Abstract
Allopurinol is the first-line agent for patients with gout, including those with moderate‐to‐severe chronic kidney disease. However, increased thyroid-stimulating hormone (TSH) levels are observed in patients with long-term allopurinol treatment. This large-scale, nested case–control, retrospective observational study analysed the association between allopurinol use and increased TSH levels. A common data model based on an electronic medical record database of 19,200,973 patients from seven hospitals between January 1997 and September 2020 was used. Individuals aged > 19 years in South Korea with at least one record of a blood TSH test were included. Data of 59,307 cases with TSH levels > 4.5 mIU/L and 236,508 controls matched for sex, age (± 5), and cohort registration date (± 30 days) were analysed. An association between the risk of increased TSH and allopurinol use in participants from five hospitals was observed. A meta-analysis (I2 = 0) showed that the OR was 1.51 (95% confidence interval: 1.32–1.72) in both the fixed and random effects models. The allopurinol intake group demonstrated that increased TSH did not significantly affect free thyroxine and thyroxine levels. After the index date, some diseases were likely to occur in patients with subclinical hypothyroidism and hypothyroidism. Allopurinol administration may induce subclinical hypothyroidism.
Subject terms: Endocrinology, Health care, Medical research, Rheumatology
Introduction
Gout is the most common form of inflammatory arthritis, caused by chronic elevation in serum uric acid levels above the saturation point of monosodium urate1. This leads to the deposition of monosodium urate crystals in the joints, tendons, and other tissues, triggering recurrent episodes of pronounced acute inflammation known as gout flares2.
In recent decades, gout incidence has increased in many countries. Although its prevalence in South Korea is lower than that in other countries, the incidence increased by 25% between 2009 and 2015, according to a study using the national health insurance claim database. Dietary factors, increasing age, and comorbid conditions were environmental influences on gout incidence2,3.
As the primary urate-lowering therapy, xanthine oxidase inhibitors (XOIs) such as allopurinol (the preferred first-line agent) or febuxostat4 are strongly recommended for all patients, including those with moderate‐to‐severe chronic kidney disease (CKD)5. According to previous studies, XOIs increase blood thyrotropin (TSH) levels6–8. Increased TSH levels (> 5.5 µIU/mL) were observed in patients on long-term treatment with allopurinol (5.8%) in a long-term, open-label extension study9. In a study by Perez-Ruiz et al.7, 88 patients receiving febuxostat and 87 patients receiving allopurinol were followed up for 12 months to measure changes in TSH levels. The authors found significant increases in TSH levels (> 5.5 µU/mL) in 3.4% of patients using allopurinol and 7.9% of patients using febuxostat. Increased TSH levels were not associated with changes in free thyroxine (FT4) levels7.
In 2017, the European Medicines Agency recommended that product information of allopurinol should be updated to include information regarding increased TSH levels in patients undergoing long-term treatment10. Information on increases in TSH appears on medication labels in Europe, including in the United Kingdom, France, Germany, Switzerland, and Italy11–15. In Korea, the label of allopurinol was updated to include this information in April 202016. Despite the update on medication labels of allopurinol, there is no known evidence that allopurinol use is related to increased TSH levels in Korea.
Observational databases have different logical organisations and physical formats, and terminologies used to describe medicinal products and clinical conditions vary across sources17. A common data model (CDM) could be used to create a standardised data structure to utilise hospital data efficiently to overcome these difficulties. The CDM is a distributed database system of encrypted and de-identified information that identifies patients and converts their information to secondary data sources in a common format. Medical records are converted into standardised data in a common format and used as secondary data sources through the distributed research network.
This CDM allows for a range of standard queries and analytic methods developed centrally to be run seamlessly in both the distributed and centralised environments, potentially leading to rapid quality-assured results18. The MOA CDM from the Korea Institute of Drug Safety and Risk Management includes the Sentinel CDM from the US Food and Drug Administration and the Observational Medical Outcomes Partnership (OMOP) CDM from Observational Health Data Sciences and Informatics (OHDSI)19. The OMOP CDM is the most widely used model, developed and managed by the OHDSI in Korea.
In this retrospective, observational study targeting a large cohort of Korean population groups in multiple centres, we performed an analysis of the association between allopurinol and increased TSH levels using the OMOP CDM.
Methods
Data source and study design
This was a nested case–control study using distributed OMOP CDM databases loaded with data records from 19,200,973 patients based on electronic medical records (EMR) from seven hospitals. These data included demographic information, medical diagnoses, prescriptions, referrals, laboratory test results, and clinical values.
The Research Ethics Committee of the seven university hospitals (Seoul St. Mary’s Hospital, Seoul Asan Hospital, St. Vincent’s Hospital, Yeouido St. Mary’s Hospital, Soonchunhyang University Hospital Seoul, Seoul National University Bundang Hospital, and Seoul National University Hospital) approved the study. This study used CDM data, which were de-identified, and involved no more than minimal risk to subjects. The requirement for written informed consent was waived by the Research Ethics Committee of the Catholic Medical Centre and this study was in accordance with relevant guidelines and regulations.
Selection of cases and controls
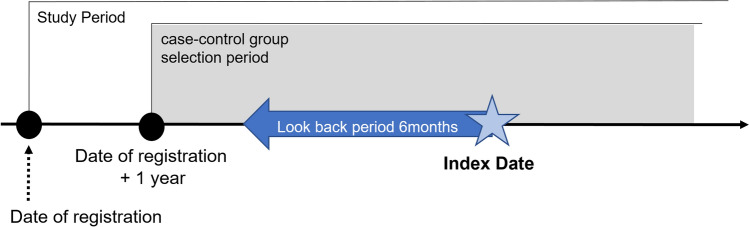
This cohort included patients aged 19 years or older with at least one record of blood TSH test registered between 1 January 1997 and 18 September 2020. The date of registration was that of the first TSH test. Those who met any exclusion criteria during the entire period from the start date of the observation period to 1 year after the registration date were excluded. We excluded patients with TSH levels < 0.5 mIU/L or > 4.5 mIU/L; those diagnosed with thyroid diseases, Graves’ disease, pituitary tumour, pituitary gland dysfunction resulting in Sheehan’s syndrome, and those who underwent thyroidectomy. Patients receiving anti-thyroid medications, levothyroxine, thyroid supplements, or radioiodine therapy were also excluded (Supplementary Table S1). Case and control patients were selected 1 year after the date of registration (Fig. 1).
Figure 1.
Study design.
To reduce bias, we collected and reviewed the source values and standard codes of each hospital in advance and selected 5 out of 108 items for TSH. We confirmed that all seven hospitals participating in the study received laboratory accreditation from the Laboratory Medicine Foundation ensure the reliability of test result. Besides, we collected the normal range for TSH from the seven hospitals participating in the study. Then, we combined and compared the values in previous studies, and decided the cut off value TSH as 0.5 to 4.5 mIU/L with the help of an endocrinology specialist.
Patients whose TSH levels were > 4.5 mIU/L after 1 year of cohort registration were allocated to the case group, and those having levels > 4.5 mIU/L at the date of the first test were assigned to the index date of the case group. The patients in the control group who had TSH levels from 0.5 to 4.5 mIU/L were matched four times with the case group for sex, age (± 5), and date of cohort registration (± 30 days) using incidence density sampling without replacement. The control index date was assigned as the case group index date of the patients matching.
Exposure
Details of medication prescriptions, including allopurinol were obtained (Supplementary Table S2). We selected the look-back period of allopurinol use for 6 months before the case–control index date and determined whether allopurinol was prescribed.
Confounding variables
Confounding variables were selected by reviewing previous studies and expert consultations based on data available on the CDM network. We considered diseases and medications that could affect TSH levels as confounding variables and analysed the records for 6 months before selecting the case and control groups.
Diseases included 17 disease groups (myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, hemiplegia or paraplegia, dementia, chronic pulmonary disease, rheumatologic disease, peptic ulcer disease, diabetes without chronic complications, diabetes with chronic complications, renal disease, any malignancy including leukaemia and lymphoma, metastatic solid tumour, mild liver disease, moderate or severe liver disease, and acquired immune deficiency syndrome/human immunodeficiency virus (AIDS/HIV) of the Charlson Comorbidity Index (CCI)20–23 and pituitary disease24,25. Medications included those that could affect TSH levels26–34 and medication used by gout patients, such as non-steroidal anti-inflammatories, acetaminophen, oxycodone, colchicine, and corticosteroids35,36 (Supplementary Table S3).
Statistical analyses
Each hospital’s database was converted to the OMOP CDM version 5 format using the same table structures and standardised data. The association between allopurinol use and increment in TSH levels was analysed using a standardised analytical code. We used conditional logistic regression37 to estimate the adjusted odds ratio (OR) that increased TSH levels because of allopurinol exposure for the confounding variables. The overall effects were evaluated using a meta-analysis method, a statistical analysis combining the results of each hospital and a forest plot. Statistical approaches (I2 value test) were used to test for heterogeneity. We performed the following additional analyses for the case group that was prescribed allopurinol: (1) changes in TSH levels; (2) changes in FT4 and thyroxine (T4) levels; and (3) diseases diagnosed after the index date of the case group (the first increase in TSH). All analyses were performed using R 4.0.2 (http://www.R-project.org), and two-sided p-values lower than 0.05 were considered statistically significant.
Results
Clinical characteristics
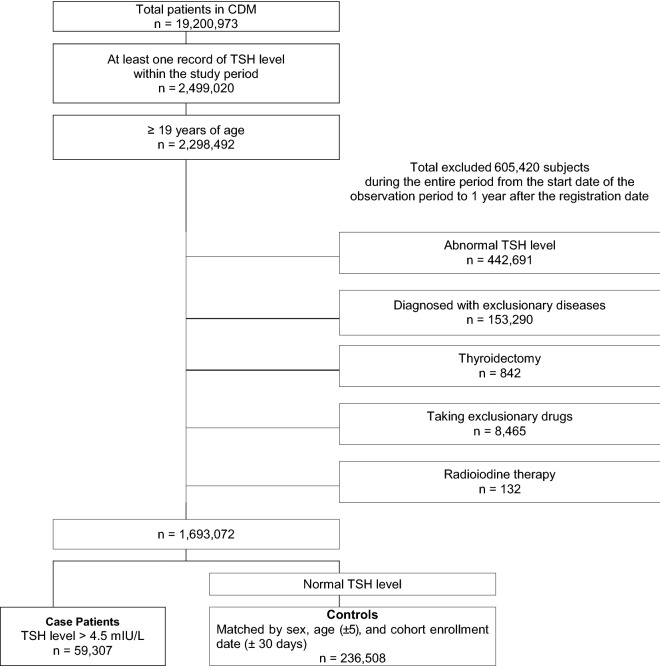
The total population of all CDM databases was 19,200,973 patients from seven hospitals. A total of 2,298,492 patients were registered in the cohort according to the inclusion criteria, and 605,420 patients were excluded. Finally, the cohort comprised 1,693,072 patients.
The case group had 59,307 case patients (TSH > 4.5 mIU/L) who were matched with 236,508 control patients for sex, age (± 5), and the date of cohort registration (± 30 days) using incidence density sampling without replacement after 1 year from the date of cohort registration. Detailed information regarding the number of cases and controls is described in Fig. 2.
Figure 2.
Flowchart of study participants in the study network. CDM Common data model, TSH Thyroid-stimulating hormone.
Table 1 presents the clinical characteristics of the study population. All medications that were considered confounding variables showed significant differences between the case and control groups. Therefore, cases were found to more likely use the medication than controls. Among the diseases considered confounding variables, all diseases showed significant differences between the case and control groups. The case group had a higher incidence of disease than the control group.
Table 1.
Characteristics of the study population.
| Total | Case patients | Controls | χ2 | ||||
|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | p value | |
| Total | 295,815 | (100) | 59,307 | (20.05) | 236,508 | (79.95) | 0.510 |
| Sex | |||||||
| Male | 114,732 | (38.79) | 23,039 | (38.85) | 91,693 | (38.77) | 0.730 |
| Female | 181,083 | (61.21) | 36,268 | (61.15) | 144,815 | (61.23) | |
| Age (years) | |||||||
| Mean (SD) | 55.23 | (13.06) | 55.63 | (13.26) | 55.13 | (13.00) | |
| 19–29 | 7435 | (2.51) | 1500 | (2.53) | 5935 | (2.51) | < 0.001 |
| 30–39 | 26,426 | (8.93) | 5031 | (8.48) | 21,395 | (9.05) | |
| 40–49 | 65,489 | (22.14) | 12,825 | (21.62) | 52,664 | (22.27) | |
| 50–59 | 88,710 | (29.99) | 17,934 | (30.24) | 70,776 | (29.93) | |
| 60–69 | 63,352 | (21.42) | 12,537 | (21.14) | 50,815 | (21.49) | |
| 70–79 | 34,569 | (11.69) | 7119 | (12.00) | 27,450 | (11.61) | |
| ≥ 80 | 9834 | (3.32) | 2361 | (3.98) | 7473 | (3.16) | |
| Medication | |||||||
| Tyrosine kinase inhibitors | 382 | (0.13) | 227 | (0.38) | 155 | (0.07) | < 0.001 |
| Cancer immunotherapy | 70 | (0.02) | 62 | (0.10) | 8 | (0.00) | < 0.001 |
| Anti-tuberculosis medications | 462 | (0.16) | 204 | (0.34) | 258 | (0.11) | < 0.001 |
| Dobutamine | 287 | (0.10) | 197 | (0.33) | 90 | (0.04) | < 0.001 |
| Octreotide | 42 | (0.01) | 18 | (0.03) | 24 | (0.01) | < 0.001 |
| Interferon-α | 133 | (0.04) | 111 | (0.19) | 22 | (0.01) | < 0.001 |
| Amiodarone | 1613 | (0.55) | 1282 | (2.16) | 331 | (0.14) | < 0.001 |
| Azathioprine | 380 | (0.13) | 135 | (0.23) | 245 | (0.10) | < 0.001 |
| Mercaptopurine | 17 | (0.01) | 7 | (0.01) | 10 | (0.00) | 0.029 |
| Warfarin | 3015 | (1.02) | 1313 | (2.21) | 1702 | (0.72) | < 0.001 |
| Dopamine | 444 | (0.15) | 271 | (0.46) | 173 | (0.07) | < 0.001 |
| Metformin | 11,099 | (3.75) | 4016 | (6.77) | 7083 | (2.99) | < 0.001 |
| NSAIDs | 28,054 | (9.48) | 10,867 | (18.32) | 17,187 | (7.27) | < 0.001 |
| Acetaminophen | 10,961 | (3.71) | 5235 | (8.83) | 5726 | (2.42) | < 0.001 |
| Oxycodone | 1346 | (0.46) | 642 | (1.08) | 704 | (0.30) | < 0.001 |
| Colchicine | 438 | (0.15) | 166 | (0.28) | 272 | (0.12) | < 0.001 |
| Corticosteroid | 10,474 | (3.54) | 4437 | (7.48) | 6037 | (2.55) | < 0.001 |
| Disease | |||||||
| Panhypopituitarism | 42 | (0.01) | 25 | (0.04) | 17 | (0.01) | < 0.001 |
| Myocardial Infarction | 1362 | (0.46) | 480 | (0.81) | 882 | (0.37) | < 0.001 |
| Congestive heart failure | 2390 | (0.81) | 1221 | (2.06) | 1169 | (0.49) | < 0.001 |
| Peripheral vascular disease | 1497 | (0.51) | 561 | (0.95) | 936 | (0.40) | < 0.001 |
| Cerebrovascular disease | 5514 | (1.86) | 1793 | (3.02) | 3721 | (1.57) | < 0.001 |
| Dementia | 2440 | (0.82) | 632 | (1.07) | 1808 | (0.76) | < 0.001 |
| Chronic pulmonary disease | 3021 | (1.02) | 1180 | (1.99) | 1841 | (0.78) | < 0.001 |
| Rheumatologic disease | 1710 | (0.58) | 597 | (1.01) | 1113 | (0.47) | < 0.001 |
| Peptic ulcer disease | 3134 | (1.06) | 1140 | (1.92) | 1994 | (0.84) | < 0.001 |
| Mild liver disease | 2840 | (0.96) | 1115 | (1.88) | 1725 | (0.73) | < 0.001 |
| Diabetes without chronic complications | 12,413 | (4.20) | 4905 | (8.27) | 7508 | (3.17) | < 0.001 |
| Diabetes with chronic complications | 7832 | (2.65) | 3199 | (5.39) | 4633 | (1.96) | < 0.001 |
| Hemiplegia or paraplegia | 292 | (0.10) | 147 | (0.25) | 145 | (0.06) | < 0.001 |
| Renal disease | 3637 | (1.23) | 1834 | (3.09) | 1803 | (0.76) | < 0.001 |
| Any malignancy | 13,631 | (4.61) | 6912 | (11.65) | 6719 | (2.84) | < 0.001 |
| Moderate or severe liver disease | 225 | (0.08) | 124 | (0.21) | 101 | (0.04) | < 0.001 |
| Metastatic solid tumour | 1726 | (0.58) | 1259 | (2.12) | 467 | (0.20) | < 0.001 |
| AIDS/HIV | 67 | (0.02) | 25 | (0.04) | 42 | (0.02) | < 0.001 |
| CCI group | |||||||
| 0 | 264,455 | (89.4) | 45,957 | (77.49) | 218,498 | (92.39) | < 0.001 |
| 1 | 8634 | (2.92) | 3010 | (5.08) | 5624 | (2.38) | |
| 2 | 15,722 | (5.31) | 6658 | (11.23) | 9064 | (3.83) | |
| > 3 | 7004 | (2.37) | 3682 | (6.21) | 3322 | (1.40) | |
SD Standard deviation, NSAIDs Nonsteroidal anti-inflammatory drugs, AIDS/HIV Acquired immune deficiency syndrome/human immunodeficiency virus, CCI Charlson comorbidity index.
Association between allopurinol use and increased TSH
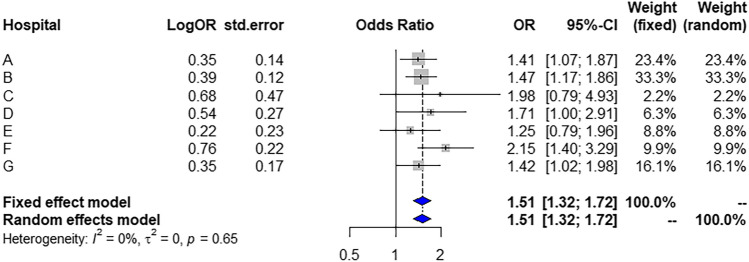
Allopurinol use was associated with an increased risk of elevated TSH in five of seven hospitals (Table 2). Figure 1 presents the adjusted OR of allopurinol use for increased TSH levels from the meta-analysis. The OR was 1.51 (95% confidence interval, 1.32–1.72) in both the fixed effects and random effects models. In the meta-analysis, the fraction of variance due to heterogeneity was estimated using the statistic I2; the I2 value was zero (Fig. 3).
Table 2.
Crude and adjusted odds ratios (ORs) showing the association between allopurinol use and increased thyroid-stimulating hormone (TSH) levels.
| Hospital | Allopurinol use | Case patients | Controls | Crude OR | Adjusted OR | ||||
|---|---|---|---|---|---|---|---|---|---|
| n = 59,307 | n = 236,508 | ||||||||
| n | % | n | % | OR | 95% CI | OR | 95% CI | ||
| A | 6646 | 26,569 | |||||||
| No | 6520 | 98.10 | 26,419 | 99.44 | |||||
| Yes | 126 | 1.90 | 150 | 0.56 | 3.40 | (2.68, 4.32) | 1.41 | (1.07, 1.87) | |
| B | 29,868 | 119,047 | |||||||
| No | 29,694 | 99.42 | 118,844 | 99.83 | |||||
| Yes | 174 | 0.58 | 203 | 0.17 | 3.43 | (2.80, 4.20) | 1.47 | (1.17, 1.86) | |
| C | 2360 | 9422 | |||||||
| No | 2347 | 99.45 | 9409 | 99.86 | |||||
| Yes | 13 | 0.55 | 13 | 0.14 | 4.01 | (1.86, 8.66) | 1.98 | (0.79, 4.93) | |
| D | 1094 | 4354 | |||||||
| No | 1049 | 95.89 | 4314 | 99.08 | |||||
| Yes | 45 | 4.11 | 40 | 0.92 | 4.63 | (3.01, 7.12) | 1.71 | (1.00, 2.91) | |
| E | 3805 | 15,082 | |||||||
| No | 3737 | 98.21 | 15,030 | 99.66 | |||||
| Yes | 68 | 1.79 | 52 | 0.34 | 5.26 | (3.66, 7.56) | 1.25 | (0.79, 1.96) | |
| F | 6882 | 27,457 | |||||||
| No | 6822 | 99.13 | 27,404 | 99.81 | |||||
| Yes | 60 | 0.87 | 53 | 0.19 | 4.55 | (3.14, 6.59) | 2.15 | (1.40, 3.29) | |
| G | 8652 | 34,577 | |||||||
| No | 8570 | 99.05 | 34,458 | 99.66 | |||||
| Yes | 82 | 0.95 | 119 | 0.34 | 2.77 | (2.09, 3.67) | 1.42 | (1.02, 1.98) | |
CI Confidence interval.
Figure 3.
Forest plot. OR Odds ratio, CI Confidence interval.
Additional analysis
We performed an additional analysis for patients who used allopurinol. The cohort end date was set as the date 1 year after the last use of allopurinol and the changes in TSH levels, TSH value at the date of cohort registration, index date of the case, maximum TSH value after the case index date, and mean TSH value after the case index date were analysed.
The mean TSH level was 2.49 mIU/L at the date of cohort registration and 10.54 mIU/L at the index date in the case group. The mean maximum TSH level was 12.52 mIU/L. The mean TSH level between the index date and cohort end date was 7.64 mIU/L in the case group (Table 3).
Table 3.
Changes in thyroid-stimulating hormone (TSH) levels.
| Hospital | Variable | Cohort registration | Index date | Max | Mean |
|---|---|---|---|---|---|
| Total | Mean ± SD | 2.49 ± 1.06 | 10.54 ± 18.11 | 12.52 ± 19.72 | 7.64 ± 15.15 |
| n | 568 | 568 | 568 | 2021 | |
| A | Mean ± SD | 2.48 ± 1.02 | 8.1 ± 10.18 | 9.12 ± 10.7 | 5.94 ± 7.93 |
| n | 126 | 126 | 126 | 413 | |
| B | Mean ± SD | 2.31 ± 1.04 | 11.91 ± 18.5 | 15.5 ± 22.18 | 9.59 ± 17.51 |
| n | 174 | 174 | 174 | 642 | |
| C | Mean ± SD | 2.28 ± 0.87 | 6.18 ± 1.93 | 6.2 ± 1.92 | 5.53 ± 2.07 |
| n | 13 | 13 | 13 | 20 | |
| D | Mean ± SD | 2.44 ± 0.93 | 11.65 ± 22.63 | 13.25 ± 22.91 | 8.25 ± 15.61 |
| n | 45 | 45 | 45 | 170 | |
| E | Mean ± SD | 2.47 ± 1.07 | 8.87 ± 12.5 | 10 ± 12.77 | 5.73 ± 8.28 |
| n | 68 | 68 | 68 | 199 | |
| F | Mean ± SD | 2.79 ± 1.46 | 7.99 ± 12.33 | 8.63 ± 13.79 | 5.67 ± 9.69 |
| n | 60 | 60 | 60 | 250 | |
| G | Mean ± SD | 2.78 ± 1.02 | 14.69 ± 39.86 | 16.92 ± 41.06 | 8.42 ± 28.85 |
| n | 82 | 82 | 82 | 327 |
SD Standard deviation.
We analysed the FT4 and T4 values before and after the index date of the case group. The mean FT4 level was within the normal reference range after the case group’s index date in all hospitals. The mean T4 level was within the normal reference range in all hospitals (Table 4).
Table 4.
Changes in free thyroxine (FT4) and thyroxine (T4) levels.
| Hospital | Variable | FT4 (ng/dL) | T4 (μg/dL) | ||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| Index date | Index date | Index date | Index date | ||
| Total | Mean ± SD | 1.23 ± 0.32 | 1.2 ± 0.2 | 8.03 ± 1.25 | 7.27 ± 1.23 |
| N | 513 | 526 | 62 | 27 | |
| A | Mean ± SD | 1.22 ± 0.21 | 1.17 ± 0.24 | 8.75 ± 2.41 | 6.82 ± 1.98 |
| n | 112 | 117 | 19 | 9 | |
| B | Mean ± SD | 1.31 ± 0.57 | 1.3 ± 0.26 | 8.12 ± 1.87 | 6.66 ± 2.49 |
| n | 163 | 167 | 29 | 13 | |
| C | Mean ± SD | 1.1 ± 0.14 | 0.9 ± 0.18 | 8.38 ± 1.76 | 10.53 |
| n | 13 | 13 | 2 | 1 | |
| D | Mean ± SD | 1.15 ± 0.29 | 1.11 ± 0.28 | ||
| n | 44 | 45 | |||
| E | Mean ± SD | 1.23 ± 0.41 | 1.2 ± 0.27 | 5.96 ± 2.16 | 6.21 ± 0.46 |
| n | 65 | 59 | 6 | 3 | |
| F | Mean ± SD | 1.29 ± 0.3 | 1.24 ± 0.18 | 7.33 ± 2.51 | 4.5 |
| n | 39 | 44 | 6 | 1 | |
| G | Mean ± SD | 1.11 ± 0.3 | 1.11 ± 0.27 | ||
| n | 77 | 81 | |||
| Reference range | FT4: 0.9–1.7 ng/dL | T4: 5.0–11.0 μg/dL | |||
SD Standard deviation, Ft4 Free thyroxine, T4 Thyroxine.
We analysed new diseases that occurred after the case group’s index date based on the International Classification of Diseases 10th Revision (ICD-10) code in the case of patients who used allopurinol. CKD (N18) was the most common (in 133 cases), followed by gastro-oesophageal reflux disease (GERD) (K21) in 117, chronic ischaemic heart disease (IHD) (I25) in 113, type 2 diabetes mellitus (T2DM) (E11) in 97, and gastritis and duodenitis (K29) in 83 (Table 5).
Table 5.
Diseases diagnosed after increased thyroid-stimulating hormone (TSH) levels.
| ICD-10 code | Disease | Total |
|---|---|---|
| N18 | Chronic kidney disease | 133 |
| K21 | Gastro-oesophageal reflux disease | 117 |
| I25 | Chronic ischaemic heart disease | 113 |
| E11 | Type 2 diabetes mellitus | 97 |
| K29 | Gastritis and duodenitis | 83 |
| E13 | Other specified diabetes mellitus | 71 |
| I48 | Atrial fibrillation and flutter | 63 |
| K59 | Other functional intestinal disorders | 62 |
| M1A | Chronic gout | 60 |
| J30 | Vasomotor and allergic rhinitis | 59 |
| E03 | Other hypothyroidism | 57 |
| G47 | Sleep disorders | 52 |
| H35 | Other retinal disorders | 50 |
| I50 | Heart failure | 50 |
| H04 | Disorders of lacrimal system | 49 |
| E87 | Other disorders of fluid, electrolyte, and acid–base balance | 44 |
| M54 | Dorsalgia | 44 |
| I10 | Essential (primary) hypertension | 43 |
| E78 | Disorders of lipoprotein metabolism and other lipidaemias | 40 |
| J06 | Acute upper respiratory infections of multiple and unspecified sites | 39 |
| Omitted 578 items below | 3462 | |
ICD-10 International classification of diseases 10th revision.
Discussion
We found an association between allopurinol use and an increased TSH level using a CDM based on the EMRs of 19,200,973 patients in the distributed databases of seven hospitals. All medications and diseases considered confounding variables showed significant differences between the case and control groups. The adjusted OR for the risk between increased TSH and allopurinol use in each hospital, after an aggregated meta-analysis, was 1.51 (95% CI; 1.32–1.72). Regarding the additional analysis of patients who used allopurinol, the mean maximum TSH level was 12.52 mIU/L and the highest level was 16.92 mIU/L at hospital G. Since free T4 levels were all within the normal reference range even after the case group index date (that is, when the TSH level increased), the possibility of subclinical hypothyroidism (SCH) could be suspected38,39.
Serum TSH measurement is the single most reliable test to diagnose all common forms of hypothyroidism and hyperthyroidism40. The best way to initially test thyroid function is to measure TSH levels in a blood sample41. Most laboratories report a normal TSH reference range of 0.4–0.5 mIU/L on the lower end and 4–5.5 mIU/L on the upper end of the range42,43. Primary hypothyroidism is manifested by elevated serum TSH with low serum FT4 levels. In SCH, although FT4 levels were within the normal reference range, there were elevations in TSH44.
Our findings are consistent with those of Perez-Ruiz et al.7 and Faisal et al.8. These studies showed that allopurinol use affected TSH levels and was associated with the onset of SCH. We identified diseases that occurred after the case group’s index date based on the ICD-10 codes for patients who used allopurinol. They were CKD, GERD, chronic IHD, T2DM, gastritis, and duodenitis, among others. These diseases have a high prevalence in patients with SCH or hypothyroidism, as reported by previous studies. These findings accorded with those of previous studies of associations between SCH or hypothyroidism and CKD45–48, GERD49,50, IHD51–54 and T2DM55,56.
Other studies have suggested that SCH is associated with an increased risk of coronary heart disease (CHD) events. In particular, CHD mortality rates increased in those with higher TSH levels, particularly in those with TSH levels of 10 mIU/L or greater52,57,58. Treatment might be needed for patients with SCH and serum TSH levels of 10 mIU/L38.
Our study has several limitations. This observational study showed associations, but it was unable to determine causality. Traditional medical record reviews provide detailed clinical information, whereas the database of the CDM deidentifies personal information to protect patient privacy. Additional information was not available in this study59. Due to the lack of laboratory results, we could not consider the thyroid autoimmune reaction and iodine status as covariates in this study. It is important to consider these in the study design60,61. Laboratory tests for a thyroid autoimmune reaction detect the presence and measure the number of specific thyroid autoantibodies in the blood. Therefore, if laboratory values are out of normal range in a patient who uses the allopurinol, we can suspect that allopurinol causes autoimmune diseases. Although the analyses accounted for a wide range of potential confounding variables, there is potential for residual confounding and indication biases in observational studies. Also a retrospective study, it is important to standardize and confirm the laboratory items using a laboratory study because the study is related to the reliability of the inspection items and results. Therefore, a further study that reflects these considerations is required.
To date, the understanding of pathogenetic effects and mechanisms of allopurinol on TSH limited. An experimental study on rats revealed decreases of both triiodothyronine and thyroxine levels in thyrotoxicosis rats receiving allopurinol compared with untreated thyrotoxicosis rats, and the authors suggested an association between allopurinol and the biosynthesis of thyroid hormones6. However, this theory has been not proven in clinical studies. Only a few reports suggested possible mechanisms in human studies. Faisal et al. guessed that allopurinol use might affect the enlarged thyroid gland, change echogenicity, and increase blood supply with nodule formation8. On the other hand, Perez-Ruiz et al. assumed that changes in TSH might be directly associated with inhibition of xanthine oxidase because a higher dose had a greater impact on TSH in their study7.
Nevertheless, we provided evidence for the safety of the medication using multicentre CDM data. Though allopurinol labels have recently changed in Korea based on previous research and approval from other countries, there has been a lack of relevant large-scale analysis to date.
SCH implies an absence of symptoms; however, it is perhaps better thought of as mild hypothyroidism62. Moreover, even mild hypothyroidism can progress to overt hypothyroidism63,64. Hypothyroidism is permanent in most patients and therefore requires hormone replacement over a lifetime65. Therefore, it is essential to provide evidence for the safety of the medication.
The risk of an increased TSH level was significantly higher in the allopurinol use group than in the allopurinol non-use group based on a CDM built using a large-scale hospital EMR, which is highly efficient. Additional analysis confirmed the potential for the development of SCH. TSH is a sensitive indicator, which detects changes in thyroid function. Therefore, we suggest that patients using allopurinol pay attention and observe changes in thyroid function. Thyroid function changes should be monitored because related diseases do not occur immediately. Continuous follow-up is necessary, even if no specific clinical symptoms appear immediately.
Supplementary Information
Acknowledgements
This project was funded by a grant from the Korea Institute of Drug Safety & Risk Management in 2020. Additional funding was supported by the Technology Innovation Program (20004927, Upgrade of CDM-based Distributed Biohealth Data Platform and Development of Verification Technology) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea).
Author contributions
W.C.: study design, manuscript draft and revision, data interpretation, Y.S.Y. and H.M.K: statistical analysis, data interpretation, D.J.C.: study design, revision of the manuscript and data acquisition, Y.W.C.: study design and data acquisition, S.Y.: revision manuscript and data acquisition, S.J.K., J.S.O., D.Y.K., H.J.Y.: data acquisition, I.Y.C.: study design, data acquisition and supervision. All authors reviewed and confirmed the manuscript.
Data availability
The data that support the findings of this study are available from each hospital. Analyses were performed locally, and the patient-level data are not readily available to be shared. The analytic code is, however, available at: https://github.com/WonaChoi/Allopurinol-TSH.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-98954-1.
References
- 1.Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat. Rev. Rheumatol. 2015;11:649–662. doi: 10.1038/nrrheum.2015.91. [DOI] [PubMed] [Google Scholar]
- 2.Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: Prevalence, incidence, treatment patterns, and risk factors. Nat. Rev. Rheumatol. 2020;16:380–390. doi: 10.1038/s41584-020-0441-1. [DOI] [PubMed] [Google Scholar]
- 3.Kim JW, et al. Prevalence and incidence of gout in Korea: Data from the national health claims database 2007–2015. Rheumatol. Int. 2017;37:1499–1506. doi: 10.1007/s00296-017-3768-4. [DOI] [PubMed] [Google Scholar]
- 4.Song JS. Recent advances in management of gout. J. Korean Med. Assoc. 2016;59:379–384. doi: 10.5124/jkma.2016.59.5.379. [DOI] [Google Scholar]
- 5.FitzGerald JD, et al. 2020 American College of Rheumatology Guideline for the Management of Gout. Arthritis Care Res. 2020;72:744–760. doi: 10.1002/acr.24180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makay O, et al. The role of allopurinol on oxidative stress in experimental hyperthyroidism. J. Endocrinol. Invest. 2009;32:641–646. doi: 10.1007/BF03345734. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Ruiz F, et al. Increase in thyroid stimulating hormone levels in patients with gout treated with inhibitors of xanthine oxidoreductase. Rheumatol. Int. 2015;35:1857–1861. doi: 10.1007/s00296-015-3355-5. [DOI] [PubMed] [Google Scholar]
- 8.Faisal IM, Merkhan MM, Almukhtar HM. Effect of chronic allopurinol therapy on thyroid function in patients with urate stones. J. Adv. Pharm. Educ. Res. 2020;10:2–5. [Google Scholar]
- 9.European Medicines Agency (EMA). EPACHMP Assessment Report for Adenuric. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000777/WC500021812.pdf (2008).
- 10.European Medicine Agency. Allopurinol: CMDh Scientific conclusions grounds variation amendments product information timetable.www.ema.europa.eu/en/documents/psusa/allopurinol-cmdh-scientific-conclusions-grounds-variation-amendments-product-information-timetable/00000095/201612_en.pdf (2017).
- 11.UK Medicines EMC. Allopurinol label. www.medicines.org.uk/emc/product/11195/smpc (2021).
- 12.Italy Torrinomedica. Product information Allopurinol. www.torrinomedica.it/schede-farmaci/allopurinolo_doc_generici_100_mg_compressa_50_cp_in_blister_pvc_pvdc_al/ (2020).
- 13.France Public Medication Database. Summary of product characteristics—ALLOPURINOL. http://base-donnees-publique.medicaments.gouv.fr/affichageDoc.php?specid=62674422&typedoc=R (2019).
- 14.AbZ Pharma. Product information of Allopurinol in Germany. https://www.abz.de/arzneimittel/details/praeparate/praeparatedaten/detail/pzn-1014636.html (2018).
- 15.Swissmedic. Product information_ALLOPURINOL.https://www.swissmedicinfo.ch/?Lang=EN (2017).
- 16.Korea Ministry of Food and Medication Safety. Allopurinol medication safety-related labeling change in Korea. https://nedrug.mfds.go.kr/pbp/CCBAQ03/getItem?totalPages=1262&limit=10&searchYn=true&page=1&title=알로푸리놀&&bbsYn=Y&orderNo=&bbscttNo=4039 (2020).
- 17.Reisinger SJ, et al. Development and evaluation of a common data model enabling active medication safety surveillance using disparate healthcare databases. J. Am. Med. Informatics Assoc. 2010;17:652–662. doi: 10.1136/jamia.2009.002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou X, et al. An evaluation of the THIN database in the OMOP common data model for active medication safety surveillance. Medicat. Saf. 2013;36:119–134. doi: 10.1007/s40264-012-0009-3. [DOI] [PubMed] [Google Scholar]
- 19.Korea Institute of Drug Safety and Risk Management. MOA CDM. https://moa.drugsafe.or.kr/ (2020).
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic in longitudinal studies: Development. J. Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Kim KH. Comorbidity adjustment in health insurance claim database. Health Policy Manag. 2016;26:71–78. doi: 10.4332/KJHPA.2016.26.1.71. [DOI] [Google Scholar]
- 22.Quan H, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011;173:676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 23.Thygesen SK, Christiansen CF, Lash TL, Christensen S, Sørensen HT. Predictive value of coding of diagnoses in the Charlson comorbidity index in the Danish national registry of patients. BMC Med. Res. Methodol. 2011;11:2–7. doi: 10.1186/1471-2288-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jostel A, Ryder WDJ, Shalet SM. The use of thyroid function tests in the diagnosis of hypopituitarism: Definition and evaluation of the TSH Index. Clin. Endocrinol. (Oxf.) 2009;71:529–534. doi: 10.1111/j.1365-2265.2009.03534.x. [DOI] [PubMed] [Google Scholar]
- 25.Fisher DA, Nelson JC, Carlton EI, Wilcox RB. Maturation of human hypothalamic-pituitary-thyroid function and control. Thyroid. 2000;10:229–234. doi: 10.1089/thy.2000.10.229. [DOI] [PubMed] [Google Scholar]
- 26.Hamnvik OPR, Larsen PR, Marqusee E. Thyroid dysfunction from antineoplastic agents. J. Natl. Cancer Inst. 2011;103:1572–1587. doi: 10.1093/jnci/djr373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee E, Chen P, Rao H, Lee J, Burmelster LA. Effect of acute high dose dobutamine administration on serum thyrotrophin (TSH) Clin. Endocrinol. (Oxf.) 1999;50:487–492. doi: 10.1046/j.1365-2265.1999.00678.x. [DOI] [PubMed] [Google Scholar]
- 28.Trip MD, Wiersinga W, Plomp TA. Incidence, predictability, and pathogenesis of amiodarone-induced thyrotoxicosis and hypothyroidism. Am J Med. 1991;91:507–511. doi: 10.1016/0002-9343(91)90187-3. [DOI] [PubMed] [Google Scholar]
- 29.Lazarus JH. The effects of lithium therapy on thyroid and thyrotropin-releasing hormone. Thyroid. 1998;8:909–913. doi: 10.1089/thy.1998.8.909. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Gao J, Fan Q, Li H, Di Y. The effect of metformin on thyroid-associated serum hormone levels and physiological indexes: A meta-analysis. Curr. Pharm. Des. 2019;25:3257–3265. doi: 10.2174/1381612825666190918162649. [DOI] [PubMed] [Google Scholar]
- 31.Cooper DS, Klibanski A, Ridgway EC. Dopaminergic modulation of TSH and its subunits: In vivo and in vitro studies. Clin. Endocrinol. (Oxf.) 1983;18:265–275. doi: 10.1111/j.1365-2265.1983.tb03211.x. [DOI] [PubMed] [Google Scholar]
- 32.Harris MD, Siegel LB, Alloway JA. Gout and hyperuricemia. Am. Fam. Physician. 1999;59:925. [PubMed] [Google Scholar]
- 33.Eskes SA, Wiersinga WM. Amiodarone and thyroid. Best Pract. Res. Clin. Endocrinol. Metab. 2009;23:735–751. doi: 10.1016/j.beem.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Torino F, Corsello SM, Longo R, Barnabei A, Gasparini G. Hypothyroidism related to tyrosine kinase inhibitors: An emerging toxic effect of targeted therapy. Nat. Rev. Clin. Oncol. 2009;6:219–228. doi: 10.1038/nrclinonc.2009.4. [DOI] [PubMed] [Google Scholar]
- 35.Emmerson BT. The management of gout. N. Engl. J. Med. 1996;334:445–451. doi: 10.1056/NEJM199602153340707. [DOI] [PubMed] [Google Scholar]
- 36.Grosser T, Smyth E, FitzGerald GA. Anti-inflammatory, Antipyretic, and Analgesic Agents; Pharmacotherapy of Gout. McGraw-Hill Companies; 2011. [Google Scholar]
- 37.Kuo CL, Duan Y, Grady J. Unconditional or conditional logistic regression model for age-matched case–control data? Front. Public Heal. 2018;6:57. doi: 10.3389/fpubh.2018.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biondi B, Cappola AR, Cooper DS. Subclinical hypothyroidism: A review. JAMA. 2019;322:153–160. doi: 10.1001/jama.2019.9052. [DOI] [PubMed] [Google Scholar]
- 39.Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379:1142–1154. doi: 10.1016/S0140-6736(11)60276-6. [DOI] [PubMed] [Google Scholar]
- 40.Ladenson PW, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch. Intern. Med. 2000;160:1573–1575. doi: 10.1001/archinte.160.11.1573. [DOI] [PubMed] [Google Scholar]
- 41.American Thyroid Association. Thyroid function tests. www.thyroid.org/thyroid-function-tests/ (2019).
- 42.Henze M, Brown SJ, Hadlow NC, Walsh JP. Rationalizing thyroid function testing: Which TSH cutoffs are optimal for testing free T4? J Clin Endocrinol Metab. 2017;102:4235–4241. doi: 10.1210/jc.2017-01322. [DOI] [PubMed] [Google Scholar]
- 43.Woodmansee, W. W. Clinical thyroidology for the public determination of optimal TSH ranges for reflex free T4 testing. American Thyroid Association Clinical Thyroidology for the Public. https://www.thyroid.org/patient-thyroid-information/ct-for-patients/february-2018/vol-11-issue-2-p-3-4/ (2018).
- 44.Almandoz JP, Gharib H. Hypothyroidism: Etiology, diagnosis, and management. Med. Clin. North Am. 2012;96:203–221. doi: 10.1016/j.mcna.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Kim EO, et al. Unresolved subclinical hypothyroidism is independently associated with progression of chronic kidney disease. Int. J. Med. Sci. 2014;11:52–59. doi: 10.7150/ijms.7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.den Hollander JG, Wulkan RW, Mantel MJ, Berghout A. Correlation between severity of thyroid dysfunction and renal function. Clin. Endocrinol. (Oxf.) 2005;62:423–427. doi: 10.1111/j.1365-2265.2005.02236.x. [DOI] [PubMed] [Google Scholar]
- 47.Iglesias P, Díez JJ. Thyroid dysfunction and kidney disease. Eur. J. Endocrinol. 2009;160:503–515. doi: 10.1530/EJE-08-0837. [DOI] [PubMed] [Google Scholar]
- 48.Baumgartner C, et al. Thyroid function within the normal range, subclinical hypothyroidism, and the risk of atrial fibrillation. Circulation. 2017;136:2100–2116. doi: 10.1161/CIRCULATIONAHA.117.028753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akyüz F, Soyer ÖM. Which diseases are risk factors for developing gastroesophageal reflux disease? Turk. J. Gastroenterol. 2017;28:S44–447. doi: 10.5152/tjg.2017.12. [DOI] [PubMed] [Google Scholar]
- 50.Hamdan A, Jabbour J, Dowli A, El Dahouk I, Azar ST. Prevalence of laryngopharyngeal reflux disease in patients diagnosed with hypothyroidism. Acta Endocrinol. (Cph.) 2012;8:239–248. [Google Scholar]
- 51.Imaizumi M, et al. Risk for ischemic heart disease and all-cause mortality in subclinical hypothyroidism. J. Clin. Endocrinol. Metab. 2004;89:3365–3370. doi: 10.1210/jc.2003-031089. [DOI] [PubMed] [Google Scholar]
- 52.Floriani C, Gencer B, Collet TH, Rodondi N. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur. Heart J. 2018;39:503–507. doi: 10.1093/eurheartj/ehx050. [DOI] [PubMed] [Google Scholar]
- 53.Rodondi N, et al. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch. Intern. Med. 2005;165:2460–2466. doi: 10.1001/archinte.165.21.2460. [DOI] [PubMed] [Google Scholar]
- 54.Rodondi N, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365–1374. doi: 10.1001/jama.2010.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han C, et al. Subclinical hypothyroidism and type 2 diabetes: A systematic review and meta-analysis. PLoS ONE. 2015;10:1–22. doi: 10.1371/journal.pone.0135233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gronich N, et al. Hypothyroidism is a risk factor for new-onset diabetes: A cohort study. Diabetes Care. 2015;38:1657–1664. doi: 10.2337/dc14-2515. [DOI] [PubMed] [Google Scholar]
- 57.Gencer B, et al. Subclinical thyroid dysfunction and the risk of heart failure events an individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126:1040–1049. doi: 10.1161/CIRCULATIONAHA.112.096024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nanchen D, et al. Subclinical thyroid dysfunction and the risk of heart failure in older persons at high cardiovascular risk. J. Clin. Endocrinol. Metab. 2012;97:852–861. doi: 10.1210/jc.2011-1978. [DOI] [PubMed] [Google Scholar]
- 59.Choi SA, et al. Analysis of antiseizure drug-related adverse reactions from the electronic health record using the common data model. Epilepsia. 2020;61:610–616. doi: 10.1111/epi.16472. [DOI] [PubMed] [Google Scholar]
- 60.Vanderpump MP, Tunbridge WM. Epidemiology and prevention of clinical and subclinical hypothyroidism. Thyroid. 2002;10:839–847. doi: 10.1089/105072502761016458. [DOI] [PubMed] [Google Scholar]
- 61.Zimmermann MB, Boelaert K. Iodine deficiency and thyroid disorders. Lancet. Diabetes Endocrinol. 2015;4:286–295. doi: 10.1016/S2213-8587(14)70225-6. [DOI] [PubMed] [Google Scholar]
- 62.Croker EE, McGrath SA, Rowe CW. Thyroid disease using diagnostic tools effectively. Thyroid. 2021;50:16–21. doi: 10.31128/AJGP-10-20-5693. [DOI] [PubMed] [Google Scholar]
- 63.Vanderpump MPJ. The epidemiology of thyroid disease. Br. Med. Bull. 2011;99:39–51. doi: 10.1093/bmb/ldr030. [DOI] [PubMed] [Google Scholar]
- 64.Rosenthal MJ, Hunt WC, Garry PJ, Goodwin JS. Thyroid failure in the elderly: Microsomal antibodies as discriminant for therapy. JAMA. 1987;258:209–213. doi: 10.1001/jama.1987.03400020051029. [DOI] [PubMed] [Google Scholar]
- 65.Park KH, Lee EJ. Recent review on medical treatment of thyroid disease. J. Korean Med. Assoc. 2012;55:1207–1214. doi: 10.5124/jkma.2012.55.12.1207. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from each hospital. Analyses were performed locally, and the patient-level data are not readily available to be shared. The analytic code is, however, available at: https://github.com/WonaChoi/Allopurinol-TSH.