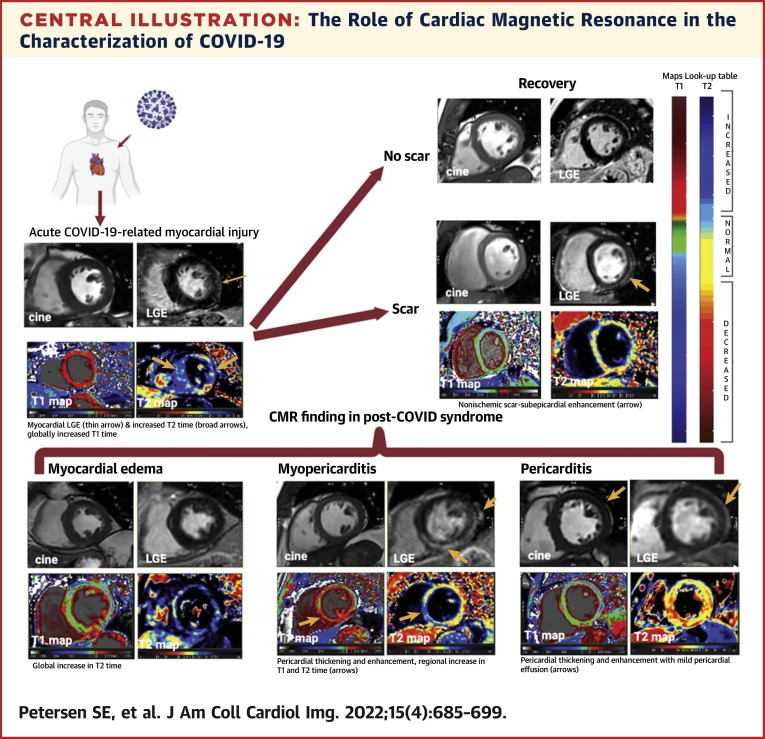
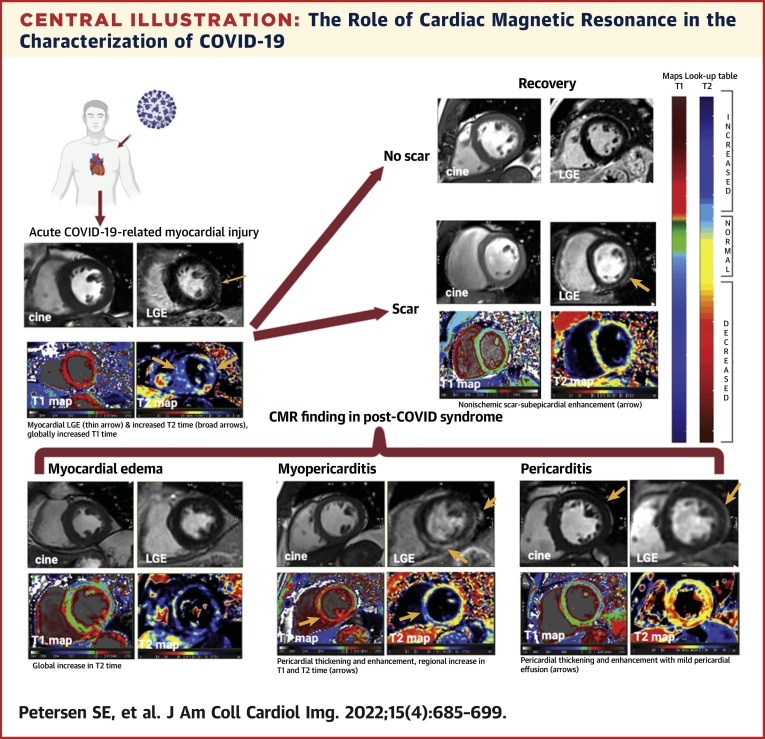
Central Illustration.
The Role of Cardiac Magnetic Resonance in the Characterization of COVID-19
In the acute setting (top left), cardiac magnetic resonance can provide a noninvasive, biopsy-like method to identify the imaging features of myocardial injury, including abnormal late gadolinium enhancement, T1 and T2 abnormalities, and pericardial abnormalities. Some patients will have persistent myocardial scar (top right) on late gadolinium enhancement images with persistent T1 and T2 mapping abnormalities. Patterns of residual myocardial abnormalities in post–COVID-19 syndrome include (bottom row) myocardial edema, myopericarditis, and isolated pericarditis.
Recommendations for the Use of CMR in Patients With COVID-19
The appropriate use of CMR and its role in the treatment of patients with COVID-19 must be considered within the multifactorial context of disease severity, availability of CMR versus other cardiovascular imaging resources, and pretest probability. Evidence-based knowledge regarding the appropriate use of CMR for patients with COVID-19 is expected to evolve over several years because the long-term complications of the disease are currently under intense study (Supplemental Table 2). To address the current gap in knowledge, a diverse group of investigators from 9 countries (principally cardiologists and radiologists with extensive experience in CMR) were assembled to offer expert opinion regarding appropriate CMR use. The opinions that follow have been informed by each researcher’s experience with COVID-19 patients and after extensive review of published reports and group discussion. Definitions of COVID-19 severity were based on established disease classifications relevant to each practitioner (82,83); 80% or higher agreement on the direction of recommendation was considered concurrence. In all discussions, the expert panel recommended consideration of CMR testing only when the test would likely affect clinical decision making, such as altering therapeutic decisions. The consensus recommendations regarding 4 distinct patient scenarios are detailed below and summarized in Table 4 .
Table 4.
CMR For Patients With COVID-19: CMR Should Be Considered Only When Results are Likely to Have an Impact on Clinical Decision Making
| Clinical Scenario | Consider CMR for the Following Patients |
|---|---|
|
High pretest probability for acute myocardial injury due to inflammation |
|
|
|
|
|
|
CMR = cardiac magnetic resonance; MIS-C = multisystem inflammatory syndrome in children.
1. CMR for patients with acute COVID-19
CMR should be considered for COVID-19 patients with high pretest probability for acute myocardial injury due to inflammation and when CMR findings are likely to have an impact on clinical decision making. In suspected acute myocardial injury, acute coronary syndrome (eg, myocardial infarction types 1 and 2 [18]) should be excluded before CMR to avoid diagnostic delay and treatment.
2. CMR for convalescent patients after recovery from COVID-19
CMR should be considered for patients with COVID-19 after recovery from COVID-19 in the following circumstances and when CMR findings are likely to have an impact on clinical decision making:
-
1.
Patients with otherwise unexplained, persisting, or recurring cardiovascular symptoms (eg, exertional dyspnea, palpitations, chest pain, fatigue, or other symptoms of myocardial injury or heart failure) as a part of a systemic inflammatory post–COVID-19 syndrome more than 4 weeks after COVID-19 recovery.
-
2.
Patients who had CMR in the acute setting who showed clinically significant acute myocardial injury. The convalescent CMR should be performed 4 weeks or more after the baseline (acute) CMR.
3. CMR for recovering high-performance athletes
-
1.Return-to-play CMR should be considered for high-performance athletes after COVID-19 recovery and before return to training in the following settings:
-
a.History of moderate COVID-19 and high-pretest probability of myocardial injury by diagnostic testing or clinical suspicion
-
b.History of severe COVID-19
-
a.
-
2.
CMR should be considered for high-performance athletes who have returned to play with new-onset cardiovascular symptoms with suspicion of myocardial injury.
See Kim et al (84) for more detail.
4. CMR for patients with suspected MIS-C
CMR should be considered for patients with MIS-C in the following settings:
-
•
Clinical suspicion of myocardial injury or with significantly diminished ventricular function during inpatient hospitalization for acute illness, particularly if not clinically improving
-
•
Approximately 1 to 6 months after the acute MIS-C presentation in patients with prior moderately or severely diminished LV systolic function or baseline abnormal CMR findings
-
•
Concern for coronary artery aneurysm
Because of the evolving clinical information, these recommendations may be revised as additional information becomes available. CMR recommendations also need to be modified based on a patient’s individual cardiovascular risk factors, change in clinical status, or unexplained symptoms. CMR practitioners should be aware of cardiac magnetic resonance parameters (Supplemental Table 3), special considerations, and clinical guidelines for certain patient populations, such as high-performance athletes (eg, Kim et al [84]) (Supplemental Table 1) and pediatric patients, including those with MIS-C (85).
Conclusions
Public health guidelines and vaccine development are expected to result in fewer incident cases of COVID-19. However, the clinical spectrum of recovery after acute COVID-19 with regard to cardiovascular disease is unresolved. Reports to date have raised the potential of sustained cardiac injury in patients who have recovered from COVID-19. CMR is a key noninvasive clinical and research tool because of its comprehensive evaluation of myocardial function, structure, and tissue composition. Given the high sensitivity of CMR, important caveats to the application of CMR include: 1) the detection of subclinical cardiac disease that may have occurred before SARS-CoV-2 infection; and 2) the detection of CMR abnormalities that may not functionally affect quality of life or increase the risk of future cardiovascular events. Longer-term studies are necessary to determine the clinical importance of CMR metrics and their association with incident health outcomes. Comparison to control groups (matched for cardiovascular risk factors and severity-matched non-COVID illness when feasible) will ultimately help determine the relationship of CMR findings to long-term patient outcomes.
Funding Support and Author Disclosures
Dr Petersen provides consultancy to and is a shareholder of Circle Cardiovascular Imaging, Inc, Calgary, Alberta, Canada. Dr Friedrich is board member, shareholder, and consultant of Circle Cardiovascular Imaging Inc. Dr Kramer is a consultant to Bristol Myers Squibb. Dr Nagel has received research support from Bayer AG, Neosoft, and Medis. Dr Pennell has received research support from Siemens. Dr Bluemke is a consultant to Bayer AG. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Dr Petersen has received support from the National Institute for Health Research Biomedical Research Centre at Barts. Dr Ferreira receives support from the British Heart Foundation, British Heart Foundation Centre of Research Excellence, Oxford, and the National Institute for Health Research Oxford Biomedical Research Centre at Oxford University Hospitals NHS Foundation Trust. Dr Raisi-Estabragh was supported by British Heart Foundation Clinical Research Training Fellowship number FS/17/81/33318.
Footnotes
Paaladinesh Thavendiranathan, MD, served as the Guest Editor for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Appendix
References
- 1.World Health Organization. Coronavirus disease Q28 (COVID-19) dashboard. Accessed July 8, 2021. https://covid19.who.int/
- 2.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi S., Qin M., Shen B., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudski L., Januzzi J.L., Rigolin V.H., et al. Multimodality imaging in evaluation of cardiovascular complications in patients with COVID-19: JACC scientific expert panel. J Am Coll Cardiol. 2020:1345–1357. doi: 10.1016/j.jacc.2020.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phelan D., Kim J.H., Elliott M.D., et al. Screening of potential cardiac involvement in competitive athletes recovering from COVID-19: an expert consensus statement. J Am Coll Cardiol Img. 2020:2635–2652. doi: 10.1016/j.jcmg.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic. Accessed April 27, 2021. https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance
- 7.Society for Cardiovascular Magnetic Resonance. Statement from SCMR on the role of CMR in patients with history of COVID-19 infection. Accessed April 23, 2021. https://scmr.org/news/526857/Statement-from-SCMR-on-the-Role-of-CMR-in-Patients-with-History-of-COVID-19-Infection.htm
- 8.Bangalore S., Sharma A., Slotwiner A., et al. ST-segment elevation in patients with Covid-19—A case series. N Engl J Med. 2020;382(25):2478–2480. doi: 10.1056/nejmc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inciardi R.M., Lupi L., Zaccone G., et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doyen D., Moceri P., Ducreux D., Dellamonica J. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet. 2020;395(10235):1516. doi: 10.1016/S0140-6736(20)30912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng Q., Hu B., Zhang Y., et al. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan, China. Int J Cardiol. 2020;311:116–121. doi: 10.1016/j.ijcard.2020.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Osch D., Asselbergs F.W., Teske A.J., et al. Takotsubo cardiomyopathy in COVID-19: a case report. Haemodynamic and therapeutic considerations. Eur Hear J Case Rep. 2020;4(FI1):1–6. doi: 10.1093/ehjcr/ytaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chitsazan M., Amin A., Chitsazan M., et al. Heart failure with preserved ejection fraction in coronavirus disease 2019 patients: the promising role of diuretic therapy in critically ill patients. ESC Heart Fail. 2021;8(2):1610–1614. doi: 10.1002/ehf2.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jirak P., Larbig R., Shomanova Z., et al. Myocardial injury in severe COVID-19 is similar to pneumonias of other origin: results from a multicentre study. ESC Heart Fail. 2021;8(1):37–46. doi: 10.1002/ehf2.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi S., Qin M., Cai Y., et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41(22):2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giustino G., Croft L.B., Stefanini G.G., et al. Characterization of myocardial injury in patients with COVID-19. J Am Coll Cardiol. 2020;76(18):2043–2055. doi: 10.1016/j.jacc.2020.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thygesen K., Alpert J.S., Jaffe A.S., et al. Fourth universal definition of myocardial infarction (2018) Eur Heart J. 2019;40(3):237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 19.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo T., Fan Y., Chen M., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lala A., Johnson K.W., Januzzi J.L., et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76(5):533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rath D., Petersen-Uribe Á., Avdiu A., et al. Impaired cardiac function is associated with mortality in patients with acute COVID-19 infection. Clin Res Cardiol. 2020;109(12):1491–1499. doi: 10.1007/s00392-020-01683-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szekely Y., Lichter Y., Taieb P., et al. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation. 2020;142(4):342–353. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dweck M.R., Bularga A., Hahn R.T., et al. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging. 2020;21(9):949–958. doi: 10.1093/ehjci/jeaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C., Huang L., Wang Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonnweber T., Sahanic S., Pizzini A., et al. Cardiopulmonary recovery after COVID-19—an observational prospective multi-center trial. Eur Respir J. 2020;57(4):2003481. doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tucker N.R., Chaffin M., Bedi K.C., et al. Myocyte-specific upregulation of ACE2 in cardiovascular disease: implications for SARS-CoV-2-mediated myocarditis. Circulation. 2020;142(7):708–710. doi: 10.1161/CIRCULATIONAHA.120.047911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basso C., Leone O., Rizzo S., et al. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020;41(39):3827–3835. doi: 10.1093/eurheartj/ehaa664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halushka M.K., Vander Heide R.S. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50:107300. doi: 10.1016/j.carpath.2020.107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindner D., Fitzek A., Bräuninger H., et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5(11):1281–1285. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bois M.C., Boire N.A., Layman A.J., et al. COVID-19–associated nonocclusive fibrin microthrombi in the heart. Circulation. 2021;143(3):230–243. doi: 10.1161/circulationaha.120.050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pellegrini D., Kawakami R., Guagliumi G., et al. Microthrombi as a major cause of cardiac injury in COVID-19: a pathologic study. Circulation. 2021;143(10):1031–1042. doi: 10.1161/circulationaha.120.051828. [DOI] [PubMed] [Google Scholar]
- 33.Ferreira V.M., Schulz-Menger J., Holmvang G., et al. cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72(24):3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 34.Lurz P., Luecke C., Eitel I., et al. Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis the MyoRacer-Trial. J Am Coll Cardiol. 2016;67(15):1800–1811. doi: 10.1016/j.jacc.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Kelle S., Bucciarelli-Ducci C., Judd R.M., et al. Society for Cardiovascular Magnetic Resonance (SCMR) recommended CMR protocols for scanning patients with active or convalescent phase COVID-19 infection. J Cardiovasc Magn Reason. 2020;22(1):61. doi: 10.1186/s12968-020-00656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokoo P., Fonseca E.K.U.N., Neto R.S., et al. COVID-19 myocarditis: a case report. Einstein (São Paulo) 2020;18(5) doi: 10.31744/einstein_journal/2020RC5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng M.Y., Ferreira V.M., Leung S.T., et al. Patients recovered from COVID-19 show ongoing subclinical myocarditis as revealed by cardiac magnetic resonance imaging. J Am Coll Cardiol Img. 2020;13(11):2476–2478. doi: 10.1016/j.jcmg.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puntmann V.O., Carerj M.L., Wieters I., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joy G., Artico J., Kurdi H., et al. Prospective case-control study of cardiovascular abnormalities 6 months following mild COVID-19 in healthcare workers. J Am Coll Cardiol Img. 2021;14(11):2155–2166. doi: 10.1016/j.jcmg.2021.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X., Wang H., Zhao R., et al. Elevated extracellular volume fraction and reduced global longitudinal strains in patients recovered from COVID-19 without clinical cardiac findings. Radiology. 2021;299(2):E230–E240. doi: 10.1148/radiol.2021203998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H., Li R., Zhou Z., et al. Cardiac involvement in COVID-19 patients: mid-term follow up by cardiovascular magnetic resonance. J Cardiovasc Magn Reason. 2021;23(1):14. doi: 10.1186/s12968-021-00710-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knight D.S., Kotecha T., Razvi Y., et al. COVID-19 myocardial injury in survivors. Circulation. 2020;142(11):1120–1122. doi: 10.1161/CIRCULATIONAHA.120.049252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kotecha T., Knight D.S., Razvi Y., et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J. 2021;42(19):1866–1878. doi: 10.1093/eurheartj/ehab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang L., Zhao P., Tang D., et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. J Am Coll Cardiol Img. 2020;13(11):2330–2339. doi: 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raman B., Cassar M.P., Tunnicliffe E.M., et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31:100683. doi: 10.1016/j.eclinm.2020.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raisi-Estabragh Z., McCracken C., Cooper J., et al. Adverse cardiovascular magnetic resonance phenotypes are associated with greater likelihood of incident coronavirus disease 2019: findings from the UK Biobank. Aging Clin Exp Res. 2021;33(4):1133–1144. doi: 10.1007/s40520-021-01808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Halle M., Binzenhöfer L., Mahrholdt H., Johannes Schindler M., Esefeld K., Tschöpe C. Myocarditis in athletes: a clinical perspective. Eur J Prev Cardiol. 2021;28:1050–1057. doi: 10.1177/2047487320909670. [DOI] [PubMed] [Google Scholar]
- 48.Kiel R.J., Smith F.E., Chason J., Khatib R., Reyes M.P. Coxsackievirus B3 myocarditis in C3H/HeJ mice: description of an inbred model and the effect of exercise on virulence. Eur J Epidemiol. 1989;5(3):348–350. doi: 10.1007/BF00144836. [DOI] [PubMed] [Google Scholar]
- 49.Marijon E., Tafflet M., Celemajer D.S., et al. Sports-related sudden death in the general population. Circulation. 2011;124(6):672–681. doi: 10.1161/CIRCULATIONAHA.110.008979. [DOI] [PubMed] [Google Scholar]
- 50.Maron B.J., Udelson J.E., Bonow R.O., et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 3: Hypertrophic Cardiomyopathy, Arrhythmogenic Right Ventricular Cardiomyopathy and Other Cardiomyopathies, and Myocarditis: a scientific statement from the American Heart Association and American College of Cardiology. Circulation. 2015;132(22):e273–e280. doi: 10.1161/CIR.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 51.Rajpal S., Tong M.S., Borchers J., et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021:116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daniels C.J., Rajpal S., Greenshields J.T., et al. Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS-CoV-2 infection. JAMA Cardiol. 2021;6(9):1078–1087. doi: 10.1001/jamacardio.2021.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brito D., Meester S., Yanamala N., et al. High prevalence of pericardial involvement in college student athletes recovering from COVID-19. J Am Coll Cardiol Img. 2021;14(3):541–555. doi: 10.1016/j.jcmg.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Małek Ł.A., Marczak M., Miłosz-Wieczorek B., et al. Cardiac involvement in consecutive elite athletes recovered from Covid-19: a magnetic resonance study. J Magn Reson Imaging. 2021;53(6):1723–1729. doi: 10.1002/jmri.27513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vago H., Szabo L., Dohy Z., Merkely B. Cardiac magnetic resonance findings in patients recovered from COVID-19: initial experiences in elite athletes. J Am Coll Cardiol Img. 2021;14(6):1279–1281. doi: 10.1016/j.jcmg.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clark D.E., Parikh A., Dendy J.M., et al. COVID-19 myocardial pathology evaluation in athletes with cardiac magnetic resonance (COMPETE CMR) Circulation. 2021;143(6):609–612. doi: 10.1161/CIRCULATIONAHA.120.052573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Starekova J., Bluemke D.A., Bradham W.S., et al. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging. JAMA Cardiol. 2021;6(8):945–950. doi: 10.1001/jamacardio.2020.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinez M.W., Tucker A.M., Bloom O.J., et al. Prevalence of inflammatory heart disease among professional athletes with prior COVID-19 infection who received systematic return-to-play cardiac screening. JAMA Cardiol. 2021;6(7):745–752. doi: 10.1001/jamacardio.2021.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hendrickson B.S., Stephens R.E., Chang J.V., et al. Cardiovascular evaluation after COVID-19 in 137 collegiate athletes: results of an algorithm-guided screening. Circulation. 2021;143(19):1926–1928. doi: 10.1161/CIRCULATIONAHA.121.053982. [DOI] [PubMed] [Google Scholar]
- 60.Moulson N., Petek B.J., Drezner J.A., et al. SARS-CoV-2 cardiac involvement in young competitive athletes. Circulation. 2021;144(4):256–266. doi: 10.1161/CIRCULATIONAHA.121.054824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Domenech-Ximenos B., Sanz-De La Garza M., Prat-González S., et al. Prevalence and pattern of cardiovascular magnetic resonance late gadolinium enhancement in highly trained endurance athletes. J Cardiovasc Magn Reason. 2020;22(1):62. doi: 10.1186/s12968-020-00660-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Royal College of Paediatrics and Child Health. Guidance: paediatric multisystem inflammatory syndrome temporally associated with COVID-19 (PIMS). Accessed August 26, 2021. https://www.rcpch.ac.uk/sites/default/files/2020-05/COVID-19-Paediatric-multisystem-%20inflammatory%20syndrome-20200501.pdf
- 63.Centers for Disease Control and Prevention Health Alert Network. Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). Accessed August 26, 2021. https://emergency.cdc.gov/han/2020/han00432.asp
- 64.World Health Organization. Multisystem inflammatory syndrome in children and adolescents with COVID-19: scientific brief. Accessed August 26, 2021. https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19
- 65.Godfred-Cato S., Bryant B., Leung J., et al. COVID-19–associated multisystem inflammatory syndrome in children—United States, March–July 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feldstein L.R., Rose E.B., Horwitz S.M., et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elias M.D., McCrindle B.W., Larios G., et al. Management of multisystem inflammatory syndrome in children associated with COVID-19: a survey from the International Kawasaki Disease Registry. CJC Open. 2020;2(6):632–640. doi: 10.1016/j.cjco.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Diorio C., Henrickson S.E., Vella L.A., et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS–CoV-2. J Clin Invest. 2020;130(11):5967–5975. doi: 10.1172/JCI140970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dufort E.M., Koumans E.H., Chow E.J., et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383(4):347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang L., Tang K., Levin M., et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020;20(11):e276–e288. doi: 10.1016/S1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feldstein L.R., Tenforde M.W., Friedman K.G., et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325(11):1074–1087. doi: 10.1001/jama.2021.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blondiaux E., Parisot P., Redheuil A., et al. Cardiac MRI in children with multisystem inflammatory syndrome associated with COVID-19. Radiology. 2020;297(3):E283–E288. doi: 10.1148/radiol.2020202288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jain S., Nolan S., Biller R., et al. Cardiovascular magnetic resonance in myocarditis related to multisystem inflammatory syndrome in children associated with COVID-19. Congenital Cardiology Today. 2020;18(8):20–22. [Google Scholar]
- 74.Theocharis P., Wong J., Pushparajah K., et al. Multimodality cardiac evaluation in children and young adults with multisystem inflammation associated with COVID-19. Eur Hear J Cardiovasc Imaging. 2021;22(8):896–903. doi: 10.1093/ehjci/jeaa212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caro-Domínguez P., Navallas M., Riaza-Martin L., et al. Imaging findings of multisystem inflammatory syndrome in children associated with COVID-19. Pediatr Radiol. 2021;51(9):1608–1620. doi: 10.1007/s00247-021-05065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sirico D., Basso A., Reffo E., et al. Early echocardiographic and cardiac MRI findings in multisystem inflammatory syndrome in children. J Clin Med. 2021;10(15):3360. doi: 10.3390/jcm10153360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bermejo I.A., Bautista-Rodriguez C., Fraisse A., et al. Short-term sequelae of multisystem inflammatory syndrome in children assessed by CMR. J Am Coll Cardiol Img. 2021;14(8):1666–1667. doi: 10.1016/j.jcmg.2021.01.035. [DOI] [PubMed] [Google Scholar]
- 78.Webster G., Patel A.B., Carr M.R., et al. Cardiovascular magnetic resonance imaging in children after recovery from symptomatic COVID-19 or MIS-C: a prospective study. J Cardiovasc Magn Reson. 2021;23(1):86. doi: 10.1186/s12968-021-00786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bartoszek M., Małek Ł.A., Barczuk-Falęcka M., Brzewski M. Cardiac magnetic resonance follow-up of children after pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV -2 with initial cardiac involvement. J Magn Reson Imaging. 2021;55:883–891. doi: 10.1002/jmri.27870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.National COVID-19 chest image database (NCCID). Accessed June 22, 2021. https://www.nhsx.nhs.uk/covid-19-response/data-and-covid-19/national-covid-19-chest-imaging-database-nccid/
- 81.SCMR COVID-19 registry. Accessed June 22, 2021. https://scmr.org/page/COVID-19Registry
- 82.World Health Organization. COVID-19 clinical management: living guidance. Accessed June 22, 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1
- 83.National Institutes of Health. COVID-19 treatment guidelines: clinical spectrum of SARSCoV-2 infection. Accessed June 22, 2021. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum [PubMed]
- 84.Kim J.H., Levine B.D., Phelan D., et al. Coronavirus disease 2019 and the athletic heart: emerging perspectives on pathology, risks, and return to play. JAMA Cardiol. 2021;6(2):219–227. doi: 10.1001/jamacardio.2020.5890. [DOI] [PubMed] [Google Scholar]
- 85.Children’s Hospital of Philadelphia. Multisystem inflammatory syndrome (MIS-C) clinical pathway—emergency, ICU and inpatient. Accessed June 22, 2021. https://www.chop.edu/clinical-pathway/multisystem-inflammatory-syndrome-mis-c-clinical-pathway
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.