Abstract
Objectives
We evaluated the efficacy and safety of dapagliflozin, a SGLT2i along with ARNI in refractory HFrEF irrespective of their diabetic status.
Methods
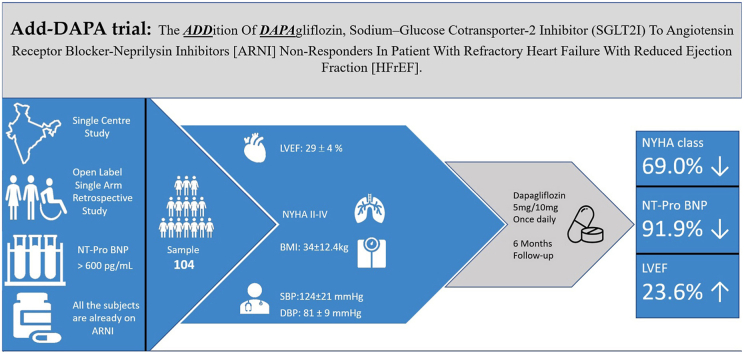
We performed a retrospective analysis of 104 symptomatic patients of HFrEF despite of optimal medical management with ARNI between January–June 2020. Despite the optimal GDMT, dapagliflozin, SGLT2i was added inpatients withrefractory heart failure. At 6-months follow-up, the primary outcome was change in left ventricular ejection fraction, and secondary outcomes included changes in NYHA functional class, vital parameters, renal function, potassium levels, and NT-pro BNP levels.
Results
The primary outcomeat 6-months follow-up was a mean change in left ventricular ejection fraction (LVEF) +9.00 ± 0.62 (p < 0.001). The secondary outcome was a significant improvement (69%) in median NYHA functional class by 2.3 (95% Confidence interval 2.245–2.355) with 92.6% of patients were in NYHA class I and 7.4% were in NYHA class II.Diabetic subgroup reached the HbA1C goal of <7%. None of them had either symptomatic hypotension, hypoglycaemia, dyselectrolaemia, and decline in renal function. The drug was well received by most of the patients.
Conclusions
Dapagliflozin, an SGLT2i, should be used in symptomatic, refractory HFrEF patients despite the use of ARNI. The combination of ARNI and SGLT2i is well tolerated, but large, randomized trials are needed to prove this hypothesis.
Keywords: Dapagliflozin, SGLT2 inhibitors, Heart failure with reduced ejection fraction, ARNI, Refractory heart failure
Abbreviations: HFrEF, Heart failure with reduced ejection fraction; ARNI, Angiotensin receptor blocker-neprilysin inhibitors; SGLT2i, Sodium-glucose cotransporter-2 inhibitor; CV, Cardiovascular; HF, Heart failure; HHF, Hospitalization for heart failure; ARB, Angiotensin receptor blocker; ACEI, Angiotensin-converting enzyme inhibitor; BB, Beta-blocker; GDMT, Guideline - directed medical therapy; MRA, Mineralocorticoid receptor antagonist; NYHA, New York Heart Association; CRT, Cardiac resynchronization therapy; ICD, Implantable cardioverter-defibrillator; T2DM, Type 2 diabetes mellitus; NT-proBNP, N-terminal prohormone brain natriuretic peptide
Graphical abstract
Highlights
•
The PARADIGM-HF trial established the therapeutic value of valsartan/sacubitril, an angiotensin receptor blocker and neprilysin inhibitor.
-
•
Despite GDMT of HFrEF, many patients continue to have symptoms that are resistant to pharmaceutical and device therapy.
-
•
Current unmet needs in the treatment of refractory HFrEF can be met by focussing on the potential function of SGLTi2.
1. Introduction
While most HFrEF patients respond to appropriate medical treatment, some patients do not respond or experience persistent and recurring symptoms, which is defined to as “refractory HF”. Those patients suffer from symptoms at rest or with limited exertion and frequently need repeated, extended hospitalizations for intensive care have a higher incidence of CV death. The first step in improving care for refractory HFrEF is to ensure that all standard GDMT, such as pharmacological therapy and device therapy, such as CRT and ICD, have been utilized optimally and that all applicable factors have been identified and controlled. The American College of Cardiology Foundation/American Heart Association (ACC/AHA) classifies these patients with chronic HF with severe symptoms despite the GDMT as having ‘Stage D’ HF.1,2
The Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) study, which included a group of HFrEF, found sacubitril/valsartan, an ARNI, to be superior to enalapril, an ACEI, in terms of the primary endpoint [CV death or first hospitalization for HHF].3 Dapagliflozin, the SGLT2i, reduced the risk of worsening HF and death in patients with HFrEF in the placebo-controlled trial, Dapagliflozin And Prevention of Adverse Heart Failure (DAPA-HF).4 In T2DM patients with or without atherosclerotic cardiovascular disease, SGLT2i reduces hospitalization due to heart failure. In patients with HFrEF, both the ARNI and dapagliflozin showed an independent reduction in CV death and HF. In the context of newer therapies using ARNI and SGLT2i, there are a few questions that need to be addressed.
-
1.
Can these benefits be extrapolated to treat patients with established heart failure?
-
2.
Are the benefits of SGLT2i glucose independent?
-
3.
Can SGLT2i be used to treat patients without T2DM?
While the DAPA-HF trial, in which dapagliflozin, 10 mg once daily, was added to standard therapy in patients with HFrEF both with and without T2DM, addressed some of these concerns, it is still unclear if SGLT2i can affect the efficacy or safety of ARNI. There is also no information on whether adding SGLT2i, dapagliflozin to valsartan/sacubitril, ARNI results in an incremental response at different tolerated doses, including the target dose.
Despite the use of ARNI, some patients experience residual symptoms. We looked at the effects of dapagliflozin in combination with other treatments, especially ARNI, in patients with refractory HFrEF.
2. Materials and methods
2.1. Study design
Our research is an open-label, retrospective study, single arm of the clinical and laboratory data collected who have prescribed the drug within the stated time and agreed to take part in this study. The Ethics Committee of our hospital gave permission and authorization for a retrospective analysis of patient data.The study group included patients with HFrEF who sought treatment with sacubitril/valsartan between January–June 2020 and who were followed up for 6-months. Our analysis was divided into two phases: symptomatic HFrEF despite GDMT initiated on ARNI and symptomatic HFrEF on GDMT including ARNI initiated on dapagliflozin.
2.2. Study patients
Inclusion criteria:
-
•
Men and women >18 years of age with HFrEF with EF ≤ 40%,
-
•
NYHA functional Class II – IV,
-
•
Patients should be on ARNI before starting SGLT2i,
-
•
Appropriately treated according to clinical recommendations with pharmacological and device therapy for HFrEF,2,5
-
•
The protocol instructed that, unless contraindicated or not tolerated BB, as well as an MRA, should be used at guideline-approved doses,
-
•
Participants were also expected to have a natriuretic peptide of the N-terminal pro-B (NT-proBNP) concentration > 600 pg/mL (>400 pg/mL if treated in preceding 12 months for HF),
-
•
Patients with atrial fibrillation or atrial flutter were expected to have an NT-proBNP level of >900 pg/mL, independent of HF hospitalization history,
-
•
Willing to give informed consent.
Exclusion Criteria.
-
•
Type 1 Diabetes mellitus,
-
•
Pregnancy,
-
•
Patients not on ARNI,
-
•
Unstable hemodynamic conditions including symptomatic hypotension, a systolic blood pressure of less than 100 mm Hg,
-
•
Hypoxia, a room air saturation less than 95%,
-
•
Ongoing myocardial ischemia requiring revascularization,
-
•
Estimated glomerular filtration rate (eGFR) below 30 mL per minute per 1.73 m2 of body-surface area,
-
•
Present or past h/o hyperkalaemia (serum potassium level of more than 5.5 mEq per litre), h/o angioedema, and multi-organ dysfunction.
2.3. Clinical and laboratory evaluation
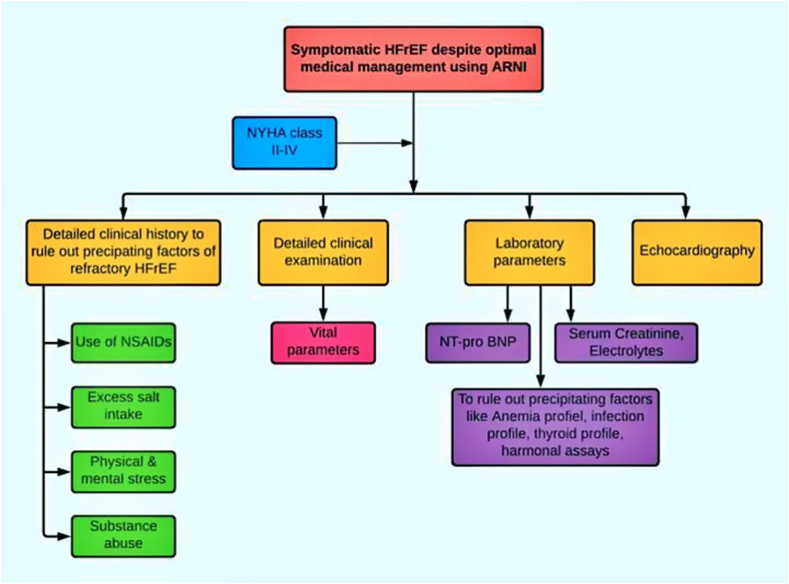
The detailed clinical history, including the use of analgesics, excess salt use, physical and mental stress, alcohol and substance abuse; detailed clinical examination, laboratory parameters, including elevated serum BNP and precipitating factors like evaluation of anaemia (iron profile, vitamin B12, folic acid levels, stool for occult blood, upper and lower endoscopies); infection profile (total differential leucocyte counts, c-reactive proteins), thyroid profile, hormonal assays in oral contraceptive pills users in females were done to rule out identifiable causes of refractory heart failure.
A single-blind echocardiography was performed as a routine case with an Epiq ultrasound system (Philips) by trained cardiologists posted in the department of echocardiography without their awareness of the patients' inclusion in this study(Fig. 1).
Fig. 1.
Flow chart showing clinical evaluation of patients with refractory heart failure with reduced ejection fraction which includes symptoms, precipitating factors, clinical examination, laboratory parameters and echocardiography.
2.4. Outcomes
The primary outcome was mean improvement of ejection fraction after 6-months of starting of dapagliflozin. The secondary outcomes at 6-months of follow-up of starting dapagliflozin were improvements in NYHA functional class, changes in vital parameters (blood pressure and heart rate), a reduction in renal function (which was defined as end-stage renal disease or as a decrease in the eGFR of at least 50% from the baseline or a decrease in the eGFR of more than 30 ml per minute per 1.73 m2,to less than 60 ml per minute per1.73 m2), changes in thepotassium levels and, the plasma NT-pro BNP levels.
2.5. Drug therapy and follow-ups
Symptomatic patients with HFrEF despite GDMT were transferred to the ARNI after 36 h of stopping the ACEI. The initial starting dose of ARNI was 24/26 mg twice daily [target dose 97/103 mg twice daily] along with other GDMT, and ARNI was only administered to patients with blood pressures of ≥110/70 mm Hg.
Despite the overall tolerated dosage of ARNI and other GDMT, the signs and symptoms of HF persisted; in those cases, an initial dose of 5 mg was administered, and after 6–8 weeks, even with no clinical improvement, the dose was increased to 10 mg.
We recorded their data in our electronic medical records (EMR), which included their demography, risk factors, past medical history, clinical presentations, vital parameters, clinical examinations, baseline investigations, and coronary angiography. Also, the adherence to the prescribed medications was ascertained.
They were asked for 1st follow-up after 1-week for the OPD visit to see the result of the administration of the combination of ARNI and dapagliflozin. Those who experienced symptomatic improvement were recommended to visit every two months for a total of 6-months. The visits were for the purpose of adjusting the doses of both drugs and other medications, including diuretics, as well as assessing tolerance and safety after the addition of dapagliflozin.
3. Sample size and statistical analysis
3.1. Sample size
Assuming a true difference in means after addition of dapagliflozin of 5.2%, a pooled standard deviation of 0.4 units, the study would require a sample size of 69 for group to achieve a power of 90% and a level of significance of 5%, for declaring that the test drug is superior at 5% margin of superiority assuming that a larger mean is desirable. A total of 104 patients taking dapagliflozin were included in the study.
3.2. Statistical analysis
The statistical analysis was performed using Windows SPSS software. The data were presented as absolute numbers with percentages in the case of nominal data and means with standard deviation in the case of continuous data. For the comparison of changes within the study group during subsequent visits, the Wilcoxon test for paired samples was used. A p-value < 0.05 was considered statistically significant.
4. Results
4.1. Study patients before initiation of ARNI
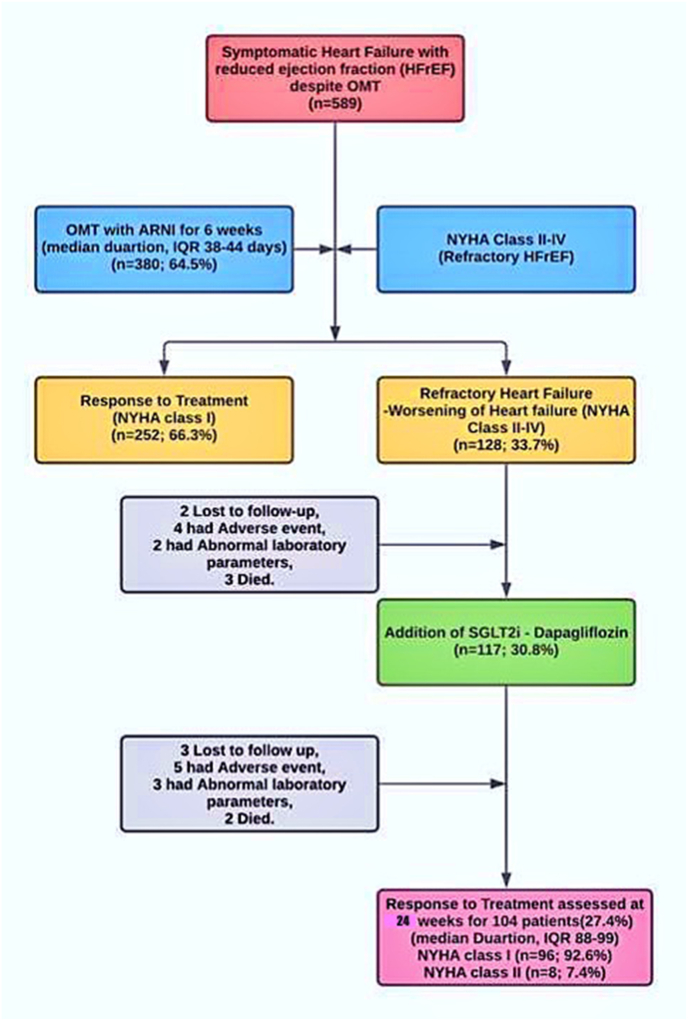
Out of 589 symptomatic patients with HFrEF despite optimal medical therapy, 380 (64.5%) of patients who did not respond to the use of ACEI/ARBs and other optimal GDMT were switched to ARNI.
Sacubitril/valsartan is available in three doses, the target dose being 97/103 mg twice daily. The starting dose listed in the ‘Drug Characteristics Summary of Sacubitril/Valsartan’ was 24/26 mg twice daily used in those who needed lower doses due to borderline blood pressure .6
In 98.2% of patients, sacubitril/valsartan was started at the lowest dose (24/26 mg twice daily).6 At consecutive visits after 2 weeks, the dose was increased to 49/51 mg twice daily in patients. Finally, in the follow-up period of 6 weeks, the dose was increased to the target dose of 97/103 mg twice daily only in 26.7% of patients. To control the symptoms, maximum tolerated doses were employed, including the target dose in a few cases when it was tolerated. 66.3% of patients were in NYHA class I at 6-week follow-up.
Before the initiation of sacubitril/valsartan, the patients received either ACEI or ARB. ACEI (enalapril, ramipril, perindopril) were used in 72.1% of patients while ARB (valsartan, losartan, telmisartan, olmesartan) was in 26.3% patients, 1.6% patients did not receive either of it. MRA was administered to 88% of patients, while BB was prescribed to 97% of patients. Detailed data on pharmacological treatment before the initiation of the sacubitril/valsartan treatment is presented in Table 2.
Table 2.
Baseline treatment using angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and other concomitant pharmacological treatment before and after the starting on angiotensin receptor blockers - neprilysin inhibitors. Angiotensin-converting enzyme inhibitor = ACEI; Angiotensin receptor blocker = ARB; Angiotensin receptor blocker-neprilysin inhibitor = ARNI.
| Treatments before the start of ARNI and SGLT2i | Number of Patients (percentage) |
|---|---|
| Guideline-driven Medical therapy | |
| Pre-study use of ACEI | 72.1 |
| Pre-study use of ARB | 26.3 |
| During the study use of ARNI | 79.01 |
| Loop diuretics | 100 |
| Beta-blockers | 97 |
| Mineralocorticoid receptor antagonist | 88 |
| Ivabradine | 58 |
| Digoxin | 56 |
| Amiodarone | 12 |
| Device- based therapy | |
| ICD | 11 |
| CRT- p | 5 |
| CRT- D | 2 |
| No- device | 82 |
4.2. Study patients beforeinitiation of SGLT2I
Even after maximum tolerated therapeutic doses, including target doses, 128 (33.7%) of these patients did not respond to ARNI. SGLT2i could not be started in 11 patients due to different circumstances such as 2 patients failed to follow-up, 4 patients experienced adverse effects such as hypotension, angioedema, intractable cough, 2 patients had abnormal laboratory test, such as decline in renal function and dyselectrolaemia, and 3 deaths occur secondary to cardiovascular causes such as worsening of HF and sudden cardiac death.
As a result, 117 (30.8%) non-responders were recommended for addition of SGLT2i, dapagliflozin as they were in NYHA class II-IV symptoms. 13 patients could not be included in the study as 3 patients were lost to follow-up, 5 patients reported adverse effects, 3 patients showed abnormal laboratory test results and 2 deaths occurred due to cardiovascular causes in the 1st week (Fig. 2).
Fig. 2.
Screening of patients with refractory heart failure with reduced ejection fraction, initiation of optimal medical therapy using angiotensin receptor blocker-neprilysin inhibitor and the addition of dapagliflozin, sodium-glucose cotransporter-2 inhibitor.
In this retrospective study, open-label study, we analysed results in patients receiving dapagliflozin in addition to therapy with ARNI. Our study included data for 104 (27.4%) patients aged 68 ± 20.5 years, of which 37.6% were females. The treatment protocol has been used in patients in NYHA class II – IV and LVEF was 18–40% (mean 29 ± 4). The mean weight was 68 ± 35.6 kg and the BMI were 34 ± 12.4 kg 32% of patients were in NYHA class II, 40% were in NYHA class III, and 28% were in NYHA class IV. Progressive breathlessness was a prominent symptom in 98% accompanied by chest pain and easy fatigue in 52% of patients. 78% of patients had a history of paroxysmal nocturnal dyspnoea while 36% had orthopnoea. The baseline characteristics are presented in Table 1.
Table 1.
Demographics and clinical features of patients prescribed on angiotensin receptor neprilysin inhibitors.
| Clinical Parameters | Mean ± SD/percentage (n = 589) |
|---|---|
| Age - years | 68 ± 20.5 |
| Female Sex – no. (%) | 37.6 |
| Weight | 68 ± 35.6 kg |
| BMI$ | 34 ± 12.4 kg/m2 |
| NYHA class# | |
| II | 32% |
| III | 40% |
| IV | 28% |
| LVEF | 18–40% (32 ± 4) |
| Risk factors | |
| Type II Diabetes Mellitus | 38.9% |
| Hypertension | 42.4% |
| Dyslipidemia | 68.8% |
| Active Smoker | 12.2% |
| Symptoms | |
| Breathlessness | 98% |
| Paroxysmal Nocturnal dyspnea | 78% |
| Orthopnea | 36% |
| Easy fatiguability | 52% |
| Chest pain | 22% |
| HF etiology | |
| Ischemic | 45% |
| Non-ischemic | 54% |
| Unknown | 1% |
| Cardiac rhythm | |
| Sinus rhythm | 84% |
| Atrial fibrillation | 14% |
| Atrial Flutter | 2% |
| Medical History | |
| Hospitalization for Heart Failure | 75.4% |
| Myocardial infarction | 44.2% |
| Stroke | 11.7% |
| Peripheral vascular disease | 4.5% |
Plus-minus values are means ± SD. $The body-mass index is the weight in kilograms divided by the square of the height in meters. #The data for New York Heart Association (NYHA) class reflect the status of patients at the time start of SGLT2i, dapagliflozin. Patients were required to have at least NYHA class II symptoms at the screening. Body mass index = BMI; New York Heart Association = NYHA; Left ventricular ejection fraction = LVEF; Implantable cardioverter-defibrillator = ICD; Cardiac resynchronization therapy pacemaker = CRT-P; Cardiac resynchronization therapy with defibrillator = CRT- D.
Mean systolic blood pressure (SBP) was 124 ± 21 mm Hg, while mean diastolic blood pressure (DBP) was 81 ± 9 mm Hg and the pulse rate of 106/min±27.7/min. The mean level of N-terminal-prohormone brain natriuretic peptide (NT-pro BNP) was 13,607 pg/mL. Cardiac resynchronization therapy with defibrillator (CRT-D) was used in 2% of patients while cardiac resynchronization therapy without defibrillator (CRT-P) in 5% of patients and implantable cardioverter-defibrillator (ICD) in 11% of patients (Table 2).
4.3. Study outcomes after addition of dapagliflozin and follow-ups
The primary outcome demonstrated a significant improvement in mean LVEF from 29 ± 4% to 38 ± 5% (+9.00 ± 0.628; p < 0.001). The secondary outcomes in the form of HF symptoms measured using the NYHA functional class decreased statistically significantly in the dapagliflozin-added patients during follow-up, with a median decline from 3.83 ± 0.17 [3.66–4.0] to 1.53 ± 0.24 [1.29–1.77](p < 0.001)and a significant decrease in the mean NT-pro BNP levels from 13,607 ± 1874 to 1100 ± 1682 pg/mL (p < 0.0001).
In the 6-months follow-up period, the first follow-up visit was held after a median duration of 11 days (7–15 days). The mean duration of follow-up after addition of dapagliflozin was 186 days (178–190 days). Following the addition of dapagliflozin, there was 100% follow-up.
4.4. Safety and adverse events
Although symptomatic hypotension was not observed there were slight decreases in mean SBP (from 124 ± 21 to 107 ± 12 mm Hg; P < 0.001), mean DBP (from 81 ± 9 to 78 ± 5 mm Hg; P = 0.0077), and heart rate (from 106 ± 27.7 to 74 ± 17.9 bpm; P < 0.0001) were observed. On the other hand, insignificant trends toward mean potassium levels (from 4.55 ± 0.4 to 4.6 ± 0.5 mEq/L; P = 0.31) and mean creatinine levels (from 0.88 ± 0.19 to 0.9 ± 0.31 mg/dL; P = 0.45) were observed (Table 3).
Table 3.
Comparison of vital parameters and laboratory values before starting therapy with angiotensin receptor neprilysin inhibitors and at 6-month follow-up.
| Clinical and laboratory parameters | Baseline value of patients who are symptomatic despite ARNI (n = 104) | Values at 6-months follow-up after initiation of SGLT2i (n = 104) | Mean/Median ± SE change in parameters from baseline after initiation of SGLTi2 at 6-months follow-up | p-Value |
|---|---|---|---|---|
| Primary Outcome | ||||
| Ejection fraction [%] | 29 ± 4 | 38 ± 5 | +9.00 ± 0.628 | <0.001 |
| Secondary Outcomes | ||||
| NYHA Class (Median) | 3.83 ± 0.17 | 1.53 ± 0.24 | −2.3 ± 0.2 | <0.001 |
| Systolic BP [mmHg] | 124 ± 21 | 107 ± 12 | −17.0 ± 2.372 | <0.001 |
| Diastolic BP [mmHg] | 81 ± 9 | 78 ± 5 | −3.0 ± 1.010 | 0.0033 |
| Heart rate [bpm] | 106 ± 7 | 74 ± 17.9 | −32.0 ± 3.234 | <0.001 |
| NT-proBNP [pg/mL] | 13,607 ± 1874 | 1100 ± 682 | −12507 ± 246.92 | <0.001 |
| Creatinine [mg/dL]@ | 0.88 ± 0.19 | 0.9 ± 0.31 | +0.02 ± 0.036 | 0.575 |
| Potassium [mEq/L] | 4.55 ± 0.4 | 4.6 ± 0.5 | +0.05 ± 0.06 | 0.426 |
@A decline in renal function was defined as end-stage renal disease or a decrease of 50% or more in the estimated glomerular filtration rate (eGFR) from the initial value or a decrease in the eGFR of more than 30 mL per minute per 1.73 m2, to less than 60 mL per minute per 1.73 m2. N-terminal pro hormone brain natriuretic peptide = NT-proBNP; Nonsignificant = NS; Beats per minute = BPM; Blood Pressure = BP; NYHA = New York Heart Association.
None of them had any hypotension, hypoglycaemia, dyselectrolaemia, and elevation of serum creatinine while 0.4% of patients had angioedema. The combination was tolerated well by 99.7% of the patients. Diabetic patients reached the HbA1C target of <7%.
In the follow-up period, 98.6% of patients with HFrEF, sacubitril/valsartan continued. 1.4% of those who did not display improvement in their symptoms after 4 weeks had noncompliance with a prescription due to medication costs. No substantial changes in HF therapy were noticed during the follow-up period, except for a half reduction in the dose of furosemide. Two patients died in the first week after starting dapagliflozin, therefore it was not regarded important. After escalating the dose of dapagliflozin in HFrEF patients for four weeks, no patient has been hospitalized or died.
5. Discussion
5.1. Guideline-directed medical therapy for heart failure
There is no doubt that the three main pharmacological therapies for HFrEF are a RAS blocker (ACE inhibitor/ARB), beta-blockers (BB) and mineralocorticoid receptor antagonists (MRA) and randomized studies showing decreased mortality and hospitalization .7, 8, 9, 10, 11 In comparison to these key treatments, three new pharmacological treatments have shown additional effectiveness over the past few years. The first one was sinus node inhibitor, ivabradine, followed by sacubitril neprilysin inhibitor, and most recently, dapagliflozin, the SGLT2i .3,12,13
In this study, we have shown that dapagliflozin not only enhances results when applied to the essential combination of RAS blocker, BB and MRA, but also has a clear advantage if ivabradine or sacubitril/valsartan are used in the background therapy. The observation that none of these agents changed the dapagliflozin reaction reinforces the belief that inhibition of SGLT2 works in a mechanistically distinct and complementary manner to other HFrEF therapies 14 Originally, established as glucose-lowering drugs for the treatment of type 2 diabetes mellitus, the DAPA-HF finding that the advantage of dapagliflozin existed in patients with, and without, diabetes indicates that this advantage is irrespective of any glucose-lowering effect .4,15,16
Different hypotheses on the mechanisms of action underlying the advantages of dapagliflozin have been suggested, including a diuretic function, enhanced renal erythropoietin production, mitigation of myocardial fibrosis and possible influence on peripheral vasculature, ion transporters, adipokines, and sympathetic activation of the nervous system.16,17
The proof-based effective doses of some RAS inhibitors and BBs are well established, and there is proof of a dose–response for RAS inhibitors, as most in terms of HF hospitalization reductions. But registry analyses consistently indicate that such evidence-based targeted doses are rarely reached in clinical practice and it is not entirely clear if this is due to higher dose intolerance.18 Vardeny et al, in a post-hoc study from PARADIGM-HF, classified patients based on whether they achieved the maximum dose during the trial or whether they had dose reductions to lower doses. The amount of advantage for patients on lower doses of sacubitril/valsartan compared to those on lower doses of enalapril was comparable to that for patients who stayed on target doses of both medications .19
Device therapy also has an significant role to play in handling the HFrEF, however as regarding medication dosing, ‘real-world’ reports indicate that systems are underused in Indian practice, with significant regional heterogeneity in use patterns, indicating the role of socio-economic considerations in understanding this difference, among others .20
5.2. Dapagliflozin and heart failure with reduced ejection fraction
In addition, given the context pharmaceutical and device therapy, we found a clear advantage of dapagliflozin on top of ARNI. Such results indicate that dapagliflozin has progressive impact and is similar to standard HFrEF therapies. If these results are actually similar to the benefits derived from other evidence based HFrEF therapies is obviously important to know. A role of SGLT2i in the treatment of HFrEF can only be documented for dapagliflozin .4,15,21 The DAPA-HF trial demonstrated that dapagliflozin can produce a significant improvement in quality of life as assessed by KCCQ in patients with HFrEF which is of high clinical value. Dapagliflozin exerts beneficial effects in HFrEF irrespective of T2DM status and it appears that the mechanism of action of dapagliflozin in HFrEF extends beyond a simple glucose-lowering effect. Dapagliflozin is the only one that can be considered in the treatment of HFrEF patients, with and without T2DM.22,23
The DAPA-HF trial was designed to investigate dapagliflozin for the treatment of HFrEF as an add on to standard of care, which included ACEIs, ARBs, beta-blockers, MRAs, and neprilysin inhibitors, in over 4700 patients with or without T2DM. The DAPA-HF trial is the first HF outcome study to report results with a dapagliflozin in the treatment of patients with HFrEF, with or without T2DM. The population enrolled in DAPA-HF is representative of patients in clinical practice, as evidenced by HF registry data, and similar to patients enrolled in other contemporary HFrEF randomized controlled trials .3,16
Solomon et al evaluated the effectiveness and safety of dapagliflozin in patients who were or were not taking sacubitril/valsartan at baseline in the DAPA-HF trial. They demonstrated that dapagliflozin was fairly safe and effective in patients who were taking sacubitril/valsartan, implying that the use of both agents in combination could also reduce morbidity and mortality in patients with HFrEF .24
The U.S. Food and Drug Administration approved in May 2020 dapagliflozin for adults with HFrEF to reduce the risk of cardiovascular death and HHF. With the approval, dapagliflozin is the first SGLT2i in this particular class of drug to be approved for treating adults with HFrEF of the NYHA functional class II-IV.25
Based on the current LIFE study data, the combination of sacubitril/valsartan was not superior to valsartan for reducing NT-proBNP and did not benefit other clinical endpoints in patients with refractory HFrEF. The patient cohort with refractory HF (ACC/AHA stage D) differs from that of patients with less severe HF (ACC/AHA stage B and C) due to end organ abnormalities. End organ changes limit the ability of the failing heart to respond to standard therapy to the same degree as patients with milder forms of HF .26,27
6. Limitations of the study
A retrospective non-randomized research was conducted, with a small sample size for the studied population. As this was a real-world retrospective data collection, we included patients on various tolerated doses, including maximum tolerated dosages of the ARNI. In addition, our results included changes in LVEF and NYHA class, which might be operator dependent and subjective. Longer follow-ups are required for hard outcomes such as mortality and hospitalization for heart failure. We emphasize multicentre clinical experience and better follow-up evidence in larger randomized controlled trials in the Indian subset.
7. What is already known?
SGLT2i are a new class of glucose-lowering drugs that, due to their particular mechanism of action, do not necessitate insulin or islet cells to induce pharmacological effects in vivo. Controlled osmotic diuresis using SGLT2i is a mechanism other than neuro-humoral modulation for the treatment of refractory heart failure. SGLT2i has shown to have strong cardiorenal therapeutic benefits so far.
8. What this study adds?
Individually or in combination, the ARNI and SGLT2i are well tolerated, with a reduced rate of treatment discontinuation. Despite the use of ARNI, dapagliflozin is a novel cost-effective treatment option for refractory HFrEF in Indian patients.SGLT2i, dapagliflozin, have shown promising results so far, and are predicted to become clinical first-line treatments for refractory HFrEF.
Funding
There is no source of funding for this article.
Contributorship statement
PJ is a treating Consultant Cardiologist, and responsible for the writing manuscript, collection, and preparation of the article. AR and HKB assisted in the review of the manuscript, statistical calculations, and grammatical and spelling corrections. AP, HKB, and AR shared their cases and reviewed manuscript independently.
Declaration of competing interest
As an author, I declare that there is no financial or non-financial conflict/competing of interests. This manuscript is not submitted to any journal before for publication as a part or complete version. I give complete consent and rights to the journal for its publication. Informed consent was obtained from a participant included in the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ihj.2021.07.005.
Contributor Information
Pankaj Jariwala, Email: pankaj_jariwala@hotmail.com.
Kartik Jadhav, Email: drkartik303@gmail.com.
Arshad Punjani, Email: arpunjani@gmail.com.
Harikishan Boorugu, Email: drharikishan@gmail.com.
Ajay Reddy Mari, Email: drajayreddy77@gmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Yancy C.W., Jessup M., Bozkurt B. ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology foundation/American heart association task force on practice guidelines. J Am Coll Cardiol. 2013;62:e147. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Yancy C.W., Jessup M., Bozkurt B. 2017. ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of Amer.https://www.ahajournals.org/doi/10.1161/CIR.0000000000000509 [Google Scholar]
- 3.McMurrayJJV, Solomon S.D., Inzucchi S.E. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 4.McMurray J.J.V., DeMets D.L., Inzucchi S.E. SS. DAPA- HF Committees and Investigators. A trial to evaluate the effect of the sodium– glucose co-transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA- HF) Eur J Heart Fail. 2019;381:665–675. doi: 10.1002/ejhf.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaul U., Das M.K., Agarwal R. Vol. 72. Indian Heart Journal; 2020. Consensus and development of document for management of stabilized acute decompensated heart failure with reduced ejection fraction in India; pp. 477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Entresto, INN-sacubitril/valstartan . 2015. SUMMARY OF PRODUCT CHARACTERISTICS. [Google Scholar]
- 7.The SOLVD Investigators Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 8.CONSENSUS Trial Study Group Effects of enalapril on mortality in severe congestive heart failure. N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 9.Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 10.Pitt B., Zannad F., Remme W.J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341 doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 11.Zannad F., McMurray J.J.V., Krum H. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 12.McMurray J.J.V., Packer M., Desai A.S. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 13.Swedberg K., Komajda M., Böhm M. Ivabradine and outcomes in chronic heart failure (SHIFT): a rando- mised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 14.Ni L., Yuan C., Chen G. Vol. 19. Cardiovascular Diabetology. BioMed Central; 2020. SGLT2i: beyond the glucose-lowering effect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Docherty K.F., Jhund P.S., Inzucchi S.E. Effects of dapagliflozin in DAPA-HF according to background heart failure therapy. Eur Heart J. 2020;41(25):2379–2392. doi: 10.1093/eurheartj/ehaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma S., McMurray J. SGLT2 inhibitors and mechanisms of cardiovascular bene- fit: a state-of-the-art review. Diabetologia. 2018;61:2108–2117. doi: 10.1007/s00125-018-4670-7. [DOI] [PubMed] [Google Scholar]
- 17.Murray S.W., McKelvey S., Heseltine T.D. Journal of Human Hypertension. Springer Nature; 2021. The “discordant doppelganger dilemma”: SGLT2i mimics therapeutic carbohydrate restriction - food choice first over pharma? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maggioni A.P., Anker S.D., Dahlström U. Heart failure association of the ESC. Are hospitalized or ambulatory patients with heart failure treated in accordance with European society of Cardiology guidelines? Evidence from 12,440 patients of the ESC heart failure long-term registry. Eur J Heart Fail. 2013 Oct;15(10):1173–1184. doi: 10.1093/eurjhf/hft134. [DOI] [PubMed] [Google Scholar]
- 19.Vardeny O., Claggett B., Packer M. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail. 2016;18(10):1228–1234. doi: 10.1002/ejhf.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schrage B., Uijl A., Benson L. Association between use of primary-prevention implantable cardioverter-defibrillators and mortality in patients with heart failure: a prospective propensity score-matched analysis from the Swedish heart failure registry. Circulation. 2019 Nov 5;140(19):1530–1539. doi: 10.1161/CIRCULATIONAHA.119.043012. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka H., Soga F., Tatsumi K. Positive effect of dapagliflozin on left ventricular longitudinal function for type 2 diabetic mellitus patients with chronic heart failure. Cardiovascular Diabetology [Internet. 2020;19(1):1–9. doi: 10.1186/s12933-019-0985-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crespo Leiro M.G., Paniagua Martín M.J. Management of advanced or refractory heart failure. Rev Esp Cardiol. 2004;57(9):869–883. [PubMed] [Google Scholar]
- 23.Lo K.B., Gul F., Ram P. vol. 10. CardioRenal Medicine. S. Karger AG; 2020. pp. 1–10. (The Effects of SGLT2 Inhibitors on Cardiovascular and Renal Outcomes in Diabetic Patients: A Systematic Review and Meta-Analysis). [DOI] [PubMed] [Google Scholar]
- 24.Solomon S.D., Jhund P.S., Claggett B.L. Effect of Dapagliflozin in Patients with HFrEF Treated with Sacubitril/Valsartan: The DAPA-HF Trial. JACC: Heart Failure [Internet] 2020 Jul doi: 10.1016/j.jchf.2020.04.008. https://linkinghub.elsevier.com/retrieve/pii/S2213177920302547 Available from: [DOI] [PubMed] [Google Scholar]
- 25.https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-type-heart-failure
- 26.Mann D.L., Greene S.J., Givertz M.M. Vol. 8. JACC: Heart Failure. Elsevier Inc.; 2020. Sacubitril/valsartan in advanced heart failure with reduced ejection fraction: rationale and design of the LIFE trial; pp. 789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LIFE Sacubitril/valsartan not superior to valsartan in advanced HFrEF - American College of Cardiology [internet] https://www.acc.org/Latest-in-Cardiology/Articles/2021/05/12/19/40/Mon-8am-LIFE-acc-2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.