Abstract
Background
Efficacy of the live-attenuated herpes zoster (HZ) vaccine (ZVL) wanes substantially over time. We evaluated immunogenicity and safety of the adjuvanted recombinant zoster vaccine (RZV) in previous ZVL recipients.
Methods
Adults aged ≥65 years who were previously vaccinated with ZVL ≥5 years earlier (n = 215) were group-matched with ZVL-naive individuals (n = 215) and vaccinated with RZV. Glycoprotein E (gE)–specific humoral and cell-mediated immune responses and the correlation between them, polyfunctional gE-specific CD4 T-cell responses, safety, and confirmed HZ cases were assessed.
Results
Through 12 months after dose 2, anti-gE antibody concentrations, gE-specific CD4 T-cell frequencies, and activation marker profiles were similar between groups. Safety outcomes were also similar. No HZ episodes were confirmed.
Conclusions
RZV induced strong humoral and polyfunctional cell-mediated immune responses that persisted above prevaccination levels through 1 year after dose 2 in adults aged ≥65 years irrespective of previous ZVL vaccination. The RZV safety profile was not affected.
Clinical Trials Registration
Keywords: Herpes zoster, Adjuvanted recombinant zoster vaccine, Live-attenuated herpes zoster vaccine, Persistence of immune response, Polyfunctionality, Safety
The adjuvanted recombinant zoster vaccine induced strong humoral and polyfunctional cell-mediated immune responses that persisted above prevaccination levels through 1 year after dose 2 and was safe in adults aged ≥65 years, irrespective of previous live-attenuated herpes zoster vaccine administration.
Varicella-zoster virus (VZV) causes herpes zoster (HZ) on reactivation [1]. Immunosenescence leads to a decrease of VZV-specific T-cell responses [2], which are thought to be the main mechanistic protection against VZV reactivation [3]. Advanced age is therefore one of the major risk factors for developing HZ, with incidences increasing from <5/1000 person-years in individuals aged <50 years to >10/1000 person-years in those aged ≥70 years [4, 5].
Vaccination represents a means to reduce HZ risk in older adults [6]. In 2006, a live-attenuated HZ vaccine (ZVL; Zostavax; Merck Sharp & Dohme) was licensed and subsequently recommended in the United States for prevention of HZ in adults aged ≥60 years [7]. However, its efficacy decreases with age [6, 8]. In addition, efficacy against HZ is statistically significant only through year 5 and becomes negligible by year 11 [9, 10]. Tseng et al [11] hypothesized that, owing to declining effectiveness over time, revaccination of previous ZVL recipients might be beneficial. However, ZVL-boosted cell-mediated immune (CMI) responses decline significantly in 3 years in reimmunized individuals, with only memory responses remaining marginally higher compared with de novo–immunized individuals [12].
Given on a 2-dose schedule, the adjuvanted recombinant zoster vaccine (RZV; Shingrix; GSK), consisting of the VZV glycoprotein E (gE) antigen and the AS01B adjuvant system, demonstrated >90% efficacy against HZ across all age strata, sustained over 4 years of follow-up [13, 14]. Since 2017, RZV has received licensure for the prevention of HZ in adults aged ≥50 years in several countries. RZV induces robust humoral and CMI responses [15], which plateau as of about 4 years after vaccination and are predicted to remain above prevaccination levels for ≥15 years after vaccination [16].
Because the efficacy of ZVL wanes during the first 5 years after vaccination [10], and because ZVL was recommended in the United States for persons aged ≥60 years when our study was designed [7], we evaluated RZV in ≥65-year-olds who had received ZVL ≥5 years earlier. After demonstration of the study’s primary objectives (ie, noninferior immune responses to RZV in previous ZVL recipients compared with ZVL-naive individuals and a similar safety profile between groups up to 1 month after dose 2 [17]), RZV was recommended in the United States to previous ZVL recipients [18].
Here we report end-of-study protocol-defined analyses, including humoral and CMI responses as well as safety of RZV and the occurrence of confirmed HZ cases up to 12 months after dose 2 in adults aged ≥65 years. In addition, we present data from post hoc analyses on the correlation between humoral and CMI responses, and polyfunctional CD4 T-cell responses.
METHODS
Study Design
In this phase III, open-label, multicenter study conducted in the United States between March 2016 and August 2017, adults aged ≥65 years previously vaccinated with ZVL ≥5 years before RZV vaccination in this study (HZ-PreVac) were group-matched with ZVL-naive individuals (HZ-NonVac), as described elsewhere [17]. Study participants were to receive 2 RZV doses intramuscularly, 2 months apart.
Study Participants
Adults aged ≥65 years were eligible for participation if they did not have an immunocompromising condition or a previous history of HZ. Detailed inclusion and exclusion criteria have been presented elsewhere [17]. All participants provided written informed consent before the start of the study. The protocol was reviewed and approved by relevant institutional review boards. The study was conducted in accordance with the Declaration of Helsinki and the principles of good clinical practice and is registered at ClinicalTrials.gov (NCT02581410).
Study Vaccines
RZV (Shingrix) contains 50 μg of VZV gE antigen and the AS01B Adjuvant System (50 μg of 3-O-desacyl-4'-monophosphoryl lipid A (MPL), 50 μg of QS-21, and liposome) per 0.5 mL of reconstituted vaccine.
Objectives and Assessments
Objectives
The co-primary objectives were to demonstrate noninferiority of anti-gE antibody responses in previous ZVL recipients as compared to ZVL-naïve study participants at 1mo post-dose 2 and the evaluation of safety and reactogenicity of RZV in both groups through 1mo post-dose 2, and were disclosed elsewhere [17]. Secondary objectives included the descriptive assessment of humoral and CMI responses through 12 months after dose 2, as well as the assessment of safety between 1 and 12 months after dose 2. The correlation between humoral and CMI responses and polyfunctional CD4 T cells were evaluated in post hoc analyses.
Assessment of Immunogenicity
Anti-gE antibody concentrations were measured by means of enzyme-linked immunosorbent assay, as described elsewhere [15]. The frequencies of gE-specific CD4 T cells expressing ≥2 activation markers (referred to as CD42+ T cells) and the frequencies of gE-specific CD4 T cells expressing each or various combinations of the assessed activation markers (including interferon [IFN] γ, interleukin 2 [IL-2], tumor necrosis factor α, and CD40 ligand [CD40L]) were determined as described elsewhere [15].
Assessment of Safety
Serious adverse events (SAEs) and potential immune-mediated diseases (pIMDs) were recorded from the first RZV dose through 12 months after dose 2. The causal relationship to vaccination was assessed by the study investigator.
Suspected HZ cases, defined as a new rash characteristic of HZ diagnosed by the investigator, were collected throughout the study. HZ cases were confirmed by means of polymerase chain reaction (PCR) or by a HZ ascertainment committee in a blinded manner if the case could not be confirmed or excluded by PCR.
Statistical Analyses
Immunogenicity was assessed in the according-to-protocol cohort, which included all participants who complied with protocol-specified procedures up to the considered time point and for whom data were available.
Anti-gE antibody geometric mean concentrations (GMCs) were calculated, along with their 2-sided 95% confidence intervals (CIs) and descriptive statistics (minimum, quarter 1, median, quarter 3, and maximum) of CD42+ T cells were tabulated. Mean frequencies and descriptive statistics (median and interquartile range) were determined for gE-specific CD4 T cells expressing 1, 2, 3, or all assessed activation markers. Spearman correlation coefficients were calculated to evaluate the correlation between anti-gE antibody concentrations and gE-specific CD42+ T-cell frequencies.
Safety was assessed in the total vaccinated cohort, which included participants who received ≥1 RZV dose, by computing the percentage of participants reporting each type of SAE or pIMD, along with the corresponding 2-sided 95% CI. No formal sample size calculations were performed for the presented analyses. Statistical analyses were performed using Statistical Analysis System (SAS) software version 9.3.
RESULTS
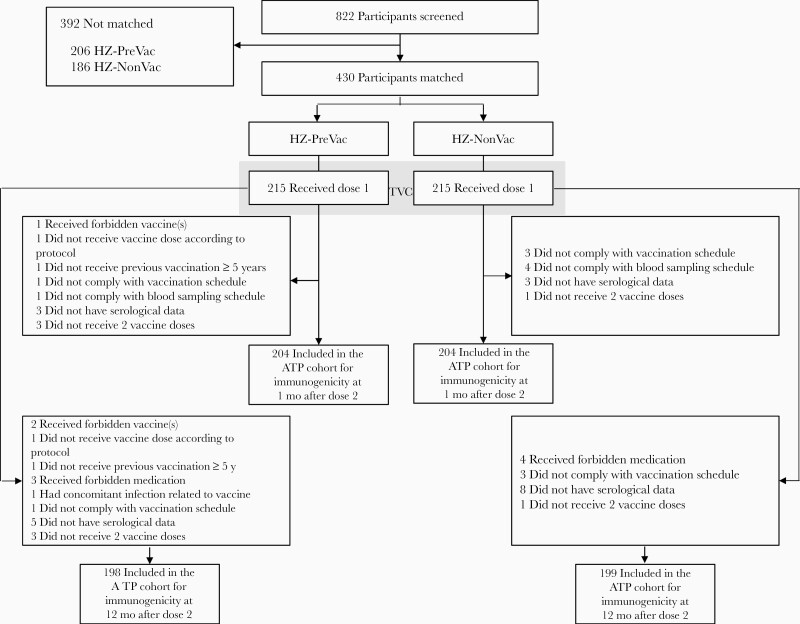
Four hundred thirty participants (215 in each study group) were matched and vaccinated [17]. At 12 months after dose 2, the according-to-protocol cohort for immunogenicity consisted of 198 participants from the HZ-PreVac group (mean age [standard deviation], 71.2 [4.6] years) and 199 from the HZ-NonVac group (mean age, 70.7 [4.7] years) (Figure 1); 51% and 50.8% of participants, respectively, were women. All participants were White/European ancestry.
Figure 1.
Participant flow diagram. Abbreviations: ATP, according-to-protocol; HZ-NonVac, no previous vaccination with live-attenuated herpes zoster (HZ) vaccine before administration of adjuvanted recombinant zoster vaccine (RZV); HZ-PreVac, previous vaccination with live-attenuated HZ vaccine ≥5 years before administration of adjuvanted RZV; TVC, total vaccinated cohort; y, years.
Humoral and CMI responses and safety through 1 month after dose 2, including solicited and unsolicited adverse event occurrence, have been described elsewhere [17]. Anti-gE antibody GMCs (Supplementary Figure 1A) and gE-specific CD42+ T-cell frequencies (Supplementary Figure 1B) remained similar between groups and above prevaccination levels through 12 months after dose 2.
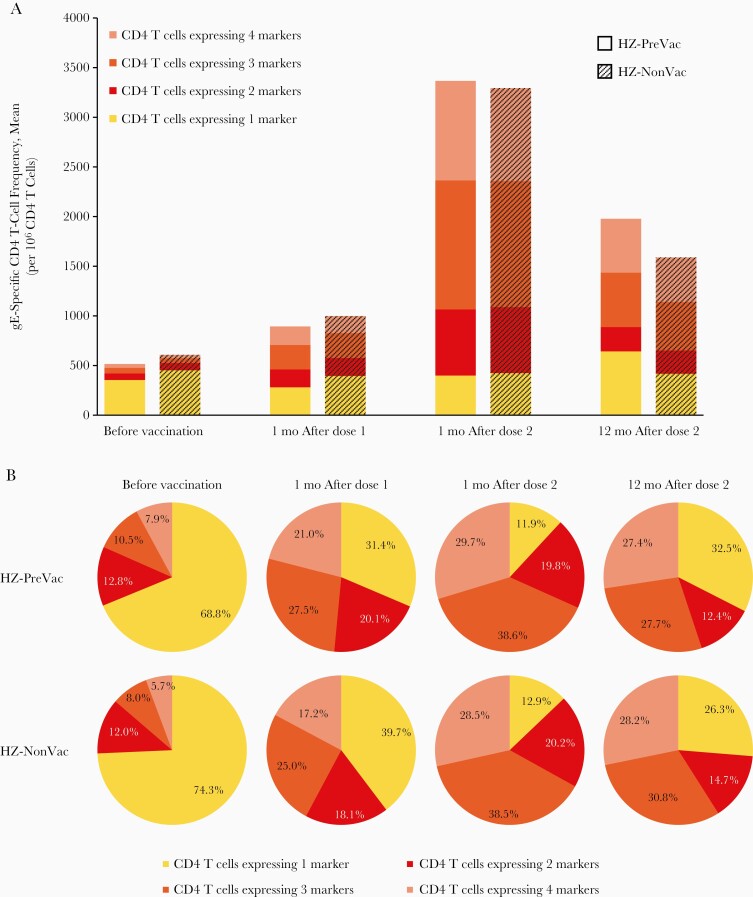
In both study groups, the mean frequencies of CD4 T cells expressing 2, 3, or 4 activation markers increased over baseline after each RZV dose and remained above prevaccination levels at 12 months after dose 2. The mean frequencies of CD4 T cells expressing only 1 marker were generally similar in both study groups from before vaccination through 12 months after dose 2 (Figure 2A).
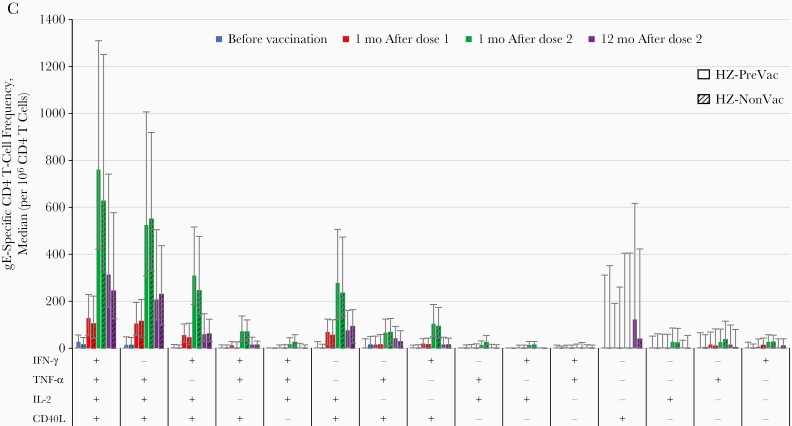
Figure 2.
Frequency of glycoprotein E (gE)–specific CD4 T cells expressing any combination of activation markers (according-to-protocol cohort for immunogenicity). A, Mean gE-specific CD4 T-cell frequencies. B, Relative mean gE-specific CD4 T-cell frequencies. C, Activation marker combinations (error bars represent interquartile ranges). Abbreviations: CD40L, CD40 ligand; HZ-NonVac, no previous vaccination with live-attenuated herpes zoster (HZ) vaccine before administration of adjuvanted recombinant zoster vaccine (RZV); HZ-PreVac, previous vaccination with live-attenuated HZ vaccine ≥5 years before administration of adjuvanted RZV; IFN-γ, interferon γ; IL-2, interleukin 2; mo, month(s); TNF-α, tumor necrosis factor α.
In both study groups, while the proportion of CD4 T cells expressing 3 or 4 activation markers (polyfunctional CD4 T cells) increased after each RZV dose and remained above prevaccination levels at 12 months after dose 2, the proportion of CD4 T cells expressing a single activation marker decreased after each RZV dose and remained below the prevaccination level at 12 months after dose 2 (Figure 2B).
Expression of activation markers was similar in the 2 study groups. At all time points, CD40L (alone or especially in combination with other markers) was expressed in the vast majority of gE-specific CD4 T cells. IFN-γ and tumor necrosis factor α were usually expressed in combination with IL-2 and/or CD40L. The frequencies of CD4 T cells expressing individual and combinations of 2, 3, or 4 activation markers peaked 1 month after dose 2 and decreased thereafter, except for the frequency of those expressing CD40L alone, which peaked 12 months after dose 2 (Figure 2C). In both study groups, exploratory analyses showed a statistically significant positive correlation between anti-gE concentrations and gE-specific CD42+ T-cell frequencies at all time points, which was highest at 1 month after dose 2 (Supplementary Table 1).
From RZV dose 1 through 12 months after dose 2, ≥1 SAE was reported by 18 (8.4%; 95% CI, 5.0%–12.9%) and 22 (10.2%; 95% CI, 6.5%–15.1%) participants from the HZ-PreVac and HZ-NonVac groups, respectively. Fatal SAEs were reported for 2 of 215 participants from the HZ-PreVac and 3 of 215 from the HZ-NonVac group. At least 1 pIMD was reported by 2 (0.9%; 95% CI, 0.1–3.3%) and 4 (1.9%; 95% CI, 0.5%–4.7%) participants from the HZ-PreVac and HZ-NonVac groups, respectively (Supplementary Table 2). None of the reported SAEs or pIMDs were assessed by the investigator to be causally related to vaccination.
During the entire study period, 1 suspected HZ episode was reported, which occurred in a HZ-PreVac group participant approximately 160 days after dose 2. Although both the investigator and the HZ ascertainment committee ascertained the case as HZ, this diagnosis was ruled out by PCR.
DISCUSSION
In adults aged ≥65 years, 2 RZV doses administered 2 months apart induced strong and persistent humoral and CMI responses, irrespective of ZVL vaccination status. Occurrences of SAEs and pIMDs after RZV were similar between previous ZVL recipients and ZVL-naive study participants.
Our end-of-study data show that both anti-gE antibody and gE-specific CD4 T-cell responses persist through 12 months after RZV dose 2, with no apparent differences between previous ZVL recipients and ZVL-naive participants. These findings indicate that persistence of immune responses to RZV, currently demonstrated for ≥10 years after vaccination [16], may also be expected in previous ZVL recipients.
Anti-gE antibody GMCs were similar between previous ZVL recipients and ZVL-naive study participants at all time points, including before vaccination. Although the humoral response to ZVL is dominated by anti-gE antibodies [19], this similarity may be explained by the rapid waning of ZVL-induced humoral immunity. When measured by glycoprotein-based enzyme-linked immunosorbent assay, VZV-specific antibody titers 12 months after ZVL administration were indeed similar to prevaccination titers [20]. Even though the results are not directly shown for anti-gE antibodies, one can assume that these follow the same trend and have decreased to pre-ZVL administration levels in the previous ZVL recipients in our study.
The mean frequencies of CD4 T cells expressing 2, 3, and 4 markers were very similar between study groups at all time points. In both study groups, the mean frequency as well as the proportion of polyfunctional CD4 T cells increased after each RZV dose. The mean frequencies and, to a lesser extent, the proportions of polyfunctional CD4 T cells decreased through 12 months after dose 2. Although the last assessment in our study was made 12 months after dose 2, previous findings in adults aged ≥50 years confirm this pattern and suggest that the proportion of polyfunctional CD4 T cells will plateau or even slightly increase through the second and third years after vaccination [15].
A single dose of ZVL was shown to boost VZV-specific memory T cells, which express the same 4 activation markers assessed in our study [21]. The high frequency of IL-2–secreting CD4 T cells (in the absence of IFN-γ production) and high proportion of polyfunctional CD4 T cells observed after the first RZV dose may suggest a mixed population of gE-specific T cells resulting from both mobilization of the naive T-cell repertoire and recall of memory cells, respectively. However, further investigations are needed to confirm this hypothesis and to evaluate the individual contributions of naive and memory T cells to the overall observed CD4 T-cell response to RZV, as previously suggested for ZVL [22].
Expression of IFN-γ, alone or in combination with IL-2, differentiates effector and memory T-cell responses [23]. In both study groups, the expression of IFN-γ, usually in combination with IL-2, is indicative of the presence and persistence of central memory (IL-2+) and effector memory (IL-2+IFN-γ +) CD4 T cells. This hypothesis is further supported by the limited ability of the AS01 adjuvant system to elicit IFN-γ–secreting T cells in naive individuals [24]. A recent study comparing CMI responses to ZVL and RZV showed that RZV induces higher peak frequencies of CD4 T cells expressing IL-2 and IFN-γ either alone or in combination, which remain higher 1 year after vaccination [25]. This study therefore suggests that, whereas ZVL generates higher effector CD4 T-cell responses than RZV, RZV generates higher memory and effector-memory CD4 T-cell peak responses than ZVL, which may account for the longer immunogenicity persistence and superior efficacy of RZV [25–27].
The similarity of the CD4 T-cell response magnitude and profile between previous ZVL recipients and ZVL-naive study participants may suggest that the CMI response to ZVL is not predominantly directed to gE, and thus does not provide the basis for RZV to recall ZVL-induced T-cell responses. It was indeed shown that gE is not the dominant T-cell antigen in ZVL, which supports the above hypothesis [21].
In a 2018 article reviewing licensed HZ vaccines, one key question was whether RZV would be efficacious in previous ZVL recipients [28]. Even though our study was not powered to evaluate efficacy, suspected HZ cases were collected prospectively throughout the study and confirmed by a HZ ascertainment committee and/or PCR in a process similar to that used in the 2 pivotal efficacy trials with RZV [13, 14]. Despite the relatively short follow-up period for HZ cases (1 year) and the small sample size to evaluate vaccine efficacy, the lack of confirmed HZ cases likely reflects the high efficacy of RZV [13, 14].
Previous trials have also shown that CD4 T-cell polyfunctionality correlates with protection after vaccination, such as against human immunodeficiency virus, tuberculosis, malaria, or melanoma [29–31]. The very similar polyfunctional CD4 T-cell responses after RZV in previous ZVL recipients and ZVL-naive individuals, along with the lack of confirmed HZ cases in either study group, suggests that previous ZVL vaccination is not likely to affect the efficacy of RZV in the prevention of HZ. Long-term follow-up studies may be required to definitively address this question.
In line with previous findings [15], we observed a statistically significant correlation between anti-gE antibody concentrations and CD42+ T-cell frequencies, which peaked at 1 month after dose 2 and were similar between study groups at all time points. These findings further confirm the similarity of immune responses to RZV between previous ZVL recipients and ZVL-naive individuals. SAE and pIMD occurrences were similar between groups and consistent with the safety profile of RZV assessed in approximately 30 000 adults aged ≥50 years, who were enrolled in 2 pivotal efficacy trials [32].
The main limitation of these results is that intervals of <5 years between a previous ZVL dose and 2 subsequent RZV doses have not been evaluated. However, there are no scientific data or theoretical concerns to indicate that RZV would be less safe or effective when administered with an interval of <5 years. Accordingly, in older individuals (aged ≥50 years), the US Centers for Disease Control and Prevention recommends administration of RZV at a shorter interval, but no sooner than 8 weeks after ZVL [18].
In conclusion, RZV induced strong humoral and polyfunctional CMI responses that persisted above prevaccination levels up to 1 year after RZV dose 2 in adults aged ≥65 years, irrespective of previous ZVL vaccination, without clinically significant differences in the RZV safety profile between study groups. Therefore, both ZVL-naive individuals and previous ZVL recipients are likely to similarly benefit from RZV vaccination.
Data Sharing
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com (study ID: 201198).
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: IDWeek 2017, San Diego, California, 3–7 October 2017.
Acknowledgments. The authors thank the study participants, investigators, and study teams involved in this trial, as well as the GSK teams, including laboratory teams who undertook the immunological assessments, clinical research and development, clinical operations, statisticians and statistical analysts, medical data reviewers, safety case management, and publications.
The authors also thank the Modis platform for editorial assistance and manuscript coordination, on behalf of GSK. Andrada Pașcu and Alpár Pöllnitz provided medical writing support, and Sander Hulsmans coordinated the manuscript development and provided editorial support.
Author contributions. The list of contributors who conceived and designed the study (NCT02581410), as well as those who performed the study can be found in the contributor sections of the active-phase report [17]. A. F. D. and G. K. helped generate the post hoc analyses data. All authors interpreted the data, helped write the manuscript, and reviewed and approved the final submitted version.
Financial support. This work was supported by GlaxoSmithKline Biologicals SA in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA also took responsibility for all costs associated with the development and publishing of the present manuscript.
Potential conflicts of interest. A. F. D., C. H., E. D. P., B. S., and A. S. are employees of the GSK group of companies. A. F. D., E. D. P., B. S., and A. S. hold shares in the GSK group of companies as part of their employee remuneration. G. K. is a freelance statistician at Keyrus Biopharma, serving as a paid consultant on behalf of the GSK group of companies. N. P. K. received grants from the GSK group of companies during the conduct of this study, as well as grants from Merck, Pfizer, Sanofi Pasteur, MedImmune, Novartis, and Protein Science outside the submitted work. J. P. certifies no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Gershon AA, Gershon MD. Pathogenesis and current approaches to control of varicella-zoster virus infections. Clin Microbiol Rev 2013; 26:728–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cohen JI. VZV: molecular basis of persistence (latency and reactivation). In: Arvin A, Campadelli-Fiume G, Mocarski E, et al, eds. Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge, United Kingdom: Cambridge University Press, 2007. [PubMed] [Google Scholar]
- 3. Weinberg A, Levin MJ. VZV T cell-mediated immunity. Curr Top Microbiol Immunol 2010; 342:341–57. [DOI] [PubMed] [Google Scholar]
- 4. Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open 2014; 4:e004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnson BH, Palmer L, Gatwood J, Lenhart G, Kawai K, Acosta CJ. Annual incidence rates of herpes zoster among an immunocompetent population in the United States. BMC Infect Dis 2015; 15:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oxman MN, Levin MJ, Johnson GR, et al. . Shingles Prevention Study Group . A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–84. [DOI] [PubMed] [Google Scholar]
- 7. Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57:1–30; quiz CE2-4. [PubMed] [Google Scholar]
- 8. Gabutti G, Valente N, Sulcaj N, Stefanati A. Evaluation of efficacy and effectiveness of live attenuated zoster vaccine. J Prev Med Hyg 2014; 55:130–6. [PMC free article] [PubMed] [Google Scholar]
- 9. Morrison VA, Johnson GR, Schmader KE, et al. . Shingles Prevention Study Group . Long-term persistence of zoster vaccine efficacy. Clin Infect Dis 2015; 60:900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schmader KE, Oxman MN, Levin MJ, et al. . Shingles Prevention Study Group . Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin Infect Dis 2012; 55:1320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tseng HF, Harpaz R, Luo Y, et al. . Declining effectiveness of herpes zoster vaccine in adults aged ≥60 years. J Infect Dis 2016; 213:1872–5. [DOI] [PubMed] [Google Scholar]
- 12. Weinberg A, Popmihajlov Z, Schmader KE, et al. . Persistence of varicella-zoster virus cell-mediated immunity after the administration of a second dose of live herpes zoster vaccine. J Infect Dis 2019; 219:335–8. [DOI] [PubMed] [Google Scholar]
- 13. Lal H, Cunningham AL, Godeaux O, et al. . ZOE-50 Study Group . Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372:2087–96. [DOI] [PubMed] [Google Scholar]
- 14. Cunningham AL, Lal H, Kovac M, et al. . ZOE-70 Study Group . Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med 2016; 375:1019–32. [DOI] [PubMed] [Google Scholar]
- 15. Cunningham AL, Heineman TC, Lal H, et al. . ZOE-50/70 Study Group . Immune responses to a recombinant glycoprotein E herpes zoster vaccine in adults aged 50 years or older. J Infect Dis 2018; 217:1750–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bastidas A, Catteau G, Volpe S, et al. . 2905. Long-term immunological persistence of the adjuvanted recombinant zoster vaccine: clinical data and mathematical modeling. Open Forum Infect Dis 2019; 6:S84–S5. [Google Scholar]
- 17. Grupping K, Campora L, Douha M, et al. . Immunogenicity and safety of the HZ/su adjuvanted herpes zoster subunit vaccine in adults previously vaccinated with a live attenuated herpes zoster vaccine. J Infect Dis 2017; 216: 1343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dooling KL, Guo A, Patel M, et al. . Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep 2018; 67:103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sullivan NL, Reuter-Monslow MA, Sei J, et al. . Breadth and functionality of varicella-zoster virus glycoprotein-specific antibodies identified after Zostavax vaccination in humans. J Virol 2018; 92:e00269–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vesikari T, Hardt R, Rümke HC, et al. . Immunogenicity and safety of a live attenuated shingles (herpes zoster) vaccine (Zostavax®) in individuals aged ≥ 70 years: a randomized study of a single dose vs. two different two-dose schedules. Hum Vaccin Immunother 2013; 9:858–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sei JJ, Cox KS, Dubey SA, et al. . Effector and central memory poly-functional CD4+ and CD8+ T Cells are Boosted upon ZOSTAVAX® vaccination. Front Immunol 2015; 6:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qi Q, Cavanagh MM, Le Saux S, et al. . Diversification of the antigen-specific T cell receptor repertoire after varicella zoster vaccination. Sci Transl Med 2016; 8: 332ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Levin MJ, Schmader KE, Pang L, et al. . Cellular and humoral responses to a second dose of herpes zoster vaccine administered 10 years after the first dose among older adults. J Infect Dis 2016; 213:14–22. [DOI] [PubMed] [Google Scholar]
- 24. Leroux-Roels G, Marchant A, Levy J, et al. . Impact of adjuvants on CD4+ T cell and B cell responses to a protein antigen vaccine: results from a phase II, randomized, multicenter trial. Clin Immunol 2016; 169:16–27. [DOI] [PubMed] [Google Scholar]
- 25. Levin MJ, Kroehl ME, Johnson MJ, et al. . Th1 memory differentiates recombinant from live herpes zoster vaccines. J Clin Invest 2018; 128:4429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weinberg A, Kroehl ME, Johnson MJ, et al. . Comparative immune responses to licensed herpes zoster vaccines. J Infect Dis 2018; 218:81–7. [DOI] [PubMed] [Google Scholar]
- 27. Levin MJ, Weinberg A. Immune responses to zoster vaccines. Hum Vaccin Immunother 2019; 15:772–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cunningham AL, Levin MJ. Herpes zoster vaccines. J Infect Dis 2018; 218:127–33. [DOI] [PubMed] [Google Scholar]
- 29. Berry N, Manoussaka M, Ham C, et al. . Role of occult and post-acute phase replication in protective immunity induced with a novel live attenuated SIV vaccine. PLoS Pathog 2016; 12:e1006083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maggioli MF, Palmer MV, Thacker TC, et al. . Increased TNF-α/IFN-γ/IL-2 and Decreased TNF-α/IFN-γ production by central memory T cells are associated with protective responses against bovine tuberculosis following BCG vaccination. Front Immunol 2016; 7:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mordmüller B, Surat G, Lagler H, et al. . Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature 2017; 542:445–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. López-Fauqued M, Campora L, Delannois F, et al. . ZOE-50/70 Study Group . Safety profile of the adjuvanted recombinant zoster vaccine: pooled analysis of two large randomised phase 3 trials. Vaccine 2019; 37:2482–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.