To the Editor,
Post-Acute Sequelae of SARS-CoV-2 infection (PASC) is the syndrome of persisting (>1 month) symptoms following COVID-19 illness [1,2]. Neuropsychiatric sequelae are most common, including central fatigue (up to 87%), cognitive dysfunction (“brain fog”), pain (e.g., headaches, myalgia), and emotional dysregulation (e.g., anxiety, depression) [1,2]. PASC can be disabling for otherwise healthy adults (e.g., preventing return to work), and there is a critical need for therapeutic interventions.
Noninvasive brain stimulation may have therapeutic application for both the acute and chronic stages of COVID-19 [3,4]. Due to its growing clinical evidence base, transcranial direct current stimulation (tDCS) has potential for therapeutic targeting of these neuropsychiatric PASC symptoms, with the advantage of portable and wearable devices for home-based access.
We have extensively developed a protocol of remotely supervised tDCS (RS-tDCS) that provides tDCS as telehealth [3,5]. We established a tDCS Clinical Telehealth Program at NYU Langone Health (using the Virtual Visit telemedicine platform) as innovative clinical care to meet the strong demand for tDCS treatment access.
The tDCS service utilizes Soterix Medical mini-CT devices, using an “unlock” code for each stimulation session, along with customized headsets and EASYpad single use pre-saturated sponge electrodes. tDCS treatment parameters are individually determined based on targeted symptom and published evidence. All stimulation is paired with an ongoing and seated rehabilitation activity, also based on targeted symptoms.
During the initial setup visit (either in person or virtual visit), patients are oriented to device use and safety precautions, and instructed in the procedure for daily at-home self-administration guided by a tDCS clinician in real-time. Safety and tolerability procedures follow closely our RS-tDCS research protocol, and rehabilitative procedures are described elsewhere [5,6]. tDCS is targeted for daily (M-F) sessions with live clinician guidance to patients at home, with safety clearance and confirmation of headset placement, followed by the provision of an “unlock” code to operate the tDCS device.
1. PASC clinical cases
Based on its potential benefit and the absence of any yet available options, a growing number of patients with PASC have sought out tDCS treatment. Here we report the approach and initial outcomes for the first two patients with PASC seen by our service.
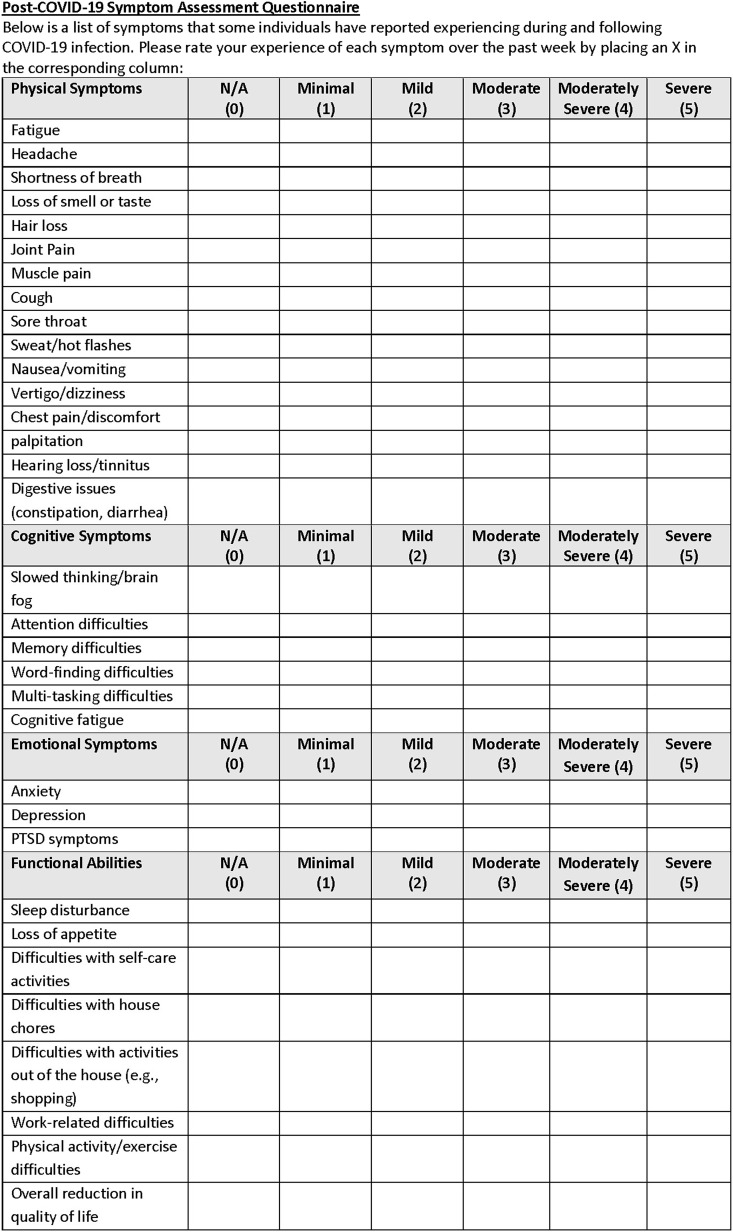
The patients were referred by their treating neurologists. Both were otherwise healthy adults who experienced persisting fatigue, cognitive dysfunction, and other symptoms following acute COVID-19 illness. Initial clinical assessment included a neuropsychological testing and self-report inventories. We also developed the Assessment of PASC (A-PASC) inventory of symptoms to match the range of cognitive, emotional, physical, and functional domains that have been reported to date ([1,2]; Fig. 1 ).
Fig. 1.
The Assessment of PASC (A-PASC) inventory.
tDCS is delivered for 30 minutes × 2.0 mA, using a left anodal dorsolateral prefrontal cortex (DLPFC) montage guided by clinical evidence of symptom response [[6], [7], [8], [9]]. Stimulation is paired with a combination of physical exercise (seated pedaling, using the Cubii Pro seated elliptical device), online adaptive computerized cognitive training with BrainHQ (Posit Science, San Francisco, California, Glenn Smith) or Lumosity (http://www.lumosity.com), and guided mindfulness meditation (The 10 Minute Mind by Monique Rhodes).
1.1. Cases
Patient 1: A 42-year-old, right-handed, Black woman presented for tDCS clinical treatment of PASC in 01/2021, approximately nine months following COVID-19 illness. She continued to experience fatigue, cognitive impairment, anxiety and depression, dyspnea, sleep disturbances, and numbness sensation in the right side of her face.
Patient started her tDCS treatment in January 2021. A comparison between initial (08/2020) and repeat (02/2021) neuropsychological evaluations following 4 weeks of treatment (15 sessions) demonstrated significant improvements (≥1 SD) in visual attention and processing speed, timed verbal fluency, and cognitive flexibility. Speeded fine motor dexterity was also improved in her left hand (and remained intact in her right hand). She also reported clinically significant improvements in cognitive functioning, depression, and fatigue. Anxiety ratings increased, indicating ongoing mild anxiety (which she attributed to her approaching return to work). Sleep remained unchanged and within normal limits. The A-PASC inventory indicated significant improvements across all affected domains. Importantly, over the course of treatment, the patient returned to her job, gradually increasing her work responsibilities.
Patient 2: A 57-year-old, right-handed, White woman presented for tDCS clinical treatment of PASC in November 2020, approximately seven months following COVID-19 illness. She experienced a constellation of persisting symptoms, including marked fatigue and “brain fog,” emotional dysregulation, intermittent numbness in her extremities, and pain.
Comparison between initial (11/2020) and repeat (06/2021) neuropsychological evaluations indicated a stable cognitive functioning (which was intact at baseline). Self-report rating scales of mood, sleep, fatigue, and pain remained overall stable as well. However, on the A-PASC inventory, patient reported experiencing significant improvements across physical, cognitive, emotional, and functional domains. She was also able to return to work following tDCS treatment, and to resume most of her prior activities.
2. Discussion
With these initial cases, we have found that tDCS, in the context of a home-based rehabilitation program, may provide a treatment option for patients with PASC. Initial case reports of the use of tDCS for COVID-19 are emerging, with more studies underway. However, to our knowledge, these are the first reports of PASC recovery using at-home tDCS. Our findings provide early support that trials of tDCS for PASC are warranted.
Recovery from other post-viral syndromes, including from other coronaviruses, can be prolonged (taking up to 4 years), with a subset of patients who never fully recover and remain functionally impaired [10]. Therefore, while a gradual recovery is expected, the significant improvements experienced by our patients in only a few months may represent an additive effect of the treatment to potentiate recovery. A controlled trial for further evaluation of tDCS will be critical, as well as further evidence to guide dosing determination.
We targeted tDCS and paired activities for PASC symptoms of cognitive dysfunction, fatigue, and mood dysregulation. Given the marked variability in PASC presentation, we have developed the A-PASC inventory to provide a metric to evaluate treatment benefit. Treatment may also ultimately be personalized to target specific PASC symptoms. Return to function may provide the best real-world indicator of treatment benefit.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. Jama. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nalbandian A., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pilloni G., et al. Update on the use of transcranial electrical brain stimulation to manage acute and chronic COVID-19 symptoms. Front Hum Neurosci. 2020;14:595567. doi: 10.3389/fnhum.2020.595567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baptista A.F., et al. Applications of non-invasive neuromodulation for the management of disorders related to COVID-19. Front Neurol. 2020;11:573718. doi: 10.3389/fneur.2020.573718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charvet L.E., et al. Supervised transcranial direct current stimulation (tDCS) at home: a guide for clinical research and practice. Brain Stimul. 2020;13(3):686–693. doi: 10.1016/j.brs.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Charvet L., et al. Remotely supervised transcranial direct current stimulation increases the benefit of at-home cognitive training in multiple sclerosis. Neuromodulation: Technology at the Neural Interface. 2018;21(4):383–389. doi: 10.1111/ner.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charvet L.E., et al. Remotely supervised transcranial direct current stimulation for the treatment of fatigue in multiple sclerosis: results from a randomized, sham-controlled trial. Mult Scler. 2018;24(13):1760–1769. doi: 10.1177/1352458517732842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayache S.S., et al. Prefrontal tDCS decreases pain in patients with multiple sclerosis. Front Neurosci. 2016;10:147. doi: 10.3389/fnins.2016.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Razza L.B., et al. A systematic review and meta-analysis on the effects of transcranial direct current stimulation in depressive episodes. Depress Anxiety. 2020;37(7):594–608. doi: 10.1002/da.23004. [DOI] [PubMed] [Google Scholar]
- 10.Islam M.F., Cotler J., Jason L.A. Post-viral fatigue and COVID-19: lessons from past epidemics. Fatigue: Biomedicine, Health & Behavior. 2020;8(2):61–69. [Google Scholar]