Abstract
Background
The increasing incidence of candidemia and emergence of drug-resistant Candida species are major concerns worldwide. Long-term surveillance studies are needed.
Methods
The Fungal Infection Network of Switzerland (FUNGINOS) conducted a 15-year (2004–2018), nationwide, epidemiological study of candidemia. Hospital-based incidence of candidemia, Candida species distribution, antifungal susceptibility, and consumption were stratified in 3 periods (2004–2008, 2009–2013, 2014–2018). Population-based incidence over the period 2009–2018 derived from the Swiss Antibiotic Resistance Surveillance System (ANRESIS).
Results
A total of 2273 Candida blood isolates were studied. Population and hospital-based annual incidence of candidemia increased from 2.96 to 4.20/100 000 inhabitants (P = .022) and 0.86 to 0.99/10 000 patient-days (P = .124), respectively. The proportion of Candida albicans decreased significantly from 60% to 53% (P = .0023), whereas Candida glabrata increased from 18% to 27% (P < .0001). Other non-albicans Candida species remained stable. Candida glabrata bloodstream infections occurred predominantly in the age group 18–40 and above 65 years. A higher proportional increase of C glabrata was recorded in wards (18% to 29%, P < .0001) versus intensive care units (19% to 24%, P = .22). According to Clinical and Laboratory Standards Institute, nonsusceptibility to fluconazole in C albicans was observed in 1% of isolates, and anidulafungin and micafungin nonsusceptibility was observed in 2% of C albicans and C glabrata. Fluconazole consumption, the most frequently used antifungal, remained stable, whereas use of mold-active triazoles and echinocandins increased significantly in the last decade (P < .0001).
Conclusions
Over the 15-year period, the incidence of candidemia increased. A species shift toward C glabrata was recently observed, concurring with increased consumption of mold-active triazoles.
Keywords: antifungals, candida, candidemia, epidemiology, resistance
The incidence of candidemia increased in Switzerland from 2004 to 2018. A species shift toward C glabrata was observed after 2013, now accounting for one fourth of all candidemia, concurring with increased consumption of mold-active triazoles.
Candida species are the most common cause of nosocomial fungal infections [1]. Candidemia is associated with substantial morbidity, mortality, and increased healthcare costs [2, 3]. The worldwide incidence of candidemia is difficult to assess, and long-term population-based surveillance data are available from few countries. Reported incidence rates vary significantly between 3.2 and 8.4 episodes/100.000 inhabitants [4–9].
In a nationwide survey of candidemia performed by the Fungal Infection Network of Switzerland (FUNGINOS) between 1991 and 2000, hospital-based incidence rates remained unchanged over the 10-year period [10]. Over the last 2 decades, new antifungal agents and new management strategies such as antifungal prophylaxis and pre-emptive therapy using triazoles or echinocandins have been recommended in high-risk hospital populations, in particular patients with hematological malignancies and critically ill patients [1]. Their larger use may have influenced the Candida species distribution and antifungal susceptibility. Although Candida albicans has been the worldwide predominant species during decades, a shift to Candida species with reduced fluconazole susceptibility, in particular Candida glabrata, has been reported from many countries [11, 12]. In addition, there are increasing reports of echinocandin-resistant Candida infections [13–15]. In Switzerland, no shift to resistant Candida species was observed in the FUNGINOS hospitals over the period 1991–2009 [10, 16]. To evaluate the trends in the epidemiology and antifungal drug susceptibility of candidemia in Switzerland, FUNGINOS conducted a prospective study over 15 years from 2004 to 2018.
METHODS
The present prospective survey of candidemia in Switzerland was conducted in 5 university and 2 tertiary care university-affiliated hospitals of the FUNGINOS research network.
Hospital and Population-Based Surveillance Over the Period 2004–2018
For hospital-based surveillance, epidemiological and microbiological data of the 7 participating hospitals were included over 2004–2018 and stratified in 3 periods (2004–2008, 2009–2013, 2014–2018). These institutions are distributed across the country and play a key role in the national healthcare system. All adult hematopoietic stem cell and solid organ transplantations are performed in these 7 hospitals. Five of seven surveyed hospitals include affiliated pediatric centers.
For population-based surveillance, microbiological data were included from the Swiss Antibiotic Resistance Surveillance System (ANRESIS) from 2009 to 2018. The ANRESIS program centralizes antibiotic and antifungal resistance data of microorganisms from a representative selection of clinical microbiology laboratories [17]. From 2009 to 2018, the annual coverage of national laboratories by ANRESIS increased from 60.9% to 80.6%. To calculate the incidence rates of candidemia per 100 000 inhabitants, annual population data were extracted from the Federal Statistical Office database and were corrected according to the reported coverage by ANRESIS [18]. The study has been approved by the Ethical Committee of the Lausanne University Hospital as the FUNGINOS national coordinating center.
Study Variables
The following variables were recorded by questionnaire survey on a yearly basis: hospital activities, number and species of the 10 most frequent bloodstream pathogens, Candida isolates from blood cultures, age and ward distribution of patients with candidemia, and consumption of antibiotics and antifungals. A Candida isolate from at least 1 blood culture set was defined as candidemia. Multiple Candida isolates of the same species from the same patient were considered as different episodes of candidemia if they occurred more than 4 weeks apart [19]. The annual questionnaires were distributed to local investigators (infectious diseases specialist(s) and clinical microbiologist(s)) at each hospital. The FUNGINOS Data Review Committee checked data for completeness and consistency and addressed queries to centers. Data on hematopoietic stem cell and solid organ transplantations derived from the Federal Office of Public Health [20].
Consumption of antimicrobial agents was calculated in Defined Daily Doses (DDD) according to the 2021 Anatomical Therapeutical Chemical/ Defined Daily Dose (ATC/DDD) Index of the World Health Organization (WHO), except for fluconazole, amphotericin B, and posaconazole. For fluconazole, DDDs were calculated on a 0.4 grams basis instead of 0.2 grams, reflecting the dose recommended in severe systemic infections. For liposomal amphotericin B, DDDs were calculated on a 0.21 grams basis (3 mg/kg for a body weight of 70 kg). Posaconazole tablets and intravenous formulations were approved in Switzerland since mid-2015. Since the licensed maintenance dose of these formulations is 300 mg/day compared with 600 mg/day for the oral suspension, DDDs for posaconazole were calculated on a 0.6 grams and 0.3 grams basis before and after mid-2015, respectively.
Candida Species Identification and Antifungal Susceptibility Testing
The 7 microbiology laboratories affiliated with participating hospitals used automated blood culture systems (Bactec [Becton Dickinson, Sparks, MD] or BacT/Alert [bioMérieux, Marcy l’Etoile, France]).
Over the study period 2004–2013, the Candida bloodstream isolates were centralized to the FUNGINOS mycology reference laboratory, Institute of Microbiology, Lausanne University Hospital (Centre hospitalier universitaire vaudois [CHUV]). Candida species were identified by standard biochemical assays in a test gallery (ATB ID 32 C(R); bioMérieux) and by Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry. In case of discordant identification between center and reference laboratory, molecular identification was performed by polymerase chain reaction amplification and sequencing of the D1/D2 region of the large subunit of the 28S ribosomal RNA gene (28S rDNA). Antifungal susceptibility testing was performed by microtiter broth dilution method using the Sensititre YeastOne test panel (TREK Diagnostic System, Cleveland, OH). Susceptibility testing included fluconazole, voriconazole, caspofungin, and amphotericin B over 2004–2013. Posaconazole was tested after 2005 and anidulafungin and micafungin were tested after 2010. Over 2014–2018, species identification was performed at local laboratories and susceptibility data were extracted from ANRESIS database. Candida species identification and susceptibility testing at local laboratories were performed by MALDI-TOF and Sensititre YeastOne or Etest, respectively. One center used VITEK (bioMérieux) for susceptibility testing. Only isolates with available minimum inhibitory concentration (MIC) were included. Interpretation (or reinterpretation) of antifungal susceptibility was performed according to Clinical and Laboratory Standards Institute (CLSI) document M60-Ed2.
Statistical Analysis
Continuous variables were compared using Student’s t test or Mann-Whitney U test, and proportions were compared using χ 2 test or Fisher’s exact test, as appropriate. Changes in Candida species distribution over time were evaluated with the χ 2 test for trend. Linear trends over time were analyzed with the Poisson regression model. All P values were based on 2-tailed tests of significance (P < .05). Statistical analysis was performed using SPSS software, version 22 (IBM SPSS Statistics for Windows, Armonk, NY).
RESULTS
Hospital Characteristics and Patient Care Activities Over the Period 2004–2018
The number of hospital beds, patient-days, admissions, and clinical activities at high risk for candidemia from the 7 participating institutions over the 5-year time periods 2004–2008, 2009–2013, and 2014–2018 are summarized in Table 1. The number of beds, admissions, and patient-days increased significantly over the 15-year period, whereas the length of hospital stay decreased significantly (P < .0001). Mean annual hematopoietic stem cell and solid organ transplantations increased by 75% and 26%, respectively.
Table 1.
Hospital Activities in the Seven Participating Hospitals Over the Period 2004–2018
| Years | P Value (r) | |||
|---|---|---|---|---|
| 2004–2008 | 2009–2013 | 2014–2018 | ||
| Admissions | 203 939 (16 456) | 233 509 (5529) | 271 017 (9136) | P < .0001, r = 0.92 |
| Patient-days | 1 596 485 (49 017) | 1 768 829 (16 784) | 1 753 755 (40 072) | P = .002, r = 0.74 |
| Length of stay, days | 7.9 (0.4) | 7.6 (0.2) | 6.5 (0.1) | P < .0001, r = −0.87 |
| Beds | 5067 (192) | 5492 (110) | 5530 (396) | P = .029, r = 0.56 |
| Solid organ transplantations | 454 (20.3) | 494 (16.6) | 571 (39.2) | P < .0001, r = 0.86 |
| Hematopoietic stem cell transplantations | 409 (26.2) | 544 (41.3) | 719 (53.8) | P < .0001, r = 0.95 |
| Allogeneic | 135 (19.4) | 182 (18.3) | 247 (12.8) | |
| Autologous | 275 (15.7) | 362 (24.1) | 472 (42.7) |
Data are reported as mean annual numbers and standard deviation.
Trends of Bloodstream Infections
Data on the most frequent isolates in bloodstream infections (BSIs) were available for 5 hospitals. The top 10 ranking of BSIs remained stable over 15 years with the exception of a proportional increase of Enterococcus spp and a decrease of Streptococcus pneumoniae. Among the 10 most frequently reported bloodstream pathogens, Candida species accounted for 2.9% in 2004–2008, 3.6% in 2009–2013, and 2.1% in 2014–2018. In 2014–2018, Candida BSI ranked 10th (range, 9–11) (Supplemental Table 1).
Candida Species Incidence and Distribution
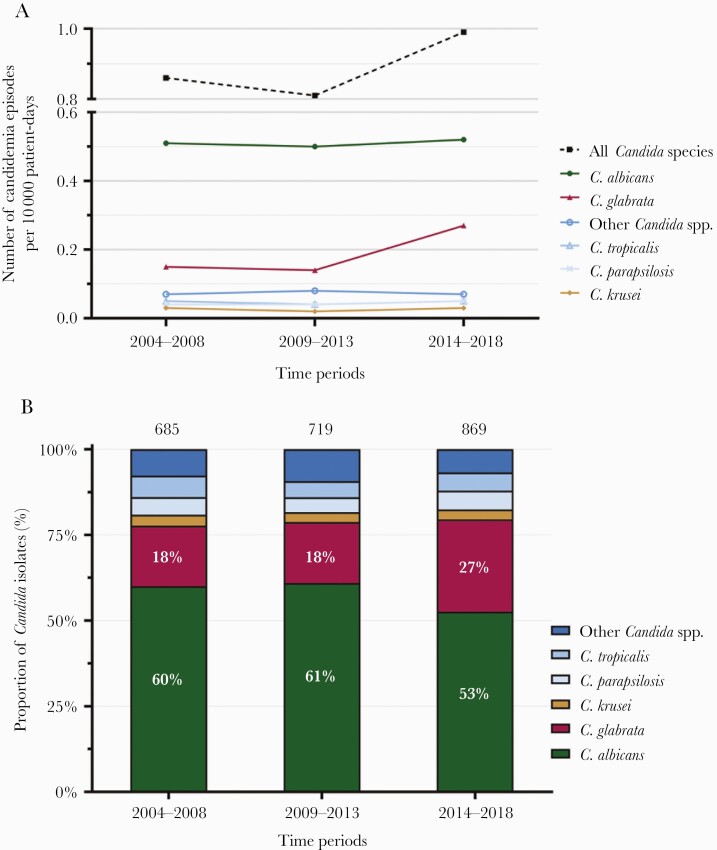
A total of 2273 Candida blood isolates were documented in the 7 centers over the period 2004–2018. A trend for increasing hospital-based incidence of candidemia was found in the last 5 years with 0.86 (standard deviation [SD] = 0.11) and 0.81 (SD = 0.12) per 10 000 patient-days in 2004–2008 and 2009–2013 and 0.99 (SD = 0.09) in 2014–2018 (P = .124) (Figure 1A). Consistently, population-based incidence of candidemia from the nation-wide retrospective ANRESIS surveillance increased significantly over the last 5 years from 2.96 (SD = 0.37) per 100 000 inhabitants in 2009–2013 to 4.20 (SD = 0.47) in 2014–2018 (P = .022).
Figure 1.
(A) Annual incidence of candidemia per 10 000 patient days in the 7 hospitals over the 3 time periods 2004–2008, 2009–2013, and 2014–2018. (B) Species distribution of Candida bloodstream isolates. Total number of Candida isolates per time period are indicated at the top.
Hospital-based incidence rates of candidemia per 10 000 patient-days remained stable for C albicans, Candida tropicalis, Candida parapsilosis, Candida krusei, and the other Candida species, whereas it increased significantly for C glabrata from 0.15 per 10 000 patient-days in 2004–2008 and 2009–2013 to 0.27 in 2013–2018 (P = .008) (Figure 1A).
Over the 15-year study period, C albicans was the most frequent species (58%), followed by C glabrata (21%), C tropicalis, and C parapsilosis (each 5%). Candida krusei remained rare with 3%. Eight percent of all isolates belonged to other Candida spp. No Candida auris was isolated during the study period. The proportion of C albicans decreased significantly from 60% in 2004–2008 to 53% in 2014–2018 (P = .0023). By contrast, the proportion of C glabrata increased from 18% to 27% (P < .0001) (Figure 1B). No change was observed in the proportions of other Candida species.
Candida Species Distribution According to Patients’ Age and Location in the Hospital
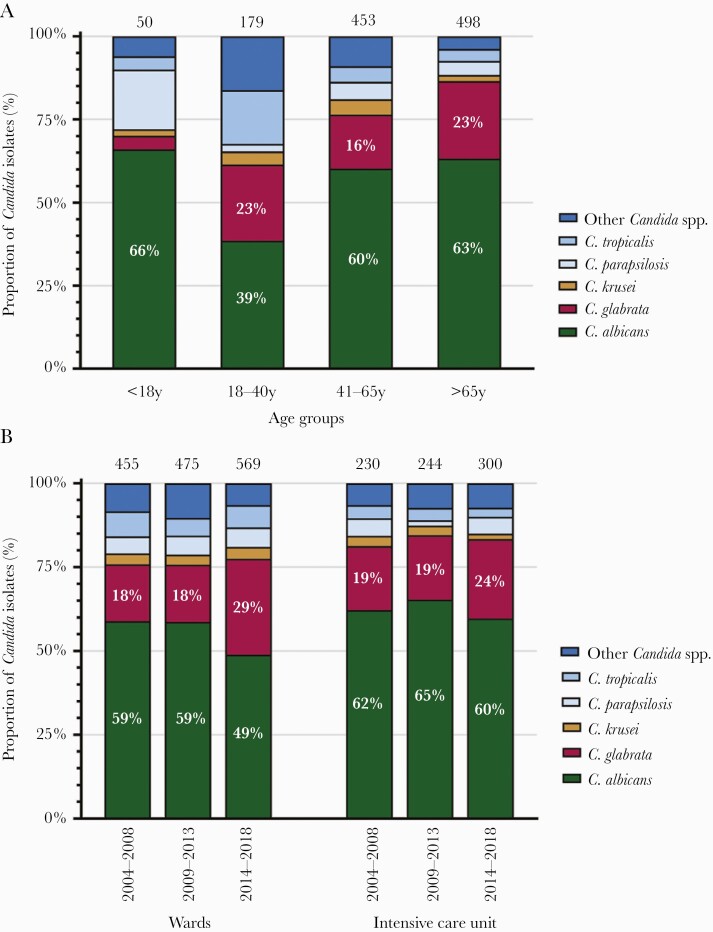
Data on Candida species distribution according to patients’ age were available from 4 hospitals, representing 52% of all candidemias. The highest proportion of C glabrata (23%) was found in the age group 18–40 and above 65 years (Figure 2A). Candida tropicalis and other Candida spp occurred proportionally more frequently in the age group 18–40 years. Although only a limited number of isolates of patients below 18 years have been investigated, a tendency to a higher proportion of C paraspilosis was observed in this age group.
Figure 2.
(A) Species distribution of Candida bloodstream isolates according to patients’ age over 2004–2018 (4 hospitals). Total number of Candida isolates per age group are indicated at the top. (B) Species distribution of Candida bloodstream isolates according to patients’ location in the hospital over 2004–2018 (7 hospitals). Total number of Candida isolates per time period and ward are indicated at the top. Other Candida spp included (total numbers): Candida dubliniensis (n = 65), C lusitaniae (n = 20), Candida pelliculosa (n = 13), Candida kefyr (n = 11), Candida guilliermondii (n = 8), C norvegensis (n = 8), Candida africana (n = 5), Candida orthopsilosis (n = 4), Candida nivariensis (n = 4), Candida fabianii (n = 3), Candida inconspicua (n = 2), Candida palmioleophila (n = 2), Candida rugosa (n = 1), Candida pulcherrima (n = 1).
Data on patients’ hospital location at the time of candidemia were available from all 7 hospitals. A higher proportional increase of C glabrata was recorded in wards, from 18% in 2004–2008 to 29% in 2014–2018 (P < .0001), than in intensive care units, from 19% to 24% (P = .22) (Figure 2B).
Antifungal Susceptibility Testing According to Clinical and Laboratory Standards Institute
Availability of susceptibility data varied during the study period. Over the whole study period, 88% (n = 1835) of Candida isolates (C albicans, C glabrata, C krusei, C parapsilosis, C tropicalis) were tested for fluconazole, 80% (n = 1681) were tested for voriconazole, 63% (n = 1319) were tested for posaconazole, 81% (n = 1697) were tested for amphotericin B, 75% (n = 1564) were tested for caspofungin, 30% (n = 626) were tested for anidulafungin, and 32% (n = 662) were tested for micafungin. Overall, 33% (n = 207) of all tested Candida isolates in 2014–2018 were classified as nonsusceptible (ie, susceptible-dose-dependent or resistant) to fluconazole, mainly due to the high proportion of C glabrata (Table 2). Fluconazole nonsusceptibility among C albicans remained at 1% over the 15-year period. In C parapsilosis, fluconazole nonsusceptibility fluctuated from 6% to 7% to 4% from 2004–2008 to 2009–2013 and 2014–2018, respectively. Micafungin and anidulafungin nonsusceptibility in C albicans and C glabrata remained below 2%.
Table 2.
Susceptibility of Candida Bloodstream Isolates to Antifungal Drugs According to CLSI Document M60-Ed2
| Species | S or WT* | I | SDD | R or Non-WT** | No. of Isolates Tested | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2004–2008 | 2009–2013 | 2014–2018 | 2004–2008 | 2009–2013 | 2014–2018 | 2004–2008 | 2009–2013 | 2014–2018 | 2004–2008 | 2009–2013 | 2014–2018 | 2004–2008 | 2009–2013 | 2014–2018 | |
| Candida albicans | |||||||||||||||
| Fluconazole | 99% | 99% | 99% | - | - | - | 0% | 0% | 1% | 1% | 1% | 0% | 411 | 426 | 330 |
| Voriconazole | 99% | 99% | 99% | 0% | 0% | 0% | - | - | - | 0% | 1% | 0% | 411 | 428 | 277 |
| Posaconazole | 99%* | 98%* | 96%* | - | - | - | - | - | - | 1%** | 2%** | 4%** | 227 | 426 | 214 |
| Caspofungin | 100% | 99% | 98% | 0% | 0% | 1% | - | - | - | 0% | 0% | 1% | 409 | 426 | 150 |
| Amphotericin B | 100%* | 100%* | 100%* | - | - | - | - | - | - | 0%** | 0%** | 0%** | 399 | 425 | 214 |
| Anidulafungin | -a | 99% | 99% | -a | 0% | 0% | - | - | - | -a | 1% | 1% | 0 | 141 | 212 |
| Micafungin | -a | 100% | 98% | -a | 0% | 0% | - | - | - | -a | 0% | 2% | 0 | 218 | 176 |
| Candida glabrata | |||||||||||||||
| Fluconazole | - | - | - | - | - | - | 90% | 92% | 91% | 10% | 8% | 9% | 117 | 124 | 176 |
| Voriconazole | 79% | 61% | 70.5%* | - | - | - | - | - | - | 21%** | 39%** | 29.5%** | 117 | 124 | 44 |
| Posaconazole | 92.5%* | 70%* | 86%* | - | - | - | - | - | - | 7.5%** | 30%** | 14%** | 67 | 124 | 43 |
| Caspofungin | 91% | 94% | 95% | 8% | 6% | 2% | - | - | - | 1% | 0% | 3% | 117 | 124 | 104 |
| Amphotericin B | 100%* | 100%* | 100%* | - | - | - | - | - | - | 0%** | 0%** | 0%** | 117 | 124 | 147 |
| Anidulafungin | -a | 100% | 98% | -a | 0% | 1% | - | - | - | -a | 0% | 1% | 0 | 40 | 124 |
| Micafungin | -a | 100% | 98% | -a | 0% | 2% | - | - | - | -a | 0% | 0% | 0 | 75 | 107 |
| Candida krusei | |||||||||||||||
| Fluconazole | - | - | - | - | - | - | - | - | - | - | - | - | |||
| Voriconazole | 81% | 100% | 91% | 14% | 0% | 9% | - | - | - | 5% | 0% | 0% | 21 | 20 | 11 |
| Posaconazole | 100%* | 100%* | 100%* | - | - | - | - | - | - | 0%** | 0%** | 0%** | 13 | 20 | 3 |
| Caspofungin | 38% | 85% | 78% | 43% | 15% | 22% | - | - | - | 19% | 0% | 0% | 21 | 20 | 9 |
| Amphotericin B | 100%* | 100%* | 100%* | - | - | - | - | - | - | 0%** | 0%** | 0%** | 21 | 20 | 16 |
| Anidulafungin | -a | 100% | 100% | -a | 0% | 0% | - | - | - | -a | 0% | 0% | 0 | 6 | 15 |
| Micafungin | -a | 100% | 100% | -a | 0% | 0% | - | - | - | -a | 0% | 0% | 0 | 12 | 4 |
| Candida parapsilosis | |||||||||||||||
| Fluconazole | 94% | 93% | 96% | - | - | - | 6% | 0% | 0% | 0% | 7% | 4% | 35 | 30 | 50 |
| Voriconazole | 100% | 100% | 95% | 0% | 0% | 2% | - | - | - | 0% | 0% | 2% | 35 | 30 | 44 |
| Posaconazole | 100%* | 97%* | 97.5%* | - | - | - | - | - | - | 0%** | 3%** | 2.5%** | 24 | 30 | 40 |
| Caspofungin | 100% | 100% | 100% | 0% | 0% | 0% | - | - | - | 0% | 0% | 0% | 35 | 30 | 19 |
| Amphotericin B | 100%* | 100%* | 97.5%* | - | - | - | - | - | - | 0%** | 0%** | 2.5%** | 35 | 30 | 40 |
| Anidulafungin | -a | 100% | 97% | -a | 0% | 3% | - | - | - | -a | 0% | 0% | 0 | 11 | 39 |
| Micafungin | -a | 100% | 97% | -a | 0% | 3% | - | - | - | -a | 0% | 0% | 0 | 14 | 34 |
| Candida tropicalis | |||||||||||||||
| Fluconazole | 90% | 82% | 80% | - | - | - | 5% | 6% | 9% | 5% | 12% | 11% | 42 | 33 | 45 |
| Voriconazole | 83% | 82% | 75% | 14% | 6% | 16% | - | - | - | 2% | 12% | 9% | 42 | 33 | 44 |
| Posaconazole | 75%* | 70%* | 74%* | - | - | - | - | - | - | 25%** | 30%** | 26%** | 20 | 33 | 35 |
| Caspofungin | 100% | 91% | 96% | 0% | 6% | 4% | - | - | - | 0% | 3% | 0% | 42 | 33 | 25 |
| Amphotericin B | 100%* | 100%* | 100%* | - | - | - | - | - | - | 0%** | 0%** | 0%** | 41 | 33 | 35 |
| Anidulafungin | -a | 100% | 97% | -a | 0% | 3% | - | - | - | -a | 0% | 0% | 0 | 6 | 32 |
| Micafungin | -a | 100% | 100% | -a | 0% | 0% | - | - | - | -a | 0% | 0% | 0 | 12 | 10 |
Abbreviations: CLSI, Clinical and Laboratory Standards Institute; I, intermediate; R, resistant; S, susceptible; SDD, susceptible, dose-dependent; WT, wild type.
Classification according to CLSI clinical breakpoints (CBP). In the absence of established CBP, CLSI epidemiological cutoff values (ECV) were used for discrimination between WT* and non-WT** isolates (CLSI document M59-Ed3).
aIsolates were not tested for the specific drug.
Antibiotic and Antifungal Drugs Consumption
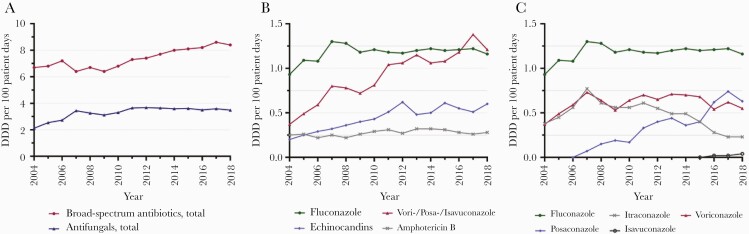
The consumption of broad-spectrum antibiotics with anti-Pseudomonas aeruginosa activity increased over the 15 years in the 7 hospitals (P < .0001). The overall antifungal drug consumption increased from 2.12 to 3.65 DDD per 100 patient-days from 2004 to 2011, and it remained stable afterwards (Figure 3A). Fluconazole use remained stable since 2007 (Figure 3B). Consumption of mold-active triazoles (voriconazole, posaconazole, isavuconazole) increased from 0.37 to 1.21 DDD per 100 patient-days during 2004–2018 (P < .0001) (Figure 3C). Voriconazole use increased from 2004 to 2007, remained stable until 2015, and decreased thereafter. Posaconazole consumption overtook voriconazole consumption in 2016 (Figure 3C). Echinocandin use increased from 0.2 to 0.6 DDD per 100 patient-days from 2004 to 2012 (P < .0001) and remained stable thereafter. Liposomal amphotericin B consumption increased (P < .0001), whereas amphotericin deoxycholate decreased (P < .0001) during 2004–2018 (data not shown).
Figure 3.
(A) Annual consumption of broad-spectrum antibiotics and antifungals in Defined Daily Doses (DDDs) per 100 patient days over 2004–2018. (B) Annual consumption of antifungals in DDDs per 100 patient days over 2004–2018. (C) Annual consumption of azoles in DDDs per 100 patient days over 2004–2018. Data of 7 hospitals are reported. Broad-spectrum antibiotics include the following: cefepime, piperacillin/tazobactam, imipenem, and meropenem. Antifungals include fluconazole, itraconazole, voriconazole, posaconazole, isavuconazole, amphotericin B deoxycholate, liposomal amphotericin B, caspofungin, and anidulafungin. The DDDs were defined as follows: cefepime 4 grams, piperacillin/tazobactam 14 grams, imipenem 2 grams, meropenem 3 grams; fluconazole 0.4 grams, itraconazole 0.2 grams, voriconazole 0.4 grams, posaconazole 0.3–0.6 grams, isavuconazole 0.2 grams, amphotericin B deoxycholate 0.07 grams, liposomal amphotericin B 0.21 grams, caspofungin 0.05 grams, and anidulafungin 0.2 grams.
DISCUSSION
This FUNGINOS study provides a longitudinal overview of the secular trends of candidemia associated with patient care activities. This survey, together with the previous 10-year FUNGINOS study, is representative for the entire country over a 30-year period and constitutes one of the largest contemporary worldwide series of candidemia. Our analysis revealed 3 major findings. The incidence of candidemia showed a significant increase. Mold-active triazole and echinocandin consumption increased significantly. A shift toward C glabrata was observed after 2013, representing 27% of all candidemias in 2014–2018.
The population-based candidemia incidence in our study is in line with reports from Sweden (4.7/100 000, 2016) and England (3.2/100 000, 2018), whereas it is higher than that reported in Australia (2.41/100 000, 2014–2015), and lower than in Denmark (8.38/100 000, 2013–2015), Spain (8.1/100 000, 2010–2011), and the United States (8.2/100 000, 2013–2017) [4–8, 11]. Multiple reasons may explain large differences in population-based candidemia incidences, which may be influenced by geographical and ecological factors, variability in monitoring and reporting systems, studied patient populations, infection prevention and control strategies, as well as clinical management and antimicrobial use practices [21].
International comparison of hospital-based incidences of candidemia is difficult, because they highly depend on the study population and time period studied, whereas changes over time within a single country, where reporting and monitoring systems remain stable, may be more accurate. In Switzerland, hospital-based incidence rates of candidemia have been studied by FUNGINOS using standardized questionnaires since 1991. Compared with our previous survey, in which incidence rates remained unchanged, we now observed an increase of candidemia from 0.49 episodes per 10 000 patient-days to 0.99 episodes over the last 3 decades [10].
Although the Candida species distribution was stable over 2 decades, we observed a significant decrease of the proportion of C albicans and an increase of C glabrata since 2014. This observation is consistent with data from the United States as well as from Australia, Denmark, and Canada from 2004 to 2015 [5, 11, 12, 14]. The recent emergence of C glabrata infections in Switzerland has occurred concomitantly with the increased use of mold-active triazoles in the last decade. The proportion of other non-albicans Candida species such as C parapsilosis and C tropicalis, which increased in southern Europe and South America, remained stable in Switzerland, ranging 4%–6% [4, 22].
Candida glabrata infections occurred predominantly in patients hospitalized on wards and in the age group 18–40 years and above 65 years. This may reflect the prolonged azole exposure for prophylaxis or therapy in patients with malignant hematological diseases or undergoing transplantation [23–26].
Overall, acquired antifungal resistance in Candida species remains rare in Switzerland. Candida auris BSI has not been detected in our country in spite of a look-back analysis to exclude misidentification [27]. Although few cases of acquired echinocandin resistance associated with FKS mutations have been reported in Switzerland since 2009, we do not have any indication of the emergence of FKS mutations based on the low echinocandin MICs observed in our survey [28]. Anidulafungin and micafungin MICs according to CLSI interpretative criteria or commercial Sensititre YeastOne echinocandins epidemiological cutoff values appear sensitive and specific for identifying FKS mutations among C albicans and glabrata isolates [29]. Although several countries have reported the emergence of azole resistance of C parapsilosis, we did not observe such a trend in our study [30–32].
Limitations of the present study include differences in clinical practices across the different centers, the lack of individual clinical data on comorbidities, the lack of clinical data to distinguish mixed infections from candidemias with a single species, previous antifungal drug exposure, and risk factors for candidemia or occurrence of infections due to non-albicans Candida species. Despite these limitations, this study, together with the previous FUNGINOS survey, provides one of the largest contemporary longitudinal overviews on candidemia from an entire country over a 30-year period. These data may add to contemporary initiatives such as ECMM CandiReg, which are essential for multinational surveillance of the epidemiology of Candida spp [33].
CONCLUSIONS
In conclusion, after 2 decades of stable epidemiology in Switzerland, we observed a recent increase of the incidence of candidemia with emergence of C glabrata, which now accounts for one fourth of bloodstream isolates. The increasing selection pressure mediated by a larger use of mold-active triazoles over the last decade most probably promoted this epidemiological shift. Continued epidemiological surveillance studies at the national level are key for monitoring the emergence of antifungal resistance and updating guidelines for antifungal therapy.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
The authors and the FUNGINOS Group warmly thank Isabel Cobos, Aurélie Guillet, Corine Guyaz, Monika Ochsner, and Annie Savoie of the Infectious Diseases Service, Department of Medicine, Lausanne University Hospital, for outstanding assistance in collecting and managing data from candidemic patients as well as Christian Durussel, Dominique Pilloud, Dr. Philippe Hauser, and colleagues for outstanding technical support in collecting Candida bloodstream isolates and performing species identification and antifungal susceptibility testing at the FUNGINOS reference mycology laboratory, Institute of Microbiology, Lausanne University Hospital.
Author contributions. O. M. and N. K. contributed to conception and design of the study. K.-M. A., N. K., and O. M. drafted the manuscript. K.-M. A., N. K., O. M., and F. L. contributed to data interpretation. M. O. and K.-M. A. contributed to statistical analysis and data management. All authors contributed to data collection. All authors revised the manuscript and approved the final version.
Disclaimer. None of the funding sources has been involved in study design and conduct, patient recruitment, data collection, analysis, and interpretation, writing of the manuscript, or decision to submit the article for publication.
Financial support. This work was funded by Schering-Plough, Gilead, Merck and Sharp and Dohme, Novartis, and Pfizer. The present project received unrestricted grant support from the Fondation pour le Progrès en Microbiologie Médicale et Maladies Infectieuses (FAMMID), Lausanne, Switzerland.
Potential conflicts of interest. R. Z., A. K., and C. Q. are members of the Swiss Antibiotic Resistance Surveillance System (ANRESIS). F. L. received research grants from Novartis, MSD, and Pfizer and speaker honoraria from Gilead. P. W. S. was supported by the academic career program “Filling the Gap” of the Medical Faculty of the University of Zurich. D. N. received research support from MSD and Pfizer and consulting fees from Roche Diagnostics, MSD, Pfizer, Basilea, and Gilead. O. M. was supported by the Leenaards Foundation and is a participant in the European Union’s Seventh Framework Program (FP7/2007-2013) under grant agreement number HEALTH-2010-260338 (ALLFUN). N. K. received research grants from MSD and Debiopharm, consulting fees from MSD, Pfizer, Basilea, and Gilead and speaker honoraria from Pfizer and MSD. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Appendix
Fungal Infection Network of Switzerland (FUNGINOS):
Investigators of the Candidemia Study
Clinical Investigators (Institutions and Persons in alphabetical order):
Thomas Bregenzer, Anna Conen, Kantonsspital, Aarau; Kai-Manuel Adam, Anna Conen, Ursula Flückiger, Nina Khanna, Christina Orasch, Michael Osthoff, University Hospital, Basel; Ulrich Heininger, Universitätskinderspital, Basel; Mario Franciolli, Ospedale San Giovanni, Ente Ospedaliero Cantonale, Bellinzona; Lauro Damonti, Stefan Zimmerli, University Hospital, Bern; Madeleine Rothen, Claudine Zellweger, Spitalzentrum, Biel; Madeleine Rothen, Philipp Tarr, Kantonsspital, Bruderholz; Felix Fleisch, Kantonsspital, Chur; Christian Chuard, Véronique Erard, Hôpital Cantonal, Fribourg; Stéphane Emonet, Jorge Garbino, Dionysios Neofytos, Christian van Delden, University Hospital, Geneva; Daniel Genne, Hôpital Communal, La-Chaux-de-Fonds; Pierre-Yves Bochud, Thierry Calandra, Lauro Damonti, Véronique Erard, Frédéric Lamoth, Oscar Marchetti, Christina Orasch, University Hospital, Lausanne; Jean-Philippe Chave, Clinique Bois-Cerf, Clinique Cécil, and Clinique La Source, Lausanne; Peter Graber, Kantonsspital, Liestal; Rita Monotti, Ospedale Regionale, Ente Ospedaliero Cantonale, Locarno; Enos Bernasconi, Ospedale Civico, Ente Ospedaliero Cantonale, Lugano; Marco Rossi, Kantonsspital, Luzern; Martin Krause, Kantonsspital, Münsterlingen; Rein-Jan Piso, Kantonsspital, Olten; Frank Bally, Nicolas Troillet, Institut Central des Hôpitaux Valaisans, Sion; Katia Boggian, Kantonsspital, Sankt Gallen; Gerhard Eich, Jacques Gubler, Kantonsspital, Winterthur; Jan Fehr, Alexander Imhof, Christian Ruef, Peter Werner Schreiber, University Hospital, Zürich; Gerhard Eich, Jacques Gubler, Stadtspital Triemli, Zürich; Christoph Berger, Universitätskinderspital, Zürich.
Microbiology Laboratory Investigators (Institutions and Persons in alphabetical order):
Hans Fankhauser, Ivo Heinzer, Kantonsspital, Aarau; Daniel Goldenberger, Reno Frei, University Hospital, Basel; Roland Hertel, Universitätskinderspital, Basel; Marisa Dolina, Orlando Petrini, Istituto Cantonale di Microbiologia, Bellinzona; Olivier Dubuis, Viollier Microbiology Laboratories, Bienne; Konrad Mühlethaler, University Hospital, Bern; Suzanne Graf, Kantonsspital, Bruderholz and Kantospital, Liestal; Martin Risch, Eva Ritzler, Kantonsspital, Chur; Dominique Fracheboud, Hôpital Cantonal, Fribourg; Arnaud Riat, Peter Rohner, Jacques Schrenzel, University Hospital, Geneva; Reto Lienhardt, Hôpital Communal, La-Chaux-de-Fonds; Jacques Bille, Frédéric Lamoth, University Hospital, Lausanne; Corinne Andreutti-Zaugg, Alberto Gallusser, Clinique La Source, Lausanne; Suzanne Graf, Kantonsspital, Liestal; Gaby Pfyffer, Kantonsspital, Luzern; Karin Herzog, Kantonsspital, Münsterlingen; Urs Schibli, Kantonsspital, Olten; Lysiane Tissière, Institut Central des Hôpitaux Valaisans, Sion; Thomas Bruderer, Detlev Schultze, Kantonsspital, Sankt Gallen; Reinhard Zbinden, University Hospital, Zürich.
Presented in part: European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), July 9–12, 2021, Vienna, Austria.
Contributor Information
Fungal Infection Network of Switzerland (FUNGINOS):
Thomas Bregenzer, Anna Conen, Kai-Manuel Adam, Anna Conen, Ursula Flückiger, Nina Khanna, Christina Orasch, Ulrich Heininger, Mario Franciolli, Ospedale San Giovanni, Lauro Damonti, Stefan Zimmerli, Madeleine Rothen, Claudine Zellweger, Madeleine Rothen, Philipp Tarr, Felix Fleisch, Christian Chuard, Véronique Erard, Stéphane Emonet, Jorge Garbino, Dionysios Neofytos, Christian van Delden, Daniel Genne, Pierre-Yves Bochud, Thierry Calandra, Lauro Damonti, Véronique Erard, Frédéric Lamoth, Oscar Marchetti, Christina Orasch, Jean-Philippe Chave, Clinique Bois-Cerf, Clinique Cécil, Clinique La Source, Peter Graber, Rita Monotti, Ospedale Regionale, Enos Bernasconi, Ospedale Civico, Marco Rossi, Martin Krause, Rein-Jan Piso, Frank Bally, Nicolas Troillet, Katia Boggian, Gerhard Eich, Jacques Gubler, Jan Fehr, Alexander Imhof, Christian Ruef, Peter Werner Schreiber, Gerhard Eich, Jacques Gubler, Christoph Berger, Hans Fankhauser, Ivo Heinzer, Daniel Goldenberger, Reno Frei, Roland Hertel, Marisa Dolina, Orlando Petrini, Olivier Dubuis, Konrad Mühlethaler, Suzanne Graf, Martin Risch, Eva Ritzler, Dominique Fracheboud, Arnaud Riat, Peter Rohner, Jacques Schrenzel, Reto Lienhardt, Jacques Bille, Frédéric Lamoth, Corinne Andreutti-Zaugg, Alberto Gallusser, Suzanne Graf, Gaby Pfyffer, Karin Herzog, Urs Schibli, Lysiane Tissière, Thomas Bruderer, and Reinhard Zbinden
References
- 1. Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol 2010; 36:1–53. [DOI] [PubMed] [Google Scholar]
- 2. Novosad SA, Fike L, Dudeck MA, et al. Pathogens causing central-line-associated bloodstream infections in acute-care hospitals-United States, 2011-2017. Infect Control Hosp Epidemiol 2020; 41:313–9. [DOI] [PubMed] [Google Scholar]
- 3. Koehler P, Stecher M, Cornely OA, et al. Morbidity and mortality of candidaemia in Europe: an epidemiologic meta-analysis. Clin Microbiol Infect 2019; 25:1200–12. [DOI] [PubMed] [Google Scholar]
- 4. Puig-Asensio M, Padilla B, Garnacho-Montero J, et al. ; CANDIPOP Project; GEIH-GEMICOMED (SEIMC); REIPI. Epidemiology and predictive factors for early and late mortality in Candida bloodstream infections: a population-based surveillance in Spain. Clin Microbiol Infect 2014; 20:O245–54. [DOI] [PubMed] [Google Scholar]
- 5. Astvad KMT, Johansen HK, Røder BL, et al. Update from a 12-year nationwide fungemia surveillance: increasing intrinsic and acquired resistance causes concern. J Clin Microbiol 2018; 56:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention. Invasive Candidiasis Statistics. Available at: https://www.cdc.gov/fungal/diseases/candidiasis/invasive/statistics.html. Accessed 1 February 2021.
- 7. Klingspor L, Ullberg M, Rydberg J, et al. Epidemiology of fungaemia in Sweden: a nationwide retrospective observational survey. Mycoses 2018; 61:777–85. [DOI] [PubMed] [Google Scholar]
- 8. Public Health England. Voluntary surveillance of candidaemia in England, Wales and Northern Ireland. Heal Prot Rep 2018; 13(25). [Google Scholar]
- 9. Rajendran R, Sherry L, Deshpande A, et al. A prospective surveillance study of Candidaemia: epidemiology, risk factors, antifungal treatment and outcome in hospitalized patients. Front Microbiol 2016; 7:915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marchetti O, Bille J, Fluckiger U, et al. ; Fungal Infection Network of Switzerland. Epidemiology of candidemia in Swiss tertiary care hospitals: secular trends, 1991-2000. Clin Infect Dis 2004; 38:311–20. [DOI] [PubMed] [Google Scholar]
- 11. Chapman B, Slavin M, Marriott D, et al. ; Australian and New Zealand Mycoses Interest Group. Changing epidemiology of candidaemia in Australia. J Antimicrob Chemother 2017; 72:1103–8. [DOI] [PubMed] [Google Scholar]
- 12. Pfaller MA, Diekema DJ, Turnidge JD, et al. Twenty years of the SENTRY antifungal surveillance program: results for Candida species from 1997-2016. Open Forum Infect Dis 2019; 6:79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coste AT, Kritikos A, Li J, et al. ; Fungal Infection Network of Switzerland (FUNGINOS). Emerging echinocandin-resistant Candida albicans and glabrata in Switzerland. Infection 2020; 48:761–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fuller J, Dingle TC, Bull A, et al. ; Canadian Antimicrobial Resistance Alliance (CARA) and CANWARD. Species distribution and antifungal susceptibility of invasive Candida isolates from Canadian hospitals: results of the CANWARD 2011-16 study. J Antimicrob Chemother 2019; 74:iv48–54. [DOI] [PubMed] [Google Scholar]
- 15. Shields RK, Nguyen MH, Press EG, et al. Rate of FKS mutations among consecutive Candida isolates causing bloodstream infection. Antimicrob Agents Chemother 2015; 59:7465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Orasch C, Marchetti O, Garbino J, et al. ; FUNGINOS. Candida species distribution and antifungal susceptibility testing according to European Committee on Antimicrobial Susceptibility Testing and new vs. old Clinical and Laboratory Standards Institute clinical breakpoints: a 6-year prospective candidaemia survey from the fungal infection network of Switzerland. Clin Microbiol Infect 2014; 20:698–705. [DOI] [PubMed] [Google Scholar]
- 17. ANRESIS. Swiss centre for antibiotic resistance. Available at: https://www.anresis.ch/. Accessed 1 February 2021.
- 18. Bundesamt für Statistik. Bevölkerung. Available at: https://www.bfs.admin.ch/bfs/de/home/statistiken/bevoelkerung.html. Accessed 1 February 2021.
- 19. Muñoz P, Vena A, Valerio M, et al. Risk factors for late recurrent candidaemia. A retrospective matched case-control study. Clin Microbiol Infect 2016; 22:277.e11–20. [DOI] [PubMed] [Google Scholar]
- 20. Bundesamt für Gesundheit. Zahlen zur Spende und Transplantation von Organen in der Schweiz. Available at: https://www.bag.admin.ch/bag/de/home/zahlen-und-statistiken/zahlen-fakten-zu-transplantationsmedizin.html. Accessed 1 February 2021.
- 21. Lamoth F, Lockhart SR, Berkow EL, Calandra T. Changes in the epidemiological landscape of invasive candidiasis. J Antimicrob Chemother 2018; 73:i4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nucci M, Queiroz-Telles F, Alvarado-Matute T, et al. ; Latin American Invasive Mycosis Network. Epidemiology of candidemia in Latin America: a laboratory-based survey. PLoS One 2013; 8:e59373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hachem R, Hanna H, Kontoyiannis D, et al. The changing epidemiology of invasive candidiasis: Candida glabrata and Candida krusei as the leading causes of candidemia in hematologic malignancy. Cancer 2008; 112:2493–9. [DOI] [PubMed] [Google Scholar]
- 24. Bodey GP, Mardani M, Hanna HA, et al. The epidemiology of Candida glabrata and Candida albicans fungemia in immunocompromised patients with cancer. Am J Med 2002; 112:380–5. [DOI] [PubMed] [Google Scholar]
- 25. Horn DL, Neofytos D, Anaissie EJ, et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis 2009; 48:1695–703. [DOI] [PubMed] [Google Scholar]
- 26. Orasch C, Mertz D, Garbino J, et al. ; Fungal Infection Network of Switzerland (FUNGINOS). Fluconazole non-susceptible breakthrough candidemia after prolonged low-dose prophylaxis: a prospective FUNGINOS study. J Infect 2018; 76:489–95. [DOI] [PubMed] [Google Scholar]
- 27. Riat A, Neofytos D, Coste A, et al. First case of Candida auris in Switzerland: discussion about preventive strategies. Swiss Med Wkly 2018; 148:w14622. [DOI] [PubMed] [Google Scholar]
- 28. Kritikos A, Neofytos D, Khanna N, et al. ; Fungal Infection Network of Switzerland (FUNGINOS). Accuracy of Sensititre YeastOne echinocandins epidemiological cut-off values for identification of FKS mutant Candida albicans and Candida glabrata: a ten year national survey of the Fungal Infection Network of Switzerland (FUNGINOS). Clin Microbiol Infect 2018; 24:1214.e1–4. [DOI] [PubMed] [Google Scholar]
- 29. Shields RK, Nguyen MH, Press EG, et al. Anidulafungin and micafungin MIC breakpoints are superior to that of caspofungin for identifying FKS mutant Candida glabrata strains and echinocandin resistance. Antimicrob Agents Chemother 2013; 57:6361–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Demirci-Duarte S, Arikan-Akdagli S, Gülmez D. Species distribution, azole resistance and related molecular mechanisms in invasive Candida parapsilosis complex isolates: increase in fluconazole resistance in 21 years. Mycoses 2021; 64:823–30. [DOI] [PubMed] [Google Scholar]
- 31. Mesini A, Mikulska M, Giacobbe DR, et al. Changing epidemiology of candidaemia: increase in fluconazole-resistant Candida parapsilosis. Mycoses 2020; 63:361–8. [DOI] [PubMed] [Google Scholar]
- 32. Siopi M, Tarpatzi A, Kalogeropoulou E, et al. Epidemiological trends of fungemia in Greece with a focus on candidemia during the recent financial crisis: a 10-year survey in a tertiary care academic hospital and review of literature. Antimicrob Agents Chemother 2020; 64(3):e01516–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koehler P, Arendrup MC, Arikan-Akdagli S, et al. ; European Confederation of Medical Mycology (ECMM). ECMM CandiReg-A ready to use platform for outbreaks and epidemiological studies. Mycoses 2019; 62:920–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.