Abstract
Patent foramen ovale closure (PFO) is an underutilized therapy, and our study explored the challenges and feasibility of PFO closure in the Indian setting.
Eighty patients with Embolic Stroke of Undetermined Source (ESUS) were screened by transcranial Doppler (TCD) for PFO. Twenty-nine patients underwent successful closure.
High-risk features of a long tunnel, inter-atrial septal aneurysm, and large defect were present in 31%, 28%, and 59%. Transcranial Doppler had a sensitivity and specificity of 78% and 53% (p = 0.02) to detect PFO. Anticoagulation was withdrawn in 85% of patients post closure. Two patients had residual shunts at follow-up of 19 (9,34) months.
Keywords: Patent foramen ovale (PFO), Cryptogenic stroke, Embolic stroke of undetermined source(ESUS)
1. Introduction
Three randomized trials proved the superiority of percutaneous PFO closure over medical therapy with antiplatelet agents in cryptogenic stroke when PFO was associated with high-risk attributes for stroke.1, 2, 3, 4 Data from anticoagulation trials using Vitamin K antagonists in the Asian population suggest increased adverse events than the Caucasian population.5 Thus, PFO closure may prove to be a superior strategy for preventing recurrent stroke in young Indian patients.
2. Methodology
The institutional review board cleared the study (Mi. No. 13438). Neurologists evaluated patients presenting with ischemic stroke for aetiology of stroke. If all other workups for ischemic stroke (Supplementary data 2.0) were negative, a TCD evaluation was performed in the stroke clinic to screen for PFO. If it was positive, and or the patient had a high risk of paradoxical embolism (RoPE)score,6 they were referred for a transoesophageal echocardiogram (TEE) study to exclude PFO.
2.1. TEE protocol
The presence of PFO was confirmed by Transoesophageal echo (TEE) Agitated Saline Contrast (ASC) study (Fig. 1). The shunt was quantified using the Soliman et algrading.7 The detailed TEE examination protocol, the high-risk attributes of the PFO (supplementary data 2.1), and the proforma used for capturing data is given in Appendix B.
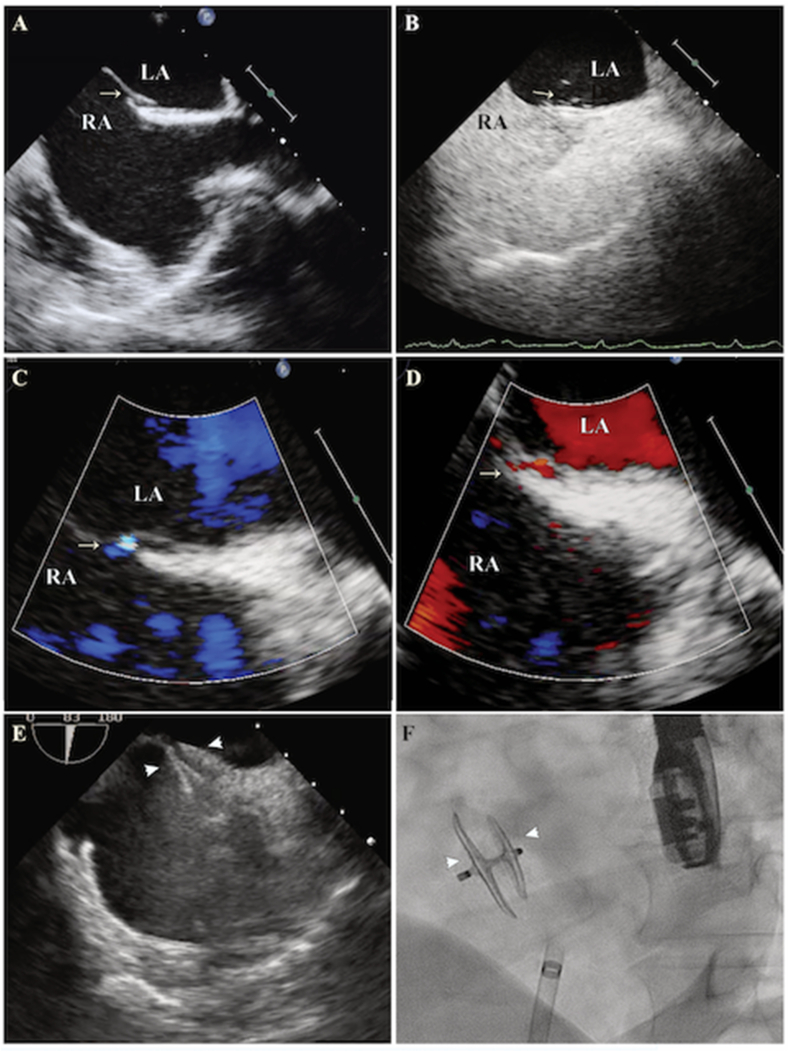
Fig. 1.
Transesophageal and fluoroscopic images of PFO closure, Legend Transesophageal echocardiographic (TEE) images at mid-esophageal level at an angle of 70° (Bicaval view) demonstrating PFO (white arrow - Panel A), Contrast bubbles in the left atrium (LA) during Valsalva maneuver (white arrow - Panel B). Left to right color flow (white arrow – Panel C) and right to left shunt (white arrow - Panel D) through the PFO. Intraprocedural images of device deployment with TEE guidance (Panel E) and fluoroscopic image of PFO device after deployment (Panel F). The white arrowheads in Panel E and F demarcate the right and left atrial disc of the device.
The 25 mm Amplatzer PFO occluder (St. Jude Medical, Zaventem, Belgium) device was used in 83% of cases. All procedures were performed under conscious sedation from the right femoral venous approach using the 9 French Amplatzer Torque Vue delivery system and TEE guidance. The details of device selection, sedation, and adjunct medications are given in Supplementary data (Sections 2.2,2.3,2.4). Post closure, all the patients received dual antiplatelet therapy for six months, followed by a single antiplatelet agent.
2.2. Statistical analysis
Categorical variables were expressed as n (%) and continuous variables as mean ± standard deviation (SD) or median with interquartile ranges (IQR) depending on the variable's distribution.
The Chi-square test or Fisher's exact test was used to ascertain the association between categorical variables. A p-value of <0.05 was taken to be statistically significant.
3. Results
Sixty-six of 88 patients referred for PFO evaluation had a PFO on TEE and were counselled to undergo closure. Thirty-one came forward, and twenty-nine underwent PFO closure between June 2014 to February 2020.
The baseline features of patients and high risk attributes of PFO are given in Table 1.
Table 1.
Clinical features and transesophageal findings of patients referred for Patent foramen ovale closure.
| Parameters | Screened for PFO closure n = 88 | PFO closure done n = 29 |
|---|---|---|
| Age in years, mean (SD) | 38 (11) | 41 (11) |
| Gender, male n (%) | 55 (63) | 16 (55) |
| Indication, n (%) | ||
| Stroke | 74 (84) | 25 (86) |
| Stroke and migraine | 11 (12) | 3 (10) |
| Othersa | 3 (3) | 1 (3) |
| RoPE Score, median (IQR) | 7 (6,8) | 6 (6,8) |
| Transcranial doppler performed, n (%) | 75 (85) | 23 (80) |
| PFO present on TEE, n (%) | 66 (75) | |
| Size of PFO, mean (SD) | 2 (1.4) | 2.4 (1.6) |
| Large PFO (>2 mm), n (%) | 31 (47) | 16 (55) |
| bModerate to large shunt by TEE, n (%) | 42 (52) | 17 (70) |
| Length of tunnel in mm, mean (SD) | 9 (4) | 9.5 (5) |
| Interatrial septal aneurysm, n (%) | 15 (23) | 8 (28) |
| PFO closure - follow up in months. median (IQR) | 19 (9,34) | |
PFO – Patent foramen ovale, RoPE – Risk of Paradoxical Embolism, TEE – Transesophageal echocardiogram.
Systemic embolism, migraine.
Moderate to large shunt was based on Soliman classification of more than 10 bubbles in a single frame within 3 consecutive heart beats on bubble contrast transesophageal echo study after entire right atrium is opacified. TEE bubble study performed in total of 80 patients.
3.1. Correlation between TCD grade and PFO size and shunting
In our study ≥30 High-intensity transient signals (HITS), 8on TCD had a sensitivity and specificity of 78% and 53% (p = 0.02), respectively. The positive and negative predictive values were 82 and 45%, respectively. The mean size of the defect (5.1 ± 4.2) mm observed in a large shunt was significantly larger than a small (1.8 ± 0.8) or moderate shunt (1.9 ± 0.9) (p < 0.05). No correlation was observed between the TCD or the TEE shunt size when the PFO defect was stratified as small < 2 mm or large ≥ 2 mm.9
3.2. Antiplatelet and anticoagulant drugs
In 85% of the patients, anticoagulation was discontinued. Eighteen per cent were free of anticoagulant or antiplatelet medications, and 54% were on a single antiplatelet agent at follow-up.
3.3. Technical success
Successful closure was achieved in 94% of patients. In two patients, PFO could not be crossed due to unfavourable anatomy. Two patients had small residual shunts at the nine-month follow-up. There were no recurrent strokes or TIA in those who underwent closure at a median follow-up of 19 (9,34) months.
3.4. Adverse events
One patient developed air embolism, which recovered without any sequelae. The second patient developed intraprocedural atrial fibrillation (AF) and was treated with direct current cardioversion. There were no periprocedural strokes, access site complications, or bleeding complications.
4. Discussion
In India, cerebrovascular disease is second only to ischemic heart disease for non-communicable disease-related mortality and in 28% of the patients with ischemic stroke, the aetiology was unknown.10 The prevalence of PFO in Indian stroke patients is not known.
TCD as a screening test for PFO in our study had fair sensitivity but had low specificity and negative predictive value. Hence in patients with strong clinical suspicion of PFO with a high RoPE score, a TEE ASC study is warranted.
Meta-analysis and systematic reviews have confirmed the superiority of PFO closure compared to medical therapy but carry a small significant risk of new-onset AF.1, 2, 3,11, 12, 13
In our case series, one patient developed periprocedural AF, which was successfully cardioverted.
Two (6.8%) of our patients had small residual shunts confirmed on TEE at nine months. One patient had a hypermobile septum with a long PFO tunnel of 15 mm, and the second patient had multiple fenestrations in the interatrial septum. In both these cases, the degree of shunting was less by two grades on TCD from baseline. Residual shunts have been identified in 22–26% of patients who undergo PFO closure.14,15 Deng et al documented an increased incidence of recurrent events with moderate to large residual shunts, but the significance of small shunts remains unknown.14
Anticoagulation could be withdrawn in 85% of the patients. Two patients were continued on anticoagulation because of small residual shunts, and another had chronic AF requiring anticoagulation. In the latter patient, the cardio-neuro team decided on PFO closure because of recurrent stroke despite anticoagulation.
PFO closure for ESUS is an attractive option for Indian patients due to the scarcity of reliable laboratories to monitor anticoagulation therapy, prohibitive costs of novel anticoagulant agents (NOAC's), and poor compliance with treatment.
The impediment to the widespread application of this therapy include;
-
1.
Lack of awareness among physicians regarding PFO related strokes.
-
2.
Lack of multidisciplinary (cardiology-neurology) team to diagnose and treat PFO related ESUS.
-
3
Cost of the device
In our institute, the formation of a heart-stroke team helped to overcome some of these challenges. The neurologists should be the gatekeepers of this therapy in choosing the appropriate patient for PFO closure since incidental PFO's occur in 25% of the population.
A concerted effort from all cardiology and neurology forums and societal bodies is required to maximize the benefit of PFO closure for young Indian patients with ESUS.
Key message
PFO closure is beneficial in patients who have high risk anatomical attributes for ESUS; establishing a cardio-neurology team is crucial in identifying and triaging patients to maximize the benefit of this therapy in the Indian setting.
Declaration of competing interest
None of the authors have any conflicts of interest and there was no sponsorship or funding received for the preparation of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ihj.2021.09.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Mas J.L., Derumeaux G., Guillon B. Patent foramen ovale closure or anticoagulation vs antiplatelets after stroke. N Engl J Med. 2017;377:1011–1021. doi: 10.1056/NEJMoa1705915. [DOI] [PubMed] [Google Scholar]
- 2.Saver J.L., Carroll J.D., Thaler D.E. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017;377:1022–1032. doi: 10.1056/NEJMoa1610057. [DOI] [PubMed] [Google Scholar]
- 3.Sondergaard L., Kasner S.E., Rhodes J.F. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2017;377:1033–1042. doi: 10.1056/NEJMoa1707404. [DOI] [PubMed] [Google Scholar]
- 4.Mojadidi M.K., Elgendy A.Y., Elgendy I.Y. Transcatheter patent foramen ovale closure after cryptogenic stroke: an updated meta-analysis of randomized trials. JACC Cardiovasc Interv. 2017;10:2228–2230. doi: 10.1016/j.jcin.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Chiang C.-E., Wang K.-L., Lip G.Y. Stroke prevention in atrial fibrillation: an Asian perspective. Thromb Haemostasis. 2014;112:789–797. doi: 10.1160/TH13-11-0948. [DOI] [PubMed] [Google Scholar]
- 6.Kent D.M., Ruthazer R., Weimar C. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology. 2013;81:619–625. doi: 10.1212/WNL.0b013e3182a08d59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soliman O.I., Geleijnse M.L., Meijboom F.J. The use of contrast echocardiography for the detection of cardiac shunts. Eur J Echocardiogr. 2007;8:s2–s12. doi: 10.1016/j.euje.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Lao A.Y., Sharma V.K., Tsivgoulis G. Detection of right-to-left shunts: comparison between the international consensus and spencer logarithmic scale criteria. J Neuroimaging. 2008;18:402–406. doi: 10.1111/j.1552-6569.2007.00218.x. [DOI] [PubMed] [Google Scholar]
- 9.Nakayama R., Takaya Y., Akagi T. Identification of high-risk patent foramen ovale associated with cryptogenic stroke: development of a scoring system. J Am Soc Echocardiogr. 2019;32:811–816. doi: 10.1016/j.echo.2019.03.021. In this issue. [DOI] [PubMed] [Google Scholar]
- 10.Sylaja P.N., Pandian J.D., Kaul S. Ischemic stroke profile, risk factors, and outcomes in India: the indo-US collaborative stroke project. Stroke. 2018;49:219–222. doi: 10.1161/STROKEAHA.117.018700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang T.K.M., Wang M.T.M., Ruygrok P. Patent foramen ovale closure versus medical therapy for cryptogenic stroke: meta-analysis of randomised trials. Heart Lung Circ. 2019;28:623–631. doi: 10.1016/j.hlc.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Nasir U.B., Qureshi W.T., Jogu H. Updated meta-analysis of closure of patent foramen ovale versus medical therapy after cryptogenic stroke. Cardiovasc Revascularization Med. 2019;20:187–193. doi: 10.1016/j.carrev.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Vaduganathan M., Qamar A., Gupta A. Patent foramen ovale closure for secondary prevention of cryptogenic stroke: updated meta-analysis of randomized clinical trials. Am J Med. 2018;131:575–577. doi: 10.1016/j.amjmed.2017.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng W., Yin S., McMullin D. Residual shunt after patent foramen ovale closure and long-term stroke recurrence: a prospective cohort study. Ann Intern Med. 2020;172:717–725. doi: 10.7326/M19-3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moon J., Kim M., Oh P.C. Residual shunt after patent foramen ovale device closure in patients with cryptogenic stroke: serial bubble contrast transesophageal echocardiography data. J Stroke Cerebrovasc Dis. 2019;28:347–353. doi: 10.1016/j.jstrokecerebrovasdis.2018.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.