Abstract
Performance as a function of polar angle at isoeccentric locations across the visual field is known as a performance field (PF) and is characterized by two asymmetries: the HVA (Horizontal-Vertical Anisotropy) and VMA (Vertical Meridian Asymmetry). Exogenous (involuntary) spatial attention does not affect the shape of the PF, improving performance similarly across polar angle. Here we investigated whether endogenous (voluntary) spatial attention, a flexible mechanism, can attenuate these perceptual asymmetries. Twenty participants performed an orientation discrimination task while their endogenous attention was either directed to the target location or distributed across all possible locations. The effects of attention were assessed either using the same stimulus contrast across locations or equating difficulty across locations using individually-titrated contrast thresholds. In both experiments, endogenous attention similarly improved performance at all locations, maintaining the canonical PF shape. Thus, despite its voluntary nature, like exogenous attention, endogenous attention cannot alleviate perceptual asymmetries at isoeccentric locations.
Keywords: Endogenous attention, Visual performance fields, Vertical meridian asymmetry, Horizontal-vertical anisotropy, Spatial vision, Contrast sensitivity
Introduction
We deploy attention routinely in our daily life – from the morning’s alarm waking us up to searching for that stapler that always seems to be missing. At any given time, the brain has limited resources for processing an overwhelming amount of information. Successful deployment of attention allows us to selectively process relevant information, can help overcome the inherent processing limitations of our sensory systems, and can improve visual performance. Spatial attention may be allocated overtly by moving one’s eyes toward a specific location, or covertly by attending to a location without directing one’s gaze toward it. Spatial covert attention improves performance in many visual detection, discrimination, and localization tasks (for reviews see Carrasco, 2011, 2014; Carrasco & Barbot, 2015) and alters appearance in many visual tasks (for review see Carrasco & Barbot, 2019).
There are two spatial covert attention systems, the effects of which on visual perception have been well characterized: Endogenous attention corresponds to our ability to voluntarily monitor information at a given location for sustained periods of time, whereas exogenous attention corresponds to involuntary capture to a location where sudden stimulation has occurred. Endogenous attention is sustained and takes about 300 ms to be deployed, whereas exogenous is transient, peaking at about 100–120 ms and decaying quickly thereafter (e.g., Müller & Rabbitt, 1989; Liu, Stevens, & Carrasco, 2007; for reviews see, Carrasco, 2011, 2014; Carrasco & Barbot, 2015). These two covert attention systems are underpinned by distinct but partially overlapping neural networks (e.g., Beck & Kastner, 2009; Corbetta & Shulman, 2002; Dugué, Merriam, Heeger & Carrasco, 2017; 2020). Selection history also plays a role in the deployment of spatial covert attention. For instance, recent experience implicitly guides attention, as if the brain assumes that the world is stable and the most relevant features a moment ago are likely to remain so (e.g., Chun & Nakayama, 2000; White, Rolfs, & Carrasco, 2013; Yashar & Lamy, 2010).
Endogenous and exogenous attention can have similar effects on both perception and appearance (for reviews, see Carrasco, 2006, 2011, 2014; Carrasco & Barbot, 2015, 2019). But, critically, both types of attention also have differential effects. Whereas exogenous attention is automatic and inflexible, endogenous attention is more flexible. For instance, the magnitude of the endogenous attention effect on perception, in terms of both discriminability and speed of processing, scales with cue validity (e.g., Giordano, McElree & Carrasco, 2009; Kinchla, 1980; Sharp, Melcher & Hickey, 2018; Sperling & Melchner, 1978), but that of exogenous attention does not; its effects on discriminability and speed of processing are constant regardless of cue validity (Giordano et al., 2009). Endogenous attention optimizes performance as a function of task demands (e.g., Barbot, Landy & Carrasco, 2012; Barbot & Carrasco, 2017; Hein, Rolke & Ulrich, 2006; Jigo & Carrasco, 2020; Sharp, Melcher & Hickey, 2018; Yeshurun, Montagna & Carrasco, 2008). For instance, endogenous attention improves performance by either increasing or decreasing spatial resolution depending on what is beneficial for the task at hand (Barbot & Carrasco, 2017).
In contrast, exogenous cues cannot be ignored even when they are known to be uninformative and irrelevant (e.g., Dugué et al., 2017; Herrmann, Montaser-Kouhsari, Carrasco & Heeger, 2010; Müller & Rabbitt, 1989; Yantis & Jonides, 1990). Moreover, exogenous attention automatically increases spatial resolution even when detrimental for task performance (e.g., Jigo & Carrasco, 2018; Yeshurun & Carrasco, 1998; Yeshurun et al., 2008). But in the same texture segmentation task, endogenous attention always improves perception (Jigo & Carrasco, 2018; Yeshurun et al., 2008) by flexibly adjusting spatial resolution (Barbot & Carrasco, 2017). Moreover, a recent study has revealed that endogenous and exogenous attention distinctly modulate activity in visuo-occipital areas; endogenous attention facilitates both the encoding and the readout of visual information whereas exogenous attention only facilitates the encoding of information (Dugué, Merriam, Heeger & Carrasco, 2020).
Discriminability and the speed of information processing across the visual field differ as a function of eccentricity (e.g., Carrasco & Frieder, 1997; Carrasco, McElree, Denisova & Carrasco, 2003) and polar angle at isoeccentric locations. The pattern of performance as a function of polar angle at isoeccentric locations, known as a performance field (PF), has been observed for fundamental visual dimensions such as contrast sensitivity (e.g., Abrams, Nizam & Carrasco, 2012; Baldwin, Meese & Baker, 2012; Cameron, Tai & Carrasco, 2002; Carrasco, Talgar & Cameron, 2001; Himmelberg, Winawer & Carrasco, 2020; Levine & McAnany, 2005; Mackeben, 1999; Pointer & Hess, 1989; Regan & Beverley, 1983; Rijsdijk, Kroon & van der Wildt, 1980; Robson & Graham, 1981; Rovamo & Virsu, 1979), contrast appearance (Fuller, Rodriguez, & Carrasco, 2008), spatial resolution (e.g., Altpeter et al., 2000; Barbot, Xue, & Carrasco, 2021; Carrasco, Williams & Yeshurun, 2002; Greenwood, Szinte, Sayim & Cavanagh, 2017; Montaser-Khousari & Carrasco, 2009; Nazir, 1992; Talgar & Carrasco, 2002), spatial crowding (Greenwood et al., 2017; Petrov & Meleshkevich, 2011; Wallis & Bex, 2012), temporal information accrual (Carrasco, Giordano & McElree, 2004), crowding (Fortenbaugh, Silver & Robertson, 2015; Greenwood et al., 2017), motion perception (Fuller & Carrasco, 2009) and visual short-term memory (Montaser-Kouhsari & Carrasco, 2009).
In a typical PF psychophysical task, stimuli are presented at isoeccentric locations around the visual field while observers maintain central fixation (monitored using an eye-tracker). The method of constant stimuli can be used to estimate psychometric functions at each location. Alternatively, adaptive methods may be used to estimate observer thresholds; all stimuli are then presented at threshold during a discrimination task. To investigate the effects of attention on PF, performance is assessed when attention is directed towards a target (valid trials), away from it (invalid trials), or equally distributed across all locations (neutral trials). Both task and stimuli are kept constant across all attention conditions. Figure 1 shows a schematic representation of the typical PF shape for adults, when stimuli are placed at isoeccentric locations along the cardinal meridians.
Fig. 1. Schematic representation of a typical visual performance field.
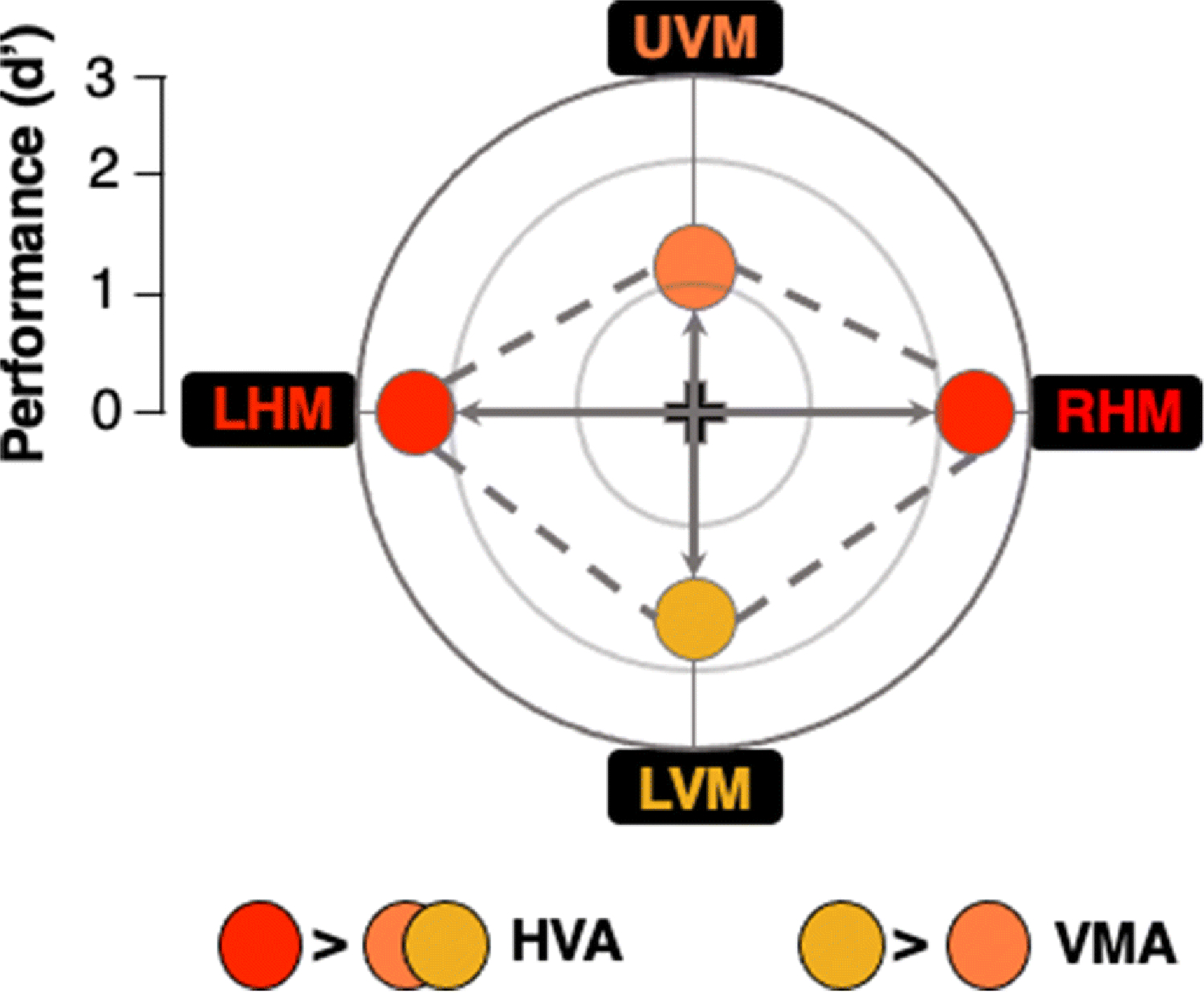
The center point (intersection of the cardinal lines) represents chance performance and more eccentric points represent higher performance. (1) Horizontal-vertical anisotropy (HVA): better performance at isoeccentric locations along the horizontal (left horizontal meridian (LHM) and right horizontal meridian (RHM) locations) than vertical (upper vertical meridian (UVM) and lower vertical meridian (LVM) locations) meridian of the visual field, and (2) vertical meridian asymmetry (VMA), better performance at the location directly below fixation (LVM) than directly above (UVM).
Performance at the cardinal locations around the visual field is characterized by two asymmetries: the HVA (horizontal-vertical anisotropy) and VMA (vertical meridian asymmetry). The HVA reflects better performance along the horizontal meridian (HM) than the vertical meridian (VM). The VMA refers to better performance along the lower vertical meridian (LVM) than along the upper vertical meridian (UVM). Typically, performance at intercardinal locations is intermediate between the horizontal and the vertical meridian (e.g., Cameron et al., 2002; Carrasco et al., 2001) and both asymmetries decrease gradually as the target location increases from the vertical meridian (Abrams et al., 2012; Barbot et al., 2020).
The HVA and VMA are pervasive; they emerge regardless of stimulus orientation, display luminance, and whether the stimuli are presented monocularly or binocularly (Barbot et al., 2020; Carrasco et al., 2001). PFs shift in line with egocentric referents, corresponding to the stimulus retinal location (Corbett & Carrasco, 2011). PF asymmetries are more pronounced as spatial frequency, eccentricity, or set size increase (Carrasco et al., 2001; Fuller, Rodriguez & Carrasco, 2008; Himmelberg et al., 2020). Although the extent of PF asymmetries varies slightly across observers, their overall shape remains reliable (e.g., Abrams et al., 2012; Cameron et al., 2002; Carrasco et al., 2001; Himmelberg et al., 2020). These asymmetries are ubiquitous in visual perception and are also preserved in short-term memory tasks (Montaser-Kouhsari & Carrasco, 2009). Note that as a result of these asymmetries, we see objects at different levels of discriminability in daily life, depending on their position in our visual field.
We have previously investigated whether PF asymmetries can be altered by attention. We used a variety of visual tasks for which exogenous attention is known to improve performance. We found that there were similar performance improvements in discriminability across isoeccentric locations on acuity (Carrasco et al., 2002), texture segmentation (Talgar & Carrasco, 2002) and contrast sensitivity tasks (e.g., Cameron et al., 2002; Carrasco et al., 2001; Himmelberg et al., 2020) for neurotypical participants, as well as for those with amblyopia (Roberts, Cymerman, Smith, Kiorpes & Carrasco, 2016) or attention-deficit hyperactivity disorder (ADHD; Roberts, Ashinoff, Castellanos & Carrasco, 2018). Thus, in all instances we have investigated, exogenous attention improves performance while preserving the PF shape.
Observers are capable of deploying endogenous covert attention across the visual field, including along the vertical meridian (e.g., Giordano et al., 2009; Ling & Carrasco, 2006); however, it is unknown whether, and how, its allocation affects visual PFs. Given its more flexible nature and that it optimizes performance as a function of task demands (e.g., Barbot, Landy & Carrasco, 2012; Barbot & Carrasco, 2017; Hein, Rolke & Ulrich, 2006; Jigo & Carrasco, 2020; Yeshurun, Montagna & Carrasco, 2008), we hypothesized that endogenous attention may play a compensatory role by differentially affecting performance at isoeccentric locations to alleviate visual asymmetries around the visual field. This result would indicate that heterogeneous performance at different locations is permeable and can be overcome by top-down attentional effects. Alternatively, endogenous attention may improve performance to a similar degree across locations even when discriminability is lower at some locations than others. This result would suggest that differential performance at various locations around the visual field is impervious to such top-down attentional factors.
In this study, we investigated how endogenous attention affects the shape of the PF under two conditions: (1) When stimulus contrast is the same at all locations, and thus discriminability across isoeccentric locations is heterogeneous, representative of real-world conditions (Experiment 1), and (2) when stimulus contrast at each location is adjusted to equate discriminability, so that attention can exert its effects relative to the same baseline level (Experiment 2).
Methods
Participants
The same 20 adults (15 F, M age = 27.3±4.7 years; two left-handed) participated in both Experiment 1 and 2, all of whom possessed normal or corrected-to-normal vision and were attending college or graduate school. We did a simulation-based power analysis using data from a study on the effects of exogenous attention on contrast sensitivity across the horizontal and vertical meridians (Cameron, Tai, & Carrasco, 2002). Individual subject data were randomly drawn from Gaussian variables that were centered on the group-average contrast threshold for each attention cue type (Neutral, Valid) and target location (HM, LVM, UVM) and had a width determined by the SEM for each condition across the group. A two-way (attention x location) repeated-measures ANOVA was used to assess statistical significance and effect size was quantified by generalized eta-squared. Given an effect size of 0.35, a sample size of 20 observers was required to detect a significant attention x location interaction with 87% power at a 0.01 significance criterion.
Apparatus and setup
Participants were tested in a dimly lit, sound-attenuated room. Stimuli were programmed on an Apple iMac MC413LL/A 21.5-in. desktop (3.06 GHz Intel Core 2 Duo) using MATLAB (Mathworks, Natick, MA, USA) in conjunction with the MGL toolbox (http://gru.stanford.edu/doku.php/mgl/overview). Stimuli were presented at a viewing distance of 57cm on a 21-in. IBM P260 CRT monitor (1280×960 pixel resolution, 90-Hz refresh rate), calibrated and linearized using a Photo Research (Chatworth, CA, USA) PR-650 SpectraScan Colorimeter. Participants performed the task using a forehead- and chinrest to ensure head stabilization. Eye movements were monitored using an EyeLink 1000 Desktop Mount eyetracker (SR Research, Ontario, Canada).
Stimuli
Observers were asked to fixate on a black, central cross (0.5° across) throughout the trial (Fig. 2). Four placeholders – each composed of four black dots (0.05° radii) arranged in a circle 0.5° from the edge of an upcoming Gabor patch stimulus (to prevent masking) – were always presented on the screen to reduce location uncertainty. The target and three distractor stimuli were 3.2° wide Gabor patches (contrast-defined sinusoidal gratings embedded in a Gaussian envelope, σ = 0.46°) with a spatial frequency of four cycles per degree (cpd), and the same mean luminance as the uniform gray background (26 cd/m2). They were centered at 6.4° eccentricity along the cardinal meridians. During each trial, the Gabors were independently and randomly tilted ±20° from vertical. To manipulate endogenous spatial attention, we presented a central precue – either a single 0.88° line or four 0.28° lines (all 0.14° thick) – 0.38° from the center of the fixation cross, indicating either one (100% valid, attention condition) or all (neutral, distributed condition) of the possible target locations. A response cue (identical to the single valid precue) indicated the target location by pointing to one placeholder and eliminated location uncertainty at response time (Pelli, 1985).
Fig. 2. Task sequence.
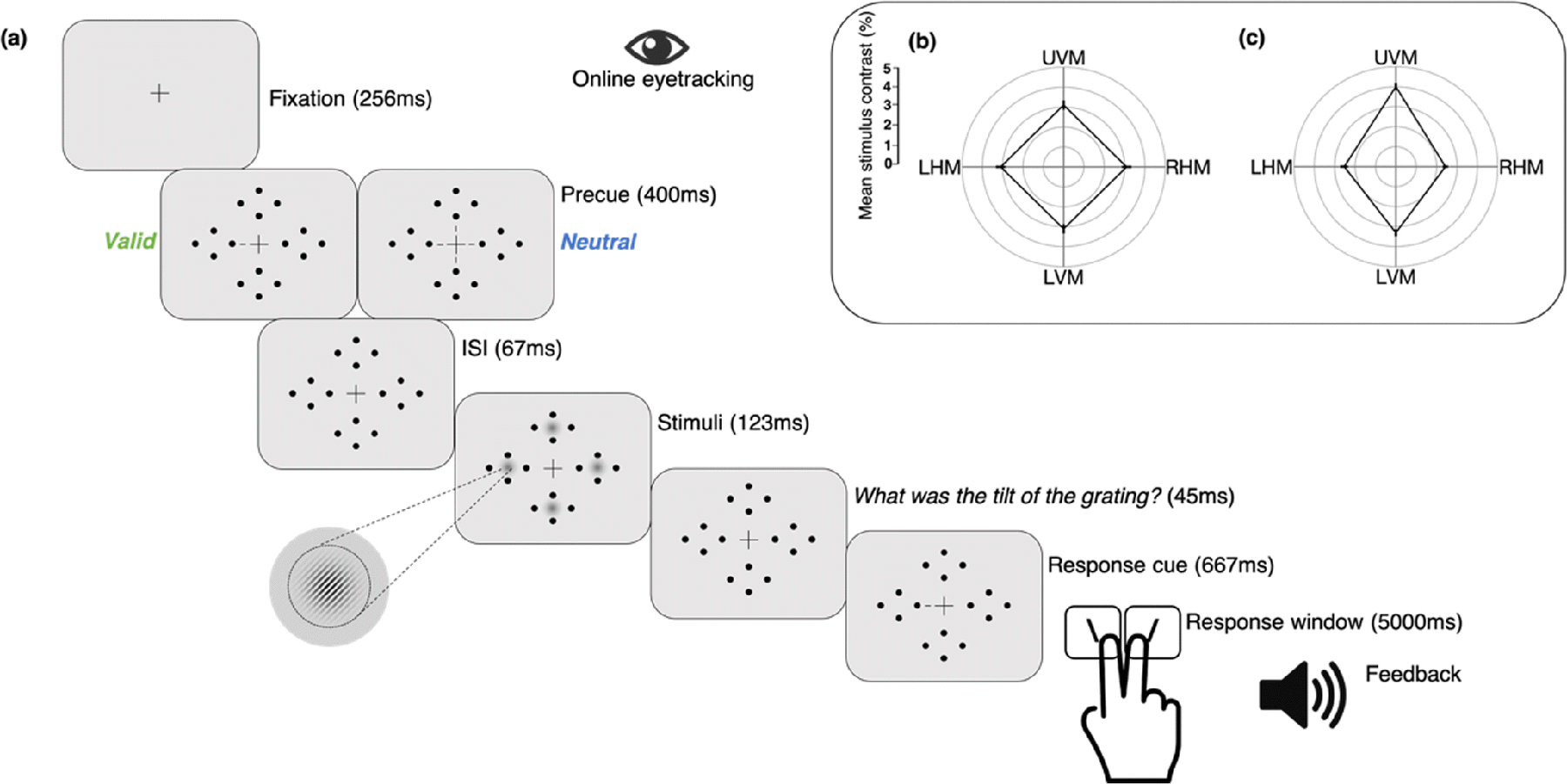
(a) Observers performed a two-alternative forced-choice (2AFC) orientation discrimination task, contingent upon contrast sensitivity, while maintaining fixation. The target could appear at any one of four isoeccentric locations along the cardinals. Participants were either cued towards a single location (Valid cue) or to all four locations (Neutral cue). On every trial, four Gabor patches briefly appeared simultaneously at all stimulus locations. A response cue indicated the target, for which participants reported the tilt (left or right). Note that Gabor tilt angle, size of placeholders, fixation point, and response cue have been exaggerated for clarity. Subpanel: Group average stimulus contrast by location was the same for valid and neutral cue condition trials in each experiment, and are shown for (b) Experiment 1, same contrast across locations resulting in different performance across locations, and (c) Experiment 2, different contrast at different locations to equate performance across locations.
Procedure
Participants underwent three sessions: (1) stimulus contrast thresholding (eight blocks, one hour), (2) the heterogeneous discriminability experiment and (3) the homogeneous discriminability experiment (both 12 blocks, ~1.5 h each). The thresholding session was always conducted first and consisted of a PEST staircase procedure in which eight interleaved three-down, one-up staircases (two staircases per location; one starting from 75% and the other at 25% contrast) estimated a single contrast threshold value (~80% accuracy overall) for each location. Only neutral cues were presented in the thresholding session, and observers were asked to report the orientation of a single target without any distractors on each trial.
Experiment sessions consisted of about 12 blocks (Exp. 1: M = 11.6, SD = 1.35; Exp. 2: M = 12.2, SD = 0.41) of 80 trials each, for a total of ~960 trials; 480 trials each in the valid and neutral cue conditions (50 −50 distribution; 120 trials for each location in each attention condition, presented in a random order).
Experiment 1: In the ‘heterogeneous discriminability across locations’ experiment, Gabors at all locations were presented at the same contrast, which was the average of the contrast thresholds independently measured at the four potential target locations (Fig. 2b). In between blocks, stimulus contrast was adjusted to maintain ~80% accuracy across all locations and cueing conditions. This allowed us to assess whether the magnitude of the endogenous attention effect differs as a function of unequal discriminability across isoeccentric cardinal locations for a given contrast. Such compensatory attentional benefits would alter the canonical PF shape.
Experiment 2: In the ‘homogeneous discriminability across locations’ experiment, we equated discriminability across the visual field by presenting stimuli of different contrasts according to the independently measured contrast thresholds of each location. In between blocks, stimulus contrast was adjusted separately for each location to maintain 80% accuracy across cueing conditions (Fig. 2c). This ensured that participants not only performed well above chance but had similar room for improvement at all locations.
The order of the two experiments was counterbalanced across participants. Throughout all sessions, if participants made an eye movement ≥1° from the fixation cross at any time between trial initiation and stimulus offset, the trial would be aborted and the text “Please fixate” would appear at the center of the screen. These trials were rerun at the end of the respective block.
Task and trial sequence
In both experiments, participants performed a two-alternative forced-choice (2AFC) orientation discrimination task binocularly while their endogenous spatial attention was manipulated via presentation of either a single (100% valid, 50% of all trials) or distributed central precue (50% of all trials; Fig. 2a). After fixating for 256 ms, the precue was presented for 400 ms, after which there was a brief blank interstimulus interval (ISI) of 67 ms. The 467 ms stimulus-onset asynchrony (SOA) between precue onset and stimulus ensured that observers had ample time to voluntarily deploy their endogenous attention to the cued location (e.g., Liu, Stevens & Carrasco, 2007). After the blank interval, the target and three distractor Gabor patches appeared simultaneously inside the placeholders for 123 ms. There was a brief 45-ms ISI between display offset and the response cue, which remained on the screen for 667 ms. A mid-frequency auditory tone indicated the beginning of the 5-s response window, in which observers had to report the target tilt (clockwise (CW) or counterclockwise (CCW) relative to vertical) using one of two keyboard presses (1 for CCW, 2 for CW) with their right hand. On every trial, observers were encouraged to respond as accurately as possible. Observer response terminated the response window, after which there was a mandatory 1-s intertrial interval. Auditory feedback was provided at the end of each trial (low-frequency tone: incorrect; high-frequency tone: correct) and visual feedback indicating observers’ accuracy and number of fixation breaks was presented onscreen at the end of each block.
Results
We conducted ANOVAs on sensitivity (d’), our main dependent variable, and median reaction times (RTs) for correct trials as our secondary variable, to ensure no speed-accuracy tradeoffs.
d’ was calculated as the z-score of the proportion of hits (target was CW, observer reported CW) minus the z-score of the proportion of false alarms (target was CCW, observer reported CW) (e.g., Jigo & Carrasco, 2020). To avoid infinite values when computing d’, we adjusted all d’ values using the conservative log-linear rule, according to which 0.5 was added to the number of hits and false alarms before computing d’ (Hautus,1995). To confirm that any nonsignificant results were not simply due to a lack of statistical power to find differences between groups, we transformed the sum of squared errors obtained from our ANOVAs to arrive at pBIC scores, which represent the posterior probabilities generated by Bayesian Information Criterion estimates of the Bayes factor (Masson, 2011). pBIC scores reflect the strength of evidence in favor of the null (H0) – a nonsignificant main- or interaction- effect – or alternative (H1) – a significant main- or interaction- effect – hypotheses given a dataset D. pBIC values between .50 −.75, .75 −.95, .95 −.99, and >.99 are considered weak, positive, strong, and very strong evidence, respectively (Masson, 2011).
All participants showed a significant overall cueing effect regardless of location. We found no significant main or interaction effects of sex in our data. We also conducted secondary analyses in which the data were weighted by an individual’s contribution to the overall dataset (i.e., total number of completed experimental blocks) and found no difference in the results. Note that whenever Mauchly’s test indicated that the assumption of sphericity had been violated, degrees of freedom were adjusted using the Greenhouse-Geisser correction.
Experiment 1: Heterogeneous discriminability across locations
When stimuli at all locations had the same contrast (M contrast = 3%, 95% CI [2.6%, 3.6%]; Fig. 2b), observers exhibited canonical performance fields on both valid and neutral trials (Fig. 3a). Participants showed pronounced and consistent HVA, i.e., higher performance accuracy along the HM than the VM (Fig. 3b), and VMA, i.e., higher accuracy at the LVM than the UVM (Fig. 3c). All observers showed an HVA in both neutral and valid conditions, and most showed a VMA in both conditions (except for three observers in the neutral and one in the valid condition). There were individual differences in the extent of both asymmetries for both neutral and valid conditions, as illustrated in the scatterplots. Notably, there were significant correlations for all participants’ performance along the HM, the UVM and the LVM between the neutral and attention conditions (r = 0.633, p < .005; Fig. 4). This correlation provides converging evidence that the attention effect was similar across locations, regardless of individual differences in the extent of performance field asymmetries.
Fig. 3. Performance in Experiment 1.
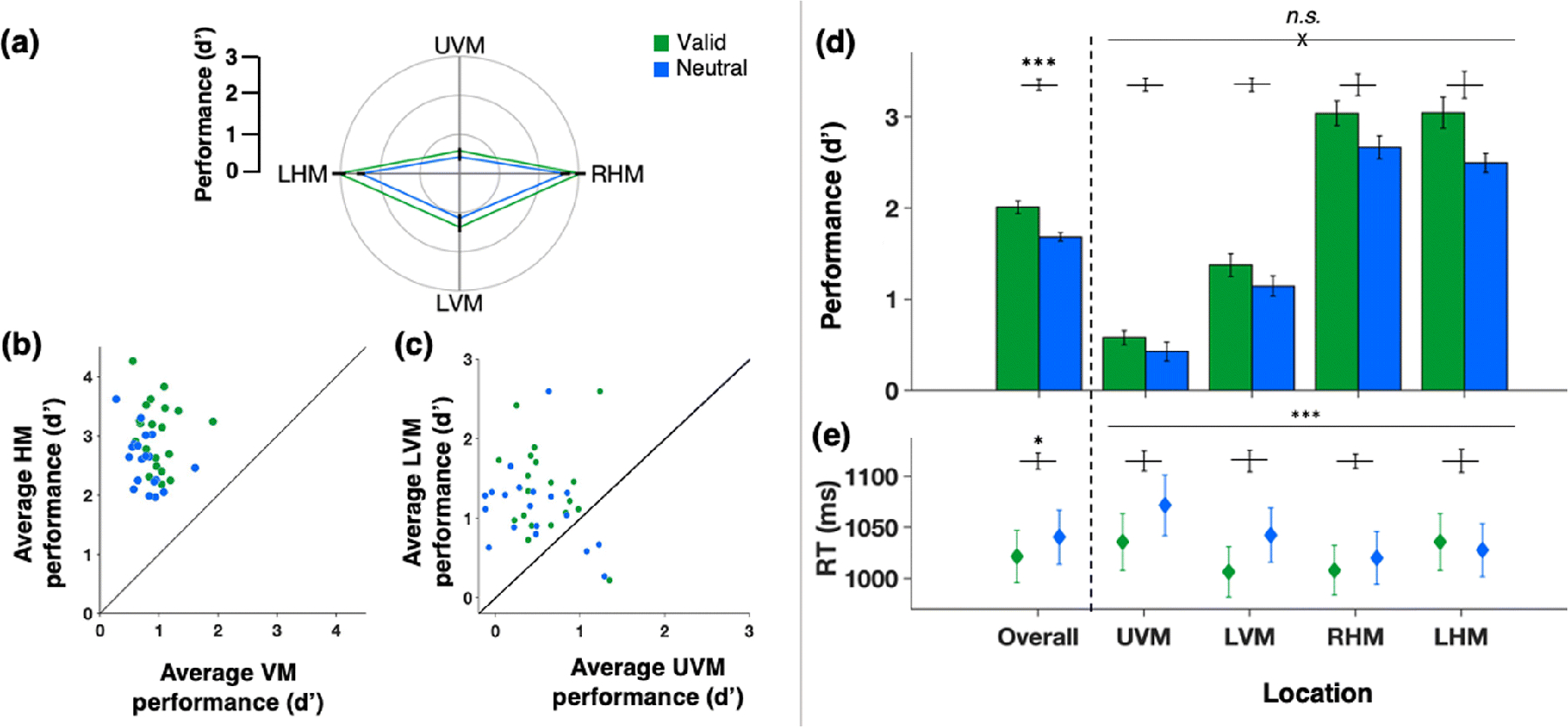
Green - valid cue condition trials, blue - neutral cue condition trials.
(a) Average d’ for each location (polar plot of performance field). (b) Scatterplot of individual d’ values of the horizontal-vertical anisotropy (HVA) (average d’ at horizontal meridian (HM) vs. average d’ at vertical meridian (VM)). (c) Scatterplot of individual d’ values of the vertical meridian asymmetry (VMA) (average d’ at lower vertical meridian (LVM) vs. average d’ at upper vertical meridian (UVM)). (d) Group average d’ (bar plots overall and by location). (e) Group average median reaction times (bar plots overall and by location). Error bars are ±1 SEM. n.s. = not significant; *p < .05; **p < .01; ***p < .001.
Fig. 4. Correlations between conditions across locations in Experiment 1.
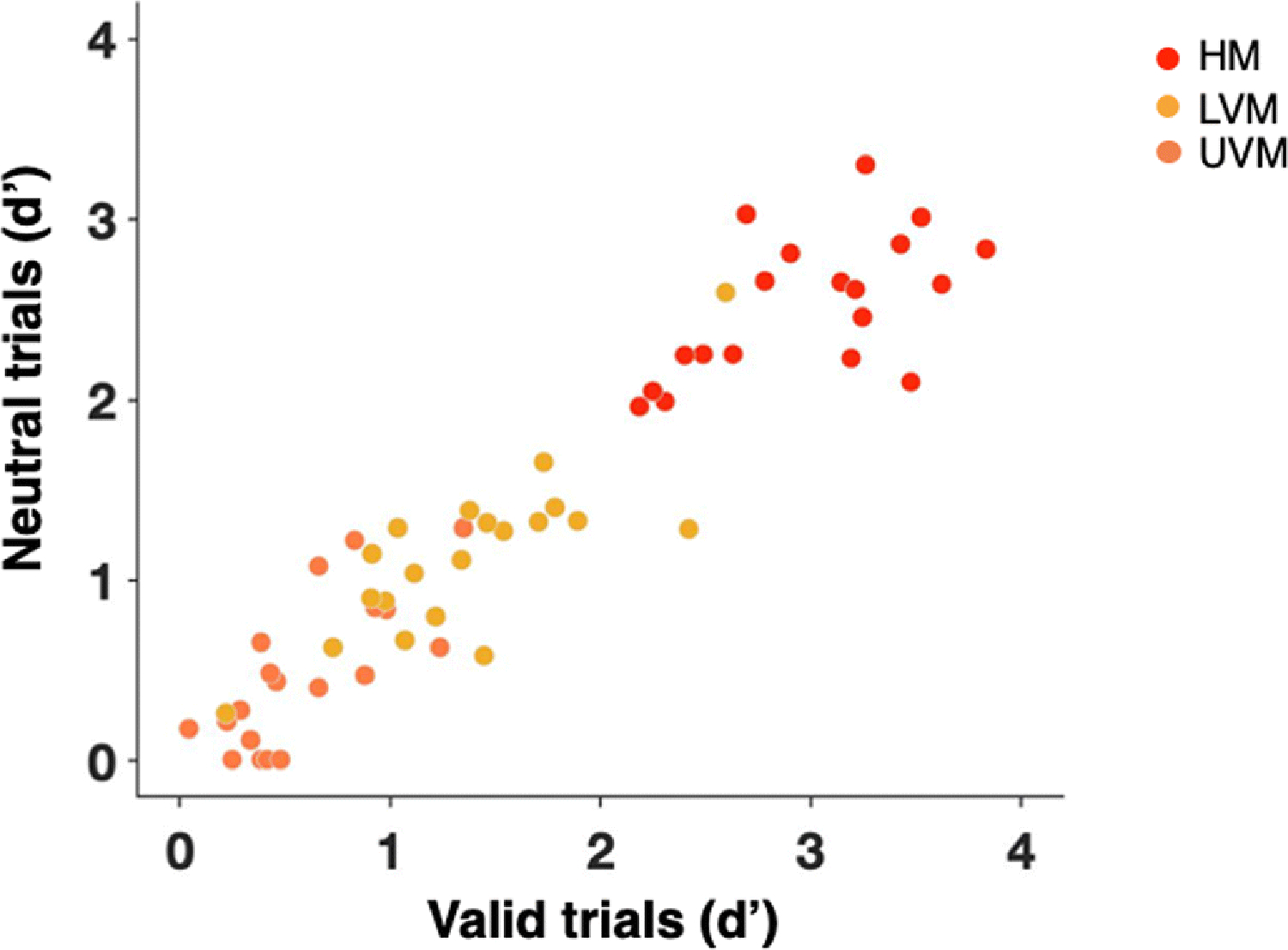
Correlations between participants’ performance in the neutral and attention conditions across locations – horizontal meridian (HM; red dots), lower vertical meridian (LVM; yellow dots) and upper vertical meridian (UVM; orange dots)
A two-way repeated-measures ANOVA of sensitivity - as measured by d’ - revealed significant main effects of location (F(3,57) = 106.555, p < .001, d = .849) and attention (F(1,19) = 28.763, p < .001, d = .602) but no significant interaction (F(2.172, 41.275) = 2.759, p = .071, d = .127, Fig. 3d). The Bayes factor analysis of the interaction between attention and location provided strong evidence in favor of the null hypothesis with an odds of 23.061 to 1: pBIC(H0|D) = .958 and pBIC(H1|D) = .042. This pattern indicates that attention improved performance at all locations to a similar extent.
Our task instructions and trial-by-trial feedback encouraged observers to be as accurate, rather than as fast, as possible. Nonetheless, a secondary two-way repeated measures ANOVA of RTs (Fig. 3e) was conducted to rule-out possible speed-accuracy trade-offs. There were significant main effects of location (F(1.572, 29.859) = 16.46, p < .001, d = .464), attention (F(1,19) = 5.751, p = .027, d = .232), and a significant interaction (F(3,57) = 9.044, p < .001, d = .322). This pattern reflected faster RTs for valid trials than neutral trials and suggests that at the locations of least discriminability (along the vertical meridian), observers took longer to respond to the neutral cue and that the valid cueing helped diminish this speed difference. However, this interaction is inconclusive as RT was a secondary variable, merely monitored to rule out any possible speed-accuracy tradeoffs; at these overall levels of accuracy (by design, around 80% accuracy), RT differences are not readily interpretable (e.g., Pachella, 1974).
Experiment 2: Homogeneous discriminability across locations
In Experiment 2, we equated stimulus discriminability by adjusting stimulus contrast, thereby ensuring that attention had equal opportunity to alter performance at all isoeccentric locations (Fig. 2c). Critically, this manipulation further ensured that all locations were reliably above chance and below ceiling. Our contrast manipulation was successful; performance was equated across all locations in the neutral condition, and attention similarly boosted performance across the visual field (Fig. 5a). The HVA (Fig. 5b) or VMA (Fig. 5c) were no longer significant for either the neutral or the valid attention condition. Most participants showed no HVA effect (individual data points fell along the diagonal), and there were some individual differences in the extent the VMA (individual data points fell along, above, and below the diagonal).
Fig. 5. Performance in Experiment 2.
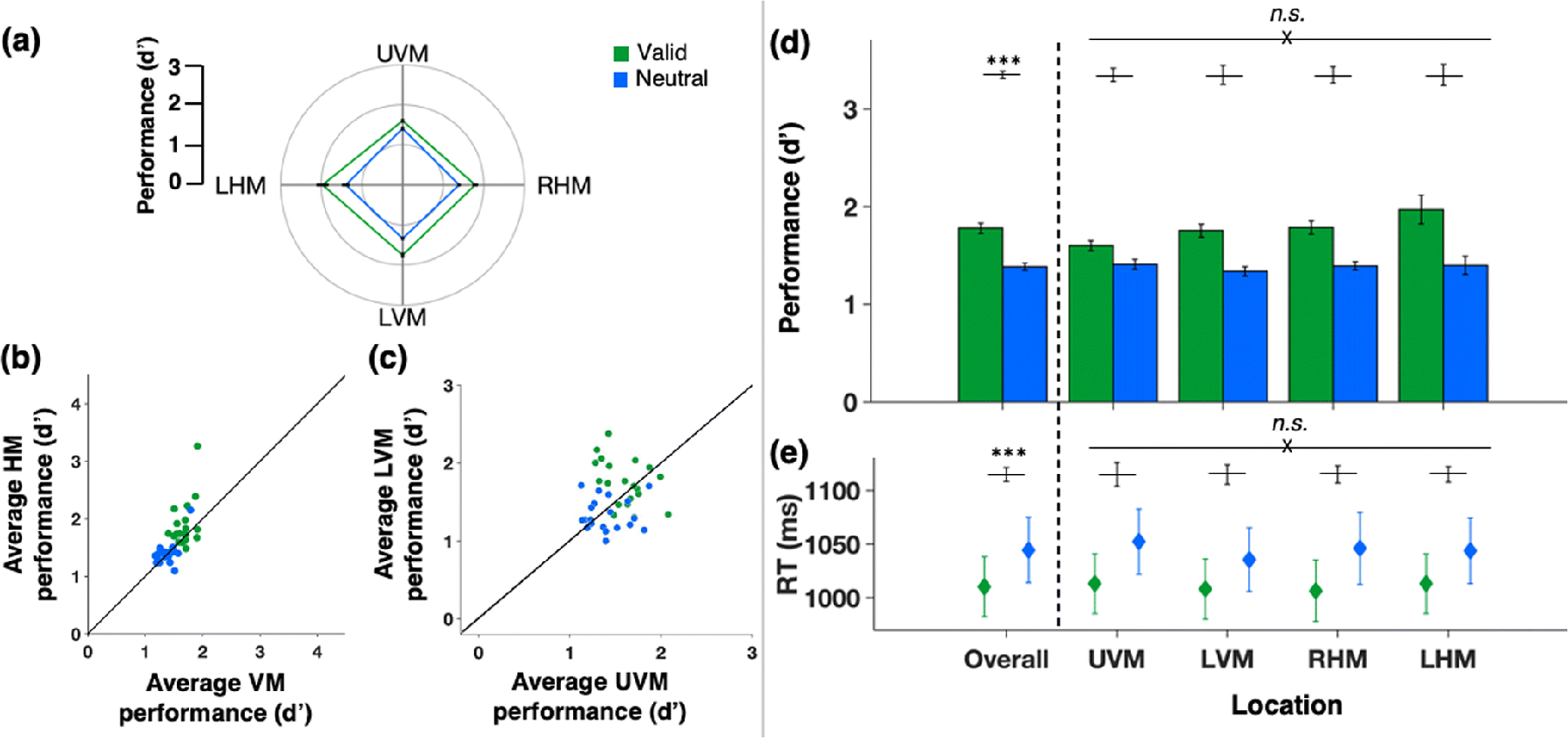
Green - valid cue condition trials, blue - neutral cue condition trials. (a) Average d’ for each location (polar plot of performance field). (b) Scatterplot of individual d’ values of the horizontal-vertical anisotropy (HVA; average d’ at horizontal meridian (HM) vs. average d’ at vertical meridian (VM)). (c) Scatterplot of individual d’ values of the vertical meridian asymmetry (VMA; average d’ at lower vertical meridian (LVM) vs. average d’ at upper vertical meridian (UVM). (d) Group average d’ (bar plots overall and by location). (e) Group average median reaction times (data points overall and by location). Error bars are ±1 SEM. n.s. = not significant; *p < .05; **p < .01; ***p < .001.
A two-way repeated measures ANOVA of d’ verified that there was a significant main effect of attention (F(1,19) = 111.554, p < .001, d = .854), but neither a significant effect of location (F(1.571, 29.845) = 1.98, p = .163, d = .094), nor a significant interaction (F(3,57) = 2.547, p = .065, d = .118; Fig. 5d). The Bayes factor analysis of the main effect of location provided positive evidence in favor of the null hypothesis with an odds of 3.906 to 1: pBIC(H0|D) = .796 and pBIC(H1|D) = .204. It also provided strong evidence for the null hypothesis regarding the interaction between attention and location with an odds of 25.425 to 1: pBIC(H0|D) = .962 and pBIC(H1|D) = .038.
Again, RTs (secondary measures) were faster for valid than neutral trials (Fig. 5e), but a two-way repeated-measures ANOVA of RTs revealed no significant main effects or any interaction (all F < 1 or p > 0.1), confirming that there were no differential effects of attention as a function of location and no speed-accuracy tradeoffs.
Discussion
The goal of this study was to investigate whether endogenous attention differentially improves performance at isoeccentric locations to compensate for performance asymmetries and alter the shape of visual PFs. In Experiment 1, all stimuli were presented at isoeccentric locations around the visual field using the same contrast level. Although there were some individual differences in the extent of the HVA and VMA and the attention effect, all observers showed the HVA and almost all observers showed the VMA. Importantly, for all participants, performance at each location was highly correlated between the neutral and valid conditions, revealing consistent individual differences as well as similar attention effects across locations, regardless of individual differences in baseline performance.
The conditions in Experiment 1 (heterogeneous discriminability across locations), which reflect typical viewing conditions and have ecological validity given that stimuli properties are constant regardless of where they appear, enabled us to assess how endogenous attention interacts with the canonical PF shape. We found marked PF, consistent with the fact that PF are particularly pronounced in the presence of distractors (Carrasco et al., 2001). When the target appeared along the VM, stimuli along the HM were highly visible and likely acted as strong “distractors” swamping out sensitivity to stimuli of similar contrast along the less discriminable vertical meridian. In contrast, when the target appeared along the horizontal meridian, the less visible stimuli along the vertical meridian acted as weak distractors and had a diminished effect on performance.
In Experiment 1 we presented stimuli at all locations at the same contrast level; consequently, their discriminability differed as a function of their location. Hence, we likely assessed performance at different parts of the contrast response function (CRF), because CRFs have a lower threshold and a shallower slope for stimuli at the HM than at the VM, and the highest threshold and steepest slope for stimuli at the upper VM (Cameron et al., 2002). Given that the largest expected endogenous attention effects occur within the dynamic range of the CRF (Herrmann et al., 2010, Jigo & Carrasco, 2020; Pestilli, Ling & Carrasco, 2009), the magnitude of the attention effect could have been compressed by testing too close to the lower or upper asymptotes. Had the contrast level we used been within the dynamic range at the HM, we could have underestimated the possible attention benefit at the VM, especially the UVM, as they would have been close to the lower asymptote. Alternatively, had the contrast level we used been within the dynamic range at the VM, we could have underestimated the possible attention benefit at the HM locations, as they would have been close to the upper asymptote.
Thus, in Experiment 2, we equated discriminability across locations to be within the dynamic range, comfortably above chance but below a performance ceiling. To do so, we varied stimulus contrast while keeping all other stimulus parameters – size, spatial frequency, orientation and eccentricity – identical to those of Experiment 1. By design, performance was similar across all locations, as each stimulus was presented at the unique ~80% contrast threshold for that location. Even then, endogenous attention had a similar effect on performance across all locations. Although there were some individual differences in the extent of the HVA and VMA and the attention effect, at the group level the patterns were clear. This uniform performance boost across locations provides further evidence that endogenous attention enhances visual perception similarly along the HM and VM, but cannot overcome the inherent asymmetries in sensory processing at isoeccentric locations around the visual field.
What seems surprising in our results is that even though endogenous attention is flexible (e.g., Barbot et al., 2012; Barbot & Carrasco, 2017; Giordano et al., 2009; Kinchla, 1980; Sperling & Melchner, 1978; Yeshurun et al., 2008), we found that it boosted performance across isoeccentric locations around the visual field to the same extent and thus preserved the canonical PF shape. This uniform boost in performance is similar to that shown by exogenous attention (Cameron et al., 2002; Carrasco et al., 2001, 2002; Roberts et al., 2016, 2018), notwithstanding the differential effects of exogenous and endogenous attention on performance (e.g., Barbot et al., 2012; Barbot & Carrasco, 2017; Jigo & Carrasco, 2018; Yeshurun & Levy, 2003; Yeshurun et al., 2008). Thus, we find that these visual asymmetries are not only a ubiquitous characteristic of visual processing, but also remarkably resilient, in that they are difficult to be overcome and compensated for low performance at disadvantaged locations.
How generalizable are the present findings? We think that the effect of endogenous attention at isoeccentric locations around the visual field would also be similar had the target appeared by itself. Endogenous attention improves performance when a target is presented alone or amidst distractors (Giordano et al., 2009), and exogenous attention does not alter the PF shape regardless of whether a target appears by itself or in the presence of distractors (e.g., Cameron et al, 2002; Carrasco et al., 2001; Roberts et al., 2016, 2018). Furthermore, we think that the effect of endogenous attention would also be similar across isoeccentric locations if the spatial frequency or the eccentricity at which the stimuli appeared had been different. This is because endogenous attention affects discriminability in a similar fashion across eccentricity, i.e., it improves sensitivity for the peak spatial frequency at each eccentricity and its lower and higher neighboring frequencies (Jigo & Carrasco, 2020).
The resilient nature of PFs and its imperviousness to attentional effects may be related to the anatomical correlates of the HVA and VMA that exist along the visual pathway. For instance, cone density is highest along the horizontal meridian, and is also higher in the superior than the inferior portions of the retina, corresponding to the lower and upper visual field (Curcio, Sloan, Packer, Hendrickson & Kalina, 1987; Curcio, Sloan, Kalina & Hendrickson, 1990). The distribution of ganglion cells in the retina follow the same pattern, but with an even greater superior-inferior asymmetry (Curcio & Allen, 1990; Perry & Cowey, 1985). A recent model, however, has shown that even though optical factors and the photoreceptor array in the retina (Kupers, Carrasco & Winawer, 2019) as well as midget retinal ganglion cells (Kupers, Benson, Carrasco & Winawer, 2019) correlate with differences in performance at isoeccentric locations around the visual field, they only account for a small fraction of observed behavioral asymmetries. Early stages of visual processing (e.g., V1) magnify these differences (Benson et al., 2020; Liu, Heeger & Carrasco, 2006) to account for a larger degree of these perceptual asymmetries.
Characterizing how visual performance varies across the visual field informs our understanding of visual perception and helps constrain models of visual perception and attention. The individual differences observed in this study, as well as in previous studies regarding the topology of spatial vision (e.g., Abrams et al., 2012; Cameron et al., 2002; Greenwood et al., 2017; Himmelberg et al., 2020), will be useful for relating behavior to biological differences in cortical representation and model development.
It is critical that we now know that the effects of covert attention cannot help to overcome these differences, as this finding has implications regarding neural correlates (e.g., Benson et al., 2020; Liu et al., 2006) and computational models (e.g., Kupers et al., 2019, 2020) of performance fields, in particular, and of visual perception, in general. Our results also have significant implications for human factors and ergonomic applications. For instance, the resilience of these perceptual asymmetries at isoeccentric locations around the visual field should be known and taken into consideration when optimizing displays for radiologists, drivers, pilots, and air traffic controllers.
To conclude, our data show that endogenous attention provides a uniform boost in performance at isoeccentric locations around the visual field under both conditions of unequal and equal discriminability. This effect parallels the effect shown by exogenous attention. These results are somewhat surprising, given that endogenous spatial attention has been shown to be more flexible than exogenous attention in many perceptual tasks. Our findings demonstrate that the visual and neural circuitry underlying PF asymmetries impose such pronounced limitations on visual processing that they may be very unlikely to be overcome.
Open Practices Statement.
The data and code for all experiments will be made available upon reasonable request via a publicly accessible online system. None of the experiments were preregistered.
Acknowledgements
This research was supported by NIH-National Eye Institute RO1-EY027401. We thank Antoine Barbot, Marc Himmelberg and Michael Jigo, as well as other Carrasco Lab members for useful comments.
References
- Abrams J, Nizam A & Carrasco M (2012). Isoeccentric locations are not equivalent: The extent of the vertical meridian asymmetry. Vision Research, 52(1), 70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altpeter E, Mackeben M & Trauzettel-Klosinski S (2000). The importance of sustained attention for patients with maculopathies. Vision Research, 40(10–12), 1539–1547. [DOI] [PubMed] [Google Scholar]
- Baldwin AS, Meese TS, & Baker DH (2012). The attenuation surface for contrast sensitivity has the form of a witch’s hat within the central visual field. Journal of Vision, 12(23) [DOI] [PubMed] [Google Scholar]
- Barbot A & Carrasco M (2017). Attention modifies spatial resolution according to task demands. Psychological Science, 28(3), 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbot A, Landy MS & Carrasco M (2012). Differential effects of exogenous and endogenous attention on second-order texture contrast sensitivity. Journal of Vision, 12(8), 6–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbot A, Xue S, & Carrasco M (2021). Spatial frequency asymmetries around the visual field. Journal of Vision. 21(1):2, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck DM & Kastner S (2009). Top-down and bottom-up mechanisms in biasing competition in the human brain. Vision Research, 49(10), 1154–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson NC, Kupers ER, Barbot A, Carrasco M, & Winawer J (2020). Cortical magnification in human visual cortex parallels task performance around the visual field. bioRxiv. [DOI] [PMC free article] [PubMed]
- Cameron EL, Tai JC & Carrasco M (2002). Covert attention affects the psychometric function of contrast sensitivity. Vision Research, 42: 949–967. [DOI] [PubMed] [Google Scholar]
- Carrasco M (2006). Covert attention increases contrast sensitivity: Psychophysical, neurophysiological and neuroimaging studies. In Progress in Brain Research (Vol. 154, pp. 33–70). Elsevier, Philadelphia, [DOI] [PubMed] [Google Scholar]
- Carrasco M (2011). Visual attention: The past 25 years. Vision Research, 51(13), 1484–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M (2014). Spatial covert attention: Perceptual modulation. The Oxford Handbook of Attention, 183–230.
- Carrasco M & Barbot A (2015). How attention affects spatial resolution. Cold Spring Harbor Symposia on Quantitative Biology, 79, 149–160, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M & Barbot A (2019). Spatial attention alters visual appearance. Current Opinion in Psychology, 29, 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M & Frieder KS (1997). Cortical magnification neutralizes the eccentricity effect in visual search. Vision Research, 37(1), 63–82. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Giordano AM, & McElree B (2004). Temporal performance fields: Visual and attentional factors. Vision Research, 44(12), 1351–1365. [DOI] [PubMed] [Google Scholar]
- Carrasco M, McElree B, Denisova K & Giordano AM (2003). Speed of visual processing increases with eccentricity. Nature Neuroscience, 6(7), 699–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Talgar CP & Cameron EL (2001). Characterizing visual performance fields: Effects of transient covert attention, spatial frequency, eccentricity, task and set size. Spatial Vision, 15(1), 61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Williams PE & Yeshurun Y (2002). Covert attention increases spatial resolution with or without masks: Support for signal enhancement. Journal of Vision, 2(6), 1351–1365. [DOI] [PubMed] [Google Scholar]
- Chun MM, & Nakayama K (2000). On the functional role of implicit visual memory for the adaptive deployment of attention across scenes. Visual Cognition, 7(1–3), 65–81. [Google Scholar]
- Corbett JE & Carrasco M (2011). Visual performance fields: Frames of reference. PLoS One, 6(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M & Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201–215. [DOI] [PubMed] [Google Scholar]
- Curcio CA & Allen KA (1990). Topography of ganglion cells in human retina. The Journal of Comparative Neurology, 300(1), 5–25. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Sloan KR, Kalina RE & Hendrickson AE (1990). Human photoreceptor topography. The Journal of Comparative Neurology, 292(4), 497–523. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Sloan KR, Packer O, Hendrickson AE & Kalina RE (1987). Distribution of cones in human and monkey retina: Individual variability and radial asymmetry. Science, 236(4801), 579–582. [DOI] [PubMed] [Google Scholar]
- Dugué L, Merriam EP, Heeger DJ & Carrasco M (2017). Specific visual subregions of TPJ mediate reorienting of spatial attention. Cerebral Cortex, 28(7), 2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugué L, Merriam EP, Heeger DJ & Carrasco M (2020). Differential impact of endogenous and exogenous activity in human visual cortex. Scientific Reports, 10(1), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortenbaugh FC, Silver MA, & Robertson LC (2015). Individual differences in visual field shape modulate the effects of attention on the lower visual field advantage in crowding. Journal of Vision, 15(2), 19–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S, & Carrasco M (2009). Perceptual consequences of visual performance fields: The case of the line motion illusion. Journal of Vision, 9(13), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S, Rodriguez RZ & Carrasco M (2008). Apparent contrast differs across the vertical meridian: Visual and attentional factors. Journal of Vision, 8(1), 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano AM, McElree B & Carrasco M (2009). On the automaticity and flexibility of covert attention: A speed-accuracy trade-off analysis. Journal of Vision, 9(3), 30–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood JA, Szinte M, Sayim B, & Cavanagh P (2017). Variations in crowding, saccadic precision, and spatial localization reveal the shared topology of spatial vision. Proceedings of the National Academy of Sciences, 114(17), E3573–E3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautus MJ (1995). Corrections for extreme proportions and their biasing effects on estimated values of d. Behavior Research Methods, Instruments, & Computers, 27(1), 46–51. [Google Scholar]
- Hein E, Rolke B, & Ulrich R (2006). Visual attention and temporal discrimination: Differential effects of automatic and voluntary cueing. Visual Cognition, 13(1), 29–50. [Google Scholar]
- Herrmann K, Montaser-Kouhsari L, Carrasco M & Heeger DJ (2010). When size matters: attention affects performance by contrast or response gain. Nature Neuroscience, 13(12), 1554–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelberg M, Winawer J & Carrasco M (2020). Stimulus-dependent contrast sensitivity asymmetries around the visual field. Journal of Vision, 20(9), 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jigo M & Carrasco M (2018). Attention alters spatial resolution by modulating second-order processing. Journal of Vision, 18(7), 2–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jigo M & Carrasco M (2020). Differential impact of exogenous and endogenous attention on the contrast sensitivity function across eccentricity. Journal of Vision, 20(6), 11–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchla RA (1980). The measurement of attention. Attention and Performance VIII, 213–238. [Google Scholar]
- Kupers ER, Carrasco M & Winawer J (2019). Modeling visual performance differences ‘around’ the visual field: A computational observer approach. PLOS Computational Biology. [DOI] [PMC free article] [PubMed]
- Kupers ER, Benson NC, Carrasco M & Winawer J (2020). Radial asymmetries around the visual field: From retina to cortex to behavior. biorXiv. [DOI] [PMC free article] [PubMed]
- Levine MW & McAnany JJ (2005). The relative capabilities of the upper and lower visual hemifields. Vision Research, 45(21), 2820–2830. [DOI] [PubMed] [Google Scholar]
- Ling S & Carrasco M (2006). Sustained and transient covert attention enhance the signal via different contrast response functions. Vision Research, 46, 1210–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Heeger DJ & Carrasco M (2006). Neural correlates of the visual vertical meridian asymmetry. Journal of Vision, 6(11), 12–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Stevens ST & Carrasco M (2007). Comparing the time course and efficacy of spatial and feature-based attention. Vision Research, 47(1), 108–113. [DOI] [PubMed] [Google Scholar]
- Mackeben M (1999). Sustained focal attention and peripheral letter recognition. Spatial Vision, 12(1), 51–72. [DOI] [PubMed] [Google Scholar]
- Masson ME (2011). A tutorial on a practical Bayesian alternative to null-hypothesis significance testing. Behavior Research Methods, 43(3), 679–690. [DOI] [PubMed] [Google Scholar]
- Montaser-Kouhsari L & Carrasco M (2009). Perceptual asymmetries are preserved in short-term memory tasks. Attention, Perception, & Psychophysics, 71(8), 1782–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller HJ & Rabbitt PM (1989). Reflexive and voluntary orienting of visual attention: time course of activation and resistance to interruption. Journal of Experimental Psychology: Human Perception and Performance, 15(2), 315. [DOI] [PubMed] [Google Scholar]
- Nazir TA (1992). Effects of lateral masking and spatial precueing on gap-resolution in central and peripheral vision. Vision Research, 32(4), 771–777. [DOI] [PubMed] [Google Scholar]
- Pachella RG (1974). The interpretation of reaction time in information processing research. In Kantowitz BH, editor.(Ed.), Human information processing: Tutorials in performance and cognition (pp. 41–82).
- Pelli DG (1985). Uncertainty explains many aspects of visual contrast detection and discrimination. Journal of the Optical Society of America, A, Optics, Image & Science, 2(9), 1508–1532. [DOI] [PubMed] [Google Scholar]
- Perry VH & Cowey A (1985). The ganglion cell and cone distributions in the monkey’s retina: Implications for central magnification factors. Vision Research, 25(12), 1795–1810. [DOI] [PubMed] [Google Scholar]
- Pestilli F, Ling S & Carrasco M (2009). A population-coding model of attention’s influence on contrast response: Estimating neural effects from psychophysical data. Vision Research, 49(10), 1144–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov Y & Meleshkevich O (2011). Asymmetries and idiosyncratic hotspots in crowding. Vision Research, 51(10), 1117–1123. [DOI] [PubMed] [Google Scholar]
- Pointer JS & Hess RF (1989). The contrast sensitivity gradient across the human visual field: With emphasis on the low spatial frequency range. Vision Research, 29(9), 1133–1151. [DOI] [PubMed] [Google Scholar]
- Regan D, & Beverley KI (1983). Visual fields described by contrast sensitivity, by acuity, and by relative sensitivity to different orientations. Investigative Ophthalmology and Visual Science, 24(6), 753–759. [PubMed] [Google Scholar]
- Rijsdijk JP, Kroon JN, & van der Wildt GJ (1980). Contrast sensitivity as a function of position on the retina. Vision Research, 20(3), 235–241. [DOI] [PubMed] [Google Scholar]
- Roberts M, Ashinoff BK, Castellanos FX & Carrasco M (2018). When attention is intact in adults with ADHD. Psychonomic Bulletin & Review, 25(4), 1423–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M, Cymerman R, Smith RT, Kiorpes L & Carrasco M (2016). Covert spatial attention is functionally intact in amblyopic human adults. Journal of Vision, 16(15), 30–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson JG & Graham N (1981). Probability summation and regional variation in contrast sensitivity across the visual field. Vision Research, 21(3), 409–418. [DOI] [PubMed] [Google Scholar]
- Rovamo J & Virsu V (1979). An estimation and application of the human cortical magnification factor. Experimental Brain Research, 37(3), 495–510. [DOI] [PubMed] [Google Scholar]
- Sharp P, Melcher D & Hickey C (2018). Endogenous attention modulates the temporal window of integration. Attention, Perception, & Psychophysics, 80(5), 1214–1228. [DOI] [PubMed] [Google Scholar]
- Sperling G & Melchner MJ (1978). The attention operating characteristic: Examples from visual search. Science, 202(4365), 315–318. [DOI] [PubMed] [Google Scholar]
- Talgar CP, & Carrasco M (2002). Vertical meridian asymmetry in spatial resolution: Visual and attentional factors. Psychonomic Bulletin & Review, 9(4), 714–722. [DOI] [PubMed] [Google Scholar]
- Yantis S, & Jonides J (1990). Abrupt visual onsets and selective attention: voluntary versus automatic allocation. Journal of Experimental Psychology: Human Perception and Performance, 16(1), 121. [DOI] [PubMed] [Google Scholar]
- Yashar A, & Lamy D (2010). Intertrial repetition facilitates selection in time: Common mechanisms underlie spatial and temporal search. Psychological Science, 21(2), 243–251. [DOI] [PubMed] [Google Scholar]
- Yeshurun Y, & Carrasco M (1998). Attention improves or impairs visual performance by enhancing spatial resolution. Nature, 396(6706), 72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun Y & Levy L (2003). Transient spatial attention degrades temporal resolution. Psychological Science, 14(3), 225–231. [DOI] [PubMed] [Google Scholar]
- Yeshurun Y, Montagna B & Carrasco M (2008). On the flexibility of sustained attention and its effects on a texture segmentation task. Vision Research, 48(1), 80–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis TS & Bex PJ (2012). Image correlates of crowding in natural scenes. Journal of Vision, 12(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AL, Rolfs M, & Carrasco M (2013). Adaptive deployment of spatial and feature-based attention before saccades. Vision Research, 85, 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


