Abstract
Of the several microsporidia that infect humans, Enterocytozoon bieneusi is known to cause a gastrointestinal disease whereas Encephalitozoon intestinalis causes both a disseminated and an intestinal disease. Although several different staining techniques, including the chromotrope technique and its modifications, Uvitex 2B, and the quick-hot Gram-chromotrope procedure, detect microsporidian spores in fecal smears and other clinical samples, they do not identify the species of microsporidia. A need for an easily performed test therefore exists. We reevaluated 120 stool samples that had been found positive for microsporidia previously, using the quick-hot Gram-chromotrope technique, and segregated them into two groups on the basis of spore size. We also screened the smears by immunofluorescence microscopy, using a polyclonal rabbit anti-E. intestinalis serum at a dilution of 1:400. Spores in 29 (24.1%) of the 120 samples fluoresced brightly, indicating that they were E. intestinalis spores. No intense background or cross-reactivity with bacteria, yeasts, or other structures in the stool samples was seen. Additionally, the numbers of spores that fluoresced in seven of these samples were substantially smaller than the numbers of spores that were present in the stained smears, indicating that these samples were probably derived from patients with mixed infections of Enterocytozoon bieneusi and E. intestinalis. Because a 1:400 dilution of this serum does not react with culture-grown Encephalitozoon hellem, Encephalitozoon cuniculi, or Vittaforma corneae or with Enterocytozoon bieneusi spores in feces, we concluded that an immunofluorescence test using this serum is a good alternative for the specific identification of E. intestinalis infections.
Microsporidia are ancient, spore-forming, mitochondrion-lacking protozoa that are known to infect patients with AIDS (8, 32, 44). Of the more than 1,000 species and as many as 100 genera of microsporidia, only 11 species included under 7 genera (i.e., Brachiola vesicularum, Encephalitozoon cuniculi, Encephalitozoon hellem, Encephalitozoon intestinalis, Enterocytozoon bieneusi, Nosema connori, Nosema ocularum, Pleistophora sp., Trachipleistophora hominis, Trachipleistophora anthropophthera, and Vittaforma corneae) are known to cause infections in humans (7, 32, 36, 39, 44). Enterocytozoon bieneusi is the most frequently identified microsporidial pathogen in fecal specimens from patients with AIDS. Recently, however, it has also been found in respiratory samples from two patients (14). E. intestinalis is the second most frequently identified microsporidial pathogen in clinical specimens, including stool samples from AIDS patients (32). According to some reports, gastrointestinal disease caused by microsporidia accounts for 30% of diarrhea in patients with AIDS (32, 44). Although the chromotrope technique of Weber et al. (43) and modifications thereof (23, 31), chemofluorescent agents such as Uvitex 2B (37), and the recently developed quick-hot Gram-chromotrope procedure (27) detect microsporidian spores in fecal smears and other clinical samples, they do not identify the species of microsporidia. Polyclonal and monoclonal antibodies (MAbs) have also been developed to identify microsporidian spores in patient specimens, including fecal samples (1, 2, 10, 19, 30, 33, 35, 40–42, 46, 47). However, with the exception of an MAb to E. hellem (10, 41), none of these reagents can specifically identify particular microsporidia. Definitive species identification can be accomplished by electron microscopy (EM) (6, 8, 32, 44) or DNA-based techniques (9, 11–13, 15, 17, 20, 21, 29, 40, 41), which are not readily available to many clinical laboratories. There exists, therefore, a need for a simple, easily performed, and reliable test to identify E. intestinalis and Enterocytozoon bieneusi, the two agents that have been found in fecal specimens from patients with diarrhea. We have reported previously the development of a polyclonal rabbit antiserum to E. intestinalis that identifies, based on an indirect immunofluorescence (IIF) technique, spores of E. intestinalis in Formalin-fixed smears of feces, urine, saliva, and nasal secretions of a patient infected with E. intestinalis (40). We therefore screened a large number of Formalin-fixed fecal samples, which had been found to be positive for microsporidia by the chromotrope stain, with this antiserum in the IIF test to identify the number and percentage of specimens that were positive for E. intestinalis. Our goal was to develop a diagnostic reagent for the routine identification of E. intestinalis in microsporidian-positive clinical specimens, including Formalin-fixed stool samples, that would be based on the use of this serum. Our additional objectives were (i) to identify cross-reactions, if any, with culture-derived microsporidia, including E. cuniculi, E. hellem, E. intestinalis, and V. corneae, or Enterocytozoon bieneusi spores in Formalin-fixed fecal specimens; (ii) to determine whether it is possible to specifically identify E. intestinalis spores in fecal samples that have been fixed in Formalin for long periods of time; and (iii) to determine the percentage of microsporidian-positive stool samples that harbor E. intestinalis spores. The results of our investigations are reported here.
MATERIALS AND METHODS
Fecal samples.
From 1991 to 1994 we monitored a cohort of 602 patients in Atlanta who were infected with human immunodeficiency virus to determine the burden of disease associated with enteric parasites (28). Forty-four (7.3%) of these patients were found to have an intestinal infection with microsporidia on at least one occasion. After the initial diagnostic tests on these patients were completed, 35 had sufficient stool specimen remaining to allow us to perform additional tests in an attempt to identify the particular species of microsporidian. This report is based on an analysis of 120 specimens from these 35 patients. These samples had been stored at room temperature (24°C) for about 3 to 5 years. Thin smears were made from each of these unconcentrated samples, and the specimens were retested for the presence of microsporidian spores by the quick-hot Gram-chromotrope technique (27).
Since two sizes of spores were visualized during a cursory microscopic examination of the stained smears, we selected 39 stained samples for accurate measurements of the spores by using a computerized image analyzer as well as a micrometer. At least 20 to 30 spores were measured separately by two individuals (H.M. and G.S.V.) in order to accurately determine the spore size.
EM.
Small aliquots of 10 of these stool samples were also processed for EM. The stool samples were first washed three times by centrifugation in phosphate-buffered saline, and then the pellets were resuspended in 2.5% glutaraldehyde buffered with 0.2 M cacodylate and allowed to stand for 3 h. The samples were then postfixed with a 1% solution of OsO4, dehydrated in ethanol, embedded in Epon 812, and processed as described previously (40–42).
Reference isolates.
E. cuniculi (CDC:V282) (13), E. hellem (CDC:0291:V213) (41), E. intestinalis (CDC:V297) (40), and V. corneae (34) were grown in monkey kidney (E6) cell cultures as described previously. Spores harvested from each of these cultures were also processed for IIF as described previously (40–42).
IIF.
IIF was performed as described previously (40–42). In brief, 10 μl of washed spores (5 × 106 per ml) from a culture was pipetted onto each well of 12-well coated slides; the slides were allowed to dry and stored at −20°C until used. Additionally, 5-μl aliquots of each of these 120 fecal specimens were pipetted onto individual wells of coated slides and allowed to dry. An IIF test was then performed with the anti-E. intestinalis (CDC:V297) serum at a 1:400 dilution and with anti-rabbit immunoglobulin G conjugated with fluorescein isothiocyanate as described previously (40). Evan’s blue was included as a counterstain. Prior to screening the stool smears with the anti-E. intestinalis (CDC:V297) serum, we titrated the serum by using several dilutions, starting with 1:100 and ending with 1:3,200, against cell culture-derived spores of E. cuniculi, E. hellem, E. intestinalis, and V. corneae along with a stool sample that was previously found to be positive for E. intestinalis by EM (40) as well as a stool sample that had been identified as positive for Enterocytozoon bieneusi by PCR (11).
RESULTS
Quick-hot Gram-chromotrope staining.
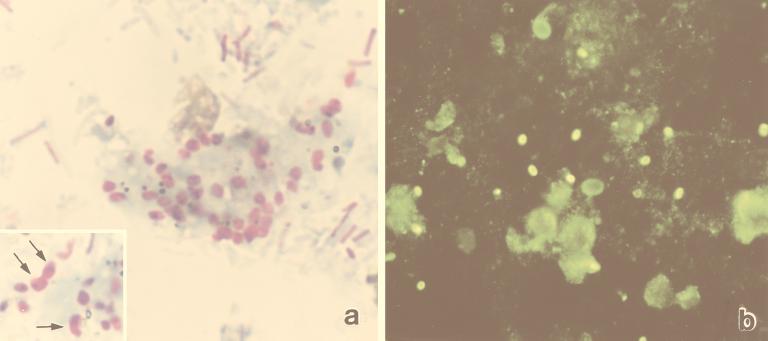
Microsporidian spores were detected in smears made from all of the 120 fecal samples that were also positive earlier. The spores stained dark violet and exhibited at least one of the characteristic features of a spore, i.e., an equatorial belt-like stripe, a vacuole, and/or the presence of gram-positive granules (Fig. 1a).
FIG. 1.
(a) Fecal smear stained by the Gram-chromotrope procedure. Note the two sizes of spores in the inset (larger spores at arrows). Magnification, ×1,250. (b) Spores in the fecal smear reacted with the anti-E. intestinalis serum at a 1:400 dilution and exhibited bright fluorescence. Note the nonspecific reaction of diplococcus-like and fungal organisms in the smear that can be easily distinguished from the specific fluorescence patterns of the brightly fluorescing spores. Magnification, ×500.
The average diameter of the spores in 11 (IIF negative) of the 39 samples that were selected for measurement was 1.43 μm (range, 0.87 to 1.98 μm). In 21 of the samples that were IIF positive, however, the spores had an average measurement of 1.59 μm (range, 0.95 to 3 μm); a few, however, measured around 1 μm (Fig. 1a). In the remaining seven samples suspected to contain both Enterocytozoon bieneusi and E. intestinalis, the spores had an average measurement of 1.56 μm (range, 0.80 to 2.56 μm).
IIF.
To ascertain the cross-reactivity of the anti-E. intestinalis serum with spores of E. intestinalis and Enterocytozoon bieneusi in stool smears and with spores of culture-grown microsporidia (E. cuniculi, E. hellem, E. intestinalis, and V. corneae), we allowed the spores of different microsporidia to react with different dilutions of the serum, starting with the 1:100 dilution. Spores of V. corneae and Enterocytozoon bieneusi showed no fluorescence even at a serum dilution of 1:100. Since spores of E. cuniculi and E. hellem showed some cross-reactivity at the lower dilutions (Table 1), we elected to use a 1:400 dilution to screen fecal samples.
TABLE 1.
Reactivities of the different dilutions of the anti-E. intestinalis serum with spores of selected microsporidial species
| Microsporidial species | Reactivity with anti-E. intestinalis serum at a dilution ofa:
|
|||||
|---|---|---|---|---|---|---|
| 1:100 | 1:200 | 1:400 | 1:800 | 1:1,600 | 1:3,200 | |
| E. intestinalis | 4+ | 4+ | 3+ | 2+ | + | − |
| E. cuniculi | + | ± | − | − | − | − |
| E. hellem | + | ± | − | − | − | − |
| V. corneae | − | − | − | − | − | − |
| Enterocytozoon bieneusi | − | − | − | − | − | − |
4+, highly reactive; 3+, moderately reactive; 2+, somewhat reactive; +, slightly reactive; ±, possible slight reactivity; −, unreactive.
In the IIF assay, spores in 29 (24.1%) of 120 smears reacted with the anti-E. intestinalis serum and produced bright fluorescence (Fig. 1b), indicating that they were E. intestinalis spores. Although in some samples diplococcus-like bacterial spores and fungal organisms exhibited dull fluorescence, they could very easily be distinguished from the microsporidian spores with the bright fluorescence (Fig. 1b). The numbers of spores that fluoresced in 7 of the 29 samples were relatively low compared with those in the corresponding Gram-chromotrope-stained smears of these samples, indicating that these specimens may have contained two different microsporidia, namely E. intestinalis and Enterocytozoon bieneusi.
The 35 patients each had between 1 and 16 stool specimens that were positive for microsporidia. Twenty-one (60.0%) of the patients were infected only with Enterocytozoon bieneusi, and four (11.4%) were infected only with E. intestinalis. Six patients had at least one stool specimen with both E. intestinalis and Enterocytozoon bieneusi, and four patients had E. intestinalis on at least one occasion and Enterocytozoon bieneusi on at least one separate occasion.
EM.
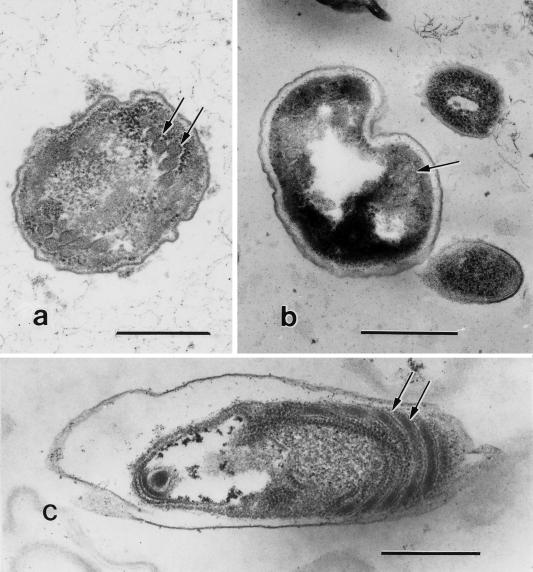
Among the 10 samples that were examined by EM, microsporidia characteristic of Enterocytozoon bieneusi (Fig. 2a and b) were observed in only five, none of which was positive in the IIF assay; 3 samples, all of which were positive in the IIF assay, had spores characteristic of E. intestinalis (Fig. 2c), while 2 samples, both positive in the IIF assay, had spores of both Enterocytozoon bieneusi and E. intestinalis. E. intestinalis spores, however, were in small numbers, as visualized by the IIF assay. Enterocytozoon bieneusi spores could be easily identified on the basis of their smaller size (∼1 μm) and double rows of polar tube coils.
FIG. 2.
(a) Electron micrograph of a fecal sample showing an Enterocytozoon bieneusi sporoblast with polar tube coils in double rows (at arrows); bar = 500 nm. (b) A mature spore of Enterocytozoon bieneusi with the characteristic double turns of the polar tubule (at arrow); bar = 500 nm. (c) Electron micrograph of a fecal sample showing an E. intestinalis spore. Note the polar tubule coils (arrows). Bar = 500 nm.
DISCUSSION
Identification of an agent of microsporidiosis in general, and gastroenteral microsporidiosis in particular, to the species level is important, since infections with E. intestinalis can be successfully treated with albendazole whereas those caused by Enterocytozoon bieneusi may be refractory to albendazole therapy (32, 44). Identification to the species level, however, can be difficult and require time-consuming and specialized techniques, including EM and PCR, which not only are tedious and time-consuming but also are currently available only in a few laboratories. It is therefore advantageous to develop species-specific serologic reagents that can be used for the identification of the species that causes the disease so that specific therapy can be instituted.
According to several reports, both polyclonal and monoclonal reagents have been developed to identify microsporidia in fecal specimens; none of them, however, can unequivocally identify microsporidia to the species level, since cross-reactivity of these reagents with other microsporidia of clinical importance has been demonstrated (1, 19, 46, 47). According to a recent report, two MAbs identified (for up to only 6 weeks) E. intestinalis spores in fresh stool specimens that were refrigerated but not in routinely Formalin-fixed stool samples (2). Another recent report, which discusses an anti-E. intestinalis antibody produced in BALB/c mice, states that the antibody can identify E. intestinalis spores in fresh stool samples fixed in ice-cold acetone but not in Formalin-fixed specimens (30).
We have also reported previously on the development of a polyclonal anti-E. intestinalis (CDC:V297) serum that can be used to identify E. intestinalis spores in clinical specimens, including Formalin-fixed stool samples (40). Further, by using the CDC:V297 serum and flow cytometry, we have recently shown that both Formalin-treated and untreated spores of E. cuniculi, E. hellem, and E. intestinalis could be clearly identified to the species level by analyzing the gated data on light-scatter profiles and fluorescence histograms (26). In the present investigation, we examined the specificity of the serum by reacting it with spores of E. hellem, E. cuniculi, E. intestinalis, and V. corneae obtained from in vitro culture as well as with spores in two Formalin-fixed stool samples (which served as positive and negative controls) as well as 120 Formalin-fixed stool samples that had been stored at room temperature for 3 to 5 years. The results of these experiments clearly indicated that this rabbit anti-E. intestinalis serum, at a dilution of 1:400, specifically identified E. intestinalis spores from cell culture and in some of the Formalin-fixed stool specimens and produced bright apple green fluorescence. It did not cross-react with E. cuniculi, E. hellem, or V. corneae from culture or with Enterocytozoon bieneusi from stool specimens (35). These results clearly indicate that E. intestinalis spores can be identified even in those samples fixed in formalin and stored at room temperature for up to 5 years. Hence, an antibody like this, if commercially available, would be very useful for screening microsporidian-positive samples for the specific identification of E. intestinalis spores even when they are present in small numbers.
In a previous study, based on EM and PCR of intestinal biopsy samples from patients with diarrhea, an overall rate of 44.1% infection with microsporidia (36.8% with Enterocytozoon bieneusi and 7.3% with E. intestinalis) was recorded (9). In another study, based on PCR of stool samples, a 10% incidence of E. intestinalis was found (24). According to a third study, based on a PCR assay, the prevalence of infection with E. intestinalis is probably higher than previously reported, because of latent infection (20). It is interesting that in the present study, spores in 29 (24.1%) of the 120 fecal specimens tested reacted intensely with the anti-E. intestinalis serum and produced a bright fluorescence. Since it was not possible to perform EM on all of the 120 specimens because of time, personnel, and cost constraints, we elected to carry out EM on 10 selected samples. The EM results for these 10 specimens confirmed that 5 of these samples that were negative in the IIF assay had only spores characteristic of Enterocytozoon bieneusi, 3 samples that were positive in the IIF test had only spores characteristic of the genus Encephalitozoon, and the 2 samples that were positive in the IIF test had spores that were characteristic of both Enterocytozoon bieneusi and Encephalitozoon spp. The number of spores that fluoresced positively with the anti-E. intestinalis serum was, however, small compared with the total number of spores in the stained smears. These results, albeit based on limited comparisons, indicate that the reagent is specific and sensitive.
Microscopic examination of the stained smears of these 29 fecal samples revealed that a majority of spores appeared to be 1.59 μm in diameter (range, 0.95 to 3 μm), which is comparable to the size of E. intestinalis spores (2, 6, 8, 16, 18, 30, 32, 38, 40, 42). On reevaluation, however, 7 of these 29 stool samples appeared to contain spores of two different sizes, namely ∼1 μm and ≥2 μm. Enterocytozoon bieneusi spores are known to be ∼1 μm in diameter, whereas those of E. intestinalis measure ∼2 μm or greater; further, the number of spores that fluoresced was considerably smaller than that in the Gram-chromotrope-stained smears. Based on these two observations, we concluded that these samples were positive for both E. intestinalis and Enterocytozoon bieneusi spores, indicating that the persons from whom the samples were obtained had mixed infections. Mixed infections with E. intestinalis and Enterocytozoon bieneusi have been reported previously (4, 38).
It is well known that E. cuniculi infects a variety of animals, including humans (8, 13, 15, 17, 22, 32, 44, 45). Enterocytozoon bieneusi was recently identified for the first time in fecal specimens from domestic pigs (15) and in simian immunodeficiency virus-inoculated macaques with hepatobiliary disease (25). E. hellem has also been identified in birds (budgerigars) for the first time (3). Further, we have recently identified spores of E. intestinalis in Formalin-fixed fecal specimens of domestic animals (i.e., pigs, a dog, a cow, a goat, and a donkey) by several techniques, including EM, PCR, and IIF (5). For the IIF assay, we used our anti-E. intestinalis serum at a 1:400 dilution as discussed here. The EM and, more importantly, the PCR data agreed with the immunofluorescence results (5). These reports therefore underscore the urgent need for the development of a simple, reliable, noninvasive technique for the identification of these microsporidia in fecal and environmental samples. Our study results are a first step in the development of species-specific reagents that can identify E. intestinalis in fecal specimens and should provide impetus for others to develop simple and reliable species-specific reagents to identify other microsporidia in stool samples as well as in the environment. Additionally, species-specific reagents would be invaluable not only in the diagnosis of microsporidiosis, so that interdiction through chemotherapeutic agents would be possible, but also in the identification of specific agents in animal and environmental samples and epidemiologic investigations.
ACKNOWLEDGMENTS
H. Moura and F. C. Sodre were recipients of scholarships from Conselho Nacional de Desenvolvimento Cientifico e Tecnologico, CNPq, Brazil. G. J. Leitch was supported in part by Public Health Service grant RR03034. F. J. Bornay-Llinares was supported in part by a grant from the Sociedad Española de Microbiología Cliníca y Enfermedades Infecciosas (SEIMC)-Bayer 1996.
REFERENCES
- 1.Aldras A M, Orenstein J O, Kotler D P, Shadduck J A, Didier E S. Detection of microsporidia by indirect immunofluorescence antibody test using polyclonal and monoclonal antibodies. J Clin Microbiol. 1994;32:608–612. doi: 10.1128/jcm.32.3.608-612.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckers P J A, Derks G J M M, van Gool T, Rietveld F J R, Sauerwein R W. Encephalitozoon intestinalis-specific monoclonal antibodies for laboratory diagnosis of microsporidiosis. J Clin Microbiol. 1996;34:282–285. doi: 10.1128/jcm.34.2.282-285.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black S S, Steinhort L A, Bertucci D C, Rogers L B, Didier E S. Encephalitozoon hellem in budgerigars. Vet Pathol. 1997;34:189–198. doi: 10.1177/030098589703400303. [DOI] [PubMed] [Google Scholar]
- 4.Blanshard B, Hollister W S, Peacock D G, Tovey D G, Ellis D S, Canning E U, Gazzard B G. Simultaneous infection with two types of intestinal microsporidia in a patient with AIDS. Gut. 1992;33:418–420. doi: 10.1136/gut.33.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bornay-Llinares F J, Da Silva A J, Moura H, Schwartz D A, Visvesvara G S, Pieniazek N J, Cruz-Lopez A, Hernandez-Jauregui P, Guerrero J, Enriquez F J. Immunologic, microscopic, and molecular evidence of Encephalitozoon intestinalis (Septata intestinalis) infection in mammals other than humans. J Infect Dis. 1998;178:820–826. doi: 10.1086/515356. [DOI] [PubMed] [Google Scholar]
- 6.Cali A, Kotler D P, Orenstein J M. Septata intestinalis, n. g., n. sp., an intestinal microsporidian associated with chronic diarrhea and dissemination in AIDS patients. J Protozool. 1992;40:101–112. doi: 10.1111/j.1550-7408.1993.tb04889.x. [DOI] [PubMed] [Google Scholar]
- 7.Cali A, Takvorian P M, Lewin S, Rendel M, Sian C S, Wittner M, Tanowitz H B, Keohane E, Weiss L M. Brachiola vesicularum, n. g., n. sp., a new microsporidium associated with AIDS and myositis. J Eukaryot Microbiol. 1998;45:240–251. doi: 10.1111/j.1550-7408.1998.tb04532.x. [DOI] [PubMed] [Google Scholar]
- 8.Canning E U, Lom J, Dykova I. The microsporidia of vertebrates. New York, N.Y: Academic Press, Inc.; 1986. [Google Scholar]
- 9.Coyle C M, Wittner M, Kotler D P, Noyer C, Orenstein J M, Tanowitz H B, Weiss L M. Prevalence of microsporidiosis due to Enterocytozoon bieneusi and Encephalitozoon (Septata) intestinalis among patients with AIDS-related diarrhea: determination by polymerase chain reaction to the microsporidian small-subunit rRNA gene. Clin Infect Dis. 1996;23:1002–1006. doi: 10.1093/clinids/23.5.1002. [DOI] [PubMed] [Google Scholar]
- 10.Croppo G P, Leitch G J, Wallace S, Visvesvara G S. Immunofluorescence and Western blot analysis of microsporidia using anti-Encephalitozoon hellem immunoglobulin G monoclonal antibodies. J Eukaryot Microbiol. 1994;41:31S. [PubMed] [Google Scholar]
- 11.da Silva A J, Schwartz D A, Visvesvara G S, de Moura H, Slemenda S B, Pieniazek N J. Sensitive PCR diagnosis of infections by Enterocytozoon bieneusi (Microsporidia) using primers based on the region coding for small-subunit rRNA. J Clin Microbiol. 1996;34:986–987. doi: 10.1128/jcm.34.4.986-987.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Da Silva A J, Slemenda S B, Visvesvara G S, Schwartz D A, Wilcox C M, Wallace S, Pieniazek N J. Detection of Septata intestinalis (Microsporidia) Cali et al. 1993 using polymerase chain reaction primers targeting the small subunit ribosomal RNA coding region. Mol Diagn. 1997;2:47–52. doi: 10.1054/MODI00200047. [DOI] [PubMed] [Google Scholar]
- 13.DeGroote M A, Visvesvara G S, Wilson M L, Pieniazek N J, Slemenda S B, Da Silva A J, Leitch G J, Bryan R T, Reves R. Polymerase chain reaction and culture confirmation of disseminated Encephalitozoon cuniculi in a patient with AIDS: successful therapy with albendazole. J Infect Dis. 1995;171:1375–1378. doi: 10.1093/infdis/171.5.1375. [DOI] [PubMed] [Google Scholar]
- 14.del Aguila C, Lopez-Velez R, Fenoy S, Turrientes C, Cobo J, Navajas R, Visvesvara G S, Croppo G P, Da Silva A J, Pieniazek N J. Identification of Enterocytozoon bieneusi spores in respiratory samples from an AIDS patient with a 2-year history of intestinal microsporidiosis. J Clin Microbiol. 1997;35:1862–1866. doi: 10.1128/jcm.35.7.1862-1866.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deplazes P, Mathis A, Muller C, Weber R. Molecular epidemiology of Encephalitozoon cuniculi and first detection of Enterocytozoon bieneusi in fecal samples of pigs. J Eukaryot Microbiol. 1996;43:93S. doi: 10.1111/j.1550-7408.1996.tb05018.x. [DOI] [PubMed] [Google Scholar]
- 16.Didier E S, Rogers L B, Orenstein J M, Baker M D, Vossbrinck C R, van Gool T, Hartskeerl R, Soave R, Beaudet L M. Characterization of Encephalitozoon (Septata) intestinalis isolates cultured from nasal mucosa and bronchoalveolar lavage fluids of two AIDS patients. J Eukaryot Microbiol. 1996;43:34–43. doi: 10.1111/j.1550-7408.1996.tb02470.x. [DOI] [PubMed] [Google Scholar]
- 17.Didier E S, Vossbrinck C R, Baker M D, Rogers L B, Bertucci D C, Shadduck J A. Identification and characterization of three Encephalitozoon cuniculi strains. Parasitology. 1995;111:411–422. doi: 10.1017/s0031182000065914. [DOI] [PubMed] [Google Scholar]
- 18.Doultree J C, Maerz A L, Ryan N J, Baird R W, Wright E, Crowe S M, Marshall J A. In vitro growth of the microsporidian Septata intestinalis from an AIDS patient with disseminated illness. J Clin Microbiol. 1995;33:463–470. doi: 10.1128/jcm.33.2.463-470.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enriquez F J, Ditrich O, Palting J D, Smith K. Simple diagnosis of Encephalitozoon sp. microsporidial infections by using a panspecific antiexospore monoclonal antibody. J Clin Microbiol. 1997;35:724–729. doi: 10.1128/jcm.35.3.724-729.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franzen C, Küppers R, Müller A, Salzberger B, Fätkenheuer G, Vetten B, Diehl V, Schrappe M. Genetic evidence for latent Septata intestinalis infection in human immunodeficiency virus-infected patients with intestinal microsporidiosis. J Infect Dis. 1996;173:1038–1040. doi: 10.1093/infdis/173.4.1038. [DOI] [PubMed] [Google Scholar]
- 21.Hartskeerl R A, van Gool T, Schuitema A R J, Didier E S, Terpstra W J. Reclassification of the microsporidian Septata intestinalis on the basis of genetic and immunological characterization. Parasitology. 1995;110:277–285. doi: 10.1017/s0031182000080860. [DOI] [PubMed] [Google Scholar]
- 22.Hollister W S, Canning E U, Colbourn N I, Aarons E J. Encephalitozoon cuniculi isolated from the urine of an AIDS patient, which differs from canine and murine isolates. J Eukaryot Microbiol. 1995;42:367–372. doi: 10.1111/j.1550-7408.1995.tb01595.x. [DOI] [PubMed] [Google Scholar]
- 23.Kokoskin E, Gyorkos T W, Camus A, Cedilotte L, Purtill T, Ward B. Modified technique for efficient detection of microsporidia. J Clin Microbiol. 1994;32:1074–1075. doi: 10.1128/jcm.32.4.1074-1075.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liguory O, David F, Sarfati C, Schuitema A R J, Hartskeerl R A, Derouin F, Modai J, Molina J-M. Diagnosis of infections caused by Enterocytozoon bieneusi and Encephalitozoon intestinalis using polymerase chain reaction in stool specimens. AIDS. 1997;11:723–726. doi: 10.1097/00002030-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Mansfield K G, Carville A, Shvetz D, Mackey J, Tzipori S, Lackner A A. Identification of an Enterocytozoon bieneusi-like microsporidian parasite in simian-immunodeficiency-virus-inoculated macaques with hepatobiliary disease. Am J Pathol. 1997;150:1395–1405. [PMC free article] [PubMed] [Google Scholar]
- 26.Moss D M, Croppo G P, Wallace S, Visvesvara G S. Flow cytometric analysis of microsporidia belonging to the genus Encephalitozoon. J Clin Microbiol. 1999;37:371–375. doi: 10.1128/jcm.37.2.371-375.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moura H, Schwartz D A, Bornay-Llinares F, Sodré F C, Wallace S, Visvesvara G S. A new and improved “quick-hot Gram-chromotrope” technique that differentially stains microsporidian spores in clinical samples, including paraffin-embedded tissue sections. Arch Pathol Lab Med. 1997;121:888–893. [PubMed] [Google Scholar]
- 28.Navin T R, Weber R, Vugia D J, Rimland D, Roberts J M, Addiss D G, Visvesvara G S, Wahlquist S P, Hogan S E, Juranek D D, Schwartz D A, Wilcox C M, Stewart J J, Thompson III S E, Bryan R T. Declining CD4+ T lymphocyte counts are associated with increased risk of enteric parasitosis and chronic diarrhea: results of a 3-year longitudinal study. J Acquir Immune Defic Syndr. 1999;20:154–159. doi: 10.1097/00042560-199902010-00007. [DOI] [PubMed] [Google Scholar]
- 29.Ombrouck C, Ciceron L, Biligui S, Brown S, Marechal P, van Gool T, Datry A, Danis M, Desportes-Livage I. Specific PCR assay for direct detection of intestinal microsporidia Enterocytozoon bieneusi and Encephalitozoon intestinalis in fecal specimens from human immunodeficiency virus-infected patients. J Clin Microbiol. 1997;35:652–655. doi: 10.1128/jcm.35.3.652-655.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ombrouck C, Desportes-Livage I, Achbarou A, Gentilini M. Specific detection of the microsporidia Encephalitozoon intestinalis in AIDS patients. C R Acad Sci Ser III Life Sci. 1996;319:39–43. [PubMed] [Google Scholar]
- 31.Ryan N J, Sutherland G, Coughlan K, Globan M, Doultree J, Marshall J, Baird R W, Pedersen J, Dwyer B. A new trichrome-blue stain for detection of microsporidial species in urine, stool, and nasopharyngeal specimens. J Clin Microbiol. 1993;31:3264–3269. doi: 10.1128/jcm.31.12.3264-3269.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz D A, Bryan R T. Microsporidia. In: Horsburgh C R Jr, Nelson A M, editors. Pathology of emerging infections. Washington, D.C: ASM Press; 1997. pp. 61–94. [Google Scholar]
- 33.Schwartz D A, Visvesvara G S, Diesenhouse M C, Weber R, Font R L, Wilson L A, Corrent G, Rosberger D F, Keenen P C, Grossniklaus H E, Hewan-Lowe K, Bryan R T. Ocular pathology of microsporidiosis: role of immunofluorescent antibody for diagnosis of Encephalitozoon hellem in biopsies, smears, and intact globes from seven AIDS patients. Am J Ophthalmol. 1993;115:285–292. doi: 10.1016/s0002-9394(14)73577-9. [DOI] [PubMed] [Google Scholar]
- 34.Silveira H, Canning E U. Vittaforma corneae n. comb. for the human microsporidium Nosema corneum Shadduck, Meccoli, Davis and Font, 1990, based on its ultrastructure in the liver of experimentally infected athymic mice. J Eukaryot Microbiol. 1995;42:158–165. doi: 10.1111/j.1550-7408.1995.tb01557.x. [DOI] [PubMed] [Google Scholar]
- 35.Sodre F C, Moura H, Wahlquist S, Bornay-Llinares F J, Visvesvara G S. Abstracts of the 97th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1997. An immunofluorescent (IF) test detects Encephalitozoon intestinalis spores in stool samples, abstr. C-225; p. 159. [Google Scholar]
- 36.Sprague V, Becnel J J, Hazard E I. Taxonomy of phylum Microspora. Crit Rev Microbiol. 1992;18:285–395. doi: 10.3109/10408419209113519. [DOI] [PubMed] [Google Scholar]
- 37.van Gool T, Snijders F, Reiss P, Eeftinck Schattenkerk J K M, van den Bergh Weerman M A, Bartelsman J F W M, Bruins J J M, Canning E U, Dankert J. Diagnosis of intestinal and disseminated microsporidial infection in patients with HIV by a new rapid fluorescence technique. J Clin Pathol. 1993;46:694–699. doi: 10.1136/jcp.46.8.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Gool T, Canning E U, Gilis H, van den Bergh Weerman M A, Eeftinck Schattenkeirk J K M, Dankert J. Septata intestinalis frequently isolated from stool of AIDS patients with a new cultivation method. Parasitology. 1994;109:281–289. doi: 10.1017/s0031182000078318. [DOI] [PubMed] [Google Scholar]
- 39.Vavra J, Yachnis A T, Shadduck J A, Orenstein J M. Microsporidia of the genus Trachipleistophora—causative agents of human microsporidiosis: description of Trachiplesitophora anthropophthera n. sp. (Protozoa: Microsporidia) J Eukaryot Microbiol. 1998;45:273–283. doi: 10.1111/j.1550-7408.1998.tb04536.x. [DOI] [PubMed] [Google Scholar]
- 40.Visvesvara G S, da Silva A J, Croppo G P, Pieniazek N J, Leitch G J, Ferguson D, de Moura H, Wallace S, Slemenda S B, Tyrrell I, Moore D F, Meador J. In vitro culture and serologic and molecular identification of Septata intestinalis isolated from urine of a patient with AIDS. J Clin Microbiol. 1995;33:930–936. doi: 10.1128/jcm.33.4.930-936.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Visvesvara G S, Leitch G J, Da Silva A J, Croppo G P, Moura H, Wallace S, Slemenda S B, Schwartz D A, Moss D, Bryan R T, Pieniazek N J. Polyclonal and monoclonal antibody and PCR-amplified small-subunit rRNA identification of a microsporidian, Encephalitozoon hellem, isolated from an AIDS patient with disseminated infection. J Clin Microbiol. 1994;32:2760–2768. doi: 10.1128/jcm.32.11.2760-2768.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Visvesvara G S, Leitch G J, Moura H, Wallace S, Weber R, Bryan R T. Culture, electron microscopy, and immunoblot studies on a microsporidian parasite isolated from the urine of a patient with AIDS. J Protozool. 1991;38:105S–111S. [PubMed] [Google Scholar]
- 43.Weber R, Bryan R T, Owen R L, Wilcox C M, Gorelkin L, Visvesvara G S. Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates. N Engl J Med. 1992;326:161–166. doi: 10.1056/NEJM199201163260304. [DOI] [PubMed] [Google Scholar]
- 44.Weber R, Bryan R T, Schwartz D A, Owen R L. Human microsporidial infections. Clin Microbiol Rev. 1994;7:426–461. doi: 10.1128/cmr.7.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weber R, Deplazes P, Flepp M, Mathis A, Baumann R, Sauer B, Kuster H, Luthi R. Cerebral microsporidiosis due to Encephalitozoon cuniculi in a patient with human immunodeficiency virus syndrome. N Engl J Med. 1997;336:474–478. doi: 10.1056/NEJM199702133360704. [DOI] [PubMed] [Google Scholar]
- 46.Weiss L M, Cali A, Levee E, Laplace D, Tanowitz H, Simon D, Wittner M. Diagnosis of Encephalitozoon cuniculi infection by Western blot and the use of cross-reactive antigens for the possible detection of microsporidiosis in humans. Am J Trop Med Hyg. 1992;47:456–462. doi: 10.4269/ajtmh.1992.47.456. [DOI] [PubMed] [Google Scholar]
- 47.Zierdt C H, Gill V J, Zierdt W S. Detection of microsporidian spores in clinical samples by indirect fluorescent-antibody assay using whole-cell antisera to Encephalitozoon cuniculi and Encephalitozoon hellem. J Clin Microbiol. 1993;31:3071–3074. doi: 10.1128/jcm.31.11.3071-3074.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]