Abstract
Background
RTS,S is the leading malaria vaccine candidate but only confers partial efficacy against malaria in children. RTS,S is based on the major Plasmodium falciparum sporozoite surface antigen, circumsporozoite protein (CSP). The induction of anti-CSP antibodies is important for protection; however, it is unclear how these protective antibodies function.
Methods
We quantified the induction of functional anti-CSP antibody responses in healthy malaria-naive adults (N = 45) vaccinated with RTS,S/AS01. This included the ability to mediate effector functions via the fragment crystallizable (Fc) region, such as interacting with human complement proteins and Fcγ-receptors (FcγRs) that are expressed on immune cells, which promote various immunological functions.
Results
Our major findings were (1) RTS,S-induced antibodies mediated Fc-dependent effector functions, (2) functional antibodies were generally highest after the second vaccine dose, (3) functional antibodies targeted multiple regions of CSP, (4) participants with higher levels of functional antibodies had a reduced probability of developing parasitemia following homologous challenge (P < .05), and (5) nonprotected subjects had higher levels of anti-CSP IgM.
Conclusions
Our data suggest a role for Fc-dependent antibody effector functions in RTS,S-induced immunity. Enhancing the induction of these functional activities may be a strategy to improve the protective efficacy of RTS,S or other malaria vaccines.
Clinical Trials Registration
Keywords: antibodies, circumsporozoite protein, complement, Fcγ-receptor, malaria, opsonic phagocytosis, Plasmodium falciparum, vaccines
The leading malaria vaccine, RTS,S, induced antibodies that mediate various Fc-dependent effector functions. High levels of antibodies with multiple functional activities were associated with protection against homologous malaria challenge. This provides valuable insights for improving RTS,S or future malaria vaccines.
After progress in reducing the burden of malaria in the past decade, the annual estimate of Plasmodium falciparum malaria cases has plateaued at approximately 200 million, which highlights the need for new antimalarial interventions such as an effective vaccine [1]. RTS,S is the leading malaria vaccine candidate, although efficacy against malaria is only approximately 30%–50% in young children and relatively short lived [2–5]. This is suboptimal compared to licensed vaccines against other pathogens [6, 7], and below the goal set by World Health Organization and funding partners to develop vaccines at least 75% efficacious against clinical malaria by 2030 [8]. Greater efficacy might be achieved by modification of RTS,S or developing new vaccines [9]. Considering that RTS,S has been subject to extensive clinical testing and can be safely coadministered with other vaccines as part of standard immunization programs [10–13], working towards improving RTS,S is an attractive option.
RTS,S targets sporozoites and therefore aims to prevent the initial asymptomatic stage of infection, and subsequent development of clinical illness and transmission to mosquitoes [14]. The vaccine is a virus-like particle expressing a fusion protein formed by the hepatitis B surface antigen and the major sporozoite surface antigen, circumsporozoite protein (CSP), administered with AS01 adjuvant [15]. The vaccine includes only the central repeat region (tandem repeat of NANP) and C-terminal region of CSP.
A tangible solution to improve vaccine efficacy is to improve the induction of functional immune responses that mediate protection, because RTS,S/AS01 already achieves very high IgG levels following a 3-dose vaccination schedule [2]. However, the mechanisms of immunity induced by RTS,S and other CSP-based vaccines are unclear, hindering the development of strategies to improve RTS,S or design next-generation vaccines. RTS,S primarily induces antibody and CD4+ T-cell responses [16]; a higher magnitude of antibodies to the central repeat region has been associated with protection in some trials [5, 17, 18] and higher-avidity IgG to the C-terminal region was associated with higher vaccine efficacy in the phase 3 trial [19]. However, antibody concentration is a broad measure that is not strongly indicative of functional activity.
Antibodies can act directly against the target cell or mediate effector functions via the fragment crystallizable (Fc) region. This includes interacting with serum complement protein, C1q, to activate the antibody-dependent classical complement pathway [20], and cross-linking Fcγ-receptors (FcγR) expressed by immune cells to mediate opsonic phagocytosis or direct killing via antibody-dependent cellular cytotoxicity [21]. These receptors include activating receptors FcγRIIa, which is widely expressed on monocytes, macrophages, and neutrophils, and FcγRIII, which is mostly expressed on neutrophils and natural killer (NK) cells [21]. Although prior studies have shown that antibodies to CSP and the central repeat region can directly inhibit hepatocyte invasion by sporozoites [22–24], there have been few studies of Fc-dependent antibody effector functions against sporozoites.
We previously showed that antibodies can recruit and activate serum complement proteins, which consequently inhibited sporozoite motility and led to cell death [25, 26]. Interestingly, children with high levels of naturally acquired complement-fixing antibodies had a significantly reduced risk of malaria [26]. Furthermore, complement-fixing antibodies were induced by RTS,S vaccination in young children in Mozambique [27]. Additionally, we identified specific roles for immune cells, particularly neutrophils, in mediating opsonic phagocytosis of P. falciparum sporozoites via interactions with FcγRIIa and FcγRIII (Feng et al, submitted). Prior studies reported that opsonic phagocytosis activity measured using the promonocytic cell line THP-1 was not a predictor of protection from experimental infection in malaria-naive volunteers; however, this cell line does not represent all FcγR-mediated functions, and the expression and functions of FcγRs vary across different cell types [21]. Conducting large-scale functional assays with viable P. falciparum sporozoites is not feasible, as they cannot be readily cultivated in standard laboratories or obtained in large numbers. To address these technical limitations, we developed high-throughput, cell-free assays that quantify the ability of anti-CSP antibodies to fix human C1q (and other downstream complement components) or interact directly with dimeric recombinant soluble FcγR complexes as a functional surrogate [26, 28, 29]. Furthermore, we used new approaches to measure opsonic phagocytosis activity with fluorescently labelled CSP-coated beads, and we have established that phagocytosis of CSP-coated beads correlates with phagocytosis of whole sporozoites (Feng et al, submitted).
Here we quantified anti-CSP antibodies that interact with C1q and FcγRs using samples from a phase 1/2a clinical trial RTS,S/AS01 conducted in healthy malaria-naive adults. We evaluated the relationship between functional antibodies and protection against controlled human malaria infection (CHMI) challenge.
METHODS
Detailed methods can be found in the Supplementary Material.
Study Participants and Ethics Approval
Healthy malaria-naive adults were administered 3 doses of RTS,S/AS01 at month 0, 1, and 2 (ClinicalTrials.gov registry number NCT00075049), and 2 weeks later underwent homologous CHMI challenge via infectious mosquito bites. Those who remained parasitemia free during the 28-day follow-up were considered protected, as previously described [30]. We tested serum samples collected at baseline on day 0 (D0, n = 45), and 2–4 weeks post vaccine dose (PV) 1 (n = 45), PV 2 (n = 43), and PV 3 (n = 33). We additionally conducted functional assays using 4 pools (each pool, n = 8) from individuals who were either not protected (NP1, NP2) or protected (P3, P4) against CHMI challenge.
All study participants had previously provided consent for future use of the samples for research, and ethics approval was also obtained by the Alfred Human Research Committee.
Antigens
The following antigens used in this study were based on P. falciparum 3D7: full-length CSP, peptides representing the central repeat (NANP) [26], and C-terminal (Pf16) [31] regions of CSP, and for some experiments a recombinant construct of the C-terminal region was used (CT) [27].
Experiments
Total immunoglobulin G (IgG) and immunoglobulin M (IgM) were measured by standard enzyme-linked immunosorbent assay (ELISA), and functional complement-fixation and FcγR-binding assays were conducted as previously described [26, 28, 32] and the optical density (OD) values were reported. Opsonic phagocytosis of CSP-coated beads by isolated neutrophils was conducted as previously described (Feng et al, submitted), and the percentage of cells containing fluorescent-positive beads was evaluated by flow cytometry and presented as phagocytosis index (PI).
Statistical Analysis
For descriptive analysis, the median and interquartile range (IQR) of OD values were shown, and the following tests were performed using GraphPad Prism 7 where appropriate: Wilcoxon matched pairs signed rank test, Mann-Whitney U test, Spearman correlation coefficient (ρ), and Gehan-Breslow-Wilcoxon test.
RESULTS
Induction of IgG and IgM in Malaria-Naive Adults by the RTS,S Vaccine
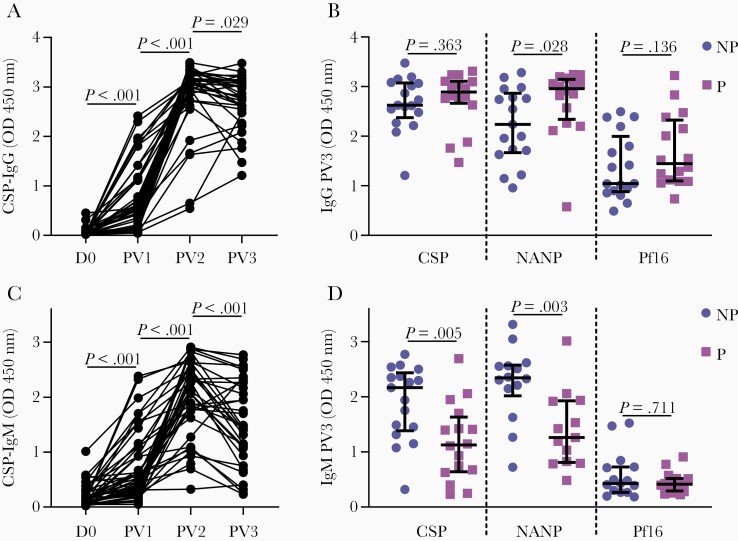
Healthy malaria-naive adults (N = 45) were vaccinated with RTS,S and serum collected at baseline (day 0, D0) and after each vaccine dose (post vaccine dose, PV) were tested for antibody reactivity to full-length CSP. Anti-CSP IgG significantly increased after the first vaccine dose and increased further after the second (OD median [IQR]: D0 = 0.034 [0.023, 0.070]; PV1 = 0.520 [0.155, 1.140]; PV2 = 3.102 [2.873, 3.250]; P < .001 for both tests). There was some decline in reactivity by the third dose compared to the second (OD median [IQR]: PV3 = 2.792 [2.513, 3.092]; P = .029) (Figure 1A). To identify which regions of CSP were targeted by antibodies, we tested serum collected at the final time point for IgG against 2 peptides representative of the central repeat and C-terminal regions of CSP (NANP and Pf16, respectively). We compared IgG responses from the day of challenge among those who were not protected (n = 17) and protected (n = 16) against developing blood-stage parasitemia. IgG reactivity to full-length CSP and Pf16 were not significantly different between groups, but anti-NANP IgG was significantly higher among protected individuals (P = .363, P = .136, and P = .028, respectively) (Figure 1B).
Figure 1.
RTS,S-induced IgG and IgM target the central and C-terminal regions of CSP. Serum from adults vaccinated with RTS,S collected at baseline (D0, n = 45), and PV1 (n = 45), PV2 (n = 43), and PV3 (n = 33) were tested for IgG (A) and IgM (C) to full-length CSP, and those collected PV3 (B, D) were also tested against peptides representing the central repeat and C-terminal regions of CSP (NANP and Pf16, respectively). Samples were tested in duplicate and the mean value was graphed, along with the group median and IQR for participants who were not protected (NP, n = 17) and protected (P, n = 16) against controlled human malaria infection challenge. Reactivity between paired samples collected at different time points was compared using the Wilcoxon matched pairs signed rank test, and reactivity between unpaired NP and P groups was compared using the Mann-Whitney U test. Abbreviations: CSP, circumsporozoite protein; IgG, immunoglobulin G; IgM, immunoglobulin M; IQR, interquartile range; OD, optical density; PV1, post vaccine dose 1; PV2, post vaccine dose 2; PV3, post vaccine dose 3.
Next, we examined the induction of anti-CSP IgM, which followed a similar pattern to the IgG response in that antibody levels peaked at PV2, and declined slightly by PV3 (OD median [IQR]: D0 = 1.123 [0.081, 0.268], PV1 = 0.514 [0.297, 1.007], PV2 = 2.137 [1.749, 2.625], PV3 = 1.454 [1.005, 2.281]) (Figure 1C). IgM to CSP and NANP were significantly lower for protected volunteers than not-protected volunteers, whereas anti-Pf16 IgM did not statistically differ between groups (P = .005, P = .003, and P = .711, respectively) (Figure 1D).
Induction of Functional Antibody Responses
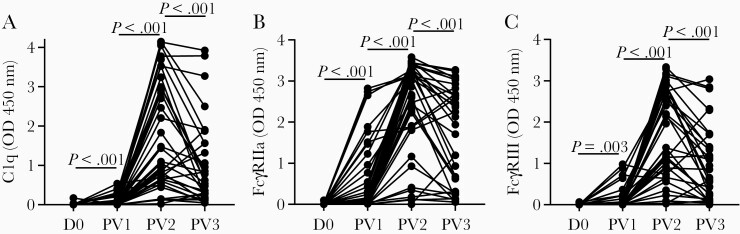
We have previously shown that anti-CSP antibodies induced by natural malaria exposure or whole sporozoite immunization can fix and activate complement, and we also identified antibody-complement interactions as a functional immune mechanism against P. falciparum sporozoites [25, 26]. Here we measured the ability of RTS,S-induced antibodies to fix C1q (the first step in classical complement activation) to full-length CSP. After 2 RTS,S vaccinations, strong levels of C1q-fixing antibodies were detected, although responses varied among participants (OD median [IQR]: D0 = 0 [0, 0], PV1 = 0.010 [0, 0.072], PV2 = 1.095 [0.649, 2.991]; P < .001) (Figure 2A). C1q-fixation responses were significantly lower after the third dose (OD median [IQR]: PV3 = 0.622 [0.233, 1.560]; P < .001) but remained substantially higher compared to baseline.
Figure 2.
Induction of functional anti-CSP antibodies following RTS,S vaccination. Serum from adults vaccinated with RTS,S collected at baseline (D0, n = 45) and PV1 (n = 45), PV2 (n = 43), and PV3 (n = 33) were tested for C1q-fixation (A), FcγRIIa (B), and FcγRIII (C) binding to full-length CSP. Samples were tested in duplicate and the mean value was graphed. Reactivity between paired samples collected at different time points was compared using the Wilcoxon matched pairs signed rank test. Abbreviations: CSP, circumsporozoite protein; OD, optical density; PV1, post vaccine dose 1; PV2, post vaccine dose 2; PV3, post vaccine dose 3.
Antibodies can also promote opsonic phagocytosis of P. falciparum sporozoites by neutrophils and monocytes, and we recently established that neutrophils play a more prominent role and involve antibody engagement of FcγRIIa and FcγRIII (Feng et al, submitted). We measured whether RTS,S-induced antibodies could promote immune complex formation to full-length CSP, and interact with dimeric recombinant soluble FcγRs [28]. After the second RTS,S vaccination, there was a strong induction of antibodies that interacted with both FcγRs. Activity was highest after 2 vaccinations and declined after the third vaccination, but remained above baseline levels (OD median [IQR]: FcγRIIa, D0 = 0 [0, 0], PV1 = 0.049 [0.003, 0.487], PV2 = 2.859 [1.877, 3.323], PV3 = 2.127 [0.469, 2.753]; FcγRIII, D0 = 0.005 [0, 0.014], PV1 = 0.013 [0.001, 0.059], PV2 = 1.378 [0.728, 2.735], PV3 = 0.655 [0.118, 1.549]; P < .003 for all tests) (Figure 2B and 2C).
Antibody Properties Associated With Functional Activity
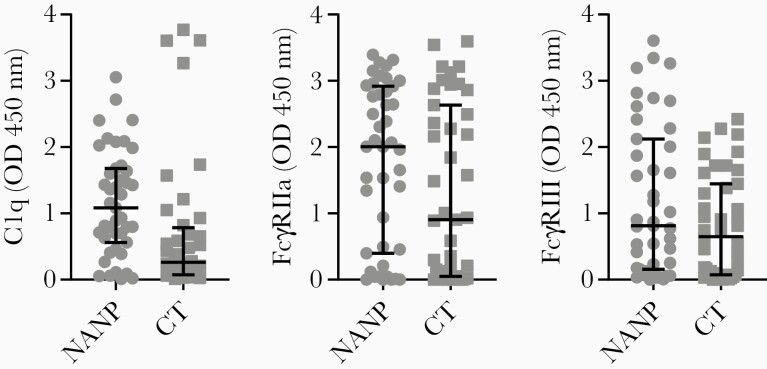
We explored the associations between antibody properties and functional activity using data obtained from samples collected after the third RTS,S dose. C1q-fixation and FcγR-binding significantly positively correlated with IgG reactivity to full-length CSP, and these associations were slightly stronger when analyses were performed with IgG responses to specific regions of CSP (Table 1). To directly confirm that the central repeat and C-terminal regions of CSP were targets of functional antibody responses, we tested samples for activity against NANP-repeat peptide and a recombinant construct representing the whole C-terminal region of CSP (CT) instead of the Pf16 peptide; note that IgG to Pf16 and CT strongly correlated (data not shown, ρ = 0.850; P < .001). For these analyses we used samples collected at PV2 due to the limited sample volumes available at the PV3 time point. Our results demonstrated that antibodies specific to both regions of CSP could interact with C1q and FcγRs (Figure 3).
Table 1.
Correlations Between Antibody Responses in Samples Collected Post Vaccine Dose 3 (n = 33)
| IgG | IgM | Function (CSP) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CSP | NANP | Pf16 | CSP | NANP | Pf16 | C1q | FcγRIIa | FcγRIII | ||
| IgG | CSP | 0.647 | 0.668 | −0.037 | 0.053 | 0.151 | 0.679 | 0.718 | 0.777 | |
| NANP | 0.505 | −0.149 | −0.206 | −0.001 | 0.763 | 0.810 | 0.792 | |||
| Pf16 | 0.096 | 0.165 | 0.410 | 0.719 | 0.752 | 0.813 | ||||
| IgM | CSP | 0.928 | 0.456 | 0.162 | 0.016 | 0.023 | ||||
| NANP | 0.475 | 0.222 | 0.114 | 0.076 | ||||||
| Pf16 | 0.204 | 0.254 | 0.192 | |||||||
| Function (CSP) | C1q | 0.893 | 0.913 | |||||||
| FcγRIIa | 0.940 | |||||||||
| FcγRIII |
Spearman correlation coefficient; significant correlations (P < .05) are in bold.
Abbreviations: CSP, circumsporozoite protein; IgG, immunoglobulin G; IgM, immunoglobulin M; NANP, CSP central repeat region; Pf16, CSP C-terminal region.
Figure 3.
Functional antibody responses to the central repeat and C-terminal regions of CSP. Serum from adults vaccinated with RTS,S collected post vaccine dose 2 (n = 43) were tested for C1q-fixation and FcγRIIa and FcγRIII-binding to antigens representing the central repeat and C-terminal regions of CSP (NANP and CT, respectively). Samples were tested in duplicate and the mean value was graphed, with the group median and IQR. Abbreviations: CSP, circumsporozoite protein; IQR, interquartile range; OD, optical density.
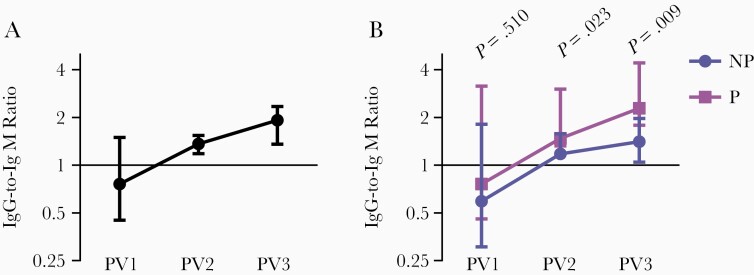
In contrast, there were no significant correlations between antibody function and IgM (Table 1). IgM does not engage FcγR; however, IgM has the potential to strongly activate complement [33], so the lack of association with C1q-fixation was unexpected. It is also noteworthy that IgM did not significantly correlate with IgG. To explore further, we quantified the relative ratio of IgG to IgM and found that while IgM responses were prominent after the first vaccine dose, antibody responses increasingly became IgG-skewed after the second and third vaccinations (Figure 4A). All participants had a similar ratio of IgG to IgM after the first vaccine dose, but after the second and third dose those who were protected against CHMI had a higher ratio of IgG to IgM than not-protected volunteers (Figure 4B).
Figure 4.
Ratio of IgG to IgM after each RTS,S dose. Serum from adults vaccinated with RTS,S collected PV1 (n = 45), PV2 (n = 43), and PV3 (n = 33) were tested for IgG and IgM to full-length CSP. The ratio of IgG to IgM was calculated at each time point, and the group median and 95% CI of the median were graphed. A, All participants and (B) those who were not protected (NP, n = 17) and protected (P, n = 16) against controlled human malaria infection are shown. The ratio of IgG to IgM (for NP and P groups) at all time points was compared using Mann-Whitney U test. Abbreviations: CI, confidence interval; CSP, circumsporozoite protein; IgG, immunoglobulin G; IgM, immunoglobulin M; PV1, post vaccine dose 1; PV2, post vaccine dose 2; PV3, post vaccine dose 3.
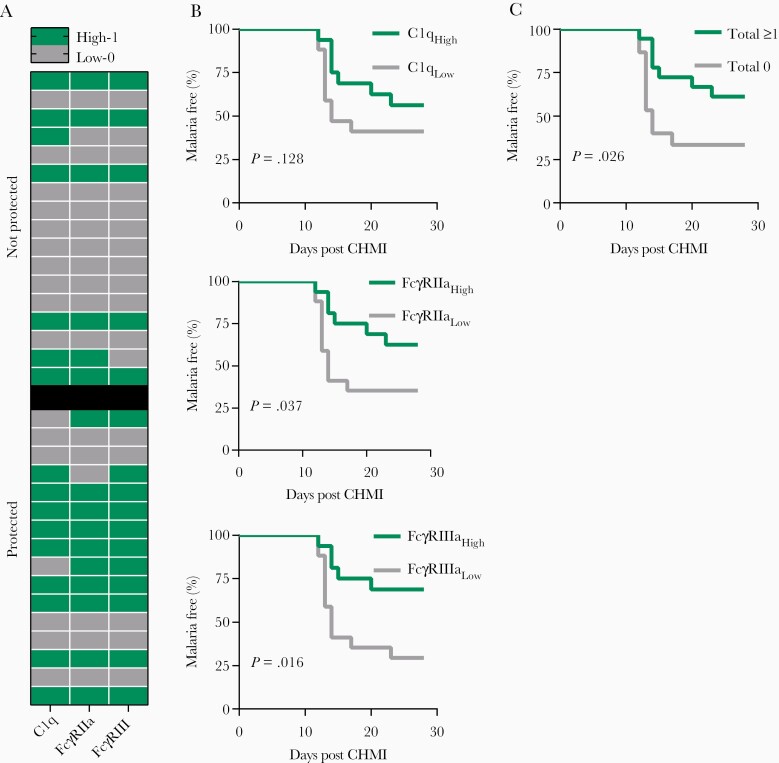
Associations Between Functional Antibodies and Protection Against CHMI
Participants were defined as having high (function score 1) or low (function score 0) activity for each functional antibody type (measured at PV3) based on whether they were above or below the median (median OD values for each response type were: C1q = 0.621, FcγRIIa = 2.127, and FcγRIII = 0.655) (Figure 5A). Participants with high activity for C1q-fixation, or binding to FcγRIIa or FcγRIII, were more likely to remain malaria free during the 28-day follow-up than those with low activity (Figure 5B). Furthermore, when we examined all 3 responses together, participants with high activity for 1 or more functional antibody types had a significantly delayed onset of parasitemia compared to those with overall low activities (total function score of ≥ 1 versus 0; P = .026) (Figure 5C).
Figure 5.
Relationship between functional antibodies and protection against CHMI. Serum from adults vaccinated with RTS,S collected post vaccine dose 3 (n = 33) were tested for C1q-fixation and FcγRIIa and FcγRIII-binding to full-length CSP. Participants were defined as having high or low activity (function scores of 1 and 0, respectively) for each functional antibody response type based on the median OD values: C1q = 0.621, FcγRIIa = 2.127, and FcγRIII = 0.655. A, Heat-map of participants with high and low antibody activity who were protected or not protected against CHMI challenge (rows represent the individual subject). B, Kaplan-Meier survival curves of individuals with high and low activity for each functional antibody response type and malaria status during 28 days follow-up, and (C) of individuals with high activity for at least 1 functional antibody response type or low for all functional responses (total function scores of 0 and ≥ 1, respectively). Kaplan-Meier survival curves were analyzed using the Gehan-Breslow-Wilcoxon test. Abbreviations: CHMI, controlled human malaria infection; CSP, circumsporozoite protein; OD, optical density.
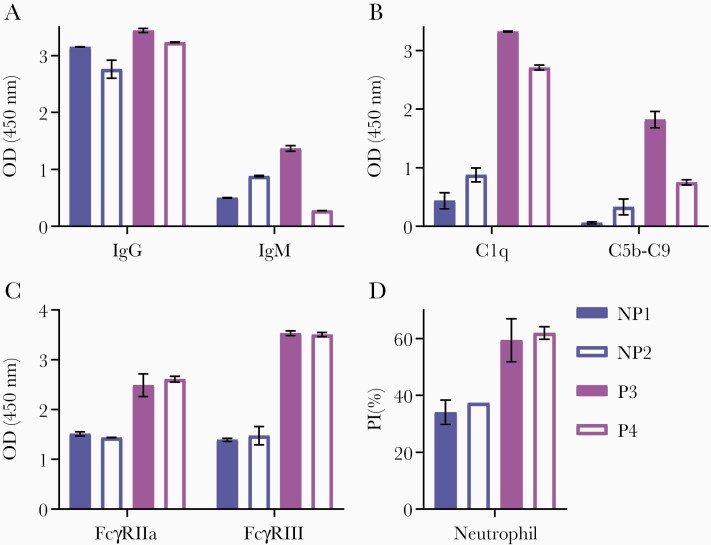
To confirm that C1q-fixation and FcγR-binding assays were indicative of complement activation and opsonic phagocytosis, respectively, we further examined the functional activities of pooled serum samples from individuals vaccinated with RTS,S (each pool, n = 8). Two pools contained individuals who were not protected against CHMI (NP1, NP2) and 2 pools contained individuals who were protected against CHMI (P3, P4). Notably IgG reactivity to full-length CSP was similar among the 4 pools, whereas IgM reactivity was variable (Figure 6A).
Figure 6.
Pooled serum activates complement and promotes opsonic phagocytosis. Pooled samples from individuals vaccinated with RTS,S (each pool, n = 8) who were not protected (NP1, NP2) or protected (P3, P4) against controlled human malaria infection challenge were tested for IgG and IgM reactivity to full-length CSP (A), C1q and C5b-C9-fixation to CSP (B), FcγRIIa and FcγRIII-binding to CSP (C), and opsonic phagocytosis of CSP-coated beads by freshly isolated neutrophils (D). Pools were tested in duplicate, and the mean and range of duplicates were graphed. Abbreviations: CSP, circumsporozoite protein; IgG, immunoglobulin G; IgM, immunoglobulin M; OD, optical density; PI, phagocytosis index.
We tested each pool for the ability to fix C1q and determined whether this led to recruitment and activation of downstream complement proteins by measuring the fixation of C5b-C9, which is involved in the terminal phase of complement and forms the membrane attack complex. Indeed, pooled samples could fix C1q and C5b-C9, and responses were substantially higher among protected pools compared to the not-protected pools (C5b-C9-fixation, OD mean [range]: P3 = 1.822 [1.683, 1.962], P4 = 0.749 [0.703, 0.795], NP1 = 0.059 [0.041, 0.077], and NP2 = 0.331 [0.197, 0.465]) (Figure 6B).
The pools were also used to confirm that RTS,S-induced antibodies could bind FcγRs and promote opsonic phagocytosis (Figure 6C and 6D). We focused on neutrophil phagocytosis as our recent studies established that neutrophils are the major cell type in blood that is active in phagocytosis of sporozoites (Feng et al, submitted). The pools were used to opsonize CSP-coated beads for phagocytic uptake by freshly isolated human neutrophils, and functional activity was greater for the protected pools than the not-protected pools (PI mean [range]: P3 = 9.35 [51.8, 66.9], P4 = 61.9 [59.7, 64.1], NP1 = 34.05 [29.8, 38.3], and NP2 = 37.3 [37.3, 37.3], respectively). Binding to FcγRIIa and FcγRIII was greater for protected pools compared to not-protected pools, which was similarly observed for C1q-fixation.
DISCUSSION
Knowledge of immune mechanisms induced by RTS,S that confer protection is limited, which hinders our capacity to enhance RTS,S or develop new malaria vaccines. Here we evaluated Fc-mediated functional antibody responses induced by RTS,S vaccination in healthy malaria-naive adults in a phase 1/2a clinical trial. Our major findings were (1) RTS,S-induced antibodies could fix and activate complement, bind to FcγRIIa and FcγRIII, and mediate opsonic phagocytosis by neutrophils; (2) functional antibodies were higher after the second vaccine dose rather than the third dose; (3) functional antibodies targeted both central repeat and C-terminal regions of CSP and correlated with IgG reactivity but not IgM; (4) participants with higher levels of functional antibodies had a reduced probability of developing parasitemia following homologous CHMI challenge (P < .05); and (5) nonprotected subjects had higher levels of anti-CSP IgM and a higher IgM to IgG ratio. These data give important insights into the mechanisms of RTS,S-induced immunity to inform future malaria vaccine development and bring us closer to identifying functional immune correlates of protection.
Antibodies to CSP induced by RTS,S were able to fix human complement proteins, including the initiation protein C1q and downstream proteins C5b-C9. We previously established antibody-complement interactions as an immune mechanism against P. falciparum sporozoites [25, 26], and demonstrated that complement-fixing antibodies could be induced by RTS,S vaccination in young children in Mozambique [27]. However, in that trial, RTS,S was formulated with the previously used AS02 adjuvant. Therefore, our current study specifically demonstrates that RTS,S/AS01 can also induce this response in malaria-naive adults. We additionally show that RTS,S-induced antibodies can mediate opsonic phagocytosis by neutrophils. While phagocytosis has been previously reported using the THP-1 promonocyte cell line [34, 35], an advantage of our study is that we used freshly isolated neutrophils, which are the most abundant leukocyte in the blood and account for the majority of phagocytosis activity in assays using whole blood (Feng et al, submitted). Furthermore, vaccine-induced antibodies could directly bind to FcγRIIa and FcγRIII, which are expressed on neutrophils, monocytes, NK cells, and other cells. This has not been previously examined in RTS,S studies, but these interactions play important roles in immunity to other pathogens and appear to be important in immunity in the partially successful RV144 vaccine trial for HIV [29, 36].
Strong levels of functional antibodies were induced after 2 vaccinations but activity then declined after the third vaccination, and this trend was also observed for total IgG and IgM. Considering that peak antibody responses were induced after the second vaccine dose, there may have been no additional benefit to receiving the third vaccination. Indeed other trials in malaria-naive adults report similar immunogenicity and efficacy (approximately 50%) when immunized with 2 or 3 RTS,S doses [37, 38]. This contrasts with studies of malaria-exposed children, whereby a 3-dose schedule gave higher IgG responses compared to a 2-dose schedule [39]. Furthermore, recent evidence suggests that a delayed fractional (reduced antigen amount) third dose improves efficacy compared to the standard 3-full–dose regimen [40], but this is yet to be confirmed in field evaluations [41]. There may be important differences in immunogenicity and efficacy following vaccination with 2- or 3-dose regimens of RTS,S among children and adults.
A key finding was that participants who acquired high levels of anti-CSP antibodies that fixed C1q or bound FcγRs had a reduced probability of malaria following CHMI. Importantly, participants with high activity for at least 1 functional antibody response type had a significantly delayed onset of parasitemia than those with overall low functional activities. Furthermore, complement and opsonic phagocytosis functional activities were substantially higher for the protected serum pools compared to the not-protected pools. These findings suggest that increasing the induction of these functional antibodies by RTS,S might be an approach to achieve higher vaccine efficacy. Because this was a phase 1/2a challenge trial the number of study subjects was relatively small, which limits the statistical power to further investigate associations with protection. Larger studies are needed to investigate antibody functional activities and target specificity in detail. An additional consideration is that participants underwent homologous CHMI challenge, and others have shown that vaccine efficacy is greater against vaccine-like strains compared to vaccine-dissimilar strains in field trials [42]. Additional studies, particularly field-based trials in target populations, are needed to confirm protective associations identified in this study, and to define correlates of protection from infection and clinical malaria.
The relationship we observed between higher functional antibody responses and protection is in some agreement with other published studies. Indeed, high levels of naturally acquired anti-CSP antibodies that fix complement have been associated with a reduced risk of malaria [26]. Opsonic phagocytosis by THP-1 cells has not been consistently associated with protection in phase 1/2a CHMI trials [34, 35]. Our recent studies demonstrated that sporozoites are mostly phagocytosed by neutrophils in whole-blood assays, and there are important differences in FcγR expression and function between THP-1 cells and neutrophils (Feng et al, submitted). In the current study, when using freshly isolated human neutrophils we found a clear differentiation between protected and not-protected pools. Another novel aspect is that we measured binding to FcγRIII, which is typically poorly expressed on THP-1 cells and resting monocytes [43] but is expressed on resting neutrophils and NK cells [21], and FcγRIII-binding strongly correlated with NK-cell activation against nonmalaria pathogens in vitro [44, 45]. Interestingly, there was a more pronounced association between protection and binding activity to FcγRIII than FcγRIIa. Kupffer cells, resident liver macrophages, have also been shown to express FcγRIII and FcγRIIa [46, 47], and may clear opsonized sporozoites [48]. It is noteworthy that FcγRIII exists as 2 forms; FcγRIIIa has a transmembrane domain whereas FcγRIIIb has a glycosylphosphatidylinositol anchor. FcγRIIIa is expressed on NK cells, neutrophils, and macrophages, whereas FcγRIIIb is expressed on neutrophils. The dimeric FcγRIII-binding assay used was a measure of pan FcγRIII-binding activity [21].
Fc-dependent responses to full-length CSP correlated strongly with IgG to the central repeat and C-terminal regions of the protein, and we directly showed that antibodies to both regions of CSP could bind FcγRIIa and FcγRIII. The relative contribution of antibodies to each epitope in relation to functional activity should be further investigated, particularly because antibody levels to the central repeat region were generally higher in protected volunteers in other RTS,S vaccine trials [5, 17, 18]. Generally, field evaluations only measure antibodies to the R32LR peptide representing the central repeat region and overlook the potential role of C-terminal antibodies [49]. However, a recent study was the first to report that RTS,S-induced antibodies to both regions of CSP of specific IgG subclasses were associated with protection against malaria in children [50]. We also measured the induction of anti-CSP IgM, which had no correlation with total IgG responses. Interestingly, protected volunteers had significantly less IgM than not-protected volunteers, which was similarly observed in another RTS,S vaccine study of malaria-exposed children [50], and tended to have higher IgG to IgM ratios compared to not-protected volunteers. While IgM does not interact with FcγR it can potently activate complement; however, IgM responses had no significant correlation with any functional antibody responses. It may be that the higher IgM levels inhibit FcγR functions involved in protection, or IgM might be reflective of other differences in immune function between groups. This observation warrants investigation in future studies.
In summary, we demonstrated the induction of antibodies that interact with complement and FcγRs following RTS,S vaccination in healthy malaria-naive adults. Participants who acquired greater levels of these antibodies generally had a reduced probability of infection or delayed onset of parasitemia following CHMI challenge. These data provide promising evidence that Fc-dependent effector functions may play a role in vaccine-induced immunity, and encourage further investigation in larger RTS,S trials, particularly in malaria-exposed cohorts. This study brings us closer to identifying immune correlates of protection, which will be crucial for developing highly efficacious vaccines. Such knowledge may shed light on why RTS,S is only partially efficacious, provide insight into how RTS,S could be modified to enhance efficacy in target populations, and aid the development of highly efficacious malaria vaccines.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgements. We thank all study participants and staff at Walter Reed Army Institute of Research (WRAIR) who were involved in the clinical trial, and Elizabeth Duncan for administrative support.
Disclaimer . The Mal-027 clinical trial (NCT00075049) was sponsored by the US Army Medical Research and Development Command in collaboration with WRAIR and GlaxoSmithKline Biologicals SA (GSK). RTS,S is a malaria vaccine candidate developed by GSK and GSK was provided the opportunity to review a preliminary version of this manuscript for factual accuracy, but the authors are solely responsible for final content and interpretation.
Financial support. This work was funded by the National Health and Medical Research Council (NHMRC) of Australia (grant to J. G. B., P. M. H., and B. D. W.; fellowships to J. G. B. and F. J. I. F.); National Institutes of Health, the Military Infectious Disease Research Program (to T. A. and E. S. B. L.); Australian Research Training Program Scholarship to L. K.; Monash Postgraduate Publication Award to L. K.; and the Australian Society for Parasitology Network Researcher Exchange, Training and Travel award to L. K. The Burnet Institute is supported by a Victorian State Government Operational Infrastructure Support grant and NHMRC Independent Research Institutes Infrastructure Support Scheme. J. G. B., F. J. I. F., L. K., M. J. B., J. A. C., and G. F. are supported by the Centre for Research Excellence in Malaria Elimination, funded by NHMRC, Australia.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Malaria Gordon Research Conference, 30 June–5 July 2019, Les Diablerets, Switzerland; Australian Society for Parasitology Annual Conference, 8–11 July 2019, Adelaide, Australia; and Malaria in Melbourne Conference, 28–29 October 2019, Melbourne, Australia.
References
- 1. World Health Organization. World malaria report 2018. Geneva, Switzerland: WHO, 2018. [Google Scholar]
- 2. RTS,S Clinical Trials Partnership. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med 2011; 365:1863–75. [DOI] [PubMed] [Google Scholar]
- 3. Olotu A, Fegan G, Wambua J, et al. Seven-year efficacy of RTS,S/AS01 malaria vaccine among young African children. N Engl J Med 2016; 374:2519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. RTSS Clinical Trial Partnerships. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 2015; 386:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. RTSS Clinical Trials Partnership. Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med 2014; 11:e1001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jefferson T, Rudin M, DiPietrantonj C. Systematic review of the effects of pertussis vaccines in children. Vaccine 2003; 21:2003–14. [DOI] [PubMed] [Google Scholar]
- 7. Bayer O, Heininger U, Heiligensetzer C, von Kries R. Metaanalysis of vaccine effectiveness in varicella outbreaks. Vaccine 2007; 25:6655–60. [DOI] [PubMed] [Google Scholar]
- 8. Malaria Funding Partners. Malaria vaccine technology roadmap. Geneva, Switzerland: World Health Organization, 2013. [Google Scholar]
- 9. Beeson JG, Kurtovic L, Dobaño C, et al. Challenges and strategies for developing efficacious and long-lasting malaria vaccines. Sci Transl Med 2019; 11:eaau1458. [DOI] [PubMed] [Google Scholar]
- 10. Aponte JJ, Aide P, Renom M, et al. Safety of the RTS,S/AS02D candidate malaria vaccine in infants living in a highly endemic area of Mozambique: a double blind randomised controlled phase I/IIb trial. Lancet 2007; 370:1543–51. [DOI] [PubMed] [Google Scholar]
- 11. Abdulla S, Salim N, Machera F, et al. Randomized, controlled trial of the long term safety, immunogenicity and efficacy of RTS,S/AS02 D malaria vaccine in infants living in a malaria-endemic region. Malar J 2013; 12:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agnandji ST, Asante KP, Lyimo J, et al. Evaluation of the safety and immunogenicity of the RTS,S/AS01E malaria candidate vaccine when integrated in the expanded program of immunization. J Infect Dis 2010; 202:1076–87. [DOI] [PubMed] [Google Scholar]
- 13. Valéa I, Adjei S, Usuf E, et al. Immune response to the hepatitis B antigen in the RTS,S/AS01 malaria vaccine, and co-administration with pneumococcal conjugate and rotavirus vaccines in African children: a randomized controlled trial. Hum Vaccin Immunother 2018; 14:1489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cohen J, Nussenzweig V, Nussenzweig R, Vekemans J, Leach A. From the circumsporozoite protein to the RTS,S/AS candidate vaccine. Hum Vaccin 2010; 6:90–6. [DOI] [PubMed] [Google Scholar]
- 15. Casares S, Brumeanu T- D, Richie TL. The RTS,S malaria vaccine. Vaccine 2010; 28:4880–94. [DOI] [PubMed] [Google Scholar]
- 16. Olotu A, Moris P, Mwacharo J, et al. Circumsporozoite-specific T cell responses in children vaccinated with RTS,S/AS01 E and protection against P falciparum clinical malaria. PLoS One 2011; 6:e25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. White MT, Verity R, Griffin JT, et al. Immunogenicity of the RTS,S/AS01 malaria vaccine and implications for duration of vaccine efficacy: secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect Dis 2015; 15:1450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alonso PL, Sacarlal J, Aponte JJ, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet 2004; 364:1411–20. [DOI] [PubMed] [Google Scholar]
- 19. Dobaño C, Sanz H, Sorgho H, et al. Concentration and avidity of antibodies to different circumsporozoite epitopes correlate with RTS,S/AS01E malaria vaccine efficacy. Nat Commun 2019; 10:2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT. Complement system part I - molecular mechanisms of activation and regulation. Front Immunol 2015; 6:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosales C. Fcγ receptor heterogeneity in leukocyte functional responses. Front Immunol 2017; 8:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Okitsu SL, Silvie O, Westerfeld N, et al. A virosomal malaria peptide vaccine elicits a long-lasting sporozoite-inhibitory antibody response in a phase 1a clinical trial. PloS One 2007; 2:e1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hollingdale MR, Nardin EH, Tharavanij S, Schwartz A, Nussenzweig R. Inhibition of entry of Plasmodium falciparum and P. vivax sporozoites into cultured cells; an in vitro assay of protective antibodies. J Immunol 1984; 132:909–13. [PubMed] [Google Scholar]
- 24. Mahajan B, Berzofsky JA, Boykins RA, et al. Multiple antigen peptide vaccines against Plasmodium falciparum malaria. Infect Immun 2010; 78:4613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Behet MC, Kurtovic L, van Gemert G-J, et al. The complement system contributes to functional antibody-mediated responses induced by immunization with Plasmodium falciparum malaria sporozoites. Infect Immun 2018; 86:e00920-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kurtovic L, Behet MC, Feng G, et al. Human antibodies activate complement against Plasmodium falciparum sporozoites, and are associated with protection against malaria in children. BMC Med 2018; 16:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kurtovic L, Agius PA, Feng G, et al. Induction and decay of functional complement-fixing antibodies by the RTS,S malaria vaccine in children, and a negative impact of malaria exposure. BMC Med 2019; 17:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wines BD, Vanderven HA, Esparon SE, Kristensen AB, Kent SJ, Hogarth PM. Dimeric FcγR ectodomains as probes of the Fc receptor function of anti-influenza virus IgG. J Immunol 2016; 197:1507–16. [DOI] [PubMed] [Google Scholar]
- 29. McLean MR, Madhavi V, Wines BD, Hogarth PM, Chung AW, Kent SJ. Dimeric Fcγ receptor enzyme-linked immunosorbent assay to study HIV-specific antibodies: A new look into breadth of Fcγ receptor antibodies induced by the RV144 vaccine trial. J Immunol 2017; 199:816–26. [DOI] [PubMed] [Google Scholar]
- 30. Kester KE, Cummings JF, Ofori-Anyinam O, et al. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis 2009; 200:337–46. [DOI] [PubMed] [Google Scholar]
- 31. Schwenk R, DeBot M, Porter M, et al. IgG2 antibodies against a clinical grade Plasmodium falciparum CSP vaccine antigen associate with protection against transgenic sporozoite challenge in mice. PLoS One 2014; 9:e111020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reiling L, Boyle MJ, White MT, et al. Targets of complement-fixing antibodies in protective immunity against malaria in children. Nat Commun 2019; 10:610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boyle M, Chan J, Handayuni I, et al. IgM in human immunity to Plasmodium falciparum malaria. Sci Adv 2019; 5:eaax4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwenk R, Asher LV, Chalom I, et al. Opsonization by antigen‐specific antibodies as a mechanism of protective immunity induced by Plasmodium falciparum circumsporozoite protein‐based vaccine. Parasite Immunol 2003; 25:17–25. [DOI] [PubMed] [Google Scholar]
- 35. Chaudhury S, Ockenhouse CF, Regules JA, et al. The biological function of antibodies induced by the RTS,S/AS01 malaria vaccine candidate is determined by their fine specificity. Malar J 2016; 15:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yates NL, Liao H-X, Fong Y, et al. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med 2014; 6:228ra39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kester KE, McKinney DA, Tornieporth N, et al. Efficacy of recombinant circumsporozoite protein vaccine regimens against experimental Plasmodium falciparum malaria. J Infect Dis 2001; 183:640–7. [DOI] [PubMed] [Google Scholar]
- 38. Kester KE, McKinney DA, Tornieporth N, et al. A phase I/IIa safety, immunogenicity, and efficacy bridging randomized study of a two-dose regimen of liquid and lyophilized formulations of the candidate malaria vaccine RTS,S/AS02A in malaria-naive adults. Vaccine 2007; 25:5359–66. [DOI] [PubMed] [Google Scholar]
- 39. Owusu-Agyei S, Ansong D, Asante K, et al. Randomized controlled trial of RTS,S/AS02D and RTS,S/AS01E malaria candidate vaccines given according to different schedules in Ghanaian children. PLoS One 2009; 4:e7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Regules JA, Cicatelli SB, Bennett JW, et al. Fractional third and fourth dose of RTS,S/AS01 malaria candidate vaccine: a phase 2a controlled human malaria parasite infection and immunogenicity study. J Infect Dis 2016; 214:762–71. [DOI] [PubMed] [Google Scholar]
- 41. Asante KP, Abdulla S, Agnandji S, et al. Safety and efficacy of the RTS,S/AS01E candidate malaria vaccine given with expanded-programme-on-immunisation vaccines: 19 month follow-up of a randomised, open-label, phase 2 trial. Lancet Infect Dis 2011; 11:741–9. [DOI] [PubMed] [Google Scholar]
- 42. Neafsey DE, Juraska M, Bedford T, et al. Genetic diversity and protective efficacy of the RTS,S/AS01 malaria vaccine. N Engl J Med 2015; 373:2025–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fleit HB, Kobasiuk CD. The human monocyte‐like cell line THP‐1 expresses FcγRI and FCγRII. J Leukoc Biol 1991; 49:556–65. [DOI] [PubMed] [Google Scholar]
- 44. Kristensen AB, Lay WN, Ana-Sosa-Batiz F, et al. Antibody responses with Fc-mediated functions after vaccination of HIV-infected subjects with trivalent influenza vaccine. J Virol 2016; 90:5724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Madhavi V, Wines BD, Amin J, et al. HIV-1 Env- and Vpu-specific antibody-dependent cellular cytotoxicity responses associated with elite control of HIV. J Virol 2017; 91:e00700- 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li X- Y, Wu L, Li S- W, et al. Effect of CD16a, the surface receptor of Kupffer cells, on the growth of hepatocellular carcinoma cells. Int J Parasitol 2016; 37:1465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ikarashi M, Nakashima H, Kinoshita M, et al. Distinct development and functions of resident and recruited liver Kupffer cells/macrophages. J Leukoc Biol 2013; 94:1325–36. [DOI] [PubMed] [Google Scholar]
- 48. Seguin M, Ballou W, Nacy C. Interactions of Plasmodium berghei sporozoites and murine Kupffer cells in vitro. J Immunol 1989; 143:1716–22. [PubMed] [Google Scholar]
- 49. Radin K, Clement F, Jongert E, et al. A monoclonal antibody-based immunoassay to measure the antibody response against the repeat region of the circumsporozoite protein of Plasmodium falciparum. Malar J 2016; 15:543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ubillos I, Ayestaran A, Nhabomba AJ, et al. Baseline exposure, antibody subclass, and hepatitis B response differentially affect malaria protective immunity following RTS,S/AS01E vaccination in African children. BMC Med 2018; 16:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.