Abstract
Background
MVC-COV1901, a recombinant protein vaccine containing pre-fusion-stabilised spike protein S-2P adjuvanted with CpG 1018 and aluminium hydroxide, has been shown to be well tolerated with a good safety profile in healthy adults aged 20–49 years in a phase 1 trial, and provided a good cellular and humoral immune responses. We present the interim safety, tolerability, and immunogenicity results of a phase 2 clinical trial of the MVC-COV1901 vaccine in Taiwan.
Methods
This is a large-scale, double-blind, randomised, placebo-controlled phase 2 trial done at ten medical centres and one regional hospital in Taiwan. Individuals aged 20 years or older who were generally healthy or had stable pre-existing medical conditions were eligible for enrolment. Exclusion criteria included (but were not limited to) travel overseas within 14 days of screening, intention to travel overseas within 6 months of the screening visit, and the absence of prespecified medical conditions, including immunosuppressive illness, a history of autoimmune disease, malignancy with risk to recur, a bleeding disorder, uncontrolled HIV infection, uncontrolled hepatitis B and C virus infections, SARS-CoV-1 or SARS-CoV-2 infections, an allergy to any vaccine, or a serious medical condition that could interfere with the study. Study participants were randomly assigned (6:1) to receive two doses of either MVC-COV1901 or placebo, administered via intramuscular injection on day 1 and day 29. MVC-COV1901 contained 15 μg of S-2P protein adjuvanted with 750 μg CpG 1018 and 375 μg aluminium hydroxide in a 0·5 mL aqueous solution, and the placebo contained the same volume of saline. Randomisation was done centrally by use of an interactive web response system, stratified by age (≥20 to <65 years and ≥65 years). Participants and investigators were masked to group assignment. The primary outcomes were to evaluate the safety, tolerability, and immunogenicity of MVC-COV1901 from day 1 (the day of the first dose) to day 57 (28 days after the second dose). Safety was assessed in all participants who received at least one dose. Immunogenicity was assessed by measuring geometric mean titres (GMTs) and seroconversion rates of neutralising antibody and antigen-specific IgG in the per-protocol population. This study is registered with ClinicalTrials.gov, NCT04695652.
Findings
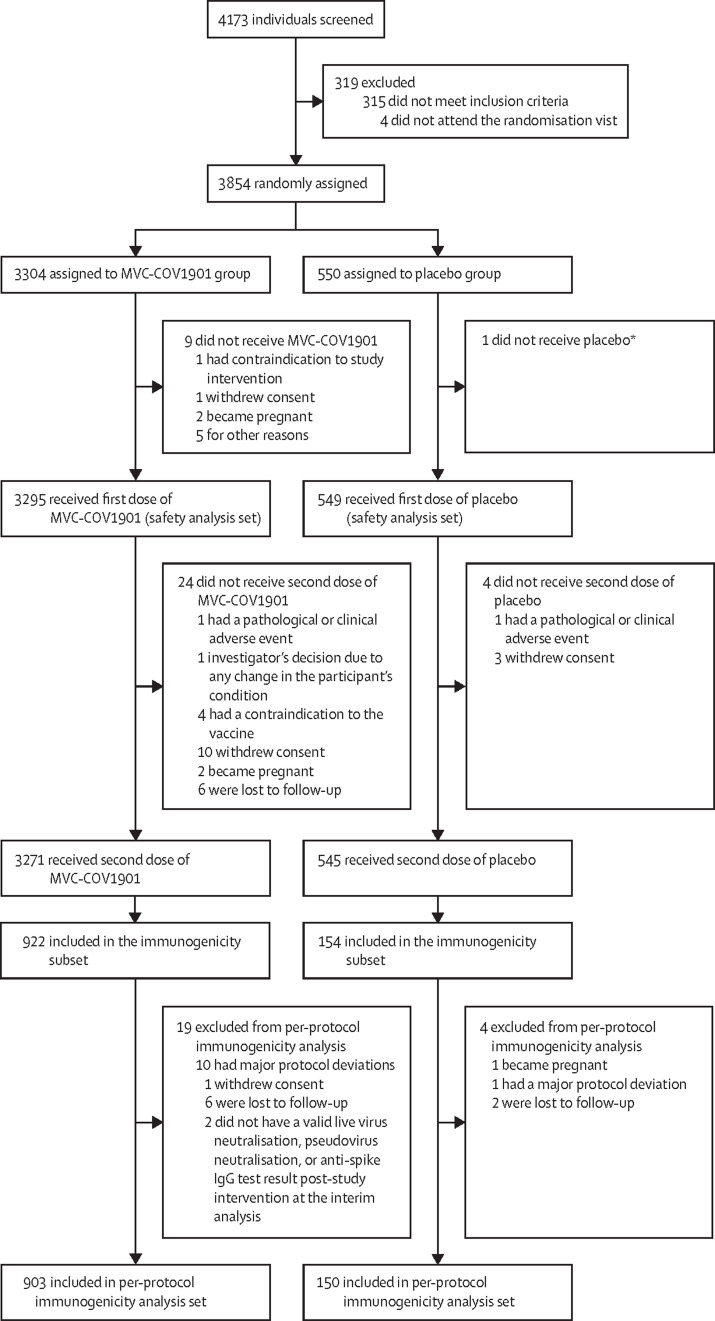
Of 4173 individuals screened between Dec 30, 2020, and April 2, 2021, 3854 were enrolled and randomly assigned: 3304 to the MVC-COV1901 group and 550 to the placebo group. A total of 3844 participants (3295 in the MVC-COV1901 group and 549 in the placebo group) were included in the safety analysis set, and 1053 participants (903 and 150) had received both doses and were included in the per-protocol immunogenicity analysis set. From the start of this phase 2 trial to the time of interim analysis, no vaccine-related serious adverse events were recorded. The most common solicited adverse events in all study participants were pain at the injection site (2346 [71·2%] of 3295 in the MVC-COV1901 group and 128 [23·3%] of 549 in the placebo group), and malaise or fatigue (1186 [36·0%] and 163 [29·7%]). Fever was rarely reported (23 [0·7%] and two [0·4%]). At 28 days after the second dose of MVC-COV1901, the wild-type SARS-CoV-2 neutralising antibody GMT was 662·3 (95% CI 628·7–697·8; 408·5 IU/mL), the GMT ratio (geometric mean fold increase in titres at day 57 vs baseline) was 163·2 (155·0–171·9), and the seroconversion rate was 99·8% (95% CI 99·2–100·0).
Interpretation
MVC-COV1901 has a good safety profile and elicits promising immunogenicity responses. These data support MVC-COV1901 to enter phase 3 efficacy trials.
Funding
Medigen Vaccine Biologics and Taiwan Centres for Disease Control, Ministry of Health and Welfare.
Research in context.
Evidence before this study
To understand the current landscape of COVID-19 vaccine development, we searched the COVID-19 vaccine tracker, which is a database maintained by the London School of Hygiene & Tropical Medicine that is updated monthly, for clinical trials (phase 1–3) of COVID-19 vaccines that use recombinant subunit protein-based technology. We searched this database on July 7, 2021, using the search term “protein subunit”, “recombinant protein subunit”, and “peptide”. We searched for clinical trials published between May 1, 2020 and July 7, 2021. The search yielded 12 publications that used the recombinant protein subunit or peptide platform. None of these protein subunit vaccines have been approved for emergency use by the US Food and Drug Administration (FDA). Five of the clinical trials are of a protein subunit vaccine in the most advanced stage of development; a nanoparticle vaccine containing recombinant SARS-CoV-2 spike glycoprotein (S protein) in a saponin-based adjuvant that is pending for emergency authorisation in the USA in the third quarter of 2021. A phase 1–2 trial of a peptide vaccine encoding immunogens of the S protein was granted approval in Russia. Three developers have based their vaccines on the receptor-binding domain of the S protein; two in China and one in Cuba. Three developers have used versions of an adjuvanted, trimerised, stabilised, pre-fusion S protein, including our MVC-COV1901 vaccine, which is based on the pre-fusion-stabilised S-2P protein adjuvanted with CpG 1018 and aluminium hydroxide. Our previous phase 1 study of MVC-COV1901 showed that two doses of vaccine administered 28 days apart at all three concentrations of S-2P (5 μg, 15 μg, or 25 μg) was safe and well tolerated. Furthermore, all participants who received the 15 μg or 25 μg doses had seroconversion and higher neutralising antibody titres than convalescent serum samples at 14 days after receiving the second dose.
Added value of this study
This study reports the results of the interim analysis of the phase 2 clinical trial of MVC-COV1901 (15 μg S-2P protein); the first large-scale trial to evaluate the safety and immunogenicity profiles of MVC-COV1901 in 3844 adults aged 20 years and older. MVC-COV1901 was shown to have a good safety and immunogenicity profile. The current data support MVC-COV1901 to enter phase 3 efficacy trials, and could enable regulatory considerations for emergency use authorisation in different countries, as has been done in Taiwan.
Implications of all the available evidence
Compared with other vaccines currently in use, MVC-COV1901 showed a good safety profile, with a low proportion of participants who had an adverse event and few participants with a febrile reaction after vaccine administration. The proposed methods of neutralising antibody conversion to international units or binding antibody units for the comparison of efficacy (to comply with the principles of correlates of protection) might serve as a starting point for other vaccine developers to predict the degree of protection of vaccine candidates in their immunobridging trials. Although the clinical efficacy or degree of protection of MVC-COV1901 remains to be validated in a standard phase 3 trial, the current phase 2 interim analysis results have given the Taiwan FDA supporting data to grant this vaccine an emergency use authorisation status. By sharing our phase 2 clinical data and the proposed correlates of protection methods with other COVID-19 vaccine developers in the community, we hope to expedite the development and approval of more COVID-19 vaccine candidates to increase global supply, and to thereby help the countries and regions most in need.
Introduction
COVID-19 was first reported in Wuhan, China in December, 2019. Identified as a severe acute respiratory syndrome-related coronavirus, it was named SARS-CoV-2.1 The disease spread rapidly, with the WHO declaring a global pandemic on March 11, 2020.2 Despite the unprecedented pace of vaccine development, COVID-19 continues to spread with over 170 million infections and 3·7 million deaths recorded worldwide as of June, 2021, according to the COVID-19 Dashboard. Even though several vaccines based on mRNA platform or adenovirus vector technology have obtained emergency use authorisation, the global supply of available vaccines are not sufficient to end this pandemic. The addition of traditional recombinant subunit protein vaccines could strengthen current vaccination efforts.
MVC-COV1901 is a recombinant protein subunit vaccine based on the stabilised pre-fusion SARS-CoV-2 spike protein S-2P, adjuvanted with CpG 1018 and aluminium hydroxide.3 The S-2P protein was developed by Wrapp and colleagues4 at the US National Institute of Allergy and Infectious Diseases, and the synthetic oligodeoxynucleotide CpG 1018 adjuvant was developed by Dynavax Technologies (Emeryville, CA, USA).3 Using animal models, we previously showed total protection in hamsters given MVC-COV1901 challenged with wild-type SARS-CoV-2.5 The dose gradients of the adjuvants were suggested by Dynavax, and later verified in this challenge study.5 The result of our phase 1 trial showed that MVC-COV1901 (containing 5 μg, 15 μg, or 25 μg of S-2P) was well tolerated and elicits robust T-cell and B-cell immune responses.6 In this interim analysis of the phase 2 trial, we assessed the safety, tolerability, and immunogenicity of the MVC-COV1901 vaccine (containing 15 μg of S-2P protein) versus placebo in healthy adults and adults with stable pre-existing medical conditions.
Methods
Study design and participants
This is an interim analysis of an ongoing large-scale, double-blind, randomised, placebo-controlled phase 2 trial being done at ten medical centres and one regional hospital in Taiwan.
Eligible participants were male or female adults aged 20 years or older, who were healthy or had stable pre-existing medical conditions. Female participants of childbearing potential were required to take effective contraception from 14 days before screening to 30 days following the last injection of study intervention. Individuals who had travelled overseas within 14 days of screening, or those who planned to travel overseas within 6 months of the screening visit were excluded. Participants were also excluded if they had any of the following prespecified medical conditions: immunosuppressive illness, a history of autoimmune disease, malignancy with risk to recur, a bleeding disorder, uncontrolled HIV infection, uncontrolled hepatitis B and C virus infections, SARS-CoV-1 or SARS-CoV-2 virus infections, an allergy to any vaccine, or a serious medical condition that could interfere with the study. A full list of inclusion and exclusion criteria is available in the appendix (appendix pp 56–58). All participants provided written informed consent.
The trial protocol (appendix pp 25–113) and informed consent form were approved by the Taiwan Food and Drug Administration (FDA) and the ethics committees at the participating sites. The main institutional review board was Chang Gung Medical Foundation (Taoyuan, Taiwan), and other institutional review boards were the following institutes in Taiwan: National Taiwan University Hospital (Taipei), Taipei Veterans General Hospital (Taipei), Tri-Service General Hospital (Taipei), Taipei Medical University Hospital (Taipei), Taipei Municipal Wanfang Hospital (Taipei), Taoyuan General Hospital Ministry of Health and Welfare (Taoyuan), China Medical University Hospital (Taichung), Changhua Christian Hospital (Changhua County), National Cheng Kung University Hospital (Tainan), and Kaoshiung Medical University Hospital (Kaoshiung). The trial was done in accordance with the principles of the Declaration of Helsinki and good clinical practice guidelines.
Randomisation and masking
Participants were randomly assigned (6:1) to receive either MVC-COV1901 or placebo. Randomisation was done centrally by use of an interactive web response system (Medidata randomisation and trial supply management; New York, NY, USA). The randomisation list by block (size 7 and 14) randomisation, stratified by age (≥20 to <65 years and ≥65 years), was generated by the randomisation biostatistician with unique randomisation identification numbers across all blocks and strata.
Participants and investigators were masked to group assignment. Since MVC-COV1901 and placebo are visually distinct, each trial site was assigned an unmasked study nurse, who was involved in the preparation, dispensing, administration, and accountability of the study intervention, but who had no involvement in the rest of the trial. To ensure that masking was achieved, study-specific training was done at all participating trial sites. Study staff who administered the intervention covered the syringes (physically by hand or with aluminium foil) to ensure participants were masked to the intervention.
Before this interim analysis, our phase 2 trial adhered to the protocol and statistical analysis plan. However, prompted by a vaccine shortage in Taiwan, this interim analysis was necessary for the regional submission of MVC-COV1901 for emergency use authorisation, which has since been granted (on July 30, 2021). The emergency use authorisation submission inevitably entailed disclosing the summary results to the authors, who were also the principal investigators at each participating site. Nevertheless, to enforce the protocol for a double-blind trial, individual treatment allocation remains masked to all team members involved in conducting this ongoing trial.
Procedures
Participants received two doses of either MVC-COV1901 or placebo at their second visit (day 1) and fourth visit (day 29) via intramuscular injection into the deltoid muscle. The 0·5-mL MVC-COV1901 prefilled syringe contained 15 μg of Chinese hamster ovary cell-derived S-2P protein, adjuvanted with 750 μg CpG 1018 and 375 μg aluminium hydroxide without any preservatives, as previously reported.7 The vaccine was produced at the Medigen Vaccine Biologics (Taipei, Taiwan) Zhubei facility in compliance with current good manufacturing practices. The placebo was 0·5 mL of saline. The duration of the study was 209 days (a 29-day treatment period and 180 days of follow-up). Participant data were collected via six on-site visits (on days −28 to 0 [screening visit], 1 [first vaccination], 29 [second vaccination], 43, 57, and 209) and three telephone calls (on days 8, 36, and 85).
Vital signs were assessed before and after each injection. Participants were observed for at least 30 min after each injection to identify any immediate adverse events. After each injection, participants were asked to record any solicited local and systemic adverse events in their diary cards for up to 7 days. Unsolicited adverse events were recorded for 28 days after each injection. All other adverse events, serious adverse events, adverse events of special interest, and vaccine-associated enhanced diseases were recorded throughout the study period. The severity of solicited and unsolicited adverse events were graded according to modified grading scales of the US FDA Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials (September 2007). Solicited adverse events are defined as those that occurred within 7 days after each dose of study intervention (appendix pp 73–75). Unsolicited adverse events are defined as any untoward medical events, other than solicited adverse events, that occurred within 28 days after each dose of study intervention. The investigator used their clinical judgement to assess the association between the study intervention and the occurrence of each solicited or unsolicited adverse event (appendix pp 77–78).
Immunogenicity was assessed by measuring neutralising antibody responses on days 1 and 57, and anti-spike IgG antibody responses on days 1, 29, 43, 57, and 209. To measure neutralising antibody titres, wild-type SARS-CoV-2, Taiwan CDC strain number 4 (hCoV-19/Taiwan/4/2020; Global Initiative on Sharing All Influenza Data accession ID EPI_ISL_411927), was titrated to calculate the 50% tissue culture infective dose (TCID50). Vero E6 cells were seeded in 96-well plates (at 1·2 × 104 cells per well) and incubated. The serum samples underwent a total of eight two-fold dilutions, starting from a 1:8 dilution to a final dilution of 1:1024. Diluted serum samples were then mixed with an equal volume of 100 TCID50 per 50 μL of virus. After incubating the serum-virus mixture at 37°C for 1 h, it was added to the wells containing Vero E6 cells. The cells were then incubated at 37°C in a 5% CO2 incubator for 4–5 days. The neutralising titre (NT50) was defined as the reciprocal of the highest dilution capable of inhibiting 50% of the cytopathic effect. The NT50 results were derived from quadruplicated tests and calculated with the Reed-Muench method.
To facilitate conversion of geometric mean titres (GMTs) to International Units (IUs/mL) or binding antibody units (BAUs/mL), we purchased WHO international standard reference panels (20/136 and 20/268 [including reference samples 20/150, 20/148, 20/144, and 20/140]) from the National Institute for Biological Standards and Control (NIBSC; Potters Bar, UK) for comparison (appendix p 3).7, 8 To convert GMTs to IU/mL, a neutralising assay was first developed to calculate the GMTs of the NIBSC serum samples. The GMTs were calculated from the titres of three repeated tests. Using the correlation between the GMTs and the assigned IU/mL of each standardised NIBSC serum sample, we established an equation for converting GMT to IU/mL (y=1·0334x + 1·0103, where y is the log2 value of IU/mL and x is the log2 value of the GMT). Finally, we applied this equation to convert the GMTs of participant serum samples to IU/mL.
For the conversion of GMTs to BAUs/mL, total serum anti-spike IgG titres were measured by use of an ELISA with customised 96-well plates coated with S-2P antigen, as previously reported.6 The WHO NIBSC 20/136 reference standard was tested with the same ELISA assay that was used for our study samples. The GMT of the anti-spike IgG titre for NIBSC 20/136 was 10 960·9, which was calculated from seven repeated tests. Since NIBSC 20/136 was assigned as 1000 BAU/mL, a conversion factor of 0·0912 (1000/10 960·9) was established to estimate the BAU/mL values from anti-Spike IgG titres. The raw data for the conversion of anti-spike IgG GMT to BAU/mL is provided in the appendix (p 4).
Outcomes
The primary safety outcomes were to evaluate the safety and tolerability of MVC-COV1901 versus placebo between day 1 and day 57 (28 days after the second dose), and included the occurrence of immediate adverse events, solicited local and systemic adverse events up to 7 days after each injection, unsolicited adverse events up to 28 days after each injection, and any other adverse events, serious adverse events, adverse events of special interest, and vaccine-associated enhanced disease that occurred throughout the study period. The primary immunogenicity outcome was to evaluate neutralising antibody titres on day 1 and day 57 in terms of GMTs, the GMT ratio, and the seroconversion rate.
The secondary safety outcome was to evaluate the safety of MVC-COV1901 over the study period. The secondary immunogenicity outcomes were to evaluate anti-spike IgG antibody titres on days 1 (the day of the first dose), 29 (the day of the second dose), 43 (14 days after the second dose), 57, and 209, the lot-to-lot consistency of MVC-COV1901 in patients aged 20 years to younger than 65 years, and the immunogenicity of MVC-COV1901 compared with placebo, in terms of antigen-specific IgG titres and neutralising antibody titres.
Statistical analysis
The sample size of our trial design meets the minimum safety requirement of 3000 study participants in the vaccine group, as recommended by the US FDA and WHO.9, 10 We planned to enrol approximately 3700 adult participants. Participants were stratified into two age groups: younger adults (aged 20–64 years) and older adults (aged ≥65 years). The older adults were to make up 20% of the participants.
The primary safety outcomes in this interim analysis were assessed in the safety set, which included all randomly assigned participants who had received at least one dose of study intervention. The per-protocol immunogenicity subset consisted of participants who had received two doses of the study intervention (administered 28 days apart), had valid immunogenicity data on day 57, and had no major protocol deviations up to day 57. The per-protocol immunogenicity subset was the primary analysis population for the primary and secondary immunogenicity outcomes in this interim analysis. The immunogenicity subset was to include the first 1090 randomly assigned participants, of whom 820 were younger adults and 270 were older adults. We calculated the sample size for the per-protocol immunogenicity subset by first determining the number of younger adults to be enrolled. Based on the assumption of achieving lot-to-lot consistency with an overall power of 98% and an assumed dropout rate of 6·4%, the lot-to-lot equivalence analysis would require 820 younger adults. To ensure the age proportion of the immunogenicity subset reflected that of the trial population (approximately 80% younger adults and 20% older adults), 270 older adults were included. Further details of the sample size calculations are provided in the appendix (pp 87–89).
Safety data of solicited adverse events are presented as stacked bar charts showing the proportions of partipants in each group according to the type and severity of adverse events. Detailed information on safety are presented in the appendix (pp 5–12). Immunogenicity data are presented as GMTs, GMT ratios, and seroconversion rates, with their associated two-sided 95% CIs. The GMT ratio is defined as the fold increase in post-study GMTs over baseline values. The seroconversion rate for both the neutralisation and anti-spike IgG assay is defined as the proportion of participants with a four-fold or higher increase in titres from baseline or from half the lower limit of detection (defined as eight for the neutralisation assay and 100 for the anti-spike IgG assay) if the baseline sample value was undetectable.
Participants from different study sites were pooled for statistical analysis. Significance tests (two-tailed; α=0·05) were done as follows: a two-sample t test or Wilcoxon rank-sum test was used for pairwise comparison of GMT and GMT ratios; Pearson's χ2 test or Fisher's exact test (in the case of small cell counts or an expected cell count of less than five) was used for calculating the significance of seroconversion rate results; and p values were rounded to four decimal places, if applicable.
All statistical analyses were done using SAS version 9.4 or later. An independent data monitoring committee (IDMC) was established to monitor data safety and trial conduct.
An interim analysis was triggered because the following conditions were met: all participants had completed the second dose of study intervention by 1 month (28 days), and half of participants had completed the second dose of study intervention by 2 months.
This trial is registered with ClinicalTrials.gov, NCT04695652.
Role of the funding source
Medigen Vaccine Biologics (the study sponsor) had a role in study design, data analysis, and data interpretation, but had no role in data collection, or writing of the report. Taiwan CDC of the Ministry of Health and Welfare had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Between Dec 30, 2020 and April 2, 2021, 4173 individuals were screened and 3854 were randomly assigned to the MVC-COV1901 group (n=3304) or the placebo group (n=550; figure 1 ). A total of 3844 participants received at least one dose of the study intervention (3295 in the MVC-COV1901 group and 549 in the placebo group) and were included in the safety set. The investigators did not exclude any eligible participants during enrolment. Up to the data cutoff date (June 2, 2021), a total of 1053 participants (903 in the MVC-COV1901 group and 150 in the placebo group) fulfilled the criteria for inclusion in the per-protocol immunogenicity subset. The mean age of participants in the safety set was 45·00 years (SD 16·27; range 20–89 years), and 2172 (56·5%) of participants were men and 1672 (43·5%) were women (table ). Only one (<0·1%) participant was non-Asian (White). Most participants (3435 [89·4%]) had a body-mass index of less than 30 kg/m2, and negative serology tests for HBsAg, anti-hepatitis C virus, and HIV at baseline. A total of 634 (16·5%) participants had comorbidities, the most common of which were higher glycated haemoglobin A1c concentrations than the normal range (517 [13·4%]), followed by cardiovascular disease (99 [2·6%]) and malignancy (42 [1·1%]).
Figure 1.
Trial profile
*The participant was assigned an incorrect randomisation number.
Table.
Demographics of the participants in the safety set
| MVC-COV1901 group (n=3295) | Placebo group (n=549) | ||
|---|---|---|---|
| Mean age, years | 45·00 (16·27) | 44·30 (16·30) | |
| Sex | |||
| Male | 1854 (56·3%) | 318 (57·9%) | |
| Female | 1441 (43·7%) | 231 (42·1%) | |
| Race | |||
| Asian | 3294 (>99·9%) | 549 (100·0%) | |
| Non-Asian | 1 (<0·1%) | 0 | |
| Mean body-mass index, kg/m2 | 24·92 (4·07) | 24·67 (4·06) | |
| Body-mass index group, kg/m2 | |||
| <30 | 2936 (89·1%) | 499 (90·9%) | |
| ≥30 | 359 (10·9%) | 50 (9·1%) | |
| HBsAg | 197 (6·0%) | 32 (5·8%) | |
| Hepatitis C virus antibodies | 43 (1·3%) | 11 (2·0%) | |
| HIV antibodies | 58 (1·8%) | 10 (1·8%) | |
| Neutralising antibodies against wild-type SARS-CoV-2 before vaccination* | 10/930 (1·1%) | 1/154 (0·6%) | |
| Comorbidities | |||
| Any | 550 (16·7%) | 84 (15·3%) | |
| Cardiovascular disease | 90 (2·7%) | 9 (1·6%) | |
| Cerebrovascular disease | 15 (0·5%) | 2 (0·4%) | |
| Chronic obstructive pulmonary disease | 14 (0·4%) | 1 (0·2%) | |
| Liver cirrhosis | 1 (<0·1%) | 0 | |
| Malignancy | 35 (1·1%) | 7 (1·3%) | |
| Glycated haemoglobin A1c higher than normal range† | 448 (13·6%) | 69 (12·6%) | |
Data are mean (SD), n (%), or n/N (%). The safety set included all participants who had received at least one dose of study intervention.
Participants who had pre-vaccination neutralising antibody concentrations above lower limit of detection were considered seropositive.
The normal range was dependent on each local laboratory where the patients glycated haemoglobin A1c was tested.
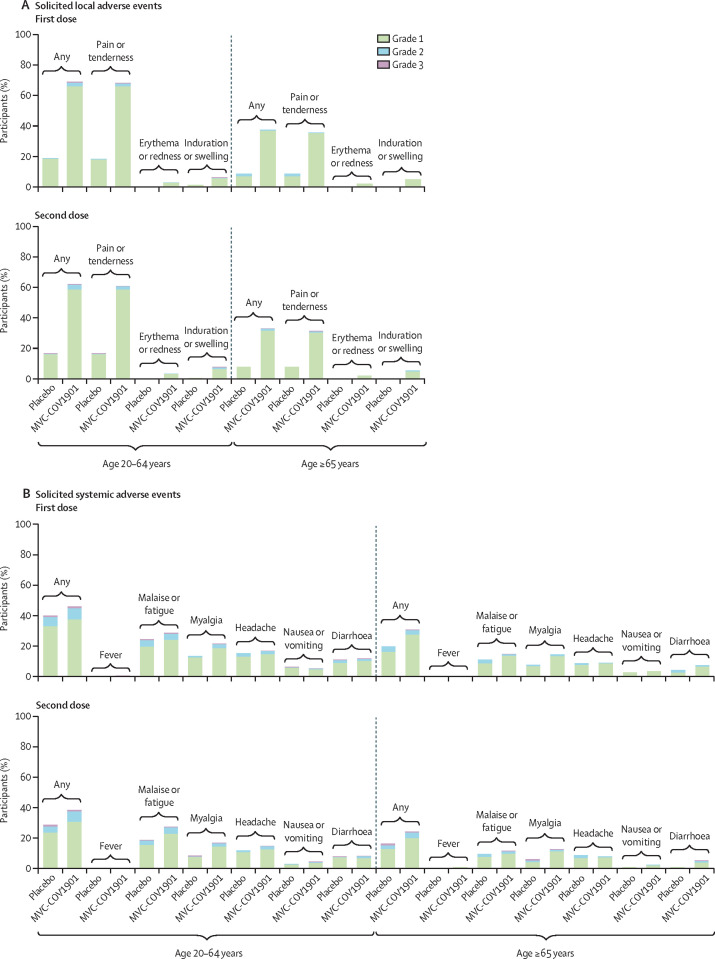
The occurrence of solicited adverse events are summarised in figure 2 and in the appendix (pp 5–13). Overall, 2510 (65·3%) of 3844 participants reported solicited local adverse events after receiving any dose of the study intervention. The MVC-COV1901 group (2381 [72·3%] of 3295) outnumbered the placebo group (129 [23·5%] of 549) in the number of solicited local adverse events reported, which were mostly mild (grade 1) to moderate (grade 2) in severity. After any dose of MVC-COV1901, the most common solicited local adverse event was pain at the injection site (2346 [71·2%] in the MVC-COV1901 group compared with 128 [23·3%] in the placebo group) and the most common solicited systemic adverse event was malaise or fatigue (1186 [36·0%] compared with 163 [29·7%]). Fever was rarely reported in either the MVC-COV1901 group (23 [0·7%] patients) or placebo group (two [0·4%]). Most of the solicited adverse events were self-limiting and resolved within 7 days, with a mean duration of less than 3 days (SD 0·4–1·5). The occurrences of both local and systemic solicited adverse events after any dose of the study intervention in the 65 years and older age group (367 [43·8%] of 838 had a solicited local adverse event and 324 [38·7%] had a solicited systemic adverse event) were slightly lower than that in the overall safety set (appendix p 11).
Figure 2.
Solicited local (A) and systemic (B) adverse events occurring within 7 days of the first and second doses of MVC-COV1901 or placebo
Adverse events were graded as mild (grade 1), moderate (grade 2), or severe (grade 3).
A total of 1081 (28·2%) of 3844 participants reported unsolicited adverse events (appendix p 14). The proportion of all participants who reported unsolicited adverse events and other adverse events in the overall safety set and by age group was similar between the MVC-COV1901 and placebo groups.
No serious adverse events were considered related to the study intervention. One adverse event of special interest (temporary facial paralysis) was reported in one (<0·1%) of 3295 participants in the MVC-COV1901 group, and was considered as possibly related to the vaccine. No deaths or vaccine-associated enhanced diseases were reported.
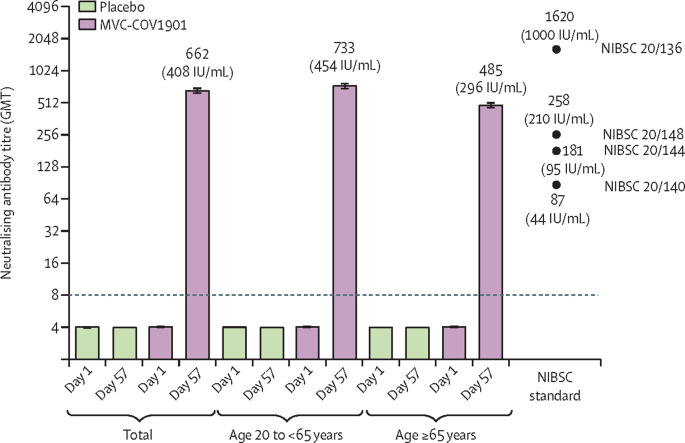
In the per-protocol immunogenicity subset of the MVC-COV1901 group, the wild-type SARS-CoV-2 neutralising antibody GMT was 662·3 (95% CI 628·7–697·8) and the GMT ratio was 163·2 (95% CI 155·0–171·9) on day 57. Both values were significantly higher (p<0·0001) than those in the placebo group (figure 3 ; appendix pp 15–16). In the MVC-COV1901 group, the GMT was higher in the younger adult group (732·9 [692·4–775·7]) than in the older adult group (484·5 [433·2–542·0]). The GMTs of the NIBSC standard 20/136 was 1619·9 (1447·3–1792·5), of 20/148 was 257·5 (207·5–307·5), of 20/144 was 181·2 (134·7–227·6), and of 20/140 was 87·1 (65·6–108·6). After conversion to IU/mL, the GMTs of neutralising antibodies on day 57 were 408·5 IU/mL in all participants in the per-protocol immunogenicity subset, 453·5 IU/mL in the younger adult group, and 295·7 IU/mL in the older adult group (figure 3).
Figure 3.
Neutralising antibody titres against live wild-type SARS-CoV-2 on day 1 and day 57
The height of the bars represent the GMTs, and the error bars represent the corresponding 95% CIs. GMT values greater than the lower limit of detection (ie, >8), indicated by the horizontal dotted line, are included above the bars. The GMT of the triplicate measurement of each NIBSC standard is indicated above, below, or immediately to the right of the filled black circles, and the name of the standard is provided on the right of each black circle. GMT=geometric mean titre. IU=international units. NIBSC=National Institute for Biological Standards and Control.
The seroconversion rate, based on the wild-type SARS-CoV-2 GMT, in the MVC-COV1901 group on day 57 was 99·8% (95% CI 99·2–100·0), with only two participants without seroconversion (appendix p 17). No seroconversion was observed in the placebo group. In both younger and older adults who received the MVC-COV1901 vaccine, almost all participants had seroconversion (99·9% [99·2–100·0] in younger adults and 99·5% [97·5–100·0] in older adults).
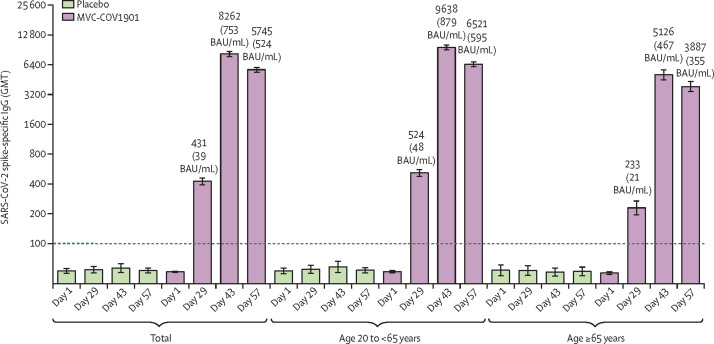
Anti-spike IgG GMTs in the per-protocol immunogenicity subset of the MVC-COV1901 group increased from 52·7 (95% CI 51·7–53·8) on day 1 to 430·5 (95% CI 398·7–464·8) on day 29, but remained at baseline levels in the placebo group (figure 4 ; appendix pp 18–21). The anti-spike IgG titre continued to increase to 8262·2 (7801·9–8,749·5]) on day 43 and remained at 5745·4 (5464·5–6040·6]) on day 57 in this group. The younger adult group had higher anti-spike IgG GMTs than the older adult group at all time points (appendix pp 18–21). After conversion to BAU/mL, anti-spike IgG GMTs on day 57 were 524·0 BAU/mL (498·4–550·9] in the whole per-protocol immunogenicity subset, 594·7 BAU/mL (564·4–626·7) in the younger adult group, and 354·5 BAU/mL (317·0–396·4) in the older adult group. Neutralising and binding antibody GMTs converted to IU/mL and BAU/mL, respectively, are shown in the appendix (p 22).
Figure 4.
SARS-CoV-2 anti-spike IgG GMTs measured by ELISA using S-2P as coated antigen on day 1, 29, 43, and 57
The height of the bars represent the GMTs, and the error bars represent the corresponding 95% CIs. GMT values greater than the lower limit of detection (ie, >100), indicated by the horizontal dotted line, are included above the bars. BAU=binding antibody unit. GMT=geometric mean titre.
The seroconversion rate of anti-spike IgG titres was 74·4% (95% CI 71·4–77·2) in the whole per-protocol immunogenicity subset of the MVC-COV1901 group on day 29, increasing to 99·7% (99·0–99·9) on day 43, and to 99·6% (98·9–99·9) on day 57 (appendix pp 23–24). On day 29, the seroconversion rate was 53·9% (47·0–60·6) in the older adult group and 80·9% (77·8–83·8) in the younger adult group. However, the seroconversion rate in both age groups was similar on day 43 (99·5% [97·5–100·0] vs 99·7% [98·9–100·0]).
Discussion
The design of this phase 2 trial followed the principle of the US FDA guidance for industry on emergency use authorisation of vaccines to prevent COVID-19, published in October, 2020.9 At the time of this interim analysis, 3295 participants had received at least one dose of MVC-COV1901. Since administering the second dose of MVC-COV1901, all participants had completed 1 month of follow-up, and the median follow-up period was 63 days (IQR 56–72). The number of participants in the vaccine group (>3000) and the accumulated safety data published in this report, meet the principles outlined by the US FDA guidance.
The design of the SARS-CoV-2 antigen used in the MVC-COV1901 vaccine involves a range of molecular modifications to the S-2P pre-fusion spike protein, a furitin cleavage site mutation, and T4 fibritin for trimerisation.3 The combination of CpG 1018 and aluminium hydroxide with this S-2P pre-fusion spike protein shows promising enhancements of both T-cell and B-cell immunity, as shown in our phase 1 trial.6 The interim analysis of MVC-COV1901 shows that two doses of vaccination is safe, well tolerated, and elicits favourable neutralising antibody responses in participants aged 20 years and older. One of the most distinct findings in our safety profile is the extremely low incidence of fever (23 [0·7%] of 3295 participants in the MVC-COV1901 group and two [0·4%] of 549 in the placebo group). Compared with other vaccines that have received emergency use authorisation,11, 12, 13 MVC-COV1901 leads to substantially fewer febrile reactions and has a good reactogenicity profile, as observed in this study and our phase 1 trial.6
The phase 2 trial began in December, 2020, when SARS-CoV-2 was not endemic in Taiwan. Therefore, the wild-type SARS-CoV-2 neutralising antibody GMT was low in all study participants at baseline (4·1 [95% CI 4·0–4·1]). However, compared with the wild-type SARS-CoV-2 neutralising antibody GMT in the placebo group on day 57, which remained low at 4·0 (4·0–4·1), the GMT for the MVC-COV1901 group increased to 662·3 (628·7–697·8), which is most likely to be caused by the vaccine.
Khoury and colleagues14 showed that the neutralising antibody titres can be compared with human convalescent serum samples to predict the clinical efficacy of vaccines across different technology platforms. However, the neutralising antibody titres of convalescent serum samples vary significantly, depending on the degree of disease severity and individual immune responses. This variation makes consecutive comparisons of different convalescent serum samples difficult, if not impossible. To facilitate comparison and avoid mis-extrapolation of the GMTs for the prediction of efficacy, we used two different sets of convalescent serum samples as parameters to estimate the ratio of neutralising antibody GMTs in study samples against the GMTs of convalescent serum samples.
Our first parameter is based on the one used in the study by Khoury and colleagues,14 which reported that 20% of convalescent serum samples (54 IU/mL) can approximate 50% vaccine efficacy. Therefore, when we set 270 IU/mL as the GMT of convalescent serum samples (54 IU/20%=270 IU/mL), the GMT on day 57 in the MVC-COV1901 group was 1·51 times higher than that of convalescent serum samples. The second parameter was based on the WHO's reference standard NIBSC 20/148, which had a GMT of 210 IU/mL in figure 3. At 210 IU/mL in convalescent serum samples (ie, the mid-level antibody titre, according to the NIBSC), the GMT on day 57 in the MVC-COV1901 group was 1·94 times higher than that of convalescent serum samples. When compared with the convalescent serum samples for both parameters, the neutralising antibody GMTs in all participants in the per-protocol immunogenicity subset on day 57 were approximately 1·51–1·94 times higher.
Another method to predict clinical efficacy is the BAU conversion model. Researchers at the University of Oxford (Oxford, UK)15 published the correlates of protection for the ChAdOx1 nCoV-19 vaccine from Oxford–AstraZeneca against symptomatic and asymptomatic SARS-CoV-2 infection, which is the only correlates of protection estimate for a COVID-19 vaccine published to date. When converted to BAUs, the report showed that if the anti-spike IgG titre is between 264 and 899 BAU/mL, the predicted vaccine efficacy is between 80% and 90%. In our study, the anti-spike IgG GMT on day 57 in participants who received the MVC-COV1901 vaccine was 524·0 BAU/mL in the whole per-protocol immunogenicity subset, 594·7 BAU/mL in the younger adult group and 354·5 BAU/mL in the older adult group. Even though the effects of cellular immunity and ethnic differences remain unclear, MVC-COV1901 yields similar predictions of efficacy using both the IU and the BAU conversion models.
For regulatory authorities to use correlates of protection for cross-vaccine comparisons to predict clinical efficacy, a standardised assay that converts GMT to IU or BAU will be a prerequisite.16, 17 This conversion assay would expedite vaccine approval, facilitate cross-checking of immunogenicity between trials, and enable data comparisons between different labs. To our knowledge, this study is the first to report neutralising antibody titres and anti-spike IgG titres using WHO IU and BAU units for a COVID-19 vaccine in a phase 2 clinical trial. We applied the IU conversion method to validate the consistency of immunogenicity between our phase 1 and phase 2 trials from different laboratories. Using the WHO IU, the GMT on day 57 in our phase 1 study was converted from 52·2 to 351·2 IU/mL, and from 663·2 to 408·5 IU/mL in our phase 2 study. The converted GMTs indicate that the immunogenicity results from our phase 1 and phase 2 trials are similar. Our results show the many benefits of standardised assays, which can facilitate the application of correlates of protection for regulatory authorities to consider.
It is paramount for a COVID-19 vaccine to provide protection for people at increased risk of severe complications. Several reports show that COVID-19 mortality rates are higher in elderly patients with chronic comorbidities than in younger patients or those without comorbidities.18, 19 In this phase 2 trial, we included older adult participants with various, but stable, chronic comorbidities, which allowed us to assess the safety and immunogenicity profiles of MVC-COV1901 in a representative sample of people in this age group. As the safety profiles, neutralising antibody titres, tolerability, and occurrence of vaccine-related serious adverse events were similar between the younger and older adult age groups, MVC-COV1901 is suitable for older adults aged 65 years and older with comorbidities.
We acknowledge the limitations of our study. The low viral transmission rate in Taiwan at the time of the study, together with the small size of the placebo group, hindered observation of both vaccine efficacy as the exploratory endpoint and the risk of vaccine-associated enhanced disease. The short duration of follow-up before the interim analysis prevented us from assessing the durability of immune responses after day 57; the long-term follow-up analysis will be available on completion of this phase 2 trial in October, 2021. In addition, the racial diversity of study participants is restricted to ethnicities in Taiwan. We currently have no available data to draw conclusions about the immune response and safety of MVC-COV1901 in children aged younger than 20 years or in pregnant or lactating women. High-risk participants and those with one or more uncontrolled comorbidities were also excluded at baseline; therefore, the efficacy of MVC-COV1901 in these individuals cannot be established. These limitations will be addressed in the ongoing study in adolescents (NCT04951388) or our phase 3 trials, which will be done in regions outside Taiwan where the prevalence of COVID-19 infection is high.
In conclusion, this interim analysis shows that the MVC-COV1901 vaccine has a good safety profile and elicits promising neutralising antibody titres. MVC-COV1901 is safe, well tolerated, and rarely causes febrile reactions in both young and older adults. MVC-COV1901 induces high neutralising antibody and anti-spike IgG titres, and it has a seroconversion rate of almost 100% by day 57. Using WHO IU and BAU conversion models, the predicted clinical efficacy for MVC-COV1901 is similar according to both methods. The phase 2 trial results support the advancement of MVC-COV1901 in subsequent phase 3 trials. MVC-COV1901 has recently been granted emergency use authorisation in Taiwan.
Data sharing
Data sharing is not applicable to this Article, as it is an interim analysis of the study; the trial is still ongoing.
Declaration of interests
CC, CEL, and I-CT are employees of Medigen Vaccine Biologics (Taipei, Taiwan) and they report receiving grants from Taiwan Centres for Disease Control, Ministry of Health and Welfare, during the conduct of the study. CC also has a patent pending relating to the MVC-COV1901 vaccine against SARS-CoV-2 (US17/351,363). All other authors declare no competing interests.
Acknowledgments
Acknowledgments
The study was funded by Medigen Vaccine Biologics (study sponsor) and the Taiwan Centres for Disease Control, Ministry of Health and Welfare. The sponsor co-designed the trial and coordinated interactions with contract Clinical Research Organization (CRO) staff and regulatory authorities. The CRO took charge of trial operation to meet the required standards of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use and good clinical practice guidelines. The IDMC oversaw the safety data and gave recommendations to the sponsor. The interim analysis was done by the CRO. We thank Stanley Chang, Meei-Yun Lin, and Luke Tzu-Chi Liu at Medigen Vaccine Biologics for drafting, editing, and revising the manuscript; all the trial participants for their dedication to this trial; the investigational staff at National Taiwan University Hospital, Taiwan Taipei Veterans General Hospital, Tri-Service General Hospital, Taipei Veterans General Hospital, Taipei Medical University Hospital, Taipei Municipal Wan Fang Hospital, Linkou Chang Gung Medical Hospital, Taoyuan General Hospital Ministry of Health and Welfare, China Medical University Hospital, Changhua Christian Hospital, National Cheng Kung University Hospital, and Kaohsiung Medical University Chung-Ho Memorial Hospital, the Clinipace Clinical Research team (Taipei, Taiwan), and Hao-Yuan Cheng, Criss Cheng, Meng-Ju Tsai at Medigen Vaccine Biologics for their involvement in conducting the trial; Barney S Graham at the Vaccine Research Centre, US National Institute of Allergy and Infectious Diseases, for the development of S-2P pre-fusion protein; Robert Janssen at Dynavax Technologies for providing important intellectual content during manuscript preparation; Dynavax Technologies for providing the CpG 1018 adjuvant; Wei-Cheng Lian, Erh-Fang Hsieh, and Yi-Jiun Lin at Medigen Vaccine Biologics for their collaboration with vaccine production, and their important contributions to the investigational new drug application and laboratory assay development; the members of the independent data monitoring committee; team members at Protech Pharmaservices (Taipei, Taiwan) for conducting the spike-specific IgG ELISA assay; Mei-Jen Hsiao, Peng-Nien Huang, Po-Wei Huang, Chia-Pei Chen, Yueh-Te Lin, Fang-Yu Lin, and Ya-Jhu Lin at the Department of Laboratory Medicine, Linkou Chang Gung Memorial Hospital (Taoyuan, Taiwan), and team members at Institute of Biomedical Sciences, Academia Sinica (Taipei, Taiwan) for conducting the neutralisation assay.
Contributors
S-MH and I-CT conceptualised and designed the study. S-MH, M-CL, Y-HC, W-SL, S-JH, S-HC, K-PH, W-CK, N-CW, and Yu-LL acquired and interpreted the data. S-MH, M-CL, Y-HC, Yu-LL, S-JH, S-HC, CEL, I-CT, and Yi-LL, drafted and prepared the manuscript. Yi-LL, C-GH, S-RS, C-CL, J-JL, and C-SC set up and did the laboratory assays, and analysed the data. CC provided administrative, technical, and material support. All authors reviewed and approved the final version of the manuscript. S-MH, T-YL, CEL, and I-CT had full access to and verified all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. S-MH, T-YL, CEL, and I-CT had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO WHO Director-General's opening remarks at the mission briefing on COVID-19–11 March 2020. World Health Organization. 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020
- 3.Kuo TY, Lin MY, Coffman RL, et al. Development of CpG-adjuvanted stable prefusion SARS-CoV-2 spike antigen as a subunit vaccine against COVID-19. Sci Rep. 2020;10:20085. doi: 10.1038/s41598-020-77077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lien CE, Lin YJ, Kuo TY, et al. CpG-adjuvanted stable prefusion SARS-CoV-2 spike protein protected hamsters from SARS-CoV-2 challenge. Sci Rep. 2021;11:1–7. doi: 10.1038/s41598-021-88283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsieh SM, Liu WD, Huang YS, et al. Safety and immunogenicity of a recombinant stabilized prefusion SARS-CoV-2 spike protein vaccine (MVC-COV1901) adjuvanted with CpG 1018 and aluminium hydroxide in healthy adults: a phase 1, dose-escalation study. EClinicalMedicine. 2021;38:100989. doi: 10.1016/j.eclinm.2021.100989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NIBSC . WHO international standard, first WHO international standard for anti-SARS-CoV-2 immunoglobulin (human), NIBSC code: 20/136, instructions for use, version 2.0. National Institute for Biological Standards and Control; Hertfordshire: 2020. [Google Scholar]
- 8.NIBSC . WHO reference panel, first WHO international reference panel for anti-SARS-CoV-2 immunoglubulin, NIBSC code: 20/268, instructions for use, version 3.0. National Institute for Biological Standards and Control; Hertfordshire: 2020. [Google Scholar]
- 9.US Food and Drug Administration Emergency use authorization for vaccines to prevent COVID-19: guidance for industry. 2020. https://downloads.regulations.gov/FDA-2020-D-1137-0019/attachment_1.pdf
- 10.WHO Guidelines on clinical evaluation of vaccines: regulatory expectations. 2020. https://cdn.who.int/media/docs/default-source/prequal/vaccines/who-trs-1004-web-annex-9.pdf?sfvrsn=9c8f4704_2&download=true
- 11.Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu L, McPhee R, Huang W, et al. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine. 2021;39:2791–2799. doi: 10.1016/j.vaccine.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 14.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 15.Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. medRxiv. 2021 doi: 10.1101/2021.06.21.21258528. published online June 24. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen J. Can immune responses predict which vaccines work best? Science. 2021;373:142–143. doi: 10.1126/science.373.6551.142. [DOI] [PubMed] [Google Scholar]
- 17.Corey L, Mascola JR, Fauci AS, Collins FS, et al. A strategic approach to COVID-19 vaccine R&D. Science. 2020;368:948–950. doi: 10.1126/science.abc5312. [DOI] [PubMed] [Google Scholar]
- 18.Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180:1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noor FM, Islam MM. Prevalence and associated risk factors of mortality among COVID-19 patients: a meta-analysis. J Community Health. 2020;45:1270–1282. doi: 10.1007/s10900-020-00920-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this Article, as it is an interim analysis of the study; the trial is still ongoing.