Summary
This patient guideline is intended for all patients at risk of or living with non-alcoholic fatty liver disease (NAFLD). NAFLD is the most frequent chronic liver disease worldwide and comes with a high disease burden. Yet, there is a lot of unawareness. Furthermore, many aspects of the disease are still to be unravelled, which has an important impact on the information that is given (or not) to patients. Its management requires a close interaction between patients and their many healthcare providers. It is important for patients to develop a full understanding of NAFLD in order to enable them to take an active role in their disease management. This guide summarises the current knowledge relevant to NAFLD and its management. It has been developed by patients, patient representatives, clinicians and scientists and is based on current scientific recommendations, intended to support patients in making informed decisions.
Abbreviations: ALD, alcohol-related or alcoholic liver disease; ASH, alcoholic steatohepatitis; BMI, body mass index; CAP, controlled attenuation parameter; CT, computed tomography; CVD, cardiovascular disease; EASD, European Association for the Study of Diabetes; EASL, European Association for the Study of the Liver; EASO, European Association for the Study of Obesity; FIB-4, fibrosis-4 index; FXR, farnesoid X receptor; GLP-1 RAs, glucagon-like receptor 1 agonists; GP, general practitioner; HCC, hepatocellular carcinoma; HDL, high-density lipoprotein; LDL, low-density lipoproteins; MRE, magnetic resonance elastography; MRI, magnetic resonance imaging; NAFL, non-alcoholic fatty liver; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; NASH CRN, NASH Clinical Research Network; NIT, non-invasive test; SMART, specific, measurable, achievable, relevant, timely; T1D, type 1 diabetes; T2D, type 2 diabetes
The purpose of this patient guideline
This guideline is intended for all patients at risk of or living with non-alcoholic fatty liver disease (NAFLD). NAFLD is a serious condition. It is important that you develop a full understanding of it. This has several advantages: first, it enables you to take an active role in your own healthcare. Second, you develop a better understanding of what the doctor is discussing with you. Third, you can monitor your condition and assess the success of various measures yourself. This guide will help you do that. It has been developed by patients, patient representatives, clinicians and scientists and is based on current scientific recommendations. It cannot and should not replace the individual consultation with your medical team but should support you in making informed decisions.
1. Introduction
a. What kind of organ is the liver? Where is it located? What is its function?
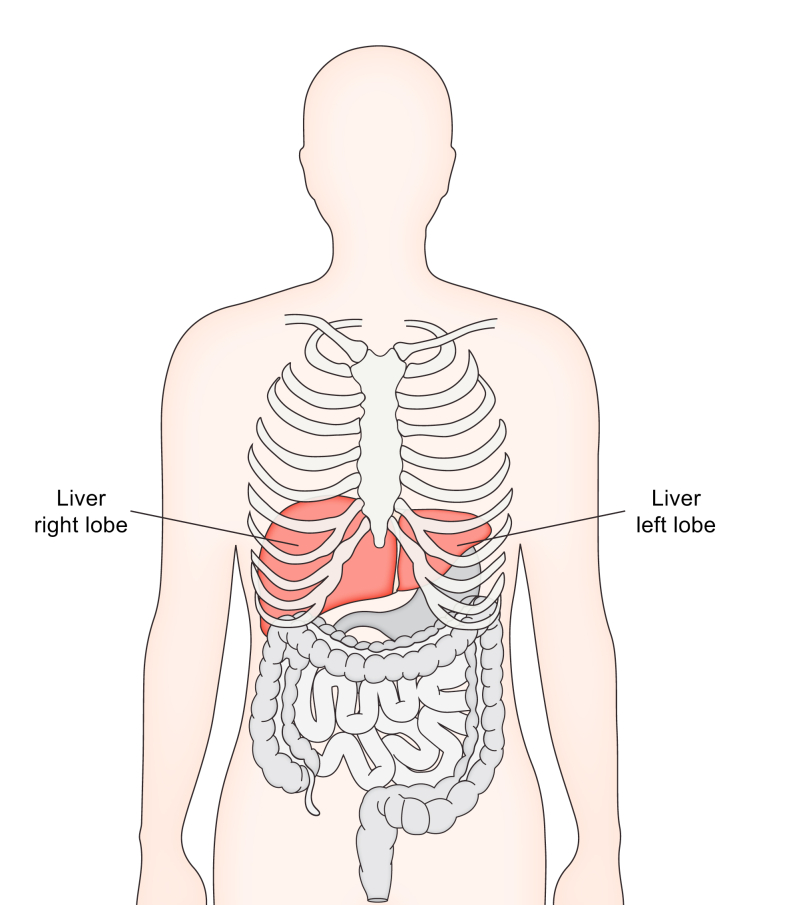
The liver is a large organ on the right-hand side of the body, located in the upper right quadrant of your abdomen. The normal weight of an adult liver is about 1,200–1,500 g. The liver is mostly covered and protected by the lower part of your ribcage (Fig. 1). About 20% of your blood volume per minute passes through the liver via the portal vein (75%) and hepatic artery (25%).
Fig. 1.
The liver is a large organ on the right-hand side of the body, located primarily in the upper right quadrant of the abdomen.
The liver is mostly covered and protected by the lower part of your ribcage.
What does your liver do? The liver, as the chemical factory of your body, performs an extraordinarily complex set of functions to keep the body in a healthy condition. It receives blood from the gut via the portal vein, which carries most of the nutrients absorbed after a meal. Thus, the liver plays an important role as the first point where nutrients are filtered and further processed. For example, the liver has a key role in handling sugars, proteins and fats. After transformation, the liver releases the building blocks for energy and growth (i.e. substrates like sugar, fat and proteins) when required by organs. When you have just eaten and you have energy substrates that are not immediately needed as fuel, the liver will process these extra sources of energy. They can then be stored in the liver and elsewhere in your body, for example in the fat tissue, until your body needs them.
This also means that the liver plays an important role in the regulation of blood sugar (glucose) and lipid levels. Lipids refer to total and several subtypes of cholesterol, but also to triglycerides, which are another type of fat molecule that circulates in the blood. The term blood lipids also encompasses lipoproteins, which are larger composite structures of fat molecules and proteins.
The liver also produces many key proteins that your body needs to function normally like albumin, a protein that acts as a carrier for many molecules that need to be transported in the blood, and proteins needed for blood clotting. Your body continuously renews most of its structures, resulting in a lot of breakdown products. Along with the kidneys, the liver helps the body to get rid of these waste products.
Furthermore, the liver produces bile, a fluid that is stored in the gallbladder. During a meal, the gallbladder will contract, and this will cause the bile to drain into the gut (Fig. 1); there the bile salts will help to break down and absorb the fat molecules in your food. Part of the bile remains in the gut and is excreted in stool. This journey of the bile allows your body to get rid of several toxic substances (including alcohol) and excess cholesterol via the liver. Bile fluid contains the breakdown product of blood, bilirubin (which has a yellow colour), and also waste products from drug and alcohol metabolism. It is a way to get rid of too much cholesterol. Bile salts play a role in the metabolism of glucose and are therefore important for health. The liver also plays a role in the breakdown of many medications and other chemicals. Finally, the liver helps fight infections by filtering harmful organisms as they circulate in the blood, especially those entering the body via the gut.
The liver thus plays a central role in total body function. Consequently, its microscopic structure is complicated. Your liver carries out these important activities in silence: there are not many pain sensors in the liver and therefore liver diseases are often not painful, which can be a reason why a chronic liver disease remains undiagnosed for a long period of time. However, some livers can be more sensitive to pain, and some patients do experience a vague uncomfortable feeling or even pain from a chronic liver problem. This is due to the pressure on the capsule of the liver that has pain sensing nerves.
b. What is non-alcoholic fatty liver disease or NAFLD? What is the difference between NAFLD and non-alcoholic steatohepatitis (NASH)?
Steatosis means accumulation of fat in the cells. When this accumulation occurs in liver cells, it is called liver steatosis or fatty liver. There are different types of fat storage in cells. The type of storage that is relevant to NAFLD is fat (mainly triglycerides) that is stored in droplets. The size of these droplets can vary, but they are mostly large. Consequently, they fill up the whole inner part of the cell, pushing other parts of the cell to the cell border. This type of steatosis is called macrovesicular steatosis.1
NAFLD is a fatty liver disease and the acronym stands for non-alcoholic fatty liver disease.1 It is a condition in which too much fat is stored in the liver cells. As explained in Section 1.a, your liver is a key organ involved in energy regulation. What your liver is not supposed to do, however, is to store excess energy in the form of fat. The liver stores only a small amount of energy, namely some carbohydrate in the form of glycogen, but not fat. In some animals there is a small amount of fat in the liver in the fasting state, but that is not the case in humans.2 Storing excess energy as fat is the role of your body’s fat tissue (adipose tissue).3 A healthy human liver hence contains few or no fat droplets. If there are fat droplets in more than 5% of the liver cells, this is considered as abnormal or pathological. In people with NAFLD, more than 5% of liver cells contain these fat droplets.
The accumulation of fat in the liver in the context of the disease called NAFLD is in most cases due to a combination of eating more calories than the body needs and leading a more sedentary (inactive) lifestyle. Therefore, it occurs most commonly, but not always, in association with being overweight/having obesity.4,5 Another group of people at risk are people living with diabetes, more often type 2 diabetes (T2D), or earlier stages of altered glucose handling in the body.6 Abnormal levels of blood lipids or high blood pressure (arterial hypertension) are also associated with an increased risk of having NAFLD.7 Abnormal levels of blood lipids can mean too many triglycerides. It can also mean unhealthy levels of cholesterol. Cholesterol is not transported as such in the blood, but is carried around in lipoproteins, those composite structures of lipid and proteins that were previously mentioned. Several types of lipoproteins circulate in the blood and they all have their specific function. The most prominent ones for the transport of cholesterol are high-density lipoproteins (HDL) and low-density lipoproteins (LDL). Your body needs both, in the right concentration, and also in the right balance. Lower HDL concentrations and/or higher LDL concentrations are harmful, in particular for your blood vessels.
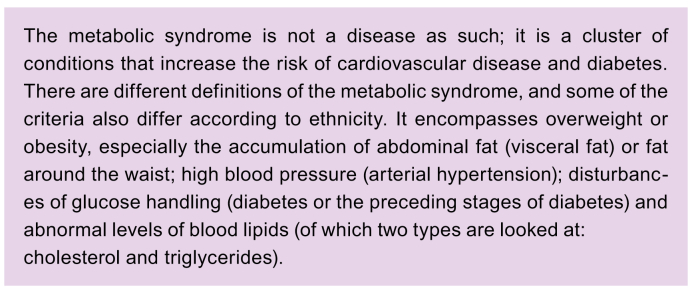
All these conditions are called metabolic factors and a combination of them is referred to as the metabolic syndrome. Several definitions of the metabolic syndrome have been developed over time and are summarised in Table 1.[8], [9], [10], [11] The metabolic syndrome is associated with an increased risk of developing numerous other health problems and diseases.
Table 1.
Definitions and criteria of the metabolic syndrome.
| Criteria | WHO (1999)8 | NCEP (2001)9 | IDF (2005)10 | Joint societies (2009)11 |
|---|---|---|---|---|
| Required for diagnosis | Impaired glucose tolerance or diabetes and/or insulin resistance | None | Central obesity as defined below | None |
| Number of features | Two other factors | ≥3 of the below | ≥2 of the below | ≥3 of the below |
| Central obesity | Waist–hip ratio of >0.9 in men, >0.85 in women or BMI ≥30 kg/m2 | Waist circumference ≥102 cm in men, ≥88 cm in women | Waist circumference ≥94 cm European men; ≥90 cm South Asian or Chinese men; ≥80 cm women | Waist circumference – population-specific definitions |
| Triglycerides | ≥150 mg/dl (1.7 mmol/L) | ≥150 mg/dl (1.7 mmol/L) | ≥150 mg/dl (1.7 mmol/L) or treatment for high triglycerides | ≥150 mg/dl (1.7 mmol/L) or treatment for high triglycerides |
| HDL-cholesterol | <40 mg/dl (1 mmol/L) in men, <50 mg/dl (1.3 mmol/L) in women | <40 mg/dl (1 mmol/L) in men, <50 mg/dl (1.3 mmol/L) in women | <40 mg/dl (1 mmol/L) in men, <50 mg/dl (1.3 mmol/L) in women | <40 mg/dl (1 mmol/L) in men, <50 mg/dl (1.3 mmol/L) in women |
| Hypertension | ≥140/90 mmHg | ≥135/85 mmHg or treated hypertension | ≥135/85 mmHg or treated hypertension | ≥135/85 mmHg or treated hypertension |
| Glucose | n.a. | 110 mg/dl (6.1 mmol/L) | ≥100 mg/dl (5.6 mmol/L) or diagnosed with type 2 diabetes mellitus | ≥100 mg/dl (5.6 mmol/L), or drug treatment for diabetes |
| Microalbuminuria | Albumin–creatinine ratio >30 mg/g; albumin excretion rate >20 μg/min | n.a. | n.a. | n.a. |
IDF, International Diabetes Federation; n.a., not applicable; NCEP, National Cholesterol Education Program; WHO, World Health Organization; BMI, body mass index; HDL, high-density lipoprotein.
It is important to highlight that steatosis is not always a result of metabolic factors. It can also be caused by alcohol.12 Furthermore, it can be caused by some drugs such as methotrexate (a drug used to treat rheumatoid arthritis). Steatosis can also be seen in some other liver diseases, such as Wilson’s disease (a very rare disease in which the body stores excess copper) and some variants of hepatitis C (a chronic inflammation of the liver caused by the hepatitis C virus).13,14
The term non-alcoholic fatty liver disease was coined in 1980, but observations of people with too much fat in their liver cells and no other cause of steatosis (e.g. alcohol consumption) were already being made in the 19th century.15 At the time, doctors did not understand the metabolic causes of the steatosis, so they named the disease according to what it was not. As alcohol was by far the most common and best-known cause of steatosis at the time, this disease was called non-alcoholic. In contrast to most diseases for which the name refers to the cause, this disease was hence named to indicate what it was not. Therefore, the name of this disease is not without problems (see Section 1d) and is under discussion.16,17
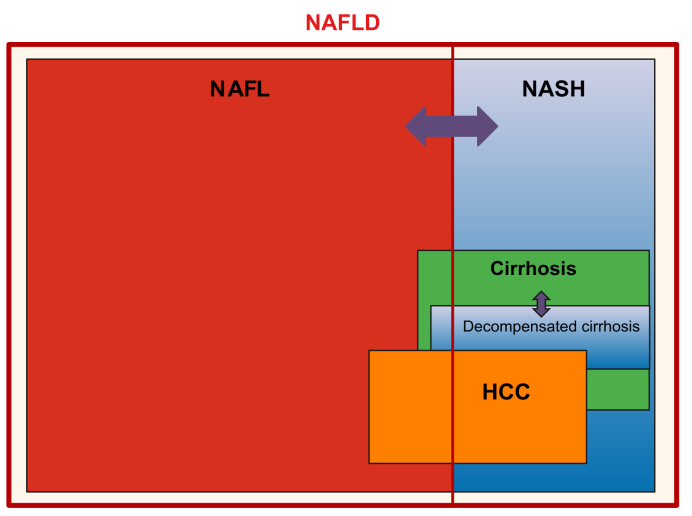
In many cases, the extra fat in the liver cells does not seem to be harmful or affect how well the liver works. This is called simple or isolated fatty liver, or non-alcoholic fatty liver (NAFL, without the “D” for disease). When the liver cells containing the fat droplets become inflamed and damaged, it is called steatohepatitis, so non-alcoholic steatohepatitis or NASH (Fig. 2).13,14 The term hepatitis refers to inflammation of the liver, whatever the cause. As outlined later, NASH is the subtype of NAFLD that carries more long-term risks (Section 2).
Fig. 2.
The different subtypes of NAFLD and their relationships with the severe consequences of the disease.
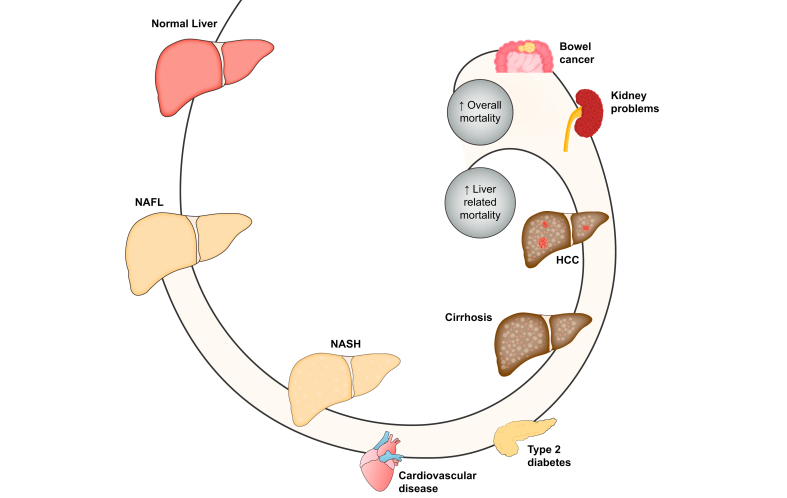
NAFLD is the overarching term. If there is mainly only steatosis, we call it simple steatosis or isolated steatosis (NAFL). If there is also liver cell damage and inflammation, we call it steatohepatitis (NASH). The separation is not static, so you can have NAFL and evolve to NASH, and also the other way around. The active disease can evolve to more severe liver injury and ultimately cirrhosis. Cirrhosis means advanced scarring of the liver, but even in these conditions, the liver can continue to function (compensated cirrhosis). Some people with cirrhosis will, however, evolve to poor liver function, which is called decompensated cirrhosis. NAFLD is also associated with the risk of developing liver cancer (HCC). As you can see from the figure, cirrhosis and decompensated cirrhosis mostly occur in association with NASH, whereas patients with NAFL have a lower (but not zero) risk. For HCC, the risk is the highest if you have cirrhosis, but there is still a risk in patients without cirrhosis and even without NASH. The magnitude of the boxes does not give any indication of the magnitude of the risk (please refer to the text for risk estimates). HCC, hepatocellular carcinoma; NAFL, non-alcoholic fatty liver; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis.
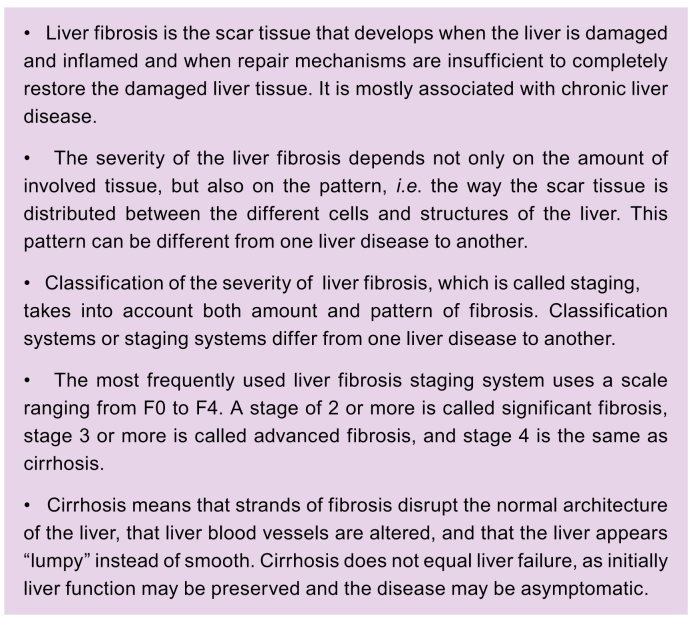
When your liver is damaged, it tries to repair itself by creating new, healthy tissue. If the damaging process continues, the liver’s ability to create enough healthy tissue and clean-up the damage may be exhausted. As a consequence, scar tissue will develop and can accumulate. This scarring is called fibrosis. Some (but not all) patients with NASH will develop fibrosis over time called progressive fibrosis. Both the amount of scar tissue and the distribution pattern (exactly where in the liver the fibrosis is located at the microscopic level) are important.1,18 Together, the amount and pattern indicate how severe the damage is. This is most commonly expressed by a scale of five stages (from 0 to 4), based on what is seen on a liver biopsy (see Section 5.c). This scale was developed by the NASH Clinical Research Network (NASH CRN) in the United States and was later incorporated into the Steatosis-Activity-Fibrosis (SAF) system.[18], [19], [20] As both the amount and the distribution pattern of the scar tissue determine the stages, this is not a linear scale: stage 2 does not mean that there is twice as much scar tissue as in stage 1. Stage 4 is called cirrhosis. Cirrhosis means that your liver tissue is becoming very scarred, with a surface that is no longer flat but instead bumpy and “nodular”. Although some scarring can still be reversible at this stage, the changes become more and more irreversible. The liver can sometimes function quite normally even with stage 4 fibrosis/cirrhosis. This is called compensated cirrhosis. Once the liver is not able to function properly, or other liver-related problems arise (e.g. liquid accumulation in the abdomen (ascites), yellowing of the skin and whites of eyes (jaundice)), it is called decompensated cirrhosis (Fig. 2).21
It should be noted that sometimes the liver does not function properly even when there is no cirrhosis, and any type of reduced liver function can have a major impact on your health and wellbeing. If the disease gets worse or better, you can change from one stage to another.22 Frequently used terms are also significant fibrosis, which means a fibrosis stage of at least 2 on a liver biopsy, and advanced fibrosis, which means a fibrosis stage of at least 3.1,18,19 Besides the most widely used staging based on the scale from 0 to 4 (referred to as F0 to F4), other classification systems with other scales exist, which can lead to confusion. If you have a diagnosis of fibrosis with a given “F” stage, your doctor should explain exactly what this means for you.
The terminology might appear confusing because of the different abbreviations used. To summarise, NAFLD is the overarching term, incorporating both NAFL and NASH, with a risk of increasing fibrosis and ultimately cirrhosis (Box 1). You can have NAFL at one time, then develop NASH but later go back to NAFL, depending on how the disease evolves and how well the risk factors are managed. Thus, NAFLD is dynamic and its activity (i.e. the extent of damage to liver cells and inflammation) can fluctuate over time (Fig. 2).22
Box 1. The terminology of non-alcoholic fatty liver disease.
c. What is the cause of NAFLD/NASH? How do you get it?
As mentioned earlier (Section 1.b), NAFLD can have many causes, so before a diagnosis can be made, other causes of steatosis should be evaluated. The most frequent of these alternative causes is related to the consumption of alcohol (the terms ALD, for alcohol-related liver disease, and ASH, alcoholic steatohepatitis, are used). NAFLD, by definition, is NOT linked to alcohol excess. This implies that you should not be diagnosed with NAFLD if you drink more than the upper limits that have been set or have had a history of past excess alcohol intake. The drinking limits are most often defined by a weekly consumption of less than 14 units for women and 21 for men (1 unit equals 8 g of alcohol, the meaning of alcohol expressed in units is explained in Box 2). These limits correspond to the amount of alcohol that is known to cause steatosis by itself.23 You should also not binge drink (binge drinking is defined as ≥4 drinks/day for women and ≥5 drinks/day for men).23 These limits do not mean that alcohol consumption below these limits is harmless. It is indeed highly questionable whether alcohol consumption at any level can be considered safe. It just means that a consumption of alcohol below these limits is probably not causing steatosis. It might, however, still carry a risk for other health problems, in particular cancer.12,24
Box 2. Alcohol consumption.
While both NAFLD and ALD cause inflammation, fibrosis, and cirrhosis, they are different diseases with different causes and different treatment options.12,13 It is important to note that you can have both at the same time.
Indeed, although other diseases that can cause fatty liver need to be ruled out when diagnosing NAFLD, you should be mindful that it is possible to live with more than one factor causing the liver damage. Examples of these other conditions that induce fatty liver are drug-associated steatohepatitis (DASH), chemotherapy-associated steatohepatitis (CASH) and toxicity-associated steatohepatitis (TASH or toxicant-associated steatohepatitis).
Over time, the medical community’s understanding of NAFLD has gradually increased. Nowadays, it is well known that metabolic factors play the most prominent role in explaining why many people develop fatty livers. There is a strong link between NAFLD and obesity.4,5 There is also a strong link with the way the body responds to insulin (T2D and pre-diabetes).3 Unhealthy levels of blood lipids and high blood pressure (hypertension) are also risk factors. As mentioned, all these elements are components of the metabolic syndrome (Box 3 & Table 1).11 These are general observations that do not always explain the full picture in a given individual. It is not always clear why fat builds up in the liver in some people. Genetic background may play a part, making some people more likely to be affected by NAFLD than others.5,[25], [26], [27]
Box 3. The metabolic syndrome.
One of the key mechanisms has to do with the fact that humans evolved over millions of years to live in conditions where there was a lack of food. That is why we are so fond of sugar, and why we have fat tissue to store extra calories. But in our modern society, food is generally available. We also need less of it because we are less physically active than our ancestors. This combination causes the quantity of our fat tissue to increase. However, there is a limit to the amount of fat that our fat tissue can store. If our food intake exceeds the storing capacity of fat tissue, the fat will build up elsewhere, including in the liver. NAFLD is hence strongly linked with unhealthy lifestyles, mainly from consuming excess calories, having an unhealthy diet and from a lack of physical activity (Fig. 3). Genetic and other factors will also determine how much fat an individual can store.28 These factors also have an influence on how vulnerable our liver is when it must deal with excess fat and its consequences.
Fig. 3.
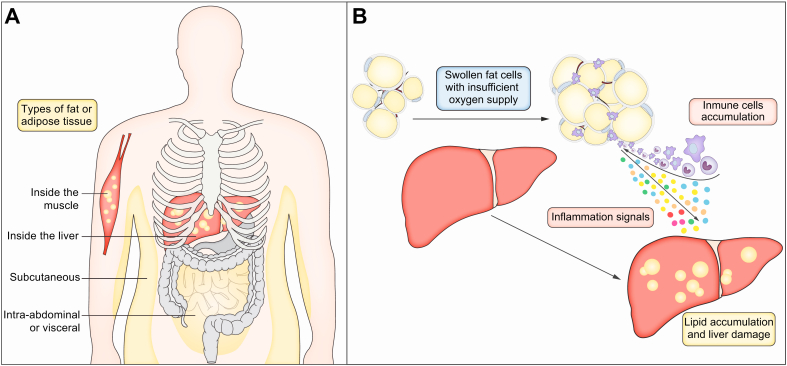
The body harbours different types of fat or adipose tissue.
(A) The fat that is inside the abdominal cavity and in close contact with both the gut and the liver is called intra-abdominal or visceral fat. The fat just beneath the skin is called subcutaneous fat. Intra-abdominal fat is more active, in terms of metabolic processes that are going on inside the cells. The intra-abdominal fat tissue is also active in the production of signals that help the body regulate its energy metabolism. It is thus not just a storage space, but also an active regulator of your body’s energy handling. (B) When this fat tissue is overwhelmed and the fat cells become very swollen, the fat tissue will become inflamed because there is not enough blood supply to and hence not enough oxygen in these too swollen fat cells. This leads to damage and dysfunction of this fat tissue. This inflamed fat tissue will release harmful substances into the blood that can then damage the liver. Subcutaneous fat is less reactive. It stores your energy reserves, which is important to protect us from the destructive consequences of calorie excess. However, there is a limit to that storage capacity too. When your excess calories exceed this storage capacity, the fat will need to go somewhere else.
There are different types of fat tissue (Fig. 3). The fat that is inside the abdominal cavity and in close contact with both the gut and the liver is called intra-abdominal fat or visceral fat. The fat just beneath the skin is called subcutaneous fat. Intra-abdominal fat is more active, in terms of metabolic processes that are going on inside the cells. The intra-abdominal fat tissue is also active in the production of signals that help the body regulate its energy metabolism.3 It is thus not just a storage organ, but also an active regulator of your body’s energy handling. This type of fat is, however, also more prone to being overwhelmed. When this happens and the fat cells become very swollen, the fat tissue will become inflamed because of insufficient blood and hence oxygen supply. This leads to damage and dysfunction of this fat tissue. As a consequence, this inflamed fat tissue will release harmful substances into the blood that can then damage the liver.3,29 Subcutaneous fat is less reactive. It stores your energy reserves, which is important to protect us from the destructive consequences of calorie excess. However, there is a limit to that storage capacity. This limit in storage capacity differs from one individual to another.
Although we now better understand the metabolic drivers of the disease, many issues remain. For the same lifestyle habits and risk factors, some people develop NAFLD, NASH and ultimately cirrhosis, while others do not.5 Additionally, some people have no or only very mild risk factors, but nevertheless develop advanced disease. The reasons behind these differences are currently poorly understood. Genetic factors only explain a fraction of the variation between individuals and much more research is needed to understand these individual differences.5,30,31
d. Why are we still calling it NAFLD/NASH? Are there alternative names?
As the understanding about NAFLD has grown, some experts argue that NAFLD/NASH are no longer appropriate names. Metabolic-associated fatty liver disease (MAFLD) has been suggested by some experts as an alternative name, to reflect the fact that a metabolic disorder is the key factor in the development of the disease.16 However, there is not yet universal support for this proposal.17 One of the reasons is that the disease also occurs and progresses in some people who do not have metabolic disorders.32
One of the problems with the term NAFLD, as explained in Section 1.b, is that the name tells you what it is not, instead of telling you what it is. Furthermore, strictly speaking, the diagnosis of NAFLD requires the other well-known causes of steatosis to be excluded.13 But metabolic risk factors for NAFLD can be present together with harmful alcohol consumption (or another well-established cause of steatosis). In this case, the fatty liver has two causes at the same time. It has also been demonstrated that both these causes together can intensify disease progression to more severe liver disease and liver cancer.[33], [34], [35], [36] In such mixed cases, it can be difficult to identify the leading cause, and it seems fair to indicate both NAFLD and ALD. This helps avoid focusing on and treating only one of the causes. It also avoids stigmatising diagnoses that are incorrect or that only tell part of the story, which would also create the risk of an incomplete and ineffective treatment plan.
For these reasons, there is support for changing the name in order to have a name that tells you what the disease stands for. MAFLD is the leading alternative, with a consensus process ongoing.
e. What is the difference between NASH and ASH?
NASH is, by definition, NOT linked to alcohol excess, whereas ASH is linked to a current and/or past history of excess alcohol consumption (see Section 1.c).13
Significant alcohol consumption can lead to steatosis, steatohepatitis, and fibrosis.12 Under the microscope, this looks very much the same as what is seen in NAFLD. As outlined in Section b, c, NAFLD has been described as the presence of steatosis and steatohepatitis, much like ALD, but occurring in people who do not drink excess alcohol.13 In most of these patients, NAFLD is related to the presence of metabolic risk factors. Those who have metabolic risk factors and drink alcohol can have both NAFLD and ALD.36 Using a terminology like MAFLD would solve the problem of “non-alcoholic” and the exclusionary nature of the definition, but the concept remains the same: both diseases can co-exist and lead to a fatty liver disease of mixed origin. Which of the factors is the major cause, is not always clear. Someone with metabolic risk factors and moderate alcohol consumption should not be labelled with ALD. Instead, they should be described as having potentially mixed cause fatty liver disease – with one or other being the main driver of damage.
2. Why is NAFLD/NASH important?
a. What is the impact in terms of liver disease? What will happen to me?
If NAFLD is detected and managed early enough, it is possible to reduce the amount of fat in your liver, which may slow down or even stop the damage, and eventually allow your liver to fully recover.
NAFLD with only fat accumulation in the liver cells and no or only minimal signs of liver cell damage or inflammation, called simple or isolated fatty liver or NAFL (Fig. 2) (you will also encounter the term ‘early NAFLD’), does not usually cause any harm, but if it is not managed, can progress to NASH, which is a more serious condition.37 Most people with NAFLD have isolated fatty liver.38
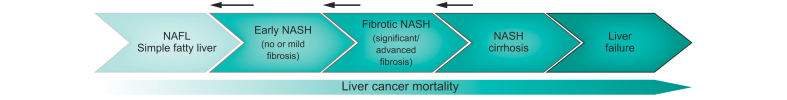
NASH is more serious than isolated fatty liver because there is inflammation in the liver, and it starts to become damaged (Fig. 3). This is sometimes referred to as early NASH. This inflammation can lead to fibrosis, which means scar tissue is forming inside the liver. This is often referred to as fibrotic NASH.
Fibrosis can lead to cirrhosis, which means that large strands of scar tissue alter the liver structure, with regenerating liver tissue in between. This is called NASH-cirrhosis. Because of these strands of fibrosis and zones of liver cell regeneration, the liver becomes very scarred and the surface becomes irregular and bumpy or “nodular” (See Section 1.b). This irregular and bumpy appearance of the liver is a hallmark of cirrhosis. In cirrhosis, the main functions of the liver can initially be preserved (compensated cirrhosis, Fig. 2). The liver will, however, struggle more and more to function properly as the disease progresses. Eventually, liver function can be so poor that problems occur (decompensated cirrhosis) to the point where liver function becomes insufficient to support life (liver failure) (Fig. 2, Fig. 3).
Liver cancer (hepatocellular carcinoma, HCC) is another potential consequence of NAFLD. This risk of developing HCC is well known in patients with cirrhosis from other causes and is now also well documented in NASH-cirrhosis.39,40 Importantly, HCC has also been reported in patients with NASH who do not have cirrhosis, and even in patients with NAFL.41,42 It is not clear how big this risk is exactly, but in general, the more severe the disease is, the higher the risk is. The highest risk is when you have cirrhosis. The presence of comorbidities like obesity, or the presence of other liver-damaging factors like alcohol, also contribute to the risk of developing HCC.39,43
b. How quickly does NAFLD progress?
The damage caused by NAFLD can take many years to progress and is most frequently described in five stages depending on the amount and distribution pattern of fibrosis found (Section b, 5):
In general, NAFLD fibrosis gets worse by one stage every 14 years.44 NASH fibrosis gets worse by one stage every seven years. These are only average figures. Progression is not linear and can be different according to the stage.22 A recent analysis of patients in clinical trials showed that 20% of patients with F3 progressed to cirrhosis in two years, while 20% of patients with cirrhosis, but with good liver function and without complications of liver disease, developed more severe cirrhosis-related liver problems (decompensated liver cirrhosis) in two years (Fig. 2).45 These figures are called the 20% ‘rule’.46 In some cases, the liver can be damaged much faster than these average figures, and up to 1-in-5 patients with fibrosis progression are rapid progressors (Fig. 5).44,47
Fig. 5.
The current understanding of NAFLD is that most patients only have fatty liver, without liver cell damage and inflammation (NAFL).
Some patients will evolve to NASH, wherein steatosis is accompanied by liver cell damage and inflammation. This can go along with the accumulation of scar tissue or fibrosis. In a subset of patients with NASH, more and more scar tissue will accumulate and ultimately result in cirrhosis. Patients with cirrhosis but with good liver function can evolve to a cirrhosis-related more severe liver problem (decompensated cirrhosis). A liver cancer (HCC) can develop at any stage, but the risk of HCC is higher when the NAFLD is more severe. Usually, the evolution of the disease is slow, but some patients can be rapid progressors. NAFLD increases the risk of developing diabetes. NAFLD also increases the risk of diseases of the heart and blood vessels (CVD). NAFLD may also increase the risk of several types of cancer (including bowel cancer) and the development of kidney problems. CVD, cardiovascular disease; HCC, hepatocellular carcinoma; NAFL, non-alcoholic fatty liver; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis.
There are major differences in the way the disease develops and progresses between individuals.5 This could be due to fluctuations in the severity of the metabolic risk factors as well as the impact of diverse (unhealthy) lifestyles, in addition to genetic factors.22,48 This is something we currently do not fully understand.
c. How does NAFLD affect general health?
Your liver is a large organ, able to carry out a complex set of functions (see Section 1.a). Large blood vessels connect your liver with the rest of the body. When your liver is not working properly, the metabolism works only to a limited extent, toxic substances may remain in the body and other damaging substances may even be produced. It is understandable that NAFLD not only damages the liver, but other organs too.
Research shows that NAFLD increases the risk of developing T2D, and if you already have T2D, it makes controlling your T2D more difficult.49 NAFLD also increases the risk of cardiovascular (heart) disease by damaging the walls of blood vessels.50 NAFLD influences the build-up of mineral deposits or calcifications on the vessel walls, which is called atherosclerosis. It also causes damage to the heart, mainly the heart muscle and the system that regulates heart rhythm. NAFLD may also increase the risk of several types of cancer (including bowel cancer)51 and the development of kidney problems (Fig. 5).52,53
d. In which stage of NAFLD do problems occur?
It is still unclear whether all patients with NAFLD are at risk for all these serious problems, or if these complications are mainly restricted to patients with NASH (Box 4). Studies have shown that the severity of liver fibrosis is the most important predictor of what will happen to someone with NAFLD.54 People with NAFLD who have more severe fibrosis were shown to have a higher chance of developing serious health problems or dying. These serious health issues obviously arise from liver disease, but also, and even more frequently, from non-liver-related diseases. Diseases of the heart and blood vessels (cardiovascular disease, CVD) are the most frequent problems encountered. Factors other than fibrosis (observed at the time of diagnosis) appear to be less predictive of health events later in life.55 Liver fibrosis does not directly cause your heart to stop working. Having liver fibrosis is, however, a sign that your liver is not able to function in a highly effective way. This leads to long-term problems for the liver and/or other organs.
Box 4. Consequences of NAFLD.
NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis.
Based on other scientific data, it is likely that the process of liver damage and inflammation (NASH) is the true driving force behind subsequent health problems.56,57 This seems to be true for the formation of fibrosis and the progression towards cirrhosis, as well as for the consequences outside of the liver.44
Because of these considerations, clinical trials to test possible treatments, especially drugs, currently focus on patients with NASH and fibrosis, and not on less severe stages of the disease.[58], [59], [60]
e. Impact of the NAFLD/NASH on health-related quality of life
The impact of NAFLD is not limited to physical issues, but also affects health-related quality of life (Box 5). The concept of health-related quality of life has been defined by the Center for Disease Control (CDC) as “an individual’s or a group’s perceived physical and mental health over time”.61 Specific questionnaires have been developed to get further insight into the health-related quality of life of patients with NAFLD/NASH.62,63
Box 5. Health-related quality of life.
NAFLD can clearly have an impact on one’s quality of life.64,65 The associated conditions of T2D, obesity, unhealthy blood lipid levels (dyslipidaemia) and CVD also negatively impact health-related quality of life.
People with NAFLD in the early stages of disease generally have no symptoms and their quality of life is not impaired. The burden of NAFLD on health-related quality of life becomes progressively more important with advancing disease, when fatigue and impaired physical conditions related to NASH (see Section a, b, 11) accumulate and have a significant impact on daily life.66,67 In the more severe stages of the disease, the physical and psychological consequences of liver disease add to those of the metabolic comorbidities (as mentioned: obesity, T2D, dyslipidaemia, CVD). So clearly, health-related quality of life deteriorates as NAFL progresses to early NASH, NASH-cirrhosis and liver failure, or liver cancer (regardless of how severe the underlying NAFLD is).
The impact of NAFLD may be made even worse by the stigma associated with obesity68 and/or T2D, problems of shame/guilt linked to presumed alcoholism, and difficulties accepting the diagnosis.
Although your day-to-day activities may not be affected for a long time, the costs of NAFLD increase linearly as fibrosis and liver damage progress.69,70 This is driven by hospital admissions, the costs of treating co-existing conditions, and personal costs (e.g. loss of employment).
3. Basic data on epidemiology and natural history
a. Who gets NAFLD/NASH?
It is estimated that between 17–46% of European adults have NAFLD (on average, around 25%). It affects people of all ages, including children.6 This condition is directly linked to chronic excess calorie consumption, lack of physical activity/exercise and being overweight/having obesity (Fig. 3).13
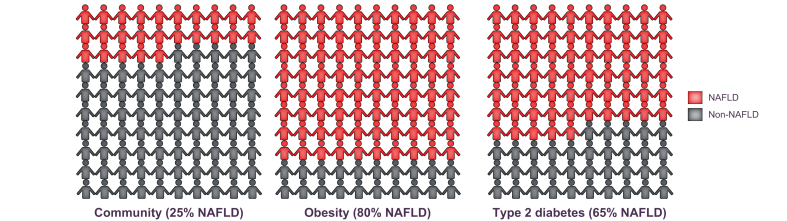
Thus, you are more likely to have NAFLD if you have obesity and T2D, with NAFLD occurring in nearly 9 out of 10 (90%) people living with obesity (depending on the severity of the excess body weight) and in 5–7 out of 10 (50–70%) people living with T2D (in relation to being overweight and having poor metabolic control,38 or if you have high lipid or LDL cholesterol levels in the blood) (Fig. 6).4,71 The number of people with NAFLD increases progressively with age. Genes and ethnic origin are also important, with more people of Asian and Spanish origin being affected than people of African origin.40,72
Fig. 6.
It is estimated that 25% of European adults have NAFLD.
You are, however, more likely to have NAFLD if you have obesity and T2D, with NAFLD occurring in nearly 8 to 9 out of 10 (80 to 90%) people living with obesity and in 5-7 out of 10 (50-70%) people living with type 2 diabetes. The number of people with NAFLD increases progressively with age. NAFLD, non-alcoholic fatty liver disease; T2D, type 2 diabetes.
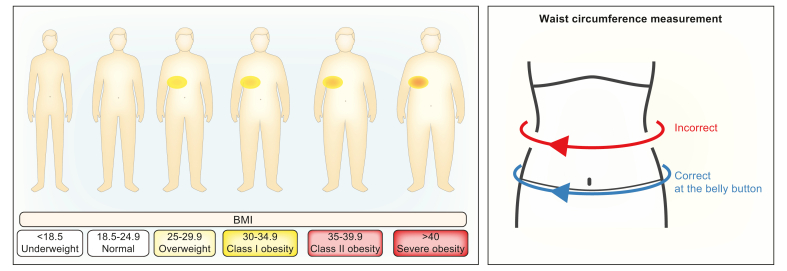
Some people with NAFLD have a normal body weight (up to 20% of NAFLD patients, which is then called lean NAFLD) or are overweight but do not have obesity (20%).32,73 These numbers vary across the world, being as low as 25% in South-East Asian countries, and up to 50% elsewhere,74 depending on how obesity is defined in each country.75 The concept of lean NAFLD is, however, somewhat misleading and simplistic. The definition of lean is based on body mass index (BMI – your weight divided by your height squared) but does not take into account how the weight is distributed in the body (fat vs. muscle, intra-abdominal fat vs. subcutaneous fat) (Box 6). It also simply draws a line at 25 kg/m2 (or 23 kg/m2 for Asian people): if you are just below that line, you are lean; if you are just above, you are overweight. In reality, BMI is a continuum. Lean people with NAFLD often have some abdominal fat accumulation or other subtle metabolic abnormalities.75 Lean NAFLD hence refers to the presence of NAFLD in people that have few obvious metabolic risk factors. They might have some excess body fat but still be lean according to the BMI criteria. We do not know why such individuals develop NAFLD. Anyhow, if the medical community can find a positive definition of the disease based on which metabolic abnormalities you must have, this oversimplified concept of lean NAFLD will disappear. For the time being, it is better to talk about NAFLD in lean people, instead of lean NAFLD.
Box 6. Body mass index.
BMI, body mass index.
b. How frequent are the complications?
Based on current scientific knowledge, it is known that in most patients with simple or isolated fatty liver, the liver remains stable over time (see also 2.a). In contrast, if you have NASH, you are at a relatively high risk of worsening liver injury.44 NASH occurs in 1 in 4-5 patients with NAFLD, i.e. between 1.5% and 6.5% of the general population, but this figure is much higher (in some studies over 60%) in patients with T2D.71 Severe fibrosis is estimated to occur in around 1.5% of the general adult population76 and in up to 10% of adults with T2D. Severe fibrosis means at least F3 on a liver biopsy, often referred to as “advanced fibrosis”. Half of these patients will ultimately develop cirrhosis.71,77
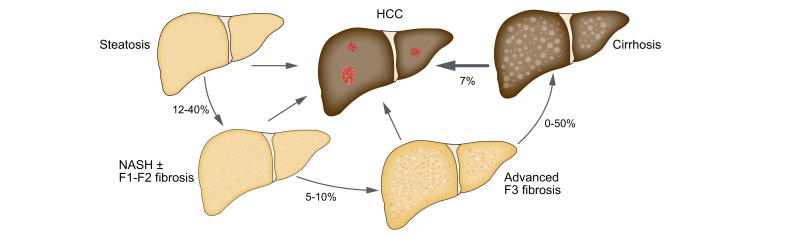
There is some understanding of how NAFLD progresses, thanks to research studies using liver biopsies. Studies using two biopsies at different time points indicate that the liver damage – in terms of fibrosis – gets worse over time if you have more active steatohepatitis, so more aggressive liver damage and inflammation.57 As mentioned in Section 2.a, on average NAFLD fibrosis worsens by one stage every 14 years and NASH fibrosis gets worse by one stage every seven years.44 The data on the progression of the disease are summarised in Fig. 7.
Fig. 7.
How does NAFLD evolve over time?
Not everybody with NAFLD will develop cirrhosis. The estimated percentages of patients who will evolve stepwise to a more severe disease stage are depicted here. F1-2-3, stage of fibrosis 1-2-3; HCC, hepatocellular carcinoma; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis.
These figures should be treated with some caution because they are based on studies that looked at the biopsies of patients and not everyone needs to have a liver biopsy. It is possible that the individuals who had liver biopsies may have already had more advanced NAFLD and so the aforementioned figures may overestimate the number of people who progress to advanced fibrosis and cirrhosis.
Studies also demonstrate that, besides patients whose disease progresses, there are also patients who improve and others who simply remain stable.57 Disease progression is hence difficult to predict in an individual case.
4. Who should be tested for NASH and how? How should tests results be interpreted?
a. Who should be screened?
The question of whether people can and should be actively screened for NAFLD is an important one. From a scientific point of view, there are several requirements that need to be fulfilled before screening can be recommended. These include the availability of cost-effective and safe screening methods, and that something can be offered to you (such as a treatment) if you screen positive for a certain condition.78 Not all these conditions are met in the field of NAFLD, which explains why there is no universal screening guideline and no universal screening algorithm everybody agrees upon.79
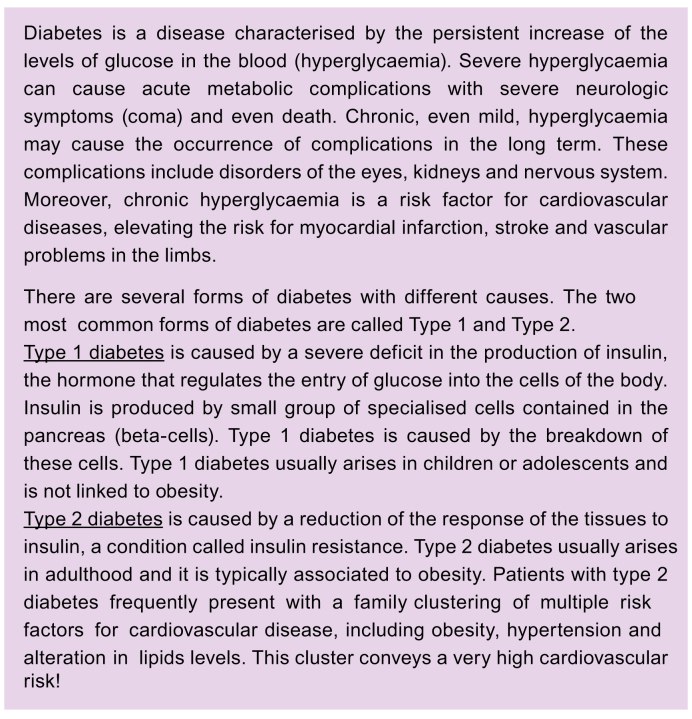
Nevertheless, although there is no global consensus, there is a growing understanding that physicians should at least consider screening patients who are at risk of having NAFLD.80 This is probably particularly true for patients at risk of developing NASH and/or fibrosis. These risk categories also include people with chronically elevated liver blood tests.13 ‘Chronically’ usually means for more than six months, so at least two blood samples six months apart are needed. It may include people living with T2D or being overweight/having obesity, people with metabolic syndrome (see Table 1 for definition and criteria of this entity), and people with CVD.13 Some of these risk categories include large groups of people. It is sometimes difficult to pinpoint who is affected: should, for example, all patients living with T2D be screened? In what frequency? Or what exactly is CVD? So, this needs fine-tuning in the future.79 Data are starting to be reported for patients living with type 1 diabetes (T1D),81 but it is probably too early to formulate a clear recommendation (Box 7). Patients with T1D who are overweight/have obesity and have other features of metabolic syndrome may also be at risk and would potentially benefit from screening.
Box 7. Diabetes, type 1 diabetes, type 2 diabetes.
b. How can screening be done?
As mentioned, there is no universal consensus on whether you need to screen, and if so, how. There are many unanswered questions to date, reflected by variations in screening policy and methodology.
As this is a slowly evolving disease, focus should probably lie on picking up the people that are most at risk of developing NAFLD-related problems in the near future. Therefore, most of the screening efforts should focus on NASH and at least F2 fibrosis, or on fibrosis alone (F2 or more, or F3 or more).
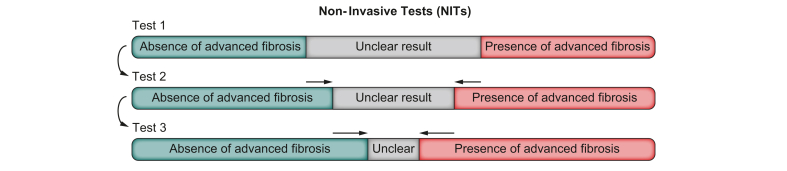
Most screening strategies rely on a blood test score, a liver ultrasound or a liver stiffness assessment, or a combination of these.82,83 The combination strategy can be deployed in one instance. It is also possible to perform tests sequentially. This means that the second test is only performed if the first one is positive or gives a result in the grey zone (Fig. 8), which signifies an unclear result. As mentioned, there is no universal strategy. Any strategy should consider available resources and how healthcare is organised in a local setting. The UK National Institute for Health and Care Excellence guideline84 or the Belgian Association for the Study of the Liver guideline85 are just two examples of how this screening algorithm can be applied, but many other strategies can work.
Fig. 8.
Screening for NAFLD can be done using different tests (non-invasive tests or NITs) and different strategies of testing.
There is no international consensus on the optimal screening strategy. Tests can be combined at one instance. Another possibility is to use several different tests sequentially: the second test is only performed if the first one is positive or gives an unclear result. NAFLD, non-alcoholic fatty liver disease.
The different tests are discussed in Section 5.
c. What to do with the result of the screening?
If you are screened according to a particular algorithm, there should be a management and follow-up plan ready to accompany the result. All patients need a plan. The intensity of the management and further work-up (which could include a liver biopsy in selected cases) can differ according to the screening results (see Section 8). The exact meaning of the results should be clearly explained to you in order to avoid misinterpretation. This is extremely important to avoid giving you unjustified reassurance or the opposite, to saddle you with unnecessary stress and fear.
d. Should tests be repeated over time?
There is no international scientific consensus on how exactly patients at risk of or with NAFLD should be monitored over time (see also Section 8).79,80 The more the screening test result indicates a higher likelihood of liver damage, the more intensive the follow-up should probably be. For example, you are overweight, and you are screened: the test result tells you that you have a very low likelihood of having NASH with at least F2 fibrosis. This is reassuring, but you should still be supported to lose weight. This is because, even though your screening result is negative this time, significant liver disease might develop later. If you remain overweight, it seems appropriate to repeat the screening at a later time. Currently, there is no precise recommendation that is universally applicable on this issue. It is, however, obvious that even if initial screening is negative, a repeat assessment at a later date is appropriate if the risk factors remain. The precise timing and modality of the repeat screening will depend on the result of the previous assessment and on local practices.
5. How can the disease and its severity be diagnosed?
a. What are the symptoms?
Most adults do not notice any symptoms in the early stages of NAFLD and find out they have it when they are being tested for something else, such as an annual check-up or during tests for other conditions such as T2D or gallstones. The liver blood tests are usually abnormal, but not always.86,87 If abnormal, they are usually only slightly elevated, which should not be considered as a sign of mild disease. Surprisingly, the degree of abnormality of the blood tests does not reliably predict the severity of liver injury. This means that liver tests may be normal or show only minor elevations and you still can have advanced disease.
You can experience fatigue or tiredness, general lethargy, and right-sided abdominal discomfort or an ache on the right side of your tummy just under the ribs. These symptoms are sometimes reported and might get worse over time as NAFLD progresses. However, other causes of these symptoms need to be ruled out. Nevertheless, evidence is accumulating that NAFLD independently contributes to fatigue and reduced physical capacity, which can be explained by the crucial role of the liver in energy metabolism.64,65
You can experience more severe symptoms when your liver becomes more severely scarred (i.e. if you have cirrhosis), such as unexplained weight loss, yellowing of the skin and the whites of the eyes (jaundice), itchy skin, and swelling in the legs or tummy.
Because NAFLD starts off without noticeable symptoms, your doctor may perform tests if you have risk factors associated with getting NAFLD (like T2D or metabolic syndrome) to see if you have it.
b. Who should diagnose NAFLD/NASH?
The diagnosis of NAFLD/NASH can be performed either by your general practitioner (GP), your doctor for internal medicine or diabetes, or your liver specialist (“hepatologist”). The GP or your doctor for internal medicine will take your medical history and can order blood tests and an ultrasound scan. These evaluations and knowledge about other conditions you may have can give a first indication of whether or not you may have NAFLD or NASH. Either your GP or doctor for internal medicine can decide if you should have an appointment with a liver specialist. A liver specialist should confirm the diagnosis, especially for those with advanced stage disease.
c. Liver biopsy as the gold standard
A liver biopsy is an invasive procedure that takes very small samples of liver tissue for investigation (about 1/50,000th of your liver). It allows the doctors to assess different areas within the liver and can provide information about the number of liver cells containing fat droplets (steatosis), the degree of liver cell damage and inflammation (the “activity” of the disease) and the degree of liver fibrosis (the “stage” of the disease).1,14
The presence of steatosis is the first criterion to define fatty liver disease. The human liver can contain a few fat droplets, but they are rare. The degree of liver steatosis on the biopsy is expressed as the number of liver cells (“hepatocytes”) that contain those droplets. A few hepatocytes (up to 5%) containing fat droplets is not considered abnormal or pathological. If more than 5% of the hepatocytes contain fat droplets, then this is no longer considered normal, such that this defines what is a fatty liver (see Section 1.b).1 If no cause such as alcohol or steatosis-inducing drugs can be identified, it is diagnosed as ‘NAFLD’.13,14 The amount of liver fat is then further graded as 1 (up to 1/3 of the cells laden with fat droplets, mild steatosis), 2 (between 1/3 and 2/3, moderate steatosis) and 3 (>2/3 of the cells laden with fat droplets, severe steatosis).19,20 Whether the amount of fat in the liver (i.e. the grade of steatosis) is important in terms of liver damage and long-term prognosis, is not completely clear. If steatosis is the only abnormality, without signs of liver damage, it is called simple or isolated steatosis. Although some worsening of the disease in terms of development of scar tissue has been described, the risk of this is much lower than in people who have liver cell damage and inflammation on top of the steatosis (Fig. 2).44,57
Liver cell damage and inflammation constitute what is called the “activity” of the disease. This is the active process that drives the development of scar tissue and the worsening of the disease towards cirrhosis and liver failure (end-stage liver disease). The latter may become an indication for liver transplantation (See Section 2.a).88 With a liver biopsy, a typical swelling of the liver cells (what is called ballooning of the liver cells) and the presence of inflammatory cells are the two most important hallmarks of liver cell damage in the context of fatty liver.1 Many other features and details can also be assessed by pathologists (these are the specialist doctors that examine tissues under the microscope) and can help to grade the severity of the underlying damaging process. Several scoring systems have been developed over time to indicate the level of disease activity.
The third cardinal feature and piece of information provided by the biopsy is the presence of scar tissue. Scar tissue formation is in fact a normal wound healing process. If the damage is, however, continuously present and if there is an imbalance between damage and repair, progressively more and more scar tissue will accumulate.22 This affects normal liver function, e.g. the exchange between the blood and the liver cells. It will also disturb, in the advanced stage, the normal microscopic architecture of the different liver cell types and blood vessels. This leads to cirrhosis. As for steatosis and activity, scoring systems have been developed over time to express the severity of the fibrosis as a figure on a scale (See Section b, a) (Box 8).19,20 This severity is not only a matter of the amount of scar tissue but also the pattern of fibrosis, i.e. how the scar tissue is located in between the different cell types and structures of the liver. The most widely used scale goes from 0 to 4, 0 meaning there is no fibrosis, whereas 4 equals cirrhosis. The scale is not linear, because both amount and pattern determine the exact stage. So, stage 2 does not mean that you have twice as much scar tissue as in stage 1: there is more scar tissue, but also the distribution pattern is different. This scale is specific for NAFLD. In other liver diseases, fibrosis can be distributed in a different pattern. Biopsy-based stage 2 in NAFLD is hence not the same as stage 2 in viral hepatitis.19,20,89
Box 8. Staging of liver fibrosis.
Cirrhosis refers to a situation where, at the microscopic level, fibrous strands breach the normal structure and create fibrous bridges (see also Section b, a). There are also changes in the blood vessels and nodules of newly formed cells emerge to replace what has been lost. All this leads to nodular reorganisation of the liver, i.e. cirrhosis. Cirrhosis is hence a structural alteration of the liver, and not per se a functional one. Liver function, at least gross liver function, can be preserved in early cirrhosis. Of course, when it becomes more severe, cirrhosis will ultimately lead to defective liver function.
Besides giving information on all these different aspects of the disease, a liver biopsy can also be very useful to exclude other liver diseases. If you have risk factors for NAFLD and you have elevated liver blood tests, you could still have another type of liver disease. A liver biopsy can be helpful to diagnose these other liver diseases as well as NAFLD.
As with every investigation in medicine, liver biopsy is not perfect. As mentioned, a classical biopsy represents only about 1/50,000th of the total amount of liver tissue. And although the liver in NAFLD is more or less diffusely damaged, there can be some differences in severity between different areas in the liver. This means that if you take a liver biopsy of two different liver regions, some differences may be observed between the two pieces under the microscope.90 This potential difference between two different areas of the liver is called sampling variability. In NAFLD, these differences are overall rather minor. For fibrosis, it is known that a difference of one stage between two biopsies is not uncommon, but a difference of two stages is rare.90 The bigger the biopsy, the more precise the scoring and the lesser the variability.91 Also, the experience of the pathologist who examines the biopsy is of importance. These limitations (and all techniques in medicine come with some limitations) must be acknowledged. Furthermore, a liver biopsy is an invasive procedure and comes with some risks.92,93 When performed by experienced doctors, the likelihood of a good quality biopsy is high and the risks are low, but never zero.
d. Can the biopsy be replaced by something else? What about other less invasive liver tests?
It is currently not possible to obtain all the information that a liver biopsy provides (degree of steatosis, activity and fibrosis, other markers of disease severity and diagnosis of other liver diseases) with a single alternative.
It is, however, to some extent possible to get an idea of some aspects of the disease using one or more non-invasive techniques (Box 9). It is important to note that liver biopsies have been used as the reference method in the development of almost all of these techniques As the liver biopsy is in itself not perfect, this approach might over- or underestimate the accuracy of a non-invasive technique. It is nevertheless currently the best way to get as close as possible to an accurate assessment of the liver abnormality that is being studied.
Box 9. Non-invasive tests.
NASH, non-alcoholic steatohepatitis.
The development of non-invasive biomarkers (non-invasive tests, NITs) in NAFLD is most advanced for the assessment of the presence and severity of steatosis and for the assessment of the severity of the fibrosis.94,95 The field is not as advanced for the non-invasive assessment of disease activity, i.e. whether there is steatohepatitis and how severe or active the NASH is.
Steatosis
The presence of steatosis can be assessed with imaging techniques. An ultrasound of your abdomen, comparing the brightness of your liver to the grey scale of your right kidney, is accurate in identifying the presence of steatosis if steatosis affects more than 30% of the liver cells.96,97 Minor degrees of steatosis can be missed on ultrasound, but moderate and severe steatosis can be diagnosed with more than 90% accuracy. The brightness of your liver can also be scored on ultrasound. This scoring correlates to some extent with the severity of steatosis on liver biopsy, although this is not very accurate.98 Ultrasound can diagnose steatosis, but not steatohepatitis and not the stage of fibrosis, and can diagnose cirrhosis if your liver looks nodular.
The Fibroscan® device (see also the next section on fibrosis) can also assess the amount of liver fat, via the determination of the controlled attenuation parameter or CAPTM.99,100
A computed tomography scan can also be used to diagnose steatosis, comparing your liver with your spleen. As with ultrasound, it cannot diagnose the presence of liver damage and inflammation, nor fibrosis.
Magnetic resonance imaging (MRI) is the most accurate technique to diagnose the presence of liver fat, and it can be used to quantify the levels of liver fat.101 Liver fat on MRI is also expressed as a percentage, but not the percentage of liver cells laden with fat droplets, rather the percentage of total liver weight that is composed of fat. Up to 5% is also considered normal. A percentage of 30% corresponds to very severe steatosis, as it is a percentage of total weight; as such, this percentage cannot be directly compared to observations made on a liver biopsy. MRI is so accurate in assessing the presence of liver fat and in quantifying it,102 that it is considered the reference method (gold standard) for this particular feature, but it is still mainly used for research purposes.
Besides imaging, there are scores based on a combination of tests, mainly blood tests that have been shown to have some value in diagnosing the presence of liver fat and its severity. Examples are the Fatty Liver Index, the SteatoTestTM or the Liver Fat Score.[103], [104], [105]
Fibrosis
Imaging is currently not able to accurately visualise scar tissue or fibrosis. Technical evolution might, however, make this possible in the future. Imaging can be used to assess liver stiffness, which is a physical characteristic of your liver. Liver stiffness is the opposite of liver elasticity, so liver stiffness measurement is called elastometry or elastography. The latter means a mapping of liver elasticity. A healthy liver is smooth in texture (elastic), but when diseased, it can become stiffer. This stiffness can be influenced by the presence of fibrosis, but also by inflammation and swelling. Also, the blood flowing through the liver must be taken into account. After a meal, when more blood goes to the gut and the liver, the liver is a little stiffer than in fasting conditions.106 All these factors can affect the stiffness of your liver. This means that liver stiffness does not simply equal fibrosis. Nevertheless, it has been shown that liver stiffness and the stage of fibrosis do correlate.87
Many factors need to be considered to correctly interpret the result of a liver stiffness measurement:107,108 Liver stiffness values have been assessed using fibrosis stage on liver biopsy as the reference method or gold standard. Scoring systems for fibrosis on liver biopsy are not the same for every liver disease. Therefore, values of liver stiffness corresponding to fibrosis stages can be different from one liver disease to another. For a given stage of fibrosis, the corresponding range of liver stiffness measurements is also broad. This means that there is a lot of overlap in liver stiffness values between two consecutive stages of fibrosis diagnosed on liver biopsy. This implies that a liver stiffness measurement cannot be interpreted as a precise measurement of the fibrosis stage on the liver biopsy.109 Because of that, your liver stiffness value is compared to threshold values or cut-offs. These cut-offs are levels that indicate a high or low risk of a given level of fibrosis severity. The interpretation depends on the values that are used as cut-offs: with a low cut-off, you can be quite sure that if you are below that value, there really is no significant fibrosis; inversely, with a high cut-off, your likelihood of having more severe fibrosis or even cirrhosis is high, but never 100%.107 The cut-offs are different whether you want to rule-in or rule-out a condition, so if you want to be sure that it is or that it is not present. The cut-offs are also different if you are looking for significant fibrosis, advanced fibrosis or cirrhosis. This is all quite complicated, and your doctor should tell you exactly what your liver stiffness value means. As explained, your liver stiffness does not tell you exactly which fibrosis stage you are in. It only tells you how likely you are to be “at least in a given stage of fibrosis”, or how certain you can be that your fibrosis is not so severe. Thus, the interpretation is quite complex and depends on multiple factors.
Several techniques to measure liver stiffness have been developed over time.94 Most of them are based on ultrasound. A series of pulses are applied to the liver. Based on the return of the pulses these instruments can measure the stiffness of the liver. The most widely used is vibration-controlled transient elastography, which is the technique in the Fibroscan® device.82 Other techniques, combined with classical ultrasound imaging of the liver or a distinct technique altogether, are available.110 The liver stiffness scores are not comparable between these techniques. Hence, each technique has its own reference values and cut-offs. When mentioning a liver stiffness value, you need to know which technique has been used to understand the meaning of the result.111
Liver stiffness can also be measured by MRI, which is called magnetic resonance elastography (MRE). MRE maps the whole liver. MRE is considered the most accurate technique for liver stiffness measurement.112 However, MRE is not widely available and is more costly and time consuming than ultrasound techniques.
Besides imaging, several individual blood parameters are available for basically the same purpose. There are several scores based on a combination of blood parameters to assess fibrosis (Box 10).82,87 Some of these scores are based on routine blood tests and can easily be calculated by the lab or using online calculators that can be found on the internet.85 Some blood tests and scores are offered commercially and come with an additional cost. Many of them are also still experimental but the interpretation of their results is comparable to liver stiffness measurements. Examples of tests and scores that are based on routine blood tests include the fibrosis-4 index (FIB-4)113 and the NAFLD fibrosis score.114 Examples of commercial tests and scores are N-terminal pro-peptide of type III collagen (Pro-C3),115 enhanced liver fibrosis (ELF)116 or a non-invasive NASH test with 4 blood markers (NIS4).117 Many other tests exist or are in development.
Box 10. Fibrosis scores.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; F0-1-2-3, stage of fibrosis 0-1-2-3; FIB-4, fibrosis-4 index; IFG, impaired fasting glycemia; IU, international units.
All these individual tests have their accuracy, depending on what you want to know. Combining test modalities clearly makes the overall result more accurate.82,108 That is why many algorithms combine a fibrosis score and a liver stiffness measurement, simultaneously or sequentially (see Section 4.b and Fig. 8).[83], [84], [85],95
Steatohepatitis
Without a biopsy, it is difficult to tell the difference between a simple or isolated fatty liver (steatosis) and a fatty liver with liver cell damage and inflammation (steatohepatitis). The assessment of this difference has been the most challenging part of developing non-invasive markers for NAFLD. This is also true for determining the severity of the steatohepatitis, what is called the activity of the NASH. One of the reasons is that the activity of the disease is closely linked to the degree of fibrosis. Changes in activity over time are linked with changes in fibrosis stage over time.57 Therefore, if you analyse a marker for its relationship with fibrosis, this relationship will also be influenced by the presence at the same moment of inflammation and cell damage. This holds true for liver stiffness measurements as well as for blood-based markers and scores.
MRI-based techniques, such as multiparametric MRI, are most promising in this regard.118,119 Some biomarkers like NIS4 also identify the combination of NASH with fibrosis.117 The FAST score, an approach combining liver stiffness measurement by Fibroscan®, steatosis assessment by CAPTM (on the same Fibroscan® device) and the liver blood test aspartate aminotransferase (AST), also seems to hold promise.120 However, further studies are required to determine the accuracy of these approaches.
Baseline assessment vs. follow-up
The techniques that have been discussed try to non-invasively assess the degree of steatosis, the stage of fibrosis or the activity of the steatohepatitis. Studies have compared these techniques with biopsies at one point in time. This type of comparison is called a cross-sectional analysis. How these tests evolve over time has not been well validated. It is also not known what a change in such a parameter over time really means. For example, if your liver stiffness goes down over time: what does that tell you about the lesions on your liver biopsy? Although it makes sense to think that the improvement in liver stiffness should correspond to a reduction in the amount of fibrosis, there is still very little scientific proof for that. This has to do with the fact that steatosis, steatohepatitis, and fibrosis are closely linked. As mentioned before, liver stiffness correlates with fibrosis, but is not determined by fibrosis alone. Liver stiffness can also be influenced by the presence of steatohepatitis.121 It might be that if the activity of your disease decreases over time, your liver stiffness will also improve.122 But the fibrosis stage can still be the same. In summary, much more research is needed to know exactly what it means if non-invasive markers change over time.
Despite these limitations and knowledge gaps, these NITs are already frequently used. More studies are urgently needed to increase our understanding of how to work with these tools in the future. For the time being, changes over time should be interpreted cautiously.109 One should also not forget the potential variability of tests. Before drawing any conclusion from an increase or a decrease of a given test over time, a repeated measurement is advisable to be sure that there is a consistent decrease or increase. And at best, any change over time can currently only be considered as a potential indicator, but by no means a proof, for a positive or negative evolution over time.
e. Algorithms
The previous sections described the different techniques that can be used to diagnose NAFLD and its severity. Many patients are at risk of NAFLD, but only a small percentage of those with NAFLD will develop more severe disease.25,44,77 It is therefore important to make an accurate diagnosis, or at least as accurate as possible. The biopsy provides the clearest overall picture, but as explained, the biopsy has its limitations. Some aspects of the disease can be diagnosed with alternative techniques. This makes it possible to have sufficiently accurate information without the need for a biopsy in many (but not all) cases.
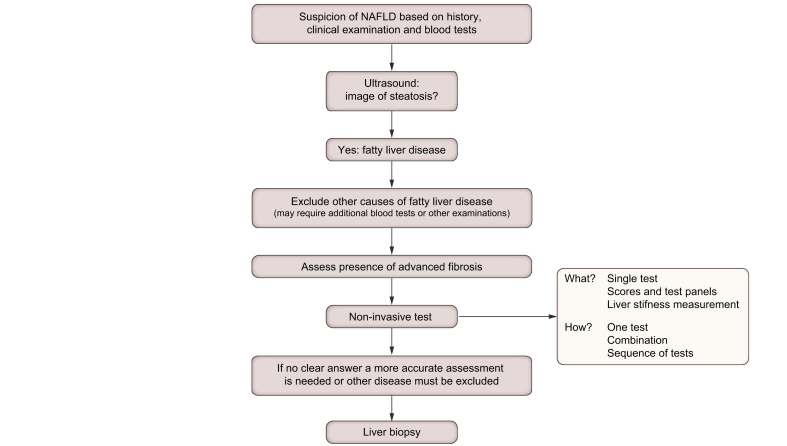
There is no unique, globally applicable scheme or algorithm to make an accurate diagnosis, but generally the following principle applies: When you are suspected of having NAFLD on the basis of clinical history, examination and blood tests, the next step is an ultrasound scan (Fig. 8).[83], [84], [85] A fatty liver usually looks brighter-than-normal on the scan. Unfortunately, neither the blood tests nor routine scans can reliably distinguish between isolated or simple fatty liver and more severe disease with inflammation or scarring/cirrhosis.
The NITs that we discussed in the previous sections are relatively new ways of ruling out advanced liver disease.94 This means that they are relatively accurate at telling you that you do not have the more severe forms of the disease. In this way, the tests may enable most people to avoid a liver biopsy. Validated scores like the FIB-4 or NAFLD fibrosis score can be used to calculate the risk of having severe disease and a liver stiffness assessment can also be performed – this also helps to predict whether a person has simple or isolated fatty liver disease or might have more advanced disease. Scores and liver stiffness measurements are often combined, or one is used as a confirmation of the other (sequential use).82,83 If these tests are negative, they can quite accurately tell you that you have little chance of having more severe disease. So, in that case, they can reassure you. The opposite, namely a positive test telling you with certainty that you have a more severe form of NAFLD, is more difficult.
If these tests do not give a reliable answer, a liver biopsy may be needed. A liver biopsy may also be necessary if a high suspicion of advanced and/or active disease needs to be confirmed, or if other liver diseases need to be excluded.
6. If a diagnosis of NAFLD is established, what is the treatment?
If you are diagnosed with NAFLD, your doctor will talk to you about making healthy diet and lifestyle choices. This is the cornerstone of NAFLD treatment and should always be the first treatment option. There are currently no medicines to specifically treat NAFLD, although research is underway to develop them. Without specific drugs for treatment, weight loss is key, and this can be achieved through a combination of dietary changes and increasing physical activity/exercise levels. These changes can help to:
-
•
Reduce the amount of fat and inflammation in your liver. Even if your liver is scarred, there can be some improvement if you make and sustain lifestyle changes, given that the liver has the ability to regenerate.
-
•
Improve your metabolic profile and thereby lower your risk of CVD, T2D and liver cancer.
a. Other liver diseases that can exist in conjunction with NAFLD should be looked for
As part of the diagnostic work-up, other causes of liver disease should be looked for and treated if they are present. If you have NAFLD you can also have another liver disease at the same time. Alcohol is discussed in Section 6.e. If multiple liver problems exist together, they often reinforce each other, and the disease may progress more rapidly. Therefore, it is important to identify other potential diseases, which can often be done with additional blood tests (see also Fig. 9) but may require a liver biopsy.
Fig. 9.
This flowchart represents a proposal of how to approach the challenge of diagnosing NAFLD if you have one or more risk factors for NAFLD.
NAFLD, non-alcoholic fatty liver disease.
b. All cardiometabolic risk factors should be checked and treated according to their proper guidelines
As mentioned, there is a strong association between the metabolic syndrome, T2D, CVD and NAFLD (see Section b, c and Table 1). Therefore, it is important to check your weight, blood glucose, blood pressure and blood lipids (cholesterol, triglycerides) on a regular basis and manage these appropriately (there are separate guidelines for this13) to reduce your risk of CVD and T2D. If necessary, your doctor may prescribe specific medications, such as those to treat high blood pressure or elevated cholesterol.
c. All other chronic diseases should be checked/excluded, including psychological diseases
NAFLD, but also the components of metabolic syndrome, are chronic diseases. They sometimes cause other chronic diseases or co-exist with other diseases. All these diseases are frequently treated by several different (specialised) physicians and other healthcare providers. Nevertheless, they are not strictly separated but frequently interact. This close entanglement of all these diseases, including psychological and psychiatric conditions, should not be minimised. Ideally, everything should be treated as part of the global management of your health (see also Section 6.g). Your family doctor and other first-line healthcare providers may help you with this and play an important role in keeping an overall view of all your health problems. If one of your health problems and diseases is not taken care of it could have a negative impact on the treatment of NAFLD or your other conditions.
d. Maximum effort should be made to improve the factors that drive the disease: this is what is meant by lifestyle modification
See Box 11.
Box 11. Lifestyle modification.
NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis.
What is the evidence for lifestyle modifications in the management of NAFLD? What exactly do I need to do?
Weight loss
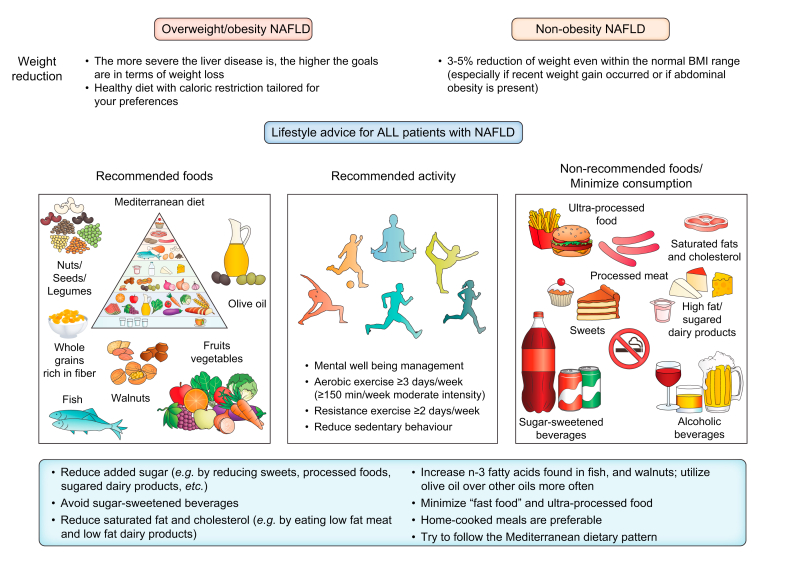
Research has shown that weight loss is an effective treatment for NAFLD across the disease spectrum.123 Weight reduction, whichever way it is achieved, leads to improvements in your liver blood tests (liver enzymes), the amount of liver fat and liver inflammation, as well as the amount of scar tissue or fibrosis.13,124 The impact of weight loss on liver improvement depends on the degree of weight reduction. A weight reduction of >5% is usually necessary to reduce liver fat, 7–10% to improve liver inflammation and >10% to improve fibrosis/scarring, although even lower reductions can be helpful.124 Therefore, the guideline, jointly written by three scientific societies (the European Association for the Study of the Liver (EASL), the European Association for the Study of Diabetes (EASD), the European Association for the Study of Obesity (EASO))recommends a weight loss target of 7–10% if you are overweight or have obesity with NAFLD.13 The favourable effects of moderate weight loss also extend to lean patients who do not have obesity-associated NAFLD. In this case, a 3% weight loss is likely to drive NAFLD remission.125 Lifestyle changes that produce even modest results, such as a sustained weight loss of 5%, can induce clinically meaningful reductions in triglycerides and blood glucose. These reductions are important to prevent heart disease and T2D, respectively.
Weight loss can be achieved by any dietary method that reduces calorie intake. Many different types of diet have been shown to be effective for inducing weight loss, though there is no “magic” diet. You can choose a diet with health benefits that you feel able to follow in the long-term, guided by your doctor and/or dietician/nutritionist. For example, a low-carbohydrate diet appears to be similarly effective as a low-fat diet in reducing liver fat and the liver enzyme alanine aminotransferase longer term, as long as a 7% weight loss is achieved.126
Dietary composition
Fat
Saturated fat has been shown in several studies to have a reinforcing effect on liver fat accumulation. For example, in a study comparing the addition of muffins high in saturated fat vs. muffins high in unsaturated fat to the habitual diet there was a similar increase in body weight across both groups. Only the muffins with the high saturated fat, however, markedly increased liver fat, liver blood tests (liver enzymes) and serum lipids.127 In another study, similar adverse effects were observed after eating saturated fat from coconut oil, butter, and high-fat blue cheese for three weeks.128 Therefore, reducing the intake of saturated fat is important. Foods rich in saturated fat include palm oil, which is found mostly in processed food, butter and high-fat dairy products. They also include high-fat meat (e.g. internal organs like liver, brain and processed meats like sausages), cakes, cookies, ice cream and other sweets. Reducing the amount of processed food that you eat can help manage your NAFLD. Fig. 10 shows some “good” and “bad” foods for the different nutrient components.
Fig. 10.
A summary of lifestyle recommendations for those living with NAFLD.
NAFLD, non-alcoholic fatty liver disease.
Sugar
Naturally occurring sugar refers to the sugar that is an integral constituent of whole fruit, vegetable, and milk products, whereas added sugar refers to sucrose (table sugar) or other refined sugars (fructose and high-fructose corn syrup) incorporated into food, fruit drinks, and other beverages.129 Sugar-sweetened beverages are the leading source of added sugar worldwide.130 Evidence from many studies shows an association between added sugars and NAFLD, which is more prominent if it comes in the form of sugar-sweetened beverages.131 Among children and adolescents, fructose consumption was independently associated with NASH (the advanced form of NAFLD).132 Importantly, reducing sugar consumption in children led to an improvement in liver fat within a few weeks.133,134 Taken together, these findings imply that the intake of sugar-sweetened beverages and foods high in added sugar should be reduced as part of the treatment of NAFLD.
Other aspects of dietary composition
Adequate amounts of fruits and vegetables may reduce the risk of NAFLD,135 attributed to the fibre, vitamins and non-vitamin antioxidants (such as phenolic compounds) they contain. Studies indicate that dietary intake of vitamin E and C and phenolic compounds might protect against NAFLD-related liver damage.136,137 The best sources of vitamins E and C are nuts, seeds, plant oils, fruit and vegetables. Phenolic compounds are abundantly present in foods such as berries, nuts, coffee, tea and whole grains.137
Dietary patterns represent the overall combination of foods that can produce synergistic health effects. One of the most studied dietary patterns is the traditional Mediterranean diet (Box 12). This diet is characterised by a high intake of olive oil (which is rich in a healthy type of fat and polyphenols that act as antioxidants), vegetables, fruits and nuts, legumes, whole grains, fish and seafood. It is also characterised by a low intake of red meat and processed foods. Several studies have shown a harmful association between high meat intake and NAFLD,138,139 specifically red (such as beef, lamb and pork) and processed meat (such as hamburger, salami, sausages and processed schnitzel) (Fig. 11).[140], [141], [142] Importantly, the Mediterranean diet is also characterised by reduced sugars and refined carbohydrates. Interestingly, one of the principles of the Mediterranean diet is to minimise processed or “fast foods” and to have more home-cooked meals (Fig. 11). The Mediterranean diet has a well-established protective role against CVD and T2D. Studies also support the protective role against NAFLD.[143], [144], [145] In two interventional studies, two months of a Mediterranean diet led to a significant decrease in liver fat independent of any weight loss.146,147 For this reason, the Mediterranean diet has been recommended for the treatment of NAFLD by the EASL-EASD-EASO Clinical Practice Guideline13 and the European Society of Clinical Nutrition and Metabolism (ESPEN) Guidelines.148 Increasing adherence to the Mediterranean diet might prove a challenge, as some of the foods in the Mediterranean diet tend to be expensive in countries outside of the Mediterranean region (fish, nuts, fruits, etc.). However, it is important to emphasise that even partial adherence to this dietary pattern (especially a reduction of sugar, saturated fat and processed food in general) and a more culturally adjusted approach can be beneficial.
Box 12. Mediterranean diet.
Fig. 11.
The Mediterranean diet.
These are general recommendations for a healthy lifestyle. Specific person-tailored recommendations should be provided to you by a nutrition expert. s, serving.
Similar foods that are “good” or “bad” for NAFLD are also related with liver cancer (Fig. 10). High intake of red meat, saturated fat,149 cholesterol150and refined sugars151 are associated with an increased liver cancer risk. High-fat dairy products and butter, but not low-fat dairy, are also associated with a higher risk of incident liver cancer.152 Consumption of omega-3 polyunsaturated fat-rich fish is inversely associated with liver cancer153 as is vegetable intake.154 Interestingly, the Mediterranean diet is associated with a lower risk of liver cancer.155,156
In summary, clinical evidence strongly supports the role of lifestyle modification as the primary therapy for the management of NAFLD. The Mediterranean diet can reduce liver fat even without weight reduction and is the most recommended dietary pattern in NAFLD.157 However, other healthy dietary patterns are also helpful, and the choice of diet should be decided by tailoring the advice of your physician or dietician to your cultural and personal preferences. Lifestyle modification should be considered as a lifetime treatment. Comprehensive weight loss interventions that provide regular follow-up meetings with a healthcare professional are recommended to successfully achieve and maintain weight loss.158
Physical activity/exercise
In NAFLD, sedentary behaviours are common.159 Sedentary behaviour refers to “any waking activity characterised by a low level of energy expenditure in a sitting or reclining posture” (e.g. watching television, using a computer, travelling by car or bus).160 Research has identified sedentary behaviour as an independent risk factor for NAFLD,[161], [162], [163], [164] modulating the development and progression of the disease. A 1.15% increase in liver fat was associated with each additional hour of time spent sedentarily per day.157,165 Decreasing overall sedentary time and breaking up sedentary time throughout the day is a useful treatment strategy for all people with NAFLD/NASH and may be more achievable initially than increasing physical activity levels (Box 13).
Box 13. Sedentary behaviour, physical activity and exercise.
Population-based studies have shown that physical activity is effective in reducing liver fat.[166], [167], [168] It is also effective in reducing the risk of other comorbid metabolic diseases such as T2D, CVD and obesity. Physical activity is also likely to reduce the loss of muscle mass that is commonly observed in NAFLD.169,170 There is increasing insight that this low muscle mass or sarcopenia plays a role in the severity of NAFLD and the complications that come with the more severe stages of the disease.[171], [172], [173]
Both aerobic and resistance training are effective, but they have different characteristics that make them suitable for different patients: resistance exercise (“strength” e.g. lifting weights or your own body, using elastic bands) might be preferred if you have poor fitness and cannot tolerate or participate in aerobic exercise (“cardio”, e.g. brisk walking, jogging, cycling, swimming, dancing).[174], [175], [176] This is good news as it allows you to select the type of exercise that appeals to you most.174 However, often a combination of aerobic and resistance exercise provides the greatest health benefits.177
Guidelines recommend over 150 min/week of moderate intensity physical activity over 3-5 sessions13 including a combination of aerobic and resistance training.160,165 The effects on liver fat follow a volume-response relationship:178 higher volumes of physical activity/exercise are also associated with greater weight loss and systematic improvement of comorbidities.179 The latter includes the possibility that even T2D can completely regress.180
Many people with NAFLD have difficulty increasing physical activity/exercise levels as a treatment for their liver disease181 and strategies to improve adherence to physical activity/exercise interventions are needed.182 Some people prefer to plan and structure their own physical activity sessions183 rather than attending supervised, group-based exercise sessions184 whereas others prefer to undertake physical activity with others in a more supervised setting.185 It is therefore important to have as many options available as possible.
Ultimately, your physical activity/exercise programme needs to be tailored to your needs and preferences, taking into account your baseline fitness and other health conditions. The more done overall throughout the day, the better, so it really is key to find something that you enjoy!
e. What should a normal-weight person with NAFLD do?
NAFLD can develop in people with a normal BMI (<25 kg/m2 BMI in Caucasian, and <23 kg/m2 in Asian individuals) (See also section 3.a). Sometimes these patients have greater “visceral” obesity (abdominal fat – an apple body shape) and decreased muscle mass,186 and thus normal-weight patients are not always metabolically healthy. So, they can benefit from increasing their physical activity/exercise levels which decreases abdominal fat and increases muscle mass independent of weight loss.174,177,187 Normal-weight patients with NAFLD will also benefit from reducing their intake of dietary fructose, sugared-sweetened beverages, saturated fats and processed foods.188 Switching to a Mediterranean diet without weight loss can help reduce liver fat and a modest 3–5% weight reduction can help to achieve NAFLD remission.125
f. How can I change my diet and increase my physical activity/exercise levels?
Dietary change and physical activity/exercise are key components of any lifestyle change package for people with NAFLD, not just to improve liver health but also to support weight loss and reduce the risk for other chronic diseases.189,190 Tailoring this to your personal preferences, daily routine and baseline fitness is essential for long-term success.187,191,192 Find something you enjoy and, if possible, find someone who will support you in making these changes – having a “buddy” is known to increase motivation and long-term adherence (Fig. 10).
Setting SMART (specific, measurable, achievable, relevant, timely) weight loss and/or physical activity goals can help you to initiate and maintain changes.187 An example of a SMART exercise goal could be to swim 10 lengths, twice a week at the local pool for the next month. Another example of a SMART dietary goal could be to stop buying sugar-sweetened beverages for the next four weeks.
Monitoring the changes you make can help to keep you on track and help you to assess whether you are meeting your personalised targets. For example, keeping a food diary and/or counting your daily calories using a smartphone app or internet website; using a pedometer/activity tracker to measure your daily step count; weighing yourself regularly to monitor weight loss.
Your healthcare professional may be able to direct you to appropriate exercise schemes, community gyms, walking groups or weight management programmes in your local area. They may also be able to advise on relevant online/digital resources to support you in making changes to your diet and/or physical activity patterns.
g. How should I maintain lifestyle modifications longer term?
The strategies to implement an intensive lifestyle intervention vary widely among studies, from mere prescription of diet and physical activity/exercise, to nutritional/exercise counselling, to cognitive behavioural treatment.193 Cognitive behavioural treatment is a treatment that has been designed to provide you with a set of principles and techniques to modify your eating and activity habits.183 This has been derived from the treatment of obesity. The intervention integrates cognitive strategies (e.g. problem solving and cognitive restructuring) with specific recommendations on diet and physical activity/exercise and is often referred to as “lifestyle modification”.194 The critical issue is engaging patients into structured intensive lifestyle intervention programmes, where strategies should include motivation, a collaborative therapeutic style, acceptance and empathy, functional analysis of pros and cons, roll with resistance and support.195 However, intensive lifestyle intervention requires a dedicated team and continuing patient/therapist interaction. Unfortunately, this is something that is not widely available and is generally poorly supported by the healthcare system.
The possibility to educate, counsel and induce permanent changes in people with NAFLD via an internet-based approach and self-management programmes have been explored in several settings.[196], [197], [198] The most recent approaches allow for interaction with therapists and with peers in discrete groups. In general, internet-based programmes are less effective than face-to-face programmes, but their effectiveness may be increased by telephone recall systems199 or text messages.200 The reduced effectiveness and larger drop-out rate are repaid by reduced costs and by the much larger audience.201,202 In young, motivated patients with NAFLD, similar weight loss targets, dietary adherence and physical activity were achieved, and maintained at 2-year follow-up, with a web-based programme or a group-based educational approach.203
Finally, very few studies have directly compared intensive lifestyle interventions and drug treatment (using drugs approved for obesity or T2D) in patients with NAFLD. A study did not demonstrate any difference between liraglutide (3 mg/day) and intensive lifestyle intervention on weight loss, liver blood tests and markers of fibrosis.204 Intensive lifestyle intervention was, however, associated with more sustained weight loss maintenance and less liver fat re-accumulation at follow-up.205
There is an increased understanding that environmental factors can make it difficult for patients to change their lifestyle and lose weight. Examples of these factors are the fact that sweets are cheap and readily available at every kiosk or supermarket, with widespread advertising on the television and internet. It is also difficult for patients living in a surrounding where several people eat and live unhealthily. Policy measures to create a healthier environment and to support a healthy lifestyle are acknowledged in a recent EASL statement on NAFLD and obesity.190 This statement aimed to inform politicians, policymakers and the general population across Europe about NAFLD and the measures required for prevention and treatment.
h. What is the role of bariatric surgery?
Bariatric surgery very effectively achieves weight loss and weight loss maintenance in patients with obesity.191 The effect of bariatric surgery on body weight largely exceeds the 10% weight loss target associated with liver fat clearance, NASH resolution and fibrosis reversal, as reported in several studies.124 Accordingly, surgery offers a possible treatment to manage NAFLD and improve clinical outcomes if you are living with severe obesity.
The agreed criteria for the surgical management of obesity and metabolic disorders (BMI ≥40 kg/m2 or BMI ≥35 kg/m2 with complicating disorders, with no resolution after medical treatment) are also applicable for NAFLD.191 Patients with a BMI of 30–35 kg/m2 who also have T2D that is not adequately controlled by medical therapy may also be candidates for surgery.206 Laparoscopic Roux-en-Y-gastric bypass and sleeve gastrectomy are the most performed surgical procedures.[207], [208], [209] Both procedures have good effects on body weight and diabetes remission.[210], [211], [212], [213], [214], [215] In addition, gastric bypass relieves gastro-oesophageal reflux, which may be worsened by sleeve gastrectomy.210,211 Long-term studies showed that bariatric surgery may reduce the risk of heart disease/stroke and improve survival.212,216
The evidence specifically supporting bariatric surgery in NAFLD is exclusively derived from observational studies.217 In these studies, liver biopsies were performed during the surgery and during follow-up.216,218 In a large series, improvement in the liver lesions of patients with NAFLD, including a reduction in fibrosis, was associated with 5-year post-surgery weight loss.209 Notably, patients whose NASH persisted one year after surgery had less weight change on average (BMI, -9.1 ± 1.5 kg/m2) than those who no longer had NASH (-12.3 ± 0.6 kg/m2). This means that liver improvement and the magnitude of the weight loss go hand-in-hand.123 Economic analyses have shown that the surgical treatment becomes cost-effective in people with a high risk of progression (F3 fibrosis).219 In a retrospective analysis of a large insurance database, patients with NAFLD and obesity who underwent bariatric surgery had a 70% reduced risk of progressing to cirrhosis vs. matched cases not receiving surgery.220 Other studies did not confirm this and suggested that alcohol consumption may undo the benefit of surgery in preventing severe liver disease.221
Cirrhosis is not an absolute contraindication to bariatric surgery, but a precise evaluation of hepatic and cardiovascular function should be performed prior to surgery.222 Considering the negative impact of obesity on liver transplantation, reports of bariatric surgery before or at the time of transplant have recently been published.223 Carefully controlled studies are needed to determine the best timing for the bariatric procedure and liver transplantation in patients with obesity and NASH-cirrhosis needing a transplant.224,225 These cases are recommended to be referred and treated in expert centres. Besides surgery, other techniques, such as endoscopic techniques, are under evaluation with the aim of reducing weight and/or improving metabolism (particularly diabetes).226 Further studies are needed to confirm the efficacy of these techniques and to determine their role in combatting NAFLD.
i. Tips on managing fatigue
We all experience tiredness at times, which can be relieved by sleep and rest. Fatigue is overwhelming tiredness that is not relieved by sleep or rest. Fatigue is a symptom of NAFLD that can be improved with weight loss.66 If you suffer with fatigue, it is important for you to make the recommended lifestyle changes that are detailed in Section 6 and keep a note of how this affects your fatigue levels. Fatigue can also be explained by other causes such as thyroid issues, anaemia and diabetes, and so it is important that these are checked and well controlled.
Tips for managing fatigue:
-
•
Losing weight can improve your ability to perform day-to-day activities more easily with less effort
-
•
Eating healthily and keeping hydrated can improve your energy levels
-
•
Keep active – it is probably the last thing you feel like doing, but regular physical activity/exercise can help improve your energy levels
-
•
Take advantage of short cuts – there are lots of things you can do to make your life easier such as online shopping or cooking extra food and freezing it, so you have effort-free meals waiting in your freezer
-
•
Plan rest time – if you have a busy day or activity ahead, plan some rest time afterwards
j. What is the role of alcohol in disease occurrence and disease progression? Is there a safe limit and if yes, what is that?
It is important to note that NAFLD is not caused by alcohol, although high alcohol consumption can worsen the problem.24,36 Anyone with cirrhosis should avoid alcohol since any regular alcohol consumption puts them at significantly greater risk of developing liver cancer.12,148,227 However, uncertainty remains regarding moderate alcohol consumption (up to 2 drinks per day) among patients without cirrhosis. At the population level, the combination of alcohol and obesity increases the probability of developing NAFLD and increases the severity of liver disease, reflecting a synergistic effect.33,36,[228], [229], [230], [231] A large cohort study from the U.S. reported that patients with NAFLD who drank more alcohol than the threshold of ≥3 drinks/day for men and ≥2 for women, were 3x more likely to have advanced fibrosis than those with NAFLD alone.34,228 In addition, overall mortality was higher in adults with NAFLD who consumed excess alcohol.34,232 Importantly, even mild-to-moderate drinking (<210 g/week) has been found to increase the risk of NASH, fibrosis, decompensated liver disease, mortality, and liver cancer.34,230,[233], [234], [235], [236], [237]
International guidelines differ in their recommendations on alcohol consumption for people with NAFLD. The EASL-EASD-EASO Clinical Practice Guideline13 for the management of NAFLD recommends keeping daily alcohol intake below the risk threshold (30 g for men; 20 g for women) and total abstinence if you have NASH-cirrhosis to reduce the risk of HCC. The ESPEN Guidelines recommend all people with NAFLD/NASH be encouraged to abstain from alcohol in order reduce the risk of comorbidities and to improve liver blood tests and liver tissue lesions.148
It is important to stay within the guidelines, not only for your liver health but especially if you are trying to lose weight, as alcoholic drinks contain many “empty” calories, which have no nutritional value. With a pint of lager containing the same number of calories as a slice of pizza, the calories in alcohol soon add up. You might be surprised to find out how many calories there are in wine and how spirits like gin could be contributing to weight gain.
You can check how many units of alcohol you drink per week (and how many calories this adds up to) using the online calculator at (1 unit = 8 g of alcohol): https://www.drinkaware.co.uk/understand-your-drinking/unit-calculator
k. What about smoking?
Smoking should be discouraged if you have NAFLD, as it has a negative influence on NAFLD[238], [239], [240] and can accelerate disease progression either by itself or synergistically with other risk factors. In current smokers, the risk of NAFLD increases with an increase in the number of cigarettes smoked in a dose-response manner.238,239,241 An interaction was observed between current heavy smoking and moderate drinking (80 to 210 g/week) on the odds of developing NAFLD. The combination of both behaviours led to the highest risk of NAFLD.[241], [242], [243]
Importantly for people with NAFLD, smoking is associated with the progression of liver scar tissue.[242], [243], [244] A large cohort study of >400,000 people living with T2D demonstrated that smoking was associated with a 60% increased risk of severe liver disease during follow-up.[244], [245], [246], [247] Smoking is also an extremely important risk factor for liver cancer,[245], [246], [247] increasing the risk by about 50% among current smokers compared to never smokers, independently of alcohol consumption.246,247 In Europe, smoking contributes to nearly half the cases of liver cancer, which is actually more than hepatitis B and C viruses.246,248 Unsurprisingly, smoking is related to an increased risk of CVD in people with NAFLD, with a 33% increased risk after taking into account other risk factors.3,248 The role of the electronic cigarette is not clear. Cases of a toxic injury to the liver have been described, but no studies in relation to NAFLD have been reported.
7. What drugs can help?
Despite intensive research and investment by drug companies, there are currently no drugs specifically approved by regulatory agencies for the treatment of NAFLD.
a. Some general considerations: why is it difficult to find drugs that are highly effective?
The mechanisms explaining why the disease occurs are very complicated. Your liver has an extraordinarily complex set of functions. Several types of cells help your liver to accomplish its role, but also help to restore your liver when it gets damaged (see Section 1.a). With all these cells and all these functions, something can go wrong. Furthermore, your liver receives signals and molecules from other organs, most notably from your fat tissue (Fig. 2). If the abdominal fat tissue is overwhelmed then excess fat will be forced to go elsewhere.3,37 Furthermore, the abdominal fat tissue gets inflamed and damaged. Consequently, it releases noxious substances that will directly reach your liver via a large connecting blood vessel, the portal vein. This portal vein also directly brings the components of the digested food absorbed by the gut to your liver. It also brings along numerous other elements, such as pieces of bacteria that are living in the gut. All this results in a very complex system of interactions between the liver and other organs, and between cells and functions within the liver (see Section 1.c).
This also means that the mechanisms leading to disease will differ between patients, which implies that a drug may work in one patient but not at all or not so well in another patient. As explained before, metabolic problems, and especially the impaired function of fat tissue, are major drivers of the disease (see section 1.c). For this reason, some drugs will focus on fat tissue function and on the mechanisms involved in the regulation of fat and glucose, so on metabolic targets. Other drugs may target inflammation or the formation of scar tissue (fibrosis).249,250 It is clear that many different drugs, with many different targets, could be of interest (Fig. 12).
Fig. 12.
The liver lesions in NAFLD are the result of an imbalance between what is damaging the liver on the one hand and leading to the formation of scar tissue (fibrogenesis) on the one hand, and the repair mechanisms on the other hand.
The imbalance is the result of upstream driving forces, pushing the progression of the disease. These driving forces are processes of cell damage and inflammation inside the liver (steatohepatitis) but also outside the liver (amongst others, the fat tissue inside the bowel cavity). These mechanisms are themselves driven by the upstream metabolic factors (excess calorie intake in combination with too little physical activity, leading to overweight and obesity, diabetes…). All these different factors can be the target of drugs that are being developed to treat NAFLD. NAFLD, non-alcoholic fatty liver disease
b. How can you test whether a drug is effective? What is required for a drug to be approved?
A drug should demonstrate what is called clinically meaningful benefit, which means that you must see an improvement in the course of the disease (in this case: less evolution towards cirrhosis and the complications of cirrhosis, less HCC or less deaths from any cause).56,251 Studies must follow stringent scientific methodology. As the evolution of this disease is rather slow, this benefit is difficult to show in studies of relatively short duration. Studies may thus take many years before this type of benefit can be unequivocally proven.
In this situation, where you need studies that take many years to prove efficacy of a given drug, the regulatory authorities can approve a drug based on so-called surrogate endpoints.58,252 Surrogate endpoints are indicators of improvement that are likely to translate, later on, into real clinical benefit for you. Examples of such surrogate endpoints could be lab tests, or, in the case of NAFLD, improvements on liver biopsy. Currently, clinical trials focus on patients with NASH and fibrosis (Box 14) (see Section 7.c). As the information that the biopsy delivers cannot entirely be replaced by accurate NITs, the efficacy of a drug will need to be proven by repeat liver biopsy. A study can rely on NITs like liver blood tests or liver stiffness or markers of fibrosis in the early phases of the investigation of a drug. These early studies help to decide whether it is interesting to go on with that drug to the next phase. But in a later phase of development of a drug, before a drug can be approved by the authorities and be brought to the market, you need a phase III trial proving, on a liver biopsy, that the drug works.
Box 14. Clinical trials for drug development NAFLD and NASH.
NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; NIT, non-invasive test.
“A drug works” can have several meanings in this case.58,250 A first possibility is that there is an improvement of the steatohepatitis to the point where it is not steatohepatitis anymore. This is called resolution of NASH. This is a positive endpoint, at least if there is also no worsening of fibrosis at the same time. Resolution of NASH also requires that the ballooning, which is the typical swelling of the liver cells as a sign of damage, has completely disappeared.58
A second possibility is that there is a reduction in the amount of fibrosis. This is called regression of fibrosis and is defined as going at least one stage down the scale. It also requires that steatohepatitis does not get worse at the same time.58
An even better goal of treatment is the combination of both these improvements in the same patient: resolution of NASH and improvement in fibrosis.
If the biopsy in phase III shows improvement according to what has been defined, approval can be applied for and, if approved, the drug can be marketed.58,250 This approval is not a definitive, final approval, but a conditional one. Why is this? It seems plausible that improvement on the liver biopsy will also lead to a lower risk of developing cirrhosis and other liver-related clinical events later on. This positive link between the improvements in liver biopsy and your long-term outcome has, however, not been scientifically proven. Therefore, the phase III trials currently go on beyond the first treatment period and first control biopsy. The long-term phase of these studies is meant to see whether patients in the end really do better and develop less disease events. But this will require many years of study. It will only be based on these end-of-study results on true clinical events that final approval can be granted.
A broad range of drugs are under investigation. This is indeed a field of very active research. As an increasing number of clinical studies are running and results are reported, recommendations may rapidly change. Information on which clinical trials are ongoing can be found on http://www.clinicaltrials.gov.
c. Should all NAFLD/NASH be treated?
In general, all metabolic factors surrounding NAFLD, and NAFLD itself, contribute to an increased risk of dying from several different causes. CVD and cancers are the most frequent causes of death (see Section a, b), adding to liver disease-related events.54,253,254 It is, however, clear that more severe NAFLD is associated with a higher risk of adverse outcomes. Furthermore, more severe NAFLD also has a greater impact on quality of life, potentially long before any overt disease develops (see Section 2.d).66 Therefore, treating all the metabolic factors associated with NAFLD, and trying to improve NAFLD using the lifestyle modifications described, is recommended in any patient regardless of disease severity.13,14,255
However, when it comes to the question of starting drug treatment specifically for NAFLD, the current consensus is the following: this is indicated in patients with NASH, particularly in those with more disease activity and with some scar tissue.13,14,56,58
In case a liver biopsy is used to decide whether a drug is needed or not, treatment is indicated when certain conditions are met. First there must be NASH. The NASH must have a certain degree of activity, so very mild disease probably does not warrant drug treatment.58 There also needs to be a certain severity, so a certain stage of scarring. This usually means a stage of F2 or more on the most widely used F0 to F4 scale, but other scales and hence other definitions can be used. The presence of scar tissue is regarded as a sign that the liver is not able to cope with the damage that has occurred.
When there is no biopsy, severity criteria based on measures of liver fat or liver stiffness are mostly used as a proxy of the liver biopsy data. This can be based on blood tests, ultrasound, MRI or other test modalities (see Section 5). Currently there is no single set of criteria that are agreed upon worldwide, and so many different (sets of) test criteria are in use.
The place of other treatment modalities, such as endoscopic226 or surgical weight-reducing interventions,208 is not clear and probably should follow the same principles as those for drug treatment. Of course, if such a procedure is indicated for an associated problem such as obesity and T2D, your liver could also benefit from this procedure, but liver disease cannot be considered the standalone reason to perform such a procedure.
d. Do we already have drugs that can be used?
Some drugs that are used to treat other conditions have also been tested for NASH. Based on their effects demonstrated on liver biopsy, the following drugs seem to have some efficacy. Vitamin E showed promise, but only in patients without cirrhosis and without T2D.256,257 Pioglitazone, which is approved for the treatment of diabetes, showed promise for NASH in patients with diabetes and pre-diabetes.[256], [257], [258], [259] Liraglutide and semaglutide are approved for the treatment of obesity and for diabetes and have also proven to be of value in NASH.[260], [261], [262]
Pioglitazone
Pioglitazone is an anti-diabetic drug able to modulate several responses, including insulin sensitivity.263 Several studies and a meta-analysis have consistently demonstrated an improvement in liver blood tests and in liver biopsy features following pioglitazone administration at doses of 30-45 mg/day vs. placebo, irrespective of the presence of T2D.257,258,[264], [265], [266] In the PIVENS trial, pioglitazone was compared with placebo and vitamin E.267 Pioglitazone significantly reduced liver blood tests, steatosis and liver cell damage and inflammation.267 Pioglitazone (45 mg/day) was particularly effective for the treatment of NASH in patients with pre-diabetes or T2D.258 In a very recent global analysis of the different studies that have been reported, pioglitazone treatment was associated with NASH resolution.3,[268], [269], [270] There was also a trend for a decrease in the amount of scar tissue. The analysis also showed that after discontinuation of the drug, the levels of liver enzymes like alanine aminotransferase increased, possibly signalling the recurrence of NASH.271 Notably, pioglitazone also has beneficial effects on the cardiovascular system.[272], [273], [274] Regarding adverse events, pioglitazone treatment induces some weight gain269 and may also increase the risk of non-osteoporotic fracture.269
The body weight increase induced by pioglitazone warrants further discussion. It may seem contradictory that losing weight can improve the condition of the liver, while a drug that increases weight can also have a positive effect on the liver. This can be explained by the balance between the calorie overload and the capacity of the fat tissue to handle this. If the calorie overload exceeds the storing capabilities of the fat, the fat tissue gets inflamed and dysfunctional. As a consequence, the fat releases substances that damage other organs and forces the fat to go elsewhere, including the liver.3 Reducing weight by lifestyle modification is one way to tackle this problem. Another way, which is what pioglitazone does, is to improve fat tissue function.275 Thanks to this effect of the drug, the “good” fat tissue, meaning the subcutaneous fat tissue, can expand. This allows fat to shift from the abdominal fat and other body areas to the subcutaneous fat. The expansion of the subcutaneous fat leads to weight gain. This weight gain is in this case a translation of metabolic improvement and goes along with improving, and not worsening, liver lesions. The aspect of weight loss or increase should hence be interpreted in the context of how the drug works.
Vitamin E
Vitamin E has anti-cell death and antioxidant properties. Therefore, it has been proposed for the treatment of NAFLD, but the results of different studies are not always in line.266 In the PIVENS trial at the dose of 800 international units (IU)/day, vitamin E was significantly better than placebo at improving steatohepatitis, but it had no significant effects on fibrosis.267 According to this data, the use of vitamin E is indicated by the U.S. guidelines in patients with biopsy-assessed NASH, without T2D or cirrhosis.14 This recommendation is not shared by the European guidelines.13 A very recent study concluded that only a combination therapy of vitamin E (800 IU/day) and pioglitazone (45 mg/day) achieved the most challenging targets of reducing liver cell damage and inflammation. However, fibrosis did not improve.257 An old global summary analysis of studies using vitamin E reported that a dose of 800 IU/day was associated with increased all-cause mortality. This potential safety issue is not supported by recent data276 that did not find such an association between vitamin E and mortality. Vitamin E remains the treatment of choice for paediatric NAFLD.277
Liraglutide, semaglutide and other glucagon-like peptide-1 receptor agonists
Liraglutide is a glucagon-like peptide-1 receptor agonist (GLP1 RA). Glucagon is an important hormone in the regulation of energy handling in your body. GLP1 RAs are a class of drugs that improve control of your blood sugar, cause weight loss and improve blood lipids. They also improve cardiovascular outcomes.278 Therefore, they are approved for the treatment of diabetes and obesity. Liraglutide is one of these GLP-1 RAs and has been tested at a daily dose of 1.8 mg in a small 1-year study.260 NASH resolved significantly more frequently in liraglutide-treated patients than placebo-treated patients. Treatment with liraglutide also led to a trend in improvement of fibrosis stage. The same holds true for semaglutide, which was already shown to improve liver blood tests in patients with T2D and obesity.261 Semaglutide was tested at several dose regimens for a treatment period of 1.5 years. NASH resolved significantly more frequently in patients treated with the higher doses of semaglutide than in patients that received placebo.262 Fibrosis also tended to improve, but this result was not strong enough to be considered as a positive endpoint of the study.
One can consider using these drugs if you meet the diagnosis for which these drugs are approved by the regulatory authorities. For example, if you are living with T2D, it could be worthwhile to consider including pioglitazone or GLP1-RAs in the treatment of your T2D, as they are also beneficial for your liver. This needs to be discussed with the doctors that treat your diabetes.
Other anti-diabetic drugs might also be of benefit, but there is currently no liver biopsy-based proof of their efficacy.279,280
e. When will we have specific anti-NASH drugs?
A lot of drugs are currently being tested in clinical trials, but none have been approved so far. It is unclear when drugs will be ready to enter the market. Some studies of molecules that looked promising were negative, meaning that they did not reach the level of efficacy that was required. Other drugs showed promise, but a successful phase III study is needed before a drug can apply for approval by the regulatory authorities. Measures of success take into account improvements of the liver as well as the side effects of the drug. So far, only one drug reached the target of fibrosis regression in phase III but has to date not been approved for the treatment of NASH.281 Many more drugs are under investigation but did not achieve the required targets as of 2021.249,250
8. How should a patient be followed/monitored after diagnosis?
a. Different paths according to severity
After diagnosis, it should be sufficiently clear which kind of fatty liver disease you have (see Section 5). Fatty liver disease can be classified into different degrees of severity (see Section 2, 5, Fig. 4). Depending on the severity of the problem, a different route of care is recommended. The following recommendations are just indicative of a general policy that could be followed, as there are no universally accepted rules on this issue.79
Fig. 4.
Non-alcoholic fatty liver disease can evolve from just a fatty liver to severe liver disease, over different stages of severity.
Many factors determine if you will develop more severe liver disease and how quick the evolution is. Luckily, many patients will not evolve to severe liver disease and many steps in the evolution are reversible with adequate management. NAFL, non-alcoholic fatty liver; NASH, non-alcoholic steatohepatitis.
b. NAFL (isolated or simple fatty liver [steatosis]):
In this case, the fatty liver should be examined periodically. As mentioned, there is no consensus yet on how frequently this should be done, but at least every two to five years seems a reasonable proposal to determine whether the fatty liver has worsened, remained the same or even improved. If you have concomitant conditions such as diabetes or heart disease, you should discuss with the doctor whether the examination interval should also take place annually. As a rule, a family doctor or a specialist can carry out the regular examination.
c. NASH with no or minimal fibrosis (fibrosis below F2):
If you have low-level inflammation and no/minimal fibrosis, a recommendation to have your liver examined at least once a year or every two years seems reasonable. If you have a concomitant disease such as diabetes or heart disease, you should discuss with the doctor whether the examination interval should instead take place more frequently, e.g. every six months. The examination should normally take place with a specialist.
d. NASH with significant fibrosis (fibrosis equal or higher than F2):
If you have severe inflammation, it is recommended that the examination interval be conducted at least once a year (probably better every 6 months). In case of diabetes or heart disease, you should discuss whether the examination interval should also be shorter. In addition, it is important that you are also regularly examined for liver cancer (HCC monitoring). The examination must be carried out by a specialist or in a specialised clinic for this purpose.
e. NASH (cirrhosis):
In the presence of cirrhosis, an examination interval of at least every six months is strictly recommended.13,14 The interval may also be shorter (e.g. every three months). In addition, it is important that you are also regularly examined for liver cancer (HCC monitoring). In fact, this should be part of the 6-monthly exam. The examination must be carried out by a specialist or in a specialised clinic for this purpose.
f. How is the monitoring performed?
The monitoring is usually carried out by blood tests (preferably combining clinical and blood tests into non-invasive surrogate markers, i.e. NITs), ultrasound and elastography. A biopsy is usually not part of the monitoring. Many NITs and monitoring methods are currently under development (see Section 5), with the objective of determining the state of the liver as precisely as possible while being non-invasive.
g. What is important between the examination intervals?
The doctor will have discussed a specific therapy with you. For the success of therapy, it is crucial that you adhere to the recommendations of the clinical care team. This is not always easy, so it is advisable to let your family, partner or friends know what the clinicians have recommended. With this information, they can help you to implement changes and motivate you to get better. It is also helpful to contact a self-help and/or patient group to look for like-minded people. Involvement of your partner or a family member can also be very helpful and supportive. Lifestyle modifications recommended by your clinical team may be easier to implement in a group than alone. Please also inform your clinicians if you are being treated for other illnesses.
Information from the internet can also be helpful, but you should always be careful who has published/written the information. Only trust the information from credible sources – if in doubt, ask your clinical team for signposting to recommended resources.
It can also be helpful to keep a diary. This allows you to record changes that you can later report to your clinicians. You can also record which recommendations were easy or difficult to implement and thus seek help in a targeted way.
h. What are the potential effects of treatment on your health-related quality of life?
Once you have started a treatment and have good adherence, the effect on your quality of life can be significant. The impact is, for example, significantly noticeable whenever weight loss is achieved. But more studies on the individual health-related quality of life must be set up to develop strategies to improve quality of life with treatment. Treatment here encompasses both non-medical (lifestyle) and medical (drug) treatment. Specific NAFLD/NASH questionnaires have been developed to get further insight into your health-related quality of life.62 Their use should be encouraged to assess and monitor quality of life, both in routine care as well as in clinical trials.
i. How can the severity of my disease be monitored over time?
We refer to Section 5.d subsection “Baseline assessment vs. follow-up”. Briefly, the techniques to non-invasively estimate the degree of steatosis, the stage of fibrosis or the activity of the steatohepatitis, have mainly been studied in what is called a cross-sectional analysis, meaning that they are tested and compared to the biopsy at one point in time. How changes in these parameters correspond to a change in liver health is not well understood. Although it makes sense to think that, for example, a decline in liver stiffness over time should correspond to a reduction in the amount of fibrosis, there is still very little scientific proof for that. Much more research is needed to know exactly what it means if non-invasive markers change over time.
Despite these limitations and knowledge gaps, these NITs are already frequently used. Until more data are available, their interpretation over time should be made with caution. One should also not forget the variability of the tests. Before drawing any conclusion based on an increase or a decrease of a given test over time, a repeated measurement is advisable. At best, any change over time can currently only be considered as a potential indicator for a positive or negative evolution over time, but not as a proof.
As mentioned in Section 8.a, it seems appropriate to check the evolution of the disease over time. So, scheduling for follow-up can be recommended, and you are advised to check with your family doctor/GP or the team taking care of your management how, with which tests and at what time interval your disease should be monitored.
j. How can I as a patient monitor whether my treatment is working?
There are two ways of monitoring the success of a treatment.
1st: your doctor will be checking regularly via blood tests, ultrasound and/or elastography (e.g. Fibroscan®) to monitor the success of treatment (see Section d, c). In this case you get an idea of a change of the liver enzymes and the fibrosis level. The exact meaning of a change in these tests in relation to the true severity of the disease is not unequivocally established. So, even if tests improve and you could logically think this means that the liver improves, this has not been scientifically proven and hence needs to be discussed with your doctor. In this regard, it is important to have a global look at different tests and not to rely on a single test to draw conclusions.
2nd: You can monitor the success of a treatment by yourself. Of course, you cannot do blood tests at home but there are many ways to observe the effect of the treatment. The simplest way is to regularly monitor your weight and your waist circumference. You can note both in a diary and observe changes. Based on this data you can also use the body mass calculator to work out your BMI (Fig. 13). Another way is to observe your symptoms. If you have symptoms like fatigue or a general discomfort you can write them down in a diary. Additionally, you can monitor improvement of your fitness level. For example, you can write in your diary how easy it is for you to go upstairs or to walk a specific distance. If you have decided to go swimming regularly, write down how many lengths you managed and how you felt afterwards.
Fig. 13.
Illustrates how you can measure your waist circumference.
It also shows you the weight classes based on BMI. You can easily calculate your BMI by dividing your weight (expressed in kg) by your height (expressed in cm) squared or you can use online calculators. BMI, body mass index.
9. Do I only need my doctor? What about a multidisciplinary team (including heart/liver/diabetes/obesity experts, dieticians/nutritionists, physiotherapists/exercise specialists, psychologists, GPs) to guide my treatment?
Around 80% of all patients with NAFLD/NASH also suffer from a metabolic disease such as obesity, T2D or CVD – all are severe diseases. Therefore, it is recommended that a multidisciplinary team forms a “NAFLD/NASH-board” to discuss the best treatment option(s) for your NAFLD/NASH and your other health conditions. Treating one condition in isolation could potentially aggravate the others. If all disciplines work closely together, you will have a more holistic treatment package. This can help you to adhere to the recommendations. Also, you do not always have to visit different healthcare professionals or report what the other doctors have advised.
The family doctor/GP should be included in the multidisciplinary team. They are important from the outset, identifying the different problems, and helping to monitor disease progression. The family doctor/GP performs a pivotal role as the “navigator” throughout your NAFLD/NASH treatment.
It is also important to consider adding a psychologist into the multidisciplinary team. Up to 20% of all patients with NASH have depression, which can be severe. This should not be managed in isolation but in line with the NASH treatment. This approach should avoid unsuccessful treatment of one problem because another problem is insufficiently treated, or because interventions on one problem do not take into account what is needed for the other. Potential side effects of drugs used to treat depression is just one of the aspects that need to be carefully looked at. Lifestyle interventions can have marked beneficial impacts on mental health. Considering psychological and psychiatric problems is crucial to achieve long-term behaviour change. Ideally, a combined approach should result in a greater and sustained improvement of both conditions.
10. Self-management: Looking after yourself when you have NAFLD
When you have NAFLD, the goal is to manage your symptoms, and to prevent your health from getting worse. Although NAFLD progresses very slowly, it is important to eat healthily and increase your physical activity/exercise levels (see Section 6). This will help to improve the health of your liver and lower your risk of worsening health.
As a person with NAFLD, you are at risk of several serious conditions including metabolic syndrome, T2D and CVD (see Section 1.c). It is important to work with your family doctor to check and manage your weight, blood glucose, blood pressure and cholesterol to reduce your risk of heart disease and T2D (see Section 6, 9).
You may also be referred to an endocrinologist/diabetologist, vascular doctor and/or cardiologist. This multidisciplinary team may also have a dietician, a physiotherapist and/or a psychologist (see Section 9). All are important for getting the NAFLD under control.
a. Improve your overall health
With a proper diet and physical activity plan, you will maximise your chances of improving your health and decrease the likelihood of missing signs of new diseases or complications (see Section 6 and Fig. 8, Fig. 9).
If you already have other health conditions, you should attend all your medical appointments and look after yourself in between appointments.
Once you are diagnosed and begin to improve your health, you can expect regular follow-up appointments to monitor your NAFLD.
b. Diagnosing NAFLD
Current health
Your doctor will perform various tests to predict how your NAFLD will develop and should adapt your treatment accordingly (see Section 5). It is impossible to guarantee that any prediction will be completely accurate, but it will be based on scientific evidence.13,14,84,85
The test results can help your doctor determine 2 main factors:
-
•
The stage your condition has reached
-
•
Improvements to your condition as a result of lifestyle changes you have made (such as liver tests in your blood) which may mean your condition is more likely to progress more slowly, with mild symptoms (see Section 5, c).
These tests can also help doctors identify patients who may be suitable to take part in clinical trials to better understand NAFLD and find potential treatments.58
You will also have a physical examination and discuss your medical history.
Assessing the stage of your condition
Your doctor will first assess the stage of your condition. At your follow-up appointments, your doctor will look for any changes and make a personalised follow-up plan according to your disease stage and the severity of your symptoms.
Tests (see Section 5 and Fig. 8, Fig. 9)
Your doctor should order some or all of the following tests, depending on the severity of your condition:
-
•
Blood tests: these should include alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl transferase, bilirubin, albumin, and platelet count. This provides your doctor with information about how your liver is functioning and signs of damage.
-
•
Ultrasound of the liver, which helps to assess structural abnormalities of the liver and is quite sensitive in detecting liver fat.
-
•
Elastography: this will provide a liver stiffness measurement, which is a way of estimating the amount of scarring in your liver.
-
•
Liver biopsy: a small sample of your liver tissue is taken. It is checked under a microscope and provides information about damage in the cells of your liver.
c. After the diagnosis
There are currently no medications that have been specifically developed and that are approved to treat NAFLD (see Section 7).250 Losing weight and doing more physical activity are critical to manage NAFLD (see Section 6.d).
Members of the multidisciplinary healthcare team such as dieticians and exercise experts will advise you on how to lose weight and do more physical activity, as this needs coaching and guidance from a variety of experts in your hospital or local area. Some hospitals offer special programmes and peer support groups.
Contact your doctor, nurse or another member of your multidisciplinary healthcare team if you lose motivation or need extra help. They may be able to signpost you to appropriate exercise schemes, community gyms, walking groups or weight management programmes in your local area. They may also be able to advise on relevant online/digital resources to support you in making changes to your diet and/or physical activity patterns. Patient organisations may also be able to provide local information and resources about managing your weight, diet and other medical conditions.
For more information about healthy eating, see section 6.d and Fig. 9, Fig. 10 .
For more information about physical activity, see section 6.d .
You and your doctor and other members of your multidisciplinary healthcare team should make decisions together and discuss the benefits and risks of all your tests, procedures and treatments.
11. Where can additional information be found?
Patient advocacy groups and patient self-help groups are excellent contact points. All of them have very well-educated consultants and those groups are available in many European countries. Most groups have a helpline which you can use without the time limitations you normally have at your doctor's appointment. You may also be able to call anonymously or contact them via e-mail. These groups can provide easy to understand information and have websites with specific explanations. Most groups also have a list of specialists so you can identify one in your own town or nearby. Also, in special cases, they have good information on NAFLD/NASH-related specialists.
A list of patient self-help groups and patient advocacy groups can be found in the appendix.
If you are more interested in medical information and you do not have a problem reading scientific medical material, then you can have a look at the websites of the national professional societies or on the webpage of regional umbrella organizations such as EASL (www.easl.eu). Unfortunately, all the material is in English only.
12. Particular situations and patient groups
a. Patients with NAFLD/NASH and psychological disorders
As already described, you as a patient have an active role in your treatment. Lifestyle changes, such as more exercise, a balanced diet and a reduction of body weight are part of therapy for NAFLD and NASH. You may be aware of many things that are perhaps less good for you, but years of everyday habits or trained behaviour are not always easy to change. If there is an additional illness that has a greater influence on the experience and the inner drive, then it may only be possible to tackle changes in a targeted manner with professional support. This is especially true for patients with depression and mental disorders. To date, this point has not yet been systematically recorded and considered in the assessment of patients diagnosed with fatty liver disease. Depression is a disease in its own right, but initial studies show that up to 25% of patients with NAFLD or NASH are affected by some level of depression and/or an eating disorder.282,283 It is important for you as a patient to know that you should speak openly with the doctors who are concerned with fatty liver disease if you know or suspect that you are suffering from a mental illness or even a disturbance of your eating habits and you need support to implement medical recommendations. At the same time, it is also important to look at possible causes of depression or psychological impairment so that you can be helped in a targeted manner. Here is just one example. Fatty liver disease can bring along a symptom of fatigue (extreme tiredness, exhaustion).66 Those who constantly feel tired and exhausted may withdraw more from social life, blame themselves, fail to cope with the demands of everyday life and develop bad thoughts and feelings. Depression is then a possible consequence of fatty liver disease. On the other hand, depression may have existed for many years.283 Eating can be a means to compensate for negative feelings, frustration, or fears. Body weight can increase and at some point, fatty liver disease results. There are certainly other ways of describing how the interaction and increase in weight, fatty liver disease and psychological factors and disease patterns are related. It is important for you to be as open with the topic as possible, to ask for support and to approach changes step by step.
b. Pregnancy in patients with NAFLD/NASH
If you have fatty liver disease and plan to become pregnant, you should discuss this with your gynaecologist and inform them that you have NAFLD or NASH.284 In the U.S., a study was conducted from 2012 to 2016 on a total of 18.5 million pregnant women. The aim was to find out how high certain pregnancy risks are in comparison groups.285 In the evaluation, a distinction was made between 3 groups of pregnant women. The first group consisted of women without liver disease. The second group had chronic liver disease (n = 115,210). The third group were women with NAFLD (n = 5,640). It was shown that pregnant women with NAFLD have a higher risk of developing pregnancy-associated (gestational) diabetes compared to the control group (23 in 100 compared to 7-8 in 100). It was also found that pregnant women with NAFLD are also more frequently affected by severe pregnancy-induced increases in blood pressure (16 out of 100 compared to 4 out of 100 if there was no liver disease), of which the HELLP syndrome is the most severe complication.
HELLP stands for haemolysis-elevated liver tests-low platelet count. This rare syndrome is a life-threatening condition during pregnancy, the course of which is difficult to predict. This leads to a dissolution of the red blood cells, increased liver enzymes and a drop in red blood platelets. HELLP syndrome can result in immediate termination of pregnancy.
Your gynaecologist should contact the doctor treating your fatty liver disease and make an assessment of your possible individual risks of pregnancy. You should be informed in detail. In addition, physicians should support you in reducing metabolic risk factors, such as the existence of fatty liver, during a planned pregnancy.
c. NAFLD/NASH in children
Various studies show that more and more children are severely overweight and that fatty liver can develop in childhood.40,277,286,287 An unhealthy lifestyle, consisting of too little exercise and a high level of sugary and fatty foods in the daily diet also promotes the development of fatty liver in children. Even though it is recommended that children, just like adults, change their physical activity and nutritional behaviour, there are nevertheless separate medical and therapeutic recommendations and measures that consider the specifics of children's metabolic and physical development.277 Therefore, the statements and recommendations in this guideline only refer to adult patients with fatty liver disease.
d. NAFLD/NASH in diabetes
Patients with fatty liver disease often have other metabolic disorders, particularly pre-diabetes or T2D.4,288,289 If you as a patient support the treatment of fatty liver disease through weight reduction, more exercise and a balanced diet, then you are also actively doing something against diabetes.289 It should be noted that a change in eating habits and regular physical activity can influence the dosage of medications you take or inject to regulate your blood sugar levels (e.g. insulin). Therefore, patients with T2D should discuss their plans and changes in exercise and eating habits with their diabetologist.
13. Questions for your doctor
It is a good idea to prepare yourself for your appointment with your doctor to ensure you get the most out of it. Take a look at the following list of questions to help you identify the most important questions you want to ask:
-
•
What is NAFLD and why have I got it?
-
•
How severe is my liver disease?
-
•
What are the complications associated with NAFLD?
-
•
What investigations will be done and why?
-
•
What treatment is available?
-
•
What can I do to help myself?
-
•
How do I know if my NAFLD is getting better/worse? Is there anything I can monitor at home?
-
•
How often will I be seen in the clinic?
-
•
Can I attend by myself or can I bring a companion?
-
•Questions about the tests:
-
○What is the purpose of the test? What will happen if I get a positive result?
-
○How long will the test take?
-
○Will the test expose me to radiation?
-
○Do I need to fast before the test or procedure?
-
○When will I get the results?
-
○Who will give the results to me?
-
○May I have a copy of the test results for my records?
-
○How often will this test or procedure be repeated?
-
○Will health insurance cover the cost of this test, and if so, how frequently?
-
○
14. Questions for your multidisciplinary team (dieticians, exercise specialist, physician) for personalised information on lifestyle change:
-
•
How much weight should I aim to lose?
-
•
Which anti-obesity medication could help me to change my lifestyle and to reduce liver fat?
-
•
What are the foods that increase liver fat?
-
•
What are the foods that reduce liver fat?
-
•
Are there recommended times for my meals?
-
•
What should I expect to see in terms of liver disease improvement if I maintain changes to my diet?
-
•
When could I expect to see liver disease improvement?
-
•
What about alcohol?
-
•
Which of my dietary habits could I change?
-
•
What are the things I need to check in the food labelling in the supermarket?
-
•
Should I take food supplements? Are there supplements I should avoid?
-
•
Is there any limit to the use of spices, vinegar, lemon juice etc. to flavour my food?
-
•
Does smoking lead to liver damage?
-
•
What can I do if I can't lose weight?
-
•
What type of physical activity or exercise is recommended for me?
-
•
How long should I exercise for and how many times per week?
-
•
How hard should I push myself?
-
•
How can I exercise without any risk for my heart or without aggravating my joints, for example?
-
•
How can I engage my family to support me in making these changes?
15. Glossary
Abdominal fat: this is fatty tissue that mainly accumulates in the abdominal region, so on the belly. It can be under the skin, or really inside the abdominal cavity, where it is called intra-abdominal or visceral fat. If the fat accumulation in your body is predominantly at the level of the belly, then this fat distribution pattern is called apple-type.
Adipose tissue or fat tissue: the fat tissue is the tissue that has the function to store energy reserves in the form of lipid molecules or fat. It is hence mainly an energy reserve, but it has other functions, including in the production of certain hormones.
Advanced fibrosis: means a fibrosis stage of at least F3 on the liver biopsy, when you use a staging system with levels ranging from F0 to F4.
Aerobic exercise: light endurance training in which the intensity is chosen so that conversation is still possible, and the effort is classified as easy to medium. For example, cycling, walking, group dancing or swimming are suitable as training.
Alanine aminotransferase or ALT: an enzyme that is produced in the liver cells. ALT is a marker of damage to the liver cells.
Albumin: the main component of blood proteins formed by the liver. With poor liver function, the albumin in the blood decreases. As a consequence, less water is bound in the blood vessels and water leaks into the tissue (oedema, ascites).
Alkaline phosphatase or ALP: one of several biochemical markers in the body that can be used to diagnose primary biliary cholangitis and track the disease. Part of the liver panel blood test that examines the health of the liver as a whole. ALP is manufactured in the liver, bones and other tissues and measured during a routine blood test. Abnormally high concentrations may indicate liver disease or bile duct congestion.
Antioxidants: substances that offer protection against so-called "free radicals". On the one hand, these free radicals are formed by the body itself during various metabolic processes; on the other hand, they are caused by harmful external influences such as cigarette smoke, environmental toxins or ultraviolet radiation from the sun. For example, vegetables are rich in antioxidants.
Apple-shape obesity: the body fat has accumulated predominantly in the abdominal region. The limbs are relatively slender.
Alcoholic fatty liver disease or alcoholic steatohepatitis (ASH): Severe liver disease caused by alcohol consumption.
“Bad” cholesterol: this is old terminology. It refers to the LDL cholesterol (low-density lipoprotein). High LDL cholesterol is one of the causes of atherosclerosis. It is, however, more the imbalance between this type of cholesterol and other types of cholesterol that is considered “good” or “bad”, because LDL cholesterol also has a normal, healthy role to play.
Bariatric surgery: is a branch of surgery that deals with surgical interventions that are intended to lead to a reduction in body weight.
Bile acid: a yellow-green liquid produced by the liver, stored in the gallbladder and transported through the bile ducts. Gall helps with the digestion of fats.
Bilirubin: the yellow degradation product of normal haem catabolism produced by the body's own cleansing of old red blood cells containing haemoglobin. Bilirubin is excreted via bile and urine. Increased values may indicate certain diseases.
Biochemistry: the connection between chemical processes in living things, metabolism, chemistry, biology and medicine.
Biopsy: removal of tissues or cells from the body organs (e.g. liver) for microscopic examination.
Blood pressure: the force that the blood exerts when it passes through to the vessels. The term is generally used to indicate blood pressure in the arteries (arterial blood pressure).
Body metabolism: a complex process in the body, that has to do with the handling of energy. It refers to the handling of carbohydrates, lipids and proteins. The metabolism has the task of keeping the body in balance and providing it with the energy needed for its numerous functions.
Capsule: in the case of the liver, it is the outer shell of the liver. The capsule encloses the liver and thus distinguishes it from surrounding organs and structures.
Carcinoma: malignant tissue degeneration, tumour.
Chemotherapy: treatment for cancer. Chemotherapy is a systemic procedure that affects the whole body.
Cirrhosis: advanced scarring of the liver that happens when the liver is damaged.
Cholesterol: one of the fat molecules in the body. It is made up of several types of cholesterol, of which LDL- and HDL-cholesterol are the most abundant. All types have their own function and importance, but they must be in balance with each other in terms of quantity: not too much LDL and enough HDL in the blood. This is supported by a healthy and balanced diet and exercise.
Clinical trials: medical study. It tests different aspects of the use of a drug or device, such as how well it works or if it is safe. A clinical trial is carried out with patients or healthy volunteers and is a prerequisite for drug approval by the authorities.
Comorbidities: additional diseases to the underlying disease. It does not have to be related to the underlying disease, but this is often the case in practice.
Contraindication: these are medical reasons or health factors in the patient that could make an operation or therapy high risk.
Coronary artery disease: the large arteries that supply the heart with oxygen are narrowed. These vessels are called "coronary arteries" or "coronary vessels".
Computed tomography or CT: is an X-ray technique in which sectional images of the body are made for diagnostic purposes.
Cardiovascular disease or CVD: diseases of the heart and the blood vessels, including the vessels in the limbs or the vessels of the brain.
Depression: is a serious condition that affects the thinking, feeling and acting of the person concerned.
Degree of liver fibrosis: severity of the scar tissue. Severity means both amount as well as the way the scar tissue is distributed in between the liver cells and structures. Usually divided into five levels, namely F0-F4. F0 means no fibrosis, F4 equals cirrhosis. There are, however, other systems with more levels.
Disciplines: different specialties in medicine (cardiologist, hepatologist, etc.).
Eating disorder: an unhealthy eating behaviour associated with mental and physical illness (e.g. binge eating disorder, characterised by eating large amounts of foods in a short time, without any possibility of restraint and accompanied by a sense of guilt).
Energy substrates: the molecules that can be used to generate energy for all the processes going on in the body.
Elastometry: a method to measure the stiffness of an organ. In case of the liver, the stiffness of the liver correlates to some extent with the degree of liver fibrosis in patients.
Fibroscan: the specific designation of a device for measuring liver stiffness.
Fibrosis: fibrosis in the liver is the scarring laid down following damage to the liver cells.
Fatigue: extreme tiredness that cannot be remedied by getting enough sleep. Feeling of exhaustion even with everyday tasks.
Gall: a yellow-green liquid produced by the liver, stored in the gallbladder and transported through the bile ducts. Gall helps with the digestion of fats.
Gallbladder: small organ, located at the inferior surface of the liver that collects the gall or bile produced by the liver. When you eat, the gallbladder will contract and empty its contents into the gut, where the bile helps to digest your meal, particularly the fats in the meal.
“Good” cholesterol: this is old terminology. It refers to the HDL cholesterol (high-density lipoprotein). It is, however, more the imbalance between the different types of cholesterol that is considered “good” or “bad”.
General practitioner: another word for family doctor. They are part of the first-line healthcare system; they take care of everyday illnesses, coordinate referral to specialised care and are often the first contact person for health complaints.
Guideline: doctors' treatment and care directive, most often issued by scientific societies.
HCC: abbreviation that stands for hepatocellular carcinoma = primary liver cell cancer, so a cancer that finds its origin in the liver itself.
Heart failure: the heart is no longer able to pump the required amount of blood through the body and supply the organs with sufficient oxygen and energy.
Hepatitis B: acute or chronic liver inflammation caused by the hepatitis B virus. Vaccination against hepatitis B can be carried out. A cure is currently not possible, but there are good treatment options to get the virus under control.
Hepatitis C: acute and then often persisting, chronic liver inflammation caused by infection with the hepatitis C virus. There is no vaccine against hepatitis C. Drug therapy is possible and offers very good chances of healing.
Hepatocellular: concerning liver cells.
Hepatologist: specialist doctor (usually gastroenterologist or specialist in internal medicine) who specialises in liver disease.
High blood pressure: when blood passes through the blood vessels (arteries), pushed forward by the heart, it exerts a force on the walls of the blood vessels, creating pressure. Blood pressure goes up and down with the beating of the heart. High arterial blood pressure means that the peak pressure (systolic pressure) goes higher than 129 mm Hg and/or the lower pressure (diastolic pressure) is higher than 79 mmHg.
Imaging techniques: include ultrasound (sonography), computed tomography (CT) and magnetic resonance imaging (MRI).
Inflammation: this is a body's own reaction to stimuli of danger and/or damage. Signs of inflammation include redness, swelling, overheating, pain and functional limitations.
Interactions: if different medications are taken, care must be taken to ensure that the active substances contained therein are compatible with each other and that the respective effect is not intensified or eliminated.
Intra-abdominal fat: this refers to the fat stored in the abdominal cavity. This fatty tissue is particularly metabolically active. It continuously releases fats into the blood. If there is too much of this fat, so if you have internal obesity (also called abdominal or visceral obesity), you are at increased risk of a number of diseases such as non-alcoholic fatty liver disease, type 2 diabetes and cardiovascular diseases.
Jaundice: yellowing of the eyes and skin, which can be caused by liver damage.
Lipoprotein: a structure composed of proteins and lipid (i.e. fat) molecules that take care of transporting fat molecules inside the cells and throughout the body.
Liver: the largest solid organ in the human body. Location: in the upper part of the abdomen on the right side. The liver helps the body digest food, store energy and break down toxic substances.
Liver failure: the liver can no longer perform its tasks and shuts down many functions. This can be caused, for example, by severe poisoning or by chronic liver diseases in the final stage of cirrhosis.
Liver function: the variety of metabolic tasks performed by the liver.
Liver stiffness: the liver is usually smooth, but when the liver gets diseased, it can become stiffer. Measuring the smoothness, or the opposite, namely the liver stiffness, is called elastography.
Macrovesicular steatosis: indicates that large fat droplets are present in the hepatocytes.
Magnetic resonance: MR, imaging procedure for the representation of tissues, vessels and organs. The MRI is used for diagnosis but also for research purposes. The representations of the images are generated with the help of strong magnetic fields.
Meta-analysis: a study that tries to statistically summarise and present the results of multiple previous research studies.
Metabolic disorder: the complex metabolic system of the body is out of balance due to a disorder, illness or malfunction.
Metabolic syndrome: metabolic syndrome is the medical term for a combination of conditions including diabetes, high blood pressure (hypertension) and obesity.
Metabolism: Refers to the complex interaction of organs, hormones, bodily fluids and tissues in the body that are important for it to be fully functional at all levels.
Non-alcoholic fatty liver or NAFL: isolated fatty liver without inflammation. Non-alcoholic fatty liver disease or NAFLD: is a fatty liver disease that usually has a metabolic cause (it is not caused by excess alcohol consumption). It covers the spectrum of NAFL to NASH. Non-alcoholic steatohepatitis or NASH: inflammatory change of an existing fatty liver, which is not caused by excess alcohol consumption.
Non-invasive tests or NITs: a test that does not require an invasive procedure like a liver biopsy or an endoscopy. Examples of this are ultrasound or blood sampling.
Obesity: excess body weight above a certain threshold due to the accumulation of fat. Obesity can be the cause of fatty liver.
Other liver disease: there are many liver diseases. They include inherited liver disease, viral liver disease, autoimmune liver disease, and liver disease caused by lifestyle, medication, poisoning or other diseases.
Oncologists: specialist in cancer diagnosis and treatment.
Oncology: specialisation in medicine dealing with cancer.
Pathologists: specialists who examine and assess, among other things, tissue samples, often using microscopy.
Physiotherapists: therapeutic specialist who helps people affected by injury, illness or disability through movement and exercise, manual therapy, education and advice.
Platelets: they are the smallest cells of the blood. In fact, they are fragments of cells, not complete cells. They play an important role in blood clotting.
Phenolic compounds: are the largest group of bioactive plant substances and have positive properties for health.
Placebo: a drug does not contain an active ingredient. The placebo effect can occur if an effective drug is assumed and the patient feels an effect even though it is a placebo. Placebos are also used in clinical studies.
Progressive: increase in scope and severity.
Proteins: universal building material of the body. Proteins from the diet are broken down in the body and converted into the body's own protein.
Pruritus: clinical term for severe itching, a common symptom of liver diseases.
Psychosocial: relationship between psychological and social factors, for example, mental problems, such as depression, can mean withdrawal from social activities.
Recurrence: the return of a disease.
Resistance exercise: training single or multiple muscle groups using weights, body weight or machines. Exercising with exercise bands is also an example of this. Professional guidance is recommended.
Sarcopenia: loss of muscle cell mass and/or muscle strength.
Self-efficacy: is the conviction of a person that they can successfully cope with difficult situations and challenges on their own.
Significant fibrosis: means a fibrosis stage of at least F2 on the 0-4 scale.
Spleen: is an organ that lies in the abdominal cavity, left to and near the stomach. It has a defensive function against foreign substances, stores the white blood cells and recycles old red blood cells.
Steatosis: refers to the presence of fat in liver cells.
Steatohepatitis: refers to the damage of liver cells and the inflammation in the liver associated with the presence of fat.
Subcutaneous fat: means fat stored below the skin. Its main function is to store excess calories as fat. It is usually regarded as healthy or good fat tissue.
Surrogate endpoints: in clinical studies, a surrogate endpoint (or surrogate marker) is a measure of effect of a specific treatment that is predictive of the fact that the patient will get better in the long run.
Symptom: side effect in case of a disease, for example the possible itching in a liver disease.
Transplantation: use of a donor organ.
Ultrasound: diagnostic process that uses sound waves. Sound waves are directed toward the relevant tissue; the reflected sound is then used to create an image for analysis.
Wilson’s disease: or Copper storage disease. In this disease, the body stores too much copper in organs such as the liver, tissues and the central nervous system, which can lead to various health disorders and damage. The disease is not curable but can be treated with medication.
Financial support
No funding was received for the production of this manuscript.
Conflict of interest
SMF has a senior clinical research mandate from the Fund for Scientific Research (FWO) Flanders (1802154 N) and has acted as advisor and/or lecturer for Roche, Gilead, Abbvie, Bayer, BMS, MSD, Janssen, Actelion, Astellas, Genfit, Inventiva, Intercept, Genentech, Galmed, Promethera, Coherus, NGM Bio and Julius Clinical. GM served as Advisory Board member for Sanofi, Novartis, Astra-Zeneca, Intercept and Pfizer, and participated in clinical trials by Sanofi, Eli Lilly, Gilead, GENFIT and Intercept. AK has received project grants from Novartis and Intercept. MW has nothing to disclose in relation to this publication. RD has received project grants from Novartis and Intercept. JVL received research grants from Gilead Sciences and MSD, and speaker fees from Genfit, Gilead Sciences, MSD, Intercept Pharmaceuticals and Janssen, outside of this work. SZS has nothing to disclose in relation to this publication. KH has nothing to disclose in relation to this publication. LB serves as an Advisory Board member for Novo Nordisk. GF has nothing to disclose in relation to this publication. DD served as Advisory Board member and lecture for Novo Nordisk, Sanofi, BI, Astra-Zeneca, and participated in clinical trials by, Novo Nordisk, Eli Lilly, Sanofi and BI. EW has nothing to disclose in relation to this publication. MK has nothing to disclose in relation to this publication. JW has nothing to disclose in relation to this publication. GK has nothing to disclose in relation to this publication. SV has nothing to disclose in relation to this publication. MU has nothing to disclose in relation to this publication. JM has nothing to disclose in relation to this publication. CL has nothing to disclose in relation to this publication.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
The authors would like to thank the patients from ECPO (European Coalition for People Living with Obesity), the patients from Deutsche Leberhilfe e.V. (German Liver Help Association), Ingo van Thiel (scientific editor of Deutsche Leberhilfe e.V), Ivan Gardini and Marco Bartoli (EpaC, Italian Liver Patients Association) and the Israelien patients who reviewed the document and provided their precious comments, as well as the Global Liver Institute.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2021.100322.
Appendix I.
List of Patient Advocacy Groups.
The following umbrella organizations represents a lot of local and national Patient Advocacy Groups. You can find details of the members on their respective webpages.
ELPA – European Liver Patient Association www.elpa.eu and contact@elpa.eu. Rue de la Loi 235/27 1040 Brussels Belgium.
LPI – Liver Patients International www.liverpatientsinternational.org and info@liverpatients.international. Dreve du Pressoir 38 1190 Brussels Belgium.
ECPO – European Coalition for People living with Obesity www.eurobesity.org and contactus@eurobesity.org, 101 Furry Park Road, Howth Road, Dublin, Ireland D05 KD52.
GLI – Global Liver Institute https://www.globalliver.org and info@globalliver.org, Washington DC, USA.
Supplementary data
The following is the supplementary data to this article:
References
- 1.Yeh M.M., Brunt E.M. Pathological features of fatty liver disease. Gastroenterology. 2014;147:754–764. doi: 10.1053/j.gastro.2014.07.056. [DOI] [PubMed] [Google Scholar]
- 2.Kwanten W.J. Role of autophagy in the pathophysiology of nonalcoholic fatty liver disease: a controversial issue. World J Gastroenterol. 2014;20:7325. doi: 10.3748/wjg.v20.i23.7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gastaldelli A., Cusi K. From NASH to diabetes and from diabetes to NASH: mechanisms and treatment options. JHEP Reports : Innovation Hepatol. 2019;1:312–328. doi: 10.1016/j.jhepr.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotronen A., Westerbacka J., Bergholm R., Pietiläinen K.H., Yki-Järvinen H. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92:3490–3497. doi: 10.1210/jc.2007-0482. [DOI] [PubMed] [Google Scholar]
- 5.Mazzotti A., Caletti M.T., Sasdelli A.S., Brodosi L., Marchesini G. Pathophysiology of nonalcoholic fatty liver disease: lifestyle-gut-gene interaction. Dig Dis (Basel, Switzerland) 2016;34(Suppl 1):3–10. doi: 10.1159/000447275. [DOI] [PubMed] [Google Scholar]
- 6.Younossi Z.M., Marchesini G., Pinto-Cortez H., Petta S. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: implications for liver transplantation. Transplantation. 2019;103:22–27. doi: 10.1097/TP.0000000000002484. [DOI] [PubMed] [Google Scholar]
- 7.Malhotra P., Gill R.K., Saksena S., Alrefai W.A. Disturbances in cholesterol homeostasis and non-alcoholic fatty liver diseases. Front Med. 2020;7:467. doi: 10.3389/fmed.2020.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . 1999. Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1: diagnosis and classification of diabetes mellitus. Geneva. [Google Scholar]
- 9.Expert Panel on Detection, Evaluation and T of HBC in A Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 10.Alberti K.G.M., Zimmet P., Shaw J. The metabolic syndrome—a new worldwide definition. The Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 11.Alberti K.G.M.M., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood Institute; American heart association; world heart federation; international. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 12.Thursz M., Gual A., Lackner C., Mathurin P., Moreno C., Spahr L. EASL clinical practice guidelines: management of alcohol-related liver disease. J Hepatol. 2018;69:154–181. doi: 10.1016/j.jhep.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Marchesini G., Day C.P., Dufour J.F., Canbay A., Nobili V., Ratziu V. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 15.Ayonrinde Oyekoya Taiwo. Historical narrative from fatty liver in the nineteenth century to contemporary NAFLD - reconciling the present with the past. JHEP Rep. 2021 doi: 10.1016/j.jhepr.2021.100261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eslam M., Newsome P.N., Sarin S.K., Anstee Q.M., Targher G., Romero-Gomez M. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 17.Ratziu V., Rinella M., Beuers U., Loomba R., Anstee Q.M., Harrison S. The times they are a-changin’ (for NAFLD as well) J Hepatol. 2020;73:1307–1309. doi: 10.1016/j.jhep.2020.08.028. [DOI] [PubMed] [Google Scholar]
- 18.Bedossa P., Poitou C., Veyrie N., Bouillot J.L., Basdevant A., Paradis V. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56:1751–1759. doi: 10.1002/hep.25889. [DOI] [PubMed] [Google Scholar]
- 19.Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 20.Bedossa P., Burt A.A., Gouw A.H.A., Lackner C., Schirmacher P., Terracciano L. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology. 2014;60:565–575. doi: 10.1002/hep.27173. [DOI] [PubMed] [Google Scholar]
- 21.Angeli P., Bernardi M., Villanueva C., Francoz C., Mookerjee R.P., Trebicka J. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 22.Schuppan D., Surabattula R., Wang X.Y. Determinants of fibrosis progression and regression in NASH. J Hepatol. 2018;68:238–250. doi: 10.1016/j.jhep.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Long M.T., Massaro J.M., Hoffmann U., Benjamin E.J., Naimi T.S. Alcohol use is associated with hepatic steatosis among persons with presumed nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2020;18 doi: 10.1016/j.cgh.2019.11.022. 1831-1841.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hagström H. Alcohol consumption in concomitant liver disease: how much is too much? Curr Hepatol Rep. 2017;16:152–157. doi: 10.1007/s11901-017-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardy T., Wonders K., Younes R., Aithal G.P., Aller R., Allison M. The European NAFLD Registry: a real-world longitudinal cohort study of nonalcoholic fatty liver disease. Contemp Clin Trials. 2020;98:106175. doi: 10.1016/j.cct.2020.106175. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y.-L., Reeves H.L., Burt A.D., Tiniakos D., McPherson S., Leathart J.B.S. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat Commun. 2014;5:4309. doi: 10.1038/ncomms5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anstee Q.M., Darlay R., Cockell S., Meroni M., Govaere O., Tiniakos D. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically characterised cohort☆. J Hepatol. 2020;73:505–515. doi: 10.1016/j.jhep.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Silventoinen K., Konttinen H. Obesity and eating behavior from the perspective of twin and genetic research. Neurosci Biobehavioral Rev. 2020;109:150–165. doi: 10.1016/j.neubiorev.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Lefere S., Tacke F. Macrophages in obesity and non-alcoholic fatty liver disease: crosstalk with metabolism. JHEP Reports : Innovation Hepatol. 2019;1:30–43. doi: 10.1016/j.jhepr.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zegers D., Verrijken A., Beckers S., Francque S., Van Camp J.K., Aerts E. Association study of PNPLA2 gene with histological parameters of NAFLD in an obese population. Clin Res Hepatol Gastroenterol. 2016;40:333–339. doi: 10.1016/j.clinre.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Anstee Q.M., Seth D., Day C.P. Genetic factors that affect risk of alcoholic and nonalcoholic fatty liver disease. Gastroenterology. 2017;150 doi: 10.1053/j.gastro.2016.01.037. 1728-1744.e7. [DOI] [PubMed] [Google Scholar]
- 32.Albhaisi S., Chowdhury A., Sanyal A.J. Non-alcoholic fatty liver disease in lean individuals. JHEP Reports : Innovation Hepatol. 2019;1:329–341. doi: 10.1016/j.jhepr.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blomdahl J., Nasr P., Ekstedt M., Kechagias S. Moderate alcohol consumption is associated with advanced fibrosis in non-alcoholic fatty liver disease and shows a synergistic effect with type 2 diabetes mellitus. Metab Clin Exp. 2021:115. doi: 10.1016/j.metabol.2020.154439. [DOI] [PubMed] [Google Scholar]
- 34.Younossi Z.M., Stepanova M., Ong J., Yilmaz Y., Duseja A., Eguchi Y. Effects of alcohol consumption and metabolic syndrome on mortality in patients with nonalcoholic and alcohol-related fatty liver disease. Clin Gastroenterol Hepatol. 2019;17 doi: 10.1016/j.cgh.2018.11.033. 1625-1633.e1. [DOI] [PubMed] [Google Scholar]
- 35.Hajifathalian K., Torabi Sagvand B., McCullough A.J. Effect of alcohol consumption on survival in nonalcoholic fatty liver disease: a national prospective cohort study. Hepatology (Baltimore, Md) 2019;70:511–521. doi: 10.1002/hep.30226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loomba R., Yang H.I., Su J., Brenner D., Barrett-Connor E., Iloeje U. Synergism between obesity and alcohol in increasing the risk of hepatocellular carcinoma: a prospective cohort study. Am J Epidemiol. 2013;177:333–342. doi: 10.1093/aje/kws252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haas J.T., Francque S., Staels B. Pathophysiology and mechanisms of nonalcoholic fatty liver disease. Annu Rev Physiol. 2016;78:181–205. doi: 10.1146/annurev-physiol-021115-105331. [DOI] [PubMed] [Google Scholar]
- 38.Rinella M., Charlton M. The globalization of nonalcoholic fatty liver disease: prevalence and impact on world health. Hepatology. 2016;64:19–22. doi: 10.1002/hep.28524. [DOI] [PubMed] [Google Scholar]
- 39.Huang D.Q., El-Serag H.B., Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2020 doi: 10.1038/s41575-020-00381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 41.Paradis V., Zalinski S., Chelbi E., Guedj N., Degos F., Vilgrain V. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49:851–859. doi: 10.1002/hep.22734. [DOI] [PubMed] [Google Scholar]
- 42.Mittal S., El-Serag H.B., Sada Y.H., Kanwal F., Duan Z., Temple S. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2017;14 doi: 10.1016/j.cgh.2015.07.019. 124-131.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanwal F., Kramer J.R., Mapakshi S., Natarajan Y., Chayanupatkul M., Richardson P.A. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155 doi: 10.1053/j.gastro.2018.08.024. 1828-1837.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh S., Allen A.M., Wang Z., Prokop L.J., Murad M.H., Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–654. doi: 10.1016/j.cgh.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanyal A.J., Harrison S.A., Ratziu V., Abdelmalek M.F., Diehl A.M., Caldwell S. The natural history of advanced fibrosis due to nonalcoholic steatohepatitis: data from the simtuzumab trials. Hepatology (Baltimore, Md) 2019;70:1913–1927. doi: 10.1002/hep.30664. [DOI] [PubMed] [Google Scholar]
- 46.Loomba R., Adams L.A. The 20% rule of NASH progression: the natural history of advanced fibrosis and cirrhosis caused by NASH. Hepatology (Baltimore, Md) 2019;70:1885–1888. doi: 10.1002/hep.30946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friedman S.L., Neuschwander-Tetri B.A., Rinella M., Sanyal A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eslam M., Valenti L., Romeo S. Genetics and epigenetics of NAFLD and NASH: clinical impact. J Hepatol. 2018;68:268–279. doi: 10.1016/j.jhep.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2017;2:901–910. doi: 10.1016/S2213-8587(14)70032-4. [DOI] [PubMed] [Google Scholar]
- 50.Francque S.M., van der Graaff D., Kwanten W.J. Non-alcoholic fatty liver disease and cardiovascular risk: pathophysiological mechanisms and implications. J Hepatol. 2016;65:425–443. doi: 10.1016/j.jhep.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Mantovani A., Petracca G., Beatrice G., Csermely A., Tilg H., Byrne C.D. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: a meta-analysis of observational cohort studies. Gut. 2021 doi: 10.1136/gutjnl-2021-324191. gutjnl-2021-324191. [DOI] [PubMed] [Google Scholar]
- 52.Musso G., Gambino R., Tabibian J.H., Ekstedt M., Kechagias S., Hamaguchi M. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PloS Med. 2014;11 doi: 10.1371/journal.pmed.1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Targher G., Francque S.M. A fatty liver leads to decreased kidney function? J Hepatol. 2017;67:1137–1139. doi: 10.1016/j.jhep.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 54.Dulai P.S., Singh S., Patel J., Soni M., Prokop L.J., Younossi Z. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65:1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hagström H., Nasr P., Ekstedt M., Hammar U., Stål P., Hultcrantz R. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67:1265–1273. doi: 10.1016/j.jhep.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 56.Ratziu V., Goodman Z., Sanyal A. Current efforts and trends in the treatment of NASH. J Hepatol. 2015;62:S65–75. doi: 10.1016/j.jhep.2015.02.041. [DOI] [PubMed] [Google Scholar]
- 57.Kleiner D.E., Brunt E.M., Wilson L.A., Behling C., Guy C., Contos M. Association of histologic disease activity with progression of nonalcoholic fatty liver disease. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.12565. e1912565–e1912565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Siddiqui M.S., Harrison S.A., Abdelmalek M.F., Anstee Q.M., Bedossa P., Castera L. Case definitions for inclusion and analysis of endpoints in clinical trials for nonalcoholic steatohepatitis through the lens of regulatory science. Hepatology. 2018;67:2001–2012. doi: 10.1002/hep.29607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Noncirrhotic nonalcoholic steatohepatitis with liver fibrosis: developing drugs for treatment. Guidance for industry. Fed Regist. 2018;83:62582–62583. [Google Scholar]
- 60.European Medicines Agency Committee for Medicinal Products for Human Use . PBC, PSC, NASH; 2018. Reflection paper on regulatory requirements for development of medicinal products for chronic non infectious liver diseases. [Google Scholar]
- 61.Prevention C for DC and. Measuring healthy days . vol. 53. Centers for Disease Control and Prevention; 2000. (Population assessment of health-related quality of life). [Google Scholar]
- 62.Doward L.C., Balp M.M., Twiss J., Slota C., Cryer D., Brass C.A. Development of a patient-reported outcome measure for non-alcoholic steatohepatitis (NASH-CHECK): results of a qualitative study. Patient. 2020 doi: 10.1007/s40271-020-00485-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Younossi Z.M., Stepanova M., Henry L., Racila A., Lam B., Pham H.T. A disease-specific quality of life instrument for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: CLDQ-NAFLD. Liv Int. 2017;37:1209–1218. doi: 10.1111/liv.13391. [DOI] [PubMed] [Google Scholar]
- 64.Dan A.A., Kallman J.B., Wheeler A., Younoszai Z., Collantes R., Bondini S. Health-related quality of life in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2007;26:815–820. doi: 10.1111/j.1365-2036.2007.03426.x. [DOI] [PubMed] [Google Scholar]
- 65.Golabi P., Otgonsuren M., Cable R., Felix S., Koenig A., Sayiner M. Non-alcoholic fatty liver disease (NAFLD) is associated with impairment of health related quality of life (HRQOL) Health Qual Life Outcom. 2016;14:18. doi: 10.1186/s12955-016-0420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Younossi Z.M., Wong V.W., Anstee Q.M., Romero-Gomez M., Trauner M.H., Harrison S.A. Fatigue and pruritus in patients with advanced fibrosis due to nonalcoholic steatohepatitis: the impact on patient-reported outcomes. Hepatol Commun. 2020;4:1637–1650. doi: 10.1002/hep4.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Younossi Z.M., Golabi P., Henry L. A comprehensive review of patient-reported outcomes in patients with chronic liver diseases. J Clin Gastroenterol. 2019;53 doi: 10.1097/MCG.0000000000001179. [DOI] [PubMed] [Google Scholar]
- 68.Rubino F., Puhl R.M., Cummings D.E., Eckel R.H., Ryan D.H., Mechanick J.I. Joint international consensus statement for ending stigma of obesity. Nat Med. 2020;26:485–497. doi: 10.1038/s41591-020-0803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Younossi Z.M., Blissett D., Blissett R., Henry L., Stepanova M., Younossi Y. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 70.O’Hara J., Finnegan A., Dhillon H., Ruiz-Casas L., Pedra G., Franks B. Cost of non-alcoholic steatohepatitis in Europe and the USA: the GAIN study. JHEP Rep. 2020;2 doi: 10.1016/j.jhepr.2020.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Younossi Z.M., Golabi P., de Avila L., Paik J.M., Srishord M., Fukui N. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71:793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 72.Estes C., Anstee Q.M., Arias-Loste M.T., Bantel H., Bellentani S., Caballeria J. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69:896–904. doi: 10.1016/j.jhep.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 73.Younossi Z.M., Stepanova M., Negro F., Hallaji S., Younossi Y., Lam B. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine. 2012;91 doi: 10.1097/MD.0b013e3182779d49. [DOI] [PubMed] [Google Scholar]
- 74.Ye Q., Zou B., Yeo Y.H., Li J., Huang D.Q., Wu Y. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:739–752. doi: 10.1016/S2468-1253(20)30077-7. [DOI] [PubMed] [Google Scholar]
- 75.Younes R., Govaere O., Petta S., Miele L., Tiniakos D., Burt A. Caucasian lean subjects with non-alcoholic fatty liver disease share long-term prognosis of non-lean: time for reappraisal of BMI-driven approach? Gut. 2021 doi: 10.1136/gutjnl-2020-322564. gutjnl-2020-322564. [DOI] [PubMed] [Google Scholar]
- 76.Brunt E.M., Janney C.G., di Bisceglie A.M., Neuschwander-Tetri B.A., Bacon B.R. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 77.McPherson S., Hardy T., Henderson E., Burt A.D., Day C.P., Anstee Q.M. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62:1148–1155. doi: 10.1016/j.jhep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 78.Zhang E., Wartelle-Bladou C., Lepanto L., Lachaine J., Cloutier G., Tang A. Cost-utility analysis of nonalcoholic steatohepatitis screening. Eur Radiol. 2015;25:3282–3294. doi: 10.1007/s00330-015-3731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lazarus J.v., Ekstedt M., Marchesini G., Mullen J., Novak K., Pericàs J.M. A cross-sectional study of the public health response to non-alcoholic fatty liver disease in Europe. J Hepatol. 2019 doi: 10.1016/j.jhep.2019.08.027. [DOI] [PubMed] [Google Scholar]
- 80.Rinella M.E., Lominadze Z., Loomba R., Charlton M., Neuschwander-Tetri B.A., Caldwell S.H. Practice patterns in NAFLD and NASH: real life differs from published guidelines. Ther Adv Gastroenterol. 2016;9:4–12. doi: 10.1177/1756283X15611581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de Vries M., Westerink J., Kaasjager K.H.A.H., de Valk H.W. Prevalence of nonalcoholic fatty liver disease (NAFLD) in patients with type 1 diabetes mellitus: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2020;105:3842–3853. doi: 10.1210/clinem/dgaa575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boursier J., de Ledinghen V., Leroy V., Anty R., Francque S., Salmon D. A stepwise algorithm using an at-a-glance first-line test for the non-invasive diagnosis of advanced liver fibrosis and cirrhosis. J Hepatol. 2017;66:1158–1165. doi: 10.1016/j.jhep.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 83.Boursier J., Tsochatzis E.A. Case-finding strategies in non-alcoholic fatty liver disease. JHEP Rep. 2021;3:100219. doi: 10.1016/j.jhepr.2020.100219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Byrne C.D., Patel J., Scorletti E., Targher G. Tests for diagnosing and monitoring non-alcoholic fatty liver disease in adults. BMJ (Online) 2018;362 doi: 10.1136/bmj.k2734. [DOI] [PubMed] [Google Scholar]
- 85.Francque S., Lanthier N., Verbeke L., Reynaert H., van Steenkiste C., Vonghia L. The Belgian association for study of the liver guidance document on the management of adult and paediatric non-alcoholic fatty liver disease. Acta Gastroenterol Belg. 2018;81:55–81. [PubMed] [Google Scholar]
- 86.Francque S.M.A., Verrijken A., Mertens I., Hubens G., Van Marck E., Pelckmans P. Noninvasive assessment of nonalcoholic fatty liver disease in obese or overweight patients. Clin Gastroenterol Hepatol. 2012;10:1162–1168. doi: 10.1016/j.cgh.2012.06.019. quiz e87. [DOI] [PubMed] [Google Scholar]
- 87.Boursier J., Vergniol J., Guillet A., Hiriart J.-B., Lannes A., Le Bail B. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J Hepatol. 2017;65:570–578. doi: 10.1016/j.jhep.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 88.Wong R.J., Aguilar M., Cheung R., Perumpail R.B., Harrison S.A., Younossi Z.M. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2017;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 89.Bedossa P., Poynard T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 90.Ratziu V., Charlotte F., Heurtier A., Gombert S., Giral P., Bruckert E. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 91.Kleiner D.E., Bedossa P. Liver histology and clinical trials for nonalcoholic steatohepatitis-perspectives from 2 pathologists. Gastroenterology. 2015;149:1305–1308. doi: 10.1053/j.gastro.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 92.Seeff L.B., Everson G.T., Morgan T.R., Curto T.M., Lee W.M., Ghany M.G. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol. 2010;8:877–883. doi: 10.1016/j.cgh.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Neuberger J., Patel J., Caldwell H., Davies S., Hebditch V., Hollywood C. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut. 2020;69:1382–1403. doi: 10.1136/gutjnl-2020-321299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Castera L., Friedrich-Rust M., Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156 doi: 10.1053/j.gastro.2018.12.036. 1264-1281.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wong V.W.-S., Adams L.A., de Lédinghen V., Wong G.L.-H., Sookoian S. Noninvasive biomarkers in NAFLD and NASH - current progress and future promise. Nat Rev Gastroenterol Hepatol. 2018;15:461–478. doi: 10.1038/s41575-018-0014-9. [DOI] [PubMed] [Google Scholar]
- 96.Ballestri S., Romagnoli D., Nascimbeni F., Francica G., Lonardo A. Role of ultrasound in the diagnosis and treatment of nonalcoholic fatty liver disease and its complications. Expert Rev Gastroenterol Hepatol. 2015;9:603–627. doi: 10.1586/17474124.2015.1007955. [DOI] [PubMed] [Google Scholar]
- 97.Schwenzer N.F., Springer F., Schraml C., Stefan N., Machann J., Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. 2009;51:433–445. doi: 10.1016/j.jhep.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 98.Saverymuttu S.H., Joseph A.E., Maxwell J.D. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J. 1986;292:13–15. doi: 10.1136/bmj.292.6512.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kwok R., Choi K.C., Wong G.L.-H., Zhang Y., Chan H.L.-Y., Luk A.O.-Y. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65 doi: 10.1136/gutjnl-2015-309265. 1359 LP – 1368. [DOI] [PubMed] [Google Scholar]
- 100.Petroff D., Blank V., Newsome P.N., Shalimar, Voican C.S., Thiele M. Assessment of hepatic steatosis by controlled attenuation parameter using the M and XL probes: an individual patient data meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:185–198. doi: 10.1016/S2468-1253(20)30357-5. [DOI] [PubMed] [Google Scholar]
- 101.Dulai P.S., Sirlin C.B., Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J Hepatol. 2017;65:1006–1016. doi: 10.1016/j.jhep.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Middleton M.S., Heba E.R., Hooker C.A., Bashir M.R., Fowler K.J., Sandrasegaran K. Agreement between magnetic resonance imaging proton density fat fraction measurements and pathologist-assigned steatosis grades of liver biopsies from adults with nonalcoholic steatohepatitis. Gastroenterology. 2017;153:753–761. doi: 10.1053/j.gastro.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bedogni G., Bellentani S., Miglioli L., Masutti F., Passalacqua M., Castiglione A. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Munteanu M., Tiniakos D., Anstee Q., Charlotte F., Marchesini G., Bugianesi E. Diagnostic performance of FibroTest, SteatoTest and ActiTest in patients with NAFLD using the SAF score as histological reference. Aliment Pharmacol Ther. 2016;44:877–889. doi: 10.1111/apt.13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Björkström K., Stål P., Hultcrantz R., Hagström H. Histologic scores for fat and fibrosis associate with development of type 2 diabetes in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2017;15:1461–1468. doi: 10.1016/j.cgh.2017.04.040. [DOI] [PubMed] [Google Scholar]
- 106.Bazerbachi F., Haffar S., Wang Z., Cabezas J., Arias-Loste M.T., Crespo J. Range of normal liver stiffness and factors associated with increased stiffness measurements in apparently healthy individuals. Clin Gastroenterol Hepatol. 2019;17 doi: 10.1016/j.cgh.2018.08.069. 54-64.e1. [DOI] [PubMed] [Google Scholar]
- 107.Patel K., Sebastiani G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Reports: Innovation Hepatol. 2020;2:100067. doi: 10.1016/j.jhepr.2020.100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boursier J., Francque S. Noninvasive tests of liver fibrosis and their combination in nonalcoholic fatty liver disease: from selected patients to real-life populations. Hepatology (Baltimore, Md) 2019;70:1500–1502. doi: 10.1002/hep.30886. [DOI] [PubMed] [Google Scholar]
- 109.Castera L. Non-invasive tests for liver fibrosis in NAFLD: creating pathways between primary healthcare and liver clinics. Liver Int. 2020;40:77–81. doi: 10.1111/liv.14347. [DOI] [PubMed] [Google Scholar]
- 110.Herrmann E., de Lédinghen V., Cassinotto C., Chu W.C.W., Leung V.Y.F., Ferraioli G. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: an individual patient data-based meta-analysis. Hepatology. 2018;67:260–272. doi: 10.1002/hep.29179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xiao G., Zhu S., Xiao X., Yan L., Yang J., Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology (Baltimore, Md) 2017;66:1486–1501. doi: 10.1002/hep.29302. [DOI] [PubMed] [Google Scholar]
- 112.Hsu C., Caussy C., Imajo K., Chen J., Singh S., Kaulback K. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol. 2019;17 doi: 10.1016/j.cgh.2018.05.059. 630-637.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McPherson S., Stewart S.F., Henderson E., Burt A.D., Day C.P. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59 doi: 10.1136/gut.2010.216077. 1265 LP – 1269. [DOI] [PubMed] [Google Scholar]
- 114.Angulo P., Hui J.M., Marchesini G., Bugianesi E., George J., Farrell G.C. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 115.Boyle M., Tiniakos D., Schattenberg J.M., Ratziu V., Bugianessi E., Petta S. Performance of the PRO-C3 collagen neo-epitope biomarker in non-alcoholic fatty liver disease. JHEP Reports: Innovation Hepatol. 2019;1:188–198. doi: 10.1016/j.jhepr.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vali Y., Lee J., Boursier J., Spijker R., Löffler J., Verheij J. Enhanced liver fibrosis test for the non-invasive diagnosis of fibrosis in patients with NAFLD: a systematic review and meta-analysis. J Hepatol. 2020;73:252–262. doi: 10.1016/j.jhep.2020.03.036. [DOI] [PubMed] [Google Scholar]
- 117.Harrison S.A., Ratziu V., Boursier J., Francque S., Bedossa P., Majd Z. A blood-based biomarker panel (NIS4) for non-invasive diagnosis of non-alcoholic steatohepatitis and liver fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:970–985. doi: 10.1016/S2468-1253(20)30252-1. [DOI] [PubMed] [Google Scholar]
- 118.Lee Y.-S., Yoo Y.J., Jung Y.K., Kim J.H., Seo Y.S., Yim H.J. Multiparametric MR is a valuable modality for evaluating disease severity of nonalcoholic fatty liver disease. Clin Translational Gastroenterol. 2020;11 doi: 10.14309/ctg.0000000000000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schaapman J.J., Tushuizen M.E., Coenraad M.J., Lamb H.J. Multiparametric MRI in patients with nonalcoholic fatty liver disease. J Magn Reson Imaging. 2020 doi: 10.1002/jmri.27292. jmri.27292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Newsome P.N., Sasso M., Deeks J.J., Paredes A., Boursier J., Chan W.-K. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:362–373. doi: 10.1016/S2468-1253(19)30383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Petta S., Maida M., Macaluso F.S., Di Marco V., Cammà C., Cabibi D. The severity of steatosis influences liver stiffness measurement in patients with nonalcoholic fatty liver disease. Hepatology. 2015;62:1101–1110. doi: 10.1002/hep.27844. [DOI] [PubMed] [Google Scholar]
- 122.Verlinden W., Francque S., Michielsen P., Vanwolleghem T. Successful antiviral treatment of chronic hepatitis C leads to a rapid decline of liver stiffness without an early effect on spleen stiffness. Hepatology (Baltimore, Md) 2016;64:1809–1810. doi: 10.1002/hep.28610. [DOI] [PubMed] [Google Scholar]
- 123.Koutoukidis D.A., Koshiaris C., Henry J.A., Noreik M., Morris E., Manoharan I. The effect of the magnitude of weight loss on non-alcoholic fatty liver disease: a systematic review and meta-analysis. Metab Clin Exp. 2021;115 doi: 10.1016/j.metabol.2020.154455. [DOI] [PubMed] [Google Scholar]
- 124.Vilar-Gomez E., Martinez-Perez Y., Calzadilla-Bertot L., Torres-Gonzalez A., Gra-Oramas B., Gonzalez-Fabian L. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149 doi: 10.1053/j.gastro.2015.04.005. 367-378.e5. [DOI] [PubMed] [Google Scholar]
- 125.Wong V.W.S., Wong G.L.H., Chan R.S.M., Shu S.S.T., Cheung B.H.K., Li L.S. Beneficial effects of lifestyle intervention in non-obese patients with non-alcoholic fatty liver disease. J Hepatol. 2018;69:1349–1356. doi: 10.1016/j.jhep.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 126.Musso G., Cassader M., Rosina F., Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885–904. doi: 10.1007/s00125-011-2446-4. [DOI] [PubMed] [Google Scholar]
- 127.Rosqvist F., Kullberg J., Ståhlman M., Cedernaes J., Heurling K., Johansson H.E. Overeating saturated fat promotes fatty liver and ceramides compared with polyunsaturated fat: a randomized trial. J Clin Endocrinol Metab. 2019;104:6207–6219. doi: 10.1210/jc.2019-00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Luukkonen P.K., Sädevirta S., Zhou Y., Kayser B., Ali A., Ahonen L. Saturated fat is more metabolically harmful for the human liver than unsaturated fat or simple sugars. Diabetes Care. 2018;41:1732–1739. doi: 10.2337/dc18-0071. American Diabetes Association Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Howard B.v., Wylie-rosett J. Sugar and cardiovascular disease: a statement for healthcare professionals from the committee on nutrition of the council on nutrition, physical activity, and metabolism of the American heart association. Circulation. 2002;106:523–527. doi: 10.1161/01.CIR.0000019552.77778.04. [DOI] [PubMed] [Google Scholar]
- 130.Gaby A.R. Adverse effects of dietary fructose. Altern Med Rev. 2005;10:294–306. [PubMed] [Google Scholar]
- 131.Jensen T., Abdelmalek M.F., Sullivan S., Nadeau K.J., Green M., Roncal C. Fructose and sugar: a major mediator of non-alcoholic fatty liver disease. J Hepatol. 2018;68:1063–1075. doi: 10.1016/j.jhep.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mosca A., Nobili V., de Vito R., Crudele A., Scorletti E., Villani A. Serum uric acid concentrations and fructose consumption are independently associated with NASH in children and adolescents. J Hepatol. 2017;66:1031–1036. doi: 10.1016/j.jhep.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 133.Schwarz J.M., Noworolski S.M., Erkin-Cakmak A., Korn N.J., Wen M.J., Tai V.W. Effects of dietary fructose restriction on liver fat, de Novo lipogenesis, and insulin kinetics in children with obesity. Gastroenterology. 2017;153:743–752. doi: 10.1053/j.gastro.2017.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schwimmer J.B., Ugalde-Nicalo P., Welsh J.A., Angeles J.E., Cordero M., Harlow K.E. Effect of a low free sugar diet vs usual diet on nonalcoholic fatty liver disease in adolescent boys: a randomized clinical trial. J Am Med Assoc. 2019;321:256–265. doi: 10.1001/jama.2018.20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Godos J., Federico A., Dallio M., Scazzina F. Mediterranean diet and nonalcoholic fatty liver disease: molecular mechanisms of protection. Int J Food Sci Nutr. 2017;68:18–27. doi: 10.1080/09637486.2016.1214239. [DOI] [PubMed] [Google Scholar]
- 136.Ivancovsky-Wajcman D., Fliss-Isakov N., Salomone F., Webb M., Shibolet O., Kariv R. Dietary vitamin E and C intake is inversely associated with the severity of nonalcoholic fatty liver disease. Dig Liver Dis. 2019;51:1698–1705. doi: 10.1016/j.dld.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 137.Salomone F., Ivancovsky-Wajcman D., Fliss-Isakov N., Webb M., Grosso G., Godos J. Higher phenolic acid intake independently associates with lower prevalence of insulin resistance and non-alcoholic fatty liver disease. JHEP Rep. 2020;2:100069. doi: 10.1016/j.jhepr.2020.100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Alferink L.J.M., Kiefte-De Jong J.C., Erler N.S., Veldt B.J., Schoufour J.D., de Knegt R.J. Association of dietary macronutrient composition and non-alcoholic fatty liver disease in an ageing population: the Rotterdam Study. Gut. 2019;68:1088–1098. doi: 10.1136/gutjnl-2017-315940. [DOI] [PubMed] [Google Scholar]
- 139.Baratta F., Pastori D., Polimeni L., Bucci T., Ceci F., Calabrese C. Adherence to mediterranean diet and non-alcoholic fatty liver disease: effect on insulin resistance. Am J Gastroenterol. 2017;112:1832–1839. doi: 10.1038/ajg.2017.371. [DOI] [PubMed] [Google Scholar]
- 140.Zelber-Sagi S., Ivancovsky-Wajcman D., Fliss Isakov N., Webb M., Orenstein D., Shibolet O. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J Hepatol. 2018;68:1239–1246. doi: 10.1016/j.jhep.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 141.Noureddin M., Zelber-Sagi S., Wilkens L.R., Porcel J., Boushey C.J., le Marchand L. Diet associations with nonalcoholic fatty liver disease in an ethnically diverse population: the multiethnic cohort. Hepatology. 2020;71:1940–1952. doi: 10.1002/hep.30967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Etemadi A., Sinha R., Ward M.H., Graubard B.I., Inoue-Choi M., Dawsey S.M. Mortality from different causes associated with meat, heme iron, nitrates, and nitrites in the NIH-AARP Diet and Health Study: population based cohort study. BMJ (Online) 2017;357 doi: 10.1136/bmj.j1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maskarinec G., Lim U., Jacobs S., Monroe K.R., Ernst T., Buchthal S.D. Diet quality in midadulthood predicts visceral adiposity and liver fatness in older ages: the multiethnic cohort study. Obesity. 2017;25:1442–1450. doi: 10.1002/oby.21868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ma J., Hennein R., Liu C., Long M.T., Hoffmann U., Jacques P.F. Improved diet quality associates with reduction in liver fat, particularly in individuals with high genetic risk scores for nonalcoholic fatty liver disease. Gastroenterology. 2018;155:107–117. doi: 10.1053/j.gastro.2018.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gepner Y., Shelef I., Schwarzfuchs D., Zelicha H., Tene L., Meir A.Y. Effect of distinct lifestyle interventions on mobilization of fat storage pools CENTRAL magnetic resonance imaging randomized controlled trial. Circulation. 2018;137:1143–1157. doi: 10.1161/CIRCULATIONAHA.117.030501. [DOI] [PubMed] [Google Scholar]
- 146.Bozzetto L., Prinster A., Annuzzi G., Costagliola L., Mangione A., Vitelli A. Liver fat is reduced by an isoenergetic MUFA diet in a controlled randomized study in type 2 diabetic patients. Diabetes Care. 2012;35:1429–1435. doi: 10.2337/dc12-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ryan M.C., Itsiopoulos C., Thodis T., Ward G., Trost N., Hofferberth S. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59:138–143. doi: 10.1016/j.jhep.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 148.Bischoff S.C., Bernal W., Dasarathy S., Merli M., Plank L.D., Schütz T. ESPEN practical guideline: clinical nutrition in liver disease. Clin Nutr. 2020;39:3533–3562. doi: 10.1016/j.clnu.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 149.Freedman N.D., Cross A.J., McGlynn K.A., Abnet C.C., Park Y., Hollenbeck A.R. Association of meat and fat intake with liver disease and hepatocellular carcinoma in the NIH-AARP cohort. J Natl Canc Inst. 2010;102:1354–1365. doi: 10.1093/jnci/djq301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ioannou G.N., Morrow O.B., Connole M.L., Lee S.P. Association between dietary nutrient composition and the incidence of cirrhosis or liver cancer in the United States population. Hepatology. 2009;50:175–184. doi: 10.1002/hep.22941. [DOI] [PubMed] [Google Scholar]
- 151.Fedirko V., Lukanova A., Bamia C., Trichopolou A., Trepo E., Nöthlings U. Glycemic index, glycemic load, dietary carbohydrate, and dietary fiber intake and risk of liver and biliary tract cancers in s. Ann Oncol. 2013;24:543–553. doi: 10.1093/annonc/mds434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yang W., Sui J., Ma Y., Simon T.G., Chong D., Meyerhardt J.A. A prospective study of dairy product intake and the risk of hepatocellular carcinoma in U.S. men and women. Int J Canc. 2020;146:1241–1249. doi: 10.1002/ijc.32423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sawada N., Inoue M., Iwasaki M., Sasazuki S., Shimazu T., Yamaji T. Consumption of n-3 fatty acids and fish reduces risk of hepatocellular carcinoma. Gastroenterology. 2012;142:1468–1475. doi: 10.1053/j.gastro.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 154.Yang Y., Zhang D., Feng N., Chen G.G., Liu J., Chen G.G. Increased intake of vegetables, but not fruit, reduces risk for hepatocellular carcinoma: a meta-analysis. Gastroenterology. 2014;147:1031–1042. doi: 10.1053/j.gastro.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 155.Turati F., Trichopoulos D., Polesel J., Bravi F., Rossi M., Talamini R. Mediterranean diet and hepatocellular carcinoma. J Hepatol. 2014;60:606–611. doi: 10.1016/j.jhep.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 156.Ma Y., Yang W., Simon T.G., Smith-Warner S.A., Fung T.T., Sui J. Dietary patterns and risk of hepatocellular carcinoma among U.S. men and women. Hepatology. 2019;70:577–586. doi: 10.1002/hep.30362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bowden Davies K.A., Sprung V.S., Norman J.A., Thompson A., Mitchell K.L., Harrold J.A. Physical activity and sedentary time: association with metabolic health and liver fat. Med Sci Sports Exerc. 2019;51:1169–1177. doi: 10.1249/MSS.0000000000001901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jensen M.D., Ryan D.H., Apovian C.M., Ard J.D., Comuzzie A.G., Donato K.A. AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63:2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 159.Hallsworth K., Thoma C., Moore S., Ploetz T., Anstee Q.M., Taylor R. Non-alcoholic fatty liver disease is associated with higher levels of objectively measured sedentary behaviour and lower levels of physical activity than matched healthy controls. Frontline Gastroenterol. 2015;6:44–51. doi: 10.1136/flgastro-2014-100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.ACSM . 5th edition. Lippincott Williams and Wilkins; 2006. ACSM’s Resource Manual for Guidelines for Exercise Testing and Prescription. [Google Scholar]
- 161.Kistler K.D., Brunt E.M., Clark J.M., Diehl A.M., Sallis J.F., Schwimmer J.B. Physical activity recommendations, exercise intensity, and histological severity of nonalcoholic fatty liver disease. Am J Gastroenterol. 2011;106:460–468. doi: 10.1038/ajg.2010.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Oh S., Shida T., Yamagishi K., Tanaka K., So R., Tsujimoto T. Moderate to vigorous physical activity volume is an important factor for managing nonalcoholic fatty liver disease: a retrospective study. Hepatology. 2015;61:1205–1215. doi: 10.1002/hep.27544. [DOI] [PubMed] [Google Scholar]
- 163.Kwak M.S., Kim D., Chung G.E., Kim W., Kim Y.J., Yoon J.H. Role of physical activity in nonalcoholic fatty liver disease in terms of visceral obesity and insulin resistance. Liver Int. 2015;35:944–952. doi: 10.1111/liv.12552. [DOI] [PubMed] [Google Scholar]
- 164.Ryu S., Chang Y., Jung H.S., Yun K.E., Kwon M.J., Choi Y. Relationship of sitting time and physical activity with non-alcoholic fatty liver disease. J Hepatol. 2015;63:1229–1237. doi: 10.1016/j.jhep.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 165.Keating S.E., George J., Johnson N.A. The benefits of exercise for patients with non-alcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2015;9:1247–1250. doi: 10.1586/17474124.2015.1075392. [DOI] [PubMed] [Google Scholar]
- 166.Perseghin G., Lattuada G., de Cobelli F., Ragogna F., Ntali G., Esposito A. Habitual physical activity is associated with intrahepatic fat content in humans. Diabetes Care. 2007;30:683–688. doi: 10.2337/dc06-2032. [DOI] [PubMed] [Google Scholar]
- 167.Gerber L., Otgonsuren M., Mishra A., Escheik C., Birerdinc A., Stepanova M. Non-alcoholic fatty liver disease (NAFLD) is associated with low level of physical activity: a population-based study. Aliment Pharmacol Ther. 2012;36:772–781. doi: 10.1111/apt.12038. [DOI] [PubMed] [Google Scholar]
- 168.Golabi P., Locklear C.T., Austin P., Afdhal S., Byrns M., Gerber L. Effectiveness of exercise in hepatic fat mobilization in nonalcoholic fatty liver disease: systematic review. World J Gastroenterol. 2016;22:6318–6327. doi: 10.3748/wjg.v22.i27.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Keating S.E., Parker H.M., Pavey T.G., Baker M.K., Caterson I.D., George J. Objectively quantified physical activity and sedentary behavior in predicting visceral adiposity and liver fat. J Obes. 2016;2016 doi: 10.1155/2016/2719014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Orci L.A., Gariani K., Oldani G., Delaune V., Morel P., Toso C. Exercise-based interventions for nonalcoholic fatty liver disease: a meta-analysis and meta-regression. Clin Gastroenterol Hepatol. 2016;14:1398–1411. doi: 10.1016/j.cgh.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 171.Kwak M.S., Kim D. Non-alcoholic fatty liver disease and lifestyle modifications, focusing on physical activity. Kor J Intern Med. 2018;33:64–74. doi: 10.3904/kjim.2017.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hong H.C., Hwang S.Y., Choi H.Y., Yoo H.J., Seo J.A., Kim S.G. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology. 2014;59:1772–1778. doi: 10.1002/hep.26716. [DOI] [PubMed] [Google Scholar]
- 173.Koo B.K., Kim D., Joo S.K., Kim J.H., Chang M.S., Kim B.G. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. 2017;66:123–131. doi: 10.1016/j.jhep.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 174.Hashida R., Kawaguchi T., Bekki M., Omoto M., Matsuse H., Nago T. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J Hepatol. 2017;66:142–152. doi: 10.1016/j.jhep.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 175.Keating S.E., Hackett D.A., George J., Johnson N.A. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157–166. doi: 10.1016/j.jhep.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 176.Berzigotti A., Saran U., Dufour J.F. Physical activity and liver diseases. Hepatology. 2016;63:1026–1040. doi: 10.1002/hep.28132. [DOI] [PubMed] [Google Scholar]
- 177.Hallsworth K., Fattakhova G., Hollingsworth K.G., Thoma C., Moore S., Taylor R. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut. 2011;60:1278–1283. doi: 10.1136/gut.2011.242073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Balducci S., Cardelli P., Pugliese L., D’Errico V., Haxhi J., Alessi E. Volume-dependent effect of supervised exercise training on fatty liver and visceral adiposity index in subjects with type 2 diabetes the Italian Diabetes Exercise Study (IDES) Diabetes Res Clin Pract. 2015;109:355–363. doi: 10.1016/j.diabres.2015.05.033. [DOI] [PubMed] [Google Scholar]
- 179.Balducci S., Zanuso S., Nicolucci A., Feo P de, Cavallo S., Cardelli P. Effect of an intensive exercise intervention strategy on modifiable cardiovascular risk factors in subjects with type 2 diabetes mellitus: a randomized controlled trial: the Italian Diabetes and Exercise Study (IDES) Arch Intern Med. 2010;170:1794–1803. doi: 10.1001/archinternmed.2010.380. [DOI] [PubMed] [Google Scholar]
- 180.MacDonald C.S., Johansen M.Y., Nielsen S.M., Christensen R., Hansen K.B., Langberg H. Dose-response effects of exercise on glucose-lowering medications for type 2 diabetes: a secondary analysis of a randomized clinical trial. Mayo Clinic Proc. 2020;95:488–503. doi: 10.1016/j.mayocp.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 181.Centis E., Moscatiello S., Bugianesi E., Bellentani S., Fracanzani A.L., Calugi S. Stage of change and motivation to healthier lifestyle in non-alcoholic fatty liver disease. J Hepatol. 2013;58:771–777. doi: 10.1016/j.jhep.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 182.Dalle Grave R., Calugi S., Centis E., el Ghoch M., Marchesini G. Cognitive-behavioral strategies to increase the adherence to exercise in the management of obesity. J Obes. 2011;2011 doi: 10.1155/2011/348293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fabricatore A.N. {A figure is presented}Behavior therapy and cognitive-behavioral therapy of obesity: is there a difference? J Am Diet Assoc. 2007;107:92–99. doi: 10.1016/j.jada.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 184.Perri M.G., Martin A.D., Leermakers E.A., Sears S.F., Notelovitz M. Effects of group- versus home-based exercise in the treatment of obesity. J Consulting Clin Psychol. 1997;65:278–285. doi: 10.1037//0022-006x.65.2.278. [DOI] [PubMed] [Google Scholar]
- 185.Mangeri F., Montesi L., Forlani G., Grave R.D., Marchesini G. A standard ballroom and Latin dance program to improve fitness and adherence to physical activity in individuals with type 2 diabetes and in obesity. Diabetology Metab Syndr. 2014;6 doi: 10.1186/1758-5996-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Younes R., Bugianesi E. NASH in lean individuals. Semin Liver Dis. 2019;39:86–95. doi: 10.1055/s-0038-1677517. [DOI] [PubMed] [Google Scholar]
- 187.Hallsworth K., Avery L., Trenell M.I. Targeting lifestyle behavior change in adults with NAFLD during a 20-min consultation: summary of the dietary and exercise literature. Curr Gastroenterol Rep. 2016;18:1–7. doi: 10.1007/s11894-016-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chen F., Esmaili S., Rogers G.B., Bugianesi E., Petta S., Marchesini G. Lean NAFLD: a distinct entity shaped by differential metabolic adaptation. Hepatology. 2020;71:1213–1227. doi: 10.1002/hep.30908. [DOI] [PubMed] [Google Scholar]
- 189.Hallsworth K., Adams L.A. Lifestyle modification in NAFLD/NASH: facts and figures. JHEP Rep. 2019;1:468–479. doi: 10.1016/j.jhepr.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.EASL Policy Statement . 2019. Obesity is feeding the rise in Non-Alcoholic Fatty Liver Disease (NAFLD) across Europe. [Google Scholar]
- 191.Durrer Schutz D., Busetto L., Dicker D., Farpour-Lambert N., Pryke R., Toplak H. European practical and patient-centred guidelines for adult obesity management in primary care. Obes Facts. 2019;12:40–66. doi: 10.1159/000496183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Avery L., Exley C., McPherson S., Trenell M.I., Anstee Q.M., Hallsworth K. Lifestyle behavior change in patients with nonalcoholic fatty liver disease: a qualitative study of clinical practice. Clin Gastroenterol Hepatol. 2017;15:1968–1971. doi: 10.1016/j.cgh.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 193.The Diabetes Prevention Program (DPP) Description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wadden T.A., McGuckin B.G., Rothman R.A., Sargent S.L. Lifestyle modification in the management of obesity. J Gastrointest Surg. 2003;7:452–463. doi: 10.1016/S1091-255X(03)00048-9. Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- 195.Grave R.D., Centis E., Marzocchi R., Ghoch M el, Marchesini G. Major factors for facilitating change in behavioral strategies to reduce obesity. Psychol Res Behav Manag. 2013;6:101–110. doi: 10.2147/PRBM.S40460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.McTigue K.M., Conroy M.B., Hess R., Bryce C.L., Fiorillo A.B., Fischer G.S. Using the internet to translate an evidence-based lifestyle intervention into practice. Telemed E-Health. 2009;15:851–858. doi: 10.1089/tmj.2009.0036. [DOI] [PubMed] [Google Scholar]
- 197.Moore T.J., Alsabeeh N., Apovian C.M., Murphy M.C., Coffman G.A., Cullum-Dugan D. Weight, blood pressure, and dietary benefits after 12 months of a web-based nutrition education program (DASH for health): longitudinal observational study. J Med Int Res. 2008;10 doi: 10.2196/jmir.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sousa P., Fonseca H., Gaspar P., Gaspar F. Controlled trial of an Internet-based intervention for overweight teens (Next.Step): effectiveness analysis. Eur J Pediatr. 2015;174:1143–1157. doi: 10.1007/s00431-015-2502-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Eagleson R., Altamirano-Diaz L., McInnis A., Welisch E., Jesus S de, Prapavessis H. Implementation of clinical research trials using web-based and mobile devices: challenges and solutions. BMC Med Res Methodol. 2017;17 doi: 10.1186/s12874-017-0324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Fortmann A.L., Gallo L.C., Garcia M.I., Taleb M., Euyoque J.A., Clark T. Dulce digital: an mHealth SMS based intervention improves glycemic control in hispanics with type 2 diabetes. Diabetes Care. 2017;40:1349–1355. doi: 10.2337/dc17-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Marchesini G., Petta S., Grave R.D. Diet, weight loss, and liver health in nonalcoholic fatty liver disease: pathophysiology, evidence, and practice. Hepatology. 2016;63:2032–2043. doi: 10.1002/hep.28392. [DOI] [PubMed] [Google Scholar]
- 202.Rasu R.S., Hunter C.M., Peterson A.L., Maruska H.M., Foreyt J.P. Economic evaluation of an Internet-based weight management program. Am J Manag Care. 2010;1:98–104. [PubMed] [Google Scholar]
- 203.Mazzotti A., Caletti M.T., Brodosi L., Domizio S di, Forchielli M.L., Petta S. An internet-based approach for lifestyle changes in patients with NAFLD: two-year effects on weight loss and surrogate markers. J Hepatol. 2018;69:1155–1163. doi: 10.1016/j.jhep.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 204.Khoo J., Hsiang J., Taneja R., Law N.M., Ang T.L. Comparative effects of liraglutide 3 mg vs structured lifestyle modification on body weight, liver fat and liver function in obese patients with non-alcoholic fatty liver disease: a pilot randomized trial. Diabetes Obes Metab. 2017;19:1814–1817. doi: 10.1111/dom.13007. [DOI] [PubMed] [Google Scholar]
- 205.Khoo J., Hsiang J.C., Taneja R., Koo S.H., Soon G.H., Kam C.J. Randomized trial comparing effects of weight loss by liraglutide with lifestyle modification in non-alcoholic fatty liver disease. Liver Int. 2019;39:941–949. doi: 10.1111/liv.14065. [DOI] [PubMed] [Google Scholar]
- 206.Busetto L. Timing of bariatric surgery in people with obesity and diabetes. Ann Translational Med. 2015;3:94. doi: 10.3978/j.issn.2305-5839.2015.03.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Peterli R., Wolnerhanssen B.K., Peters T., Vetter D., Kroll D., Borbely Y. Effect of laparoscopic sleeve gastrectomy vs laparoscopic roux-en-y gastric bypass onweight loss in patients with morbid obesity the sm-boss randomized clinical trial. J Am Med Assoc. 2018;319:255–265. doi: 10.1001/jama.2017.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lassailly G., Caiazzo R., Buob D., Pigeyre M., Verkindt H., Labreuche J. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology. 2015;149:379–388. doi: 10.1053/j.gastro.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 209.Caiazzo R., Lassailly G., Leteurtre E., Baud G., Verkindt H., Raverdy V. Roux-en-Y gastric bypass versus adjustable gastric banding to reduce nonalcoholic fatty liver disease: a 5-Year Controlled Longitudinal Study. Ann Surg. 2014;260:893–899. doi: 10.1097/SLA.0000000000000945. Lippincott Williams and Wilkins. [DOI] [PubMed] [Google Scholar]
- 210.Toolabi K., Golzarand M., Farid R. Laparoscopic roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy in terms of efficacy and safety: a comparative study during 11-year experience. Obes Surg. 2021 doi: 10.1007/s11695-021-05313-0. [DOI] [PubMed] [Google Scholar]
- 211.Gu L., Chen B., Du N., Fu R., Huang X., Mao F. Relationship between bariatric surgery and gastroesophageal reflux disease: a systematic review and meta-analysis. Obes Surg. 2019;29:4105–4113. doi: 10.1007/s11695-019-04218-3. [DOI] [PubMed] [Google Scholar]
- 212.Nguyen T., Alzahrani T., Mandler A., Alarfaj M., Panjrath G., Krepp J. Relation of bariatric surgery to inpatient cardiovascular outcomes (from the national inpatient sample) Am J Cardiol. 2021;144:143–147. doi: 10.1016/j.amjcard.2020.12.049. [DOI] [PubMed] [Google Scholar]
- 213.Sjöström L., Narbro K., Sjöström C.D., Karason K., Larsson B., Wedel H. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/nejmoa066254. [DOI] [PubMed] [Google Scholar]
- 214.Sjöström L., Peltonen M., Jacobson P., Sjöström C.D., Karason K., Wedel H. Bariatric surgery and long-term cardiovascular events. J Am Med Assoc. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 215.Sjöholm K., Pajunen P., Jacobson P., Karason K., Sjöström C.D., Torgerson J. Incidence and remission of type 2 diabetes in relation to degree of obesity at baseline and 2 year weight change: the Swedish Obese Subjects (SOS) study. Diabetologia. 2015;58:1448–1453. doi: 10.1007/s00125-015-3591-y. [DOI] [PubMed] [Google Scholar]
- 216.Klebanoff M.J., Corey K.E., Chhatwal J., Kaplan L.M., Chung R.T., Hur C. Bariatric surgery for nonalcoholic steatohepatitis: a clinical and cost-effectiveness analysis. Hepatology. 2017;65:1156–1164. doi: 10.1002/hep.28958. [DOI] [PubMed] [Google Scholar]
- 217.Lassailly G., Caiazzo R., Ntandja-Wandji L.C., Gnemmi V., Baud G., Verkindt H. Bariatric surgery provides long-term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterology. 2020;159 doi: 10.1053/j.gastro.2020.06.006. 1290-1301.e5. [DOI] [PubMed] [Google Scholar]
- 218.Laursen T.L., Hagemann C.A., Wei C., Kazankov K., Thomsen K.L., Knop F.K. Bariatric surgery in patients with non-alcoholic fatty liver disease - from pathophysiology to clinical effects. World J Hepatol. 2019;11:138–249. doi: 10.4254/wjh.v11.i2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hagström H., Ekstedt M., Olbers T., Peltonen M., Carlsson L. Bariatric surgery versus standard obesity treatment and the risk of severe liver disease: data from the Swedish Obese Subjects study. Clin Gastroenterol Hepatology. 2020 doi: 10.1016/j.cgh.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 220.Wirth K.M., Sheka A.C., Kizy S., Irey R., Benner A., Sieger G. Bariatric surgery is associated with decreased progression of nonalcoholic fatty liver disease to cirrhosis: a retrospective cohort analysis. Ann Surg. 2020;272:32–39. doi: 10.1097/SLA.0000000000003871. [DOI] [PubMed] [Google Scholar]
- 221.Hagström H., Ekstedt M., Olbers T., Peltonen M., Carlsson L. Bariatric surgery versus standard obesity treatment and the risk of severe liver disease: data from the Swedish Obese Subjects study. Clin Gastroenterol Hepatol. 2020 doi: 10.1016/j.cgh.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 222.Goh G.B.B., Schauer P.R., McCullough A.J. Considerations for bariatric surgery in patients with cirrhosis. World J Gastroenterol. 2018;24:3112–3119. doi: 10.3748/wjg.v24.i28.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ahmed S., Pouwels S., Parmar C., Kassir R., de Luca M., Graham Y. Outcomes of bariatric surgery in patients with liver cirrhosis: a systematic review. Obes Surg. 2021:31. doi: 10.1007/s11695-021-05289-x. [DOI] [PubMed] [Google Scholar]
- 224.Tsochatzis E., Coilly A., Nadalin S., Levistky J., Tokat Y., Ghobrial M. International liver transplantation consensus statement on end-stage liver disease due to nonalcoholic steatohepatitis and liver transplantation. Transplantation. 2019;103:45–56. doi: 10.1097/TP.0000000000002433. [DOI] [PubMed] [Google Scholar]
- 225.Mikolasevic I., Filipec-Kanizaj T., Mijic M., Jakopcic I., Milic S., Hrstic I. Nonalcoholic fatty liver disease and liver transplantation - where do we stand? World J Gastroenterol. 2018;24:1491–1506. doi: 10.3748/wjg.v24.i14.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mingrone G., van Baar A.C.G., Devière J., Hopkins D., Moura E., Cercato C. Safety and efficacy of hydrothermal duodenal mucosal resurfacing in patients with type 2 diabetes: the randomised, double-blind, sham-controlled, multicentre REVITA-2 feasibility trial. Gut. 2021 doi: 10.1136/gutjnl-2020-323608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ascha M.S., Hanouneh I.A., Lopez R., Tamimi T.A.R., Feldstein A.F., Zein N.N. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 228.Khoudari G., Singh A., Noureddin M., Fritze D., Lopez R., Asaad I. Characterization of patients with both alcoholic and nonalcoholic fatty liver disease in a large United States cohort. World J Hepatol. 2019;11:710–718. doi: 10.4254/wjh.v11.i10.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Loomba R., Bettencourt R., Barrett-Connor E. Synergistic association between alcohol intake and body mass index with serum alanine and aspartate aminotransferase levels in older adults: the Rancho Bernardo Study. Aliment Pharmacol Ther. 2009;30:1137–1149. doi: 10.1111/j.1365-2036.2009.04141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hart C.L., Morrison D.S., Batty G.D., Mitchell R.J., Smith G.D. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. Br Med J (Online) 2010;340:634. doi: 10.1136/bmj.c1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Mahli A., Hellerbrand C. Alcohol and obesity: a dangerous association for fatty liver disease. Dig Dis. 2016;34:32–39. doi: 10.1159/000447279. [DOI] [PubMed] [Google Scholar]
- 232.Hajifathalian K., Sagvand B.T., McCullough A.J. Effect of alcohol consumption on survival in nonalcoholic fatty liver disease: a national prospective cohort study. Hepatology. 2019;70:511–521. doi: 10.1002/hep.30226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Åberg F., Puukka P., Salomaa V., Männistö S., Lundqvist A., Valsta L. Risks of light and moderate alcohol use in fatty liver disease: follow-up of population cohorts. Hepatology. 2020;71:835–848. doi: 10.1002/hep.30864. [DOI] [PubMed] [Google Scholar]
- 234.Åberg F., Helenius-Hietala J., Puukka P., Jula A. Binge drinking and the risk of liver events: a population-based cohort study. Liver Int. 2017;37:1373–1381. doi: 10.1111/liv.13408. [DOI] [PubMed] [Google Scholar]
- 235.Ruhl C.E., Everhart J.E. Joint effects of body weight and alcohol on elevated serum alanine aminotransferase in the United States population. Clin Gastroenterol Hepatol. 2005;3:1260–1268. doi: 10.1016/S1542-3565(05)00743-3. [DOI] [PubMed] [Google Scholar]
- 236.Ekstedt M., Franzén L.E., Holmqvist M., Bendtsen P., Mathiesen U.L., Bodemar G. Alcohol consumption is associated with progression of hepatic fibrosis in non-alcoholic fatty liver disease. Scand J Gastroenterol. 2009;44:366–374. doi: 10.1080/00365520802555991. [DOI] [PubMed] [Google Scholar]
- 237.Ajmera V., Belt P., Wilson L.A., Gill R.M., Loomba R., Kleiner D.E. Among patients with nonalcoholic fatty liver disease, modest alcohol use is associated with less improvement in histologic steatosis and steatohepatitis. Clin Gastroenterol Hepatol. 2018;16 doi: 10.1016/j.cgh.2018.01.026. 1511-1520.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kim N.H., Jung Y.S., Hong H.P., Park J.H., Kim H.J., Park D il. Association between cotinine-verified smoking status and risk of nonalcoholic fatty liver disease. Liver Int. 2018;38:1487–1494. doi: 10.1111/liv.13701. [DOI] [PubMed] [Google Scholar]
- 239.Okamoto M., Miyake T., Kitai K., Furukawa S., Yamamoto S., Senba H. Cigarette smoking is a risk factor for the onset of fatty liver disease in nondrinkers: a longitudinal cohort study. PloS One. 2018;13 doi: 10.1371/journal.pone.0195147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Rezayat A.A., Moghadam M.D., Nour M.G., Shirazinia M., Ghodsi H., Zahmatkesh M.R.R. Association between smoking and non-alcoholic fatty liver disease: a systematic review and meta-analysis. SAGE Open Med. 2018;6 doi: 10.1177/2050312117745223. 205031211774522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Liu P., Xu Y., Tang Y., Du M., Yu X., Sun J. Independent and joint effects of moderate alcohol consumption and smoking on the risks of non-alcoholic fatty liver disease in elderly Chinese men. PloS One. 2017;12 doi: 10.1371/journal.pone.0181497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Jung H.S., Chang Y., Kwon M.J., Sung E., Yun K.E., Cho Y.K. Smoking and the risk of non-alcoholic fatty liver disease: a cohort study. Am J Gastroenterol. 2019;114:453–463. doi: 10.1038/s41395-018-0283-5. [DOI] [PubMed] [Google Scholar]
- 243.Zein C.O., Unalp A., Colvin R., Liu Y.C., McCullough A.J. Smoking and severity of hepatic fibrosis in nonalcoholic fatty liver disease. J Hepatol. 2011;54:753–759. doi: 10.1016/j.jhep.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Björkström K., Franzén S., Eliasson B., Miftaraj M., Gudbjörnsdottir S., Trolle-Lagerros Y. Risk factors for severe liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2019;17 doi: 10.1016/j.cgh.2019.04.038. 2769-2775.e4. [DOI] [PubMed] [Google Scholar]
- 245.Saran U., Humar B., Kolly P., Dufour J.F. Hepatocellular carcinoma and lifestyles. J Hepatol. 2016;64:203–214. doi: 10.1016/j.jhep.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 246.Trichopoulos D., Bamia C., Lagiou P., Fedirko V., Trepo E., Jenab M. Hepatocellular carcinoma risk factors and disease burden in a european cohort: a nested case-control study. J Natl Canc Inst. 2011;103:1686–1695. doi: 10.1093/jnci/djr395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lee Y.C.A., Cohet C., Yang Y.C., Stayner L., Hashibe M., Straif K. Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. Int J Epidemiol. 2009;38:1497–1511. doi: 10.1093/ije/dyp280. [DOI] [PubMed] [Google Scholar]
- 248.Hagström H., Nasr P., Ekstedt M., Hammar U., Stål P., Askling J. Cardiovascular risk factors in non-alcoholic fatty liver disease. Liver Int. 2019;39:197–204. doi: 10.1111/liv.13973. [DOI] [PubMed] [Google Scholar]
- 249.Konerman M.A., Jones J.C., Harrison S.A. Pharmacotherapy for NASH: current and emerging. J Hepatol. 2018;68:362–375. doi: 10.1016/j.jhep.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 250.Francque S., Vonghia L. Pharmacological treatment for non-alcoholic fatty liver disease. Adv Ther. 2019;36 doi: 10.1007/s12325-019-00898-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Sanyal A.J., Friedman S.L., Mccullough A.J., Dimick-Santos L. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American association for the study of liver diseases-U.S. food and drug administration joint workshop. Hepatology. 2015;61:1392–1405. doi: 10.1002/hep.27678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sanyal A.J., Brunt E.M., Kleiner D.E., Kowdley K.v., Chalasani N., Lavine J.E. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344–353. doi: 10.1002/hep.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Targher G., Byrne C.D., Lonardo A., Zoppini G., Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65:589–600. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 254.Angulo P., Kleiner D.E., Dam-Larsen S., Adams L.A., Bjornsson E.S., Charatcharoenwitthaya P. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149 doi: 10.1053/j.gastro.2015.04.043. 389-397.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Glass O., Filozof C., Noureddin M., Berner-Hansen M., Schabel E., Omokaro S.O. Standardisation of diet and exercise in clinical trials of NAFLD-NASH: recommendations from the Liver Forum. J Hepatol. 2020;73:680–693. doi: 10.1016/j.jhep.2020.04.030. [DOI] [PubMed] [Google Scholar]
- 256.Hoofnagle J.H., van Natta M.L., Kleiner D.E., Clark J.M., Kowdley K.v., Loomba R. Vitamin e and changes in serum alanine aminotransferase levels in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2013;38:134–143. doi: 10.1111/apt.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Bril F., Biernacki D.M., Kalavalapalli S., Lomonaco R., Subbarayan S.K., Lai J. Role of Vitamin E for nonalcoholic steatohepatitis in patients with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2019;42:1481–1488. doi: 10.2337/dc19-0167. [DOI] [PubMed] [Google Scholar]
- 258.Cusi K., Orsak B., Bril F., Lomonaco R., Hecht J., Ortiz-Lopez C. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus a randomized trial. Ann Intern Med. 2016;165:305–315. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 259.Bril F., Sanchez P.P., Lomonaco R., Orsak B., Hecht J., Tio F. Liver safety of statins in prediabetes or T2DM and nonalcoholic steatohepatitis: post hoc analysis of a randomized trial. J Clin Endocrinol Metab. 2017;102:2950–2961. doi: 10.1210/jc.2017-00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Armstrong M.J., Gaunt P., Aithal G.P., Barton D., Hull D., Parker R. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. The Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 261.Newsome P., Francque S., Harrison S., Ratziu V., van Gaal L., Calanna S. Effect of semaglutide on liver enzymes and markers of inflammation in subjects with type 2 diabetes and/or obesity. Aliment Pharmacol Ther. 2019;50:193–203. doi: 10.1111/apt.15316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Newsome P.N., Buchholtz K., Cusi K., Linder M., Okanoue T., Ratziu V. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2020 doi: 10.1056/nejmoa2028395. [DOI] [PubMed] [Google Scholar]
- 263.Francque S., Szabo G., Abdelmalek M.F., Byrne C.D., Cusi K., Dufour J.F. Nonalcoholic steatohepatitis: the role of peroxisome proliferator-activated receptors. Nat Rev Gastroenterol Hepatol. 2021;18:24–39. doi: 10.1038/s41575-020-00366-5. [DOI] [PubMed] [Google Scholar]
- 264.Belfort R., Harrison S.A., Brown K., Darland C., Finch J., Hardies J. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/nejmoa060326. [DOI] [PubMed] [Google Scholar]
- 265.Aithal G.P., Thomas J.A., Kaye P.v., Lawson A., Ryder S.D., Spendlove I. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176–1184. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 266.Musso G., Gambino R., Cassader M., Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79–104. doi: 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- 267.Sanyal A.J., Chalasani N., Kowdley K.v., McCullough A., Diehl A.M., Bass N.M. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/nejmoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Panunzi S., Maltese S., Verrastro O., Labbate L., de Gaetano A., Pompili M. Pioglitazone and bariatric surgery are the most effective treatments for non-alcoholic steatohepatitis: a hierarchical network meta-analysis. Diabetes Obes Metab. 2021;23:980–990. doi: 10.1111/dom.14304. [DOI] [PubMed] [Google Scholar]
- 269.Budd J., Cusi K. Role of agents for the treatment of diabetes in the management of nonalcoholic fatty liver disease. Curr Diab Rep. 2020;20 doi: 10.1007/s11892-020-01349-1. [DOI] [PubMed] [Google Scholar]
- 270.Musso G., Cassader M., Paschetta E., Gambino R. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: a meta-analysis. JAMA Intern Med. 2017;177:633–640. doi: 10.1001/jamainternmed.2016.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Bril F., Lomonaco R., Kalavalapalli S., Lai J., Cusi K. Pioglitazone discontinuation in patients with nonalcoholic steatohepatitis (NASH) is associated with disease recurrence. Diabetes. 2019;68 doi: 10.1111/dom.14936. 223-OR. [DOI] [PubMed] [Google Scholar]
- 272.Liao H.W., Saver J.L., Wu Y.L., Chen T.H., Lee M., Ovbiagele B. Pioglitazone and cardiovascular outcomes in patients with insulin resistance, pre-diabetes and type 2 diabetes: a systematic review and meta-analysis. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-013927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Dormandy J.A., Charbonnel B., Eckland D.J.A., Erdmann E., Massi-Benedetti M., Moules I.K. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial in macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 274.Kernan W.N., Viscoli C.M., Furie K.L., Young L.H., Inzucchi S.E., Gorman M. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321–1331. doi: 10.1056/nejmoa1506930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Gastaldelli A., Harrison S.A., Belfort-Aguilar R., Hardies L.J., Balas B., Schenker S. Importance of changes in adipose tissue insulin resistance to histological response during thiazolidinedione treatment of patients with nonalcoholic steatohepatitis. Hepatology. 2009;50:1087–1093. doi: 10.1002/hep.23116. [DOI] [PubMed] [Google Scholar]
- 276.Abner E.L., Schmitt F.A., Mendiondo M.S., Marcum J.L., Kryscio R.J. Vitamin E and all-cause mortality: a meta-analysis. Curr Aging Sci. 2012;4:158–170. doi: 10.2174/1874609811104020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Vos M.B., Abrams S.H., Barlow S.E., Caprio S., Daniels S.R., Kohli R. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) J Pediatr Gastroenterol Nutr. 2017;64:319–334. doi: 10.1097/MPG.0000000000001482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Pi-Sunyer X., Astrup A., Fujioka K., Greenway F., Halpern A., Krempf M. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11–22. doi: 10.1056/nejmoa1411892. [DOI] [PubMed] [Google Scholar]
- 279.Kuchay M.S., Krishan S., Mishra S.K., Farooqui K.J., Singh M.K., Wasir J.S. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT Trial) Diabetes Care. 2018;41:1801–1808. doi: 10.2337/dc18-0165. [DOI] [PubMed] [Google Scholar]
- 280.Joy T.R., McKenzie C.A., Tirona R.G., Summers K., Seney S., Chakrabarti S. Sitagliptin in patients with non-alcoholic steatohepatitis: a randomized, placebo-controlled trial. World J Gastroenterol. 2017;23:141–150. doi: 10.3748/wjg.v23.i1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Younossi Z.M., Ratziu V., Loomba R., Rinella M., Anstee Q.M., Goodman Z. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. The Lancet. 2019;394:2184–2196. doi: 10.1016/S0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]
- 282.Funuyet-Salas J., Pérez-San-Gregorio M.Á., Martín-Rodríguez A., Romero-Gómez M. Psychological biomarkers and fibrosis: an innovative approach to non-alcoholic fatty liver disease. Front Med. 2020;7 doi: 10.3389/fmed.2020.585425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Soto-Angona Ó., Anmella G., Valdés-Florido M.J., de Uribe-Viloria N., Carvalho A.F., Penninx B.W.J.H. Non-alcoholic fatty liver disease (NAFLD) as a neglected metabolic companion of psychiatric disorders: common pathways and future approaches. BMC Med. 2020;18 doi: 10.1186/s12916-020-01713-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Azzaroli F., Mazzella G., Marchesini G., Brodosi L., Petroni M.L. Fatty liver in pregnancy: a narrative review of two distinct conditions. Expert Rev Gastroenterol Hepatol. 2020;14:127–135. doi: 10.1080/17474124.2020.1715210. [DOI] [PubMed] [Google Scholar]
- 285.Sarkar M., Grab J., Dodge J.L., Gunderson E.P., Rubin J., Irani R.A. Non-alcoholic fatty liver disease in pregnancy is associated with adverse maternal and perinatal outcomes. J Hepatol. 2020;73:516–522. doi: 10.1016/j.jhep.2020.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Vajro P., Lenta S., Socha P., Dhawan A., McKiernan P., Baumann U. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN hepatology committee. J Pediatr Gastroenterol Nutr. 2012;54:700–713. doi: 10.1097/MPG.0b013e318252a13f. [DOI] [PubMed] [Google Scholar]
- 287.Nobili V., Mantovani A., Cianfarani S., Alisi A., Mosca A., Sartorelli M.R. Prevalence of prediabetes and diabetes in children and adolescents with biopsy-proven non-alcoholic fatty liver disease. J Hepatol. 2019;71:802–810. doi: 10.1016/j.jhep.2019.06.023. [DOI] [PubMed] [Google Scholar]
- 288.Adams L.A., Anstee Q.M., Tilg H., Targher G. Non-Alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138–1153. doi: 10.1136/gutjnl-2017-313884. [DOI] [PubMed] [Google Scholar]
- 289.Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901–910. doi: 10.1016/S2213-8587(14)70032-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.